The Synthesis of Copper Nanoparticles for Printed Electronic Materials Using Liquid Phase Reduction Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Copper Nanoparticles
- CuSO4·5H2O is weighed and dissolved in ethylene glycol at room temperature, with magnetic stirring, to prepare a copper source precursor solution with a concentration of 0.1 M;
- Ascorbic acid is weighed and dissolved in ethylene glycol at room temperature, with magnetic stirring, to prepare a reducing agent precursor solution with a concentration of 0.2 M;
- PVP is weighed and dissolved in ethylene glycol at 150 °C, with vigorous magnetic stirring, to prepare a coating agent precursor solution with a concentration of 0.1 M;
- The copper source precursor solution and the reducing agent precursor solution obtained in steps 1 and 2 are co-added to the PVP precursor solution from step 3 at a rate of 1 mL/min, and the mixture is stirred in an oil bath at 150 °C for 120 min;
- After the reaction is completed, the reaction system is allowed to cool down to room temperature naturally, and the resulting product is washed three times with ethanol and deionized water at a speed of 9000 rpm, then dried under vacuum at 40 °C.
2.3. Preparation of Conductive Ink
2.4. Homemade Conductive Ink Pen
2.5. Electrical Properties of Copper Nanoparticles after Sintering
2.6. Analysis and Characterization
3. Results
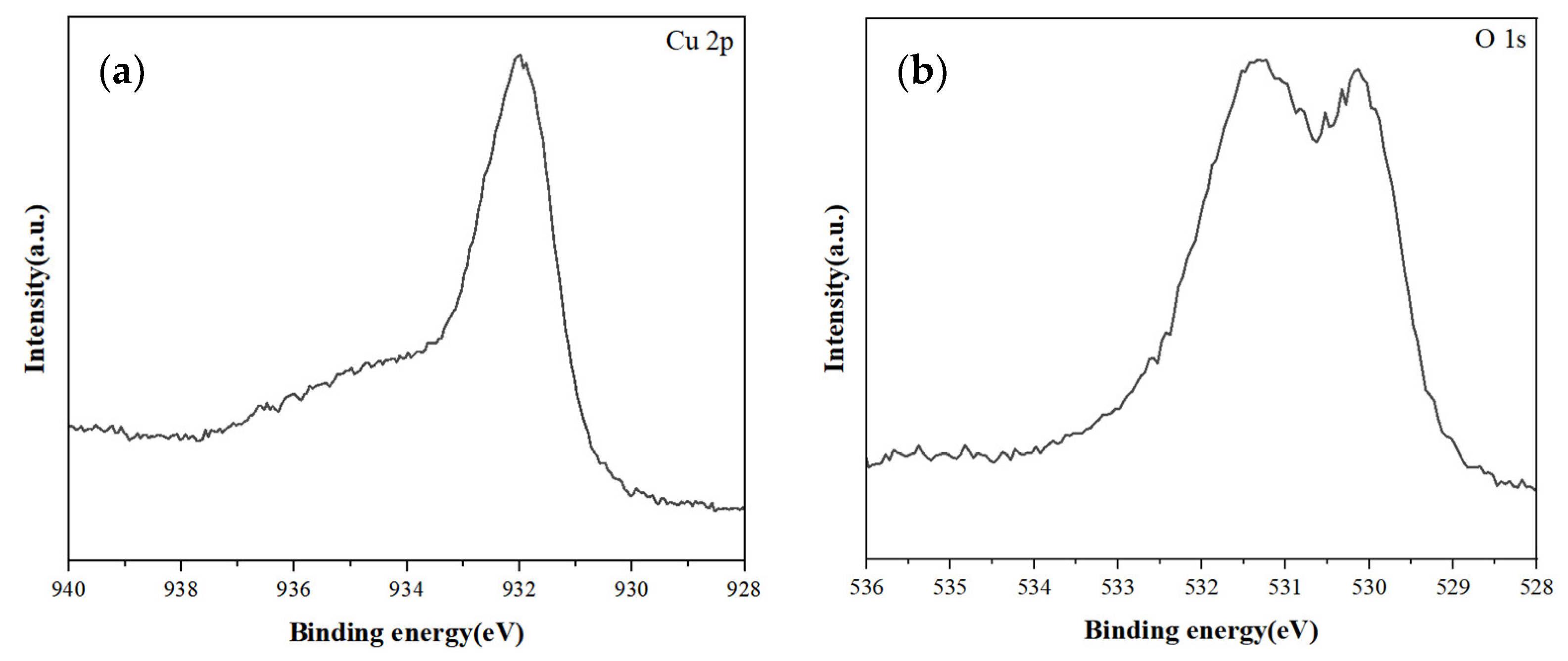
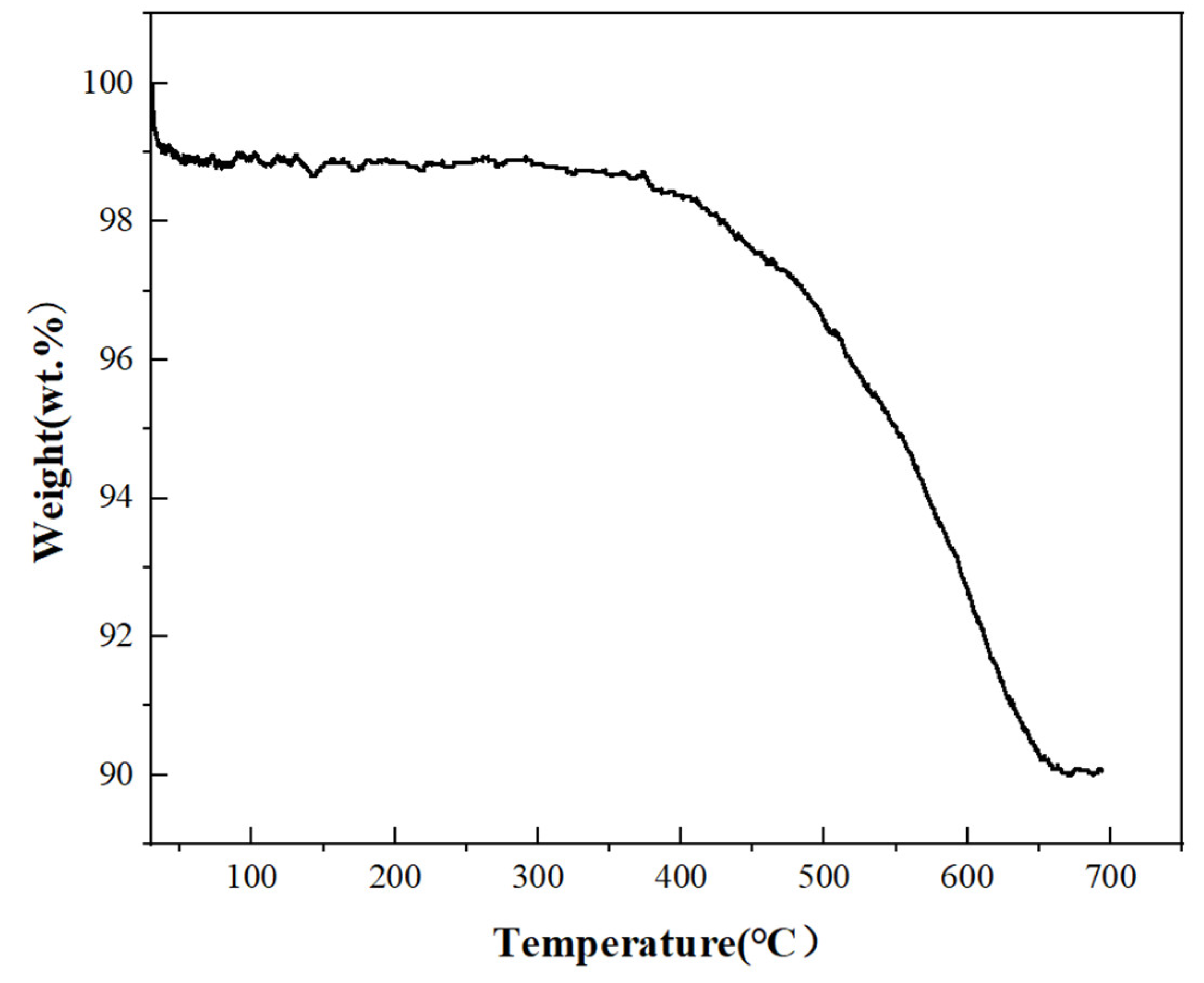
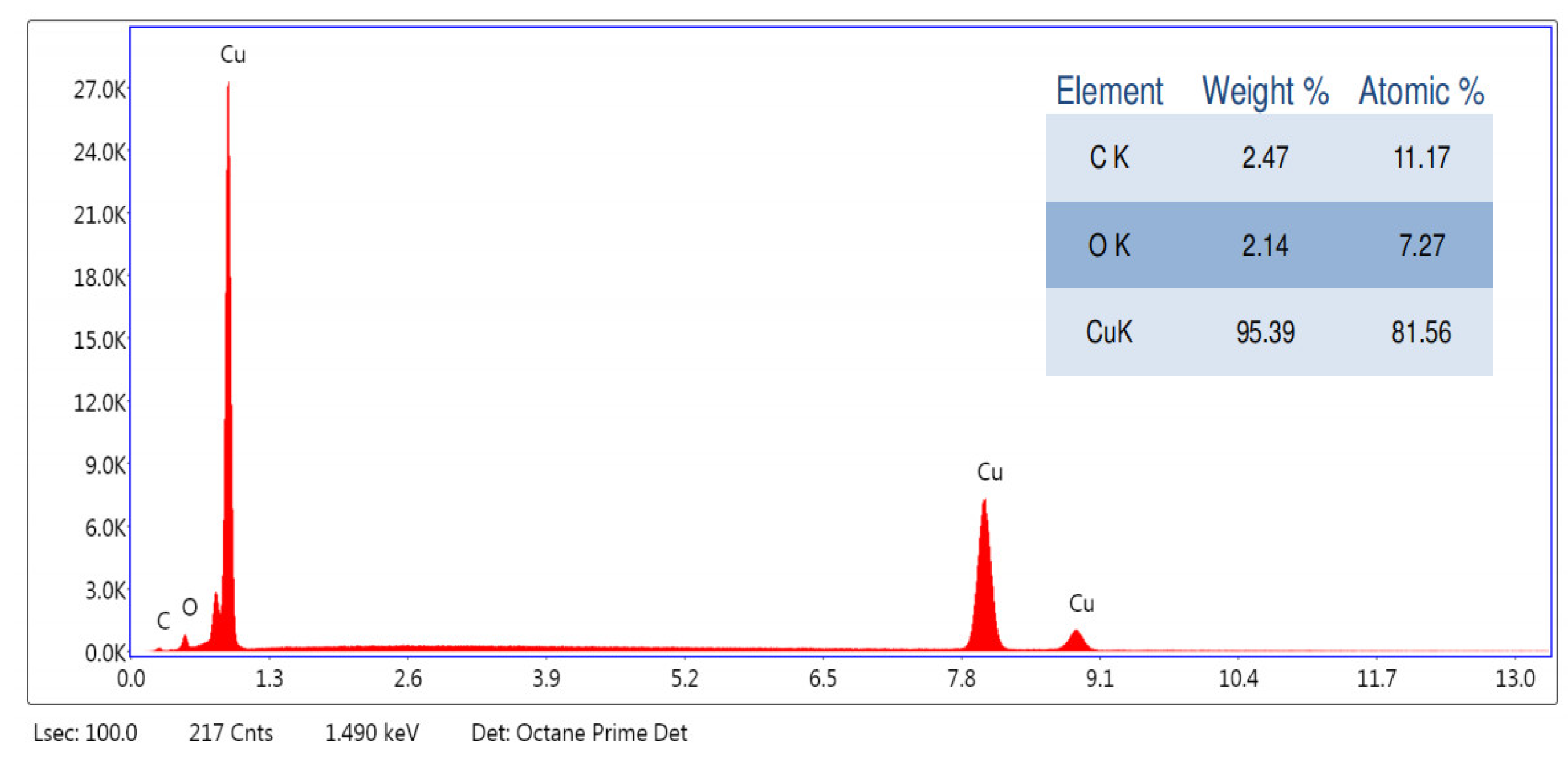
3.1. Characterization of Copper Nanoparticles
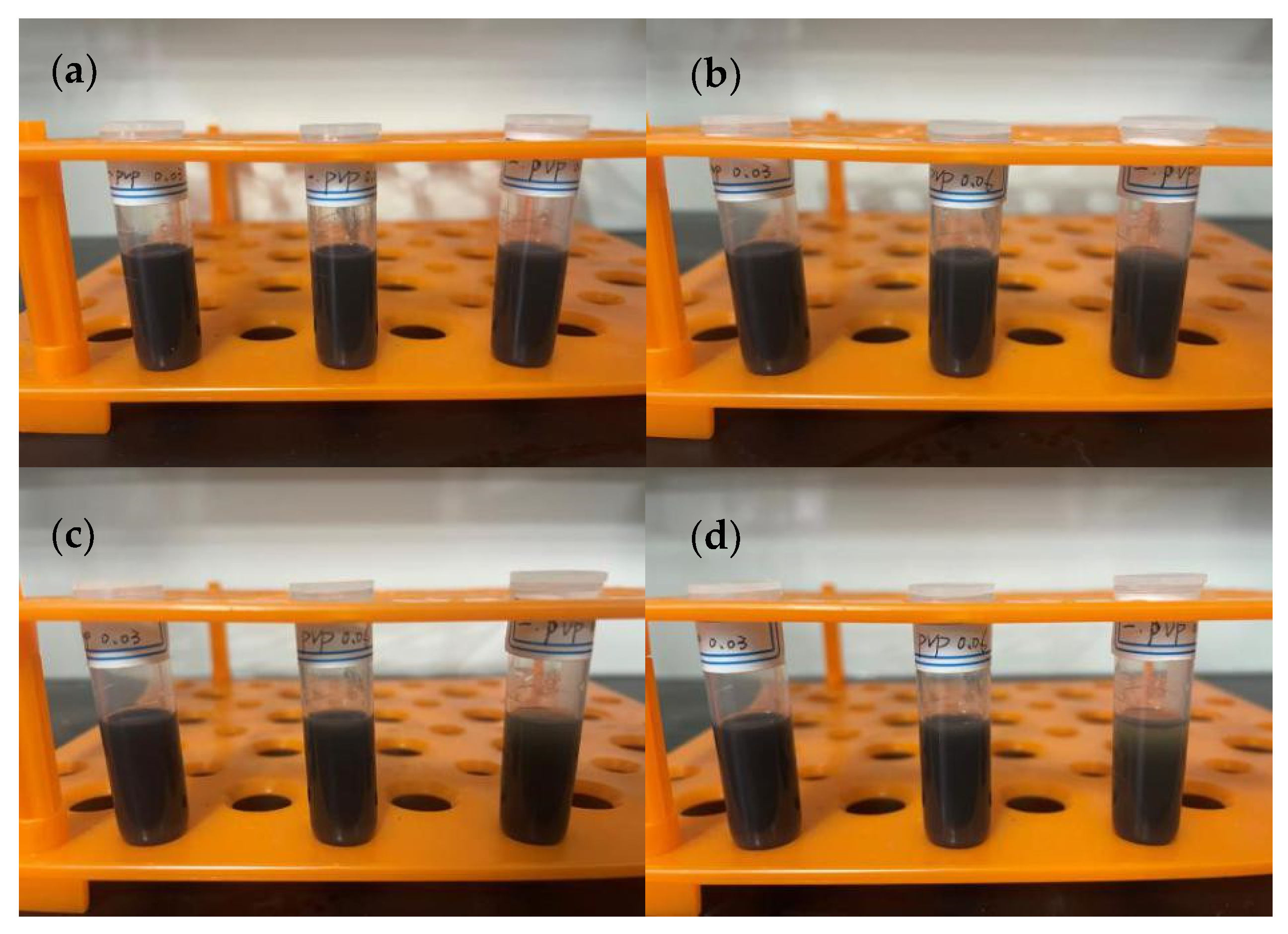
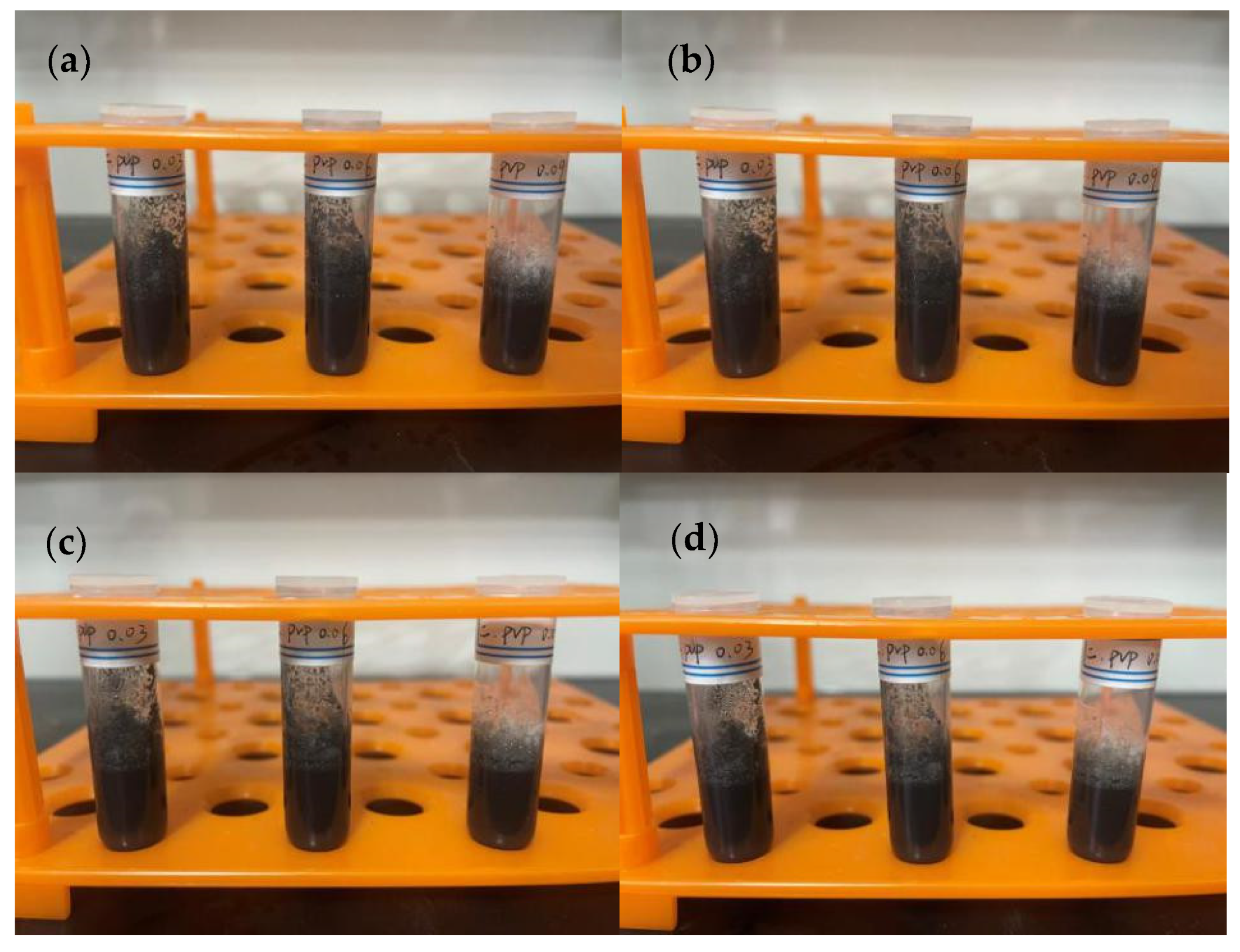
3.2. Preparation of Conductive Ink
3.3. Electrical Properties of Copper Nanoparticles after Sintering
4. Discussion
4.1. Characterization of Copper Nanoparticles
4.2. Preparation of Conductive Ink
4.3. Electrical Properties of Copper Nanoparticles after Sintering
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, J.; Yu, W.; Zhang, N.; Yang, X.; Zhang, H.; Zhao, W. A Survey on Internet of Things: Architecture, Enabling Technologies, Security and Privacy, and Applications. IEEE Internet Things J. 2017, 4, 1125–1142. [Google Scholar] [CrossRef]
- Swart, J. Identifying the Sub-Components of Intellectual Capital: A Literature Review and Development of Measures; University of Bath School of Management: Bath, UK, 2022. [Google Scholar]
- Andriole, S.J. Social Media Analytics, Wearable Technology, and the Internet-of-Things. IT Prof. 2019, 21, 11–15. [Google Scholar] [CrossRef]
