Ammonothermal Crystal Growth of Functional Nitrides for Semiconductor Devices: Status and Potential
Abstract
:1. Introduction
1.1. Potential of Functional Nitrides for Semiconductor Devices
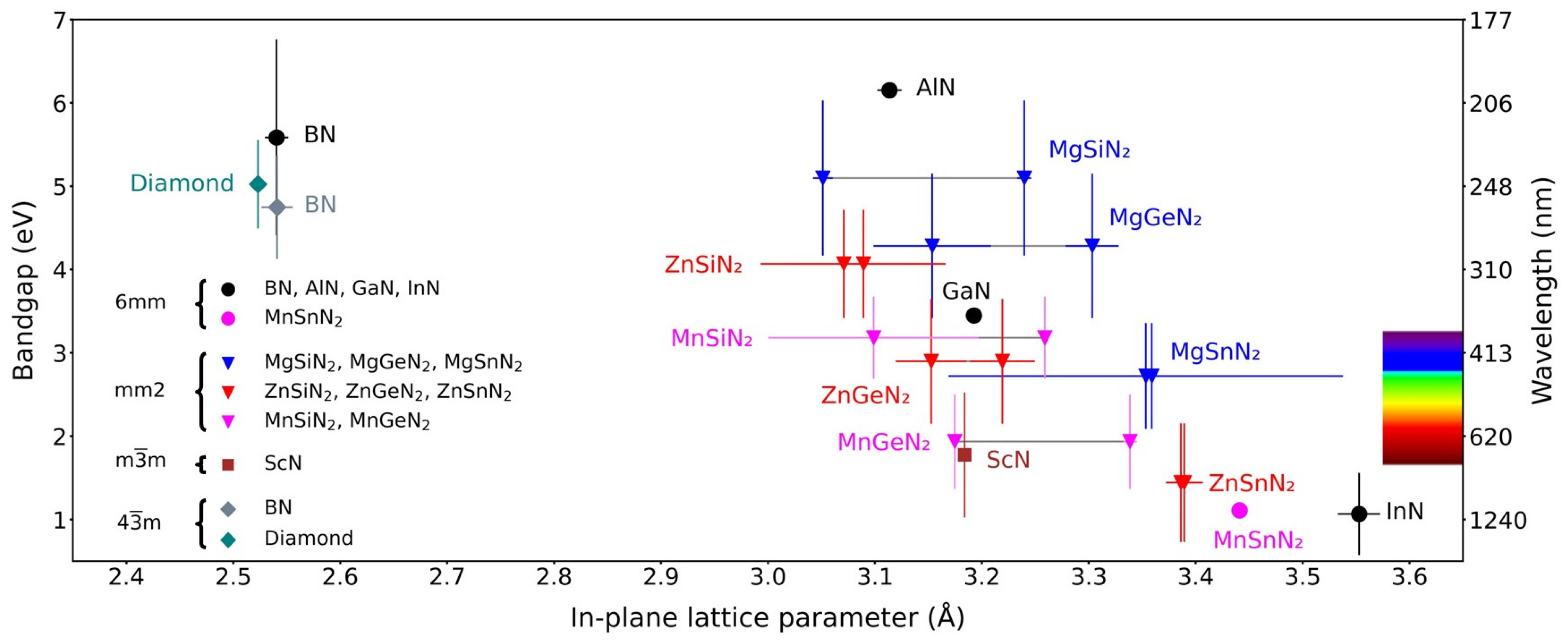
1.2. Brief Overview of Methods for Nitride Synthesis and Crystal Growth
1.3. Potential of the Ammonothermal Method for the Synthesis of Functional Nitrides Materials
2. Ammonothermal Method

3. Reactivity and Solubility of Elements under Ammonothermal Conditions
- Category C1 denotes elements or compounds exhibiting exceptional resistance to corrosion within the ammonothermal environment, either in their elemental or compound forms. Negative R and S values are assigned, as the available information suggests a lack of reactivity and solubility;
- Category C2 represents elements or compounds for which no data regarding their reactivity or solubility under ammonothermal conditions are available;
- Category C3 represents compounds or elementals that do undergo reactions under ammonothermal conditions, but no clear indication of significant transport was found. Typical examples are the corrosion of elements or compounds, or the formation of compounds without indications of transport, as is often the case for the synthesis of compounds in nano- or microcrystalline form;
- Category C4 indicates the formation of compounds that are soluble in liquid ammonia. Typical examples are the compounds that were synthesized under ammonothermal conditions and were reported to likely represent soluble intermediates during ammonothermal synthesis and crystal growth, but at times room temperature or even lower temperatures, but on the whole, ranging from 197–873 K [119];
- Category C5 represents elements for which a clear indication of transport under ammonothermal conditions was found. Examples are mineralizer cation in the case of ammonobasic growth, mineralizer anion in the case of ammonoacidic growth, impurities incorporated in grown crystals (mostly GaN, as there are hardly any investigations on impurities in other materials grown via solute transport), elements for which dedicated solubility studies are available, as well as nitrogen and hydrogen as ubiquitous elements in ammonothermal fluids and ammonothermally grown crystals.
- Ammonoacidic conditions: acidic mineralizers such as the ammonium halides NH4X (X = F, Cl, Br, I) are present, enhancing the concentration of NH4+ ions;
- Ammonobasic conditions: basic mineralizers such as alkali and alkaline earth metals or amides or azides thereof are present, enhancing the concentration of NH2− ions;
- Ammononeutral conditions: the concentration of NH4+ ions and NH2− ions is balanced, either in supercritical ammonia without mineralizers, or due to the balanced presence of different compounds increasing the concentrations of the NH4+ ions, as well as that of the NH2− ions, resulting in an ammononeutral milieu.
3.1. Ammonoacidic Conditions
3.2. Ammonobasic Conditions
3.3. Ammononeutral Conditions
4. Synthesis and Crystal Growth of Nitrides
4.1. III-Nitrides
4.1.1. GaN (Overview of Available Literature)
4.1.2. AlN
Solubility Results Will Be Discussed in the Following
Crystallization Results Will Be Discussed in the Following
4.1.3. AlGaN
4.1.4. InN
4.1.5. BN
4.2. Novel Nitrides and Related Materials beyond Group III-Nitrides
5. Generation of Point Defects and Their Impact on Crystal Properties
5.1. Native Point Defects and Related Defect Complexes
5.2. Impurities
|
|
|
|
|
|
5.3. Effect of Point Defects on Electrical, Oprical and Structural Properties
5.3.1. Electrical Properties
5.3.2. Optical Properties
5.3.3. Structural Properties
6. Advances in Reactor Technology
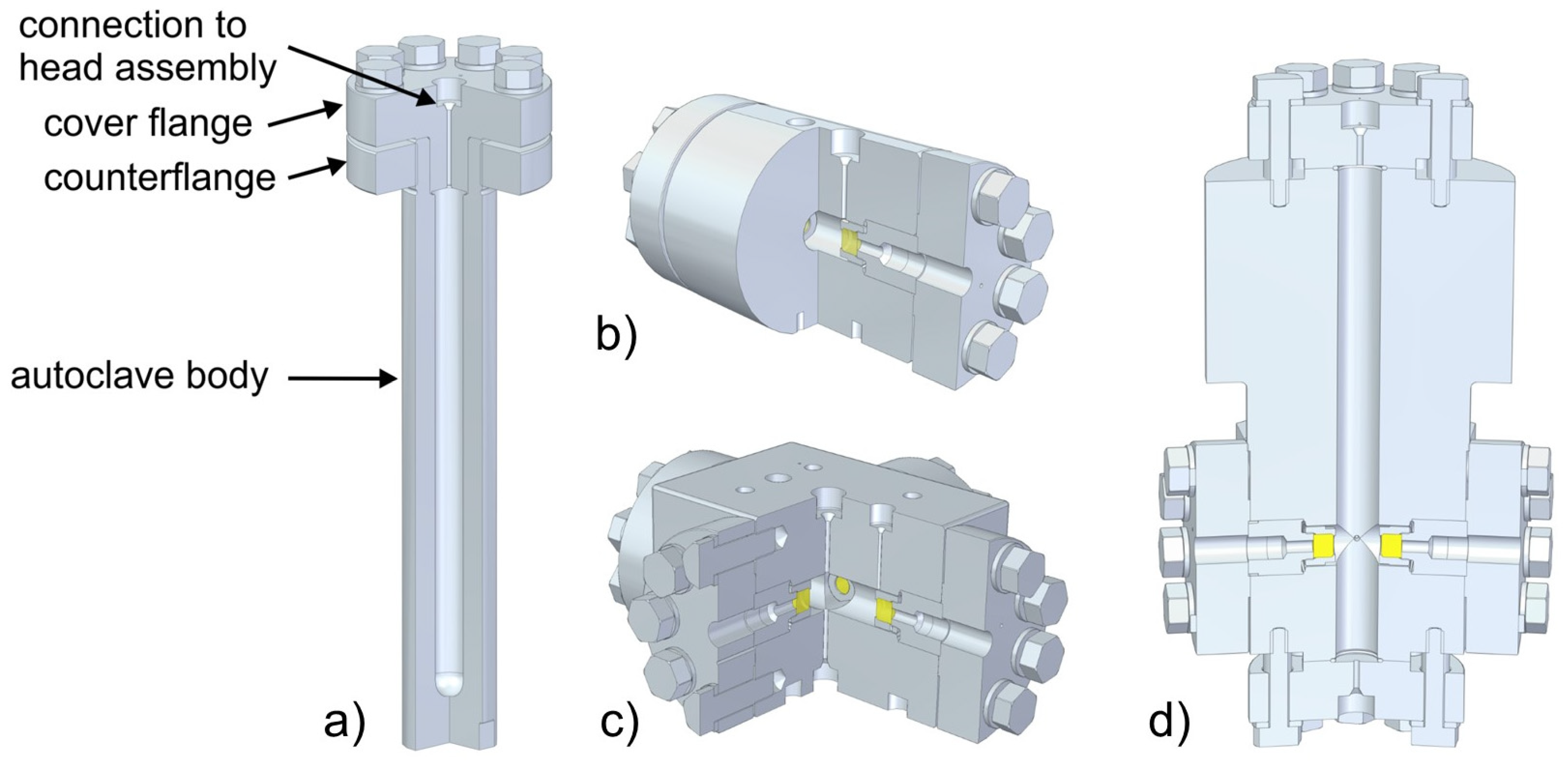
6.1. General Design Considerations
6.2. Reactor Lifetime and Crystal Purity
- They more likely mentioned in their publications which materials they used, contrary to other groups;
- Their publication record is wide, from the first publication on the topic for the basic environment and nearly on the first publication from the acidic environment, until now (contrary to other groups).
6.3. In Situ Monitoring
7. Emerging Technologies for In Situ Monitoring
7.1. Internal Temperature Measurements
7.2. Imaging Techniques
7.2.1. Video Optical Measurements
7.2.2. X-ray Imaging and Computed Tomography
7.3. Spectroscopic Techniques
7.3.1. UV/Vis Spectroscopy
7.3.2. Raman Spectroscopy
7.4. Ultrasonic Velocity Measurements
7.5. Rolling Ball Viscosimeter
7.6. X-ray Diffraction
8. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Ammonoacidic Conditions
| Element Symbol | IUPAC Group | CAS | Reactivity and Solubility Value | Mineralizer | Reference |
|---|---|---|---|---|---|
| Ag | 11 | IB | −1 | NH4F | [156] |
| Ag | 11 | IB | 2 | NH4Cl | [147] |
| Ag | 11 | IB | 6 | NH4Br | [157] |
| Al | 13 | IIIA | 4 | NH4I | [162] |
| Al | 13 | IIIA | 4 | NH4F | [161] |
| Al | 13 | IIIA | 4 | NH4Cl | [159] |
| Al | 13 | IIIA | 7 | NH4Cl | [159,223] |
| Au | 11 | IB | −1 | NH4Cl | [147] |
| Au | 11 | IB | 6 | NH4Br | [157] |
| B | 13 | IIIA | 6 | NH4F | [163] |
| Bi | 15 | VA | 6 | NH4Br | [157] |
| Br | 17 | VIIA | 5 | NH4Br | [152,154,373] |
| C | 14 | IVA | 6 | NH4Cl | [164] |
| Ca | 2 | IIA | 4 | NH4F | [158] |
| Cl | 17 | VIIA | 5 | NH4Cl | [103,285,374] |
| Co | 9 | VIIIB | 2 | NH4Cl | [147] |
| Co | 9 | VIIIB | -2 | NH4Cl | [147] |
| Cr | 6 | VIB | 6 | NH4C | [164] |
| Cu | 11 | IB | 6 | NH4Br | [157] |
| F | 17 | VIIA | 5 | NH4F | [84,102,103] |
| Fe | 8 | VIIIB | 6 | NH4Cl | [87,164] |
| Ga | 13 | IIIA | 4 | NH4F | [133] |
| Ga | 13 | IIIA | 7 | NH4F | [84,102,103,149,150,330] |
| Ga | 13 | IIIA | 7 | NH4Cl | [103,150,285,319,374,375] |
| Ga | 13 | IIIA | 7 | NH4Br | [154] |
| Ga | 13 | IIIA | 7 | NH4I | [154] |
| Ga | 13 | IIIA | 4 | NH4I | [285,376] |
| Gd | 3 | IIIB | 4 | NH4I | [167] |
| Ge | 14 | IVA | 6 | NH4Br | [157] |
| H | 1 | IA | 8 | NH4F | [84,102,103] |
| H | 1 | IA | 8 | NH4Cl | [103,285,374] |
| H | 1 | IA | 8 | NH4Br | [152,154,373] |
| H | 1 | IA | 8 | NH4I | [103,155,377] |
| I | 17 | VIIA | 5 | NH4I | [103,155,377] |
| Mg | 2 | IIA | 2 | NH4Cl | [119,147] |
| Mg | 2 | IIA | 1 | NH4Cl | [147] |
| Mo | 6 | VIB | −1 | NH4Cl | [147] |
| Mo | 6 | VIB | −2 | NH4Cl | [147] |
| Nb | 5 | VB | −1 | NH4Cl | [147] |
| Ni | 10 | VIIIB | 2 | NH4Cl | [147,165,166] |
| N | 15 | VA | 8 | NH4F | [84,102,103] |
| N | 15 | VA | 8 | NH4Cl | [103,285,374] |
| N | 15 | VA | 8 | NH4Br | [152,154,373] |
| N | 15 | VA | 8 | NH4I | [103,155,377] |
| O | 16 | VIA | 6 | NH4F | [87,103] |
| O | 16 | VIA | 6 | NH4Cl | [87,103] |
| O | 16 | VIA | 6 | NH4Br | [87,103] |
| O | 16 | VIA | 6 | NH4I | [87,103] |
| Pd | 10 | VIIIB | −1 | NH4Cl | [147] |
| Pt | 10 | VIIIB | −1 | NH4Cl | [147] |
| Pt | 10 | VIIIB | −2 | NH4Cl | [147] |
| Sc | 3 | IIIB | 4 | NH4I | [167] |
| Si | 14 | IVA | −1 | NH4Cl | [147] |
| Si | 14 | IVA | 6 | NH4F | [103] |
| Si | 14 | IVA | 6 | NH4Cl | [103] |
| Si | 14 | IVA | 6 | NH4Br | [103] |
| Si | 14 | IVA | 6 | NH4I | [103] |
| Sn | 14 | IVA | 6 | NH4Br | [157] |
| Ta | 5 | VB | 2 | NH4Cl | [147] |
| Ti | 4 | IVB | 2 | NH4Cl | [147] |
| Ti | 4 | IVB | 6 | NH4Cl | [164] |
| V | 5 | VB | 2 | NH4Cl | [147] |
| W | 6 | VIB | −1 | NH4Cl | [147] |
| W | 6 | VIB | −2 | NH4Cl | [147] |
| Y | 3 | IIB | 2 | NH4Cl | [147] |
| Y | 3 | IIIB | 4 | NH4I | [168,378] |
| Zr | 4 | IVB | 2 | NH4Cl | [147] |
| Zr | 4 | IVB | 6 | NH4Cl | [164] |
Appendix B. Ammonobasic Conditions
| Element Symbol | IUPAC Group | CAS | Reactivity and Solubility Value | Mineralizer | Reference |
|---|---|---|---|---|---|
| Ag | 11 | IB | −1 | NaNH2 | [147] |
| Al | 13 | IIIA | 7 | NaNH2 | [147] |
| Al | 13 | IIIA | 7 | KNH2 | [173,220] |
| Al | 13 | IIIA | 7 | KN3 | [174] |
| Al | 13 | IIIA | 7 | LiNH2 | [169,220] |
| Au | 11 | IB | 2 | NaNH2 | [147] |
| B | 13 | IIIA | 7 | NaNH2 | [111] |
| Ba | 2 | IIA | 5 | Ba(NH2)2 | [172] |
| Be | 2 | IIA | 4 | NaN3 | [175] |
| C | 14 | IVA | 6 | NaNH2 | [145] |
| C | 14 | IVA | 6 | KNH2 | [176] |
| Ca | 2 | IIA | 3 | NaN3 | [143,177] |
| Ca | 2 | IIA | 3 | LiNH2 | [178] |
| Cd | 12 | IIB | 3 | KNH2 | [119] |
| Co | 9 | VIIIB | −1 | NaNH2 | [147] |
| Co | 9 | VIIIB | −2 | NaNH2 | [147] |
| Cr | 6 | VIB | 3 | KNH2 | [179] |
| Cs | 1 | IA | 5 | CsNH2 | [171,186,348] |
| Fe | 8 | VIIIB | 6 | Na | [87,140] |
| Ga | 13 | IIIA | 4 | Ba(NH2)2 | [172,379] |
| Ga | 13 | IIIA | 4 | LiNH2 | [96,134,205] |
| Ga | 13 | IIIA | 4 | NaNH2 | [134] |
| Ga | 13 | IIIA | 6 | NaNH2 | [95,324,380] |
| Ga | 13 | IIIA | 6 | LiNH2 | [95] |
| Ga | 13 | IIIA | 6 | KNH2 | [170,381] |
| Ga | 13 | IIIA | 7 | NaNH2 | [149,374,382] |
| Ga | 13 | IIIA | 7 | KNH2 | [109,382] |
| Ga | 13 | IIIA | 4 | KNH2 | [205,381] |
| Gd | 3 | IIIB | 4 | NaNH2 | [167,192] |
| Ge | 14 | IVA | 3 | LiNH2 | [42] |
| Ge | 14 | IVA | 3 | NaNH2 | [42] |
| Ge | 14 | IVA | 3 | KNH2 | [42] |
| Ge | 14 | IVA | 2 | NaNH2 | [147] |
| H | 1 | IA | 8 | NaNH2 | [84,102,103] |
| H | 1 | IA | 8 | KNH2 | [170,173,381] |
| H | 1 | IA | 8 | RbNH2 | [383] |
| H | 1 | IA | 8 | CsNH2 | [171,186,348] |
| Ir | 9 | VIIIB | 3 | NaNH2 | [147] |
| K | 1 | IA | 5 | KNH2 | [170,173,381] |
| Li | 1 | IA | 5 | LiNH2 | [169,220] |
| Mg | 2 | IIA | 2 | NaNH2 | [119,147] |
| Mg | 2 | IIA | 2 | KNH2 | [119,147] |
| Mg | 2 | IIA | 1 | NaNH2 | [147] |
| Mg | 2 | IIA | 4 | NaNH2 | [180,191] |
| Mg | 2 | IIA | 4 | NaN3 | [181,191] |
| Mg | 2 | IIA | 6 | Na | [87,140] |
| Mn | 7 | VIIB | 4 | NaNH2 | [182,384] |
| Mn | 7 | VIIB | 6 | NaNH2 | [183] |
| Mo | 6 | VIB | −1 | NaNH2 | [147] |
| Mo | 6 | VIB | −2 | NaNH2 | [147] |
| Mo | 6 | VIB | 6 | Na | [87,140] |
| N | 15 | VA | 8 | NaNH2 | [84,102,103] |
| N | 15 | VA | 8 | KNH2 | [170,173,381] |
| N | 15 | VA | 8 | CsNH2 | [171,186,348] |
| N | 15 | VA | 8 | RbNH2 | [383] |
| Na | 1 | IA | 5 | NaNH2 | [84,102,103] |
| Nb | 5 | VB | −1 | NaNH2 | [147] |
| Ni | 10 | VIIIB | −1 | NaNH2 | [147,165] |
| Ni | 10 | VIIIB | −2 | NaNH2 | [147] |
| Ni | 10 | VIIIB | 4 | NaNH2 | [184] |
| O | 16 | VIA | 6 | NaNH2 | [145,146] |
| O | 16 | VIA | 6 | KNH2 | [176] |
| P | 15 | VA | 3 | NaN3 | [185,305] |
| Pd | 10 | VIIIB | 1 | NaNH2 | [147] |
| Pt | 10 | VIIIB | 2 | NaNH2 | [147] |
| Pt | 10 | VIIIB | −2 | NaNH2 | [147] |
| Rb | 1 | IA | 5 | RbNH2 | [383] |
| Si | 14 | IVA | 4 | KNH2 | [119,385] |
| Si | 14 | IVA | 1 | NaNH2 | [147] |
| Si | 14 | IVA | 3 | KNH2 | [36,42] |
| Si | 14 | IVA | 6 | Na | [87,140] |
| Ta | 5 | VB | 2 | NaNH2 | [147] |
| Ta | 5 | VB | 3 | NaN3 | [186] |
| Ta | 5 | VB | 3 | KN3 | [186] |
| Ta | 5 | VB | 3 | RbNH2 | [186] |
| Ti | 4 | IVB | 1 | NaNH2 | [147] |
| V | 5 | VB | 2 | NaNH2 | [147] |
| W | 6 | VIB | −1 | NaNH2 | [147] |
| W | 6 | VIB | −2 | NaNH2 | [147] |
| Y | 3 | IIIB | 2 | NaNH2 | [147] |
| Y | 3 | IIIB | 3 | KNH2 | [187] |
| Zn | 12 | IIB | 4 | KNH2 | [188] |
| Zn | 12 | IIB | 6 | KNH2 | [189] |
| Zr | 4 | IVB | 2 | NaNH2 | [147] |
Appendix C. Ammononeutral Conditions
| Element Symbol | IUPAC Group | CAS | R and S Value | Mineralizer | Reference |
|---|---|---|---|---|---|
| Al | 13 | IIIA | 4 | None | [190,386,387] |
| Al | 13 | IIIA | 4 | None | [173] |
| Au | 11 | IB | −2 | None | [147] |
| Ba | 2 | IIA | 4 | None | [181,194,195,388] |
| Be | 2 | IIA | 4 | None | [191] |
| Ca | 2 | IIA | 4 | None | [195,196,197,198,199,389] |
| Ce | 3 | IIIB | 4 | None | [192,390,391] |
| Co | 9 | VIIIB | −1 | None | [147] |
| Co | 9 | VIIIB | −2 | None | [147] |
| Cs | 1 | IA | 4 | None | [378,392] |
| Cs | 1 | IA | 5 | n(InI3):n(CsNH2) = 1:3 | [200] |
| Cu | 11 | IB | 4 | None | [193,393] |
| Eu | 3 | IIIB | 4 | None | [187,195,199,201,210] |
| Fe | 8 | VIIIB | 4 | None | [202,394,395] |
| Ga | 13 | IIIA | 4 | None | [133,134,396] |
| Ge | 14 | IVA | 4 | None | [147] |
| Gd | 3 | IIIB | 4 | None | [192,203] |
| H | 1 | IA | 8 | None | [111,141,182,200,348,397] |
| H | 1 | IA | 6 | None | [241,254] |
| In | 13 | IIIA | 6 | n(InF3):n(KNH2) = 1:3.12 | [144] |
| In | 13 | IIIA | 7 | n(InCl3):n(KNH2) = 1:3 | [141,144,200] |
| In | 13 | IIIA | 7 | n(InI3):n(CsNH2) = 1:3 | [200] |
| K | 1 | IA | 5 | n(InCl3):n(KNH2) = 1:3 | [141,200] |
| K | 1 | IA | 5 | None | [193,397] |
| La | 3 | IIIB | 4 | None | [192,398,399,400,401] |
| La | 3 | IIIB | 2 | None | [147] |
| Li | 1 | IA | 4 | None | [206,402] |
| Mg | 2 | IIA | 4 | None | [180,207,208] |
| Mn | 7 | VIIB | 4 | None | [182,205,211,384,394] |
| Mo | 6 | VIB | −1 | None | [147] |
| Mo | 6 | VIB | −2 | None | [147] |
| N | 15 | VA | 8 | Neutral with InX3 and KNH2 | [200,397] |
| N | 15 | VA | 8 | None | [111,182,348] |
| N | 15 | VA | 6 | None | [403,404] |
| Na | 1 | IA | 4 | None | [195,196] |
| Nb | 5 | VB | −1 | None | [147] |
| Nd | 3 | IIIB | 4 | None | [192,391,400] |
| Ni | 10 | VIIIB | −2 | None | [147] |
| Pd | 10 | VIIIB | −1 | None | [147] |
| Pt | 10 | VIIIB | −2 | None | [147] |
| Rb | 1 | IA | 4 | None | [195,198,208] |
| Sc | 3 | IIIB | −1 | None | [147] |
| Si | 14 | IVA | −1 | None | [147] |
| Si | 14 | IVA | −2 | None | [147] |
| Sm | 3 | IIIB | 4 | None | [192,209,391] |
| Sr | 2 | IIA | 4 | None | [195,196,199] |
| Ta | 5 | VB | 3 | None | [147] |
| Th | 3 | IIIB | 4 | None | [181,405] |
| V | 5 | VB | 3 | None | [147] |
| W | 6 | VIB | −1 | None | [147] |
| W | 6 | VIB | −2 | None | [147] |
| Y | 3 | IIIB | 4 | None | [168,187,203,400,406] |
| Yb | 3 | IIIB | 4 | None | [168,187,201,203,210,400] |
| Zn | 12 | IIB | 4 | None | [211] |
| Zr | 4 | IVB | −1 | None | [147] |
| Zr | 4 | IVB | 4 | None | [212,407] |
References
- Roccaforte, F.; Fiorenza, P.; Greco, G.; Lo Nigro, R.; Giannazzo, F.; Iucolano, F.; Saggio, M. Emerging trends in wide band gap semiconductors (SiC and GaN) technology for power devices. Microelectron. Eng. 2018, 187–188, 66–77. [Google Scholar] [CrossRef]
- Doolittle, W.A.; Matthews, C.M.; Ahmad, H.; Motoki, K.; Lee, S.; Ghosh, A.; Marshall, E.N.; Tang, A.L.; Manocha, P.; Yoder, P.D. Prospectives for AlN electronics and optoelectronics and the important role of alternative synthesis. Appl. Phys. Lett. 2023, 123, 070501. [Google Scholar] [CrossRef]
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; van de Walle, C.G.; Bellotti, E.; et al. Ultrawide-Bandgap Semiconductors: Research Opportunities and Challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef]
