Fire Properties of Paper Sheets Made of Cellulose Fibers Treated with Various Retardants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Sheets
2.3. Impregnation and Pressing of the Sheets
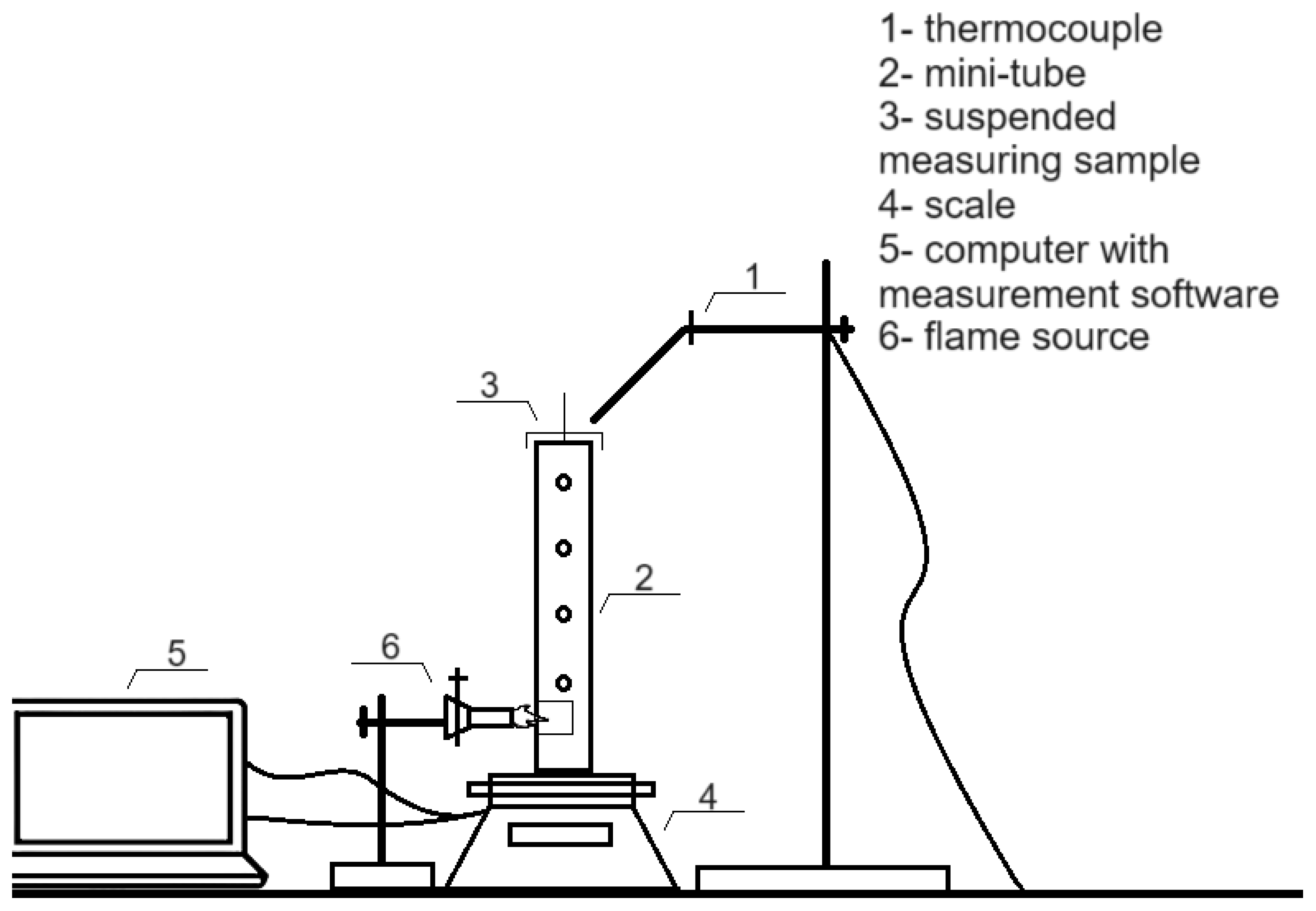
2.4. Flammability Properties Assessment
2.4.1. Mini Fire Tube
2.4.2. Mass Loss Calorimeter
2.4.3. Oxygen Index
3. Results and Discussion
3.1. Fire Properties
3.1.1. Mini Fire Tube
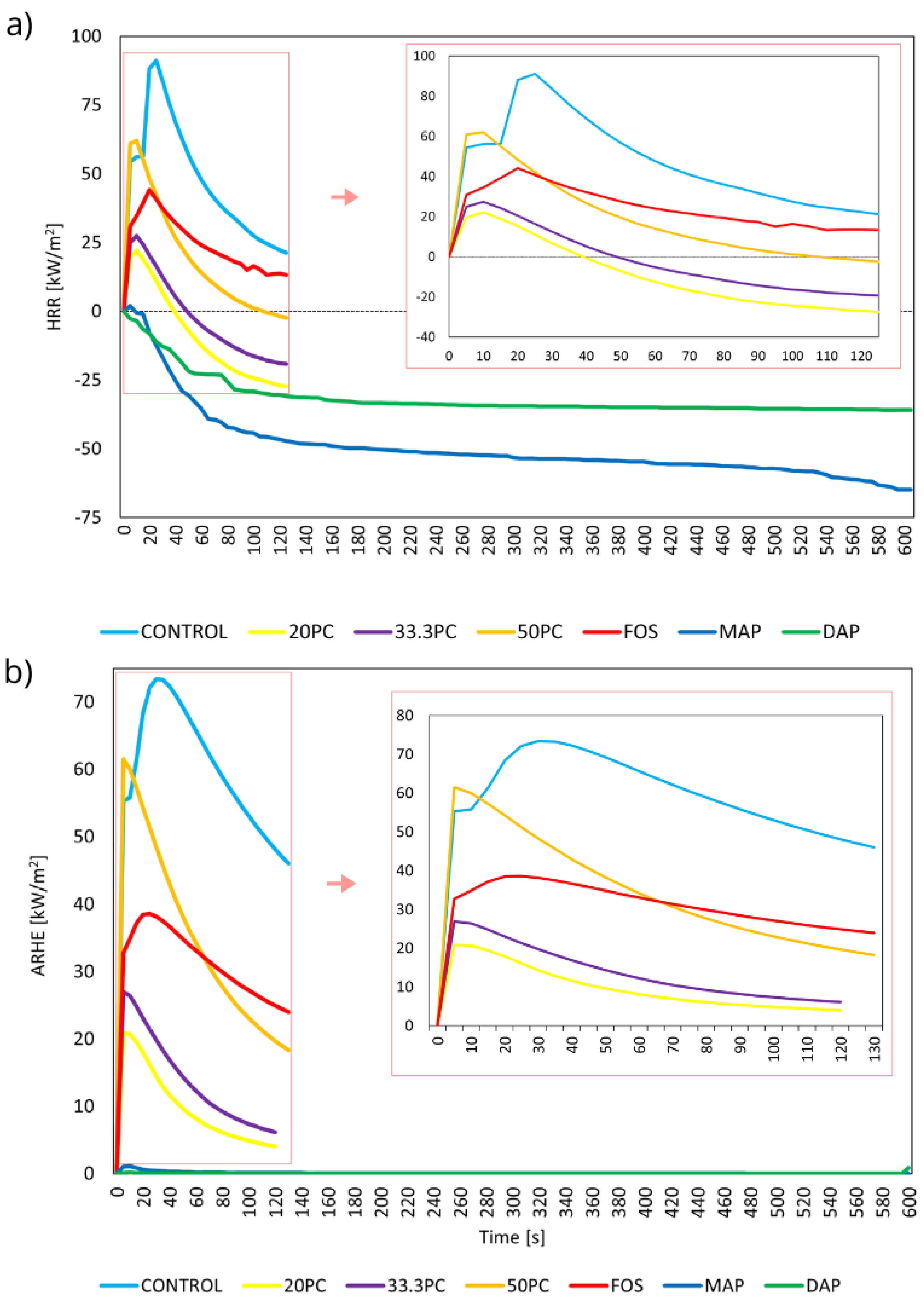
3.1.2. Mass Loss Calorimeter
3.1.3. Oxygen Index
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alam, I.; Sharma, C. Degradation of Paper Products Due to Volatile Organic Compounds. Sci. Rep. 2023, 13, 6426. [Google Scholar] [CrossRef] [PubMed]
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wågberg, L. Phosphorylated Cellulose Nanofibrils: A Renewable Nanomaterial for the Preparation of Intrinsically Flame-Retardant Materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef] [PubMed]
- Stark, N.; White, R.; Mueller, S.; Osswald, T. Evaluation of Various Fire Retardants for Use in Wood Flour–Polyethylene Composites. Polym. Degrad. Stab. 2010, 95, 1903–1910. [Google Scholar] [CrossRef]
- Kamal, I.; Ashaari, Z.; Rashid, A.M.; Abood, F.; Saad, M.J.; Zharif, A.T.; Masseat, K. Fire Propagation and Strength Performance of Fire Retardant-Treated Hibiscus Cannabinus Particleboard. Asian J. Appl. Sci. 2009, 2, 446–455. [Google Scholar] [CrossRef]
- Kozakiewicz, P.; Laskowska, A.; Drożdżek, M.; Zawadzki, J. Influence of Thermal Modification in Nitrogen Atmosphere on Physical and Technological Properties of European Wood Species with Different Structural Features. Coatings 2022, 12, 1663. [Google Scholar] [CrossRef]