- Pastorelli, F.; Schmidt, T.M.; Hösel, M.; Søndergaard, R.R.; Jørgensen, M.; Krebs, F.C. The Organic Power Transistor: Roll-to-Roll Manufacture, Thermal Behavior, and Power Handling When Driving Printed Electronics. Adv. Eng. Mater. 2015, 18, 51–55. [Google Scholar] [CrossRef]
- Nie, B.; Liu, S.; Qu, Q.; Zhang, Y.; Zhao, M.; Liu, J. Bio-Inspired Flexible Electronics for Smart E-Skin. Acta Biomater. 2022, 139, 280–295. [Google Scholar] [CrossRef]
- Cui, Z. Printed Electronics: Materials, Technologies and Applications; Wiley: Hoboken, NJ, USA, 2016; pp. 339–341. [Google Scholar] [CrossRef]
- Gao, W.; Ota, H.; Kiriya, D.; Takei, K.; Javey, A. Flexible Electronics toward Wearable Sensing. Acc. Chem. Res. 2019, 52, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Remskar, M.; Mrzel, A.; Sanjines, R.; Cohen, H.; Lévy, F. Metallic Sub-Nanometer MOS2–Xiy Nanotubes. Adv. Mater. 2003, 15, 237–240. [Google Scholar] [CrossRef]
- Delogu, F. Thermodynamic Phase Transitions in Nanometer-Sized Metallic Systems. Mater. Sci. Forum 2010, 653, 31–53. [Google Scholar] [CrossRef]
- Liu, T.; Guo, R.; Fu, Y.; Zhao, J.; Ning, H.; Fang, Z.; Liang, Z.; Wei, X.; Yao, R.; Peng, J. Morphological Regulation of Printed Low-Temperature Conductive Ink. Langmuir 2022, 38, 9955–9966. [Google Scholar] [CrossRef]