- Akiyama, M.; Kamohara, T.; Kano, K.; Teshigahara, A.; Takeuchi, Y.; Kawahara, N. Enhancement of Piezoelectric Response in Scandium Aluminum Nitride Alloy Thin Films Prepared by Dual Reactive Cosputtering. Adv. Mater. 2009, 21, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, S.; Wolff, N.; Lofink, F.; Kienle, L.; Wagner, B. AlScN: A III-V semiconductor based ferroelectric. J. Appl. Phys. 2019, 125, 114103. [Google Scholar] [CrossRef]
- Chu, D.; Huang, Y.; Xie, C.; Tikhonov, E.; Kruglov, I.; Li, G.; Pan, S.; Yang, Z. Unbiased Screening of Novel Infrared Nonlinear Optical Materials with High Thermal Conductivity: Long-neglected Nitrides and Popular Chalcogenides. Angew. Chem. Int. Ed. 2023, 62, e202300581. [Google Scholar] [CrossRef]
- Lee, C.-W.; Ud Din, N.; Yazawa, K.; Brennecka, G.L.; Zakutayev, A.; Gorai, P. Emerging Materials and Design Principles for Wurtzite-Type Ferroelectrics. Matter 2024, 7, 1644–1659. [Google Scholar] [CrossRef]
- Jena, D.; Page, R.; Casamento, J.; Dang, P.; Singhal, J.; Zhang, Z.; Wright, J.; Khalsa, G.; Cho, Y.; Xing, H.G. The new nitrides: Layered, ferroelectric, magnetic, metallic and superconducting nitrides to boost the GaN photonics and electronics eco-system. Jpn. J. Appl. Phys. 2019, 58, SC0801. [Google Scholar] [CrossRef]
- Mallmann, M.; Niklaus, R.; Rackl, T.; Benz, M.; Chau, T.G.; Johrendt, D.; Minár, J.; Schnick, W. Solid Solutions of Grimm-Sommerfeld Analogous Nitride Semiconductors II-IV-N2 (II=Mg, Mn, Zn; IV=Si, Ge): Ammonothermal Synthesis and DFT Calculations. Chemistry 2019, 25, 15887–15895. [Google Scholar] [CrossRef]
- Lyu, S.; Skachkov, D.; Kash, K.; Blanton, E.W.; Lambrecht, W.R.L. Band Gaps, Band-Offsets, Disorder, Stability Region, and Point Defects in II-IV-N 2 Semiconductors. Phys. Status Solidi (a) 2019, 216, 1800875. [Google Scholar] [CrossRef]
- Wang, P.; Wang, D.; Mondal, S.; Hu, M.; Liu, J.; Mi, Z. Dawn of nitride ferroelectric semiconductors: From materials to devices. Semicond. Sci. Technol. 2023, 38, 43002. [Google Scholar] [CrossRef]
- Wang, D.; Yang, S.; Liu, J.; Wang, D.; Mi, Z. Perspectives on nitride ferroelectric semiconductors: Challenges and opportunities. Appl. Phys. Lett. 2024, 124, 150501. [Google Scholar] [CrossRef]
- Startt, J.; Quazi, M.; Sharma, P.; Vazquez, I.; Poudyal, A.; Jackson, N.; Dingreville, R. Unlocking AlN Piezoelectric Performance with Earth-Abundant Dopants. Adv. Electron. Mater. 2023, 9, 2201187. [Google Scholar] [CrossRef]
- Zha, X.-H.; Ma, X.; Luo, J.-T.; Fu, C. Enhanced piezoelectric response of AlN via alloying of transitional metals, and influence of type and distribution of transition metals. Nano Energy 2023, 111, 108390. [Google Scholar] [CrossRef]
- Wang, F.; Ye, Q.; He, X.; Luo, K.; Ran, X.; Zheng, X.; Liao, C.; Li, R. Piezoelectric response enhancement of w-AlN by Hf (or Zr) and Sc co-alloying: A first principles study. Phys. B Condens. Matter 2024, 673, 415470. [Google Scholar] [CrossRef]
- Urban, D.F.; Ambacher, O.; Elsässer, C. First-principles calculation of electroacoustic properties of wurtzite (Al,Sc)N. Phys. Rev. B 2021, 103, 115204. [Google Scholar] [CrossRef]
- Greenaway, A.L.; Loutris, A.L.; Heinselman, K.N.; Melamed, C.L.; Schnepf, R.R.; Tellekamp, M.B.; Woods-Robinson, R.; Sherbondy, R.; Bardgett, D.; Bauers, S.; et al. Combinatorial Synthesis of Magnesium Tin Nitride Semiconductors. J. Am. Chem. Soc. 2020, 142, 8421–8430. [Google Scholar] [CrossRef]
- Ambacher, O.; Mihalic, S.; Yassine, M.; Yassine, A.; Afshar, N.; Christian, B. Review: Structural, elastic, and thermodynamic properties of cubic and hexagonal ScxAl1−xN crystals. J. Appl. Phys. 2023, 134, 160702. [Google Scholar] [CrossRef]
- Bader, S.J.; Lee, H.; Chaudhuri, R.; Huang, S.; Hickman, A.; Molnar, A.; Xing, H.G.; Jena, D.; Then, H.W.; Chowdhury, N.; et al. Prospects for Wide Bandgap and Ultrawide Bandgap CMOS Devices. IEEE Trans. Electron Devices 2020, 67, 4010–4020. [Google Scholar] [CrossRef]
- Leveillee, J.; Poncé, S.; Adamski, N.L.; van de Walle, C.G.; Giustino, F. Anisotropic-strain-enhanced hole mobility in GaN by lattice matching to ZnGeN2 and MgSiN2. Appl. Phys. Lett. 2022, 120, 202106. [Google Scholar] [CrossRef]
- Ogura, M.; Han, D.; Pointner, M.M.; Junkers, L.S.; Rudel, S.S.; Schnick, W.; Ebert, H. Electronic properties of semiconducting Zn(Si,Ge,Sn)N2 alloys. Phys. Rev. Mater. 2021, 5, 024601. [Google Scholar] [CrossRef]
- Santic, B. On the hole effective mass and the free hole statistics in wurtzite GaN. Semicond. Sci. Technol. 2003, 18, 219–224. [Google Scholar] [CrossRef]
- Adamski, N.L.; Wickramaratne, D.; van de Walle, C.G. Band alignments and polarization properties of the Zn-IV-nitrides. J. Mater. Chem. C 2020, 8, 7890–7898. [Google Scholar] [CrossRef]
- Kudrawiec, R.; Hommel, D. Bandgap engineering in III-nitrides with boron and group V elements: Toward applications in ultraviolet emitters. Appl. Phys. Rev. 2020, 7, 041314. [Google Scholar] [CrossRef]
- Ahmed, R.; Fazal-e-Aleem; Hashemifar, S.J.; Akbarzadeh, H. First principles study of structural and electronic properties of different phases of boron nitride. Phys. B Condens. Matter 2007, 400, 297–306. [Google Scholar] [CrossRef]
- Ziembicki, J.; Scharoch, P.; Polak, M.P.; Wiśniewski, M.; Kudrawiec, R. Band parameters of group III–V semiconductors in wurtzite structure. J. Appl. Phys. 2022, 132, 225701. [Google Scholar] [CrossRef]
- Vurgaftman, I.; Meyer, J.R. Band parameters for nitrogen-containing semiconductors. J. Appl. Phys. 2003, 94, 3675–3696. [Google Scholar] [CrossRef]
- Ambacher, O. Growth and applications of Group III-nitrides. J. Phys. D Appl. Phys. 1998, 31, 2653–2710. [Google Scholar] [CrossRef]
- Dreyer, C.E.; Janotti, A.; van de Walle, C.G.; Vanderbilt, D. Correct Implementation of Polarization Constants in Wurtzite Materials and Impact on III-Nitrides. Phys. Rev. X 2016, 6, 021038. [Google Scholar] [CrossRef]
- Rom, C.L.; Smaha, R.W.; Melamed, C.L.; Schnepf, R.R.; Heinselman, K.N.; Mangum, J.S.; Lee, S.-J.; Lany, S.; Schelhas, L.T.; Greenaway, A.L.; et al. Combinatorial Synthesis of Cation-Disordered Manganese Tin Nitride MnSnN2 Thin Films with Magnetic and Semiconducting Properties. Chem. Mater. 2023, 35, 2936–2946. [Google Scholar] [CrossRef]
- Råsander, M.; Quirk, J.B.; Wang, T.; Mathew, S.; Davies, R.; Palgrave, R.G.; Moram, M.A. Structure and lattice dynamics of the wide band gap semiconductors MgSiN2 and MgGeN2. J. Appl. Phys. 2017, 122, 085705. [Google Scholar] [CrossRef]
- Quirk, J.B.; Råsander, M.; McGilvery, C.M.; Palgrave, R.; Moram, M.A. Band gap and electronic structure of MgSiN2. Appl. Phys. Lett. 2014, 105, 112108. [Google Scholar] [CrossRef]
- Råsander, M.; Moram, M.A. Elastic constants of the II–IV nitride semiconductors MgSiN2, MgGeN2 and MgSnN2. J. Phys. D Appl. Phys. 2018, 51, 375101. [Google Scholar] [CrossRef]
- Jaroenjittichai, A.P.; Lambrecht, W.R.L. Electronic band structure of Mg−IV−N2 compounds in the quasiparticle-self-consistent GW approximation. Phys. Rev. B 2016, 94, 125201. [Google Scholar] [CrossRef]
- Huang, J.Y.; Tang, L.-C.; Lee, M.H. Ab initio study of the structural and optical properties of orthorhombic ternary nitride crystals. J. Phys. Condens. Matter 2001, 13, 10417–10431. [Google Scholar] [CrossRef]
- Häusler, J.; Niklaus, R.; Minár, J.; Schnick, W. Ammonothermal Synthesis and Optical Properties of Ternary Nitride Semiconductors Mg-IV-N2, Mn-IV-N2 and Li-IV2-N3 (IV=Si, Ge). Chem.–A Eur. J. 2018, 24, 1686–1693. [Google Scholar] [CrossRef]
- Kaewmeechai, C.; Laosiritaworn, Y.; Jaroenjittichai, A.P. HSE hybrid functional calculation of band gap deformation potential in MgGeN2. J. Phys. Conf. Ser. 2018, 1144, 12045. [Google Scholar] [CrossRef]
- Dumre, B.B.; Gall, D.; Khare, S.V. Stability, and electronic and optical properties of ternary nitride phases of MgSnN2: A first-principles study. J. Phys. Chem. Solids 2021, 153, 110011. [Google Scholar] [CrossRef]
- Chinnakutti, K.K.; Kirubaharan, A.M.K.; Patra, L.; Pandey, R.; Theerthagiri, J.; Vengatesh, P.; Salammal, S.T.; Paramasivam, N.; Sambandam, A.; Kasemchainan, J.; et al. Modulating the Combinatorial Target Power of MgSnN2 via RF Magnetron Sputtering for Enhanced Optoelectronic Performance: Mechanistic Insights from DFT Studies. ACS Appl. Mater. Interfaces 2023, 15, 14546–14556. [Google Scholar] [CrossRef]
- Makin, R.A.; York, K.; Durbin, S.M.; Senabulya, N.; Mathis, J.; Clarke, R.; Feldberg, N.; Miska, P.; Jones, C.M.; Deng, Z.; et al. Alloy-Free Band Gap Tuning across the Visible Spectrum. Phys. Rev. Lett. 2019, 122, 256403. [Google Scholar] [CrossRef]
- Punya, A.; Lambrecht, W.R.L.; van Schilfgaarde, M. Quasiparticle band structure of Zn-IV-N2 compounds. Phys. Rev. B 2011, 84, 165204. [Google Scholar] [CrossRef]
- Häusler, J.; Schimmel, S.; Wellmann, P.; Schnick, W. Ammonothermal Synthesis of Earth-Abundant Nitride Semiconductors ZnSiN2 and ZnGeN2 and Dissolution Monitoring by In Situ X-ray Imaging. Chemistry 2017, 23, 12275–12282. [Google Scholar] [CrossRef] [PubMed]
- Zeman, O.E.O.; von Rohr, F.O.; Neudert, L.; Schnick, W. Facile One-step Synthesis of Zn1–xMnxSiN2 Nitride Semiconductor Solid Solutions via Solid-state Metathesis Reaction. Z. Anorg. Allg. Chem. 2020, 646, 228–233. [Google Scholar] [CrossRef]
- Bai, Y.; Luo, G.; Meng, L.; Zhang, Q.; Xu, N.; Zhang, H.; Wu, X.; Kong, F.; Wang, B. Single-layer ZnMN2 (M = Si, Ge, Sn) zinc nitrides as promising photocatalysts. Phys. Chem. Chem. Phys. 2018, 20, 14619–14626. [Google Scholar] [CrossRef] [PubMed]
- Paudel, T.R.; Lambrecht, W.R.L. First-principles study of phonons and related ground-state properties and spectra in Zn-IV-N2 compounds. Phys. Rev. B 2008, 78, 115204. [Google Scholar] [CrossRef]
- Senabulya, N.; Feldberg, N.; Makin, R.A.; Yang, Y.; Shi, G.; Jones, C.M.; Kioupakis, E.; Mathis, J.; Clarke, R.; Durbin, S.M. Stabilization of orthorhombic phase in single-crystal ZnSnN2 films. AIP Adv. 2016, 6, 075019. [Google Scholar] [CrossRef]
- Kawamura, F.; Yamada, N.; Imai, M.; Taniguchi, T. Synthesis of ZnSnN2 crystals via a high-pressure metathesis reaction. Cryst. Res. Technol. 2016, 51, 220–224. [Google Scholar] [CrossRef]
- Kautzsch, L.; Georgescu, A.B.; Puggioni, D.; Kent, G.; Taddei, K.M.; Reilly, A.; Seshadri, R.; Rondinelli, J.M.; Wilson, S.D. Canted antiferromagnetism in polar MnSiN2 with high Néel temperature. Phys. Rev. Mater. 2023, 7, 104406. [Google Scholar] [CrossRef]
- Esmaeilzadeh, S.; Hålenius, U.; Valldor, M. Crystal Growth, Magnetic, and Optical Properties of the Ternary Nitride MnSiN2. Chem. Mater. 2006, 18, 2713–2718. [Google Scholar] [CrossRef]
- Koltsov, V.B.; Mikhailova, M.S. Effect of the 3d Electron Self-Interaction Correction on the Electronic and Magnetic Properties of CoGeN2, CrGeN2, MnSiN2, and MnGeN2. Russ. J. Inorg. Chem. 2021, 66, 1868–1872. [Google Scholar] [CrossRef]
- Lazarov, V.K.; Li, L.; Weinert, M.; Gajdardziska-Josifovska, M. Structure Determination of a Magnetic Semiconductor: MnGeN2. Microsc. Microanal. 2004, 10, 516–517. [Google Scholar] [CrossRef]
- Riane, R.; Boussahla, Z.; Matar, S.F.; Zaoui, A. Structural and Electronic Properties of Zinc Blende-type Nitrides BxAl1–xN. Z. Naturforschung B 2008, 63, 1069–1076. [Google Scholar] [CrossRef]
- Dreyer, C.E.; Lyons, J.L.; Janotti, A.; van de Walle, C.G. Corrigendum: “Band alignments and polarization properties of BN polymorphs” [Appl. Phys. Express 7 031001 (2014)]. Appl. Phys. Express 2020, 13, 19301. [Google Scholar] [CrossRef]
- Al-Atabi, H.A.; Zhang, X.; He, S.; Chen, C.; Chen, Y.; Rotenberg, E.; Edgar, J.H. Lattice and electronic structure of ScN observed by angle-resolved photoemission spectroscopy measurements. Appl. Phys. Lett. 2022, 121, 182102. [Google Scholar] [CrossRef]
- Zhang, X.W. Doping and electrical properties of cubic boron nitride thin films: A critical review. Thin Solid Films 2013, 544, 2–12. [Google Scholar] [CrossRef]
- Wellmann, P.J. Power Electronic Semiconductor Materials for Automotive and Energy Saving Applications—SiC, GaN, Ga2O3, and Diamond. Z. Anorg. Allg. Chem. 2017, 643, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, A.; Hammock, I.N.; Estes, J.; Jin, Y.; Bernien, H.; High, A.A.; Galli, G. Donor-acceptor pairs in wide-bandgap semiconductors for quantum technology applications. NPJ Comput. Mater. 2024, 10, 7. [Google Scholar] [CrossRef]
- Mallmann, M.; Maak, C.; Niklaus, R.; Schnick, W. Ammonothermal Synthesis, Optical Properties, and DFT Calculations of Mg2PN3 and Zn2PN3. Chemistry 2018, 24, 13963–13970. [Google Scholar] [CrossRef]
- Wintenberger, M.; Guyader, J.; Maunaye, M. Etude cristallographique et magnetique de MnGeN2 par diffraction neutronique. Solid State Commun. 1972, 11, 1485–1488. [Google Scholar] [CrossRef]
- Fu, H.; Goodrich, J.C.; Ogidi-Ekoko, O.; Tansu, N. Power electronics figure-of-merit of ScAlN. Appl. Phys. Lett. 2021, 119, 072101. [Google Scholar] [CrossRef]
- Kneissl, M.; Seong, T.-Y.; Han, J.; Amano, H. The emergence and prospects of deep-ultraviolet light-emitting diode technologies. Nat. Photonics 2019, 13, 233–244. [Google Scholar] [CrossRef]
- Lang, D.V.; Logan, R.A.; Jaros, M. Trapping characteristics and a donor-complex (DX ) model for the persistent-photoconductivity trapping center in Te-doped AlxGa1−xAs. Phys. Rev. B 1979, 19, 1015–1030. [Google Scholar] [CrossRef]
- Chadi, D.J.; Chang, K.J. Theory of the atomic and electronic structure of DX centers in GaAs and AlxGa1−xAs alloys. Phys. Rev. Lett. 1988, 61, 873–876. [Google Scholar] [CrossRef]
- Lyons, J.L.; Wickramaratne, D.; Janotti, A. Dopants and defects in ultra-wide bandgap semiconductors. Curr. Opin. Solid State Mater. Sci. 2024, 30, 101148. [Google Scholar] [CrossRef]
- Gordon, L.; Lyons, J.L.; Janotti, A.; van de Walle, C.G. Hybrid functional calculations of D X centers in AlN and GaN. Phys. Rev. B 2014, 89, 85204. [Google Scholar] [CrossRef]
- Ahmad, H.; Lindemuth, J.; Engel, Z.; Matthews, C.M.; McCrone, T.M.; Doolittle, W.A. Substantial P-Type Conductivity of AlN Achieved via Beryllium Doping. Adv. Mater. 2021, 33, e2104497. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Engel, Z.; Matthews, C.M.; Doolittle, W.A. p-type AlN based heteroepitaxial diodes with Schottky, Pin, and junction barrier Schottky character achieving significant breakdown performance. J. Appl. Phys. 2021, 130, 195702. [Google Scholar] [CrossRef]
- Ahmad, H.; Engel, Z.; Matthews, C.M.; Lee, S.; Doolittle, W.A. Realization of homojunction PN AlN diodes. J. Appl. Phys. 2022, 131, 175701. [Google Scholar] [CrossRef]
