Constructing Stiff β-Sheet for Self-Reinforced Alginate Fibers
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Secondary Structure Regulation of Sodium Alginate by Ethanol
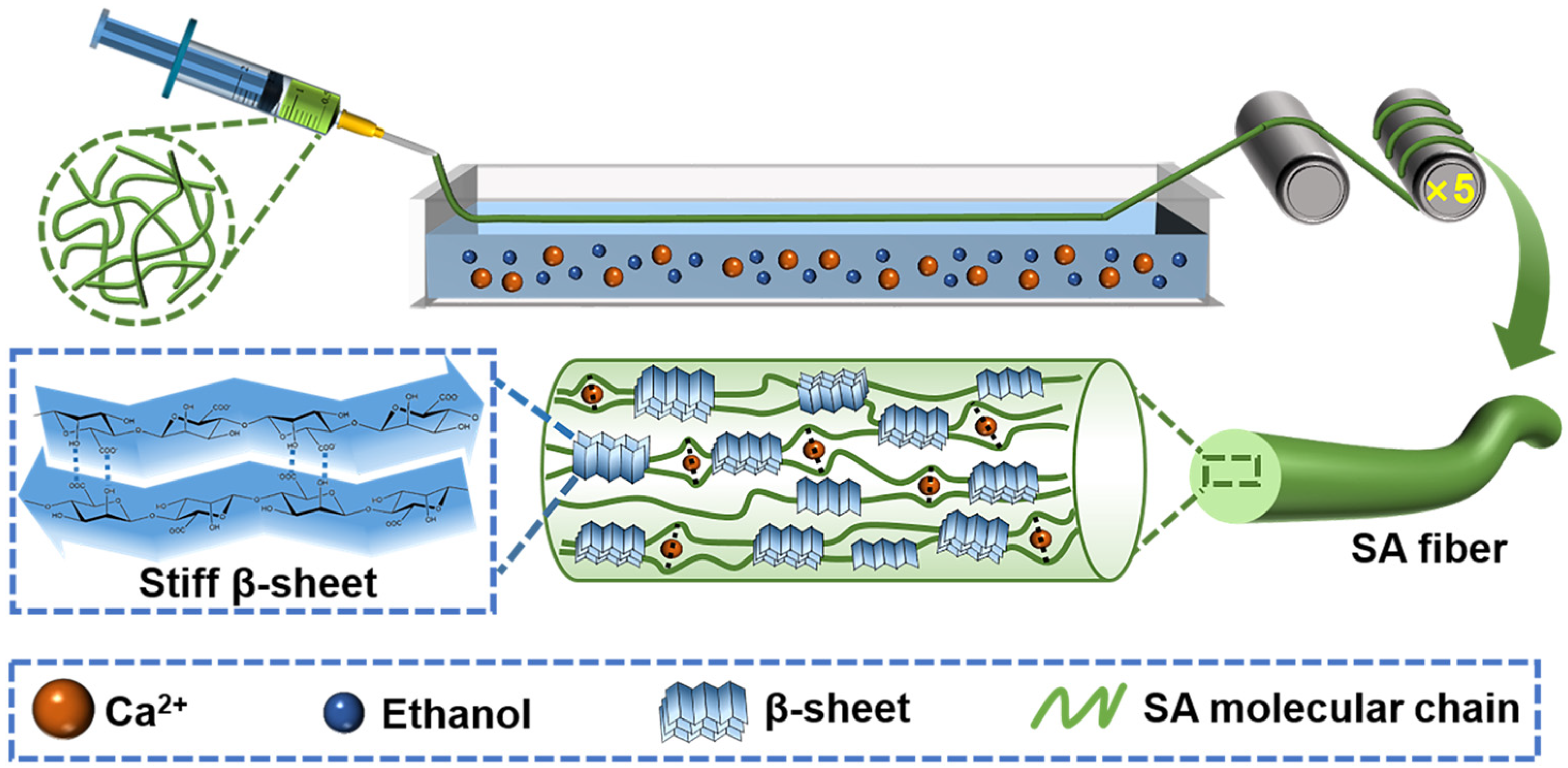
2.3. Preparation of Self-Reinforced Alginate Fibers
2.4. Characterizations
3. Results and Discussion
3.1. Constructing β-Sheet in SA Using Ethanol Treatment
3.2. Rheological Measurement for Self-Reinforcing Mechanism
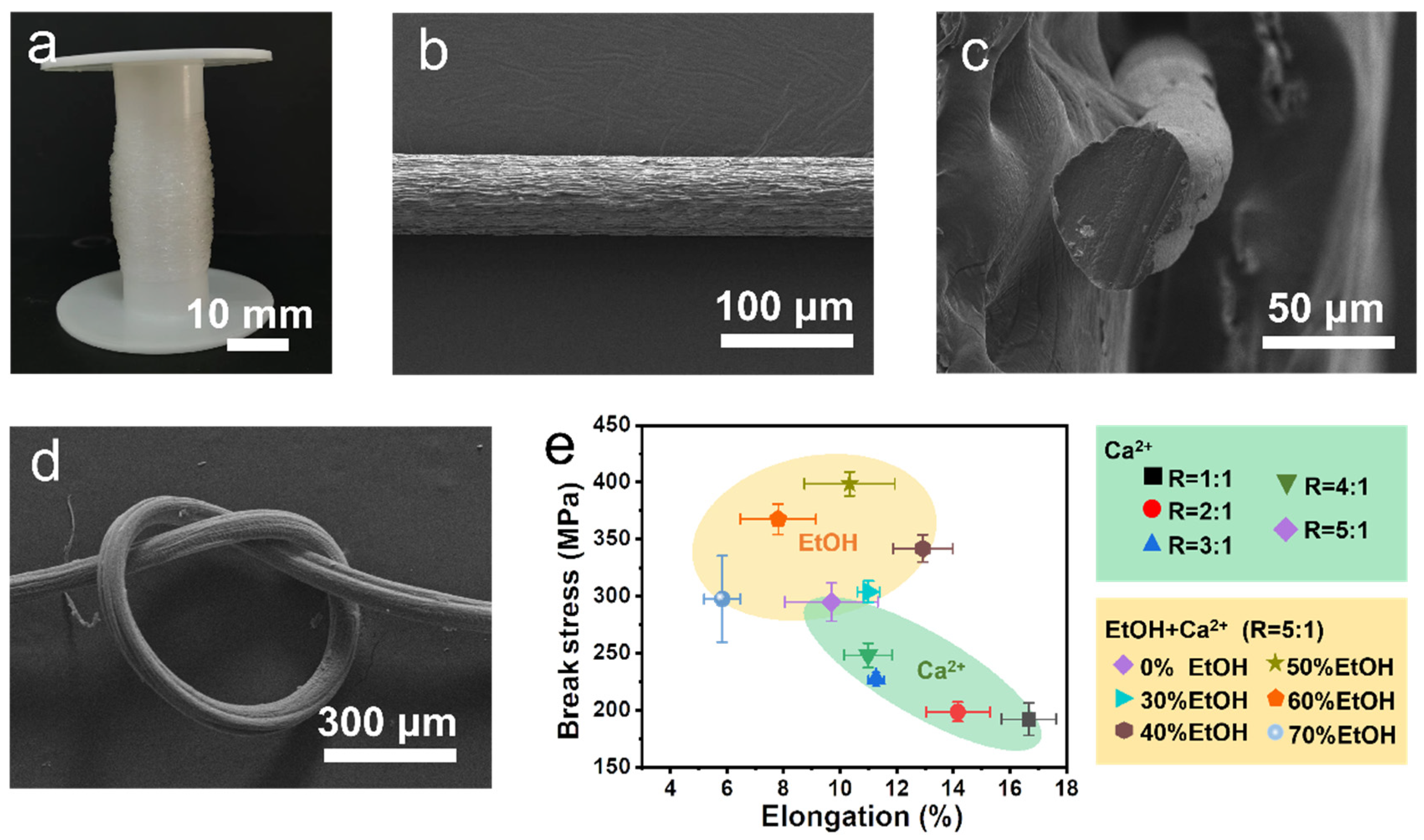
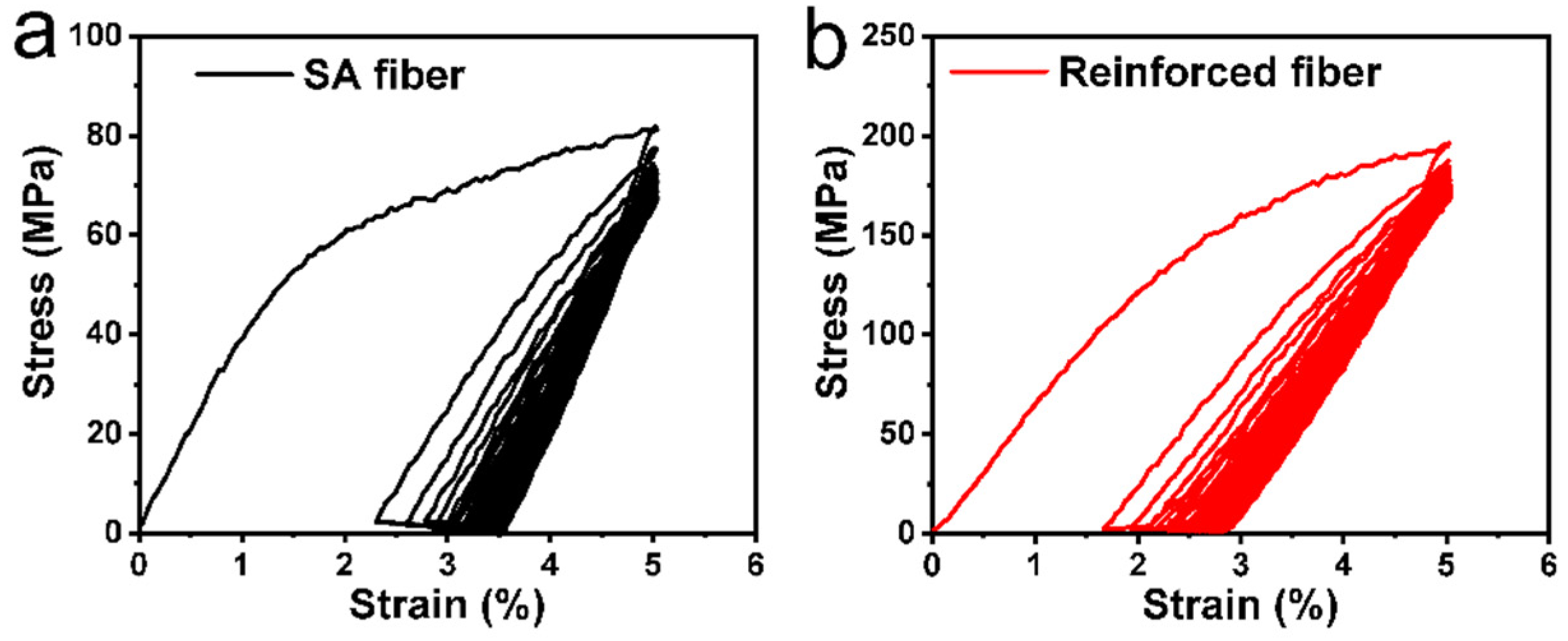
3.3. Fabrication and Application of Self-Reinforced Alginate Fibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koutsopoulos, S. Molecular fabrications of smart nanobiomaterials and applications in personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Gong, C.; Wu, S.; Cui, Z.; Zheng, Y.; Li, Z.; Zhu, S.; Liu, X. Instant protection spray for anti-Infection and accelerated healing of empyrosis. Adv. Mater. 2023, 36, 2306589. [Google Scholar] [CrossRef]
- Huang, C.; Xie, T.; Liu, Y.; Yan, S.; Ou Yang, F.; Zhang, H.; Lei, L.; He, D.; Wei, H.; Yu, C.Y. A sodium alginate-based multifunctional nanoplatform for synergistic chemo-immunotherapy of hepatocellular carcinoma. Adv. Mater. 2023, 35, 2301352–2301366. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.-X.; Hu, J.-J.; Zou, C.-Y.; Jiang, Y.-L.; Zhao, L.-M.; Zhang, X.-Z.; Li, Y.-X.; Peng, A.-N.; Song, Y.-T.; Huang, L.-P.; et al. Multifunctional two-component in-situ hydrogel for esophageal submucosal dissection for mucosa uplift, postoperative wound closure and rapid healing. Bioact. Mater. 2023, 27, 461–473. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr. Polym. 2021, 266, 118100–118109. [Google Scholar] [CrossRef]
- Yi, Y.; Song, J.; Zhou, P.; Shu, Y.; Liang, P.; Liang, H.; Liu, Y.; Yuan, X.; Shan, X.; Wu, X. An ultrasound-triggered injectable sodium alginate scaffold loaded with electrospun microspheres for on-demand drug delivery to accelerate bone defect regeneration. Carbohydr. Polym. 2024, 334, 122039–122054. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Choi, G.; Joo, K.I.; Cha, H.J.; Kim, J. Embolization of vascular malformations via In situ photocrosslinking of mechanically reinforced alginate microfibers using an optical-fiber-integrated microfluidic device. Adv. Mater. 2021, 33, 2006759. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhu, H.; Meng, X.; Wang, J.; Zheng, C.; Xia, Y.; Xiong, Z. Amphiphilic calcium alginate carbon aerogels: Broad-spectrum adsorbents for ionic and solvent dyes with multiple functions for decolorized oil–water separation. ACS Sustain. Chem. Eng. 2020, 8, 12755–12767. [Google Scholar] [CrossRef]
