Effect of Temperature on the Removal of Interferences in the Voltammetric Procedure for the Determination of Cr(VI)
Abstract
1. Introduction
2. Materials and Experimental Work
2.1. Reagents
2.2. Equipment
2.3. Measumerent Procedure
2.4. Course of Voltammetric Measurement
3. Results and Discussion
3.1. Speciation and the Influence of Temperature on Cr(VI) Voltammetric Signal
3.2. Surfactants
3.3. Effect of Temperature on Removal of Interference from Surfactants
3.3.1. Triton X-100 Nonionic Surfactant
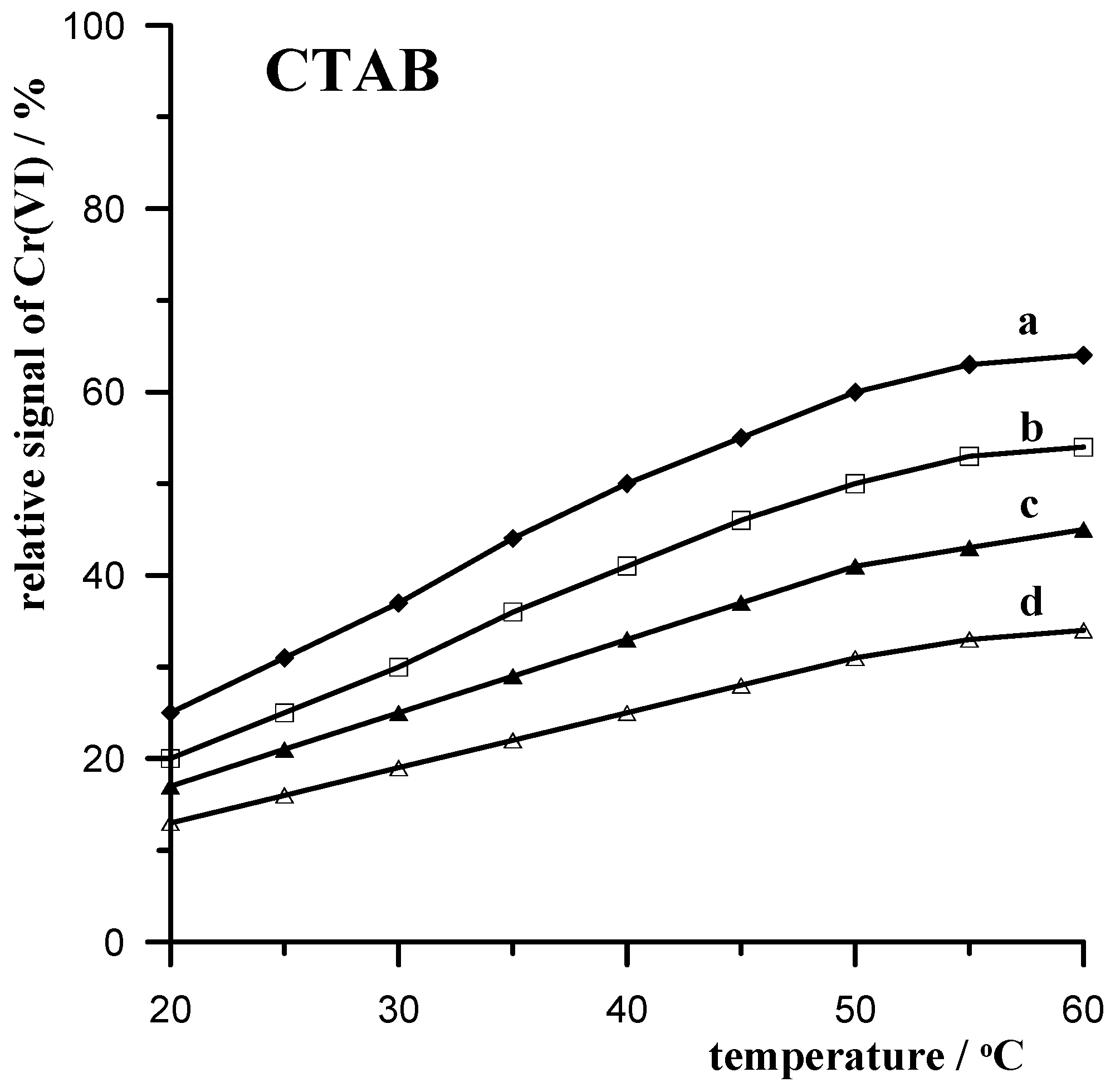
3.3.2. CTAB Cationic Surfactant
3.3.3. SDS Anionic Surfactant
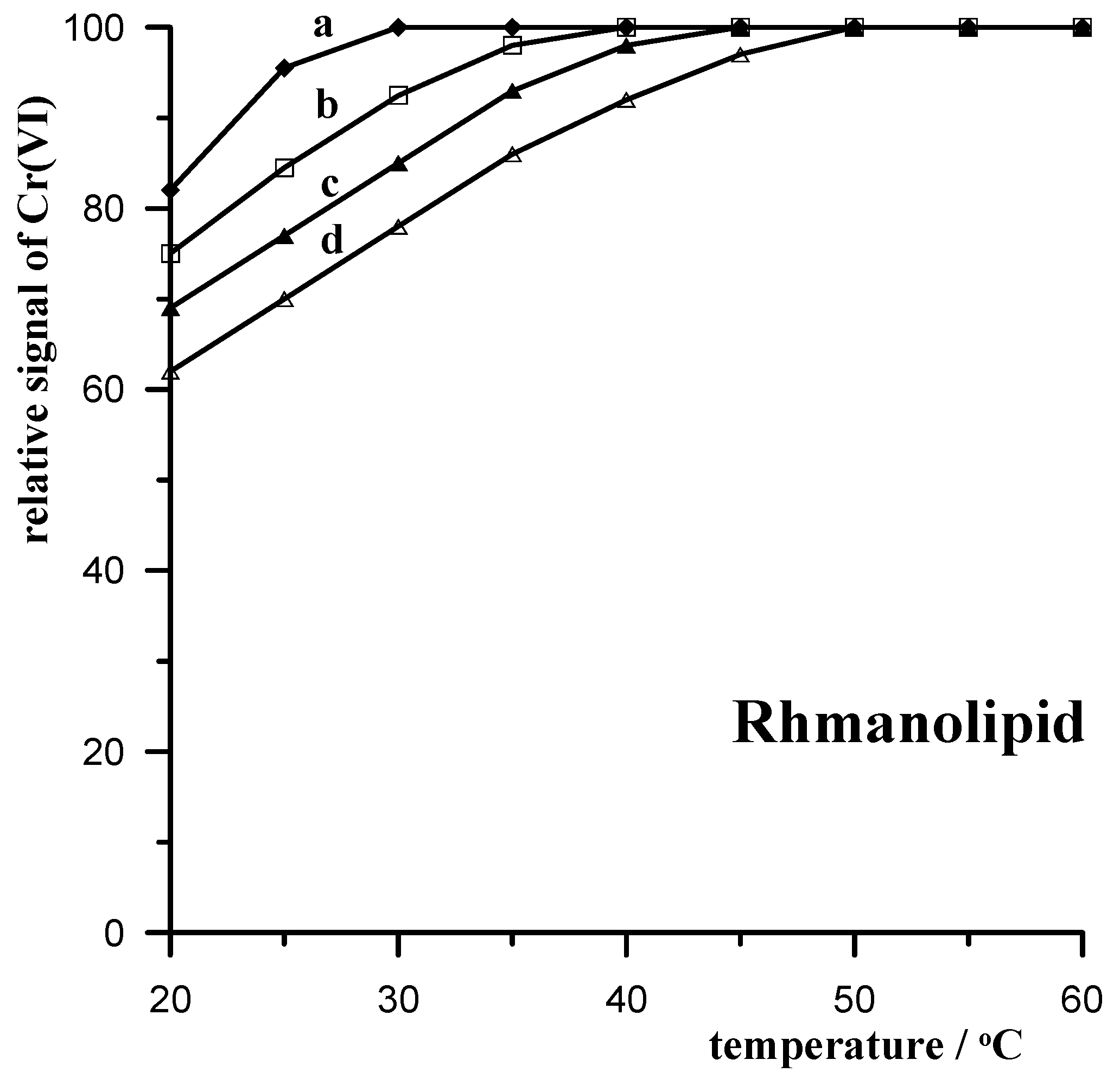
3.3.4. Rhamnolipid Biosurfactant
3.4. Analysis of Aqueous Environmental Samples for the Presence of Surfactants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruzzoniti, M.C.; Sarzanini, C.; Mentasti, E. Preconcentration of contaminants in water analysis. J. Chrom. A 2000, 902, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Borah, P.; Kumar, M.; Devi, P. Types of inorganic pollutants: Metals/metalloids, acids, and organic forms. In Inorganic Pollutants in Water; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Helaluddin, A.B.M.; Khalid, R.S.; Alaama, M.; Abbas, S.A. Main Analytical Techniques Used for Elemental Analysis in Various Matrices. Trop. J. Pharm. Res. 2016, 15, 427. [Google Scholar] [CrossRef]
- Porada, R.; Jedlińska, K.; Lipińska, J.; Baś, B. Review—Voltammetric Sensors with Laterally Placed Working Electrodes: A Review. J. Electrochem. Soc. 2020, 167, 037536. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324. [Google Scholar] [CrossRef]
- Arino, C.; Banks, C.E.; Bobrowski, A.; Crapnell, R.D.; Economou, A.; Królicka, A.; Perez-Rafols, C.; Soulis, D.; Wang, J. Electrochemical stripping analysis. Nat. Rev. Methods Primers 2022, 2, 62. [Google Scholar] [CrossRef]
- Hoyer, B.; Jansen, N. Suppression of surfactant interferences in anodic stripping voltammetry by sodium dodecyl sulfate. Electrochem. Commun. 2003, 5, 759. [Google Scholar] [CrossRef]
- Lange, B.; van den Berg, C.M.G. Determination of selenium by catalytic cathodic stripping voltammetry. Anal. Chim. Acta 2000, 418, 33. [Google Scholar] [CrossRef]
- Louis, Y.; Cmuk, P.; Omanović, D.; Garnier, C.; Lenoble, V.; Mounier, S.; Pižeta, I. Speciation of trace metals in natural waters: The influence of an adsorbed layer of natural organic matter (NOM) on voltammetric behaviour of copper. Anal. Chim. Acta 2008, 606, 37. [Google Scholar] [CrossRef] [PubMed]