- Yoshida, S.; Tsuchii, T.; Matsumoto, M.; Morita, K.; Maruyama, T. Conductive Gold Thin Film Prepared by the Two-Dimensional Assembly of Gold Nanoparticles on a Plastic Surface. ACS Appl. Electron. Mater. 2024, 6, 1113–1121. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Liu, Y.; Wang, Z.; Yan, C.; Song, C.; Gao, C.; Wu, Y. A NIR Laser Induced Self-Healing PDMS/Gold Nanoparticles Conductive Elastomer for Wearable Sensor. J. Colloid Interface Sci. 2021, 599, 360–369. [Google Scholar] [CrossRef]
- Ahmad, S.; Subhani, K.; Rasheed, A.; Ashraf, M.; Afzal, A.; Ramzan, B.; Sarwar, Z. Development of Conductive Fabrics by Using Silver Nanoparticles for Electronic Applications. J. Electron. Mater. 2020, 49, 1330–1337. [Google Scholar] [CrossRef]
- Ahn, B.Y.; Lorang, D.J.; Lewis, J.A. Transparent Conductive Grids via Direct Writing of Silver Nanoparticle Inks. Nanoscale 2011, 3, 2700–2702. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhang, X.; Huang, Q.; Xu, Q.; Song, W. Preparation of Solid Silver Nanoparticles for Inkjet Printed Flexible Electronics with High Conductivity. Nanoscale 2014, 6, 1622–1628. [Google Scholar] [CrossRef] [PubMed]
