Preparation of Al@FTCS/P(VDF-HFP) Composite Energetic Materials and Their Reaction Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
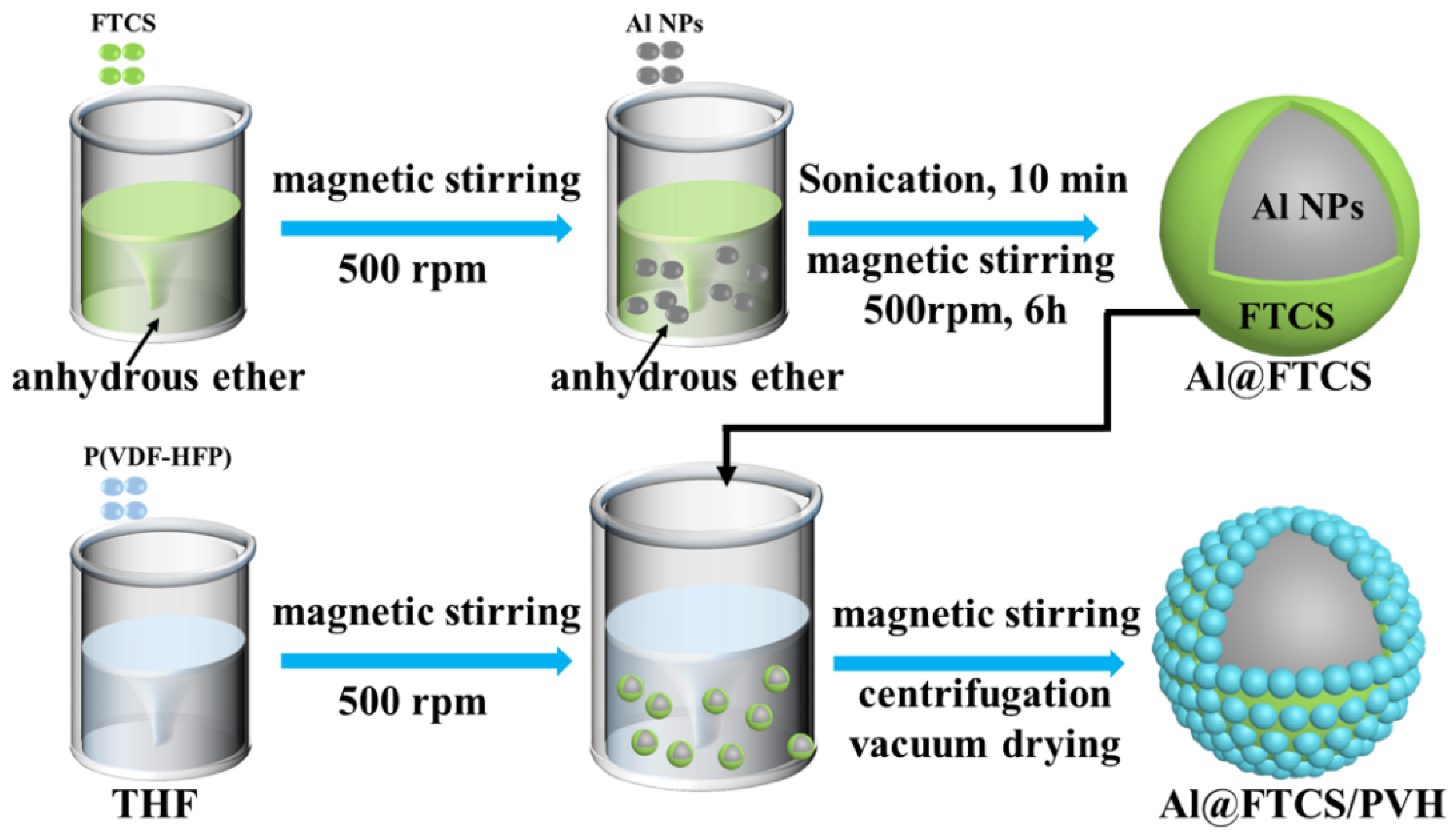
2.2. Sample Preparation
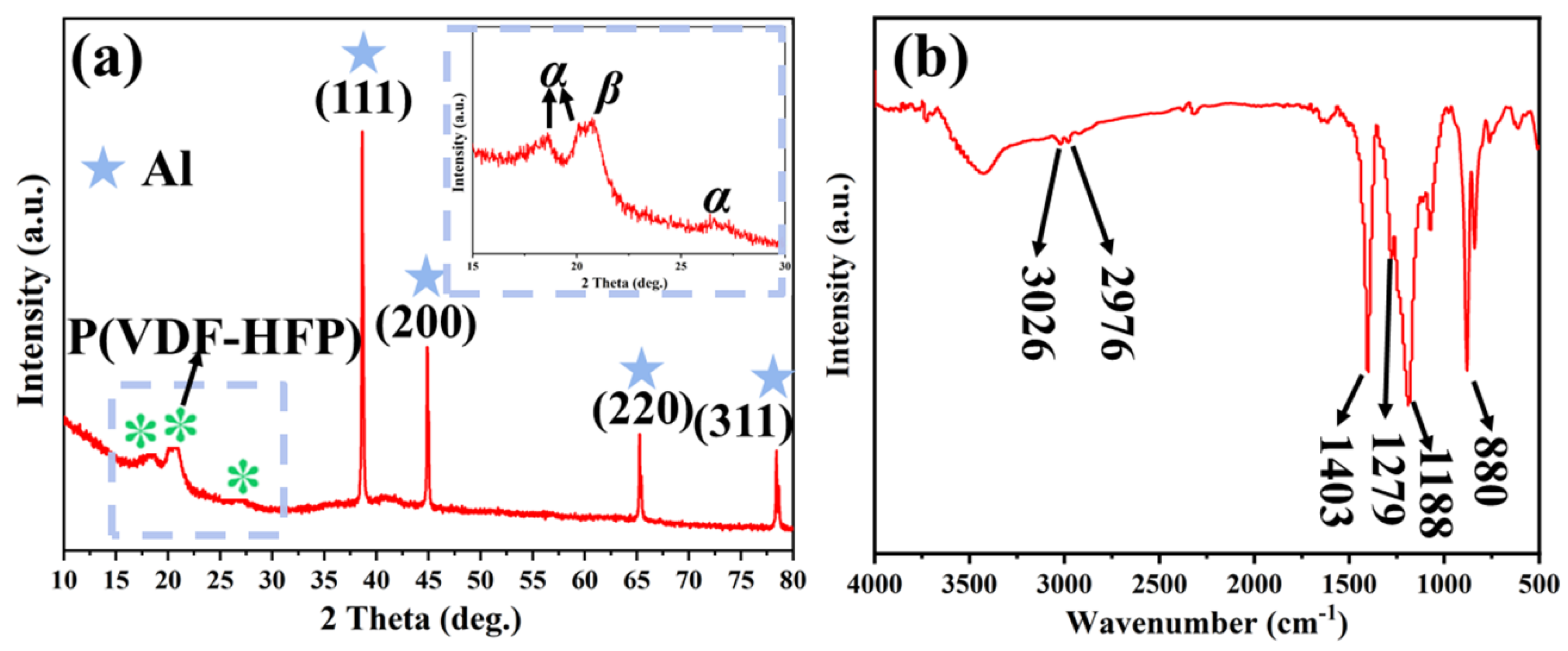
2.3. Morphology and Structural Characterization
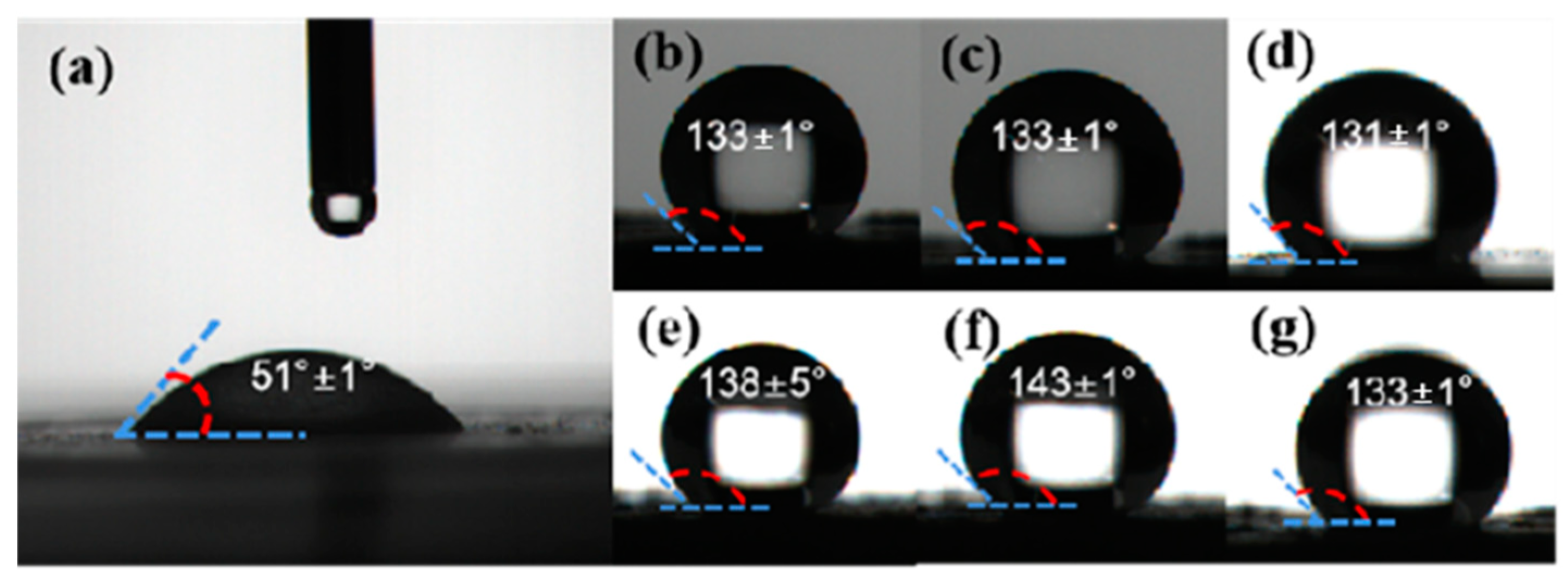
2.4. Water Contact Angle Measurement
2.5. Thermal Analysis
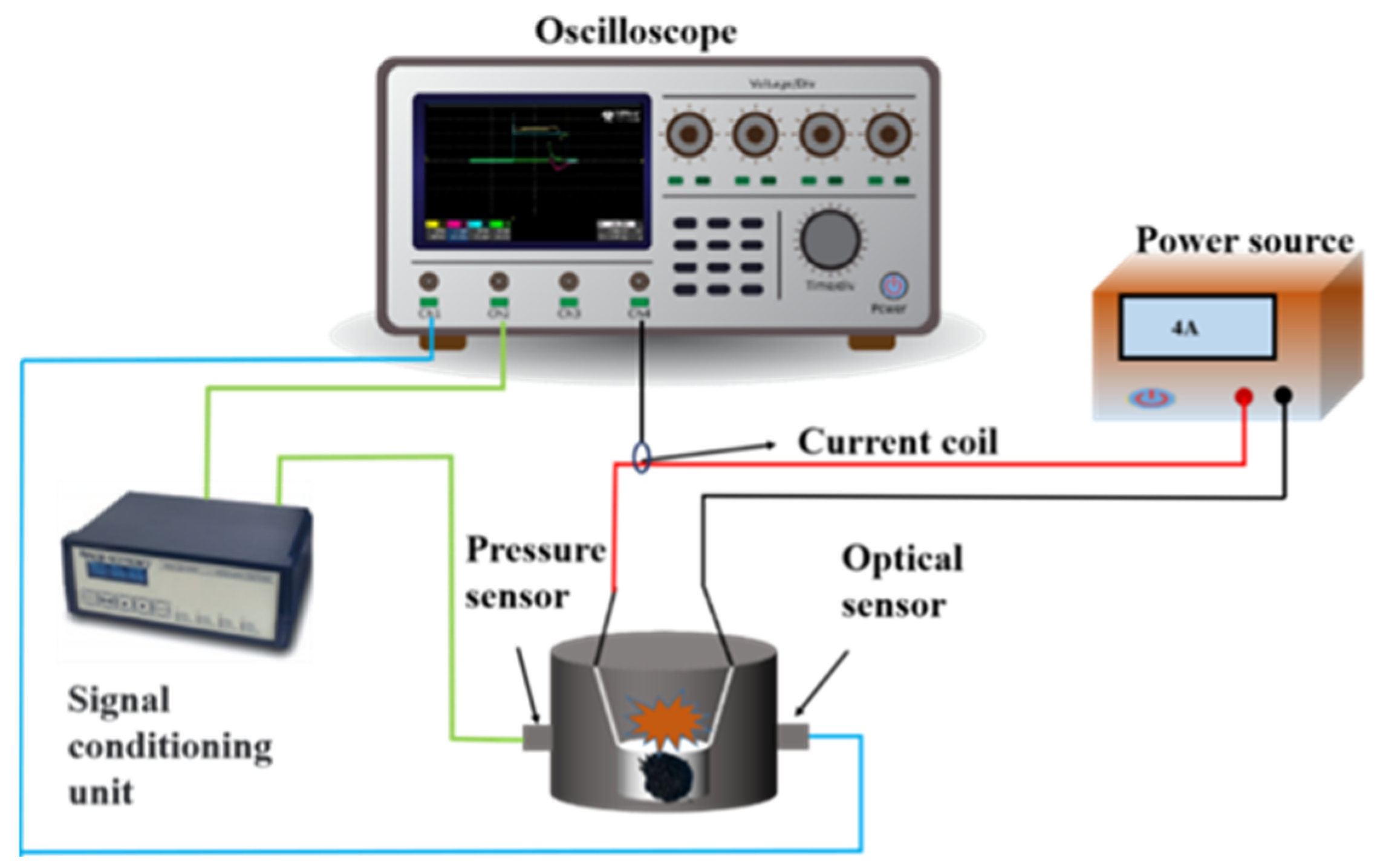
2.6. Electric Ignition and Constant-Volume Combustion Experiments
3. Results and Discussion
3.1. Hydrophobicity Test of Al, Al@FTCS, and Al@FTCS/PVH
3.2. Morphology and Composition Structure Characterizations of Al@FTCS/PVH
3.3. Thermal Analysis
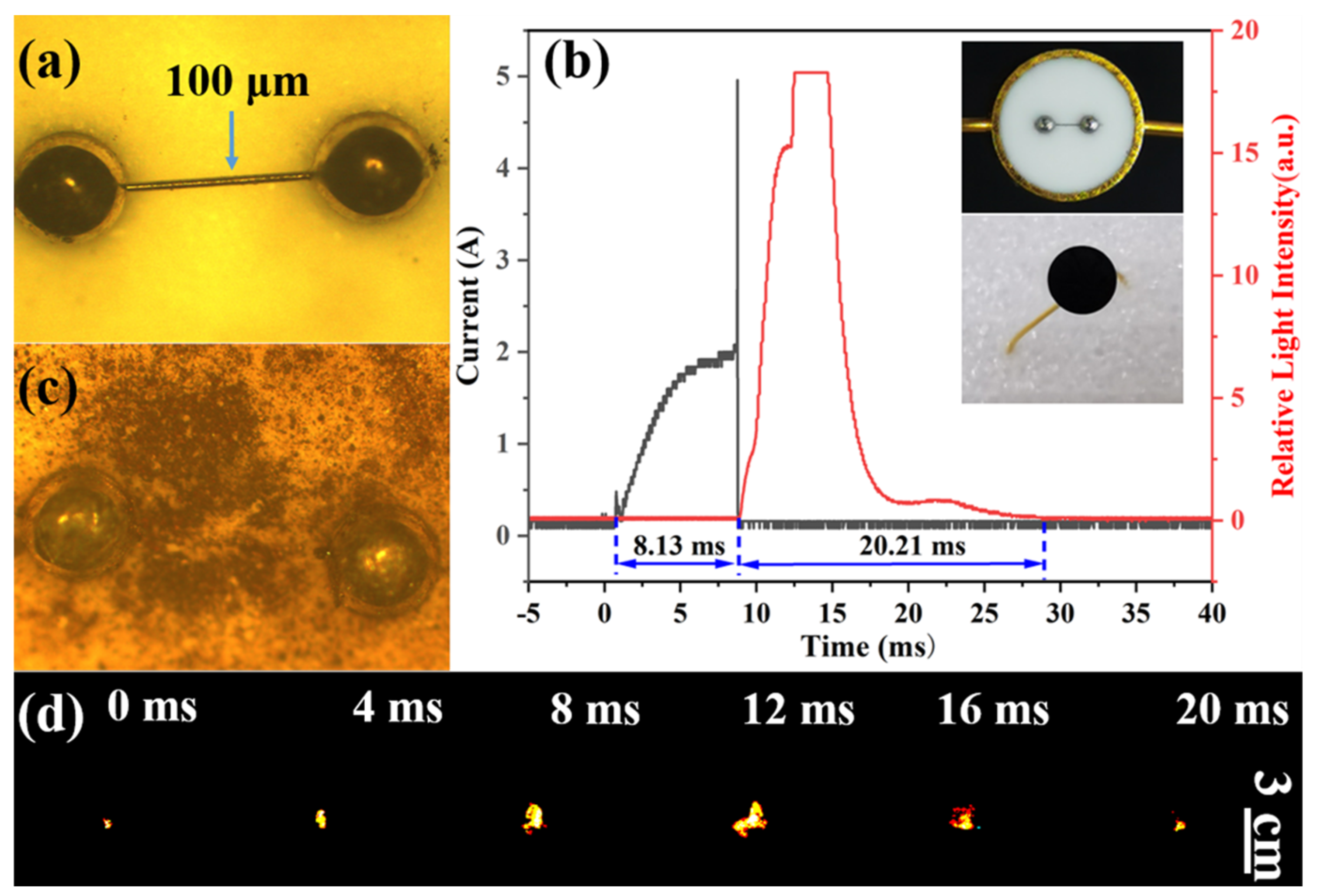
3.4. Ignition and Combustion Performances of Al@FTCS/PVH and Al/PVH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–157. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Cohen, A.; Petrutik, N.; Shlomovich, A.; Burstein, L.; Pang, S.-P.; Gozin, M. Highly insensitive and thermostable energetic coordination nanomaterials based on functionalized graphene oxides. J. Mater. Chem. A 2016, 4, 9941–9948. [Google Scholar] [CrossRef]
- Zhou, X.; Ke, X.; Jiang, W. Aluminum/copper oxide nanostructured energetic materials prepared by solution chemistry and electrophoretic deposition. RSC Adv. 2016, 6, 93863–93866. [Google Scholar] [CrossRef]
