Enhancing Concrete Durability and Strength with Fly Ash, Steel Slag, and Rice Husk Ash for Marine Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cementitious Materials
2.2. Mortar Mixtures and Description of Tests
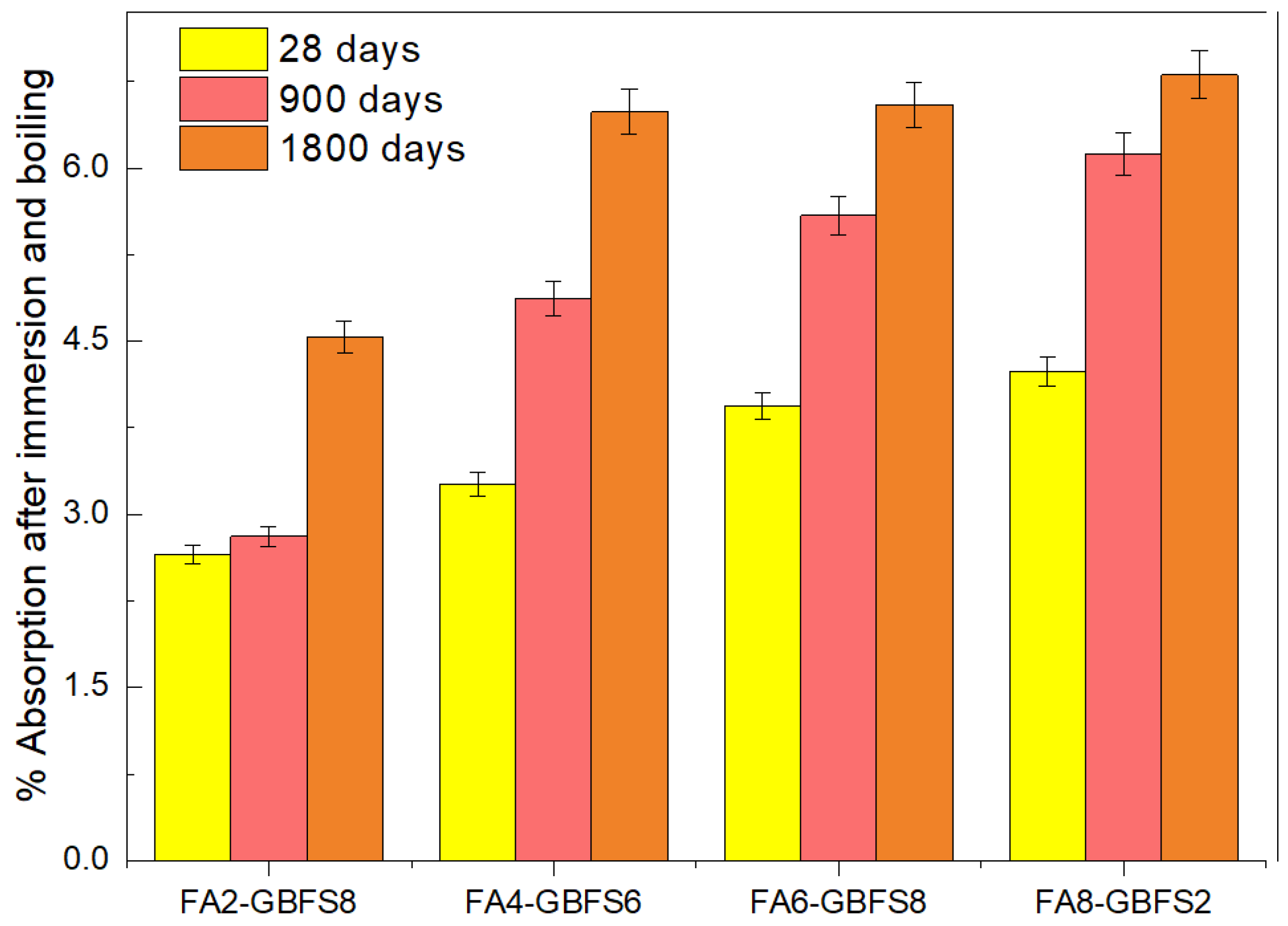
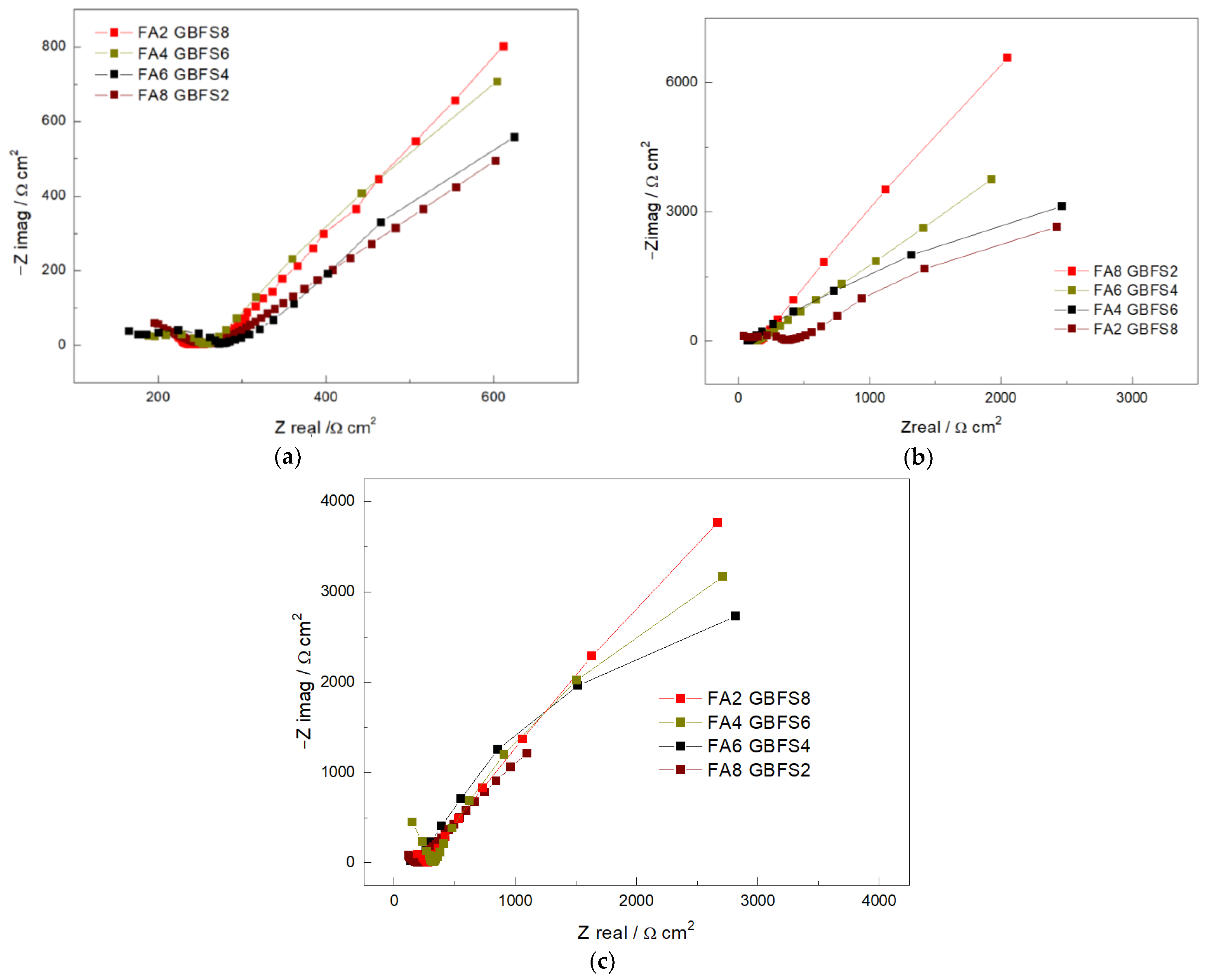
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griffiths, S.; Sovacool, B.K.; Furszyfer Del Rio, D.D.; Foley, A.M.; Bazilian, M.D.; Kim, J.; Uratani, J.M. Decarbonizing the cement and concrete industry: A systematic review of socio-technical systems, technological innovations, and policy options. Renew. Sustain. Energy Rev. 2023, 180, 113291. [Google Scholar] [CrossRef]
- Junaid, M.F.; Rehman, Z.; Kuruc, M.; Medveď, I.; Bačinskas, D.; Čurpek, J.; Čekon, M.; Ijaz, N.; Ansari, W.S. Lightweight concrete from a perspective of sustainable reuse of waste by-products. Constr. Build. Mater. 2022, 319, 126061. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, C. Circular economy in action: Case studies about the transition from the linear economy in the chemical, mining, textile, agriculture, and water treatment industries. In The Circular Economy; Sillanpää, M., Ncibi, C., Eds.; Academic Press: Helsinki, Finland, 2019; Volume 1, pp. 111–206. [Google Scholar] [CrossRef]
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Hemachandra, K. Plant fiber based bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Rissman, J.; Bataille, C.; Masanet, E.; Aden, N.; Morrow, W.R.; Zhou, N.; Elliott, N.; Dell, R.; Heeren, N.; Huckestein, B.; et al. Technologies and policies to decarbonize global industry: Review and assessment of mitigation drivers through 2070. Appl. Energy 2020, 266, 114848. [Google Scholar] [CrossRef]
- Ngab, A.S. Structural Engineering and Concrete Technology in Developing Countries: An Overview. In Structural Engineering, Mechanics and Computation; Zingoni, A., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2001; Volume 2, pp. 1339–1348. [Google Scholar] [CrossRef]