- Zhao, X.; Ding, M.; Xu, C.; Zhang, X.; Liu, S.; Lin, X.; Wang, L.; Xia, Y. A self-reinforcing strategy enables the intimate interface for anisotropic alginate composite hydrogels. Carbohydr. Polym. 2021, 251, 117054. [Google Scholar] [CrossRef]
- Wan, F.; Ping, H.; Wang, W.; Zou, Z.; Xie, H.; Su, B.-L.; Liu, D.; Fu, Z. Hydroxyapatite-reinforced alginate fibers with bioinspired dually aligned architectures. Carbohydr. Polym. 2021, 267, 118167. [Google Scholar] [CrossRef]
- Lu, H.; Jian, M.; Yin, Z.; Xia, K.; Shi, S.; Zhang, M.; Wang, H.; Liang, X.; Ma, W.; Zhang, X.; et al. Silkworm silk fibers with multiple reinforced properties obtained through feeding Ag nanowires. Adv. Fiber Mater. 2022, 4, 547–555. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Tang, P.; Deng, Z.; He, P.; Chen, M.-J.; Yu, Z.-Z.; Zhang, H.-B. Silk fibroin reinforced graphene fibers with outstanding electrical conductivity and mechanical strength. Carbon 2023, 203, 886–894. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, J.; Rajabpour, S.; Joshi, K.; Kowalik, M.; Croom, B.; Schwab, Y.; Zhang, L.; Bumgardner, C.; Brown, K.R.; et al. Graphene reinforced carbon fibers. Sci. Adv. 2020, 6, eaaz4191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, C.; Zhang, M.; Jian, M.; Zhang, Y. Feeding single-walled carbon nanotubes or graphene to silkworms for reinforced silk fibers. Nano Lett. 2016, 16, 6695–6700. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chandra Biswas, M.; Ford, E. Dual roles of sodium polyacrylate in alginate fiber wet-spinning: Modify the solution rheology and strengthen the fiber. Carbohydr. Polym. 2022, 297, 120001. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, W.J. The Influence of Cellulose Nanocrystal Characteristics on Regenerative Silk Composite Fiber Properties. Materials 2023, 16, 2323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, H.; Liu, Y.; Zou, X.; Shi, J.; Zhao, Y.; Ye, Y.; Yu, Y.; Guo, J. Preparation of calcium alginate/polyethylene glycol acrylate double network fiber with excellent properties by dynamic molding method. Carbohydr. Polym. 2019, 226, 115277. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Q.; Zhong, Y.; Wei, P.; Yu, X.; Huang, J.; Cai, J. Super-strong and super-stiff chitosan filaments with highly ordered hierarchical structure. Adv. Funct. Mater. 2021, 31, 2104368. [Google Scholar] [CrossRef]
- Boroujeni, F.M.; Fioravanti, G.; Kander, R. Synthesis and Characterization of Cellulose Microfibril-Reinforced Polyvinyl Alcohol Biodegradable Composites. Materials 2024, 17, 526. [Google Scholar] [CrossRef]
