The Reduction Reaction Behavior of Steelmaking Dusts with Lignin under Different Atmospheres
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Material Characterization of Raw Dust
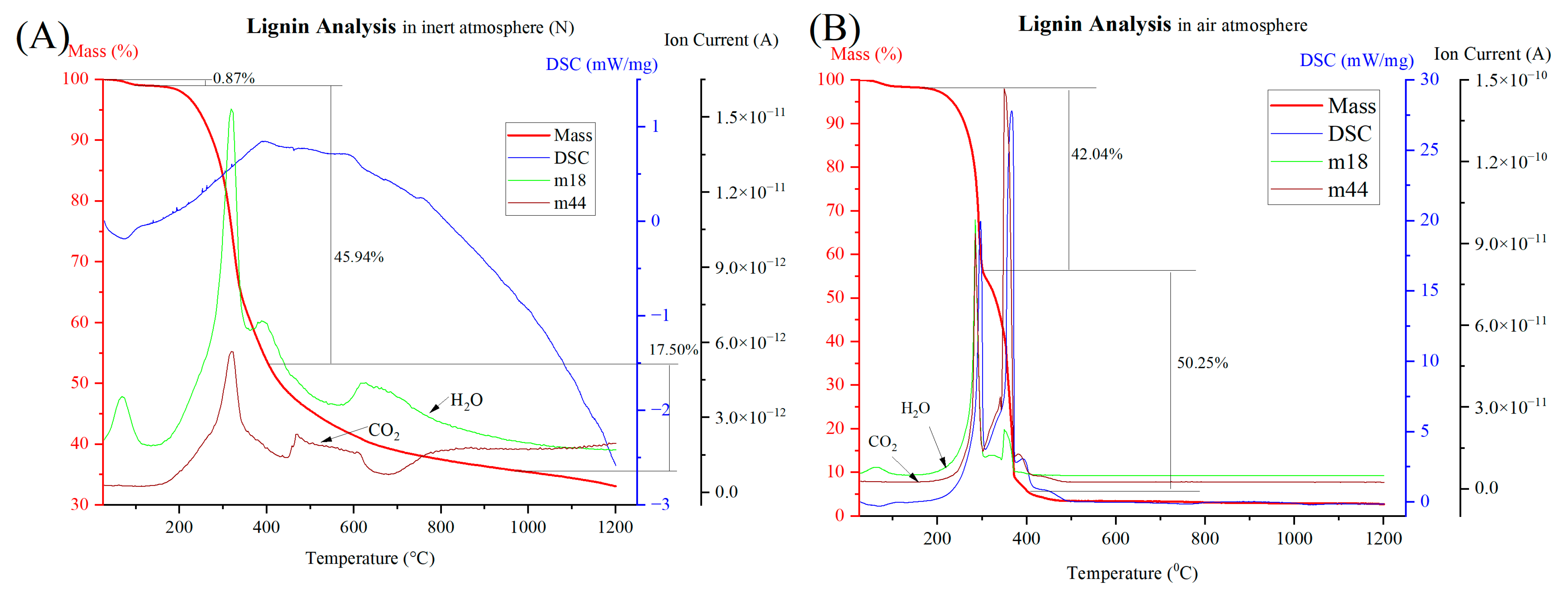
3.2. Thermal Analysis of Raw Dust
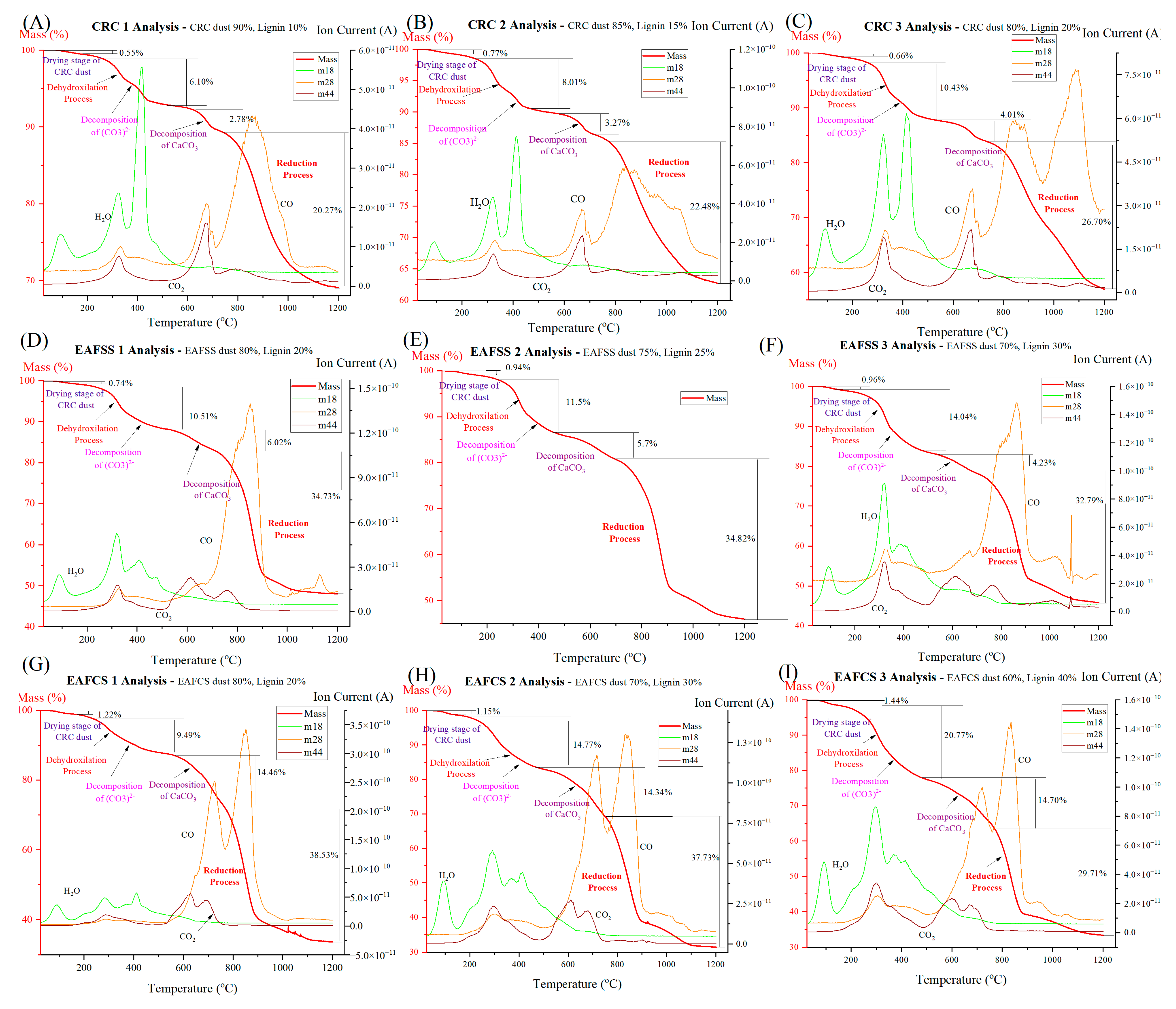
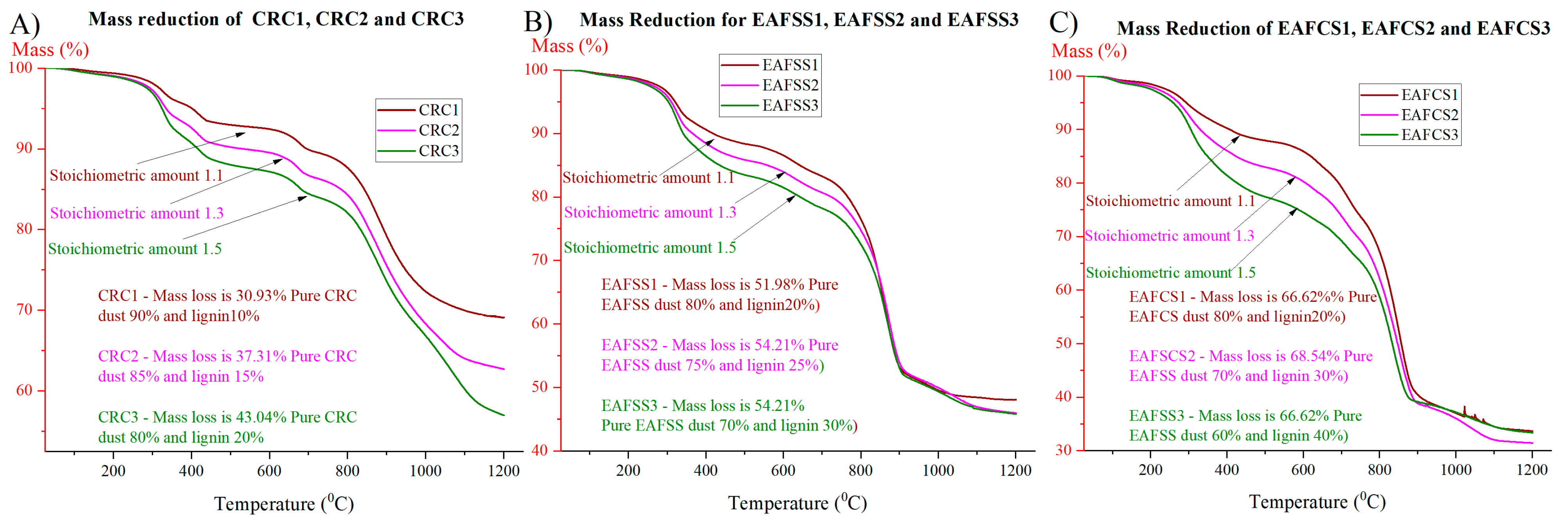
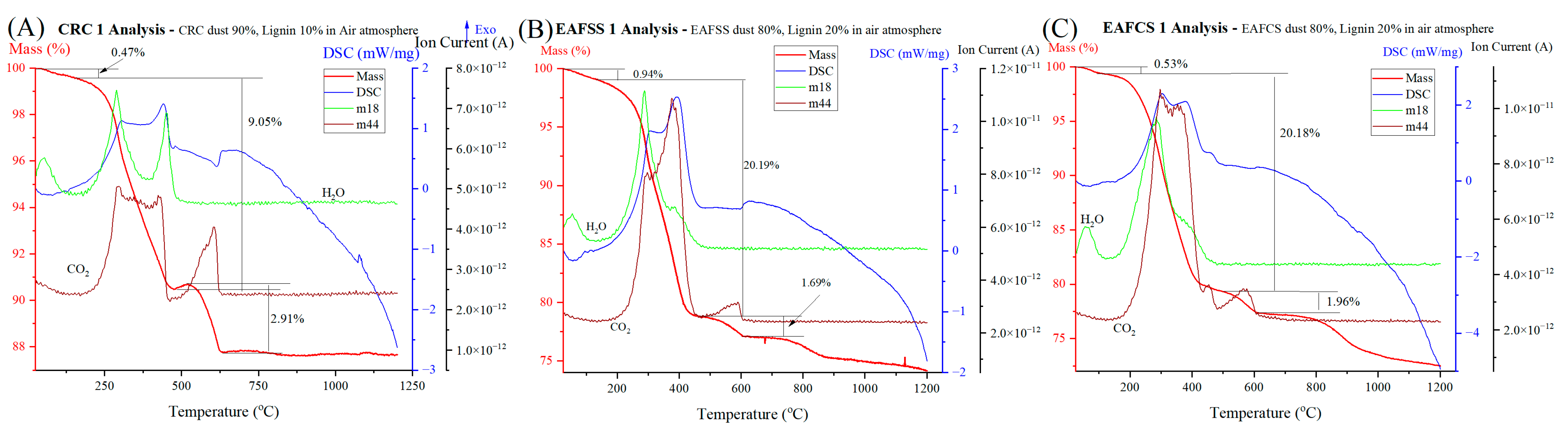
3.3. Effect of Mixing Proportion of Lignin as a Reducing Agent
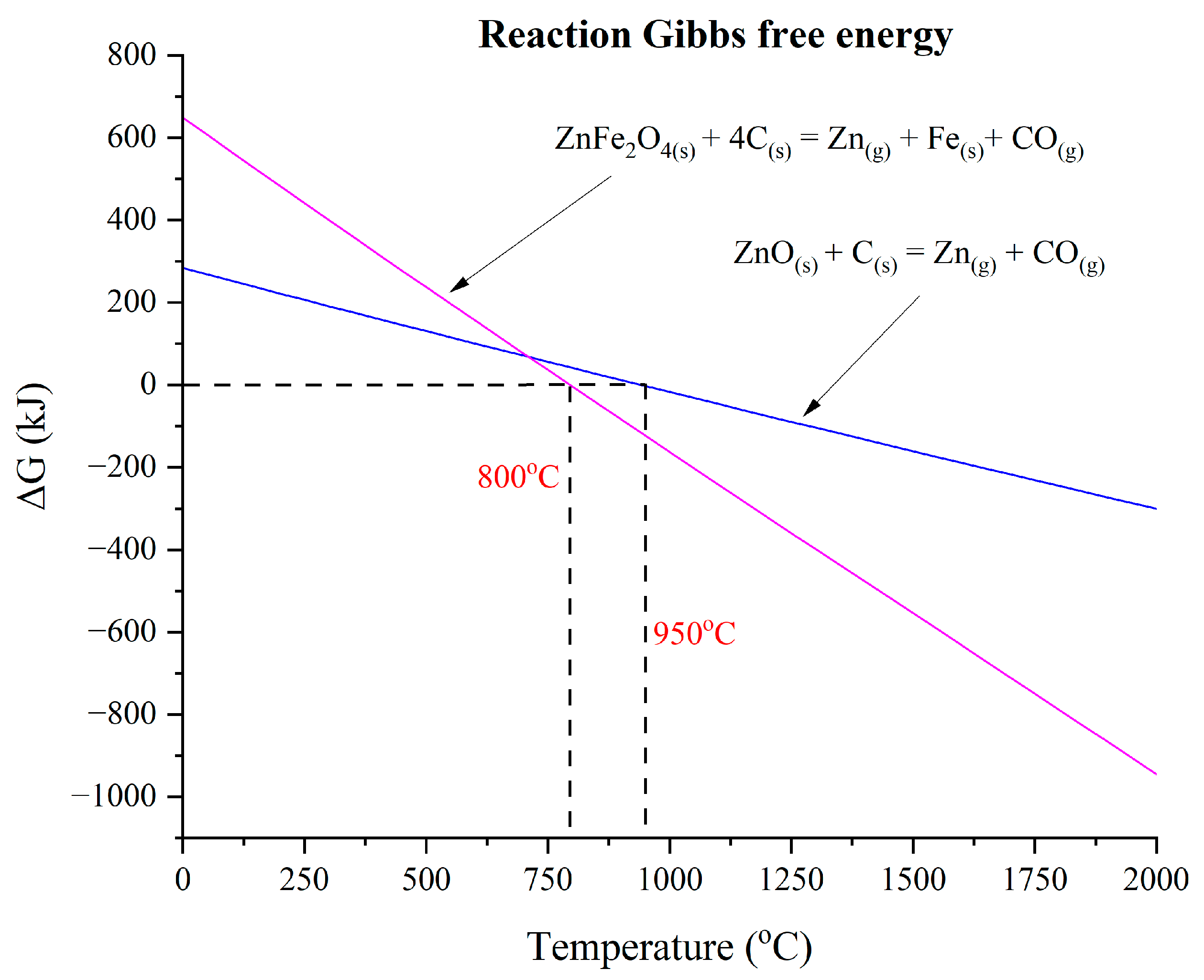
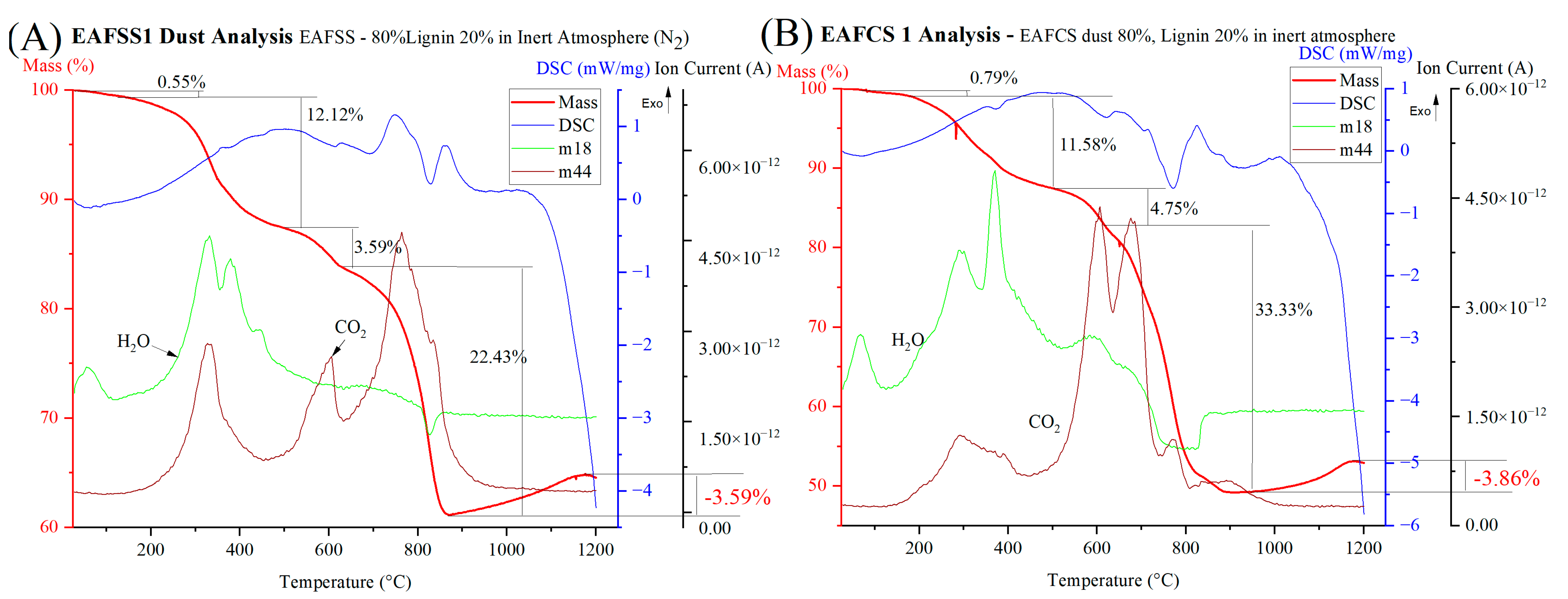
3.4. Atmospheric Impact on Steel Dust–Lignin Mixture
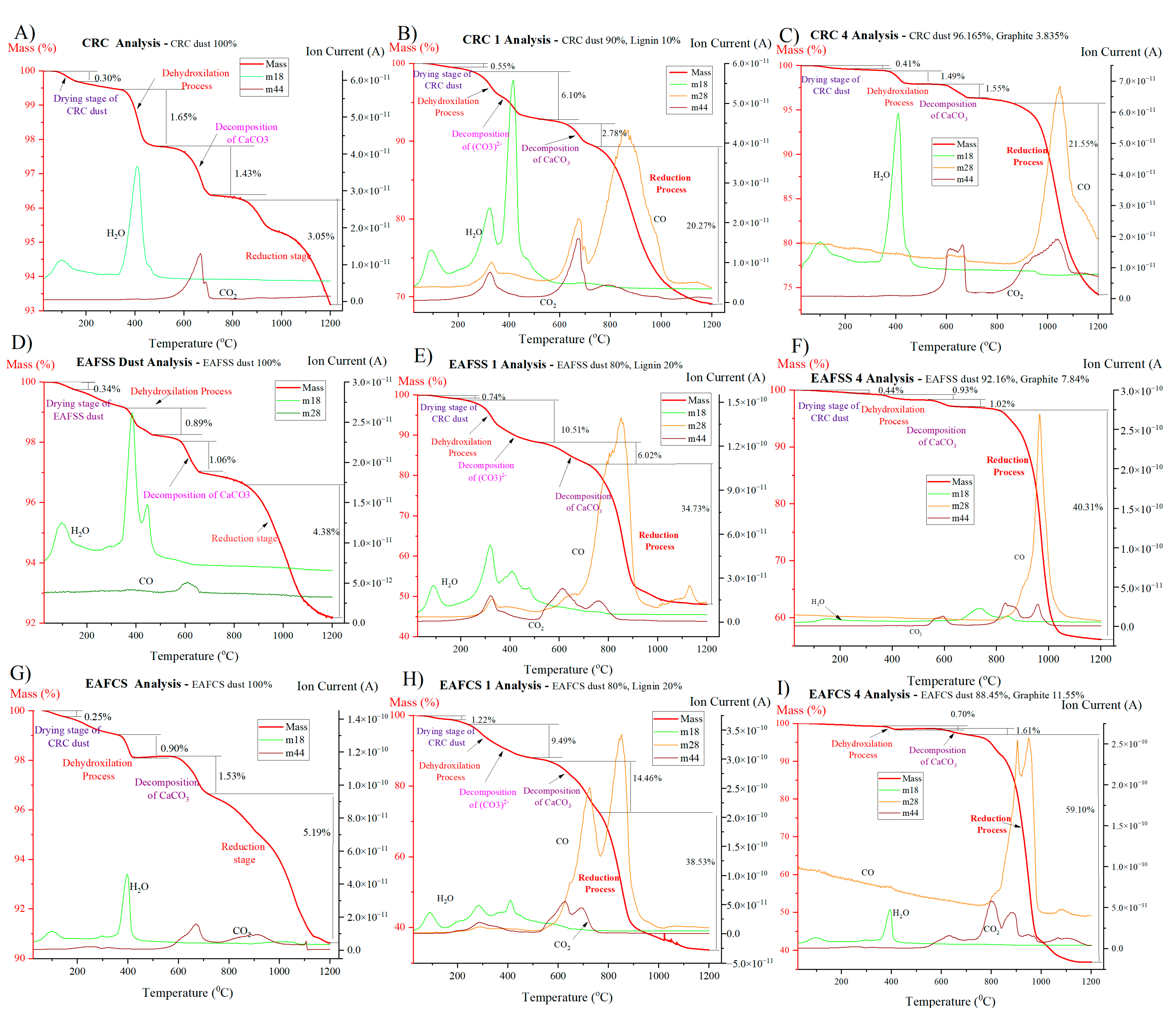
3.5. Effect of Lignin as a Reducing Agent Compared to Graphite
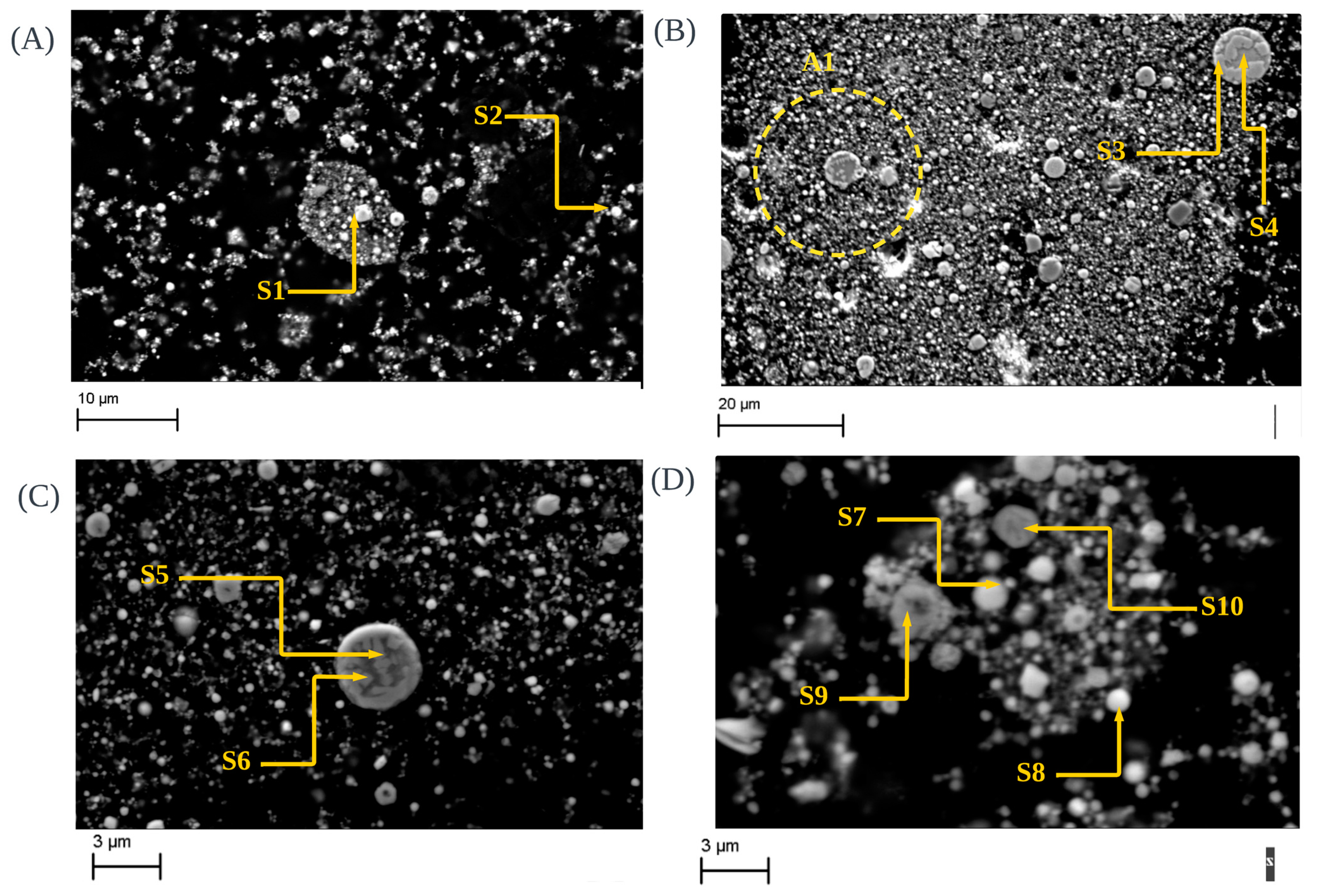
3.6. Morphological Observations of CRC1, EAFSS1 and EAFCS1 Dust under Inert Atmosphere
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- World Steel Association. World Steel Association Steel Statistical Yearbook, 2010; World Steel Association: Brussels, Belgium, 2010. [Google Scholar]
- Omran, M.; Fabritius, T.; Yu, Y.; Heikkinen, E.P.; Chen, G.; Kacar, Y. Improving Zinc Recovery from Steelmaking Dust by Switching from Conventional Heating to Microwave Heating. J. Sustain. Metall. 2021, 7, 15–26. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Electric Arc Furnace (EAF) Dust: Application of Air Classification for Improved Zinc Enrichment in in-Plant Recycling. J. Clean. Prod. 2017, 174, 1–6. [Google Scholar] [CrossRef]
- Sofilić, T.; Rastovčan-Mioč, A.; Cerjan-Stefanović, Š.; Novosel-Radović, V.; Jenko, M. Characterization of Steel Mill Electric-Arc Furnace Dust. J. Hazard. Mater. 2004, 109, 59–70. [Google Scholar] [CrossRef]