- Segura, I.P.; Ranjbar, N.; Damø, A.J.; Jensen, L.S.; Canut, M.; Jensen, P.A. A review: Alkali-activated cement and concrete production technologies available in the industry. Heliyon 2023, 9, e15718. [Google Scholar] [CrossRef]
- Jamora, J.B.; Go, A.W.; Gudia, S.E.L.; Giduquio, M.B.; Loretero, M.E. Evaluating the use of rice residue ash in cement-based industries in the Philippines—Greenhouse gas reduction, transportation, and cost assessment. J. Clean. Prod. 2023, 398, 136623. [Google Scholar] [CrossRef]
- Poojalakshmi, E.S.; Patel, J.; Sunantha, B.; Thomas, B.S.; Ramaswamy, K.P.; Khan, R.A. Effect of mechanical activation on the properties of rice husk ash-based one part geopolymer. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; Duan, H. Effect of rice husk ash on properties of slag based geopolymer pastes. J. Build. Eng. 2023, 76, 107035. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, H.; Li, H.; Liu, T.; Guo, H. Comparative study on the effects of rice husk ash and silica fume on the freezing resistance of metakaolin-based geopolymer. Constr. Build. Mater. 2021, 293, 123486. [Google Scholar] [CrossRef]
- Moutaoukil, G.; Sobrados, I.; Alehyen, S.; Taibi, M. Monitoring the Geopolymerization Reaction of Geopolymer Foams Using 29Si and 27Al MAS NMR. Minerals 2024, 14, 516. [Google Scholar] [CrossRef]
- Alouani, M.E.; Saufi, H.; Aouan, B.; Bassam, R.; Alehyen, S.; Rachdi, Y.; Hadki, H.E.; Hadki, A.E.; Mabrouki, J.; Belaaouad, S.; et al. A comprehensive review of synthesis, characterization, and applications of aluminosilicate materials-based geopolymer. Environ. Adv. 2024, 16, 100524. [Google Scholar] [CrossRef]
- Siyal, A.A.; Mohamed, R.M.S.; Shamsuddin, R.; Ridzuan, M.B. A comprehensive review of synthesis kinetics and formation mechanism of geopolymers. RSC Adv. 2024, 14, 446–462. [Google Scholar] [CrossRef]
- Li, C.; Fu, Y.; Cheng, H.; Wang, Y.; Jia, D.; Liu, H. Green and Low-Cost Modified Pisha Sandstone Geopolymer Gel Materials for Ecological Restoration: A Phase Review. Gels 2024, 10, 302. [Google Scholar] [CrossRef]