- Wang, L.L.; Munir, Z.A.; Maximoy, Y.M. Thermite reactions: Their utilization in the synthesis and processing of materials. J. Mater. Sci. 1993, 28, 3693–3708. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Zhao, F.-Q.; Kuo, K.K.; Zhang, X.-H.; Zeman, S.; DeLuca, L.T. Catalytic effects of nano additives on decomposition and combustion of RDX-, HMX-, and AP-based energetic compositions. Prog. Energy Combust. Sci. 2016, 57, 75–136. [Google Scholar] [CrossRef]
- He, W.; Liu, P.; He, G.; Gozin, M.; Yan, Q. Highly Reactive Metastable Intermixed Composites (MICs): Preparation and Characterization. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef] [PubMed]
- Fahd, A.; Baranovsky, A.; Dubois, C.; Chaouki, J.; Wen, J.Z. Superior performance of quaternary NC/GO/Al/KClO4 nanothermite for high speed impulse small-scale propulsion applications. Combust. Flame 2021, 232, 111527. [Google Scholar] [CrossRef]
- Wang, H.; Rehwoldt, M.; Kline, D.J.; Wu, T.; Wang, P.; Zachariah, M.R. Comparison study of the ignition and combustion characteristics of directly-written Al/PVDF, Al/Viton and Al/THV composites. Combust. Flame 2019, 201, 181–186. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Zhan, C.B.; Jin, H.B.; Chen, K.X. Novel method of thermite welding. Sci. Technol. Weld. Join. 2010, 15, 54–58. [Google Scholar] [CrossRef]
