The Influence of Functional Composite Coatings on the Properties of Polyester Films before and after Accelerated UV Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. PLA/PHBV Blend Films’ Extrusion
2.2.2. Extracts Preparation
2.2.3. Preliminary Tests
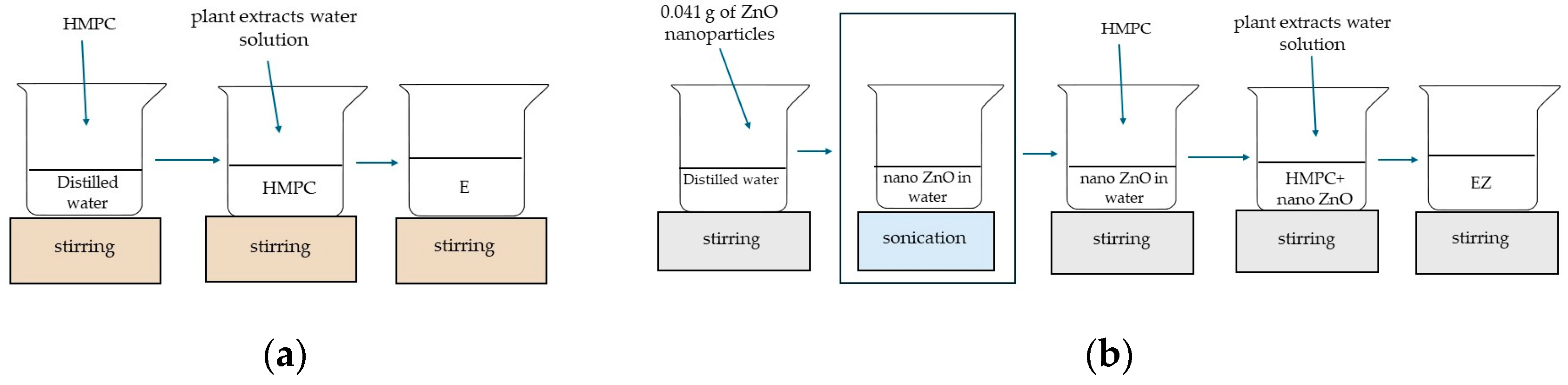
2.2.4. Preparation of the Coating Formulation and Application onto PLA/PHBV Film Surface
2.2.5. Q-SUN Irradiation
2.2.6. Antimicrobial Analysis
2.2.7. SEM Analysis
2.2.8. FTIR-ATR Analysis
2.2.9. Color Measurement of the Films
3. Results and Discussion
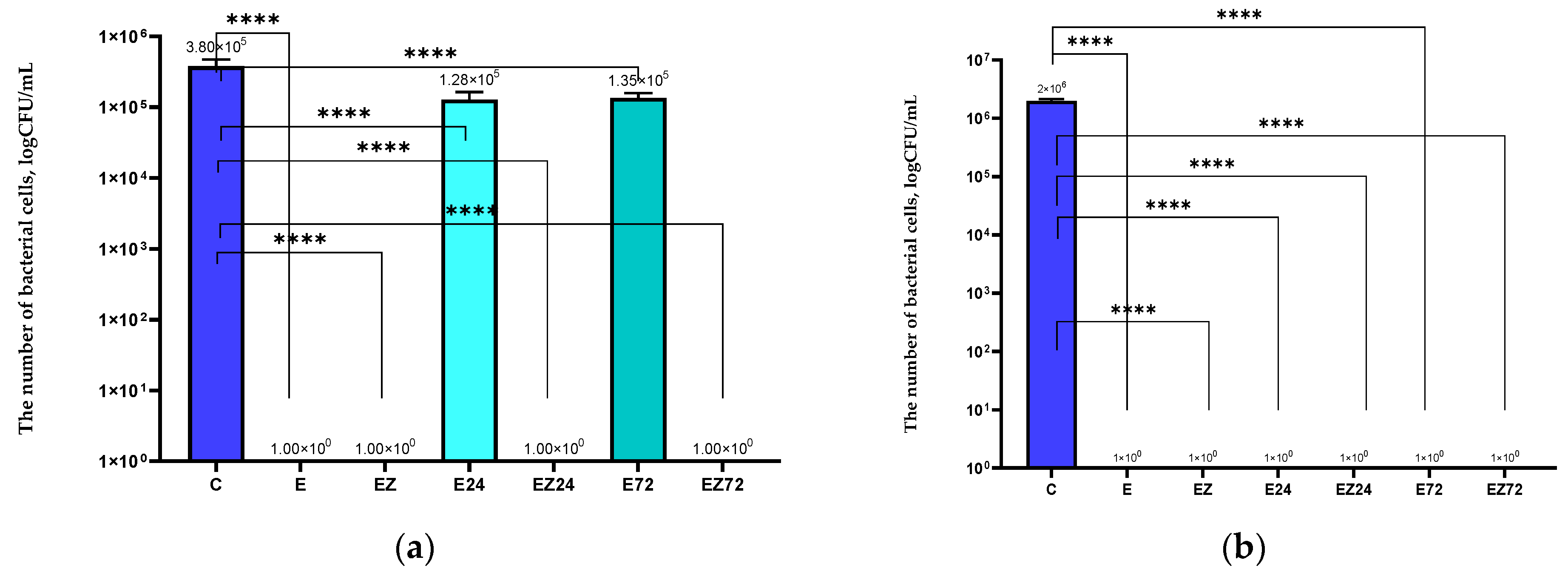
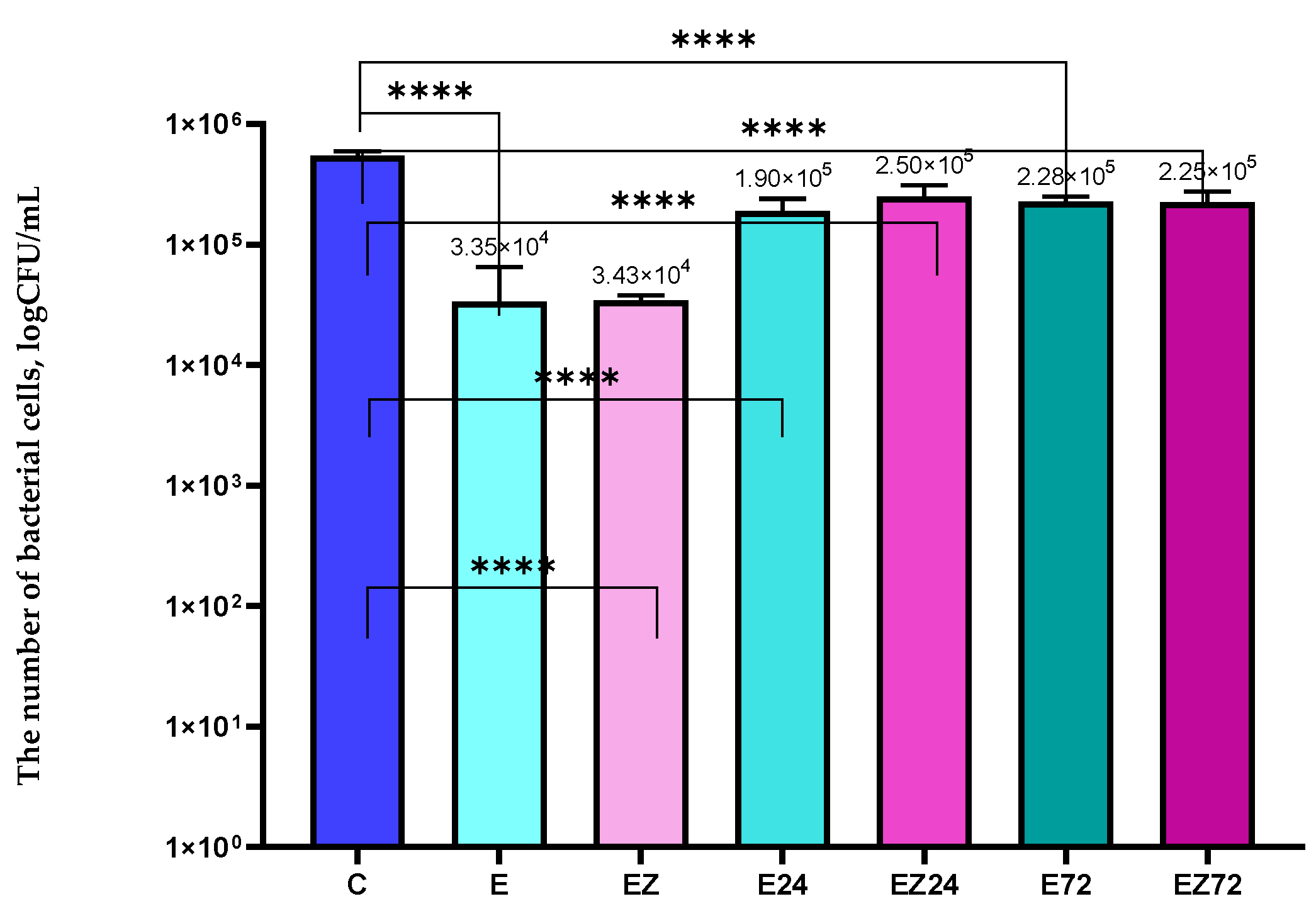
3.1. Antimicrobial Properties Analysis
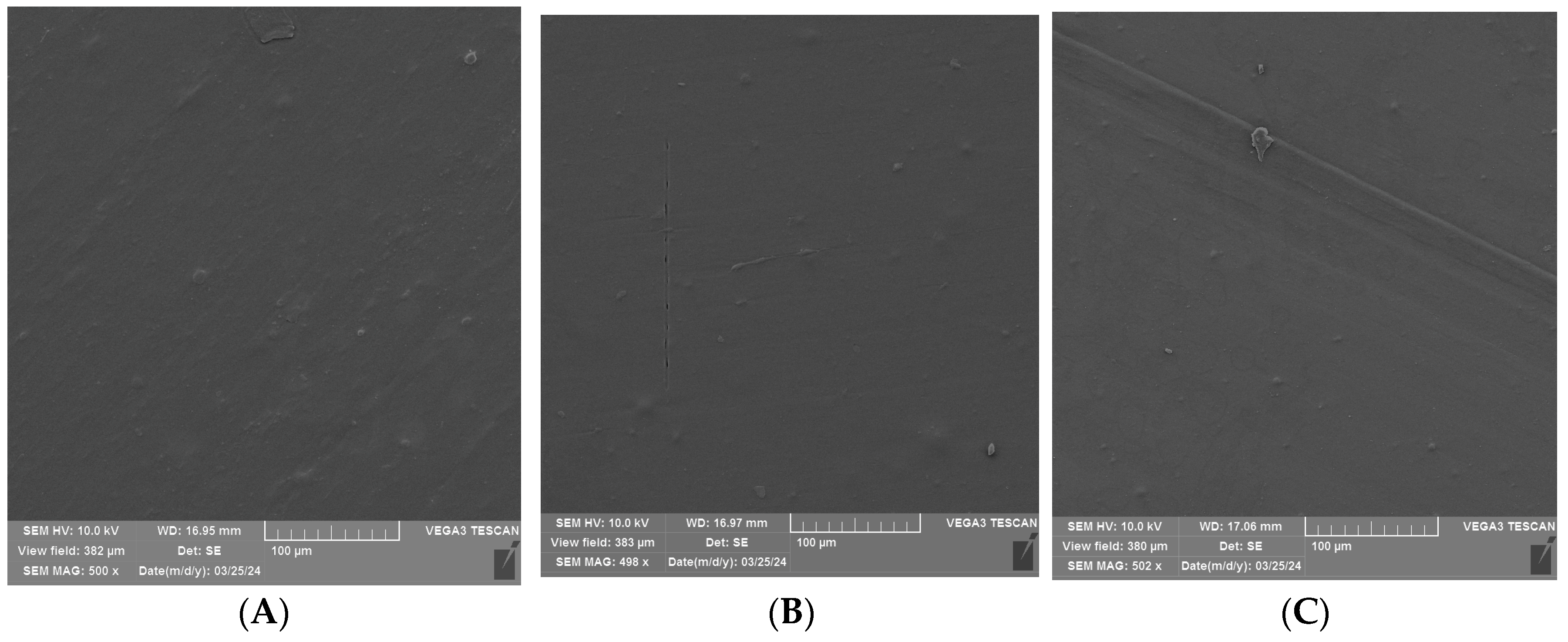
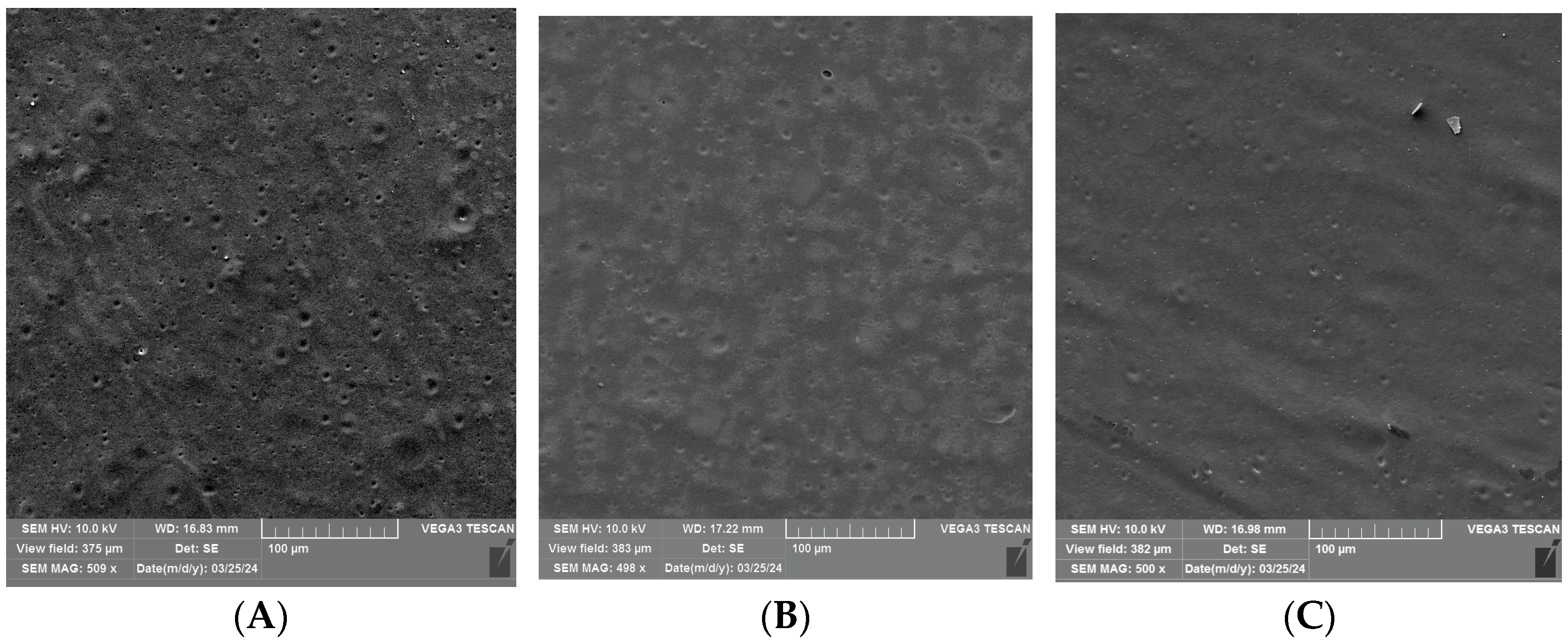
3.2. SEM Analysis of Uncoated and Coated PLA/PHBV Films
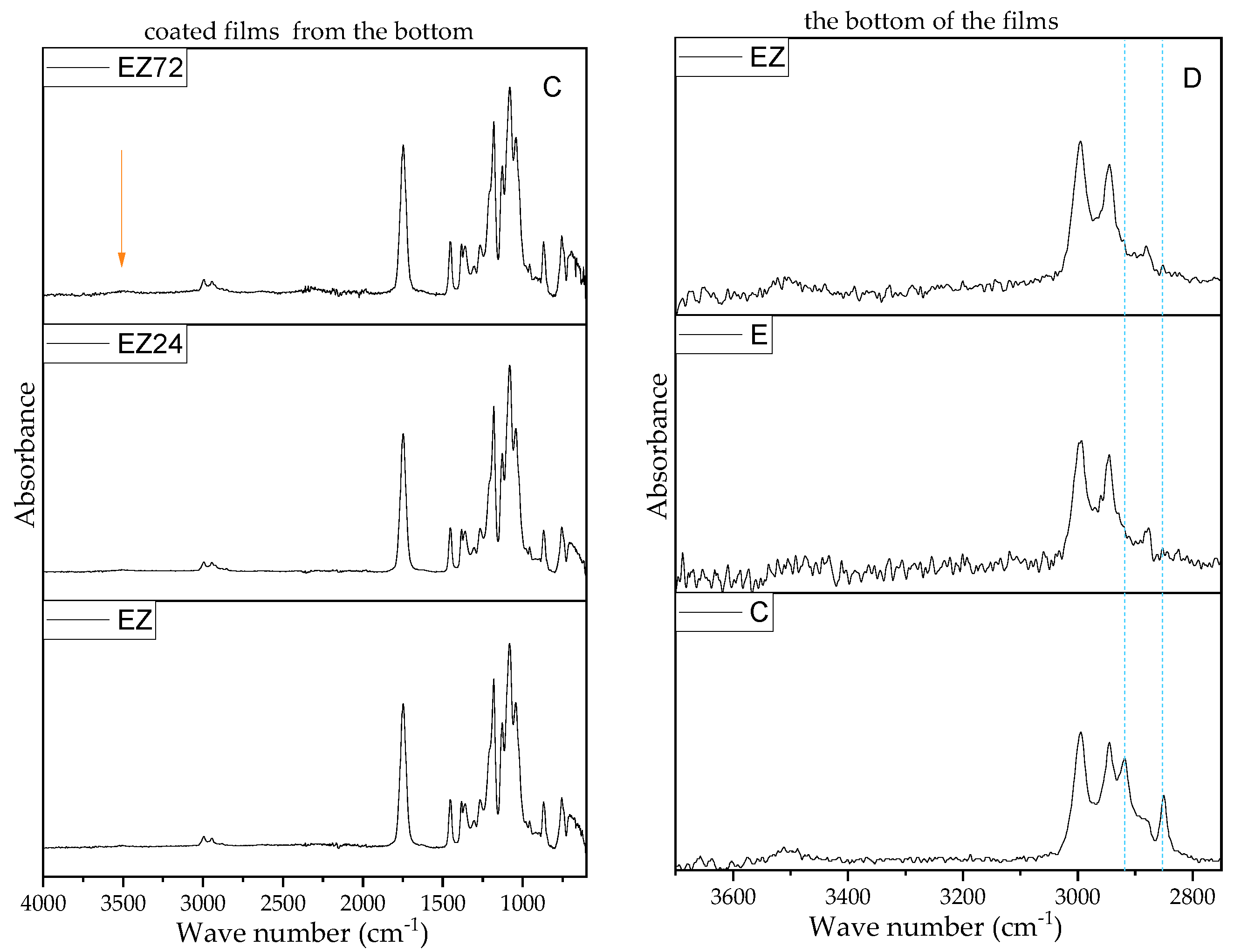
3.3. Results of FTIR-ATR Analysis
3.4. CIELab Measurement Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lajarrige, A.; Gontard, N.; Gaucel, S.; Peyron, S. Evaluation of the Food Contact Suitability of Aged Bio-Nanocomposite Materials Dedicated to Food Packaging Applications. Appl. Sci. 2020, 10, 877. [Google Scholar] [CrossRef]
- De Luca, S.; Milanese, D.; Gallichi-Nottiani, D.; Cavazza, A.; Sciancalepore, C. Poly(lactic acid) and Its Blends for Packaging Application: A Review. Clean Technol. 2023, 5, 1304–1343. [Google Scholar] [CrossRef]
