Impact of Lime Saturation Factor on Alite-Ye’Elimite Cement Synthesis and Hydration
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
2.2.1. X-ray Diffraction and Rietveld Quantitative Analysis
2.2.2. Microscopy
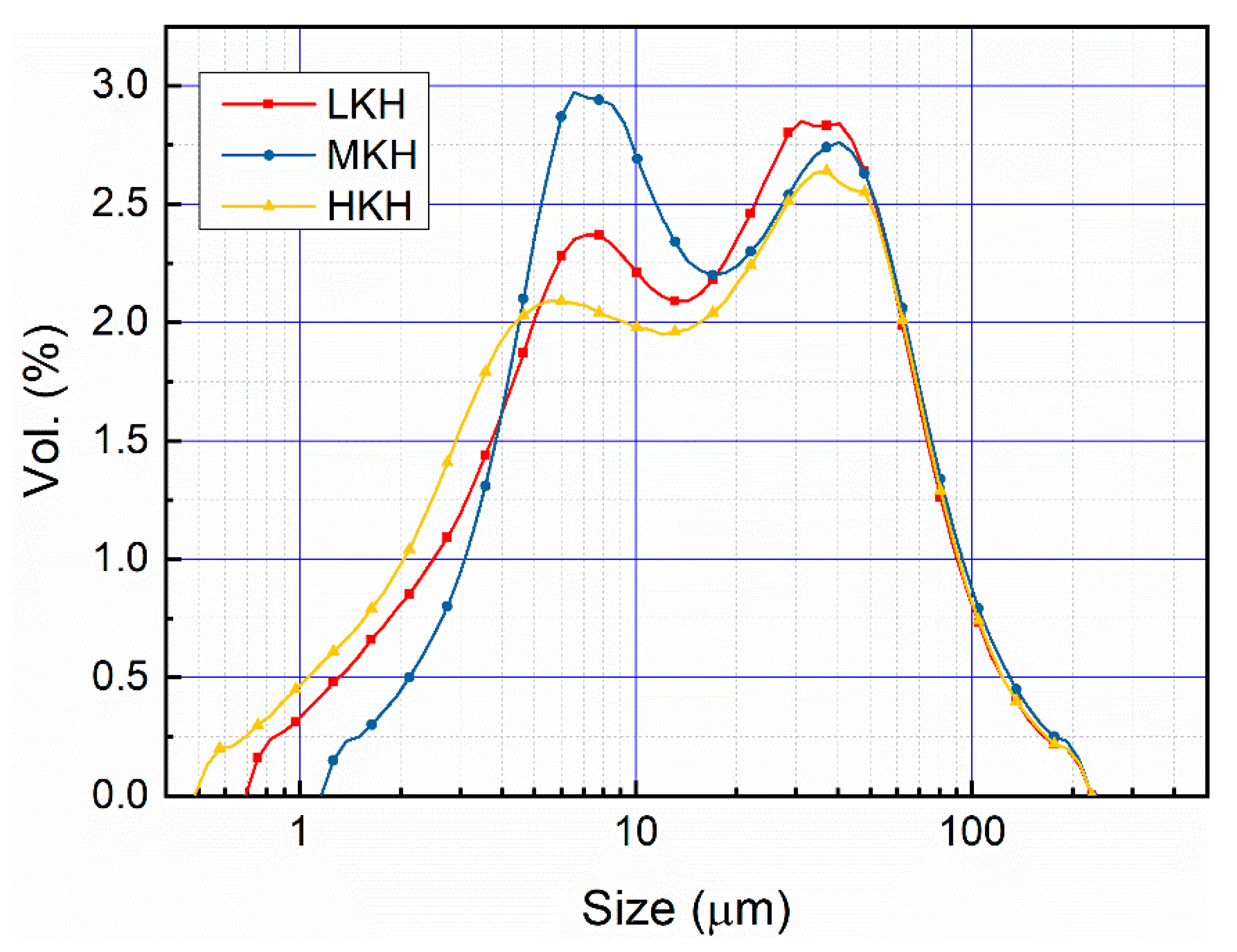
2.2.3. Particle Size
2.2.4. Compressive Strength Tests
2.2.5. Calorimetry
2.2.6. Mercury Intrusion Porosimetry (MIP)
2.2.7. Scanning Electron Microscope (SEM)