- Mościcki, A.; Smolarek-Nowak, A.; Felba, J.; Kinart, A. Ink for Ink-Jet Printing of Electrically Conductive Structures on Flexible Substrates with Low Thermal Resistance. J. Electron. Mater. 2017, 46, 4100–4108. [Google Scholar] [CrossRef]
- Bobsin, A.; Rodrigues, T.C.; Fernandes, I.J.; Ferreira, S.B.; Peter, C.R.; Hasenkamp, W.; Moraes, C.A.M. Copper and Silver Microparticles for High-Performance Conductive Inks in Electronic Chip Shielding. Mater. Chem. Phys. 2024, 315, 129007. [Google Scholar] [CrossRef]
- Nyabadza, A.; Plouze, A.; Heidarinassab, S.; Vazquez, M.; Brabazon, D. Screen-Printed Electrodes on Paper Using Copper Nano- and Micro-Particles. J. Mater. Res. Technol. 2024, 29, 5189–5197. [Google Scholar] [CrossRef]
- Chowdhury, R.; Werther, C.; Young, K.; Jang, S. A Comparative Study of Single and Dual Sintering Processes of Metal Nanoparticles for Flexible Electronics Application. Adv. Eng. Mater. 2024, 2301730. [Google Scholar] [CrossRef]
- Ghosh, R.; Liu, X.; Yates, M.Z. Flexible Copper Metal Circuits via Desktop Laser Printed Masks. Adv. Mater. Technol. 2022, 7, 2200400. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, D.; Jeong, S.; Moon, J.; Kim, J.S. Direct Writing of Copper Conductive Patterns by Ink-Jet Printing. Thin Solid Films 2007, 515, 7706–7711. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.; Li, G.; Zhao, T.; Fu, X.; Sun, R.; Zhou, F.; Wong, C. Facile Preparation of Monodisperse, Impurity-Free, and Antioxidation Copper Nanoparticles on a Large Scale for Application in Conductive Ink. ACS Appl. Mater. Interfaces 2014, 6, 560–567. [Google Scholar] [CrossRef]
- Shahrokh, A.; Seyyed Fakhrabadi, M.M. Effects of Copper Nanoparticles on Elastic and Thermal Properties of Conductive Polymer Nanocomposites. Mech. Mater. 2021, 160, 103958. [Google Scholar] [CrossRef]
- Faseela, K.P.; Ajmal, C.M.; Cha, S.; Baik, S. In Situ Regeneration of Oxidized Copper Flakes Forming Nanosatellite Particles for Non-Oxidized Highly Conductive Copper Nanocomposites. Adv. Funct. Mater. 2023, 33, 2304776. [Google Scholar] [CrossRef]
- Ibrahem, I.; Iqbal, M.N.; Verho, O.; Eivazihollagh, A.; Olsén, P.; Edlund, H.; Tai, C.W.; Norgren, M.; Johnston, E.V. Copper Nanoparticles on Controlled Pore Glass and TEMPO for the Aerobic Oxidation of Alcohols. ChemNanoMat 2018, 4, 71–75. [Google Scholar] [CrossRef]
- Nishida, N.; Miyashita, A.; Tsukuda, T.; Tanaka, H. Production of Oxidation-Resistant Copper Nanoparticles on Carbon Nanotubes by Photoreduction. Chem. Lett. 2013, 42, 168–170. [Google Scholar] [CrossRef]