- Madbouly, S.A. Bio-based polyhydroxyalkanoates blends and composites. In Biopolymers and Composites: Processing and Characterization; Madbouly, S.A., Zhang, C., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021; p. 235. [Google Scholar] [CrossRef]
- Wang, Q.; Bobadilla, S.; Espert, M.; Sanz, T.; Salvador, A. Shortening replacement by hydroxypropyl methylcellulose-based oleogels obtained by different indirect approaches. Texture and sensory properties of baked puff pastry. Food Hydrocoll. 2024, 153, 109936. [Google Scholar] [CrossRef]
- Jayalakshmi, K.; Ismayil; Hegde, S.; Monteiro, J. Investigating the properties of hydroxy propyl methyl cellulose based magnesium ion-conducting solid polymer electrolytes for primary battery applications. J. Energy Storage 2024, 89, 111575. [Google Scholar] [CrossRef]
- Ordon, M.; Burdajewicz, W.; Sternal, J.; Okręglicki, M.; Mizielińska, M. The Antibacterial Effect of the Films Coated with the Layers Based on Uncaria tomentosa and Formitopsis betulina Extracts and ZnO Nanoparticles and Their Influence on the Secondary Shelf-Life of Sliced Cooked Ham. Appl. Sci. 2023, 13, 8853. [Google Scholar] [CrossRef]
- Ordon, M.; Nawrotek, P.; Stachurska, X.; Schmidt, A.; Mizielińska, M. Mixtures of Scutellaria baicalensis and Glycyrrhiza L. Extracts as Antibacterial and Antiviral Agents in Active Coatings. Coatings 2021, 11, 1438. [Google Scholar] [CrossRef]
- Mizielińska, M.; Nawrotek, P.; Stachurska, X.; Ordon, M.; Bartkowiak, A. Packaging Covered with Antiviral and Antibacterial Coatings Based on ZnO Nanoparticles Supplemented with Geraniol and Carvacrol. Int. J. Mol. Sci. 2021, 22, 1717. [Google Scholar] [CrossRef]