- Almogbel, A.S.; Zollner, C.J.; Saifaddin, B.K.; Iza, M.; Wang, J.; Yao, Y.; Wang, M.; Foronda, H.; Prozheev, I.; Tuomisto, F.; et al. Growth of highly conductive Al-rich AlGaN:Si with low group-III vacancy concentration. AIP Adv. 2021, 11, 095119. [Google Scholar] [CrossRef]
- Tuomisto, F.; Prozheeva, V.; Makkonen, I.; Myers, T.H.; Bockowski, M.; Teisseyre, H. Amphoteric Be in GaN: Experimental Evidence for Switching between Substitutional and Interstitial Lattice Sites. Phys. Rev. Lett. 2017, 119, 196404. [Google Scholar] [CrossRef]
- Freitas, J.A.; Culbertson, J.C.; Glaser, E.R. Characterization of Defects in GaN: Optical and Magnetic Resonance Techniques. Crystals 2022, 12, 1294. [Google Scholar] [CrossRef]
- Nanishi, Y.; Yamaguchi, T. Plasma-excited MBE—Proposal and achievements through R&D of compound semiconductor materials and devices. Jpn. J. Appl. Phys. 2022, 61, SA0810. [Google Scholar] [CrossRef]
- Hatate, A.; Horiuchi, T.; Ishiyama, T.; Toko, K.; Amemiya, K.; Suemasu, T. X-ray magnetic circular dichroism of Mn4−xGaxN epitaxial thin films confirming ferrimagnetic-ferromagnetic phase transition by nonmagnetic Ga doping. J. Magn. Magn. Mater. 2024, 597, 171973. [Google Scholar] [CrossRef]
- Hardy, M.T.; Downey, B.P.; Nepal, N.; Storm, D.F.; Katzer, D.S.; Meyer, D.J. Epitaxial ScAlN grown by molecular beam epitaxy on GaN and SiC substrates. Appl. Phys. Lett. 2017, 110, 162104. [Google Scholar] [CrossRef]
- Leone, S.; Streicher, I.; Prescher, M.; Straňák, P.; Kirste, L. Metal-Organic Chemical Vapor Deposition of Aluminum Yttrium Nitride. Phys. Rapid Res. Lett. 2023, 17, 2300091. [Google Scholar] [CrossRef]
- Leone, S.; Ligl, J.; Manz, C.; Kirste, L.; Fuchs, T.; Menner, H.; Prescher, M.; Wiegert, J.; Žukauskaitė, A.; Quay, R.; et al. Metal-Organic Chemical Vapor Deposition of Aluminum Scandium Nitride. Phys. Rapid Res. Lett. 2020, 14, 1900535. [Google Scholar] [CrossRef]
- Izyumskaya, N.; Avrutin, V.; Ding, K.; Özgür, Ü.; Morkoç, H.; Fujioka, H. Emergence of high quality sputtered III-nitride semiconductors and devices. Semicond. Sci. Technol. 2019, 34, 93003. [Google Scholar] [CrossRef]
- Matthews, C.M.; Engel, Z.; Motoki, K.; Doolittle, W.A. Kinetic Model for Ternary III-Nitride Epitaxy: The Role of Vertical Segregation on Phase Separation. Cryst. Growth Des. 2023, 23, 8856–8864. [Google Scholar] [CrossRef]
- Doppalapudi, D.; Basu, S.N.; Ludwig, K.F.; Moustakas, T.D. Phase separation and ordering in InGaN alloys grown by molecular beam epitaxy. J. Appl. Phys. 1998, 84, 1389–1395. [Google Scholar] [CrossRef]
- Mori, Y.; Imanishi, M.; Murakami, K.; Yoshimura, M. Recent progress of Na-flux method for GaN crystal growth. Jpn. J. Appl. Phys. 2019, 58, SC0803. [Google Scholar] [CrossRef]
- Kucharski, R.; Sochacki, T.; Lucznik, B.; Bockowski, M. Growth of bulk GaN crystals. J. Appl. Phys. 2020, 128, 050902. [Google Scholar] [CrossRef]
- Mikawa, Y.; Ishinabe, T.; Kagamitani, Y.; Mochizuki, T.; Ikeda, H.; Iso, K.; Takahashi, T.; Kubota, K.; Enatsu, Y.; Tsukada, Y.; et al. Recent progress of large size and low dislocation bulk GaN growth. In Gallium Nitride Materials and Devices XV, Proceedings of the Volume 11280, Gallium Nitride Materials and Devices XV, San Francisco, CA, USA, 1–6 February 2020; Morkoç, H., Fujioka, H., Schwarz, U.T., Eds.; SPIE: Bellingham, WA, USA, 2020; p. 1. ISBN 9781510633230. [Google Scholar] [CrossRef]
- Hartmann, C.; Kabukcuoglu, M.P.; Richter, C.; Klump, A.; Schulz, D.; Juda, U.; Bickermann, M.; Hänschke, D.; Schröder, T.; Straubinger, T. Efficient diameter enlargement of bulk AlN single crystals with high structural quality. Appl. Phys. Express 2023, 16, 75502. [Google Scholar] [CrossRef]
- Kurimoto, K.; Bao, Q.; Mikawa, Y.; Shima, K.; Ishiguro, T.; Chichibu, S.F. Low-pressure acidic ammonothermal growth of 2-inch-diameter nearly bowing-free bulk GaN crystals. Appl. Phys. Express 2022, 15, 55504. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Sitar, Z. Advances in Bulk Crystal Growth of AlN and GaN. MRS Bull. 2009, 34, 259–265. [Google Scholar] [CrossRef]
- Stoddard, N.; Pimputkar, S. Progress in Ammonothermal Crystal Growth of Gallium Nitride from 2017–2023: Process, Defects and Devices. Crystals 2023, 13, 1004. [Google Scholar] [CrossRef]
- Suihkonen, S.; Pimputkar, S.; Sintonen, S.; Tuomisto, F. Defects in single crystalline ammonothermal gallium nitride. Adv. Electron. Mater. 2017, 3, 1600496. [Google Scholar] [CrossRef]
- Hashimoto, T.; Letts, E.R.; Key, D. Progress in Near-Equilibrium Ammonothermal (NEAT) Growth of GaN Substrates for GaN-on-GaN Semiconductor Devices. Crystals 2022, 12, 1085. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Kagamitani, Y.; Fukuda, T.; Orito, F.; Kawabata, S.; Katano, K.; Terada, S. Reviewing recent developments in the acid ammonothermal crystal growth of gallium nitride. J. Cryst. Growth 2008, 310, 3902–3906. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Pakalapati, R.T.; Kamber, D.S.; Jiang, W.; Pocius, D.W.; Downey, B.C.; McLaurin, M.; D’Evelyn, M.P. High Quality, Low Cost Ammonothermal Bulk GaN Substrates. Jpn. J. Appl. Phys. 2013, 52, 08JA01. [Google Scholar] [CrossRef]
- Tomida, D.; Saito, M.; Bao, Q.; Ishiguro, T.; Chichibu, S.F. Innovative Techniques for Fast Growth and Fabrication of High Purity GaN Single Crystals. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 65–76. ISBN 978-3-030-56304-2. [Google Scholar]
- Häusler, J.; Schnick, W. Ammonothermal Synthesis of Nitrides: Recent Developments and Future Perspectives. Chemistry 2018, 24, 11864–11879. [Google Scholar] [CrossRef] [PubMed]
- Mallmann, M.; Cordes, N.; Schnick, W. Explorative Synthesis of Novel Nitride Compounds by Ammonothermal Synthesis. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 205–225. ISBN 978-3-030-56304-2. [Google Scholar]
- Watanabe, T.; Nonaka, K.; Li, J.; Kishida, K.; Yoshimura, M. Low temperature ammonothermal synthesis of europium-doped SrAlSiN3 for a nitride red phosphor. J. Ceram. Soc. Jpn. 2012, 120, 500–502. [Google Scholar] [CrossRef]
- Dwilinski, R.; Wysmolek, A.; Baranowski, J.; Kaminska, M.; Doradzinski, R.; Garczyński, J.; Sierzputowski, L.; Jacobs, H. GaN synthesis by ammonothermal method. Acta Phys. Pol. A 1995, 88, 833–836. [Google Scholar] [CrossRef]
- Dwiliński, R.; Baranowski, J.M.; Kamińska, M.; Doradziński, R.; Garczyński, J.; Sierzputowski, L. On GaN Crystallization by Ammonothermal Method. Acta Phys. Pol. A 1996, 90, 763–766. [Google Scholar] [CrossRef]
- Grabianska, K.; Kucharski, R.; Puchalski, A.; Sochacki, T.; Bockowski, M. Recent progress in basic ammonothermal GaN crystal growth. J. Cryst. Growth 2020, 547, 125804. [Google Scholar] [CrossRef]
- Boćkowski, M.; Grzegory, I. Recent Progress in Crystal Growth of Bulk GaN. Acta Phys. Pol. A 2022, 141, 167–174. [Google Scholar] [CrossRef]
- Grabianska, K.; Jaroszynski, P.; Sidor, A.; Bockowski, M.; Iwinska, M. GaN single crystalline substrates by ammonothermal and HVPE methods for electronic devices. Electronics 2020, 9, 1342. [Google Scholar] [CrossRef]
- Pimputkar, S. Gallium nitride 11. In Single Crystals of Electronic Materials: Growth and Properties; Elsevier: London, UK, 2018; p. 351. [Google Scholar] [CrossRef]
- Dwiliński, R.; Doradziński, R.; Garczyński, J.; Sierzputowski, L.P.; Puchalski, A.; Kanbara, Y.; Yagi, K.; Minakuchi, H.; Hayashi, H. Excellent crystallinity of truly bulk ammonothermal GaN. J. Cryst. Growth 2008, 310, 3911–3916. [Google Scholar] [CrossRef]
- Bao, Q.; Saito, M.; Hazu, K.; Kagamitani, Y.; Kurimoto, K.; Tomida, D.; Qiao, K.; Ishiguro, T.; Yokoyama, C.; Chichibu, S.F. Ammonothermal growth of GaN on a self-nucleated GaN seed crystal. J. Cryst. Growth 2014, 404, 168–171. [Google Scholar] [CrossRef]
- Bao, Q.; Saito, M.; Hazu, K.; Furusawa, K.; Kagamitani, Y.; Kayano, R.; Tomida, D.; Qiao, K.; Ishiguro, T.; Yokoyama, C.; et al. Ammonothermal Crystal Growth of GaN Using an NH4F Mineralizer. Cryst. Growth Des. 2013, 13, 4158–4161. [Google Scholar] [CrossRef]
- D’Evelyn, M.P.; Hong, H.C.; Park, D.-S.; Lu, H.; Kaminsky, E.; Melkote, R.R.; Perlin, P.; Lesczynski, M.; Porowski, S.; Molnar, R.J. Bulk GaN crystal growth by the high-pressure ammonothermal method. J. Cryst. Growth 2007, 300, 11–16. [Google Scholar] [CrossRef]
- Zajac, M.; Kucharski, R.; Grabianska, K.; Gwardys-Bak, A.; Puchalski, A.; Wasik, D.; Litwin-Staszewska, E.; Piotrzkowski, R.; Domagala, J.Z.; Bockowski, M. Basic ammonothermal growth of Gallium Nitride–State of the art, challenges, perspectives. Prog. Cryst. Growth Charact. Mater. 2018, 64, 63–74. [Google Scholar] [CrossRef]
- Bockowski, M.; Iwinska, M.; Amilusik, M.; Fijalkowski, M.; Lucznik, B.; Sochacki, T. Challenges and future perspectives in HVPE-GaN growth on ammonothermal GaN seeds. Semicond. Sci. Technol. 2016, 31, 93002. [Google Scholar] [CrossRef]
- Kirste, L.; Grabianska, K.; Kucharski, R.; Sochacki, T.; Lucznik, B.; Bockowski, M. Structural Analysis of Low Defect Ammonothermally Grown GaN Wafers by Borrmann Effect X-ray Topography. Materials 2021, 14, 5472. [Google Scholar] [CrossRef] [PubMed]
- Fujikura, H.; Yoshida, T.; Shibata, M.; Otoki, Y. Recent progress of high-quality GaN substrates by HVPE method. In Gallium Nitride Materials and Devices XII, Proceedings of the SPIE OPTO, San Francisco, CA, USA, 28 January 2017; Chyi, J.-I., Fujioka, H., Morkoç, H., Nanishi, Y., Schwarz, U.T., Shim, J.-I., Eds.; SPIE: Bellingham, WA, USA, 2017; p. 1010403. [Google Scholar]
- Wang, B.; Callahan, M.J.; Rakes, K.D.; Bouthillette, L.O.; Wang, S.-Q.; Bliss, D.F.; Kolis, J.W. Ammonothermal growth of GaN crystals in alkaline solutions. J. Cryst. Growth 2006, 287, 376–380. [Google Scholar] [CrossRef]
- Kucharski, R.; Zajac, M.; Puchalski, A.; Sochacki, T.; Bockowski, M.; Weyher, J.L.; Iwinska, M.; Serafinczuk, J.; Kudrawiec, R.; Siemiątkowski, Z. Ammonothermal growth of GaN crystals on HVPE-GaN seeds prepared with the use of ammonothermal substrates. J. Cryst. Growth 2015, 427, 1–6. [Google Scholar] [CrossRef]
- Dooley, J.; Stoddard, N.; Landskron, K.; Pimputkar, S. On the solubility of boron nitride in supercritical ammonia-sodium solutions. J. Cryst. Growth 2023, 621, 127381. [Google Scholar] [CrossRef]
- Zaja̧c, M.; Doradziński, R.; Gosk, J.; Szczytko, J.; Lefeld-Sosnowska, M.; Kamińska, M.; Twardowski, A.; Palczewska, M.; Grzanka, E.; Gȩbicki, W. Magnetic and optical properties of GaMnN magnetic semiconductor. Appl. Phys. Lett. 2001, 78, 1276–1278. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Hoshino, N.; Kagamitani, Y.; Yoshikawa, A.; Fukuda, T.; Itoh, H.; Kawabata, S. Temperature effect of ammonium halogenides as mineralizers on the phase stability of gallium nitride synthesized under acidic ammonothermal conditions. J. Mater. Chem. 2007, 17, 886–893. [Google Scholar] [CrossRef]
- Makiuchi, K.; Kawamura, F.; Jia, J.; Song, Y.; Yata, S.; Tampo, H.; Murata, H.; Yamada, N. Pressure-Induced Transition from Wurtzite and Epitaxial Stabilization for Thin Films of Rocksalt MgSnN2. Chem. Mater. 2023, 35, 2095–2106. [Google Scholar] [CrossRef]
- Yassine, M.; Nair, A.; Fammels, J.; Wade, E.; Fu, Z.; Yassine, A.; Kirste, L.; Ambacher, O. Influence of structural properties on the ferroelectric behavior of hexagonal AlScN. J. Appl. Phys. 2022, 132, 114101. [Google Scholar] [CrossRef]
- Gorczyca, I.; Suski, T.; Perlin, P.; Grzegory, I.; Staszczak, G.; Aktas, M. Special role of indium nitride in the properties of related compounds and quantum structures. AIP Adv. 2024, 14, 040704. [Google Scholar] [CrossRef]
- Karpiński, J.; Jun, J.; Porowski, S. Equilibrium pressure of N2 over GaN and high pressure solution growth of GaN. J. Cryst. Growth 1984, 66, 1–10. [Google Scholar] [CrossRef]
- Leszczynski, M.; Grzegory, I.; Teisseyre, H.; Suski, T.; Bockowski, M.; Jun, J.; Baranowski, J.M.; Porowski, S.; Domagala, J. The microstructure of gallium nitride monocrystals grown at high pressure. J. Cryst. Growth 1996, 169, 235–242. [Google Scholar] [CrossRef]
- Richter, T.; Niewa, R. Chemistry of Ammonothermal Synthesis. Inorganics 2014, 2, 29–78. [Google Scholar] [CrossRef]
- Pritula, I.; Sangwal, K. Fundamentals of Crystal Growth from Solutions. In Handbook of Crystal Growth, 2nd ed.; Rudolph, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1185–1227. ISBN 978-0-444-63303-3. [Google Scholar]
- Byrappa, K. Handbook of Hydrothermal Technology, 2nd ed.; William Andrew: Oxford, UK, 2013; ISBN 9781437778366. [Google Scholar]
- Hervey, P.R.; Foise, J.W. Synthetic quartz crystal—A review. Min. Metall. Explor. 2001, 18, 1–4. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Fukuda, T. The Ammonothermal Crystal Growth of Gallium Nitride—A Technique on the Up Rise. Proc. IEEE 2010, 98, 1316–1323. [Google Scholar] [CrossRef]
- Eckert, C.A.; Knutson, B.L.; Debenedetti, P.G. Supercritical fluids as solvents for chemical and materials processing. Nature 1996, 383, 313–318. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Eckert, C.A. Phase equilibria for supercritical fluid process design. AIChE J. 1989, 35, 1409–1427. [Google Scholar] [CrossRef]
- Knox, D.E. Solubilities in supercritical fluids. Pure Appl. Chem. 2005, 77, 513–530. [Google Scholar] [CrossRef]
- Schimmel, S.; Kobelt, I.; Heinlein, L.; Kimmel, A.-C.L.; Steigerwald, T.G.; Schlücker, E.; Wellmann, P. Flow Stability, Convective Heat Transfer and Chemical Reactions in Ammonothermal Autoclaves—Insights by In Situ Measurements of Fluid Temperatures. Crystals 2020, 10, 723. [Google Scholar] [CrossRef]
- Schimmel, S.; Tomida, D.; Ishiguro, T.; Honda, Y.; Chichibu, S.; Amano, H. Numerical Simulation of Ammonothermal Crystal Growth of GaN—Current State, Challenges, and Prospects. Crystals 2021, 11, 356. [Google Scholar] [CrossRef]
- Rendel, P.M.; Mountain, B.W. Solubility of quartz in supercritical water from 375 °C to 600 °C and 200–270 bar. J. Supercrit. Fluids 2023, 196, 105883. [Google Scholar] [CrossRef]
- Reinsch, T.; Dobson, P.; Asanuma, H.; Huenges, E.; Poletto, F.; Sanjuan, B. Utilizing supercritical geothermal systems: A review of past ventures and ongoing research activities. Geotherm. Energy 2017, 5, 16. [Google Scholar] [CrossRef]
- Lemmon, E.W.; McLinden, M.O.; Friend, D.G. Thermophysical Properties of Fluid Systems: NIST Chemistry WebBook. NIST Standard Reference Database Number 69. Available online: http://webbook.nist.gov/chemistry (accessed on 5 June 2017).