- Gopal, R.; Swarnkar, R.K.; Singh, S.C. Influence of surfactant on shape and oxidation of copper nanoparticles. In Proceedings of the 2010 Conference on Lasers and Electro-Optics (CLEO) and Quantum Electronics and Laser Science Conference (QELS), San Jose, CA, USA, 16–21 May 2010. [Google Scholar]
- Lin, R.H.; Wang, F.Y.; Li, S.Y.; Wang, G.Y. Phenol-Formaldehyde Resins Modified by Copper Nanoparticles. Plast. Rubber Compos. 2007, 36, 423–427. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Stoeckel, M.-A.; Ruoko, T.-P.; Wu, H.-Y.; Liu, X.; Kolhe, N.B.; Wu, Z.; Puttisong, Y.; Musumeci, C.; Massetti, M.; et al. A High-Conductivity n-Type Polymeric Ink for Printed Electronics. Nat. Commun. 2021, 12, 2354. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.J. Sintering Neck Growth Mechanism of Fe Nanoparticles: A Molecular Dynamics Simulation. Chem. Eng. Sci. 2020, 218, 115583. [Google Scholar] [CrossRef]
- Ko, S.H.; Pan, H.; Grigoropoulos, C. The Neck Growth Mechanisms in Low Energy Laser Sintering of Gold Nanoparticles: A Molecular Dynamics Simulation Study. Proc. SPIE 2007, 6458, 253–257. [Google Scholar]
- Hu, A.; Guo, J.Y.; Alarifi, H.; Patane, G.; Zhou, Y.; Compagnini, G. Low Temperature Sintering of Ag Nanoparticles for Flexible Electronics Packaging. Appl. Phys. Lett. 2010, 97, 153117. [Google Scholar] [CrossRef]