- Frański, R.; Beszterda-Buszczak, M. Comment on Villalva et al. Antioxidant, Anti-Inflammatory, and Antibacterial Properties of an Achillea millefolium L. Extract and Its Fractions Obtained by Supercritical Anti-Solvent Fractionation against Helicobacter pylori. Antioxidants 2022, 11, 1849. Antioxidants 2023, 12, 1226. [Google Scholar] [CrossRef] [PubMed]
- Ivanović, M.; Grujić, D.; Cerar, J.; Islamčević Razboršek, M.; Topalić-Trivunović, L.; Savić, A.; Kočar, D.; Kolar, M. Extraction of Bioactive Metabolites from Achillea millefolium L. with Choline Chloride Based Natural Deep Eutectic Solvents: A Study of the Antioxidant and Antimicrobial Activity. Antioxidants 2022, 11, 724. [Google Scholar] [CrossRef]
- Daniel, P.S.; Lourenco, E.L.B.; Sete da Cruz, R.M.; de Souza Goncalves, C.H.; Marques Das Almas, L.R.; Hoscheid, J.; da Silva, C.; Jacomassi, E.; Brum, L.; Alberton, O. Composition and antimicrobial activity of essential oil of yarrow (’Achillea millefolium’ L.). Aust. J. Crop Sci. 2020, 14, 545. Available online: https://search.informit.org/doi/10.3316/informit.123462086183285 (accessed on 16 June 2024). [CrossRef]
- Radulović, N.S.; Dekić, M.S.; Ranđelović, P.J.; Stojanović, N.M.; Zarubica, A.R.; Stojanović-Radić, Z.Z. Toxic essential oils: Anxiolytic, antinociceptive and antimicrobial properties of the yarrow Achillea mbellate Sibth. Et Sm. (Asteraceae) volatiles. Food Chem. Toxicol. 