- Gao, H.-L.; Zhao, R.; Cui, C.; Zhu, Y.-B.; Chen, S.-M.; Pan, Z.; Meng, Y.-F.; Wen, S.-M.; Liu, C.; Wu, H.-A.; et al. Bioinspired hierarchical helical nanocomposite macrofibers based on bacterial cellulose nanofibers. Natl. Sci. Rev. 2020, 7, 73–83. [Google Scholar] [CrossRef]
- Li, D.-L.; Shi, S.-C.; Lan, K.-Y.; Liu, C.-Y.; Li, Y.; Xu, L.; Lei, J.; Zhong, G.-J.; Huang, H.-D.; Li, Z.-M. Enhanced dielectric properties of all-cellulose composite film via modulating hydroxymethyl conformation and hydrogen bonding network. ACS Macro Lett. 2023, 12, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fan, Z.; Wu, G.; Shao, Y.; Xia, Z.; Wei, C.; Shen, F.; Tong, X.; Yu, J.; Chen, K.; et al. Assembly of nanofluidic MXene fibers with enhanced ionic transport and capacitive charge storage by flake orientation. ACS Nano 2021, 15, 7821–7832. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, W.; Mei, G.; Wang, S.; Wen, K.; Wang, M.; Feng, D.; Qian, D.; Zhu, M.; Zhou, X.; et al. Artificial spider silk with buckled sheath by nano-pulley combing. Adv. Mater. 2023, 35, 2212112. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xiong, J.; Sun, Z.; Wang, L.; Yu, J.; Qin, X. A general strategy to electrospin nanofibers with ultrahigh molecular chain orientation. Engineering 2022, 29, 179–187. [Google Scholar] [CrossRef]
- Fang, G.; Lu, H.; Rodriguez de la Fuente, L.; Law, A.M.K.; Lin, G.; Jin, D.; Gallego-Ortega, D. Mammary tumor organoid culture in non-adhesive alginate for luminal mechanics and high-throughput drug screening. Adv. Sci. 2021, 8, 2102418. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Jia, T.; Qi, Z.; Lu, S. Regenerated Fiber’s Ideal Target: Comparable to Natural Fiber. Materials 2024, 17, 1834. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Patil, A.; Hu, F.; Liu, X.Y. Hierarchical Structure of Silk Materials Versus Mechanical Performance and Mesoscopic Engineering Principles. Small 2019, 15, 1903948. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xia, K.; Jian, M.; Liang, X.; Yin, Z.; Zhang, M.; Wang, H.; Wang, H.; Li, S.; Zhang, Y. Mechanically reinforced silkworm silk fiber by hot stretching. Research 2022, 2022, 9854063. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Jiang, Y.; Hu, J. Scalable spider-silk-like supertough fibers using a pseudoprotein polymer. Adv. Mater. 2019, 31, 1904311. [Google Scholar] [CrossRef]
- Wang, J.; Fan, T.; Li, X.; Hu, X.; Huang, W.; Yuan, W.; Lin, Z. Artificial superstrong silkworm silk surpasses natural spider silks. Matter 2022, 5, 4396–4406. [Google Scholar] [CrossRef]