- Olkowska, E.; Polkowska, Ż.; Namieśnik, J. Analytics of Surfactants in the Environment: Problems and Challenges. Chem. Rev. 2011, 111, 5667. [Google Scholar] [CrossRef]
- Palmer, M.; Hatley, H. The role of surfactants in wastewater treatment: Impact, removal and future techniques: A critical review. Water Res. 2018, 147, 60. [Google Scholar] [CrossRef]
- Nunes, R.S.; Teixeira, A.C.S.C. An overview on surfactants as pollutants of concern: Occurrence, impacts and persulfate-based remediation technologies. Chermosphere 2022, 300, 134507. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, M.; Koper, A. How to Determine Uranium Faster and Cheaper by Adsorptive Stripping Voltammetry in Water Samples Containing Surface Active Compounds. Electroanalysis 2011, 23, 1442. [Google Scholar] [CrossRef]
- Piech, R.; Baś, B.; Kubiak, W.W. The cyclic renewable mercury film silver based electrode for determination of molybdenum (VI) traces using adsorptive stripping voltammetry. Talanta 2008, 76, 295. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Khayamian, T.; Khaloo, S.S. Simultaneous determination of trace amounts of vanadium and molybdenum in water and foodstuff samples using adsorptive cathodic stripping voltammetry. Int. J. Food Sci. Technol. 2008, 43, 416. [Google Scholar] [CrossRef]
- Sander, S. Simultaneous adsorptive stripping voltammetric determination of molybdenum (VI), uranium (VI), vanadium (V), and antimony (III). Anal. Chim. Acta 1999, 394, 81. [Google Scholar] [CrossRef]
- Grad, O.A.; Ciopec, N.; Negrea, A.; Duteanu, N.; Negrea, P.; Voda, R. Evaluation of Performance of Functionalized Amberlite XAD7 with Dibenzo-18-Crown Ether-6 for Palladium Recovery. Materials 2021, 14, 1003. [Google Scholar] [CrossRef] [PubMed]
- Zając, P.; Kluczniak, G.; Bobrowski, A.; Królicka, A.; Zarębski, J. Voltammetric methods for chromium(VI) determination in extracts from coal fly ash and cement. Chem. Listy 2014, 108, 197. [Google Scholar]
- Krasnodębska-Ostręga, B.; Sadowska, M.; Biaduń, E. Sample Pretreatment for Trace Speciation Analysis. Phys. Sci. Rev. 2018, 2, 2017805. [Google Scholar]