3. Results and Discussion
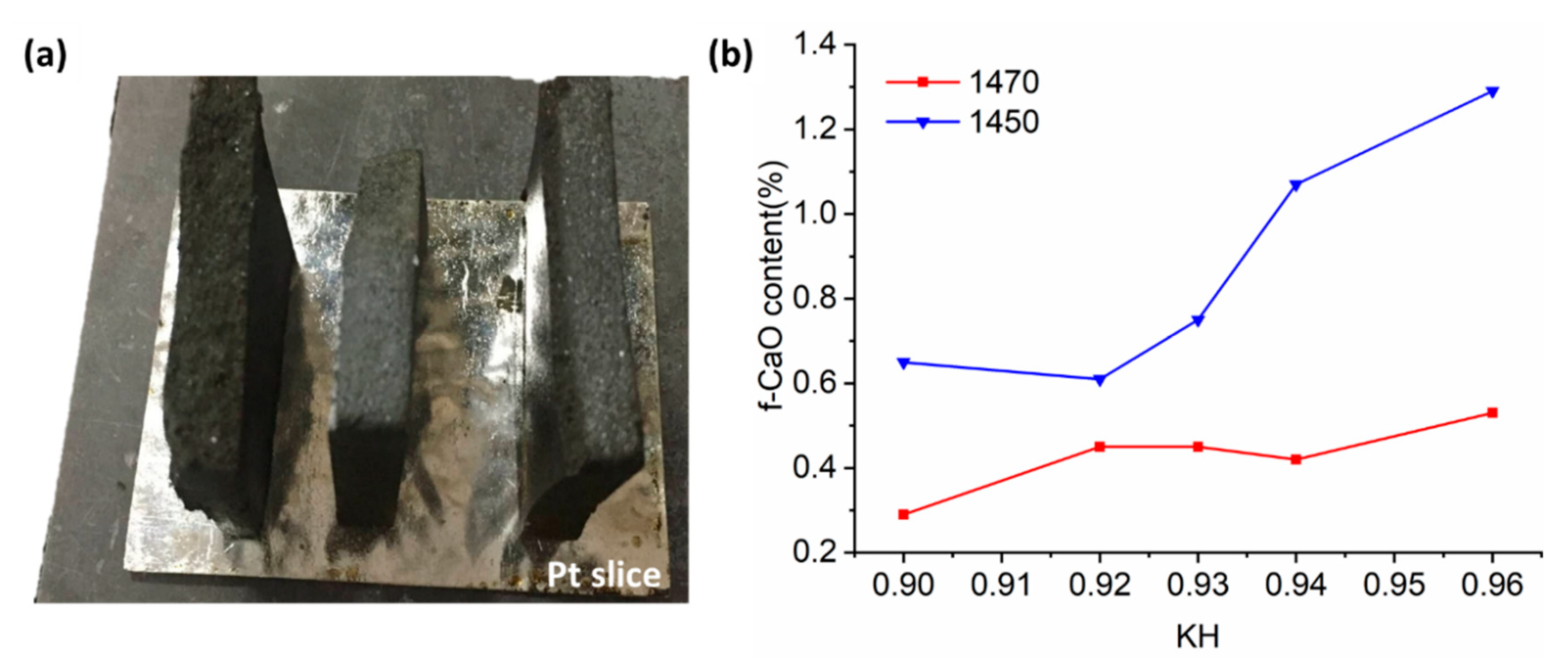
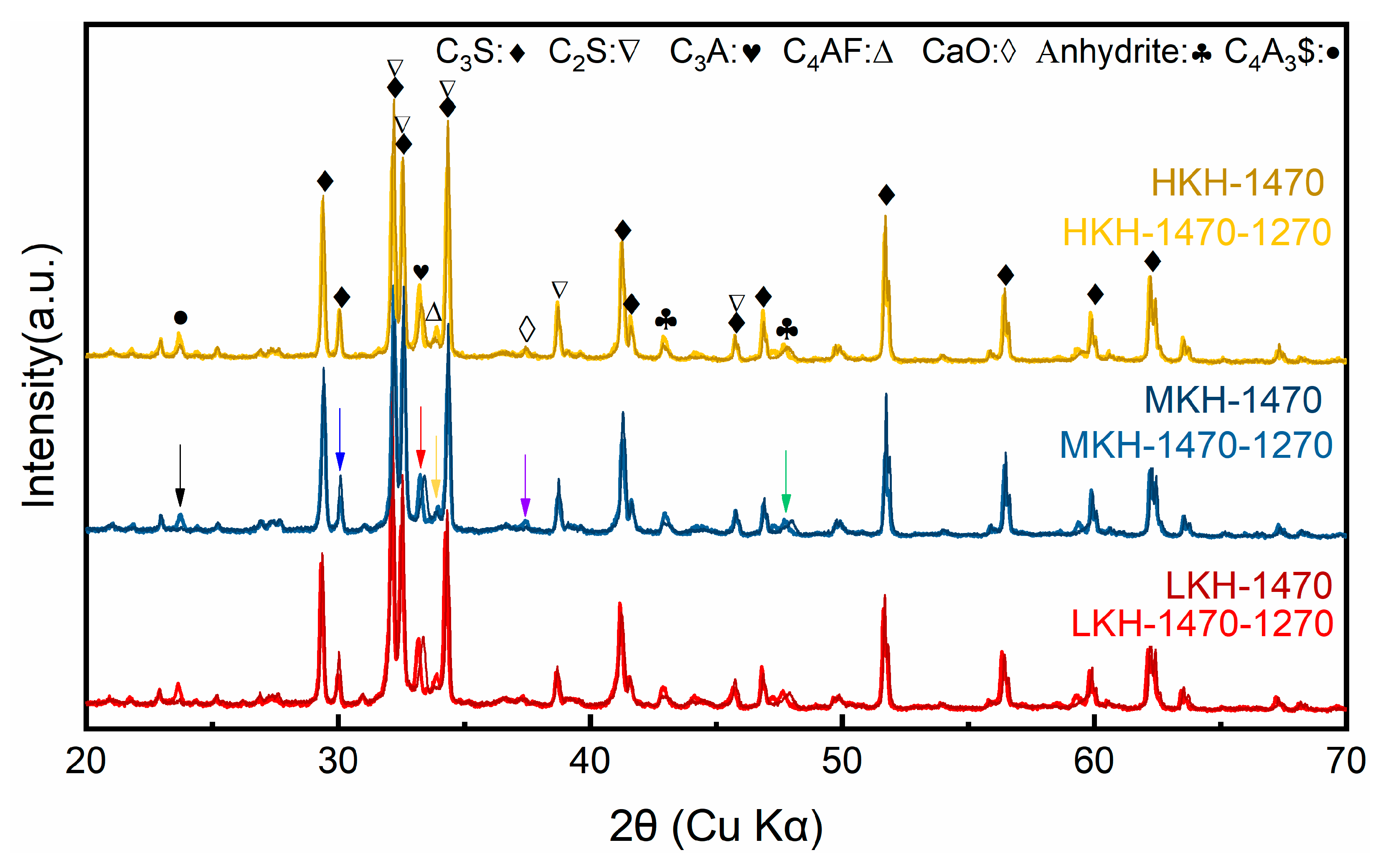
3.1. Clinkers Sintering
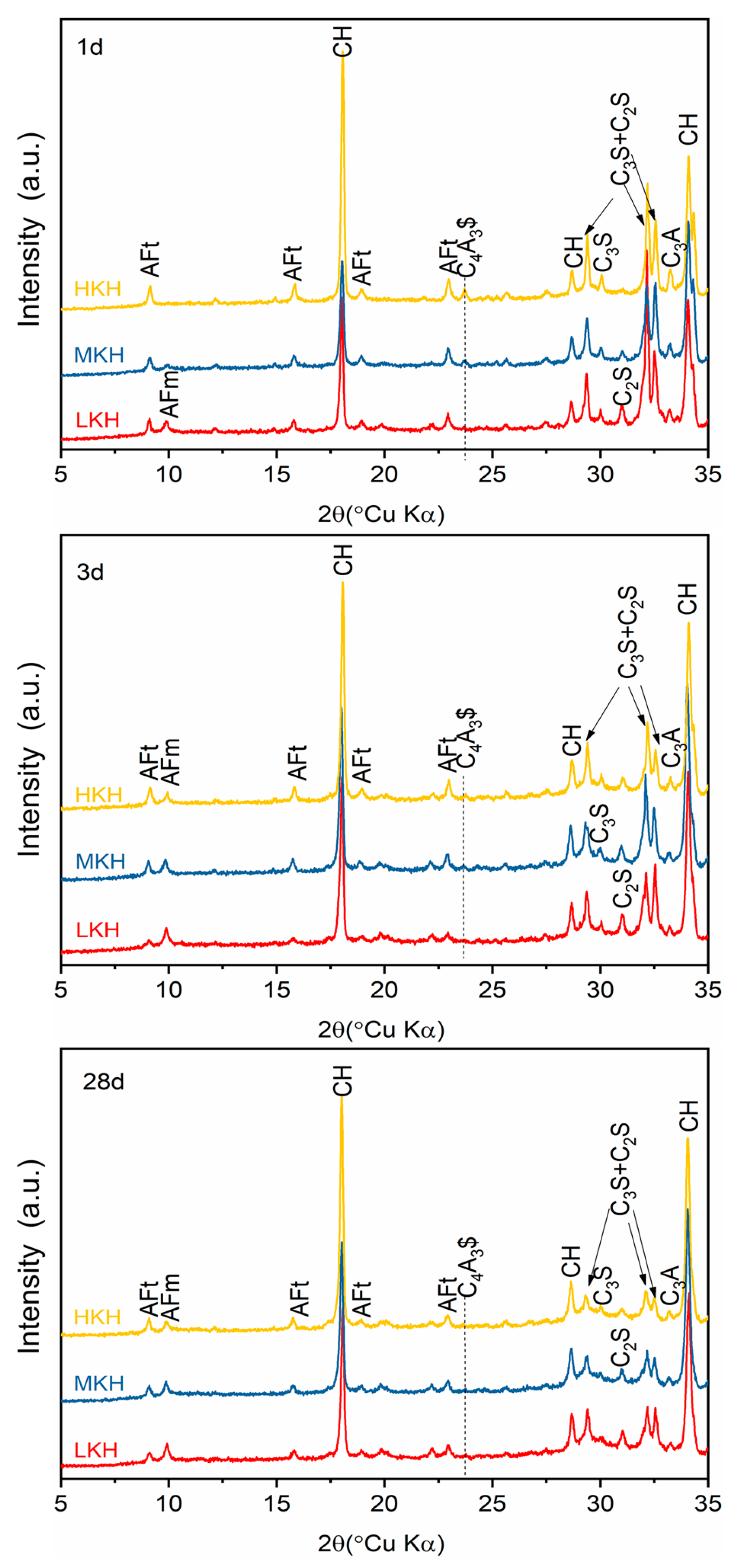
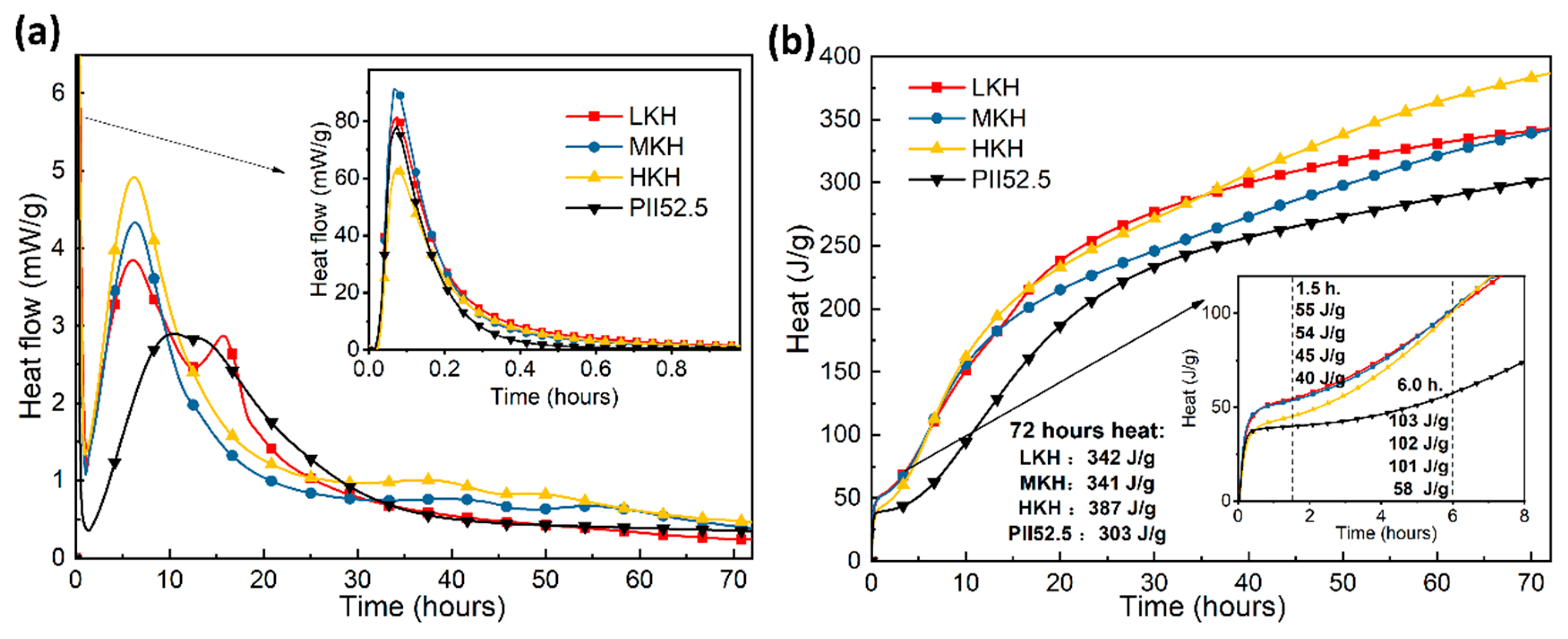