- Tsugita, Y. Problems and Prospects of Halogen Element Contained Dust Treatment in Recycling. Mater. Trans. 2003, 44, 2422–2426. [Google Scholar] [CrossRef]
- Peng, B.; Peng, J.; Zhang, C.F.; Lobel, J.; Kozinski, J.A. Thermodynamics Calculation on the Oxidation and Sulfur Removal Abilities of Slag in EAF Dust Pellet Reduction Process. J. Cent. South Univ. Technol. Engl. Ed. 2001, 8, 64–68. [Google Scholar] [CrossRef]
- Bae, H.R.; Barna, R.; Méhu, J.; van der Sloot, H.; Moszkowicz, P.; Desnoyers, C. Assessment of Chemical Sensitivity of Waelz Slaga. Stud. Environ. Sci. 1997, 71, 647–660. [Google Scholar]
- Xue, Y.; Hao, X.; Liu, X.; Zhang, N. Recovery of Zinc, and Iron from Steel Mill Dust—An Overview of Available Technologies. Materials 2022, 15, 4127. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Peng, Z.; Yan, J.; Li, Z.; Hwang, J.; Zhang, Y.; Li, G.; Giang, T. Pyrometallurgical Recycling of Electric Arc Furnace Dust. J. Clean. Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- Caravaca, C.; Cobo, A.; Alguacil, F.J. Considerations about the Recycling of EAF Flue Dusts as Source for the Recovery of Valuable Metals by Hydrometallurgical Processes. Resour. Conserv. Recycl. 1994, 10, 35–41. [Google Scholar] [CrossRef]
- Voloshin, Y.; Belaya, I.; Krämer, R. General Considerations: Caging Ligands and Cage Complexes: Main Classes and Subclasses, Types and Subtypes. In The Encapsulation Phenomenon: Synthesis, Reactivity and Applications of Caged Ions and Molecules; Springer: Midtown Manhattan, NY, USA, 2016; pp. 1–4. [Google Scholar]