- Adamczyk, M.; Grabarczyk, M.; Leszko, W. A voltammetric approach to the quantification of tungsten in environmental waters using a solid bismuth microelectrode. Measurement 2022, 194, 111089. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Korolczuk, M. Development of a simple and fast voltammetric procedure for determination of trace quantity of Se(IV) in natural lake and river water samples. J. Hazard. Mater. 2010, 175, 1007. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Wardak, C.; Piech, R.; Wawruch, A. An Electrochemical Sensor for the Determination of Trace Concentrations of Cadmium, Based on Spherical Glassy Carbon and Nanotubes. Materials 2023, 16, 3252. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, M. A catalytic adsorptive stripping voltammetric procedure for trace determination of Cr(VI) in natural samples containing high concentrations of humic substances. Anal. Bioanal. Chem. 2008, 390, 979. [Google Scholar] [CrossRef]
- Grabarczyk, M. Ultraselective and sensitive determination of Cr(VI) in the presence of a high excess of Cr(III) in natural waters with a complicated matrix. Electroanalysis 2008, 20, 1495. [Google Scholar] [CrossRef]
- Shin, D.Y.; Lee, S.M.; Jang, Y.; Lee, J.; Lee, C.M.; Cho, E.M.; Seo, Y.R. Adverse human health effects of chromium by exposure route: A comprehensive review based on toxicogenomic approach. Int. J. Mol. Sci. 2023, 24, 3410. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The Double Face of Metals: The Intriguing Case of Chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- Islam, M.M.; Mohana, A.A.; Rahman, M.A.; Rahman, M.; Nairu, R.; Rahman, M.M. A Comprehensive Review of the Current Progress of Chromium Removal Methods from Aqueous Solution. Toxics 2023, 11, 252. [Google Scholar] [CrossRef]
- Frutos-Puerto, S.; Miro, C.; Pinilla-Gil, E. Nafion-Protected Sputtered-Bismuth Screen-Printed Electrode for On-Site Voltammetric Measurements of Cd(II) and Pb(II) in Natural Water Samples. Sensors 2019, 19, 279. [Google Scholar] [CrossRef]
- Herberi, E.E.; Alexandru, P.; Busila, M. Cyclic Voltammetry of Screen-Printed Carbon Electrode Coated with Ag-ZnO Nanoparticles in Chitosan Matrix. Materials 2023, 16, 3266. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Nguyen, M.T.N.; Lee, J.S. Carbon-Based Materials and Their Applications in Sensing by Electrochemical Voltammetry. Inorganics 2023, 11, 81. [Google Scholar] [CrossRef]
- Jorge, E.O.; Rocha, M.M.; Fonseca, I.T.E.; Neto, M.M.M. Studies on the stripping voltammetric determination and speciation of chromium at a rotating-disc bismuth film electrode. Talanta 2010, 81, 556. [Google Scholar] [CrossRef]
- Lin, L.; Lawrence, N.S.; Thongngamdee, S.; Wang, J.; Lin, Y. Catalytic adsorptive stripping determination of trace chromium (VI) at the bismuth film electrode. Talanta 2005, 65, 144. [Google Scholar] [CrossRef] [PubMed]