- Tavakoli, M.; Ghasemian, A.; Dehghani-Firouzabadi, M.R.; Mazela, B. Cellulose and Its Nano-Derivatives as a Water-Repellent and Fire-Resistant Surface: A Review. Materials 2021, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Anthony, G.; Carty, P. Introduction: Polymer Combustion, Condensed Phase Pyrolysis and Smoke Formation; Elsevier: Amsterdam, The Netherlands, 2001; pp. 1–30. [Google Scholar]
- Grześkowiak, W.Ł. Evaluation of the Effectiveness of the Fire Retardant Mixture Containing Potassium Carbonate Using a Cone Calorimeter. Fire Mater. 2012, 36, 75–83. [Google Scholar] [CrossRef]
- He, Y.; Jin, X.; Li, J.; Qin, D. Mechanical and Fire Properties of Flame-Retardant Laminated Bamboo Lumber Glued with Phenol Formaldehyde and Melamine Urea Formaldehyde Adhesives. Polymers 2024, 16, 781. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Fage, J.; Liang, S.; Gaan, S. An Overview of Mode of Action and Analytical Methods for Evaluation of Gas Phase Activities of Flame Retardants. Polymers 2015, 7, 504–526. [Google Scholar] [CrossRef]
- Mazela, B.; Batista, A.; Grześkowiak, W. Expandable Graphite as a Fire Retardant for Cellulosic Materials—A Review. Forests 2020, 11, 755. [Google Scholar] [CrossRef]
- Chen, H.; Tang, Z.; Liu, B.; Chen, W.; Hu, J.; Chen, Y.; Yang, H. The New Insight about Mechanism of the Influence of K2CO3 on Cellulose Pyrolysis. Fuel 2021, 295, 120617. [Google Scholar] [CrossRef]