- Ismail, M.F.; Islam, M.A.; Khorshidi, B.; Tehrani-Bagha, A.; Sadrzadeh, M. Surface characterization of thin-film composite membranes using contact angle technique: Review of quantification strategies and applications. Adv. Colloid Interface Sci. 2022, 299, 102524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, W.; Qi, P.; Yang, J.; Xie, X.; Lin, M.; Xia, Y.; Nie, Z.; Sui, K. Hierarchical nano-helix as a new reinforcing unit for simultaneously ultra-strong and super-tough alginate fibers. Carbohydr. Polym. 2022, 297, 119998. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, L.; Tian, Y.; Li, R.; Zhu, C.; Zhao, G.; Cheng, Y. A novel low-alkali konjac gel induced by ethanol to modulate sodium release. Food Hydrocoll. 2020, 103, 105653. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.W.; Raines, R.T. The n→π* Interaction. Acc. Chem. Res. 2017, 50, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Mishra, K.K.; Sharma, N.; Das, A. Direct spectroscopic evidence for an n→π* interaction. Angew. Chem. Int. Ed. 2016, 55, 7801–7805. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Wang, S.; Yu, X.; Wang, B.; Chen, G.; Ren, L.; Li, J.; Jin, M.; Yu, J. Confining carbon dots in amino-functionalized mesoporous silica: N→π* interaction triggered deep-red solid-state fluorescence. Nano Res. 2022, 16, 4170–4177. [Google Scholar] [CrossRef]
- Hawe, A.; Friess, W.; Sutter, M.; Jiskoot, W. Online fluorescent dye detection method for the characterization of immunoglobulin G aggregation by size exclusion chromatography and asymmetrical flow field flow fractionation. Anal. Biochem. 2008, 378, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lv, B.; Wang, S.; Liu, Z.; Chen, X.D.; Cheng, Y. Study of molecular interaction and texture characteristics of hydrocolloid-mixed alginate microspheres: As a shell to encapsulate multiphase oil cores. Carbohydr. Polym. 2024, 326, 121603. [Google Scholar] [CrossRef]
- Su, L.; Mosquera, J.; Mabesoone, M.F.J.; Schoenmakers, S.M.C.; Muller, C.; Vleugels, M.E.J.; Dhiman, S.; Wijker, S.; Palmans, A.R.A.; Meijer, E.W. Dilution-induced gel-sol-gel-sol transitions by competitive supramolecular pathways in water. Science 2022, 377, 213–218. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Wang, Z.; Liu, Z.; Xu, X.; Liu, H.; Wang, D.; Tian, Z. Temperature-mediated phase separation enables strong yet reversible mechanical and adhesive hydrogels. ACS Nano 2023, 17, 13948–13960. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Lin, M.; Qi, P.; Shi, J.; Song, G.; Fan, W.; Sui, K.; Gao, C. Interfacial and build-in electric fields rooting in gradient polyelectrolyte hydrogel boosted heavy metal removal. Chem. Eng. J. 2022, 444, 136541. [Google Scholar] [CrossRef]