2012, 50, 2016. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, N.; Olas, B. Selected Seeds as Sources of Bioactive Compounds with Diverse Biological Activities. Nutrients 2023, 15, 187. [Google Scholar] [CrossRef]
- Netreba, N.; Sandulachi, E.; Macari, A.; Popa, S.; Ribintev, I.; Sandu, I.; Boestean, O.; Dianu, I. A Study on the Fruiting and Correlation between the Chemical Indicators and Antimicrobial Properties of Hippophae rhamnoides L. Horticulturae 2024, 10, 137. [Google Scholar] [CrossRef]
- Sadowska, B.; Budzyńska, A.; Stochmal, A.; Żuchowski, J.; Różalska, B. Novel properties of Hippophae rhamnoides L. twig and leaf extracts anti-virulence action and synergy with antifungals studied in vitro on Candida spp. Model. Microb. Pathog. 2017, 107, 372. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Batool, S.; Khan, S.W.; Hussain, A.; Din, S.U.; Nafees, M.A.; Ali, S.; Faizi, M.A.; Ullah, A. Antimicrobial Study of Selected Medicinal Plants (Datura stramonium L. and Hippophae rhamnoides L.) of Hunza Valley, Gilgit-Baltistan: Antimicrobial Study of Selected Medicinal Plants. Biol. Sci.-PJSIR 2021, 64, 251–255. [Google Scholar] [CrossRef]
- Dastan, S.D. Chemical and functional composition and biological activities of Anatolian Hypericum scabrum L. plant. J. Mol. Struct. 2023, 1275, 134561. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Rahnama, G.H.; Malekpoor, F.; Broujeni, H.R. Variation in antibacterial activity and phenolic content of Hypericum scabrum L. populations. J. Med. Plant. Res. 2011, 5, 4119. Available online: http://www.academicjournals.org/JMPR (accessed on 16 June 2024).