- Niewa, R. Significance of Ammonothermal Synthesis for Nitride Materials. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–12. ISBN 978-3-030-56304-2. [Google Scholar]
- Zhang, S.; Hintze, F.; Schnick, W.; Niewa, R. Intermediates in Ammonothermal GaN Crystal Growth under Ammonoacidic Conditions. Eur. J. Inorg. Chem. 2013, 2013, 5387–5399. [Google Scholar] [CrossRef]
- Zhang, S.; Alt, N.S.; Schlücker, E.; Niewa, R. Novel alkali metal amidogallates as intermediates in ammonothermal GaN crystal growth. J. Cryst. Growth 2014, 403, 22–28. [Google Scholar] [CrossRef]
- Glasson, D.R.; Jayaweera, S.A.A. Formation and reactivity of nitrides I. Review and introduction. J. Appl. Chem. 1968, 18, 65–77. [Google Scholar] [CrossRef]
- Pimputkar, S.; Nakamura, S. Decomposition of supercritical ammonia and modeling of supercritical ammonia–nitrogen–hydrogen solutions with applicability toward ammonothermal conditions. J. Supercrit. Fluids 2016, 107, 17–30. [Google Scholar] [CrossRef]
- Bao, Q.; Hashimoto, T.; Sato, F.; Hazu, K.; Saito, M.; Kagamitani, Y.; Ishinabe, T.; Kayano, R.; Tomida, D.; Qiao, K.; et al. Acidic ammonothermal growth of GaN crystals using GaN powder as a nutrient. CrystEngComm 2013, 15, 5382. [Google Scholar] [CrossRef]
- Zak, M.; Kempisty, P.; Lucznik, B.; Grabianska, K.; Kucharski, R.; Iwinska, M.; Bockowski, M. Modeling of convective transport in crystallization of gallium nitride by basic ammonothermal method. J. Cryst. Growth 2024, 627, 127525. [Google Scholar] [CrossRef]
- Grabianska, K.; Kucharski, R.; Sochacki, T.; Weyher, J.L.; Iwinska, M.; Grzegory, I.; Bockowski, M. On Stress-Induced Polarization Effect in Ammonothermally Grown GaN Crystals. Crystals 2022, 12, 554. [Google Scholar] [CrossRef]
- Pimputkar, S.; Kawabata, S.; Speck, J.S.; Nakamura, S. Improved growth rates and purity of basic ammonothermal GaN. J. Cryst. Growth 2014, 403, 7–17. [Google Scholar] [CrossRef]
- Hertrampf, J.; Becker, P.; Widenmeyer, M.; Weidenkaff, A.; Schlücker, E.; Niewa, R. Ammonothermal Crystal Growth of Indium Nitride. Cryst. Growth Des. 2018, 18, 2365–2369. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Bockowski, M. High-pressure, high-temperature solution growth and ammonothermal synthesis of gallium nitride crystals. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 577–619. [Google Scholar]
- Häusler, J.; Neudert, L.; Mallmann, M.; Niklaus, R.; Kimmel, A.-C.L.; Alt, N.S.A.; Schlücker, E.; Oeckler, O.; Schnick, W. Ammonothermal Synthesis of Novel Nitrides: Case Study on CaGaSiN3. Chemistry 2017, 23, 2583–2590. [Google Scholar] [CrossRef]
- Becker, P.; Cekovski, T.B.; Niewa, R. Two Intermediates in Ammonothermal InN Crystal Growth: [In(NH3)5Cl]Cl2 and InF2(NH2). Z. Anorg. Allg. Chem. 2021, 647, 2006–2014. [Google Scholar] [CrossRef]
- Sintonen, S.; Kivisaari, P.; Pimputkar, S.; Suihkonen, S.; Schulz, T.; Speck, J.S.; Nakamura, S. Incorporation and effects of impurities in different growth zones within basic ammonothermal GaN. J. Cryst. Growth 2016, 456, 43–50. [Google Scholar] [CrossRef]
- Sintonen, S.; Wahl, S.; Richter, S.; Meyer, S.; Suihkonen, S.; Schulz, T.; Irmscher, K.; Danilewsky, A.N.; Tuomi, T.O.; Stankiewicz, R.; et al. Evolution of impurity incorporation during ammonothermal growth of GaN. J. Cryst. Growth 2016, 456, 51–57. [Google Scholar] [CrossRef]
- Pimputkar, S.; Malkowski, T.F.; Griffiths, S.; Espenlaub, A.; Suihkonen, S.; Speck, J.S.; Nakamura, S. Stability of materials in supercritical ammonia solutions. J. Supercrit. Fluids 2016, 110, 193–229. [Google Scholar] [CrossRef]
- Schimmel, S.; Künecke, U.; Meisel, M.; Hertweck, B.; Steigerwald, T.G.; Nebel, C.; Alt, N.S.; Schlücker, E.; Wellmann, P. Chemical stability of carbon-based inorganic materials for in situ X-ray investigations of ammonothermal crystal growth of nitrides. J. Cryst. Growth 2016, 456, 33–42. [Google Scholar] [CrossRef]
- Schimmel, S.; Koch, M.; Macher, P.; Kimmel, A.-C.L.; Steigerwald, T.G.; Alt, N.S.; Schlücker, E.; Wellmann, P. Solubility and dissolution kinetics of GaN in supercritical ammonia in presence of ammonoacidic and ammonobasic mineralizers. J. Cryst. Growth 2017, 479, 59–66. [Google Scholar] [CrossRef]
- Schimmel, S.; Lindner, M.; Steigerwald, T.G.; Hertweck, B.; Richter, T.M.; Künecke, U.; Alt, N.S.; Niewa, R.; Schlücker, E.; Wellmann, P.J. Determination of GaN solubility in supercritical ammonia with NH4F and NH4Cl mineralizer by in situ X-ray imaging of crystal dissolution. J. Cryst. Growth 2015, 418, 64–69. [Google Scholar] [CrossRef]
- Griffiths, S.; Pimputkar, S.; Speck, J.S.; Nakamura, S. On the solubility of gallium nitride in supercritical ammonia–sodium solutions. J. Cryst. Growth 2016, 456, 5–14. [Google Scholar] [CrossRef]
- Tomida, D.; Kuroda, K.; Nakamura, K.; Qiao, K.; Yokoyama, C. Temperature dependent control of the solubility of gallium nitride in supercritical ammonia using mixed mineralizer. Chem. Cent. J. 2018, 12, 127. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Ohshima, E.; Fukuda, T.; Tsuji, H.; Oshima, K. Crystal growth of GaN by ammonothermal method. J. Cryst. Growth 2004, 260, 67–72. [Google Scholar] [CrossRef]
- Tomida, D.; Kuribayashi, T.; Suzuki, K.; Kagamitani, Y.; Ishiguro, T.; Fukuda, T.; Yokoyama, C. Effect of halogen species of acidic mineralizer on solubility of GaN in supercritical ammonia. J. Cryst. Growth 2011, 325, 52–54. [Google Scholar] [CrossRef]
- Tomida, D.; Kagamitani, Y.; Bao, Q.; Hazu, K.; Sawayama, H.; Chichibu, S.F.; Yokoyama, C.; Fukuda, T.; Ishiguro, T. Enhanced growth rate for ammonothermal gallium nitride crystal growth using ammonium iodide mineralizer. J. Cryst. Growth 2012, 353, 59–62. [Google Scholar] [CrossRef]
- Tomida, D.; Bao, Q.; Saito, M.; Osanai, R.; Shima, K.; Kojima, K.; Ishiguro, T.; Chichibu, S.F. Ammonothermal growth of 2 inch long GaN single crystals using an acidic NH4F mineralizer in a Ag-lined autoclave. Appl. Phys. Express 2020, 13, 55505. [Google Scholar] [CrossRef]
- Purdy, A.P.; Case, S.; George, C. Ammonothermal Crystal Growth of Germanium and Its Alloys: Synthesis of a Hollow Metallic Crystal. Cryst. Growth Des. 2003, 3, 121–124. [Google Scholar] [CrossRef]
- Tomida, D.; Bao, Q.; Saito, M.; Kurimoto, K.; Sato, F.; Ishiguro, T.; Chichibu, S.F. Effects of extra metals added in an autoclave during acidic ammonothermal growth of m -plane GaN single crystals using an NH4F mineralizer. Appl. Phys. Express 2018, 11, 91002. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, X.; Cao, Y.; Xu, Y.; Xun, L.; Xu, T.; Liang, J. Low-temperature synthesis and photoluminescence of AlN. J. Cryst. Growth 1999, 207, 247–250. [Google Scholar] [CrossRef]
- Peters, D. Preparation of aluminium nitride by ammonolysis of hexammine aluminium iodide: Ceramic Powder Processing Science. In Ceramic Powder Processing Science, Proceedings of the Second International Conference, FRG, Berchtesgaden, Germany, 12–14 October 1988; Hausner, H., Messing, G.L., Hirano, S., Eds.; Deutsche Keramische Gesellschaft: Köln, Germany, 2015; pp. 181–188. [Google Scholar]
- Ketchum, D.R.; Schimek, G.L.; Pennington, W.T.; Kolis, J.W. Synthesis of new Group III fluoride–ammonia adducts in supercritical ammonia: Structures of AlF3(NH3)2 and InF2(NH2)(NH3). Inorganica Chim. Acta 1999, 294, 200–206. [Google Scholar] [CrossRef]
- Peters, D.; Bock, J.; Jacobs, H. Hexaaminaluminiumiodidmonoammoniakat—[Al(NH3)6]I3NH3—Darstellung und kristallstruktur. J. Less Common Met. 1989, 154, 243–250. [Google Scholar] [CrossRef]
- Kobayashi, T. Solvent effects of fluorides in cubic BN high pressure synthesis. Mater. Res. Bull. 1979, 14, 1541–1551. [Google Scholar] [CrossRef]
- Malkowski, T.F.; Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Acidic ammonothermal growth of gallium nitride in a liner-free molybdenum alloy autoclave. J. Cryst. Growth 2016, 456, 21–26. [Google Scholar] [CrossRef]
- Hertweck, B.; Steigerwald, T.G.; Alt, N.S.; Schluecker, E. Different corrosion behaviour of autoclaves made of nickel base alloy 718 in ammonobasic and ammonoacidic environments. J. Supercrit. Fluids 2014, 95, 158–166. [Google Scholar] [CrossRef]
- Kimmel, A.-C.; Malkowski, T.F.; Griffiths, S.; Hertweck, B.; Steigerwald, T.G.; Freund, L.P.; Neumeier, S.; Göken, M.; Speck, J.S.; Schluecker, E. High-temperature corrosion of Inconel®Alloy 718, Haynes®282®Alloy and CoWAlloy1&2 in supercritical ammonia/ammonium chloride solution. J. Cryst. Growth 2018, 498, 289–300. [Google Scholar] [CrossRef]
- Linde, G.; Juza, R. Amidometallate von Lanthan und Gadolinium und Umsetzung von Lanthan, Gadolinium und Scandium mit Ammoniak. Z. Anorg. Allg. Chem. 1974, 409, 191–198. [Google Scholar] [CrossRef]
- Stuhr, A.; Jocobs, H.; Juza, R. Amide des Yttriums. Z. Anorg. Allg. Chem. 1973, 395, 291–300. [Google Scholar] [CrossRef]
- Dwiliński, R.; Doradziński, R.; Garczyński, J.; Sierzputowski, L.; Baranowski, J.; Kamińska, M. AMMONO method of GaN and AlN production. Diam. Relat. Mater. 1998, 7, 1348–1350. [Google Scholar] [CrossRef]
- Adekore, B.T.; Callahan, M.J.; Bouthillette, L.; Dalmau, R.; Sitar, Z. Synthesis of erbium-doped gallium nitride crystals by the ammonothermal route. J. Cryst. Growth 2007, 308, 71–79. [Google Scholar] [CrossRef]
- Bäucker, C.; Bauch, S.; Niewa, R. Synthesis and Characterization of the Amidomanganates Rb2[Mn(NH2)4] and Cs2[Mn(NH2)4]. Crystals 2021, 11, 676. [Google Scholar] [CrossRef]
- Hertrampf, J.; Alt, N.; Schlücker, E.; Knetzger, M.; Meissner, E.; Niewa, R. Ammonothermal synthesis of GaN using Ba(NH2)2 as mineralizer. J. Cryst. Growth 2016, 456, 2–4. [Google Scholar] [CrossRef]
- Peters, D. Ammonothermal synthesis of aluminum nitride. J. Cryst. Growth 1990, 104, 411–418. [Google Scholar] [CrossRef]
- Adekore, B.T.; Rakes, K.; Wang, B.; Callahan, M.J.; Pendurti, S.; Sitar, Z. Ammonothermal synthesis of aluminum nitride crystals on group III-nitride templates. J. Electron. Mater. 2006, 35, 1104–1111. [Google Scholar] [CrossRef]
- Jacobs, H.; Juza, R. Darstellung und Eigenschaften von Berylliumamid und -imid. Z. Anorg. Allg. Chem. 1969, 370, 248–253. [Google Scholar] [CrossRef]
- Xu, L.; Li, T.; Ren, G.; Su, X.; Gao, X.; Zheng, S.; Wang, H.; Xu, K. Study of lateral growth regions in ammonothermal c-plane GaN. J. Cryst. Growth 2021, 556, 125987. [Google Scholar] [CrossRef]
- Mallmann, M.; Maak, C.; Schnick, W. Ammonothermal Synthesis and Crystal Growth of the Chain-type Oxonitridosilicate Ca1+xY1–xSiN3–xOx (x > 0). Z. Anorg. Allg. Chem. 2020, 646, 1539–1544. [Google Scholar] [CrossRef]
- Häusler, J.; Eisenburger, L.; Oeckler, O.; Schnick, W. Ammonothermal Synthesis and Crystal Structure of the Nitridoalumogermanate Ca1–xLixAl1–xGe1+xN3 (x ≈ 0.2). Eur. J. Inorg. Chem. 2018, 2018, 759–764. [Google Scholar] [CrossRef]
- Bäucker, C.; Niewa, R. Exchange of ammine- and fluorido-ligands in complex salts: The series [Cr(NH3)6][AlF6], [Cr(NH3)5F][SiF6] and K2[Cr(NH3)4F2][Si(NH3)0.5F5.5]2. Z. Anorg. Allg. Chem. 2022, 648, e2200209. [Google Scholar] [CrossRef]
- Jacobs, H.; Juza, R. Darstellung und Eigenschaften von Magnesiumamid und -imid. Z. Anorg. Allg. Chem. 1969, 370, 254–261. [Google Scholar] [CrossRef]
- Juza, R.; Jacobs, H.; Gerke, H. Ammonothermalsynthese von Metallamiden und Metallnitriden. Berichte Bunsenges. Phys. Chem. 1966, 70, 1103–1105. [Google Scholar] [CrossRef]
- Kreiner, G.; Jacobs, H. Magnetische struktur von η-Mn3N2. J. Alloys Compd. 1992, 183, 345–362. [Google Scholar] [CrossRef]
- Heikkinen, T.; Pavlov, J.; Ceponis, T.; Gaubas, E.; Zając, M.; Tuomisto, F. Effect of Mn and Mg dopants on vacancy defect formation in ammonothermal GaN. J. Cryst. Growth 2020, 547, 125803. [Google Scholar] [CrossRef]
- Leineweber, A.; Jacobs, H.; Hull, S. ChemInform Abstract: Ordering of Nitrogen in Nickel Nitride Ni3N Determined by Neutron Diffraction. ChemInform 2002, 33, 201004. [Google Scholar] [CrossRef]
- Wendl, S.; Mallmann, M.; Strobel, P.; Schmidt, P.J.; Schnick, W. Ammonothermal Synthesis of Ba2PO3 N—An Oxonitridophosphate with Non-Condensed PO3N Tetrahedra. Eur. J. Inorg. Chem. 2020, 2020, 841–846. [Google Scholar] [CrossRef]
- Cordes, N.; Niklaus, R.; Schnick, W. Ammonothermal Crystal Growth of ATaN2 with A = Na, K, Rb, and Cs and Their Optical and Electronic Properties. Cryst. Growth Des. 2019, 19, 3484–3490. [Google Scholar] [CrossRef]
- Jacobs, H.; Kockelkorn, J. Über kalium- und rubidiumamidometallate des Europiums, Yttriums und Ytterbiums, K3M(NH2)6 und Rb3M(NH2)6. J. Less Common Met. 1982, 85, 97–110. [Google Scholar] [CrossRef]