3.2. Hydration Properties of AC$A Cement
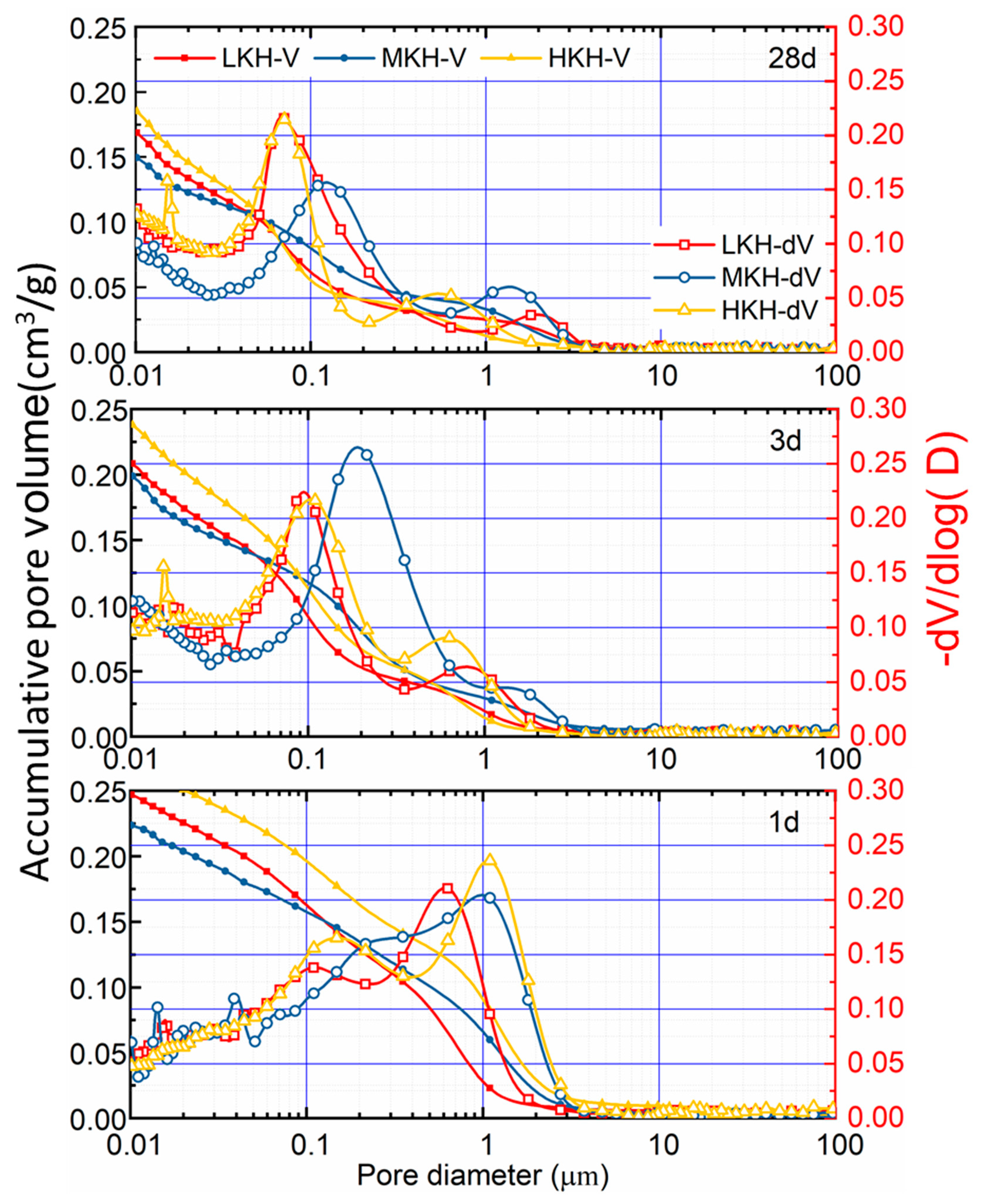
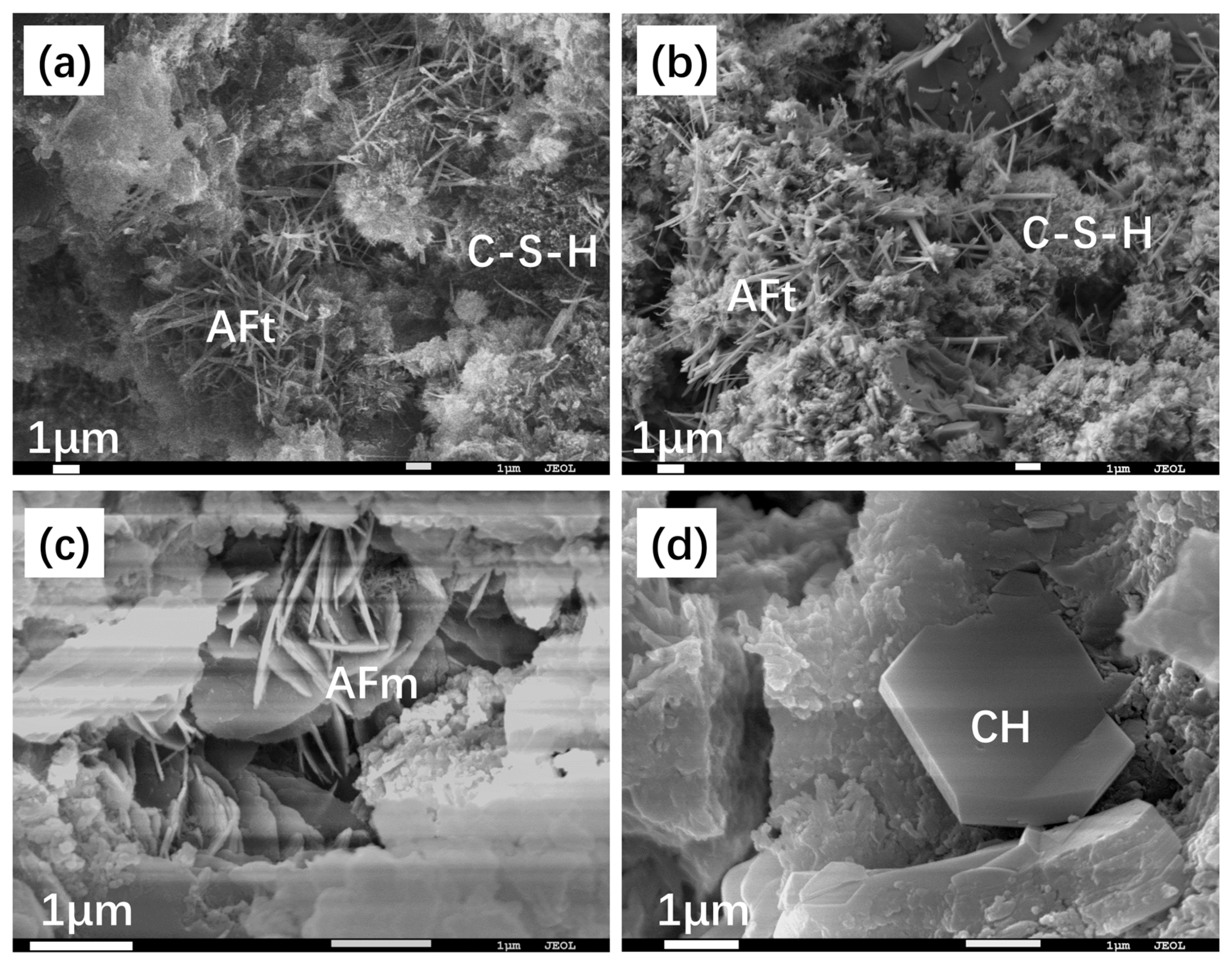
3.3. Microscopy Structure of Hydration Pastes
4. Conclusions
- (1)
- A great amount of C3S (64.88%) and C4A3$ (2.06%) can coexist in the resulting clinker with the sintering temperature at 1470 °C and secondary heat treatment temperature at 1270 °C, while the content of f-CaO is qualified.