- Poças, F.; Franz, R. Overview on European Regulatory Issues, Legislation, and EFSA Evaluations of Nanomaterials. In Nanotechnology for Food Packaging: Materials, Processing Technologies, and Safety Issues; Cerqueira, M., Maria Lagaron, J., Pastrana Castro, L., Vicente., A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Akbar, A.; Anal, A.K. Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in readyto-eat poultry meat. Food Control 2014, 38, 88–95. [Google Scholar] [CrossRef]
- Mizielińska, M.; Łopusiewicz, Ł.; Mężyńska, M.; Bartkowiak, A. The Influence of Accelerated UV-A and Q-SUN Irradiation on the Antimicrobial Properties of Coatings Containing ZnO Nanoparticles. Molecules 2017, 22, 1556. [Google Scholar] [CrossRef]
- Mizielińska, M.; Bartkowiak, A. The Influence of the Q-SUN and UV-B Irradiation on the Antiviral Properties of the PP Films Covered with the Coatings Based on ZnO Nanoparticles and TiO2. Coatings 2024, 14, 125. [Google Scholar] [CrossRef]
- Ran, X.; Qu, Y.; Wang, Y.; Cui, B.; Shen, Y.; Li, Y. Enhanced UV-Blocking Capabilities of Polylactic Acid Derived from Renewable Resources for Food and Drug Packaging: A Mini-Review. J. Compos. Sci. 2023, 7, 410. [Google Scholar] [CrossRef]