- Richter, T.T.; Zhang, S.; Niewa, R. Ammonothermal synthesis of dimorphic K2[Zn(NH2)4]. Z. Krist.-Cryst. Mater. 2013, 228, 351–358. [Google Scholar] [CrossRef]
- Zajac, M.; Konczewicz, L.; Litwin-Staszewska, E.; Iwinska, M.; Kucharski, R.; Juillaguet, S.; Contreras, S. p-type conductivity in GaN:Zn monocrystals grown by ammonothermal method. J. Appl. Phys. 2021, 129, 135702. [Google Scholar] [CrossRef]
- Jacobs, H.; Schröder, F.O. Penta-Ammoniates of Aluminium Halides: The Crystal Structures of AlX3·5NH3 with X = Cl, Br, IProfessor Joachim Strähle zum 65. Geburtstag gewidmet. Z. Anorg. Allg. Chem. 2002, 628, 951. [Google Scholar] [CrossRef]
- Juza, R.; Jacobs, H. Ammonothermal Synthesis of Magnesium and Beryllium Amides. Angew. Chem. Int. Ed. Engl. 1966, 5, 247. [Google Scholar] [CrossRef]
- Jacobs, H.; Schmidt, D. ChemInform Abstract: High-pressure ammonolysis in solid-state chemistry. Chem. Informationsdienst 1983, 14, 381–427. [Google Scholar] [CrossRef]
- Zachwieja, U.; Jacobs, H. Ammonothermalsynthese von kupfernitrid, Cu3N. J. Less Common Met. 1990, 161, 175–184. [Google Scholar] [CrossRef]
- Jacobs, H.; Birkenbeul, J.; Kockelkorn, J. Darstellung und eigenschaften des caesiumbarium-amids, CsBa(NH2)3: Strukturverwandtschaft zum NH4CdCl3-Typ. J. Less Common Met. 1982, 85, 71–78. [Google Scholar] [CrossRef]
- Jacobs, H.; Kockelkorn, J.; Birkenbeul, J. Struktur und eigenschaften der ternären metallamide NaCa(NH2)3, KBa(NH2)3, RbBa(NH2)3, RbEu(NH2)3 und RbSr(NH2)3. J. Less Common Met. 1982, 87, 215–224. [Google Scholar] [CrossRef]
- Jacobs, H.; Fink, U. Über natrium- und kaliumamidometallate des calciums, strontiums und europiums. J. Less Common Met. 1979, 63, 273–286. [Google Scholar] [CrossRef]
- Jacobs, H.; Fink, U. Darstellung und Kristallstruktur von KCa(NH2)3. Z. Anorg. Allg. Chem. 1977, 435, 137–145. [Google Scholar] [CrossRef]
- Jacobs, H.; Kockelkorn, J. Darstellung und KristallStruktur des Rubidiumcalciumamids, RbCa(NH2)3. Z. Anorg. Allg. Chem. 1979, 456, 147–154. [Google Scholar] [CrossRef]
- Jacobs, H.; Kockelkorn, J. Über cäsiumamidometallate (CsM(NH2)3) des calciums, strontiums und europiums; verbindungen mit der struktur “hexagonaler perowskite”. J. Less Common Met. 1981, 81, 143–154. [Google Scholar] [CrossRef]
- Becker, P.; Niewa, R. Progress in ammonothermal crystal growth of indium nitride. J. Cryst. Growth 2022, 581, 126480. [Google Scholar] [CrossRef]
- Jacobs, H.; Fink, U. Untersuchung des Systems Kalium/Europium/Ammoniak. Z. Anorg. Allg. Chem. 1978, 438, 151–159. [Google Scholar] [CrossRef]
- Jacobs, H.; Rechenbach, D.; Zachwieja, U. Structure determination of γ′-Fe4N and ϵ-Fe3N. J. Alloys Compd. 1995, 227, 10–17. [Google Scholar] [CrossRef]
- Jacobs, H.; Peters, D.; Hassiepen, K. Caesiumamidometallate des gadoliniums, ytterbiums und yttriums mit perowskitverwandten Atomanordnungen Cs3M2(NH2)9. J. Less Common Met. 1986, 118, 31–41. [Google Scholar] [CrossRef]
- Hadenfeldt, C.; Gieger, B.; Jacobs, H. Darstellung und Kristallstruktur von K3La(NH2)6. Z. Anorg. Allg. Chem. 1974, 403, 319–326. [Google Scholar] [CrossRef]
- Zając, M.; Gosk, J.; Grzanka, E.; Stelmakh, S.; Palczewska, M.; Wysmołek, A.; Korona, K.; Kamińska, M.; Twardowski, A. Ammonothermal synthesis of GaN doped with transition metal ions (Mn, Fe, Cr). J. Alloys Compd. 2008, 456, 324–338. [Google Scholar] [CrossRef]
- Jacobs, H.; Harbrecht, B. Schichtenweise Substitution von Kationen im Lithiumamid: Kaliumtrilithiumamid, KLi3(NH2)4 und Kaliumheptalithiumamid, KLi7(NH2)8. Z. Anorg. Allg. Chem. 1984, 518, 87–100. [Google Scholar] [CrossRef]
- Jacobs, H.; Birkenbeul, J.; Schmitz, D. Strukturverwandtschaft des dicaesiumamidomagnesats, Cs2[Mg(NH2)4], zum β-K2SO4-Typ. J. Less Common Met. 1982, 85, 79–86. [Google Scholar] [CrossRef]
- Jacobs, H.; Birkenbeul, J.; Kockelkorn, J. Darstellung und eigenschaften der amidomagnesate des kaliums und rubidiums K2[Mg(NH2)4]- und Rb2[Mg(NH2)4]-Verbindungen mit isolierten [Mg(NH2)4]2−-tetraedern. J. Less Common Met. 1984, 97, 205–214. [Google Scholar] [CrossRef]
- Jacobs, H.; Kistrup, H. Über das System Kalium/Samarium/Ammoniak. Z. Anorg. Allg. Chem. 1977, 435, 127–136. [Google Scholar] [CrossRef]
- Hadenfeldt, C.; Jacobs, H.; Juza, R. Über die Amide des Europiums und Ytterbiums. Z. Anorg. Allg. Chem. 1970, 379, 144–156. [Google Scholar] [CrossRef]
- Froehling, B.; Kreiner, G.; Jacobs, H. ChemInform Abstract: Synthesis and Crystal Structure of Manganese(II) and Zinc Amides, Mn(NH2)2 and Zn(NH2)2. ChemInform 1999, 30, 211–216. [Google Scholar] [CrossRef]
- Juza, R. Über die Amide der 1. und 2. Gruppe des periodischen Systems. Metallamide. I. Mitteilung. Z. Anorg. Allg. Chem. 1937, 231, 121–135. [Google Scholar] [CrossRef]
- Sun, D.; Liu, L.; Wang, G.; Yu, J.; Li, Q.; Tian, G.; Wang, B.; Xu, X.; Zhang, L.; Wang, S. Research Progress in Liquid Phase Growth of GaN Crystals. Chem.–A Eur. J. 2023, 30, e202303710. [Google Scholar] [CrossRef]
- Kucharski, R.; Sochacki, T.; Lucznik, B.; Amilusik, M.; Grabianska, K.; Iwinska, M.; Bockowski, M. Ammonothermal and HVPE Bulk Growth of GaN. Wide Bandgap Semicond. Power Electron. Mater. Devices Appl. 2021, 2, 529–554. [Google Scholar]
- Scholz, F.; Bockowski, M.; Grzanka, E. GaN-Based Materials: Substrates, Metalorganic Vapor-Phase Epitaxy, and Quantum Well Properties. In Nitride Semiconductor Technology: Power Electronics and Optoelectronic Devices; Wiley-VCH: Weinheim, Germany, 2020; pp. 41–98. [Google Scholar]
- Xu, K.; Wang, J.-F.; Ren, G.-Q. Progress in bulk GaN growth. Chin. Phys. B 2015, 24, 66105. [Google Scholar] [CrossRef]
- Brodt-Giles, D.; Gomach, S.; American-Made Team. Spring 2024 Alumni Book of the AMERICAN-MADE; U.S. Department of Energy: Washington, DC, USA, 2024.
- Kirste, L.; Tran Thi Caliste, T.N.; Weyher, J.L.; Smalc-Koziorowska, J.; Zajac, M.A.; Kucharski, R.; Sochacki, T.; Grabianska, K.; Iwinska, M.; Detlefs, C.; et al. Large-Scale Defect Clusters with Hexagonal Honeycomb-like Arrangement in Ammonothermal GaN Crystals. Materials 2022, 15, 6996. [Google Scholar] [CrossRef]
- Meissner, E.; Niewa, R. Ammonothermal Synthesis and Crystal Growth of Nitrides; Springer International Publishing: Cham, Switzerland, 2021; Volume 10, ISBN 978-3-030-56305-9. [Google Scholar]
- Dwili, R.; Doradzi, R.; Garczy, J.; Sierzputowski, L.; Palczewska, M.; Wysmolek, A.; Kami, M. AMMONO method of BN, AlN and GaN synthesis and crystal growth. MRS Internet J. Nitride Semicond. Res. 1998, 3, e25. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, X.; Lan, Y.; Li, J.; Xu, Y.; Xu, T.; Liu, Q.; Liang, J. Blue emission and Raman scattering spectrum from AlN nanocrystalline powders. J. Cryst. Growth 2000, 213, 198–202. [Google Scholar] [CrossRef]
- Cao, Y.G.; Chen, X.L.; Lan, Y.C.; Li, J.Y.; Zhang, Y.; Yang, Z.; Liang, J.K. Synthesis and Raman characteristics of hexagonal AlxGa1-xN alloy nanocrystalline solids through ammonothermal routes. Appl. Phys. A 2001, 72, 125–127. [Google Scholar] [CrossRef]
- Motchanyy, A.I.; Reu, A.A.; Kovalenko, V.S.; Balakirev, V.G. Ammonothermal Crystallization of AlN Crystals. MRS Proc. 2005, 878, Y3.3. [Google Scholar] [CrossRef]
- Dwilinski, R.; Doradzinski, R.; Garczynski, J.; Sierzputowski, L.; Kanbara, Y. Method and Equipment for Manufacturing Aluminum Nitride Bulk Single Crystal. U.S. Patent No. 7,374,615, 20 May 2008. [Google Scholar]
- Wang, B.; Callahan, M.J. Ammonothermal Synthesis of III-Nitride Crystals. Cryst. Growth Des. 2006, 6, 1227–1246. [Google Scholar] [CrossRef]
- Dwilinski, R.; Doradzinski, R.; Garczynski, J.; Sierzputowski, L.P.; Kanbara, Y. Process for Obtaining of Bulk Monocrystalline Gallium-Containing Nitride. U.S. Patent 7,364,619, 29 April 2008. [Google Scholar]
- Kukushkin, S.A.; Sharofidinov, S.S. A New Method of Growing AlN, GaN, and AlGaN Bulk Crystals Using Hybrid SiC/Si Substrates. Phys. Solid State 2019, 61, 2342–2347. [Google Scholar] [CrossRef]
- Jander, G.; Spandau, H.; Addison, C.C. Chemistry in Nonaequeous Ionizing Solvents; Friedr. Vieweg & Sohn: Braunschweig, Germany, 1966. [Google Scholar]
- Izyumskaya, N.; Demchenko, D.O.; Das, S.; Özgür, Ü.; Avrutin, V.; Morkoç, H. Recent Development of Boron Nitride towards Electronic Applications. Adv. Electron. Mater. 2017, 3, 1600485. [Google Scholar] [CrossRef]
- Taniguchi, T.; Watanabe, K. Synthesis of high-purity boron nitride single crystals under high pressure by using Ba–BN solvent. J. Cryst. Growth 2007, 303, 525–529. [Google Scholar] [CrossRef]
- Chilleri, J.; Siddiqua, P.; Shur, M.S.; O’Leary, S.K. Cubic boron nitride as a material for future electron device applications: A comparative analysis. Appl. Phys. Lett. 2022, 120, 122105. [Google Scholar] [CrossRef]
- Naclerio, A.E.; Kidambi, P.R. A Review of Scalable Hexagonal Boron Nitride (h-BN) Synthesis for Present and Future Applications. Adv. Mater. 2023, 35, e2207374. [Google Scholar] [CrossRef]
- Yankowitz, M.; Ma, Q.; Jarillo-Herrero, P.; LeRoy, B.J. van der Waals heterostructures combining graphene and hexagonal boron nitride. Nat. Rev. Phys. 2019, 1, 112–125. [Google Scholar] [CrossRef]
- Maruyama, Y.; Kurozumi, T.; Omori, K.; Otsubo, H.; Sato, T.; WATANABE, T. Ammonothermal synthesis of rhombohedral boron nitride. Mater. Lett. 2018, 232, 110–112. [Google Scholar] [CrossRef]
- Jacobs, H.; Pinkowski, E. von. Synthese ternärer nitride von alkalimetallen: Verbindungen mit tantal, MTaN2 MIT M ≡ Na, K, RbundCs. J. Less Common Met. 1989, 146, 147–160. [Google Scholar] [CrossRef]
- Brokamp, T.; Jacobs, H. Synthese und Kristallstruktur eines gemischtvalenten Lithium-Tantalnitrids Li2Ta3N5. J. Alloys Compd. 1991, 176, 47–60. [Google Scholar] [CrossRef]
- Jacobs, H. Preparation and crystal structure of a sodium silicon nitride, NaSi2N3. Eur. J. Solid State Inorg. Chem. 1993, 30, 45–53. [Google Scholar]
- Jacobs, H.; Nymwegen, R. Darstellung und Kristallstruktur eines Kaliumnitridophosphats, K3P6N11. Z. Anorg. Allg. Chem. 1997, 623, 429–433. [Google Scholar] [CrossRef]
- Mallmann, M.; Wendl, S.; Strobel, P.; Schmidt, P.J.; Schnick, W. Sr3P3N7: Complementary Approach by Ammonothermal and High-Pressure Syntheses. Chemistry 2020, 26, 6257–6263. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, F. Vacancy Defects in Bulk and Quasi-Bulk GaN Crystals. Crystals 2022, 12, 1112. [Google Scholar] [CrossRef]
- Suihkonen, S.; Pimputkar, S.; Speck, J.S.; Nakamura, S. Infrared absorption of hydrogen-related defects in ammonothermal GaN. Appl. Phys. Lett. 2016, 108, 202105. [Google Scholar] [CrossRef]
- Shima, K.; Kurimoto, K.; Bao, Q.; Mikawa, Y.; Saito, M.; Tomida, D.; Uedono, A.; Ishibashi, S.; Ishiguro, T.; Chichibu, S.F. Improved midgap recombination lifetimes in GaN crystals grown by the low-pressure acidic ammonothermal method. Appl. Phys. Lett. 2024, 124, 181103. [Google Scholar] [CrossRef]
- Zajac, M.; Kaminski, P.; Kozlowski, R.; Litwin-Staszewska, E.; Piotrzkowski, R.; Grabianska, K.; Kucharski, R.; Jakiela, R. Formation of Grown-In Nitrogen Vacancies and Interstitials in Highly Mg-Doped Ammonothermal GaN. Materials 2024, 17, 1160. [Google Scholar] [CrossRef] [PubMed]
- Chichibu, S.F.; Shima, K.; Uedono, A.; Ishibashi, S.; Iguchi, H.; Narita, T.; Kataoka, K.; Tanaka, R.; Takashima, S.; Ueno, K.; et al. Impacts of vacancy complexes on the room-temperature photoluminescence lifetimes of state-of-the-art GaN substrates, epitaxial layers, and Mg-implanted layers. J. Appl. Phys. 2024, 135, 185701. [Google Scholar] [CrossRef]
- Freysoldt, C.; Grabowski, B.; Hickel, T.; Neugebauer, J.; Kresse, G.; Janotti, A.; van de Walle, C.G. First-principles calculations for point defects in solids. Rev. Mod. Phys. 2014, 86, 253–305. [Google Scholar] [CrossRef]
- Lyons, J.L.; Wickramaratne, D.; van de Walle, C.G. A first-principles understanding of point defects and impurities in GaN. J. Appl. Phys. 2021, 129, 111101. [Google Scholar] [CrossRef]
- Baker, J.N.; Bowes, P.C.; Harris, J.S.; Collazo, R.; Sitar, Z.; Irving, D.L. Complexes and compensation in degenerately donor doped GaN. Appl. Phys. Lett. 2020, 117, 102109. [Google Scholar] [CrossRef]
- van de Walle, C.G. Interactions of hydrogen with native defects in GaN. Phys. Rev. B 1997, 56, R10020. [Google Scholar] [CrossRef]
- Wright, A.F. Interaction of hydrogen with gallium vacancies in wurtzite GaN. J. Appl. Phys. 2001, 90, 1164–1169. [Google Scholar] [CrossRef]
- Dreyer, C.E.; Alkauskas, A.; Lyons, J.L.; Speck, J.S.; van de Walle, C.G. Gallium vacancy complexes as a cause of Shockley-Read-Hall recombination in III-nitride light emitters. Appl. Phys. Lett. 2016, 108, 141101. [Google Scholar] [CrossRef]