- Ma, X.; Fei, W.; Liu, J.; Zhang, X.; Ji, J.; Zhou, X. Energetic characteristics of highly reactive Si nanoparticles prepared by magnesiothermic reduction of mesoporous SiO2. Chem. Eng. J. 2024, 481, 148542. [Google Scholar] [CrossRef]
- Petrantoni, M.; Rossi, C.; Salvagnac, L.; Conédéra, V.; Estève, A.; Tenailleau, C.; Alphonse, P.; Chabal, Y.J. Multilayered Al/CuO thermite formation by reactive magnetron sputtering: Nano versus micro. J. Appl. Phys. 2010, 108, 084323. [Google Scholar] [CrossRef]
- Ji, J.; Liang, L.; Xu, H.; Xiang, G.; Li, H.; Li, P.; Zhou, X.; Guo, X. Facile solvent evaporation synthesis of core-shell structured Al@PVDF nanoparticles with excellent corrosion resistance and combustion properties. Combust. Flame 2022, 238, 111925. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhang, K.L. Fabrication and reaction mechanism study of Co(OH)F@Al nanowirearrays: A functional fluorine-containing metastable intermolecular composite. Combust. Flame 2023, 255, 112872. [Google Scholar] [CrossRef]
- Sippel, T.R.; Son, S.F.; Groven, L.J. Aluminum agglomeration reduction in a composite propellant using tailored Al/PTFE particles. Combust. Flame 2014, 161, 311–321. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, D.W.; Kim, C.K.; Choi, Y.J. A facile synthesis and efficient thermal oxidation of polytetrafluoroethylene-coated aluminum powders. Mater. Lett. 2016, 167, 262–265. [Google Scholar] [CrossRef]
- Xiao, F.; Liang, T.X. Preparation of hierarchical core-shell Al-PTFE@TA and Al-PTFE@TA-Fe architecture for improving the combustion and ignition properties of aluminum. Surf. Coat. Technol. 2021, 412, 127073. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.; Zhang, L.; Qiao, Z.; Gao, B.; Yang, G.; Huang, H. Design and fabrication of energetic superlattice like-PTFE/Al with superior performance and application in functional micro-initiator. Nano Energy 2015, 12, 597–605. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Z.; Yang, Y.; Shen, J.; Long, Z.; Li, Z.; Cui, X.; Yang, G. Core-Shell Al-Polytetrafluoroethylene (PTFE) Configurations to Enhance Reaction Kinetics and Energy Performance for Nanoenergetic Materials. Chem. A Eur. J. 2016, 22, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Guo, S.; Zhang, G.; Zhou, X.; Xiao, L.; Hao, G.; Wang, N.; Jiang, W. Safe preparation, energetic performance and reaction mechanism of corrosion-resistant Al/PVDF nanocomposite films. J. Mater. Chem. A 2018, 6, 17713–17723. [Google Scholar] [CrossRef]