- Arndt, T.; Greco, G.; Schmuck, B.; Bunz, J.; Shilkova, O.; Francis, J.; Pugno, N.M.; Jaudzems, K.; Barth, A.; Johansson, J.; et al. Engineered spider silk proteins for biomimetic spinning of fibers with toughness equal to dragline silks. Adv. Funct. Mater. 2022, 32, 2200986. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Li, X.; Niu, S.; Zhou, X.; Lu, Y.; Yang, Y.; Sun, Y.; Meng, Q. High-strength and ultra-tough supramolecular polyamide spider silk fibers assembled via specific covalent and reversible hydrogen bonds. Acta Biomater. 2024, 176, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Hu, W.; Li, X.; Zhao, P.; Xia, Q. Bending–spinning produces silkworm and spider silk with enhanced mechanical properties. Macromolecules 2023, 56, 1199–1212. [Google Scholar] [CrossRef]
- Cao, G.S.; Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. A novel strategy for producing high-performance continuous regenerated fibers with wool-like structure. SusMat 2022, 2, 90–103. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Li, M.; Su, Y.; Zhang, Q.; Zhang, S.; Hu, J. Reconstructed hierarchically structured keratin fibers with shape-memory features based on reversible secondary-structure transformation. Adv. Mater. 2023, 35, 2304725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ma, N.; Li, S.; Zhang, L.; Tong, X.; Shao, Y.; Shen, C.; Wen, Y.; Jian, M.; Shao, Y.; et al. Reinforced wool keratin fibers via dithiol chain re-bonding. Adv. Funct. Mater. 2023, 33, 2213644. [Google Scholar] [CrossRef]
- Azam, F.; Ahmad, F.; Ahmad, S.; Zafar, M.S.; Ulker, Z. Synthesis and characterization of natural fibers reinforced alginate hydrogel fibers loaded with diclofenac sodium for wound dressings. Int. J. Biol. Macromol. 2023, 241, 124623. [Google Scholar] [CrossRef]
- Zhu, K.; Tu, H.; Yang, P.; Qiu, C.; Zhang, D.; Lu, A.; Luo, L.; Chen, F.; Liu, X.; Chen, L.; et al. Mechanically strong chitin fibers with nanofibril structure, biocompatibility, and biodegradability. Chem. Mater. 2019, 31, 2078–2087. [Google Scholar] [CrossRef]
- Mittal, N.; Jansson, R.; Widhe, M.; Benselfelt, T.; Hakansson, K.M.O.; Lundell, F.; Hedhammar, M.; Soderberg, L.D. Ultrastrong and bioactive nanostructured bio-based composites. ACS Nano 2017, 11, 5148–5159. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Chen, S.; Chen, Y.; Wang, B.; Pei, Q.; Wang, H. Macrofibers with high mechanical performance based on aligned bacterial cellulose nanofibers. ACS Appl. Mater. Interfaces 2017, 9, 20330–20339. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Xue, Z.; Liu, J.; Yan, M.; Xia, Y.; Wang, B. Preparation of carrageenan fibers with extraction of Chondrus via wet spinning process. Carbohydr. Polym. 2018, 194, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, J.; Guan, F.; Yin, J.; Yang, Q.; Zhang, S.; Tian, J.; Zhang, Y.; Ding, M.; Wang, W. Oxidized sodium alginate cross-linked calcium alginate/antarctic krill protein composite fiber for improving strength and water resistance. Colloids Surf. A 2023, 656, 130317. [Google Scholar] [CrossRef]
- He, Y.; Zhang, N.; Gong, Q.; Qiu, H.; Wang, W.; Liu, Y.; Gao, J. Alginate/graphene oxide fibers with enhanced mechanical strength prepared by wet spinning. Carbohydr. Polym. 2012, 88, 1100–1108. [Google Scholar] [CrossRef]
- Torres-Rendon, J.G.; Schacher, F.H.; Ifuku, S.; Walther, A. Mechanical performance of macrofibers of cellulose and chitin nanofibrils aligned by wet-stretching: A critical comparison. Biomacromolecules 2014, 15, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.; Aitomaki, Y.; Norberg, N.; Mathew, A.P.; Oksman, K. Dry-spun single-filament fibers comprising solely cellulose nanofibers from bioresidue. ACS Appl. Mater. Interfaces 2015, 7, 13022–13028. [Google Scholar] [CrossRef]
- Fu, X.; Liang, Y.; Wu, R.; Shen, J.; Chen, Z.; Chen, Y.; Wang, Y.; Xia, Y. Conductive core-sheath calcium alginate/graphene composite fibers with polymeric ionic liquids as an intermediate. Carbohydr. Polym. 2019, 206, 328–335. [Google Scholar] [CrossRef]
- Chen, Z.; Song, J.; Xia, Y.; Jiang, Y.; Murillo, L.L.; Tsigkou, O.; Wang, T.; Li, Y. High strength and strain alginate fibers by a novel wheel spinning technique for knitting stretchable and biocompatible wound-care materials. Mater. Sci. Eng. C 2021, 127, 112204–112215. [Google Scholar] [CrossRef]
- Bert, C.W.; Malik, M. Differential quadrature: A powerful new technique for analysis of composite structures. Compos. Struct. 1997, 39, 179–189. [Google Scholar] [CrossRef]
- Kabir, H.; Aghdam, M.M. A generalized 2D Bézier-based solution for stress analysis of notched epoxy resin plates reinforced with graphene nanoplatelets. Thin-Walled Struct. 2021, 169, 108484. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. J. Appl. Phycol. 2009, 22, 131–137. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arabian J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Gomez, C.G.; Pérez Lambrecht, M.V.; Lozano, J.E.; Rinaudo, M.; Villar, M.A. Influence of the extraction–purification conditions on final properties of alginates obtained from brown algae (Macrocystis pyrifera). Int. J. Biol. Macromol. 2009, 44, 365–371. [Google Scholar] [CrossRef]