- Lawrynowicz, A.; Palo, E.; Nizamov, R.; Miettunen, K. Self-cleaning and UV-blocking cotton—Fabricating effective ZnO structures for photocatalysis. J. Photochem. Photobiol. A Chem. 2024, 450, 115420. [Google Scholar] [CrossRef]
- Kubra, I.R.; Kumar, D.; Rao, L.J.M. Effect of microwave-assisted extraction on the release of polyphenols from ginger (Zingiber officinale). Int. J. Food Sci. Tech. 2013, 48, 1828–1833. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction—An Innovative and Promising Extraction Tool for Medicinal Plant Research. Pharmacogn. Rev. 2007, 1, 7–18. Available online: http://www.phcogrev.com (accessed on 16 June 2024).
- E 2180-01: 2002; ASTM Standard Test Method for Determining the Activity of Incorporated Antimicrobial Agent(s) in Polymeric or Hydrophobic Materials. ASTM: West Conshohocken, PA, USA, 2002. Available online: https://standards.globalspec.com/std/4472809/astm-e2180-18 (accessed on 16 June 2024).
- Bhetwal, A.; Maharjan, A.; Shakya, S.; Satyal, D.; Ghimire, S.; Khanal, P.R.; Parajuli, N.P. Isolation of Potential Phages against Multidrug-Resistant Bacterial Isolates: Promising Agents in the Rivers of Kathmandu, Nepal. BioMed Res. Int. 2017, 2017, 3723254. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, N.; Rojas, M.J.; Cruz, G.N.F.; Hung, S.H.; Rohwer, F.; Barr, J.J. Phage on tap–a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ 2016, 4, 2261. [Google Scholar] [CrossRef]
- ISO 22196-2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2011. Available online: https://www.iso.org/standard/54431.html (accessed on 16 June 2024).