- Osborne, D.T.; Pantoya, M.L. Effect of Al Particle Size on the Thermal Degradation of Al/Teflon Mixtures. Combust. Sci. Technol. 2007, 179, 1467–1480. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Xu, J.; Liu, Q.; Shi, W.; Tang, W. Al@Polytannic Acid/Polyvinylidene Fluoride Nanoenergetic Films for Controlled Combustion. ACS Appl. Nano Mater. 2024, 7, 13347–13357. [Google Scholar] [CrossRef]
- Ke, X.; Guo, S.; Gou, B.; Wang, N.; Zhou, X.; Xiao, L.; Hao, G.; Jiang, W. Superhydrophobic Fluorine-Containing Protective Coating to Endow Al Nanoparticles with Long-Term Storage Stability and Self-Activation Reaction Capability. Adv. Mater. Interfaces 2019, 6, 1901025. [Google Scholar] [CrossRef]
- Fei, W.; Ma, X.; Zhang, X.; Ji, J.; Zhou, X. Perfluorotetradecanoic acid-directed precipitation of P(VDF-HFP) around nano-Al for the improved ignition and combustion characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133141. [Google Scholar] [CrossRef]
- Wang, C.; Qin, M.; Yi, Z.; Deng, H.; Wang, J.; Sun, Y.; Luo, G.; Shen, Q. Oxidation Mechanism of Core-Shell Structured Al@PVDF Powders Synthesized by Solvent/Non-Solvent Method. Materials 2022, 15, 3036. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, L.; Li, X.; Zhu, L.; Xiang, Z.; Xu, J.; Xue, D.; Deng, Z.; Su, X.; Zou, M. High-Performance Aluminum Fuels Induced by Monolayer Self-Assembly of Nano-Sized Energetic Fluoride Vesicles on the Surface. Adv. Sci. 2024, 2401564. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.J.; Colavita, P.E.; Murphy, D.M.; Platts, J.A.; Wallis, J.D. Fluorine–fluorine Interactions in the Solid State: An Experimental and Theoretical Study. J. Phys. Chem. A 2012, 116, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Lyu, J.-Y.; Tang, D.-Y.; He, G.-Q.; Liu, P.-J.; Yan, Q.-L. Control the combustion behavior of solid propellants by using core-shell Al-based composites. Combust. Flame 2020, 221, 441–452. [Google Scholar] [CrossRef]
- Cui, R.; Zhang, X.; Mao, H.; Zhang, C.; Ji, J.; Zhou, X. Reactive Characteristics of Novel Core-Shell Al-CuO Microspheres Prepared by Alcohol-Thermal Treatment of Cu(OH)2. Propellants Explos. Pyrotech. 2022, 48, e202200157. [Google Scholar] [CrossRef]
- Raza, M.A.; Kooij, E.S.; van Silfhout, A.; Poelsema, B. Superhydrophobic Surfaces by Anomalous Fluoroalkylsilane Self-Assemblyon Silica Nanosphere Arrays. Am. Chem. Soc. 2010, 26, 12962–12972. [Google Scholar]
- Kalimuldina, G.; Turdakyn, N.; Abay, I.; Medeubayev, A.; Nurpeissova, A.; Adair, D.; Bakenov, Z. A Review of Piezoelectric PVDF Film by Electrospinning and Its Applications. Sensors 2020, 20, 5214. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, Z.; Liang, T.; Yang, R.; Li, J.; Luo, P. Establishing the interface layer on the aluminum surface through the self-assembly of tannic acid (TA): Improving the ignition and combustion properties of aluminum. Chem. Eng. J. 2021, 420, 130523. [Google Scholar] [CrossRef]



| Sample | Q (J/g) | T0 (°C) | Tp (°C) | Mass Loss (%) |
|---|---|---|---|---|
| Al/PVH | 289 | 485 | 496 | 47.3 |
| F2 | 1529 | 437 | 493 | 31.2 |
| F4 | 1935 | 428 | 485 | 27.5 |
| F8 | 573 | 442 | 476 | 34.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, X.; Deng, L.; Wang, Y.; Tang, K.; Xiao, L.; Hao, G.; Li, P.; Zhou, X. Preparation of Al@FTCS/P(VDF-HFP) Composite Energetic Materials and Their Reaction Properties. Materials 2024, 17, 3046. https://doi.org/10.3390/ma17133046
Ke X, Deng L, Wang Y, Tang K, Xiao L, Hao G, Li P, Zhou X. Preparation of Al@FTCS/P(VDF-HFP) Composite Energetic Materials and Their Reaction Properties. Materials. 2024; 17(13):3046. https://doi.org/10.3390/ma17133046
Chicago/Turabian StyleKe, Xiang, Lifang Deng, Yanping Wang, Kai Tang, Lei Xiao, Gazi Hao, Peili Li, and Xiang Zhou. 2024. "Preparation of Al@FTCS/P(VDF-HFP) Composite Energetic Materials and Their Reaction Properties" Materials 17, no. 13: 3046. https://doi.org/10.3390/ma17133046