- Martínez-Martínez, S.; Bouguermouh, K.; Bouzidi, N.; Mahtout, L.; Sánchez-Soto, P.J.; Pérez-Villarejo, L. Preparation of Geopolymeric Materials from Industrial Kaolins, with Variable Kaolinite Content and Alkali Silicates Precursors. Materials 2024, 17, 1839. [Google Scholar] [CrossRef]
- Davidovits, J. Solid phase synthesis of a mineral blockpolymer by low temperature polycondensation of aluminosilicate polymers. In IUPAC International Symposium on Macromolecules, Topic III, New Polymers of High Stability; The Royal Institute of Technology: Stockholm, Sweden, 1976; pp. 145–152. [Google Scholar]
- Ali, A.; Khan, Q.; Mehboob, S.S.; Tayyab, A.; Hayyat, K.; Khan, D.; Haq, I.U.; Qureshi, Q. Enhancing multi-objective mix design for GGBS-based geopolymer concrete with natural mineral blends under ambient curing: A Taguchi-Grey relational optimization. Ain Shams Eng. J. 2024, 15, 102708. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. 17—Alkali-activated based concrete. In Woodhead Publishing Series in Civil and Structural Engineering, Eco-Efficient Concrete; Pacheco-Torgal, F., Jalali, S., Labrincha, J., John, V.M., Eds.; Woodhead Publishing: Boca Raton, FL, USA, 2013; pp. 439–487. [Google Scholar] [CrossRef]
- Antzaras, A.N.; Papalas, T.; Heracleous, E.; Kouris, C. Techno–economic and environmental assessment of CO2 capture technologies in the cement industry. J. Clean. Prod. 2023, 428, 139330. [Google Scholar] [CrossRef]
- ASTM C618-22; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASM International: West Conshohocken, PA, USA, 2022.
- ASTM C192/C192M-14; Standard Practice for Making and Curing Concrete Test Specimens in the Laboratory. ASM International: West Conshohocken, PA, USA, 2014.
- ASTM C1202-19; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. ASM International: West Conshohocken, PA, USA, 2019.
- ASTM C642-21; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASM International: West Conshohocken, PA, USA, 2021.