- Havlík, T.; Vidor E Souza, B.; Moura Bernardes, A.; André, I.; Schneider, H.; Miškufová, A. Hydrometallurgical Processing of Carbon Steel EAF Dust. J. Hazard. Mater. 2006, 135, 311–318. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T. Effect of Steelmaking Dust Characteristics on Suitable Recycling Process Determining: Ferrochrome Converter (CRC) and Electric Arc Furnace (EAF) Dusts. Powder Technol. 2017, 308, 47–60. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Kemppainen, A.; Haapakangas, J.; Fabritius, T. Extensive Review of the Opportunities to Use Biomass-Based Fuels in Iron and Steelmaking Processes. J. Clean. Prod. 2017, 148, 709–734. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Koskela, A.; Suopajärvi, H.; Mattila, O.; Uusitalo, J.; Fabritius, T. Lignin from Bioethanol Production as a Part of a Raw Material Blend of a Metallurgical Coke. Energies 2019, 12, 1533. [Google Scholar] [CrossRef]
- Luo, H.; Abu-Omar, M.M. Chemicals from Lignin. Sustain. Technol. 2017, 3, 573–585. [Google Scholar]
- Hu, J.; Zhang, Q.; Lee, D.J. Kraft Lignin Biorefinery: A Perspective. Bioresour. Technol. 2018, 247, 1181–1183. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. (Charles) Global Lignin Supply Overview and Kraft Lignin Potential as an Alternative for Petroleum-Based Polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Oyj, O. Stainless Steel Production. In Handbook of Stainless Steel; Outokumpu Oyj, Ed.; Outokumpu Oyj: Helsinki, Finland, 2013; pp. 24–29. [Google Scholar]
- Omran, M.; Fabritius, T.; Heikkinen, E.P. Selective Zinc Removal from Electric Arc Furnace (EAF) Dust by Using Microwave Heating. J. Sustain. Metall. 2019, 5, 331–340. [Google Scholar] [CrossRef]