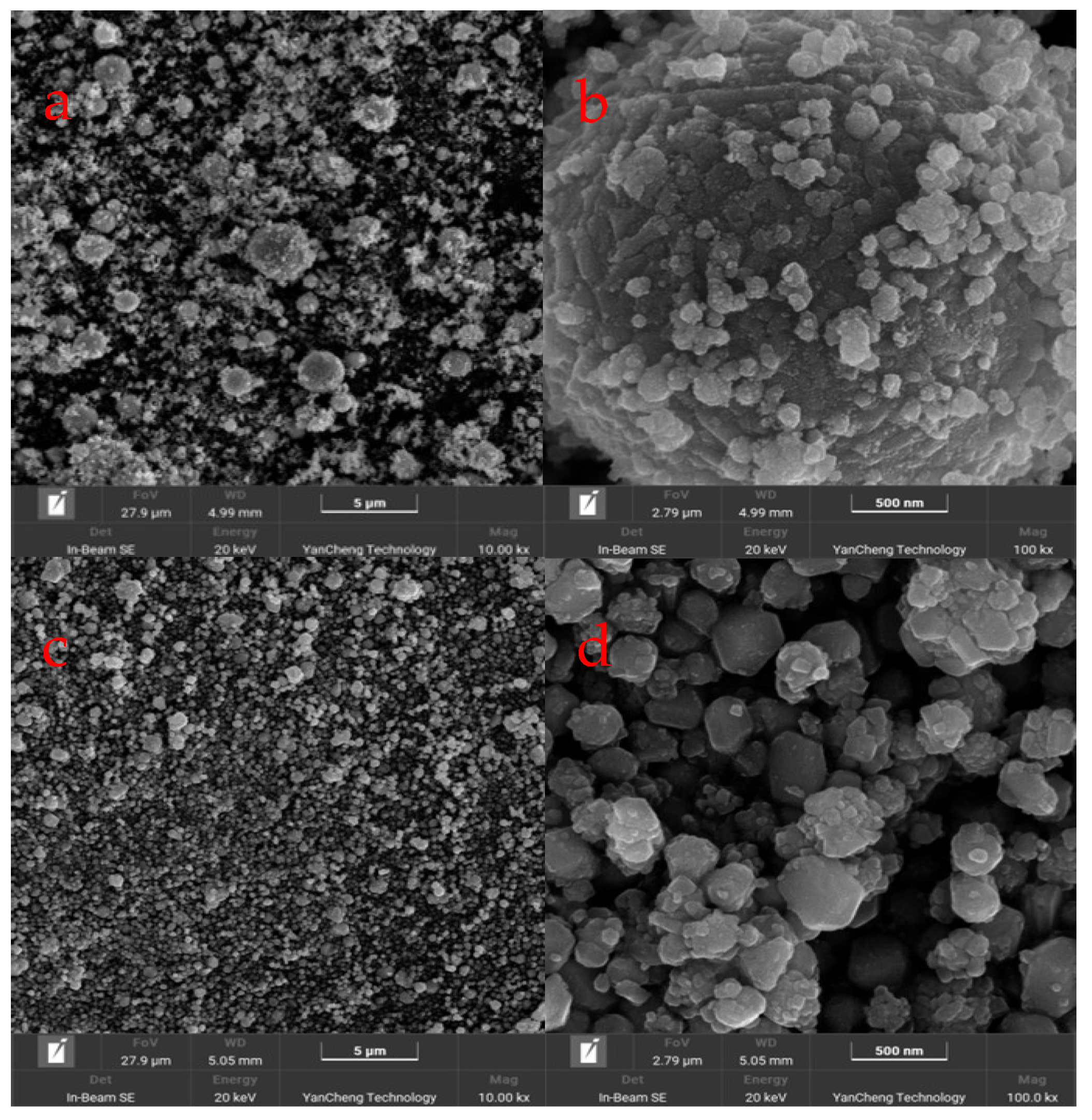





| 10 min | 30 min | 60 min | 90 min | 120 min | 150 min | |
|---|---|---|---|---|---|---|
| 200 °C | - | - | 956.9 | 208.2 | 100.7 | 80.9 |
| 250 °C | 398.3 | 105.9 | 72.4 | 53.6 | 40.8 | 35.4 |
| 300 °C | 99.7 | 42.8 | 31.3 | 26.9 | - | - |
| 350 °C | 32.4 | 20.1 | 15.5 | - | - | - |
| 10 min | 30 min | 60 min | 90 min | 120 min | 150 min | |
|---|---|---|---|---|---|---|
| 200 °C | - | - | 574.1 | 127.1 | 59.8 | 47.8 |
| 250 °C | 234.5 | 62.3 | 42.6 | 31.9 | 23.5 | 20.5 |
| 300 °C | 58.2 | 24.9 | 18.5 | 15.5 | - | - |
| 350 °C | 18.2 | 11.2 | 8.1 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Jiang, X. The Synthesis of Copper Nanoparticles for Printed Electronic Materials Using Liquid Phase Reduction Method. Materials 2024, 17, 3069. https://doi.org/10.3390/ma17133069
Li K, Jiang X. The Synthesis of Copper Nanoparticles for Printed Electronic Materials Using Liquid Phase Reduction Method. Materials. 2024; 17(13):3069. https://doi.org/10.3390/ma17133069
Chicago/Turabian StyleLi, Kai, and Xue Jiang. 2024. "The Synthesis of Copper Nanoparticles for Printed Electronic Materials Using Liquid Phase Reduction Method" Materials 17, no. 13: 3069. https://doi.org/10.3390/ma17133069