- (2)
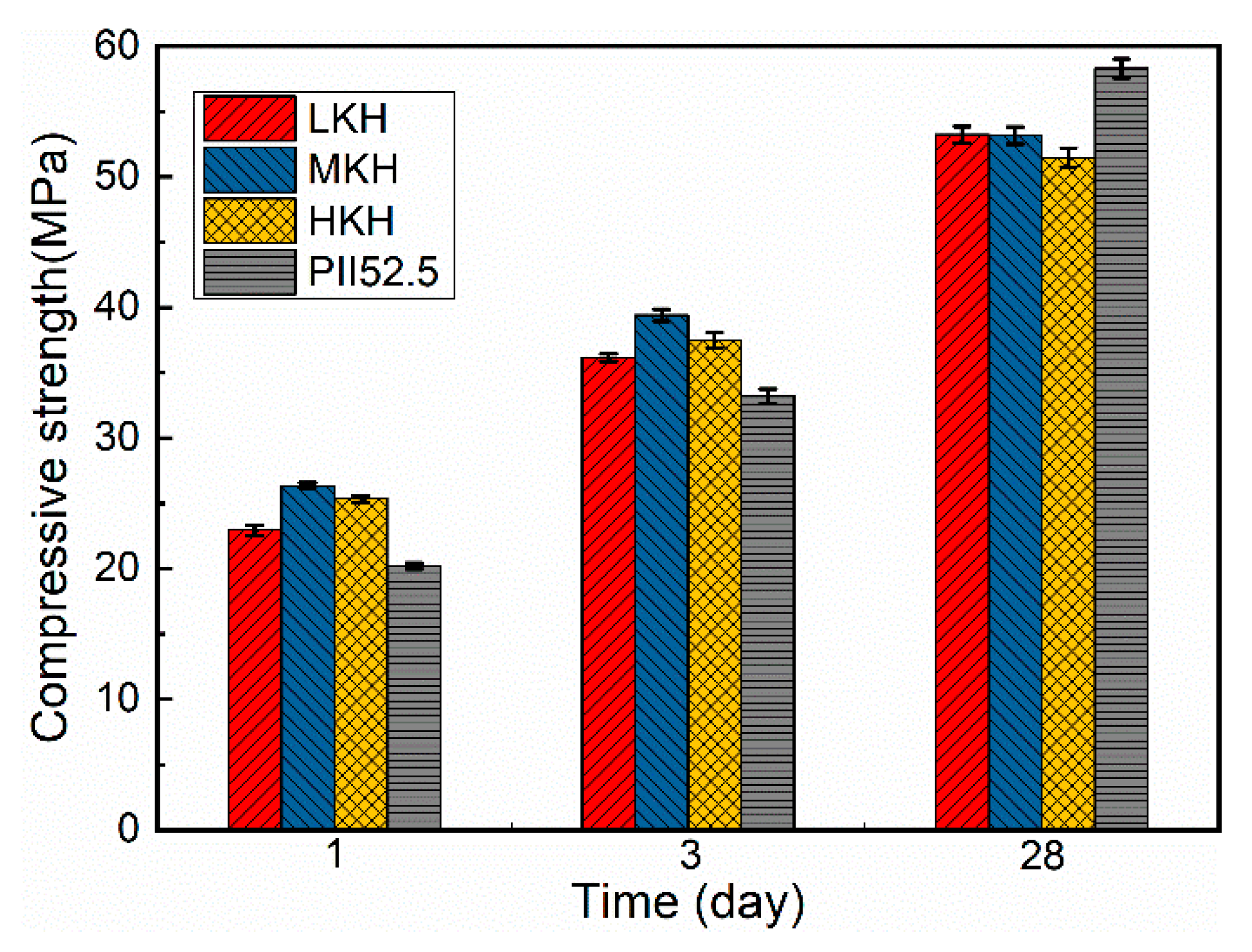
- Increasing KH leads to higher C3S content and lower C2S content, while the presence of ye’elimite shows minimal correlation with KH. Heat release shows that as the KH value increases, the peak for AFm formation gradually obscures. In this study, based on the phase components in the clinker and mechanical strength of the mortar, the MKH labeled sample with a KH value of 0.93 was chosen for optimizing AC$A clinker sintering.