- Reshchikov, M.A.; Demchenko, D.O.; Ye, D.; Andrieiev, O.; Vorobiov, M.; Grabianska, K.; Zajac, M.; Nita, P.; Iwinska, M.; Bockowski, M. The effect of annealing on photoluminescence from defects in ammonothermal GaN. J. Appl. Phys. 2022, 131, 035704. [Google Scholar] [CrossRef]
- Jiang, W.; Nolan, M.; Ehrentraut, D.; D’Evelyn, M.P. Electrical and optical properties of gallium vacancy complexes in ammonothermal GaN. Appl. Phys. Express 2017, 10, 75506. [Google Scholar] [CrossRef]
- Tuomisto, F.; Mäki, J.-M.; Zając, M. Vacancy defects in bulk ammonothermal GaN crystals. J. Cryst. Growth 2010, 312, 2620–2623. [Google Scholar] [CrossRef]
- Tuomisto, F.; Kuittinen, T.; Zając, M.; Doradziński, R.; Wasik, D. Vacancy–hydrogen complexes in ammonothermal GaN. J. Cryst. Growth 2014, 403, 114–118. [Google Scholar] [CrossRef]
- Uedono, A.; Tsukada, Y.; Mikawa, Y.; Mochizuki, T.; Fujisawa, H.; Ikeda, H.; Kurihara, K.; Fujito, K.; Terada, S.; Ishibashi, S. Vacancies and electron trapping centers in acidic ammonothermal GaN probed by a monoenergetic positron beam. J. Cryst. Growth 2016, 448, 117–121. [Google Scholar] [CrossRef]
- Reshchikov, M.A.; Demchenko, D.O.; McNamara, J.D.; Fernández-Garrido, S.; Calarco, R. Green luminescence in Mg-doped GaN. Phys. Rev. B 2014, 90, 035207. [Google Scholar] [CrossRef]
- Myers, S.M.; Wright, A.F.; Petersen, G.A.; Seager, C.H.; Wampler, W.R.; Crawford, M.H.; Han, J. Equilibrium state of hydrogen in gallium nitride: Theory and experiment. J. Appl. Phys. 2000, 88, 4676–4687. [Google Scholar] [CrossRef]
- Myers, S.M.; Wright, A.F.; Petersen, G.A.; Wampler, W.R.; Seager, C.H.; Crawford, M.H.; Han, J. Diffusion, release, and uptake of hydrogen in magnesium-doped gallium nitride: Theory and experiment. J. Appl. Phys. 2001, 89, 3195–3202. [Google Scholar] [CrossRef]
- Saarinen, K.; Suski, T.; Grzegory, I.; Look, D.C. Thermal stability of isolated and complexed Ga vacancies in GaN bulk crystals. Phys. Rev. B 2001, 64, 233201. [Google Scholar] [CrossRef]
- Horibuchi, K.; Yamaguchi, S.; Kimoto, Y.; Nishikawa, K.; Kachi, T. Formation of helical dislocations in ammonothermal GaN substrate by heat treatment. Semicond. Sci. Technol. 2016, 31, 34002. [Google Scholar] [CrossRef]
- Tuomisto, F.; Mäki, J.-M.; Rauch, C.; Makkonen, I. On the formation of vacancy defects in III-nitride semiconductors. J. Cryst. Growth 2012, 350, 93–97. [Google Scholar] [CrossRef]
- Tomida, D.; Chichibu, S.F.; Kagamitani, Y.; Bao, Q.; Hazu, K.; Simura, R.; Sugiyama, K.; Yokoyama, C.; Ishiguro, T.; Fukuda, T. Improving the purity of GaN grown by the ammonothermal method with in-autoclave gas-phase acidic mineralizer synthesis. J. Cryst. Growth 2012, 348, 80–84. [Google Scholar] [CrossRef]
- Shim, J.B.; Kim, G.H.; Lee, Y.K. Basic ammonothermal growth of bulk GaN single crystal using sodium mineralizers. J. Cryst. Growth 2017, 478, 85–88. [Google Scholar] [CrossRef]
- Neugebauer, J.; van de Walle, C.G. Chemical trends for acceptor impurities in GaN. J. Appl. Phys. 1999, 85, 3003–3005. [Google Scholar] [CrossRef]
- Götz, W.; Johnson, N.M.; Chen, C.; Liu, H.; Kuo, C.; Imler, W. Activation energies of Si donors in GaN. Appl. Phys. Lett. 1996, 68, 3144–3146. [Google Scholar] [CrossRef]
- Matsubara, M.; Bellotti, E. A first-principles study of carbon-related energy levels in GaN. II. Complexes formed by carbon and hydrogen, silicon or oxygen. J. Appl. Phys. 2017, 121, 195702. [Google Scholar] [CrossRef]
- Lee, J.-H.; Hahm, S.-H.; Lee, J.-H.; Bae, S.-B.; Lee, K.-S.; Cho, Y.-H.; Lee, J.-L. Effect of Al doping in GaN films grown by metalorganic chemical vapor deposition. Appl. Phys. Lett. 2003, 83, 917–919. [Google Scholar] [CrossRef]
- Lyons, J.L.; Janotti, A.; van de Walle, C.G. Shallow versus deep nature of Mg acceptors in nitride semiconductors. Phys. Rev. Lett. 2012, 108, 156403. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, J.; van de Walle, C.G. Hydrogen in GaN: Novel aspects of a common impurity. Phys. Rev. Lett. 1995, 75, 4452–4455. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.L.; van de Walle, C.G. Computationally predicted energies and properties of defects in GaN. npj Comput. Mater. 2017, 3, 12. [Google Scholar] [CrossRef]
- Lee, D.; Mitchell, B.; Fujiwara, Y.; Dierolf, V. Thermodynamics and Kinetics of Three Mg−H−VN Complexes in Mg:GaN from Combined First-Principles Calculation and Experiment. Phys. Rev. Lett. 2014, 112, 205501. [Google Scholar] [CrossRef]
- Wickramaratne, D.; Shen, J.-X.; Dreyer, C.E.; Alkauskas, A.; van de Walle, C.G. Electrical and optical properties of iron in GaN, AlN, and InN. Phys. Rev. B 2019, 99, 205202. [Google Scholar] [CrossRef]
- Reshchikov, M.A. Photoluminescence from defects in GaN. In Gallium Nitride Materials and Devices XVIII, Proceedings of the Gallium Nitride Materials and Devices XVIII, San Francisco, CA, USA, 28 January–3 February 2023; Morkoç, H., Fujioka, H., Schwarz, U.T., Eds.; SPIE: Bellingham, WA, USA, 2023; p. 17. ISBN 9781510659476. [Google Scholar]
- Lyons, J.L.; Janotti, A.; van de Walle, C.G. Impact of Group-II Acceptors on the Electrical and Optical Properties of GaN. Jpn. J. Appl. Phys. 2013, 52, 08JJ04. [Google Scholar] [CrossRef]
- Cui, X.Y.; Delley, B.; Freeman, A.J.; Stampfl, C. Neutral and charged embedded clusters of Mn in doped GaN from first principles. Phys. Rev. B 2007, 76, 045201. [Google Scholar] [CrossRef]
- Korotkov, R.Y.; Wessels, B.W. Electrical Properties of Oxygen Doped GaN Grown by Metalorganic Vapor Phase Epitaxy. MRS Internet J. Nitride Semicond. Res. 2000, 5, 301–307. [Google Scholar] [CrossRef]
- Wickramaratne, D.; Dreyer, C.E.; Monserrat, B.; Shen, J.-X.; Lyons, J.L.; Alkauskas, A.; van de Walle, C.G. Defect identification based on first-principles calculations for deep level transient spectroscopy. Appl. Phys. Lett. 2018, 113, 192106. [Google Scholar] [CrossRef]
- Dwiliński, R.; Doradziński, R.; Garczyński, J.; Sierzputowski, L.; Baranowski, J.; Kamińska, M. Exciton photo-luminescence of GaN bulk crystals grown by the AMMONO method. Mater. Sci. Eng. B 1997, 50, 46–49. [Google Scholar] [CrossRef]
- Friedrich, J.; von Ammon, W.; Müller, G. 2—Czochralski Growth of Silicon Crystals. In Handbook of Crystal Growth, 2nd ed.; Rudolph, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 45–104. ISBN 978-0-444-63303-3. [Google Scholar]
- Lu, W.; Li, T.; Ren, G.; Xia, Z.; Xie, K.; Li, S.; Shen, L.; Xu, K. Study on Raman scattering spectroscopy of Mn-doped GaN grown by the ammonothermal method. CrystEngComm 2024, 26, 2166–2171. [Google Scholar] [CrossRef]
- Wang, B.; Bliss, D.; Suscavage, M.; Swider, S.; Lancto, R.; Lynch, C.; Weyburne, D.; Li, T.; Ponce, F.A. Ammonothermal growth of high-quality GaN crystals on HVPE template seeds. J. Cryst. Growth 2011, 318, 1030–1033. [Google Scholar] [CrossRef]
- Pimputkar, S.; Suihkonen, S.; Imade, M.; Mori, Y.; Speck, J.S.; Nakamura, S. Free electron concentration dependent sub-bandgap optical absorption characterization of bulk GaN crystals. J. Cryst. Growth 2015, 432, 49–53. [Google Scholar] [CrossRef]
- Chyi, J.-I.; Fujioka, H.; Morkoç, H. (Eds.) Gallium Nitride Materials and Devices X; SPIE OPTO; SPIE: San Francisco, CA, USA, 2015. [Google Scholar]
- Mikawa, Y.; Ishinabe, T.; Kawabata, S.; Mochizuki, T.; Kojima, A.; Kagamitani, Y.; Fujisawa, H. Ammonothermal growth of polar and non-polar bulk GaN crystal. In Gallium Nitride Materials and Devices X; Chyi, J.-I., Fujioka, H., Morkoç, H., Eds.; SPIE OPTO; SPIE: San Francisco, CA, USA, 2015; p. 936302. [Google Scholar]
- Yoshida, K.; Aoki, K.; Fukuda, T. High-temperature acidic ammonothermal method for GaN crystal growth. J. Cryst. Growth 2014, 393, 93–97. [Google Scholar] [CrossRef]
- Letts, E.; Hashimoto, T.; Hoff, S.; Key, D.; Male, K.; Michaels, M. Development of GaN wafers via the ammonothermal method. J. Cryst. Growth 2014, 403, 3–6. [Google Scholar] [CrossRef]
- Amilusik, M.; Zajac, M.; Fijalkowski, M.; Iwinska, M.; Sochacki, T.; Wlodarczyk, D.; Somakumar, A.K.; Jakiela, R.; Suchocki, A.; Bockowski, M. Role of carbon in n-type bulk GaN crystals. J. Cryst. Growth 2024, 632, 127641. [Google Scholar] [CrossRef]
- Jiang, W.; Ehrentraut, D.; Downey, B.C.; Kamber, D.S.; Pakalapati, R.T.; Yoo, H.D.; D’Evelyn, M.P. Highly transparent ammonothermal bulk GaN substrates. J. Cryst. Growth 2014, 403, 18–21. [Google Scholar] [CrossRef]
- Trivellin, N.; Meneghini, M.; Zanoni, E.; Orita, K.; Yuri, M.; Tanaka, T.; Ueda, D.; Meneghesso, G. A review on the reliability of GaN-based laser diodes. In Proceedings of the 2010 IEEE International Reliability Physics Symposium, Garden Grove (Anaheim), CA, USA, 2–6 May 2010; IEEE: Piscataway, NJ, USA, 2010. [Google Scholar]
- Wen, P.Y.; Zhang, S.M.; Li, D.Y.; Liu, J.P.; Zhang, L.Q.; Zhou, K.; Feng, M.X.; Tian, A.Q.; Zhang, F.; Gao, X.D.; et al. Identification of degradation mechanisms of blue InGaN/GaN laser diodes. J. Phys. D Appl. Phys. 2015, 48, 415101. [Google Scholar] [CrossRef]
- Leszczynski, M.; Prystawko, P.; Suski, T.; Lucznik, B.; Domagala, J.; Bak-Misiuk, J.; Stonert, A.; Turos, A.; Langer, R.; Barski, A. Lattice parameters of GaN single crystals, homoepitaxial layers and heteroepitaxial layers on sapphire. J. Alloys Compd. 1999, 286, 271–275. [Google Scholar] [CrossRef]
- Krysko, M.; Sarzynski, M.; Domagała, J.; Grzegory, I.; Łucznik, B.; Kamler, G.; Porowski, S.; Leszczyński, M. The influence of lattice parameter variation on microstructure of GaN single crystals. J. Alloys Compd. 2005, 401, 261–264. [Google Scholar] [CrossRef]
- Letts, E.; Key, D.; Hashimoto, T. Reduction of crack density in ammonothermal bulk GaN growth. J. Cryst. Growth 2016, 456, 27–32. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Effects of impurities on the lattice parameters of GaN. Phys. Rev. B 2003, 68, 165209. [Google Scholar] [CrossRef]
- Tanaka, A.; Nagamatsu, K.; Usami, S.; Kushimoto, M.; Deki, M.; Nitta, S.; Honda, Y.; Bockowski, M.; Amano, H. V-shaped dislocations in a GaN epitaxial layer on GaN substrate. AIP Adv. 2019, 9, 095002. [Google Scholar] [CrossRef]
- Erlekampf, J.; Seebeck, J.; Savva, P.; Meissner, E.; Friedrich, J.; Alt, N.; Schlücker, E.; Frey, L. Numerical time-dependent 3D simulation of flow pattern and heat distribution in an ammonothermal system with various baffle shapes. J. Cryst. Growth 2014, 403, 96–104. [Google Scholar] [CrossRef]
- Alt, N.S.A. Entwicklung Spezieller Hochdruckapparate und Methoden zur Durchführung von In-Situ Messungen Beim Ammonothermalen Prozess; FAU Erlangen-Nürnberg: Erlangen, Germany, 2010. [Google Scholar]
- Alt, N.; Meissner, E.; Schlücker, E.; Frey, L. In situ monitoring technologies for ammonthermal reactors. Phys. Status Solidi C 2012, 9, 436–439. [Google Scholar] [CrossRef]
- Schimmel, S. In Situ Visualisierung des Ammonothermalen Kristallisationsprozesses Mittels Röntgenmesstechnik; Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU): Erlangen, Germany, 2018. [Google Scholar]
- Schimmel, S.; Wellmann, P. In Situ Visualization of the Ammonothermal Crystallization Process by X-ray Technology. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 171–190. ISBN 978-3-030-56304-2. [Google Scholar]
- Schlücker, E.; Kimmel, A.-C.L. Technological Challenges of Autoclave Design for Ammonothermal Syntheses. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 27–44. ISBN 978-3-030-56304-2. [Google Scholar]
- Schlücker, E. High-Pressure Technology. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; pp. 1–49. ISBN 9783527306732. [Google Scholar]
- Hertweck, B.; Steigerwald, T.G.; Alt, N.S.A.; Schluecker, E. Corrosive Degeneration of Autoclaves for the Ammonothermal Synthesis: Experimental Approach and First Results. Chem. Eng. Technol. 2014, 37, 1903–1906. [Google Scholar] [CrossRef]
- Schimmel, S.; Salamon, M.; Tomida, D.; Neumeier, S.; Ishiguro, T.; Honda, Y.; Chichibu, S.F.; Amano, H. High-Energy Computed Tomography as a Prospective Tool for In Situ Monitoring of Mass Transfer Processes inside High-Pressure Reactors-A Case Study on Ammonothermal Bulk Crystal Growth of Nitrides including GaN. Materials 2022, 15, 6165. [Google Scholar] [CrossRef] [PubMed]
- Mallmann, M.; Wendl, S.; Schnick, W. Crystalline Nitridophosphates by Ammonothermal Synthesis. Chemistry 2020, 26, 2067–2072. [Google Scholar] [CrossRef]
- Cordes, N.; Schnick, W. Ammonothermal Synthesis of Crystalline Oxonitride Perovskites LnTaON2 (Ln = La, Ce, Pr, Nd, Sm, Gd). Chemistry 2017, 23, 11410–11415. [Google Scholar] [CrossRef]
- NeoNickel. A 286 Technical Datasheet. Available online: https://www.neonickel.com/alloys/all-alloys/a286 (accessed on 25 April 2024).