- Singh, R. Production of Steel. In Applied Welding Engineering Processes, Codes, and Standards; Guerin, B., Ed.; Mathew Deans: Oxford, UK, 2020; pp. 35–52. [Google Scholar]
- Madias, J. Electric Arc Furnace. In Ironmaking and Steelmaking Processes: Greenhouse Emissions, Control, and Reduction; Cavaliere, P., Ed.; Springer International Publishing: New York, NY, USA, 2016; pp. 267–281. [Google Scholar]
- Guézennec, A.G.; Huber, J.C.; Patisson, F.; Sessiecq, P.; Birat, J.P.; Ablitzer, D. Dust Formation in Electric Arc Furnace: Birth of the Particles. Powder Technol. 2005, 157, 2–11. [Google Scholar] [CrossRef]
- Simonyan, L.M.; Alpatova, A.A.; Demidova, N.V. The EAF Dust Chemical and Phase Composition Research Techniques. J. Mater. Res. Technol. 2019, 8, 1601–1607. [Google Scholar] [CrossRef]
- GMS Machado, J.; Andrade Brehm, F.; Alberto Mendes Moraes, C.; Alberto dos Santos, C.; Cezar Faria Vilela, A.; Batista Marimon da Cunha, J. Chemical, Physical, Structural and Morphological Characterization of the Electric Arc Furnace Dust. J. Hazard. Mater. 2006, 136, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.M.; Manoel, J.; Neto, R.; Jorge Da Cunha, C. Mineral Phases of Weathered and Recent Electric Arc Furnace Dust. J. Hazard. Mater. 2008, 154, 417–425. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal Degradation of Lignin—A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Ranzi, E. Detailed Kinetic Modeling of the Thermal Degradation of Lignins. Biomass Bioenergy 2010, 34, 290–301. [Google Scholar] [CrossRef]