- Torres, M.R.; Sousa, A.P.A.; Silva Filho, E.A.T.; Melo, D.F.; Feitosa, J.P.A.; de Paula, R.C.M.; Lima, M.G.S. Extraction and physicochemical characterization of Sargassum vulgare alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074. [Google Scholar] [CrossRef]
- Larsen, B.; Salem, D.M.S.A.; Sallam, M.A.E.; Mishrikey, M.M.; Beltagy, A.I. Characterization of the alginates from algae harvested at the Egyptian Red Sea coast. Carbohydr. Res. 2003, 338, 2325–2336. [Google Scholar] [CrossRef]




| Fiber type | β-Sheet (%) | Random coil (%) | Helix (%) | Stress (MPa) | Modulus (GPa) | Toughness (MJ m−3) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| SA fibers | SA fibers | 23.8 ± 1.6 | 55.9 ± 3.6 | 6.5 ± 1.2 | 295.1 ± 16.7 | 12.3 ± 0.8 | 18.2 ± 1.2 | This work |
| 50% EtOH | 32.8 ± 0.4 | 44.2 ± 0.2 | 8.9 ± 0.4 | 410.2 ± 9.5 | 15.4 ± 0.7 | 31.3 ± 1.9 | ||
| Spider silk fibers | SUMO-NTA72S-R-CTC92S | 29 | 17 | 17.8 | 103.0 ± 10.0 | 4.0 ± 0.9 | 10.0 ± 1.0 | [44] |
| SUMO-NT-R-CT | 35.6 | 17.5 | 19.2 | 1007 ± 77 | 36.7 ± 9.0 | 191 ± 26 | ||
| A15-A14 | 40.1 | 40.2 | 44.1 ± 19.6 | 1.7 ± 0.6 | 18.2 ± 20.3 | [43] | ||
| (A3I)3-A14 | 43.2 | 30.3 | 131.6 ± 31.9 | 3.5 ± 0.9 | 145.6 ± 42.2 | |||
| Regenerated silk fibroins fibers | Bending angle 0° | 25.9 ± 1.7 | 62.7 ± 1.4 | 1205 ± 78 | 11.1 ± 1.6 | 130.9 ± 24.2 | [45] | |
| Bending angle 90° | 33.2 ± 0.9 | 56.5 ± 2.1 | 1304 ± 178 | 13.4 ± 1.5 | 122.3 ± 38.6 | |||
| RSF-SN | 55.8 ± 1.6 | 14.8 ± 2.2 | 14.6 ± 1.0 | 2054 ± 177 | 43 ± 6 | 155.0 ± 34 | [30] | |
| RSF-Pa | 51.3 ± 1.1 | 17.3 ± 0.8 | 17.3 ± 0.3 | 816 ± 124 | 21 ± 3 | 215.8 ± 29 | ||
| B. mori silk | 44.2 ± 1.1 | 22.1 ± 0.6 | 16.1 ± 0.3 | 610 ± 84 | 16 ± 4 | 55.3 ± 12.4 | ||
| Silkworm Silk fibers | B. mori silk | 45 ± 2.5 | 38 ± 3.5 | 560 ± 80 | 11.0 ± 1.7 | 69 ± 25 | [28] | |
| Hot-stretched silkFibers (7.5%-S) | 49 ± 5 | 32.5 ± 7.5 | 770 ± 130 | 21.6 ± 2.8 | 80 ± 46 | |||
| Regenerated wool keratin fiber | Dry conditions | 65.4 a | -- | 34.6 a | 22.6 ± 2.7 | -- | -- | [47] |
| Wet conditions | 34.5 a | -- | 65.5 a | 2.71 ± 0.3 | -- | -- | ||
| 5 wt% PEGDA and 40 wt% cortical cells | 60.5 | 39.5 | 8.3 ± 1.0 cN/tex | -- | -- | [46] | ||
| GA/MDI crosslinked regenerated fibers | 63.3 | 36.7 | 10.0 ± 1.2 cN/tex | -- | -- | |||
| Drawing ratio 2.4 | 17.8 a | -- | 82.2 a | 144.9 ± 4.9 | 5 | 13.4 | [48] | |
| Drawing ratio 4.8 | 39.4 a | -- | 60.6 a | 186.1 ± 7.1 | 7.4 | 10.9 | ||