- Larson, F.R.; Miller, J. A Time-Temperature Relationship for Rupture and Creep Stresses. J. Fluids Eng. 1952, 74, 765–771. [Google Scholar] [CrossRef]
- Guštin, A.Z.; Žužek, B.; Podgornik, B. Creep Life Prediction of 10CrMo9-10 Steel by Larson-Miller Model. Materials 2022, 15, 4431. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Esaka, H.; Shinozuka, K. Stress and Temperature Dependence of Time to Rupture of Heat Resisting Steels. ISIJ Int. 1999, 39, 380–387. [Google Scholar] [CrossRef]
- Sherby, O.D.; Burke, P.M. Mechanical behavior of crystalline solids at elevated temperature. Prog. Mater. Sci. 1968, 13, 323–390. [Google Scholar] [CrossRef]
- Metzger, F.; Rienzi, V.; Mascetti, C.; Nguyen, T.; Pimputkar, S. Properties of Titanium Zirconium Molybdenum Alloy after Exposure to Indium at Elevated Temperatures. Materials 2022, 15, 5270. [Google Scholar] [CrossRef]
- Jiang, W.; Ehrentraut, D.; Cook, J.; Kamber, D.S.; Pakalapati, R.T.; D’Evelyn, M.P. Transparent, conductive bulk GaN by high temperature ammonothermal growth. Phys. Status Solidi (b) 2015, 252, 1069–1074. [Google Scholar] [CrossRef]
- Kimmel, A.-C.L.; Schlücker, E. Corrosive Degeneration of Process Equipment and Technical Solutions for Corrosion Protection Under Ammonothermal Conditions. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 191–201. ISBN 978-3-030-56304-2. [Google Scholar]
- Hertweck, B.; Schimmel, S.; Steigerwald, T.G.; Alt, N.S.; Wellmann, P.J.; Schluecker, E. Ceramic liner technology for ammonoacidic synthesis. J. Supercrit. Fluids 2015, 99, 76–87. [Google Scholar] [CrossRef]
- Becker, P.; Wonglakhon, T.; Zahn, D.; Gudat, D.; Niewa, R. Approaching Dissolved Species in Ammonoacidic GaN Crystal Growth: A Combined Solution NMR and Computational Study. Chemistry 2020, 26, 7008–7017. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Wu, F.; Speck, J.S.; Nakamura, S. Growth of Bulk GaN Crystals by the Basic Ammonothermal Method. Jpn. J. Appl. Phys. 2007, 46, L889. [Google Scholar] [CrossRef]
- Kagamitani, Y.; Ehrentraut, D.; Yoshikawa, A.; Hoshino, N.; Fukuda, T.; Kawabata, S.; Inaba, K. Ammonothermal Epitaxy of Thick GaN Film Using NH 4 Cl Mineralizer. Jpn. J. Appl. Phys. 2006, 45, 4018. [Google Scholar] [CrossRef]
- Tomida, D.; Kuroda, K.; Hoshino, N.; Suzuki, K.; Kagamitani, Y.; Ishiguro, T.; Fukuda, T.; Yokoyama, C. Solubility of GaN in supercritical ammonia with ammonium chloride as a mineralizer. J. Cryst. Growth 2010, 312, 3161–3164. [Google Scholar] [CrossRef]
- Purdy, A.P.; Jouet, R.J.; George, C.F. Ammonothermal Recrystallization of Gallium Nitride with Acidic Mineralizers. Cryst. Growth Des. 2002, 2, 141–145. [Google Scholar] [CrossRef]
- Hertweck, B.; Zhang, S.; Steigerwald, T.G.; Alt, N.S.A.; Niewa, R.; Schluecker, E. Applicability of Metals as Liner Materials for Ammonoacidic Crystal Growth. Chem. Eng. Technol. 2014, 37, 1835–1844. [Google Scholar] [CrossRef]
- Malkowski, T.F.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. An exploratory study of acidic ammonothermal growth in a TZM autoclave at high temperatures. J. Cryst. Growth 2018, 499, 85–89. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; Nakamura, S. Basic ammonothermal GaN growth in molybdenum capsules. J. Cryst. Growth 2016, 456, 15–20. [Google Scholar] [CrossRef]
- Griffiths, S.; Pimputkar, S.; Kearns, J.; Malkowski, T.F.; Doherty, M.F.; Speck, J.S.; Nakamura, S. Growth kinetics of basic ammonothermal gallium nitride crystals. J. Cryst. Growth 2018, 501, 74–80. [Google Scholar] [CrossRef]
- Steigerwald, T.G.; Schlücker, E. Direct Determination of Viscosity of Supercritical Solutions. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 117–153. ISBN 978-3-030-56304-2. [Google Scholar]
- Schlücker, E.; Hertweck, B.; Schimmel, S.; Wellmann, P. Special Equipment for Ammonothermal Processes. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer Series in Materials Science; Springer: Cham, Switzerland, 2021; Volume 304. [Google Scholar] [CrossRef]
- MacKay, R.A.; Gabb, T.P.; Smialek, J.L.; Nathal, M.V. A new approach of designing superalloys for low density. JOM 2010, 62, 48–54. [Google Scholar] [CrossRef]
- Schlücker, E. Technical Solutions for In Situ Monitoring of Ammonothermal Processes. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 47–64. ISBN 978-3-030-56304-2. [Google Scholar]
- Schimmel, S.; Tomida, D.; Ishiguro, T.; Honda, Y.; Chichibu, S.F.; Amano, H. Temperature Field, Flow Field, and Temporal Fluctuations Thereof in Ammonothermal Growth of Bulk GaN-Transition from Dissolution Stage to Growth Stage Conditions. Materials 2023, 16, 2016. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, S.; Duchstein, P.; Steigerwald, T.G.; Kimmel, A.-C.; Schlücker, E.; Zahn, D.; Niewa, R.; Wellmann, P. In situ X-ray monitoring of transport and chemistry of Ga-containing intermediates under ammonothermal growth conditions of GaN. J. Cryst. Growth 2018, 498, 214–223. [Google Scholar] [CrossRef]
- William, S. The viscosity of gases and molecular force. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1893, 36, 507–531. [Google Scholar] [CrossRef]
- Lencka, M.M.; Anderko, A.; Sanders, S.J.; Young, R.D. Modeling Viscosity of Multicomponent Electrolyte Solutions. Int. J. Thermophys. 1998, 19, 367–378. [Google Scholar] [CrossRef]
- Jones, G.; Dole, M. The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J. Am. Chem. Soc. 1929, 51, 2950–2964. [Google Scholar] [CrossRef]
- Saeed, R.; Uddin, F.; Masood, S.; Asif, N. Viscosities of ammonium salts in water and ethanol+water systems at different temperatures. J. Mol. Liq. 2009, 146, 112–115. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Marcus, Y. Viscosity B-Coefficients of Ions in Solution. Chem. Rev. 1995, 95, 2695–2724. [Google Scholar] [CrossRef]
- Steigerwald, T.G.; Balouschek, J.; Hertweck, B.; Kimmel, A.-C.L.; Alt, N.S.; Schluecker, E. In situ investigation of decomposing ammonia and ammonobasic solutions under supercritical conditions via UV/vis and Raman Spectroscopy. J. Supercrit. Fluids 2018, 134, 96–105. [Google Scholar] [CrossRef]
- Baser, H.; Schwieger, W.; Freitag, D.; Steigerwald, T.G.; Schluecker, E. Solubility Studies of Sodium Azide in Liquid Ammonia by In Situ Ultrasonic Velocity Measurement. Chem. Eng. Technol. 2017, 40, 1101–1106. [Google Scholar] [CrossRef]
- Schimmel, S.; Tomida, D.; Saito, M.; Bao, Q.; Ishiguro, T.; Honda, Y.; Chichibu, S.; Amano, H. Boundary Conditions for Simulations of Fluid Flow and Temperature Field during Ammonothermal Crystal Growth—A Machine-Learning Assisted Study of Autoclave Wall Temperature Distribution. Crystals 2021, 11, 254. [Google Scholar] [CrossRef]
- Chervin, J.C.; Syfosse, G.; Besson, J.M. Mechanical strength of sapphire windows under pressure. Rev. Sci. Instrum. 1994, 65, 2719–2725. [Google Scholar] [CrossRef]
- Ockenfels, T.; Vewinger, F.; Weitz, M. Sapphire optical viewport for high pressure and temperature applications. Rev. Sci. Instrum. 2021, 92, 65109. [Google Scholar] [CrossRef] [PubMed]
- Grunwaldt, J.-D.; Wandeler, R.; Baiker, A. Supercritical Fluids in Catalysis: Opportunities of In Situ Spectroscopic Studies and Monitoring Phase Behavior. Catal. Rev. 2003, 45, 1–96. [Google Scholar] [CrossRef]
- Alt, N.S.; Meissner, E.; Schluecker, E. Development of a novel in situ monitoring technology for ammonothermal reactors. J. Cryst. Growth 2012, 350, 2–4. [Google Scholar] [CrossRef]
- Spieß, L.; Teichert, G.; Schwarzer, R.; Behnken, H.; Genzel, C. (Eds.) Erzeugung und Eigenschaften von Röntgenstrahlung. In Moderne Röntgenbeugung; Vieweg+Teubner: Wiesbaden, Germany, 2009; pp. 5–40. ISBN 978-3-8351-0166-1. [Google Scholar]
- Schimmel, S.; Künecke, U.; Baser, H.; Steigerwald, T.G.; Hertweck, B.; Alt, N.S.A.; Schlücker, E.; Schwieger, W.; Wellmann, P. Towards X-ray in-situ visualization of ammonothermal crystal growth of nitrides. Phys. Status Solidi C 2014, 11, 1439–1442. [Google Scholar] [CrossRef]
- Advances in Imaging and Electron Physics Volume 171; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780123942975.
- Berger, M.J.; Hubbell, J.H.; Seltzer, S.M.; Chang, J.; Coursey, J.S.; Sukumar, R.; Zucker, D.S.; Olsen, K. XCOM: Photon Cross Sections Database, NIST Standard Reference Database 8 (XGAM). Available online: https://physics.nist.gov/PhysRefData/Xcom/html/xcom1.html (accessed on 13 May 2022).
- Richter, T.M.M.; Alt, N.S.A.; Schlücker, E.; Niewa, R. Ammonothermal Synthesis and Characterization of Li4[Zn(NH2)4](NH2)2. Z. Anorg. Allg. Chem. 2015, 641, 1016–1023. [Google Scholar] [CrossRef]
- Richter, T.M.M.; Alt, N.S.A.; Schlücker, E.; Niewa, R. Ammonothermal Synthesis and Characterization of Cs2[Zn(NH2)4]. Z. Anorg. Allg. Chem. 2016, 642, 1207–1211. [Google Scholar] [CrossRef]
- Braeuer, A. In Situ Spectroscopic Techniques at High Pressure; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780444634207. [Google Scholar]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Das, R.S.; Agrawal, Y.K. Raman spectroscopy: Recent advancements, techniques and applications. Vib. Spectrosc. 2011, 57, 163–176. [Google Scholar] [CrossRef]
- Klein, T.; Wu, W.; Rausch, M.H.; Giraudet, C.; Koller, T.M.; Fröba, A.P. Influence of Liquid Structure on Fickian Diffusion in Binary Mixtures of n-Hexane and Carbon Dioxide Probed by Dynamic Light Scattering, Raman Spectroscopy, and Molecular Dynamics Simulations. J. Phys. Chem. B 2018, 122, 7122–7133. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, A.; Schweiger, G. Fluid Phase Equilibria of Binary Mixtures with Supercritical Solvents with in-situ Concentration Measurements by Raman Spectroscopy. In Supercritical Fluids as Solvents and Reaction Media; Elsevier: Amsterdam, The Netherlands, 2004; pp. 85–120. ISBN 9780444515742. [Google Scholar]
- Braeuer, A. Chapter 3—Raman Spectroscopy from an Engineering Point of View. In Supercritical Fluid Science and Technology: Spectroscopic Techniques at High Pressure; Braeuer, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 193–281. ISBN 2212-0505. [Google Scholar]
- Stegemann, D.; Raj, B.; Bhaduri, A. NDT for Analysis of Microstructures and Mechanical Properties of Metallic Materials. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780128035818. [Google Scholar] [CrossRef]
- Schwieger, W.; Baser, H. Ultrasound Measurement as a Tool for In Situ Determination of Filling Degree Under Extreme Conditions. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 105–116. ISBN 978-3-030-56304-2. [Google Scholar]