- Skaradzińska, A.; Ochocka, M.; Śliwka, P.; Kuźmińska-Bajora, M.; Skaradziński, G.; Friese, A.; Roschanski, N.; Murugaiyan, J.; Roesler, U. Bacteriophage amplification–A comparison of selected methods. J. Virol. Methods 2020, 282, 113856. [Google Scholar] [CrossRef] [PubMed]
- Saberi, B.; Thakur, R.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Optimization of physical and optical properties of biodegradable edible films based on pea starch and guar gum. Ind. Crops Prod. 2016, 86, 342–352. [Google Scholar] [CrossRef]
- Zdanowicz, M. Influence of Epilobium parviflorum Herbal Extract on Physicochemical Properties of Thermoplastic Starch Films. Polymers 2024, 16, 64. [Google Scholar] [CrossRef]
- Ong, G.; Kasi, R.; Subramaniam, R. A review on plant extracts as natural additives in coating applications. Prog. Org. Coat. 2021, 151, 106091. [Google Scholar] [CrossRef]
- Brobbey, K.J.; Saarinen, J.; Alakomi, H.; Yang, B.; Toivakka, M. Efficacy of natural plant extracts in antimicrobial packaging systems. J. Appl. Packag. Res. 2017, 9, 60–71. Available online: http://scholarworks.rit.edu/japr/vol9/iss1/6 (accessed on 16 June 2024).
- Mizielińska, M.; Kowalska, U.; Salachna, P.; Łopusiewicz, Ł.; Jarosz, M. The Influence of Accelerated UV-A and Q-SUN Irradiation on the Antibacterial Properties of Hydrophobic Coatings Containing Eucomis comosa Extract. Polymers 2018, 10, 421. [Google Scholar] [CrossRef]
- Kairyte, K.; Kadys, A.; Luksiene, Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. Biol. 2013, 128, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, H.; Shahdadi, F.; Sardoei, A.S.; Hatami, M.; Ghorbanpour, M. Investigation of physio-mechanical, antioxidant and antimicrobial properties of starch–zinc oxide nanoparticles active films reinforced with Ferula gummosa Boiss essential oil. Sci. Rep. 2024, 14, 5789. [Google Scholar] [CrossRef] [PubMed]
- Mizielińska, M.; Ordon, M.; Burdajewicz, W.; Nawrotek, P.; Sternal, J.; Okręglicki, M. The Antifungal and Antiviral Activity of Coatings Containing Zinc Oxide Nanoparticles and Verbascum L. or Formitopsis betulina Extracts and Their Influence on the Quality of Strawberries after Storage. Coatings 2024, 14, 260. [Google Scholar] [CrossRef]
- Donn, P.; Barciela, P.; Perez-Vazquez, A.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Bioactive Compounds of Verbascum sinuatum L.: Health Benefits and Potential as New Ingredients for Industrial Applications. Biomolecules 2023, 13, 427. [Google Scholar] [CrossRef]
- Okasha, Y.M.; Fathy, F.I.; Fathy Soliman, F.; Fayek, N.M. The untargeted phytochemical profile of Verbascum hapsus L. with potent antiviral, antibacterial and anticancer activities. S. Afr. J. Bot. 2023, 156, 334. [Google Scholar] [CrossRef]
- Sofrenić, I.; Anđelković, B.; Todorović, N.; Stanojković, T.; Vujisić, L.; Novaković, M.; Milosavljević, S.; Tešević, V. Cytotoxic triterpenoids and triterpene sugar esters from the medicinal mushroom Fomitopsis betulina. Phytochemistry 2021, 181, 112580. [Google Scholar] [CrossRef]
- Herrera, D.R.; Durand-Ramirez, J.E.; Falcăo, A.; Nogueira da Silva, E.J.L.; dos Santos, E.B.; Figueiredo de Almeida Gomes, B.P. Antimicrobial activity and substantivity of Uncaria tomentosa in infected root, canal dentin. Braz. Oral Res. 2016, 30, 61. [Google Scholar] [CrossRef]
- Cahuana-Vasquez, R.A.; Soléo Ferreira dos Santos, S.; Koga-Ito, C.Y.; Cardoso Jorge, A.O. Antimicrobial activity of Uncaria tomentosa against oral human pathogens. Braz. Oral Res. 2007, 46, 46. [Google Scholar] [CrossRef]
- Mirzapoor, A.; Ghashghaee, E. Nano engineered synthesis layer by layer and transparent synergistic antimicrobial coating based on Se/Ag/TiO2/ZnO hybrid nanostructures. Inorg. Chem. Commun. 2023, 153, 110840. [Google Scholar] [CrossRef]