| Fiber Type | Additives | Control Group | Reinforced Fibers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Elongation (%) | Strength (MPa) | Toughness (MJ m−3) | Elongation (%) | Strength (MPa) | Toughness (MJ m−3) | Strength Increment (%) | Ref. | ||
| This work | -- | 9.7 | 295.1 | 18.2 | 11.2 | 410.2 | 31.3 | 39.0 | This work |
| Alginate fibers | Hydroxyapatite | 23.5 | 121.4 | 15.7 | 20.6 | 153.8 | 18.5 | 26.7 | [10] |
| Sodium polyacrylate | 11.8 | 0.57 cN/dtex | 5.6 | 7.0 | 0.64 cN/dtex | 3.5 | 12.3 | [15] | |
| Antarctic krill protein | 12.0 | 1.8 cN/dtex | 21.9 | 9.5 | 2.6 cN/dtex | 26.8 | 44.4 | [54] | |
| Kapok fibers | 2.1 | 135 cN | -- | 1.2 | 174 cN | -- | 28.9 | [49] | |
| Hemp fibers | 2.1 | 135 cN | -- | 1.5 | 185 cN | -- | 37.0 | ||
| Graphene oxide fibers | 16.8 | 320 | -- | 14.3 | 620 | -- | 93.8 | [55] | |
| Graphene fibers | 7.6 | 199 | -- | 5.6 | 203.8 | -- | 2.4 | [58] | |
| Alginate fibers | 16.0 | 130 | 22.0 | 18.0 | 173 | 23.0 | 33.1 | [59] | |
| Bacterial cellulose | 7.9 | 394.2 | 16.0 | 12.2 | 535.4 | 38.3 | 35.8 | [20] | |
| 7.9 | 394.2 | 16.0 | 13.0 | 504.2 | 37.1 | 27.9 | |||
| Chitin nanofibril fibers | -- | 9.5 | 132.0 | -- | 3.5 | 223 | -- | 68.9 | [56] |
| Chitin nanofibril fibers | -- | 8.6 | 1.7 cN/dtex | -- | 5.2 | 2.3 cN/dtex | -- | 35.3 | [50] |
| Cellulose nanofibers | -- | 5.8 | 131 | -- | 2.9 | 222 | -- | 69.5 | [57] |
| Bacterial cellulose | -- | 4.5 | 198 | -- | 3.8 | 248.6 | -- | 25.6 | [52] |
| Cellulose nanofibers | Spider silk proteins | 6.1 | 830 | 43.0 | 10.5 | 1015 | 55.0 | 22.3 | [51] |
| Silkworm silk | Ag Nanowires | 17.8 | 430 | 58.1 | 24.5 | 590 | 98.1 | 37.2 | [11] |
| Silkworm silk | -- | 16.4 | 560 | 69.0 | 11.9 | 770 | 80.0 | 37.5 | [28] |
| Carrageenan fibers | -- | 6.6 | 0.75 cN/dtex | -- | 9.6 | 0.73 cN/dtex | -- | −2.7 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Cui, M.; Wang, T.; Yang, J.; Li, W.; Wang, K.; Lin, M. Constructing Stiff β-Sheet for Self-Reinforced Alginate Fibers. Materials 2024, 17, 3047. https://doi.org/10.3390/ma17133047
Xie X, Cui M, Wang T, Yang J, Li W, Wang K, Lin M. Constructing Stiff β-Sheet for Self-Reinforced Alginate Fibers. Materials. 2024; 17(13):3047. https://doi.org/10.3390/ma17133047
Chicago/Turabian StyleXie, Xuelai, Min Cui, Tianyuan Wang, Jinhong Yang, Wenli Li, Kai Wang, and Min Lin. 2024. "Constructing Stiff β-Sheet for Self-Reinforced Alginate Fibers" Materials 17, no. 13: 3047. https://doi.org/10.3390/ma17133047
APA StyleXie, X., Cui, M., Wang, T., Yang, J., Li, W., Wang, K., & Lin, M. (2024). Constructing Stiff β-Sheet for Self-Reinforced Alginate Fibers. Materials, 17(13), 3047. https://doi.org/10.3390/ma17133047