- Navarrete, I.; Valdes, J.; Lopez, M.; Vargas, F. Replacement of pozzolanic blended cement by supplementary cementitious materials: Mechanical and environmental approach. Constr. Build. Mater. 2023, 94, 132263. [Google Scholar] [CrossRef]
- Bonifacio, A.L.; Archbold, P. The effect of calcination conditions on oat husk ash pozzolanic activity. Mater. Today Proc. 2022, 65, 622–628. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Huang, D.; Chen, Q.; Tu, M.; Wu, K.; Zhang, Z. Pozzolanic activity and environmental risk assessment of water-based drilling cuttings of shale gas. Constr. Build. Mater. 2022, 348, 128657. [Google Scholar] [CrossRef]
- Kang, S.; Kang, H.; Lee, N.; Kwon, Y.; Moon, J. Development of cementless ultra-high performance fly ash composite (UHPFC) using nucleated pozzolanic reaction of low Ca fly ash. Cem. Concr. Compos. 2022, 132, 104650. [Google Scholar] [CrossRef]
- Patangia, J.; Saravanan, T.J.; Kabeer, K.I.; Bisht, K. Study on the utilization of red mud (bauxite waste) as a supplementary cementitious material: Pathway to attaining sustainable development goals. Constr. Build. Mater. 2023, 375, 131005. [Google Scholar] [CrossRef]
- Wang, F.; Long, G.; Zhou, J.L. Deep insight into green remediation and hazard-free disposal of electrolytic manganese residue-based cementitious material. Sci. Total Environ. 2023, 894, 165049. [Google Scholar] [CrossRef]
- Tokareva, A.; Kaassamani, S.; Waldmann, D. Fine demolition wastes as Supplementary cementitious materials for CO2 reduced cement production. Constr. Build. Mater. 2023, 392, 131991. [Google Scholar] [CrossRef]