- Brodin, I.; Sjö Holm, B.; Gö Ran Gellerstedt, E. The Behavior of Kraft Lignin during Thermal Treatment. J. Anal. Appl. Pyrolysis 2010, 87, 70–77. [Google Scholar] [CrossRef]
- Koskela, A.; Heikkilä, A.; Bergna, D.; Salminen, J.; Fabritius, T. Effects of Briquetting and High Pyrolysis Temperature on Hydrolysis Lignin Char Properties and Reactivity in CO-CO2-N2 Conditions. Minerals 2021, 11, 187. [Google Scholar] [CrossRef]
- SFS-EN 14774-2; Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 2: Total Moisture—Simplified Procedure. Finnish Standards Association: Helsinki, Finland, 2010.
- CEN/TS 15414-2; Solid Recovered Fuels—Determination of Moisture Content Using the Oven Dry Method—Part 2: Determination of Total Moisture by a Simplified Method. CEN: Brussels, Belgium, 2010.
- ISO 589; Hard Coal—Determination of Total Moisture. ISO: Geneva, Switzerland, 2008.
- SFS-EN ISO 16948; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen, and Nitrogen—Instrumental Methods. Finnish Standards Association: Helsinki, Finland, 2015.
- SFS-EN 15407; Solid Recovered Fuels—Methods for the Preparation of the Test Sample from the Laboratory Sample. Finnish Standards Association: Helsinki, Finland, 2011.
- ISO 29541; Solid Mineral Fuels—Determination of Total Carbon, Hydrogen and Nitrogen Content—Instrumental Methods. ISO: Geneva, Switzerland, 2010.
- SFS-EN ISO 16993; Solid Biofuels—Determination of Bulk Density. Finnish Standards Association: Helsinki, Finland, 2016.
- ASTM D4239-18; Standard Test Method for Sulfur in the Analysis Sample of Coal and Coke Using High-Temperature Tube Furnace Combustion. ASTM International: West Conshohocken, PA, USA, 2018.