- Hue, N.T.; Hop, N.V.; Long, H.T.; Phong, N.H.; Uyen, T.H.; Hung, L.Q.; Phuong, N.N. Determination of Chromium in Natural Water by Adsorptive Stripping Voltammetry Using In Situ Bismuth Film Electrode. J. Environ. Public Health 2020, 2020, 1347836. [Google Scholar]
- Kowalski, Z. Method of and Apparatus for Making a Thin-Layer Mercury Electrod. Polish Patent No. P-319 984 1997, 31 July 2002. [Google Scholar]
- Baś, B.; Kowalski, Z. Preparation of silver surface for mercury film electrode of prolonged analytical application. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2002, 14, 1067. [Google Scholar] [CrossRef]
- Boussemart, M.; van den Berg, C.M.G.; Ghaddaf, M. The determination of the chromium speciation in sea water using catalytic cathodic stripping voltammetry. Anal. Chim. Acta 1992, 262, 103. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Korolczuk, M. Modyfication of catalytic adsorptive stripping voltammetric method of hexavalent chromium determination in the presence of DTPA and nitrate. Anal. Bioanal. Chem. 2003, 376, 1115. [Google Scholar] [CrossRef]


| Sample | Surfactant Added | Recovery of Cr(VI) (%) | RSD (n = 5) (%) | |
|---|---|---|---|---|
| Type | Concentration (mg L−1) | |||
| Bystrzyca river water | Triton X-100 | 2.5 | 95.3 | 3.9 |
| 5 | 97.1 | 4.6 | ||
| CTAB | 1 | 96.7 | 4.8 | |
| 2.5 | 94.7 | 4.2 | ||
| SDS | 10 | 98.8 | 3.7 | |
| 15 | 101.4 | 3.8 | ||
| Rhamnolipid | 20 | 100.7 | 4.3 | |
| 25 | 98.0 | 3.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabarczyk, M.; Wardak, C. Effect of Temperature on the Removal of Interferences in the Voltammetric Procedure for the Determination of Cr(VI). Materials 2024, 17, 3050. https://doi.org/10.3390/ma17133050
Grabarczyk M, Wardak C. Effect of Temperature on the Removal of Interferences in the Voltammetric Procedure for the Determination of Cr(VI). Materials. 2024; 17(13):3050. https://doi.org/10.3390/ma17133050
Chicago/Turabian StyleGrabarczyk, Malgorzata, and Cecylia Wardak. 2024. "Effect of Temperature on the Removal of Interferences in the Voltammetric Procedure for the Determination of Cr(VI)" Materials 17, no. 13: 3050. https://doi.org/10.3390/ma17133050
APA StyleGrabarczyk, M., & Wardak, C. (2024). Effect of Temperature on the Removal of Interferences in the Voltammetric Procedure for the Determination of Cr(VI). Materials, 17(13), 3050. https://doi.org/10.3390/ma17133050