- Song, B.; Liu, S.; Hu, X.; Ouyang, K.; Li, G.; Shi, C. Compressive strength, water and chloride transport properties of early CO2-cured Portland cement-fly ash-slag ternary mortars. Cem. Concr. Compos. 2022, 134, 104786. [Google Scholar] [CrossRef]
- Laxmi, G.; Patil, S.; Hossiney, N.; Thejas, H.K. Effect of hooked end steel fibers on strength and durability properties of ambient cured geopolymer concrete. Case Stud. Constr. Mater. 2023, 18, e02122. [Google Scholar] [CrossRef]
- Alyousef, R.; Abbass, W.; Aslam, F.; Gillani, S.A.A. Characterization of high-performance concrete using limestone powder and supplementary fillers in binary and ternary blends under different curing regimes. Case Stud. Constr. Mater. 2023, 18, e02058. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, H.; Jun, Y.; Jeong, J.H.; Oh, J.E. Microstructural verification of the strength performance of ternary blended cement systems with high volumes of fly ash and GGBFS. Constr. Build. Mater. 2015, 95, 96–107. [Google Scholar] [CrossRef]
- Lawrence, P.; Cyr, M.; Ringot, E. Mineral admixtures in mortars: Effect of inert materials on short-term hydration. Cem. Concr. Res. 2003, 33, 1939–1947. [Google Scholar] [CrossRef]
- Li, D.; Shen, J.; Chen, Y.; Cheng, L.; Wu, X. Study of properties of fly ash–slag complex cement. Cem. Concr. Res. 2000, 30, 1381–1387. [Google Scholar] [CrossRef]
- Klemczak, B.; Gołaszewski, J.; Smolana, A.; Gołaszewska, M.; Cygan, G. Shrinkage behaviour of self-compacting concrete with a high volume of fly ash and slag experimental tests and analytical assessment. Constr. Build. Mater. 2023, 400, 132608. [Google Scholar] [CrossRef]