- SFS-EN ISO 16994; Solid Biofuels—Determination of Particle Size Distribution. Finnish Standards Association: Helsinki, Finland, 2019.
- SFS-EN ISO 18123; Solid Biofuels—Determination of Ash Content. Finnish Standards Association: Helsinki, Finland, 2016.
- SFS-EN 15402; Solid Biofuels—Determination of Moisture Content. Finnish Standards Association: Helsinki, Finland, 2014.
- ISO 562; Hard Coal and Coke—Determination of Volatile Matter. International Organization for Standardization: Geneva, Switzerland, 1998.
- SFS-EN ISO 18122; Solid Biofuels—Determination of Ash Content. Finnish Standards Association: Helsinki, Finland, 2016.
- SFS-EN 15403; Solid Recovered Fuels—Determination of Ash Content. Finnish Standards Association: Helsinki, Finland, 2011.
- ISO 1171; Solid Mineral Fuels—Determination of Ash. International Organization for Standardization: Geneva, Switzerland, 2010.
- Trifunović, V.; Milić, S.; Avramović, L.; Jonović, R.; Gardić, V.; Đorđievski, S.; Dimitrijević, S. Investigation of Hazardous Waste—A Case Study of Electric Arc Furnace Dust Characterization; Association of the Chemical Engineers of Serbia: Beograd, Serbia, 2022; Volume 76, pp. 237–249. [Google Scholar]
- Makkonen, J.; Marsh, L.A.; Vihonen, J.; O’Toole, M.D.; Armitage, D.W.; Jarvi, A.; Peyton, A.J.; Visa, A. Determination of Material and Geometric Properties of Metallic Objects Using the Magnetic Polarisability Tensor. In Proceedings of the SAS 2015—2015 IEEE Sensors Applications Symposium, Proceedings, Zadar, Croatia, 13–15 April 2015. [Google Scholar]
- Navarro, C.; Díaz, M.; Villa-García, M.A. Physico-Chemical Characterization of Steel Slag. Study of Its Behavior under Simulated Environmental Conditions. Environ. Sci. Technol. 2010, 44, 5383–5388. [Google Scholar] [CrossRef] [PubMed]
- El Moustaqim, M.; El Kaihal, A.; El Marouani, M.; Men-La-Yakhaf, S.; Taibi, M.; Sebbahi, S.; El Hajjaji, S.; Kifani-Sahban, F. Thermal and Thermomechanical Analyses of Lignin. Sustain. Chem. Pharm. 2018, 9, 63–68. [Google Scholar] [CrossRef]



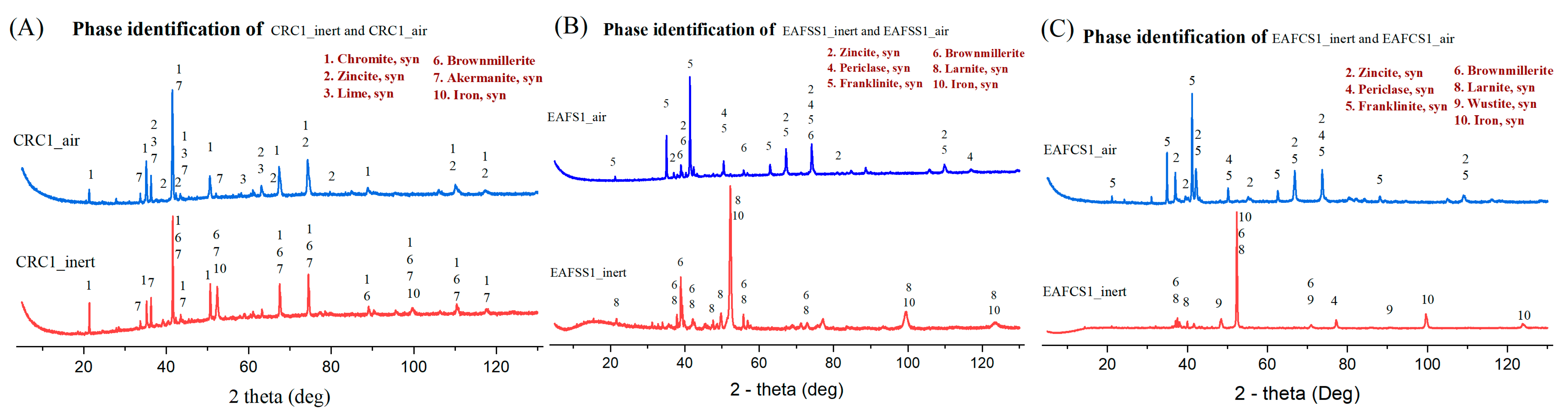
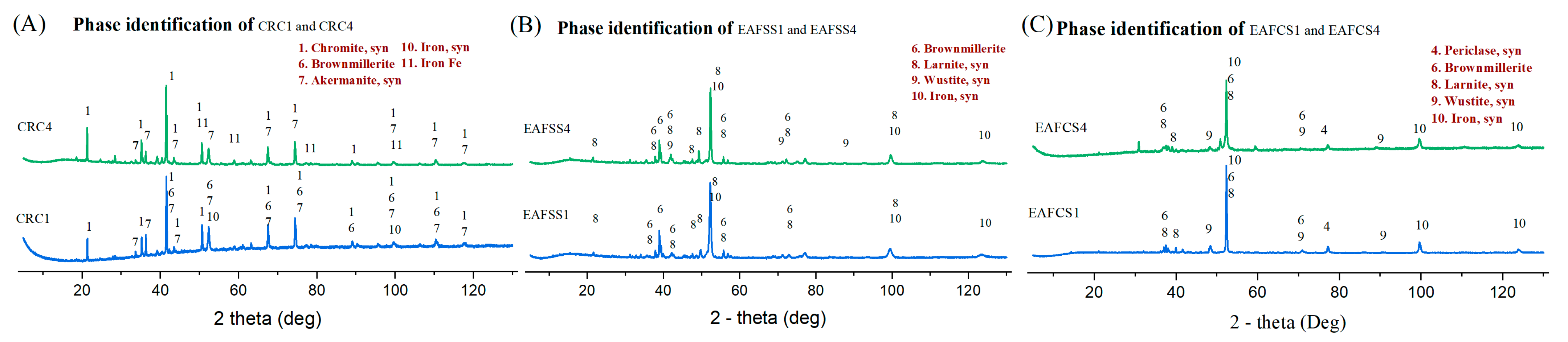
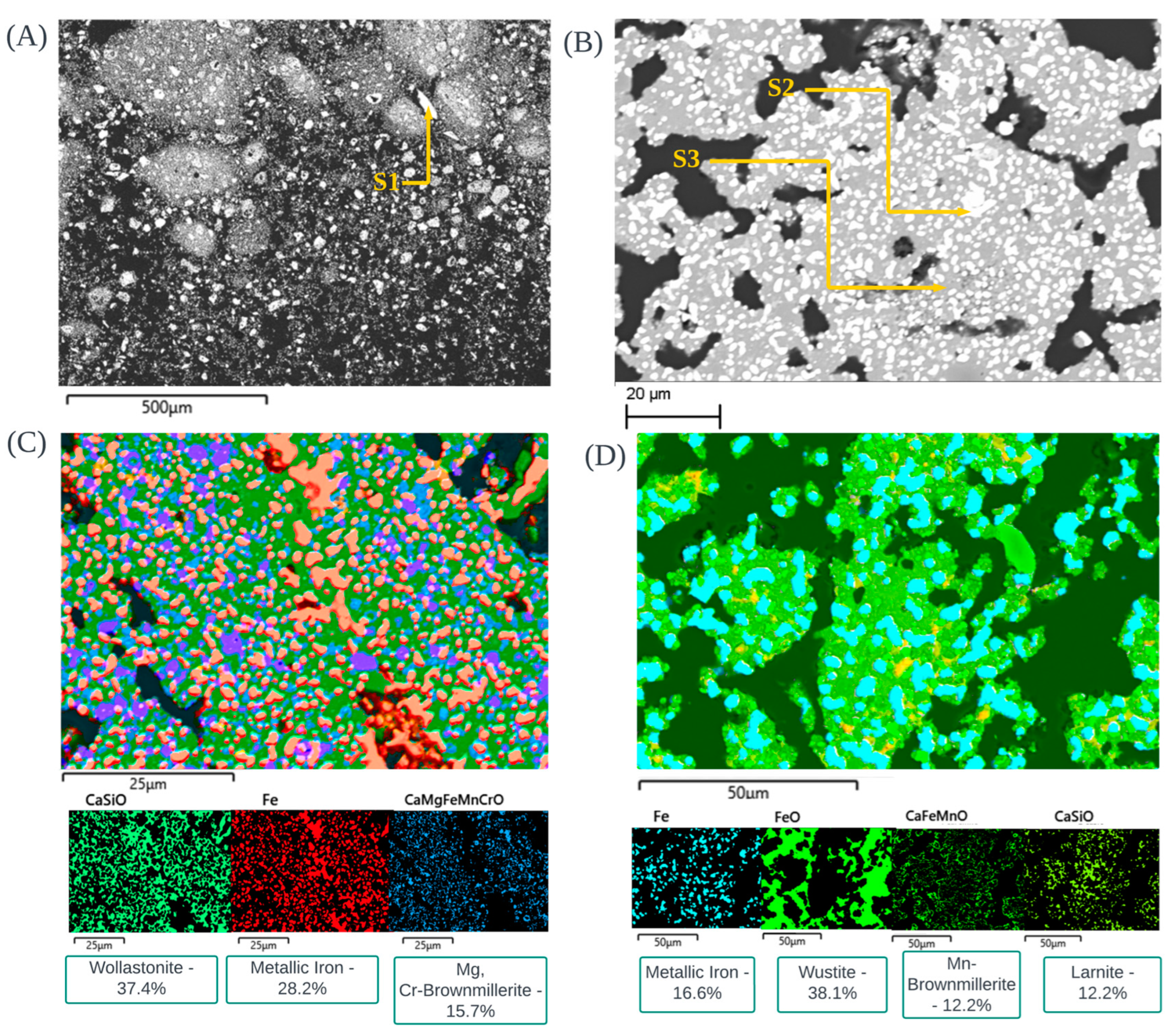
| Type | Sample | Reducing Agent | Stoichiometric Amount |
|---|---|---|---|
| CRC | CRC | - | - |
| CRC1 | lignin | 1.1 | |
| CRC2 | lignin | 1.3 | |
| CRC3 | lignin | 1.5 | |
| CRC4 | graphite | 1.3 | |
| EAFSS | EAFSS | - | - |
| EAFSS1 | lignin | 1.1 | |
| EAFSS2 | lignin | 1.3 | |
| EAFSS3 | lignin | 1.5 | |
| EAFSS4 | graphite | 1.3 | |
| EAFCS | EAFCS | - | - |
| EAFCS1 | lignin | 1.1 | |
| EAFCS2 | lignin | 1.3 | |
| EAFCS3 | lignin | 1.5 | |
| EAFCS4 | graphite | 1.3 |
| CRC | EAFSS | EAFCS | |
|---|---|---|---|
| Element | Major Elements (wt.%) | ||
| C | 0.3 | 0.5 | 1.5 |
| Zn | 10.83 | 19.84 | 35.76 |
| Cr | 20.88 | 3.19 | 0.47 |
| Fe | 18.74 | 23.70 | 23.50 |
| MgO | 9.76 | 7.21 | 1.07 |
| MnO | 1.56 | 5.82 | 3.99 |
| CaO | 14.27 | 11.91 | 5.93 |
| Al2O3 | 0.80 | 0.87 | 0.27 |
| K2O | 0.74 | 1.49 | 3.21 |