- Carvalho, P.; Sampaio, P.; Azevedo, S.; Vaz, C.; Espinós, J.P.; Teixeira, V.; Carneiro, J.O. Influence of thickness and coatings morphology in the antimicrobial performance of zinc oxide coatings. Appl. Surf. Sci. 2024, 307, 548–557. [Google Scholar] [CrossRef]
- Nascimento da Silva, M.; de Matos Fonseca, J.; Kirchner Feldhaus, H.; Santos Soares, L.; Valencia, G.A.; Maduro de Campos, C.E.; Di Luccio, M.; Monteiro, A.R. Physical and morphological properties of hydroxypropyl methylcellulose films with curcumin polymorphs. Food Hydrocoll. 2019, 97, 105217. [Google Scholar] [CrossRef]
- Brütting, C.; Dreier, J.; Bonten, C.; Altstädt, V.; Ruckdäschel, H. Sustainable Immiscible Polylactic Acid (PLA) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) Blends: Crystallization and Foaming Behavior. ACS Sustain. Chem. Eng. 2023, 11, 6676–6687. [Google Scholar] [CrossRef]
- Perez-Martinez, V.; Bello-Rocha, L.; Rodríguez-Rodriguez, C.; Sierra, C.A.; Castellanos, D.A. Obtention and characterization of PLA/PHBV thin sheets by solvent casting and extrusion with application in food packaging. Bull. Mater. Sci. 2024, 47, 47. [Google Scholar] [CrossRef]
- Tubio, C.R.; Valle, X.; Carvalho, E.; Moreira, J.; Costa, P.; Correia, D.M.; Lanceros-Mendez, S. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Blends with Poly(caprolactone) and Poly(lactic acid): A Comparative Study. Polymers 2023, 15, 4566. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, C.; Sun, J.; Zhu, Q.; Liu, J. Structure and Properties of Polylactic Acid Biocomposite Films Reinforced with Cellulose Nanofibrils. Molecules 2020, 25, 3306. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhu, C.; Xiong, P.; Gong, J.; Zhao, K. Calcium alginate-coated electrospun polyhydroxybutyrate/carbon nanotubes composite nanofibers as nanofiltration membrane for dye removal. J. Mater. Sci. 2018, 53, 14801–14820. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Villani, F.; Rizzo, A.; Caputo, I.; Paolella, G.; Vigliotta, G. Antibacterial Al-doped ZnO coatings on PLA films. J. Mater. Sci. 2020, 55, 4830–4847. [Google Scholar] [CrossRef]
- Dos Santos, F.A.; Tavares, M.I.B. Development and characterization of hybrid materials based on biodegradable PLA matrix, microcrystalline cellulose and organophilic silica. Polímeros 2014, 24, 561–566. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties. Foods 2020, 9, 1572. [Google Scholar] [CrossRef]




| Sample | L* | a* | b* | ∆E | YI | C* |
|---|---|---|---|---|---|---|
| C | 97.3 (0.04) | 0.04 (0.01) | 0.37 (0.05) | - | 0.543 | 0.372 |
| C24 | 97.3 (0.25) | 0.06 (0.01) | 0.34 (0.04) | 0.036 | 0.499 | 0.345 |
| C72 | 97.5 (0.06) | 0.09 (0.02) | 0.37 (0.04) | 0.206 | 0.542 | 0.381 |
| E | 96.8 (0.09) | −0.15 (0.03) | 1.81 (0.25) | 1.532 | 2.739 | 1.866 |
| E24 | 96.7 (0.15) | −0.18 (0.03) | 2.41 (0.04) | 2.090 | 3.553 | 2.417 |
| E72 | 96.7 (0.04) | −0.29 (0.06) | 2.62 (0.22) | 2.367 | 3.951 | 2.695 |
| EZ | 96.8 (0.07) | −0.15 (0.01) | 1.81 (0.07) | 1.101 | 2.131 | 1.458 |
| EZ24 | 96.9 (0.05) | −0.16 (0.03) | 1.86 (0.13) | 1.575 | 2.730 | 1.857 |
| EZ72 | 97.0 (0.06) | −0.18 (0.03) | 1.89 (0.21) | 1.625 | 2.845 | 1.938 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizielińska, M.; Zdanowicz, M.; Tarnowiecka-Kuca, A.; Bartkowiak, A. The Influence of Functional Composite Coatings on the Properties of Polyester Films before and after Accelerated UV Aging. Materials 2024, 17, 3048. https://doi.org/10.3390/ma17133048
Mizielińska M, Zdanowicz M, Tarnowiecka-Kuca A, Bartkowiak A. The Influence of Functional Composite Coatings on the Properties of Polyester Films before and after Accelerated UV Aging. Materials. 2024; 17(13):3048. https://doi.org/10.3390/ma17133048
Chicago/Turabian StyleMizielińska, Małgorzata, Magdalena Zdanowicz, Alicja Tarnowiecka-Kuca, and Artur Bartkowiak. 2024. "The Influence of Functional Composite Coatings on the Properties of Polyester Films before and after Accelerated UV Aging" Materials 17, no. 13: 3048. https://doi.org/10.3390/ma17133048