- Nakum, A.V.; Arora, N.K. Fresh and mechanical characterization of fly ash/slag by incorporating steel fiber in self-compacted geopolymer concrete. Constr. Build. Mater. 2023, 368, 130481. [Google Scholar] [CrossRef]
- Kuranlı, Ö.F.; Uysal, M.; Abbas, M.T.; Cosgun, T.; Niş, A.; Aygörmez, Y.; Canpolat, O.; Al-mashhadani, M.M. Evaluation of slag/fly ash based geopolymer concrete with steel, polypropylene and polyamide fibers. Constr. Build. Mater. 2022, 325, 126747. [Google Scholar] [CrossRef]
- Mocharla, I.R.; Selvam, R.; Govindaraj, V.; Muthu, M. Performance and life-cycle assessment of high-volume fly ash concrete mixes containing steel slag sand. Constr. Build. Mater. 2022, 341, 127814. [Google Scholar] [CrossRef]
- Li, L.; Ye, T.; Li, Y.; Wang, Z.; Feng, Z.; Liu, Z. Improvement of microporous structure and impermeability of cement mortars using fly ash and blast furnace slag under low curing pressures. Constr. Build. Mater. 2023, 401, 132890. [Google Scholar] [CrossRef]
- Wang, X.Y.; Lee, H.S. Modeling the hydration of concrete incorporating fly ash or slag. Cem. Concr. Res. 2010, 40, 984–996. [Google Scholar] [CrossRef]
- Gesoğlu, M.; Özbay, E. Effects of mineral admixtures on fresh and hardened properties of self-compacting concretes: Binary, ternary and quaternary systems. Mater. Struct. 2007, 40, 923–937. [Google Scholar] [CrossRef]
- Aperador, W.; Mejía de Gutiérrez, R.; Bastidas, D.M. Steel corrosion behaviour in carbonated alkali-activated slag concrete. Corros. Sci. 2009, 51, 2027–2033. [Google Scholar] [CrossRef]