| P2O5 | 0.02 | 0.08 | 0.13 |
| Cl | 0.29 | 1.25 | 1.69 |
| F | 0.31 | 0.51 | 0.37 |
| TiO2 | 0.09 | 0.09 | 0.05 |
| Pb | 0.11 | 0.62 | 2.15 |
| Ni | 0.25 | 0.37 | 0.038 |
| S | 0.14 | 0.48 | 1.22 |
| Cu | 0.032 | 0.30 | 0.25 |
| pH | 10–11 | 11 | 10–11 |
| Moisture % | 0.4 | 0.8 | 0.98 |
| Properties of Hydrolysis Lignin | Standard/Analysis Methods | |||
|---|---|---|---|---|
| Total moisture (105 °C) | [m%] | 5.3 | SFS-EN 14774-2 [32], CEN/TS 15414-2 [33], ISO 589 [34] | |
| Ultimate analysis | C | %, d. b. | 61 | SFS-EN ISO 16948 [35], SFS-EN 15407 [36], ISO 29541 [37] |
| H | %, d. b. | 6.1 | SFS-EN ISO 16948 [35], SFS-EN 15407 [36], ISO 29541 [37] | |
| O | %, d. b. | 31.9 | SFS-EN ISO 16993 [38] | |
| N | %, d. b. | 0.69 | SFS-EN ISO 16948 [35], SFS-EN 15407 [36], ISO 29541 [37] | |
| S | %, d. b. | 0.12 | ASTM D 4239 (mod) [39], SFS-EN ISO 16994 [40] | |
| Proximate analysis | Volatile matter | %, d. b. | 70.9 | SFS-EN ISO 18123 [41], SFS-EN 15402 [42], ISO 562 [43] |
| Ash content | %, d. b. | 0.2 | SFS-EN ISO 18122 [44], SFS-EN 15403 [45], ISO 1171 [46] | |
| Fixed Carbon | %, d. b. | 28.9 | Determined by difference | |
| Ash Content | ||
|---|---|---|
| Element | unit | amount |
| Cl | %, d. b. | 0.002 |
| F | %, d. b. | <0.001 |
| Br | %, d. b. | <0.001 |
| Ca | mg/kg, d. b. | 290 |
| Mg | mg/kg, d. b. | 51 |
| Na | mg/kg, d. b. | <10.000 |
| K | mg/kg, d. b. | 130 |
| P | mg/kg, d. b. | 74 |
| S | mg/kg, d. b. | 1100 |
| Fe | mg/kg, d. b. | 810 |
| Al | mg/kg, d. b. | 14 |
| Si | mg/kg, d. b. | <10.000 |
| Ti | mg/kg, d. b. | <1.000 |
| Mn | mg/kg, d. b. | 41 |
| Ba | mg/kg, d. b. | 4.8 |
| Cr | mg/kg, d. b. | 15 |
| Cu | mg/kg, d. b. | <1.000 |
| Ni | mg/kg, d. b. | 12 |
| Zn | mg/kg, d. b. | 12 |
| Pb | mg/kg, d. b. | 1.3 |
| V | mg/kg, d. b. | <1.000 |
| Ar | mg/kg, d. b. | <0.500 |
| Elements | wt% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | |
| Mg-Mn-Si- Chromite | Zincite | Franklinite-Glass Sphere | Franklinite-Glass Sphere | Ca-Franklinite | Ca-Franklinite | Zincite | Zincite | Franklinite | Franklinite | |
| O | 31.29 | 21.40 | 32.19 | 30.51 | 32.06 | 29.07 | 20.73 | 22.37 | 24.83 | 26.64 |
| Zn | 33.82 | 11.58 | 19.2 | 8.29 | 14.46 | 58.36 | 62.45 | 19.38 | 19.87 | |
| Ca | 21.21 | 16.22 | 9.25 | |||||||
| Fe | 13.86 | 3.34 | 10.14 | 12.92 | 32.63 | 37.13 | 32.13 | 34.45 | ||
| Mg | 3.42 | 19.55 | ||||||||
| Cr | 40.54 | 6.23 | ||||||||
| Mn | 1.21 | |||||||||
| Na | 3.67 | 1.98 | ||||||||
| C | 28.79 | |||||||||
| Sample | Dihydroxylation and Partial Decomposition | Decomposition of Carbonates | Reduction | Total Mass Loss % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Start °C | End °C | Mass Loss % | Start °C | End °C | Mass Loss % | Start °C | End °C | Mass Loss % | ||
| CRC1 | 257 | 520 | 6.10 | 627 | 745 | 2.78 | 745 | 1200 | 20.27 | 30.93 |
| CRC2 | 248 | 457 | 8.01 | 573 | 717 | 3.27 | 778 | 1200 | 22.48 | 37.31 |
| CRC3 | 265 | 519 | 10.43 | 519 | 739 | 4.01 | 739 | 1200 | 26.70 | 43.04 |