- (3)
- AC$A paste showed significantly improved compressive strength development during the early curing period than PII 52.5 due to the rapid hydration of C4A3$ to form needle-shaped AFt crystals. These crystals then combine with the C-S-H gel to create a crystal–gel network structure. MKH exhibits higher compressive strength at 1, 3, and 28 days, which can be confirmed by the pore structure analysis revealing consistently lower accumulative pore volume in MKH paste compared to other pastes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amato, I. Concrete Solutions. Nature 2013, 494, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, L.; Liu, T.; Hao, L.; Li, Y.; Liu, P.; Zhu, T. A review of low-carbon technologies and projects for the global cement industry. J. Environ. Sci. 2024, 136, 682–697. [Google Scholar] [CrossRef]
- Tan, C.; Yu, X.; Guan, Y. A technology-driven pathway to net-zero carbon emissions for China’s cement industry. Appl. Energy 2022, 325, 119804. [Google Scholar] [CrossRef]
- Beerling, D.J.; Kantzas, E.P.; Lomas, M.R.; Wade, P.; Eufrasio, R.M.; Renforth, P.; Sarkar, B.; Andrews, M.G.; James, R.H.; Pearce, C.R.; et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 2020, 583, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Zea-Garcia, J.D.; De la Torre, A.G.; Aranda, M.A.G.; Santacruz, I. Processing and characterisation of standard and doped alite-belite-ye’elimite ecocement pastes and mortars. Cem. Concr. Res. 2020, 127, 105911. [Google Scholar] [CrossRef]
- Wang, X.; Guo, M.-Z.; Yue, G.; Li, Q.; Ling, T.-C. Synthesis of high belite sulfoaluminate cement with high volume of mixed solid wastes. Cem. Concr. Res. 2022, 158, 106845. [Google Scholar] [CrossRef]
- Tao, X.; Li, P.; Wang, G.; Li, W.; Ma, S. Preparation and hydration of Belite-Ye’elimite-Ternesite clinker based on industrial solid waste. Case Stud. Constr. Mater. 2023, 18, e01922. [Google Scholar] [CrossRef]
- Li, W.; Ji, D.; Shi, F.; Huang, X.; Ji, X.; Ma, S. Study on the synthesis of belite-ye’elimite-ternesite clinker. Constr. Build. Mater. 2022, 319, 126022. [Google Scholar] [CrossRef]
- Zhou, H.; Gu, X.; Sun, J.; Yu, Z.; Huang, H.; Wang, Q.; Shen, X. Research on the formation of M1-type alite doped with MgO and SO3—A route to improve the quality of cement clinker with a high content of MgO. Constr. Build. Mater. 2018, 182, 156–166. [Google Scholar] [CrossRef]
- Dvořák, K.; Všianský, D.; Ravaszová, S.; Jančíků, A. Synthesis of M1 and M3 alite polymorphs and accuracy of their quantification. Cem. Concr. Res. 2023, 163, 107016. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, W.; Ye, J. FTIR study on the polymorphic structure of tricalcium silicate. Cem. Concr. Res. 2017, 99, 129–136. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Shen, X.; Wang, Q.; Pan, Z. Kinetics of calcium sulfoaluminate formation from tricalcium aluminate, calcium sulfate and calcium oxide. Cem. Concr. Res. 2014, 55, 79–87. [Google Scholar] [CrossRef]
- Ben Haha, M.; Winnefeld, F.; Pisch, A. Advances in understanding ye’elimite-rich cements. Cem. Concr. Res. 2019, 123, 105778. [Google Scholar] [CrossRef]
- Tao, Y.; Rahul, A.V.; Mohan, M.K.; De Schutter, G.; Van Tittelboom, K. Recent progress and technical challenges in using calcium sulfoaluminate (CSA) cement. Cem. Concr. Compos. 2023, 137, 104908. [Google Scholar] [CrossRef]
- Hanein, T.; Duvallet, T.Y.; Jewell, R.B.; Oberlink, A.E.; Robl, T.L.; Zhou, Y.; Glasser, F.P.; Bannerman, M.N. Alite calcium sulfoaluminate cement: Chemistry and thermodynamics. Adv. Cem. Res. 2019, 31, 94–105. [Google Scholar] [CrossRef]
- Kothari, A.; Tole, I.; Hedlund, H.; Ellison, T.; Cwirzen, A. Partial replacement of OPC with CSA cements—Effects on hydration, fresh and hardened properties. Adv. Cem. Res. 2023, 35, 207–224. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Scholten, T.; Scrivener, K.L.; Ben Haha, M.; Wolter, A. Formation, composition and stability of ye’elimite and iron-bearing solid solutions. Cem. Concr. Res. 2020, 131, 106009. [Google Scholar] [CrossRef]
- Abir, F.-Z.; El Hafiane, Y.; Smith, A.; Kondo, Y.; Sakai, Y.; Asaka, T.; Fukuda, K.; Mesnaoui, M.; Abouliatim, Y.; Nibou, L. Chemical synthesis and crystallographic data on iron doped cubic ye’elimite. Cem. Concr. Res. 2023, 173, 107257. [Google Scholar] [CrossRef]
- Wu, S.; Ren, C.; Sun, Y.; Wang, W. Synthesis, structure, and hydration of stoichiometric ye’elimite and iron-bearing ye’elimite. J. Sustain. Cem.-Based Mater. 2024, 13, 164–177. [Google Scholar] [CrossRef]
- Hu, Y.; Li, W.; Ma, S.; Wang, Q.; Zou, H.; Shen, X. The composition and performance of alite-ye’elimite clinker produced at 1300 °C. Cem. Concr. Res. 2018, 107, 41–48. [Google Scholar] [CrossRef]
- Staněk, T.; Sulovský, P. The influence of the alite polymorphism on the strength of the Portland cement. Cem. Concr. Res. 2002, 32, 1169–1175. [Google Scholar] [CrossRef]
- Segata, M.; Marinoni, N.; Galimberti, M.; Marchi, M.; Cantaluppi, M.; Pavese, A.; De la Torre, Á.G. The effects of MgO, Na2O and SO3 on industrial clinkering process: Phase composition, polymorphism, microstructure and hydration, using a multidisciplinary approach. Mater. Charact. 2019, 155, 109809. [Google Scholar] [CrossRef]
- Zhang, W.; Luan, Z.; Ren, X.; Ye, J.; Shi, D.; Zhang, H. Influence of alumina modulus on formation of high-magnesium clinker and morphological evolution of MgO. Cem. Concr. Res. 2022, 162, 106986. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Li, X.; Shen, X.; Xu, J.; Li, X.; Ma, S. Hydration properties of the alite-ye’elimite cement clinker synthesized by reformation. Constr. Build. Mater. 2015, 99, 254–259. [Google Scholar] [CrossRef]
- GB/T 176-2017; Methods for Chemical Analysis of Cement. China Standard Press: Beijing, China, 2017.