- Huo, Y.; Huang, J.; Lu, D.; Han, X.; Sun, H.; Liu, T.; Wang, J.; Wang, F.; Tan, P.; Wang, M.; et al. Durability of alkali-activated slag concrete incorporating silica fume and rice husk ash. J. Build. Eng. 2023, 78, 107637. [Google Scholar] [CrossRef]
- Abbass, M.; Singh, G. Durability of rice husk ash and basalt fiber based sustainable geopolymer concrete in rigid pavements. Mater. Today Proc. 2022, 61, 558–570. [Google Scholar] [CrossRef]
| Chemical Composition (wt.%) | |||
|---|---|---|---|
| Compound | FA% | GBFS% | RHA% |
| SiO2 | 54.30 | 33.70 | 90.93 |
| Al2O3 | 28.8 | 12.80 | 0.11 |
| Fe2O3 | 5.30 | 0.48 | 0.19 |
| CaO | 6.40 | 45.40 | 0.36 |
| MgO | 0.80 | 1.00 | 0.33 |
| Na2O | 0.90 | 0.12 | 0.02 |
| K2O | 0.70 | 1.50 | 1.97 |
| P2O5 | 0.70 | - | - |
| TiO2 | 1.20 | 0.50 | - |
| MnO | 0.01 | - | - |
| SO3 | 0.92 | - | 0.15 |
| SiO2/Al2O3 | 1.88 | 2.63 | - |
| Unburnt | 6.50 | - | 4.10 |
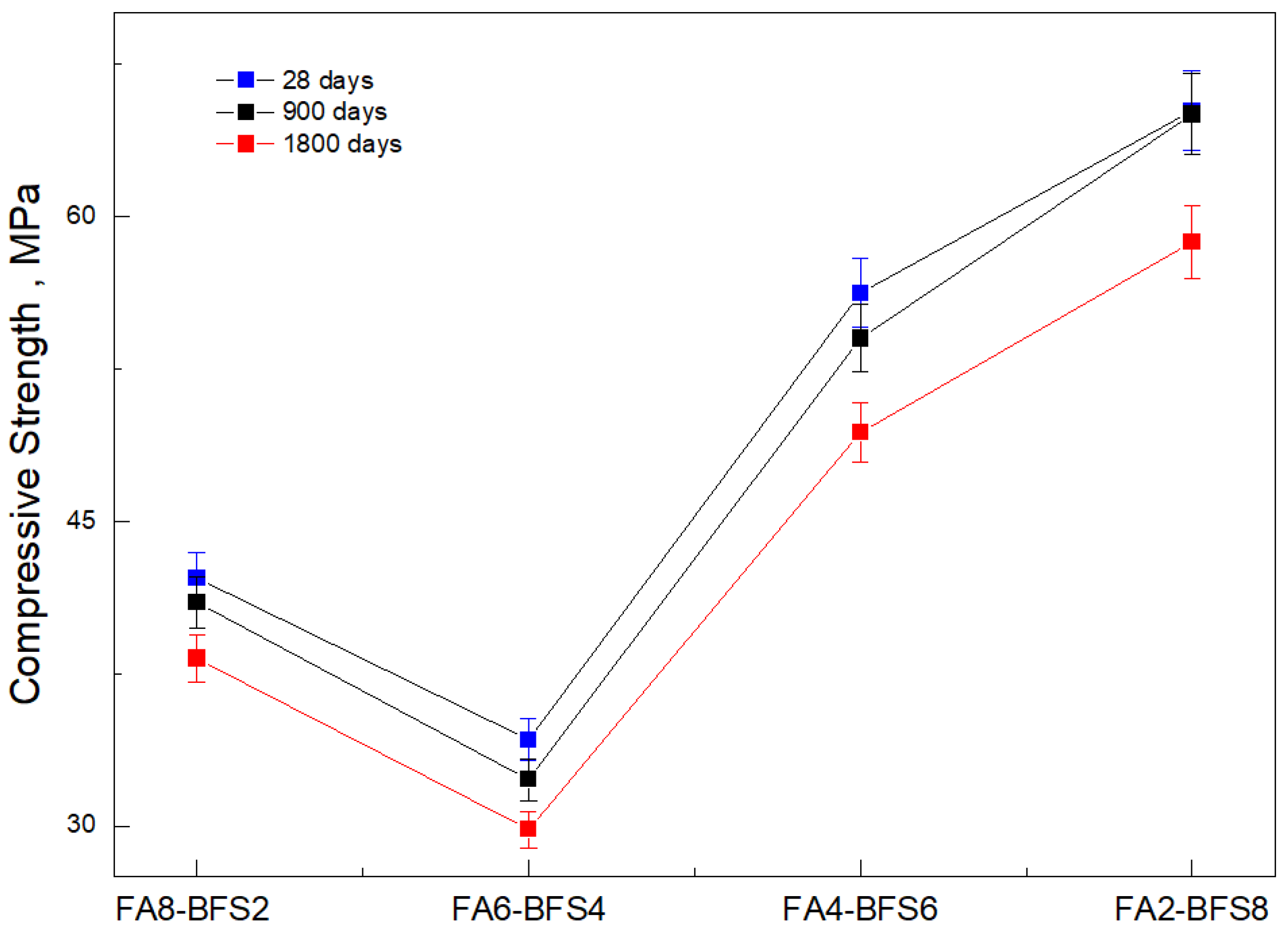
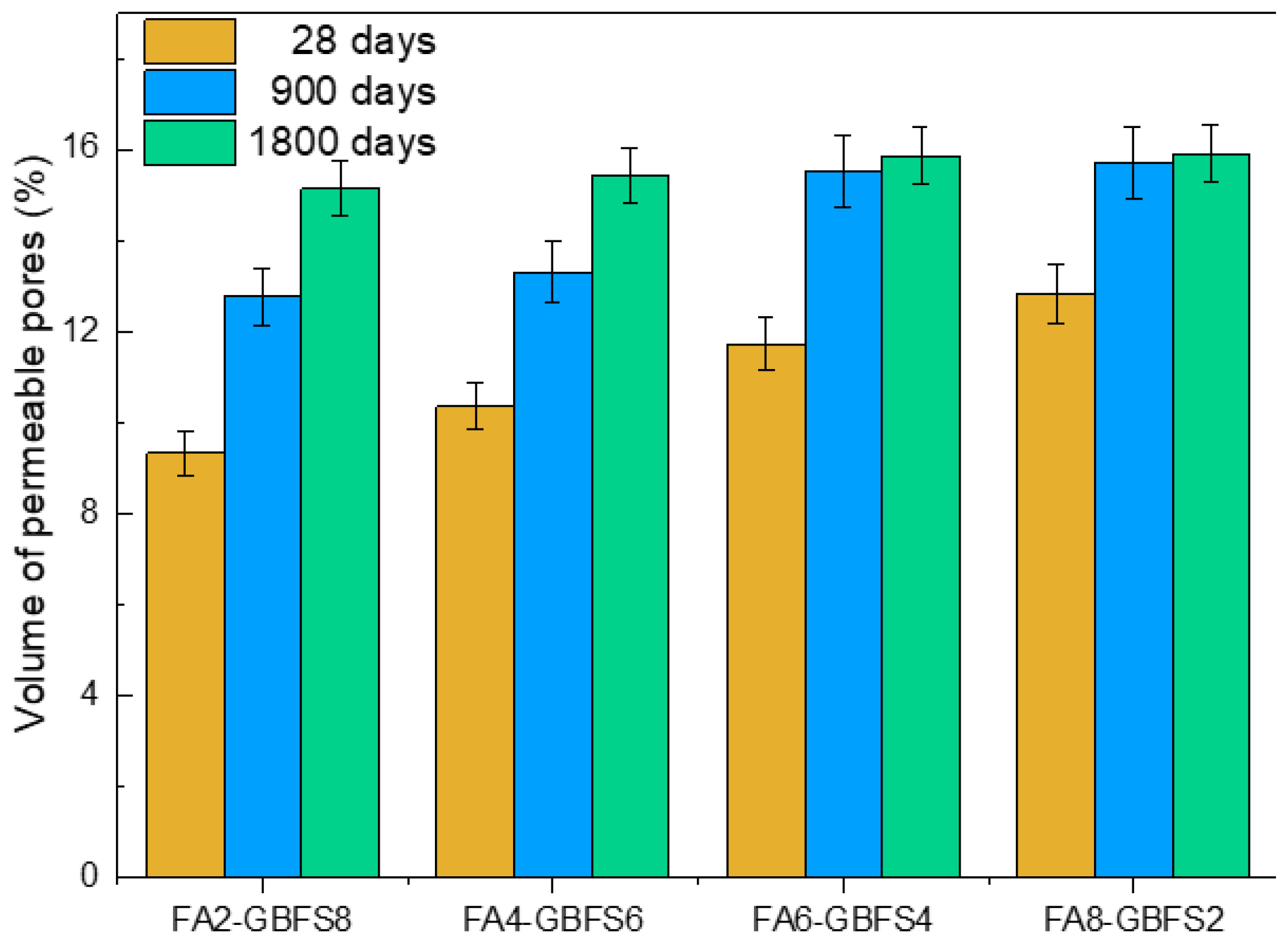
| Sample | FA (%) | GBFS (%) |
|---|---|---|
| FA8-GBFS2 | 80 | 20 |
| FA6-GBFS4 | 60 | 40 |
| FA4-GBFS6 | 40 | 60 |
| FA2-GBFS8 | 20 | 80 |
| Ratio of Activator | Sample | FA | GBFS | Fine Aggregate | RHA | Coarse Aggregates | NaOH | Water Additional |
|---|---|---|---|---|---|---|---|---|
| (kg/m3) | ||||||||
| 85% NaOH 15% RHA | FA8-GBFS2 | 360 | 90 | 675 | 37 | 950 | 210 | 1 |
| FA6-GBFS4 | 270 | 180 | 675 | 37 | 950 | 210 | 5 | |
| FA4-GBFS6 | 180 | 270 | 675 | 37 | 950 | 210 | 8 | |
| FA2-GBFS8 | 90 | 360 | 675 | 37 | 950 | 210 | 11 | |
| Molar Ratios | |||||
|---|---|---|---|---|---|
| Activator | Sample | SiO2/Al2O3 | Na2O/Al2O3 | H2O/Na2O | CaO/SiO2 |