- Sharma, V.; Agarwal, S.; Mathur, A.; Singhal, S.; Wadhwa, S. Advancements in Nanomaterial Based Flame-Retardants for Polymers: A Comprehensive Overview. J. Ind. Eng. Chem. 2024, 133, 38–52. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Liu, Y.; Wang, Q. Properties and Mechanisms of Different Guanidine Flame Retardant Wood Pulp Paper. J. Anal. Appl. Pyrolysis 2017, 128, 224–231. [Google Scholar] [CrossRef]
- Gao, M.; Ling, B.; Yang, S.; Zhao, M. Flame Retardance of Wood Treated with Guanidine Compounds Characterized by Thermal Degradation Behavior. J. Anal. Appl. Pyrolysis 2005, 73, 151–156. [Google Scholar] [CrossRef]
- ISO 2470; Paper, Board and Pulps—Measurement of Diffuse Blue Reflectance Factor—Part 2: Outdoor Daylight Conditions (D65 Brightness). Technical Committee ISO/TC 6: Geneva, Switzerland, 2008.
- ISO 5350-2; Pulps — Estimation of Dirt and Shives Part 2: Inspection of Mill Sheeted Pulp by Transmitted Light. Technical Committee ISO/TC 6: Geneva, Switzerland, 2006.
- ISO 5351; Pulps—Determination of Limiting Viscosity Number in Cupriethylenediamine (CED) Solution. Technical Committee ISO/TC 6: Geneva, Switzerland, 2010.
- ISO 6588-1; Paper, Board and Pulps—Determination of pH of Aqueous Extracts. Technical Committee ISO/TC 6: Geneva, Switzerland, 2020.
- Lv, Y.F.; Thomas, W.; Chalk, R.; Singamneni, S. Flame Retardant Polymeric Materials for Additive Manufacturing. Mater. Today Proc. 2020, 33, 5720–5724. [Google Scholar] [CrossRef]
- Mazela, B.; Bajstok, M.; Siemińska, I.; Perdoch, W. Molded Pulp Packaging Made of Recycled Lignocellulose Fibres. In Proceedings of the International Panel Products Symposium 2023, Llandudno, UK, 3–4 October 2023. [Google Scholar]
- ASTM E69; Standard Test Method for Combustible Properties of Treated Wood by the Fire-Tube Apparatus. ASTM International: West Conshohocken, PA, USA, 2022.