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR; Los Alamos National Laboratory: Los Alamos, NM, USA, 1994; pp. 86–748. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. China Standard Press: Beijing, China, 2011.
- GB/T 17671-2021; Test Method of Cement Mortar Strenth (ISO Method). China Standard Press: Beijing, China, 2021.
- Ludwig, H.-M.; Zhang, W. Research review of cement clinker chemistry. Cem. Concr. Res. 2015, 78, 24–37. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Tang, M.; Li, X. Stability of Tricalcium Silicate and Other Primary Phases in Portland Cement Clinker. Ind. Eng. Chem. Res. 2014, 53, 1954–1964. [Google Scholar] [CrossRef]
- Lv, L.; Luo, S.; Savija, B.; Zhang, H.; Li, L.; Ueda, T.; Xing, F. Effect of particle size distribution on the pre-hydration, hydration kinetics, and mechanical properties of calcium sulfoaluminate cement. Constr. Build. Mater. 2023, 398, 132497. [Google Scholar] [CrossRef]
- Cuesta, A.; Álvarez-Pinazo, G.; Sanfélix, S.G.; Peral, I.; Aranda, M.A.G.; De la Torre, A.G. Hydration mechanisms of two polymorphs of synthetic ye’elimite. Cem. Concr. Res. 2014, 63, 127–136. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]

| Composition | LOSS | SiO2 | Al2O3 | Fe2O3 | CaO | MgO |
|---|---|---|---|---|---|---|
| Limestone | 43.31 | 0.34 | 0.24 | 0.25 | 54.93 | 0.13 |
| Fly ash | 1.96 | 54.15 | 31.49 | 3.38 | 1.98 | 1.27 |
| Clay | 17.02 | 30.75 | 31.24 | 15.32 | 2.35 | 0.65 |
| Silica | 0.36 | 93.95 | 2.38 | 1.96 | 0.48 | 0.32 |
| Label | KH | LOSS | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | MgO * | K2O * | SO3 * |
|---|---|---|---|---|---|---|---|---|---|---|
| LKH | 0.90 | 35.07 | 43.48 | 14.25 | 4.32 | 1.39 | 0.26 | 2.00 | 0.80 | 3.00 |
| MKH | 0.93 | 35.25 | 43.73 | 13.95 | 4.22 | 1.36 | 0.26 | 2.00 | 0.80 | 3.00 |
| HKH | 0.96 | 35.42 | 43.98 | 13.65 | 4.13 | 1.33 | 0.25 | 2.00 | 0.80 | 3.00 |
| Label | C3S | C2S | C3A | C4AF | f-CaO | f-MgO | C4A3$ | Anhydrite | Rwp |
|---|---|---|---|---|---|---|---|---|---|
| LKH-1470 | 61.86 | 22.99 | 10.14 | 1.73 | 0.40 | 2.21 | 0.32 | 0.33 | 10.82 |
| LKH-1470-1270 | 53.42 | 26.18 | 8.25 | 5.90 | 0.29 | 3.61 | 2.06 | 0.28 | 11.8 |
| MKH-1470 | 67.27 | 14.92 | 9.95 | 3.33 | 0.31 | 2.77 | 1.09 | 0.37 | 10.99 |
| MKH1470-1270 | 64.88 | 14.46 | 9.88 | 4.89 | 0.45 | 3.24 | 2.06 | 0.15 | 9.58 |
| HKH-1470 | 75.35 | 7.2 | 9.59 | 2.69 | 0.92 | 2.47 | 1.44 | 0.34 | 11.12 |
| HKH-1470-1270 | 68.22 | 9.41 | 9.29 | 5.46 | 0.53 | 3.64 | 2.86 | 0.48 | 10.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ma, B.; Ji, W.; Dou, S.; Zhou, H.; Zhang, H.; Wang, J.; Hu, Y.; Shen, X. Impact of Lime Saturation Factor on Alite-Ye’Elimite Cement Synthesis and Hydration. Materials 2024, 17, 3035. https://doi.org/10.3390/ma17123035
Li X, Ma B, Ji W, Dou S, Zhou H, Zhang H, Wang J, Hu Y, Shen X. Impact of Lime Saturation Factor on Alite-Ye’Elimite Cement Synthesis and Hydration. Materials. 2024; 17(12):3035. https://doi.org/10.3390/ma17123035
Chicago/Turabian StyleLi, Xiaodong, Bing Ma, Wenqian Ji, Shang Dou, Hao Zhou, Houhu Zhang, Jiaqing Wang, Yueyang Hu, and Xiaodong Shen. 2024. "Impact of Lime Saturation Factor on Alite-Ye’Elimite Cement Synthesis and Hydration" Materials 17, no. 12: 3035. https://doi.org/10.3390/ma17123035
APA StyleLi, X., Ma, B., Ji, W., Dou, S., Zhou, H., Zhang, H., Wang, J., Hu, Y., & Shen, X. (2024). Impact of Lime Saturation Factor on Alite-Ye’Elimite Cement Synthesis and Hydration. Materials, 17(12), 3035. https://doi.org/10.3390/ma17123035