| EAFSS1 | 220 | 519 | 10.51 | 519 | 732 | 6.02 | 732 | 1200 | 34.73 | 51.98 |
| EAFSS2 | 243 | 464 | 11.50 | 464 | 692 | 5.70 | 692 | 1200 | 34.82 | 54.21 |
| EAFSS3 | 236 | 478 | 14.04 | 538 | 686 | 4.23 | 686 | 1200 | 32.79 | 54.21 |
| EAFCS1 | 247 | 502 | 9.49 | 565 | 765 | 14.46 | 765 | 1200 | 38.53 | 66.62 |
| EAFCS2 | 184 | 474 | 14.77 | 474 | 754 | 14.34 | 754 | 1200 | 37.73 | 68.54 |
| EAFCS3 | 243 | 481 | 20.77 | 481 | 771 | 14.70 | 771 | 1200 | 29.71 | 66.62 |
| Sample | CRC1 | CRC2 | CRC3 | EAFSS1 | EAFSS2 | EAFSS3 | EAFCS1 | EAFCS2 | EAFCS3 |
| C% (wt) | 0.10 | 0.18 | 0.81 | 0.20 | 3.94 | 8.59 | 0.25 | 12.23 | 28.87 |
| Sample | Combustion of Hydrocarbon | Decomposition of Carbonates | ||||||
|---|---|---|---|---|---|---|---|---|
| Start °C | End °C | Mass Loss % | DSC | Start °C | End °C | Mass Loss % | DSC | |
| CRC1 | 155 | 477 | 9.05 | exothermic | 521 | 632 | 2.91 | endothermic |
| EAFSS1 | 100 | 456 | 20.19 | exothermic | 456 | 610 | 1.69 | endothermic |
| EAFCS1 | 97 | 468 | 20.18 | exothermic | 468 | 607 | 1.96 | endothermic |
| Sample | Reducing Agent | Dihydroxylation and Partial Decomposition | Decomposition of Carbonates | Reduction | Total Mass Loss % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Start °C | End °C | Mass Loss % | Start °C | End °C | Mass Loss % | Start °C | End °C | Mass Loss % | |||
| CRC | no | 100 | 494 | 1.65 | 494 | 717 | 1.43 | 845 | 1200 | 3.05 | 6.82 |
| CRC1 | lignin | 257 | 520 | 6.10 | 627 | 745 | 2.78 | 745 | 1200 | 20.27 | 30.93 |
| CRC4 | graphite | 211 | 470 | 1.49 | 470 | 688 | 1.55 | 666 | 1200 | 21.55 | 25.82 |
| EAFSS | no | 357 | 474 | 0.89 | 583 | 658 | 1.06 | 843 | 1200 | 4.38 | 7.82 |
| EAFSS1 | lignin | 220 | 519 | 10.51 | 519 | 732 | 6.02 | 732 | 1200 | 34.73 | 51.98 |
| EAFSS4 | graphite | 355 | 530 | 0.93 | 530 | 320 | 1.02 | 806 | 1200 | 40.31 | 43.84 |
| EAFCS | no | 366 | 433 | 0.90 | 562 | 719 | 1.53 | 719 | 1200 | 5.19 | 9.40 |
| EAFCS1 | lignin | 247 | 502 | 9.49 | 565 | 765 | 14.46 | 765 | 1200 | 38.53 | 66.62 |
| EAFCS4 | graphite | 367 | 413 | 0.70 | 538 | 698 | 1.61 | 698 | 1200 | 59.10 | 63.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Don, D.M.W.W.; Fabritius, T.; Omran, M. The Reduction Reaction Behavior of Steelmaking Dusts with Lignin under Different Atmospheres. Materials 2024, 17, 3106. https://doi.org/10.3390/ma17133106
Don DMWW, Fabritius T, Omran M. The Reduction Reaction Behavior of Steelmaking Dusts with Lignin under Different Atmospheres. Materials. 2024; 17(13):3106. https://doi.org/10.3390/ma17133106
Chicago/Turabian StyleDon, Danuka Maduranga Wawita Widanalage, Timo Fabritius, and Mamdouh Omran. 2024. "The Reduction Reaction Behavior of Steelmaking Dusts with Lignin under Different Atmospheres" Materials 17, no. 13: 3106. https://doi.org/10.3390/ma17133106
APA StyleDon, D. M. W. W., Fabritius, T., & Omran, M. (2024). The Reduction Reaction Behavior of Steelmaking Dusts with Lignin under Different Atmospheres. Materials, 17(13), 3106. https://doi.org/10.3390/ma17133106