- EN ISO 13927:2015; Plastics—Simple Heat Release Test Using a Conical Radiant Heater and a Thermopile Detector (ISO 13927:2015). ISO: Geneva, Switzerland, 2015. Available online: https://standards.iteh.ai/catalog/standards/cen/0eda501e-b7fb-42e7-bd3b-fe193efa216c/en-iso-13927-2015 (accessed on 8 May 2024).
- PN-76 C-89020; Plastics. Oxygen Index Flammability Test. Wydawnictwa Normalizacyjne: Warsaw, Poland, 1976.
- ISO 4589; Tworzywa Sztuczne-Oznaczanie Zapalności Metodą Wskaźnika Tlenowego-Część 2: Badanie w Temperaturze Pokojowej. KT 141, Tworzyw Sztucznych: Warszawa, Polska, 2017.
- Grzeskowiak, W.L.; Bartkowiak, M.; Wachowiak, T. Fire Properties of Exotic Wood Species Covered with Protective Oils on the Basis of the Oxygen Ratio. Ann. Wars. Univ. Life Sci. -SGGW. For. Wood Technol. 2014, 85, 76–80. [Google Scholar]
- White, R.H. Oxygen Index Evaluation of Fire-Retardant-Treated Wood; Forest Products Laboratory: Madison, WI, USA, 1979. [Google Scholar]
- Palność Polimerów i Materiałów Polimerowych—Władysław Przygocki, Andrzej Włochowicz, Grażyna Janowska—ebook. Available online: https://www.ibuk.pl/fiszka/38593/palnosc-polimerow-i-materialow-polimerowych.html (accessed on 8 May 2024).
- Gilman, J. Flammability and Thermal Stability Studies of Polymer Layered-Silicate (Clay) Nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Fabijański, M. Palność materiałów polimerowych stosowanych w transporcie szynowym, opóźniacze palenia. Probl. Kolejnictwa 2009, Z. 149, 53–66. [Google Scholar]
- Salmeia, K.A.; Jovic, M.; Ragaisiene, A.; Rukuiziene, Z.; Milasius, R.; Mikucioniene, D.; Gaan, S. Flammability of Cellulose-Based Fibers and the Effect of Structure of Phosphorus Compounds on Their Flame Retardancy. Polymers 2016, 8, 293. [Google Scholar] [CrossRef]
- Nishimura, M.; Iwasaki, S.; Horio, M. The Role of Potassium Carbonate on Cellulose Pyrolysis. J. Taiwan Inst. Chem. Eng. 2009, 40, 630–637. [Google Scholar] [CrossRef]
- Liodakis, S.; Fetsis, I.; Agiovlasitis, I. The Fire-Retarding Effect of Inorganic Phosphorus Compounds on the Combustion of Cellulosic Materials. J. Therm. Anal. Calorim. 2009, 98, 285–291. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Biodegradable Flame Retardants for Biodegradable Polymer. Biomolecules 2020, 10, 1038. [Google Scholar] [CrossRef]
- Niu, F.; Wu, N.; Yu, J.; Ma, X. Gelation, Flame Retardancy, and Physical Properties of Phosphorylated Microcrystalline Cellulose Aerogels. Carbohydr. Polym. 2020, 242, 116422. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, Y.; Homma, I.; Watanabe, T. Properties of Phosphorylated Cellulose Nanofiber Dispersions under Various Conditions. Cellulose 2020, 27, 2029–2040. [Google Scholar] [CrossRef]
- Li, L.; Hu, H.; Guo, C.; Hu, H. The Synergistic Effect of Boric Acid and Ammonium Polyphosphate on the Thermal Degradation and Flammability of Pine-Needles. Drewno 2014, 57, 37–49. [Google Scholar] [CrossRef]
- Hasburgh, L.; White, R.; Dietenberger, M.; Boardman, C. Comparison of the Heat Release Rate from the Mass Loss Calorimeter to the Cone Calorimeter for Wood-Based Materials. In Proceedings of the Fire and Materials 2015, San Francisco, CA, USA, 2–4 February 2015. [Google Scholar]