| 85% NaOH 15% RHA | FA8-BFS2 | 4.48 | 1.84 | 4.18 | 0.22 |
| FA6-BFS4 | 4.54 | 2.00 | 4.32 | 0.43 | |
| FA4-BFS6 | 4.62 | 2.19 | 4.45 | 0.69 | |
| FA2-BFS8 | 4.70 | 2.43 | 4.47 | 0.98 | |
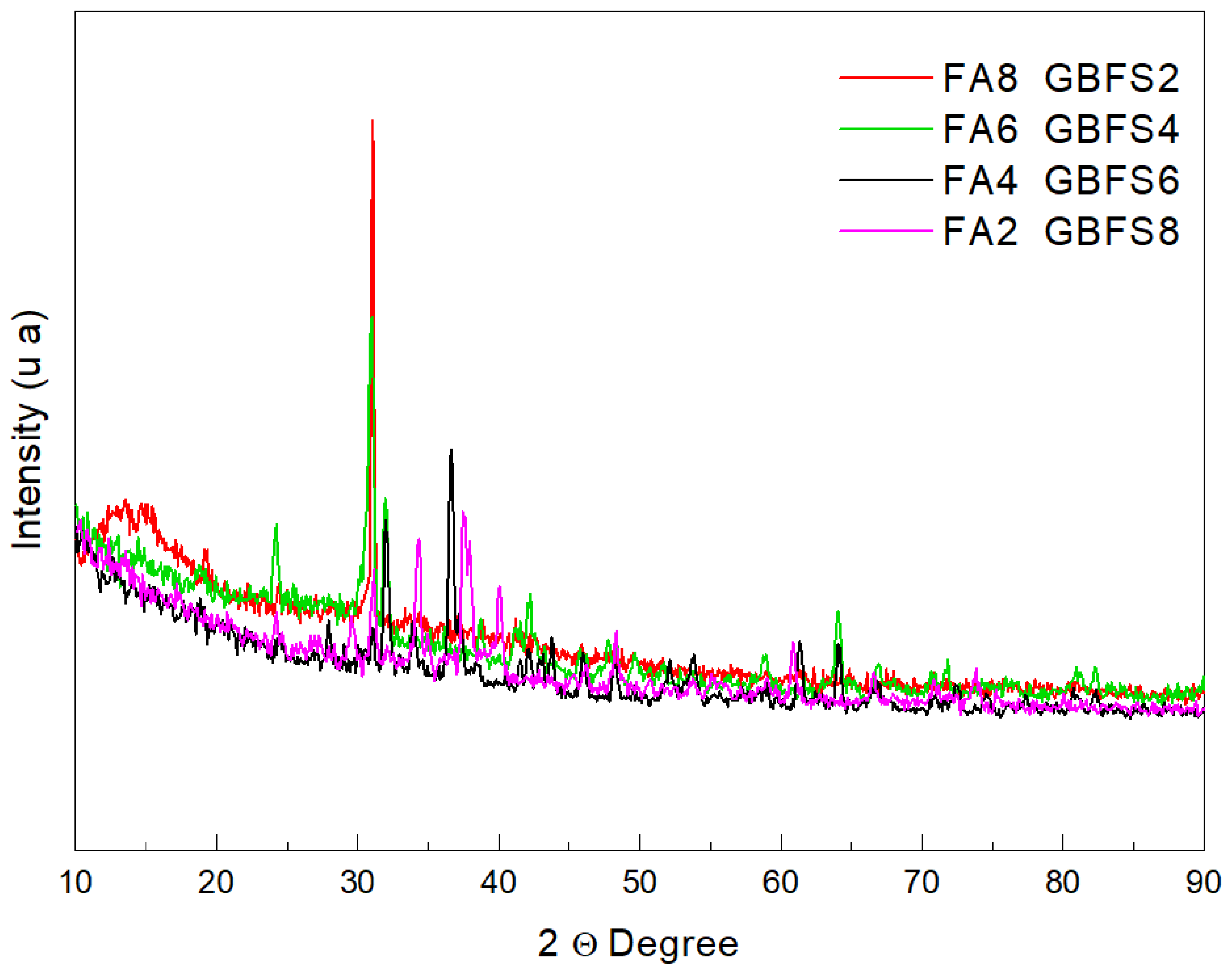
| Crystalline Phases (%) | Sample | |||
|---|---|---|---|---|
| FA8-GBFS2 | FA6-GBFS4 | FA4-GBFS6 | FA2-GBFS8 | |
| Quartz (SiO2) | 4.8 | 3.3 | 3 | 2.2 |
| Mullite (Al4O8Si) | 3.6 | 1.7 | 1.7 | 0.7 |
| Calcite (CaCO3) | 0.1 | 0.2 | 0.6 | 0.9 |
| Akermatite (Ca2MgSi2O7) | 0.1 | 0.1 | 0.3 | 0.4 |
| Graphite (C4) | 1.1 | 2.3 | 0.9 | 0.4 |
| Nosean-Carbonate (Na8Al6Si6O24-CO3) | 0.2 | 0.1 | 0.2 | 1 |
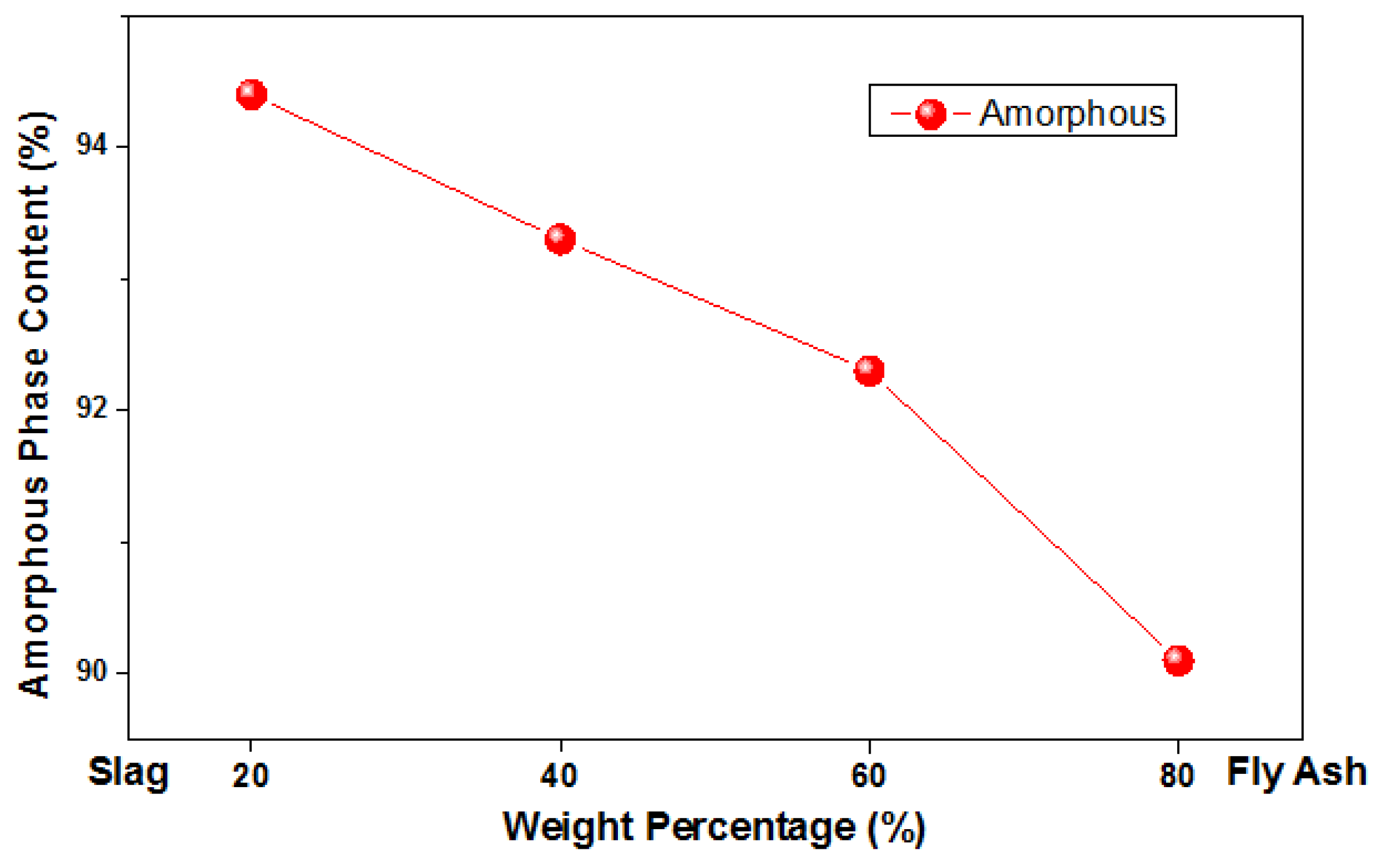
| Total Crystalline Phases (%) | 9.9 | 7.7 | 6.7 | 5.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barragán-Ramírez, R.; González-Hernández, A.; Bautista-Ruiz, J.; Ospina, M.; Aperador Chaparro, W. Enhancing Concrete Durability and Strength with Fly Ash, Steel Slag, and Rice Husk Ash for Marine Environments. Materials 2024, 17, 3001. https://doi.org/10.3390/ma17123001
Barragán-Ramírez R, González-Hernández A, Bautista-Ruiz J, Ospina M, Aperador Chaparro W. Enhancing Concrete Durability and Strength with Fly Ash, Steel Slag, and Rice Husk Ash for Marine Environments. Materials. 2024; 17(12):3001. https://doi.org/10.3390/ma17123001
Chicago/Turabian StyleBarragán-Ramírez, Rodolfo, Andrés González-Hernández, Jorge Bautista-Ruiz, Michel Ospina, and Willian Aperador Chaparro. 2024. "Enhancing Concrete Durability and Strength with Fly Ash, Steel Slag, and Rice Husk Ash for Marine Environments" Materials 17, no. 12: 3001. https://doi.org/10.3390/ma17123001