- Terzi, E.; Kartal, S.; White, R.; Shinoda, K.; Imamura, Y. Fire Performance and Decay Resistance of Solid Wood and Plywood Treated with Quaternary Ammonia Compounds and Common Fire Retardants. Holz Als Roh Werkst. 2009, 69, 41–51. [Google Scholar] [CrossRef]
- Akbulut, T.; Dundar, T.; White, R.; Mengeloglu, F.; Büyüksarı, Ü.; Candan, Z.; Avci, E. Effect of Boron and Phosphate Compounds on Physical, Mechanical, and Fire Properties of Wood-Polypropylene Composites. Constr. Build. Mater. 2012, 33, 63–69. [Google Scholar] [CrossRef]
- Grześkowiak, W.Ł. Effectiveness of New Wood Fire Retardants Using a Cone Calorimeter. J. Fire Sci. 2017, 35, 565–576. [Google Scholar] [CrossRef]
- Babrauskas, V. Book Review: SFPE Handbook of Fire Protection Engineering. J. Fire Sci. 2016, 34, 164–167. [Google Scholar] [CrossRef]
- Kicko-Walczak, E.; Jankowski, P. Wpływ bezhalogenowej modyfikacji żywic epoksydowych na ich poziom uniepalnienia. Polimery 2005, 50, 860–867. [Google Scholar] [CrossRef]

| Treatment Type | WPG [%] | Dry Mass of the Retardant [g/m2] |
|---|---|---|
| CONTROL | 0 | 0 |
| 20PC | 11.38 ± 0.13 | 22.33 ± 1.28 |
| 33.3PC | 13.54 ± 0.20 | 29.00 ± 0.82 |
| 50PC | 14.91 ± 0.12 | 23.33 ± 0.93 |
| FOS | 15.90 ± 0.21 | 30.00 ± 0.60 |
| MAP | 12.19 ± 0.14 | 20.00 ± 1.18 |
| DAP | 14.79 ± 0.33 | 39.33 ± 1.21 |
| Treatment Type | Maximum Temp. of Exhaust Gases (°C) | Time to Reach the Max. Exhaust Gases Temperature (s) | Mass Loss [%] |
|---|---|---|---|
| CONTROL | 326.20 ± 46.27 | 12.00 ± 0.83 | 99.70 ± 0.32 |
| 20PC | 119.50 ± 18.61 | 44.88 ± 16.80 | 29.44 ± 1.87 |
| 33.3PC | 122.38 ± 11.54 | 45.80 ± 11.77 | 19.07 ± 1.83 |
| 50PC | 137.28 ± 20.19 | 54.20 ± 6.58 | 24.32 ± 4.28 |
| FOS | 100.44 ± 12.80 | 50.20 ± 9.22 | 17.61 ± 2.84 |
| MAP | 156.06 ± 12.79 | 35.20 ± 5.50 | 15.30 ± 2.43 |
| DAP | 144.83 ± 15.94 | 55.00 ± 4.97 | 15.12 ± 2.67 |
| Parameters | CONTROL | 20PC | 33.3PC | 50PC | FOS | MAP | DAP |
|---|---|---|---|---|---|---|---|
| Total Heat Release | 5.98 (130 *) | 0.49 (60) | 0.59 (80) | 2.38 (130) | 3.12 (130) | 0.01 (15) | 0.01 (10) |
| Peak of Heat-Release Rate | 56.39 (10 *) | 22.15 (5) | 28.19 (5) | 50.70 (10) | 30.10 (10) | 11.35 (10) | 22.29 (5) |
| Effective heat of combustion | 15.09 (10 *) | 9.46 (10) | 1.10 (5) | 2 (10) | 1.58 (10) | 0.13 (10) | 0 (0) |
| Time to ignition (s) | 6 | 5 | 4 | X ** | 6 | X | X |
| Time to flameout (s) | 14 | 12 | 7 | X ** | 10 | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szubert, Z.; Mazela, B.; Tomkowiak, K.; Grześkowiak, W. Fire Properties of Paper Sheets Made of Cellulose Fibers Treated with Various Retardants. Materials 2024, 17, 3074. https://doi.org/10.3390/ma17133074
Szubert Z, Mazela B, Tomkowiak K, Grześkowiak W. Fire Properties of Paper Sheets Made of Cellulose Fibers Treated with Various Retardants. Materials. 2024; 17(13):3074. https://doi.org/10.3390/ma17133074
Chicago/Turabian StyleSzubert, Zuzanna, Bartłomiej Mazela, Karolina Tomkowiak, and Wojciech Grześkowiak. 2024. "Fire Properties of Paper Sheets Made of Cellulose Fibers Treated with Various Retardants" Materials 17, no. 13: 3074. https://doi.org/10.3390/ma17133074