- Viswanath, D.S.; Ghosh, T.K.; Prasad, D.H.L.; Dutt, N.V.; Rani, K.Y. Viscometers. In Viscosity of Liquids; Springer: Dordrecht, The Netherlands, 2007; pp. 9–107. ISBN 978-1-4020-5481-5. [Google Scholar]
- Gupta, S.V. Oscillating Viscometers. In Viscometry for Liquids; Gupta, S.V., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 107–136. ISBN 978-3-319-04857-4. [Google Scholar]
- Gupta, S.V. New Trends in Viscometers. In Viscometry for Liquids; Gupta, S.V., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 137–170. ISBN 978-3-319-04857-4. [Google Scholar]
- Cohen-Tenoudji, F.; Pardee, W.J.; Tittmann, B.R.; Ahlberg, L.A.; Elsley, R.K. A shear wave rheology sensor. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1987, 34, 263–269. [Google Scholar] [CrossRef]
- Kleis, S.J.; Sanchez, L.A. Dependence of speed of sound on salinity and temperature in concentrated NaCl solutions. Sol. Energy 1990, 45, 201–206. [Google Scholar] [CrossRef]
- Natarajan, S.; Randolph, T.W. Ultrasonic velocity measurements in supercritical jet fuel. J. Supercrit. Fluids 1997, 10, 65–70. [Google Scholar] [CrossRef]
- Sheen, S.H.; Chien, H.-T.; Raptis, A.C. An in-Line Ultrasonic Viscometer. In Review of Progress in Quantitative Nondestructive Evaluation; Thompson, D.O., Chimenti, D.E., Eds.; Springer: New York, NY, USA, 1995; pp. 1151–1158. ISBN 978-1-4613-5819-0. [Google Scholar]
- McSkimin, H.J.; Andreatch, P. Measurement of Dynamic Shear Impedance of Low Viscosity Liquids at Ultrasonic Frequencies. J. Acoust. Soc. Am. 1967, 42, 248–252. [Google Scholar] [CrossRef]
- Balasubramaniam, K.; Shah, V.V.; Costley, R.D.; Boudreaux, G.; Singh, J.P. High temperature ultrasonic sensor for the simultaneous measurement of viscosity and temperature of melts. Rev. Sci. Instrum. 1999, 70, 4618–4623. [Google Scholar] [CrossRef]
- Moore, R.S.; McSkimin, H.J. Dynamic Shear Properties of Solvents and Polystyrene Solutions from 20 to 300 MHz. In Physical Acoustics; Mason, W.P., Thurston, R.N., Eds.; Elsevier: Amsterdam, The Netherlands, 1970; pp. 167–242. ISBN 9780123956668. [Google Scholar] [CrossRef]
- Carmichael, L.T.; Sage, B.H. Viscosity of Liquid Ammonia at High Pressures. Ind. Eng. Chem. 1952, 44, 2728–2732. [Google Scholar] [CrossRef]
- Dobson, D.P.; Jones, A.P.; Rabe, R.; Sekine, T.; Kurita, K.; Taniguchi, T.; Kondo, T.; Kato, T.; Shimomura, O.; Urakawa, S. In-situ measurement of viscosity and density of carbonate melts at high pressure. Earth Planet. Sci. Lett. 1996, 143, 207–215. [Google Scholar] [CrossRef]
- Suzuki, A.; Ohtani, E.; Funakoshi, K.; Terasaki, H.; Kubo, T. Viscosity of albite melt at high pressure and high temperature. Phys. Chem. Miner. 2002, 29, 159–165. [Google Scholar] [CrossRef]
- Funakoshi, K.; Nozawa, A. Development of a method for measuring the density of liquid sulfur at high pressures using the falling-sphere technique. Rev. Sci. Instrum. 2012, 83, 103908. [Google Scholar] [CrossRef]
- Steigerwald, T.G.; Alt, N.S.; Hertweck, B.; Schluecker, E. Feasibility of density and viscosity measurements under ammonothermal conditions. J. Cryst. Growth 2014, 403, 59–65. [Google Scholar] [CrossRef]
- Schlücker, E.; Hertweck, B.; Schimmel, S.; Wellmann, P. Special Equipment for Ammonothermal Processes. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 317–328. ISBN 978-3-030-56304-2. [Google Scholar]
- Richter, T.; Strobel, S.; Alt, N.; Schlücker, E.; Niewa, R. Ammonothermal Synthesis and Crystal Structures of Diamminetriamidodizinc Chloride [Zn2(NH3)2(NH2)3]Cl and Diamminemonoamidozinc Bromide [Zn(NH3)2(NH2)]Br. Inorganics 2016, 4, 41. [Google Scholar] [CrossRef]
- Hashimoto, T.; Saito, M.; Fujito, K.; Wu, F.; Speck, J.S.; Nakamura, S. Seeded growth of GaN by the basic ammonothermal method. J. Cryst. Growth 2007, 305, 311–316. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Kagamitani, Y.; Yokoyama, C.; Fukuda, T. Physico-chemical features of the acid ammonothermal growth of GaN. J. Cryst. Growth 2008, 310, 891–895. [Google Scholar] [CrossRef]
- Jouet, R.J.; Purdy, A.P.; Wells, R.L.; Janik, J.F. Preparation of Phase Pure Cubic Gallium Nitride, c-GaN, by Ammonothermal Conversion of Gallium Imide, {Ga(NH)3/2} n. J. Clust. Sci. 2002, 13, 469–486. [Google Scholar] [CrossRef]
- Bao, Q.; Sawayama, H.; Hashimoto, T.; Sato, F.; Hazu, K.; Kagamitani, Y.; Ishinabe, T.; Saito, M.; Kayano, R.; Tomida, D.; et al. Powder synthesis and ammonothermal crystal growth of GaN from metallic Ga in the presence of NH4I. CrystEngComm 2012, 14, 3351. [Google Scholar] [CrossRef]
- Harbrecht, B.; Jacobs, H. Hochdrucksynthese von Caesiumamidazid, Cs2(NH2)N3 aus Caesiummetall und Ammoniak. Z. Anorg. Allg. Chem. 1983, 500, 181–187. [Google Scholar] [CrossRef]
- Hertrampf, J.; Alt, N.S.A.; Schlücker, E.; Niewa, R. Three Solid Modifications of Ba[Ga(NH2)4]2: A Soluble Intermediate in Ammonothermal GaN Crystal Growth. Eur. J. Inorg. Chem. 2017, 2017, 902–909. [Google Scholar] [CrossRef]
- Hashimoto, T.; Wu, F.; Saito, M.; Fujito, K.; Speck, J.S.; Nakamura, S. Status and perspectives of the ammonothermal growth of GaN substrates. J. Cryst. Growth 2008, 310, 876–880. [Google Scholar] [CrossRef]
- Ketchum, D.R.; Kolis, J.W. Crystal growth of gallium nitride in supercritical ammonia. J. Cryst. Growth 2001, 222, 431–434. [Google Scholar] [CrossRef]
- Schwieger, W.; Baser, H. Determination of Solubility of GaN in Ammonobasic Systems. In Ammonothermal Synthesis and Crystal Growth of Nitrides; Meissner, E., Niewa, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 155–170. ISBN 978-3-030-56304-2. [Google Scholar]
- Bäucker, C.; Niewa, R. A New Modification of Rb[Al(NH2)4] and Condensation in Solid State. Crystals 2020, 10, 1018. [Google Scholar] [CrossRef]
- Jacobs, H.; Stüve, C. Hochdrucksynthese der η-phase im system Mn-N:Mn3N2. J. Less Common Met. 1984, 96, 323–329. [Google Scholar] [CrossRef]
- Peters, D.; Jacobs, H. Ammonothermalsynthese von kristallinem siliciumnitridimid, Si2N2NH. J. Less Common Met. 1989, 146, 241–249. [Google Scholar] [CrossRef]
- Pust, P.; Schmiechen, S.; Hintze, F.; Schnick, W. Ammonothermal Synthesis and Crystal Structure of BaAl2(NH2)8·2NH3. Z. Anorg. Allg. Chem. 2013, 639, 1185–1187. [Google Scholar] [CrossRef]
- Hadenfeldt, C.; Jacobs, H. Darstellung, Eigenschaften und Kristallstruktur von Na3[Yb(NH2)6]. Z. Anorg. Allg. Chem. 1972, 393, 111–125. [Google Scholar] [CrossRef]
- Jacobs, H.; Hadenfeldt, C. Die Kristallstruktur von Bariumamid, Ba(NH2)2. Z. Anorg. Allg. Chem. 1975, 418, 132–140. [Google Scholar] [CrossRef]
- Senker, J.; Jacobs, H.; Müller, M.; Press, W.; Müller, P.; Mayer, H.M.; Ibberson, R.M. Reorientational Dynamics of Amide Ions in Isotypic Phases of Strontium and Calcium Amide. 1. Neutron Diffraction Experiments. J. Phys. Chem. B 1998, 102, 931–940. [Google Scholar] [CrossRef]
- Jacobs, H.; Kablitz, D. Untersuchung des Systems Kalium/Cer/Ammoniak. Z. Anorg. Allg. Chem. 1979, 454, 35–42. [Google Scholar] [CrossRef]
- Jacobs, H.; Schmidt, D. Struktur und eigenschaften von perowskitartigen cäsiumamidometallaten des cers, neodyms und samariums Cs3Ln2(NH2)9. J. Less Common Met. 1980, 76, 227–244. [Google Scholar] [CrossRef]
- Jacobs, H.; SCHMIDT, D.; Schmitz, D.; Fleischhauer, J.; Schleker, W. Struktur und eigenschaften der cäsiumamidolanthanatmonoammoniakate Cs3La(NH2)6·NH3 und Cs4La(NH2)7·NH3. J. Less Common Met. 1981, 81, 121–133. [Google Scholar] [CrossRef]
- Jacobs, H.; Zachwieja, U. Kupferpalladiumnitride, Cu3PdxN mit x = 0,020 und 0,989, Perowskite mit “bindender 3d10-4d10-Wechselwirkung”. J. Less Common Met. 1991, 170, 185–190. [Google Scholar] [CrossRef]
- Jacobs, H.; Bock, J.; Stüve, C. Röntgenographische strukturbestimmung und IR-spektroskopische Untersuchungen an Hexaammindiiodiden, [M(NH3)6]I2, von Eisen und Mangan. J. Less Common Met. 1987, 134, 207–214. [Google Scholar] [CrossRef]
- Jacobs, H.; Bock, J. Einkristallzüchtung von γ′-Fe4N in überkritischem ammoniak. J. Less Common Met. 1987, 134, 215–220. [Google Scholar] [CrossRef]
- Jacobs, H.; Nöcker, B. Neubestimmung von Struktur und Eigenschaften isotyper Natriumtetraamidometallate des Aluminiums und Galliums. Z. Anorg. Allg. Chem. 1993, 619, 381–386. [Google Scholar] [CrossRef]
- Becker, P.; Cekovski, T.B.; Niewa, R. Indium Ammoniates from Ammonothermal Synthesis: InAlF6(NH3)2, [In(NH3)6][AlF6], and [In2F(NH3)10]2[SiF6]5∙2NH3. Crystals 2021, 11, 679. [Google Scholar] [CrossRef]
- Hadenfeldt, C.; Gieger, B.; Jacobs, H. Darstellung und Kristallstruktur von KLa2(NH2)7. Z. Anorg. Allg. Chem. 1974, 408, 27–36. [Google Scholar] [CrossRef]
- Jacobs, V.H.; Scholze, H. Untersuchung des Systems Na/La/NH3. Z. Anorg. Allg. Chem. 1976, 427, 8–16. [Google Scholar] [CrossRef]
- Jacobs, H.; Stüve, C. Rubidiumhexaamidolanthanat und -neodymat, Rb3[La(NH2)6] und Rb3[Nd(NH2)6]; Strukturverwandtschaft zu K3[Cr(OH6)] und K4CdCl6. Z. Anorg. Allg. Chem. 1987, 546, 42–47. [Google Scholar] [CrossRef]
- Jacobs, H.; Schmidt, D. Über ein cäsiumheptaamidodilanthanat CsLa2(NH2)7. J. Less Common Met. 1981, 78, 51–59. [Google Scholar] [CrossRef]
- Kraus, F.; Korber, N. K2Li(NH2)3 and K2Na(NH2)3—Synthesis and crystal structure of two crystal-chemically isotypic mixed-cationic amides. J. Solid State Chem. 2005, 178, 1241–1246. [Google Scholar] [CrossRef]
- Jakiela, R.; Sierakowski, K.; Sochacki, T.; Iwinska, M.; Fijalkowski, M.; Barcz, A.; Bockowski, M. Investigation of diffusion mechanism of beryllium in GaN. Phys. B Condens. Matter 2020, 594, 412316. [Google Scholar] [CrossRef]
- Schipper, F.; Kubo, S.; Fellinger, T.-P. Nitrogen-doped porous carbon via ammonothermal carbonization for supercapacitors. J. Sol-Gel Sci. Technol. 2019, 89, 101–110. [Google Scholar] [CrossRef]
- Blunck, H.; Juza, R. über Verbindungen des Thoriums mit Stickstoff und Wasserstoff. Z. Anorg. Allg. Chem. 1974, 410, 9–20. [Google Scholar] [CrossRef]
- Jacobs, H.; Peters, D. Übergang von dichter anionenpackung zu perowskitartiger struktur bei kalium- und rubidiumamidoyttriat, KY(NH2)4 und RbY(NH2)4. J. Less Common Met. 1986, 119, 99–113. [Google Scholar] [CrossRef]
- Blunck, H.; Juza, R. Über Ein Nitridamid des Zirkoniums. Z. Anorg. Allg. Chem. 1974, 406, 145–152. [Google Scholar] [CrossRef]
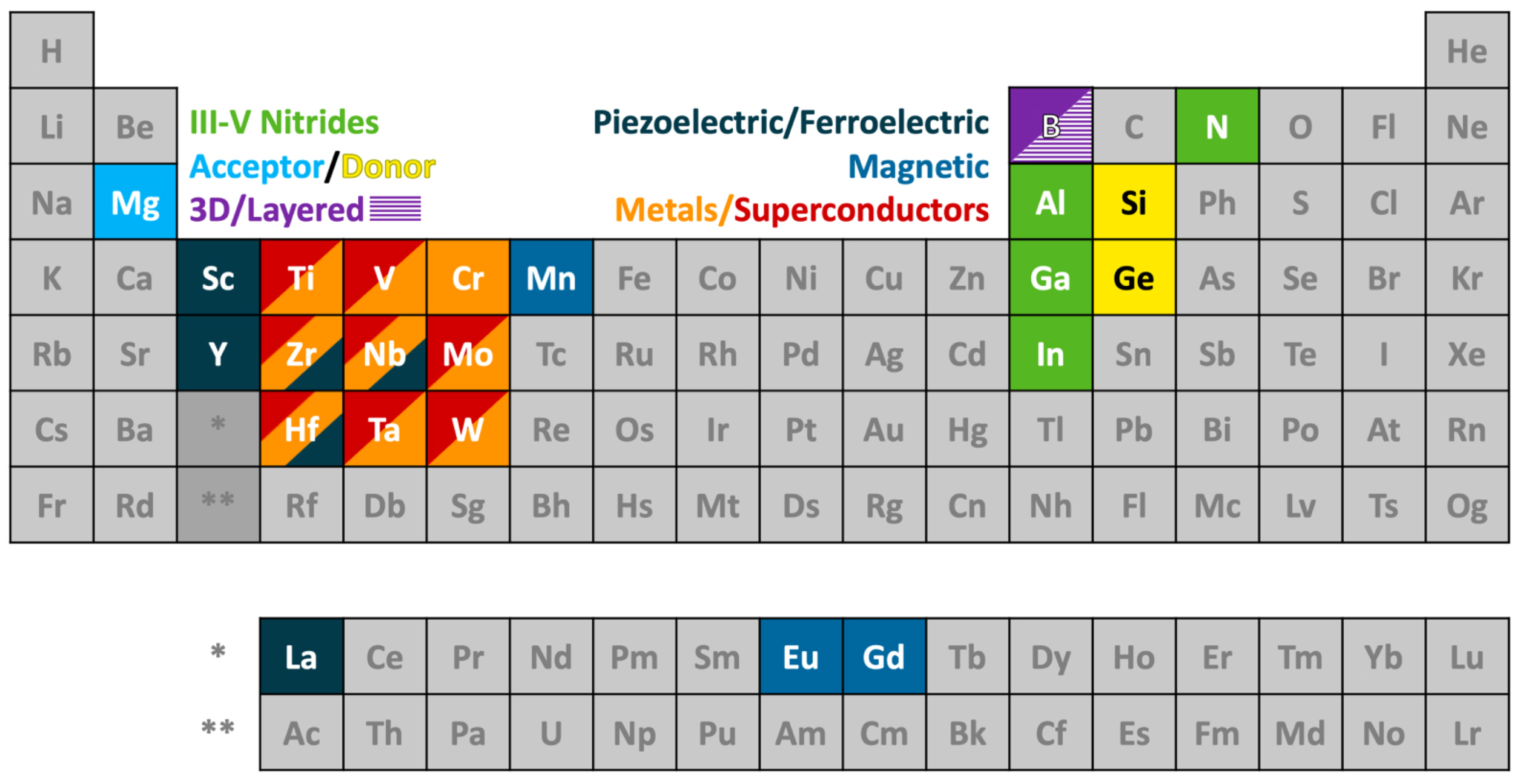

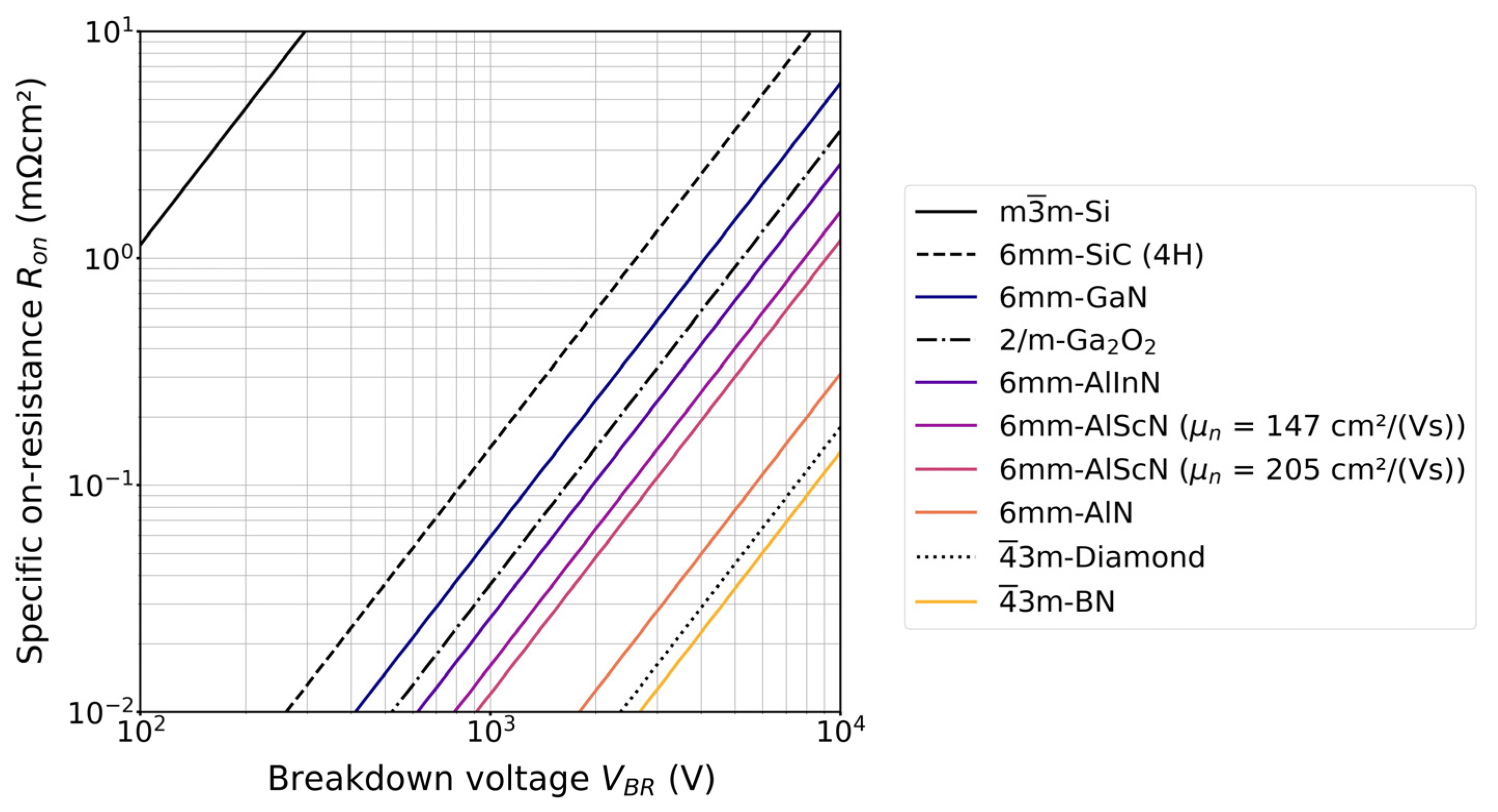
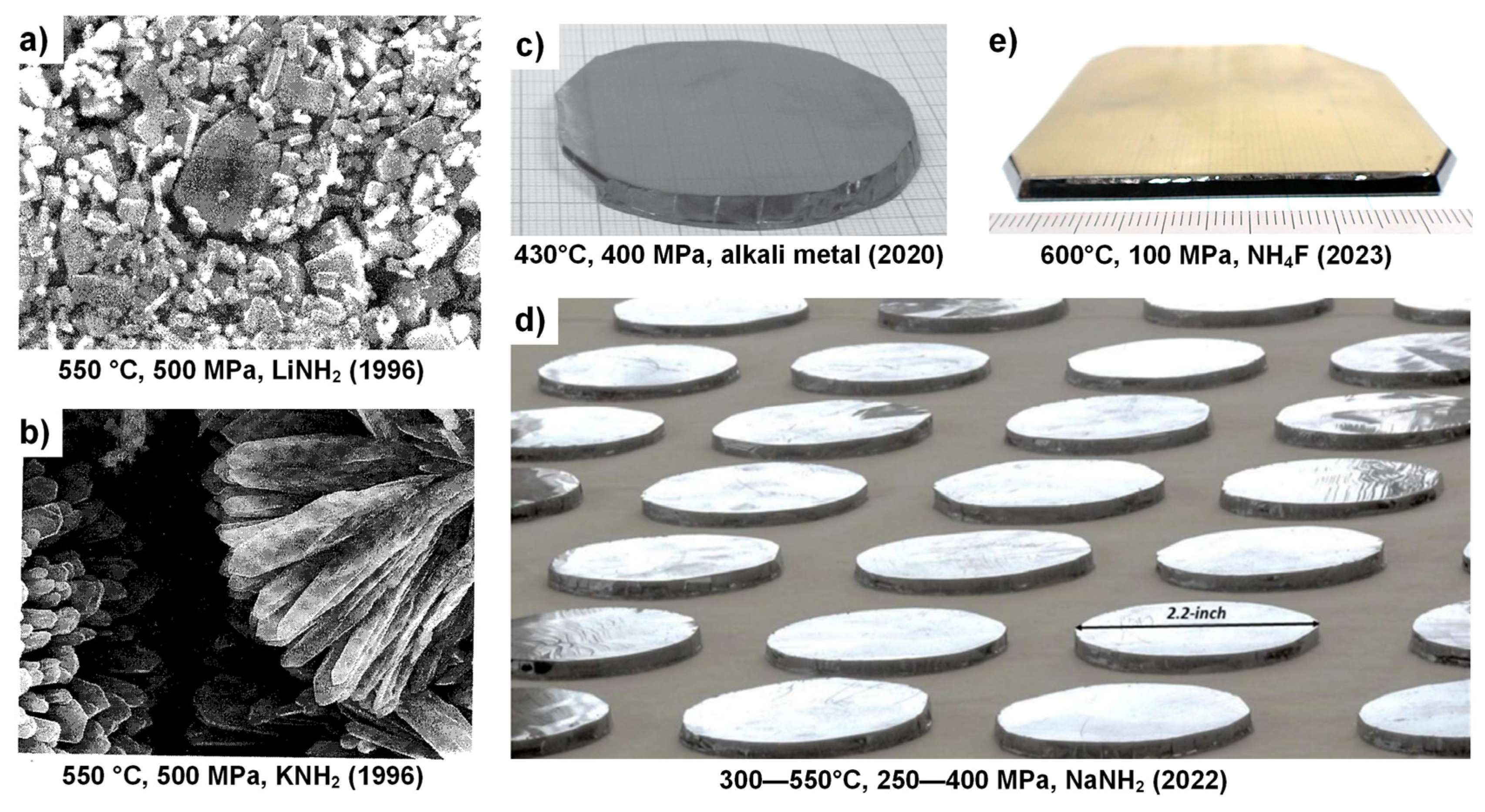
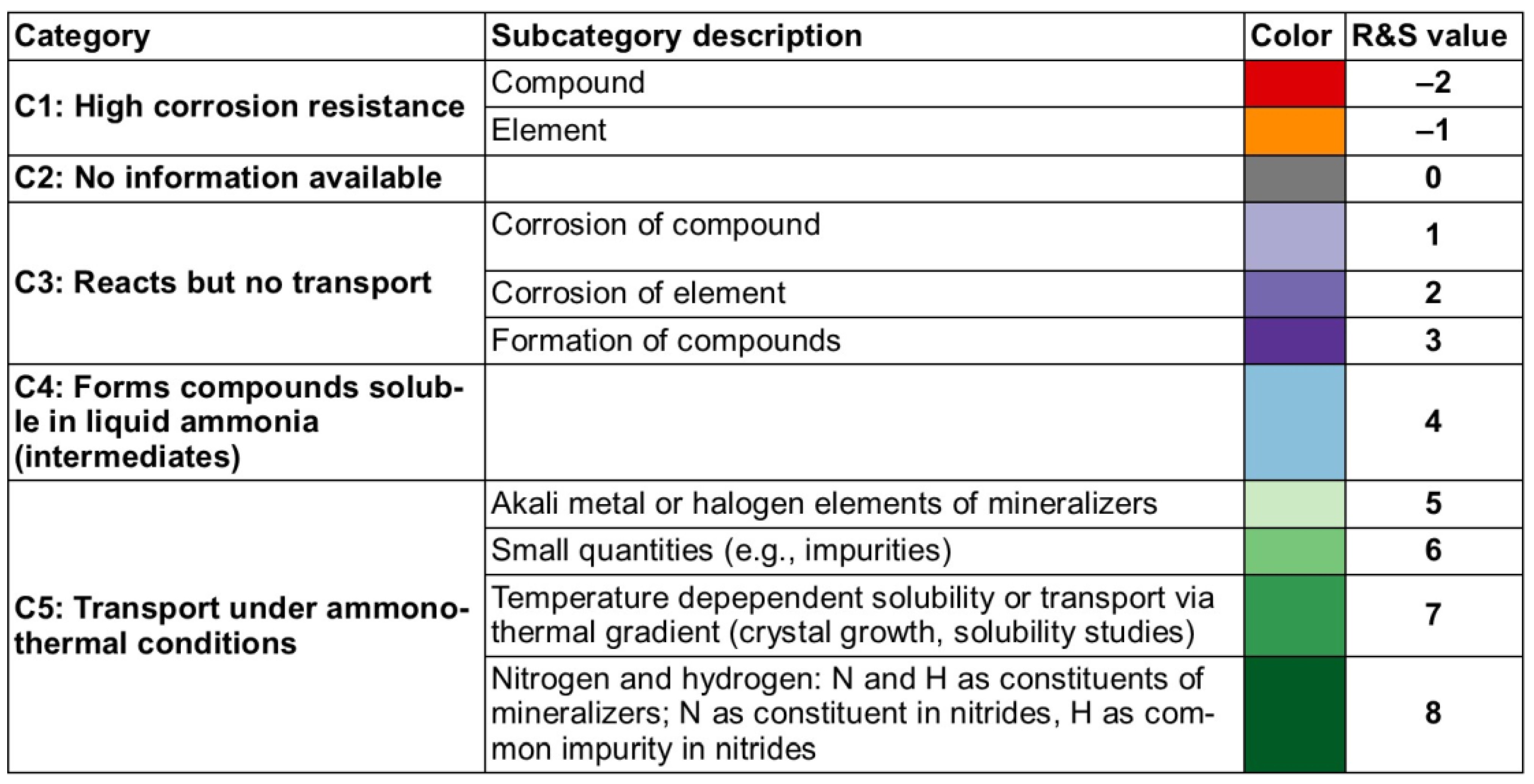
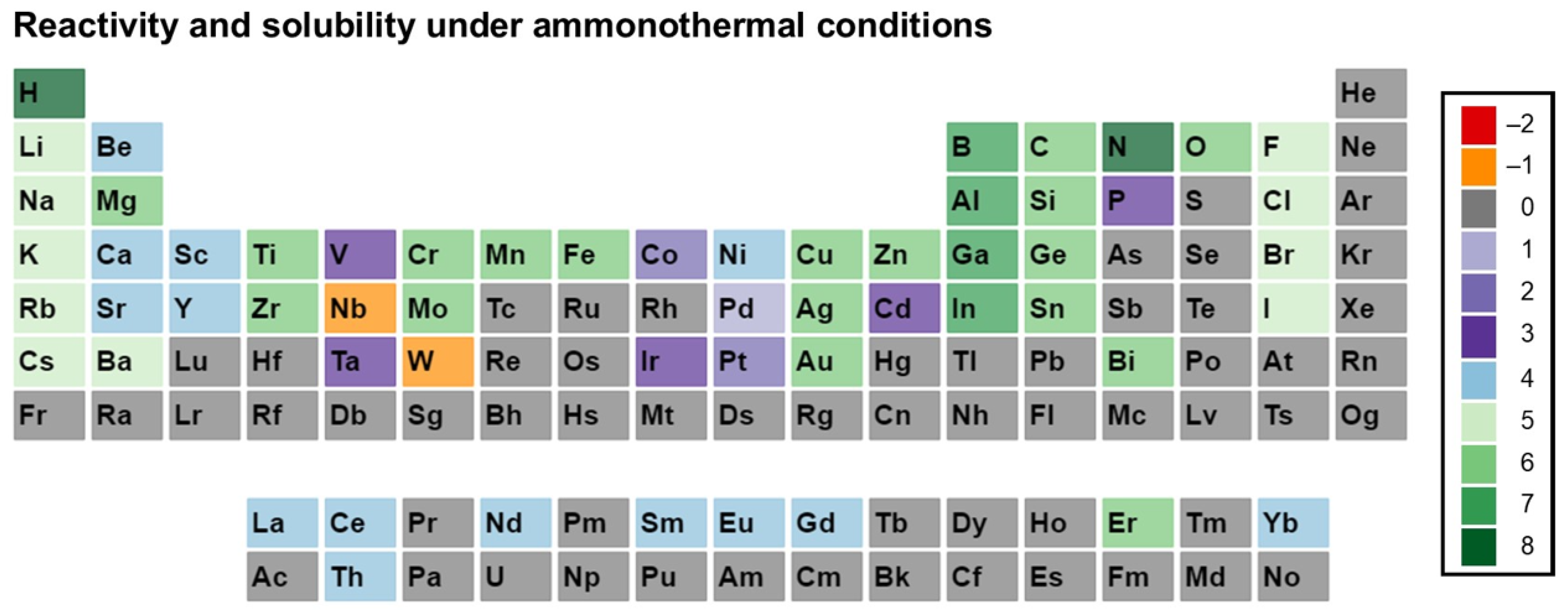
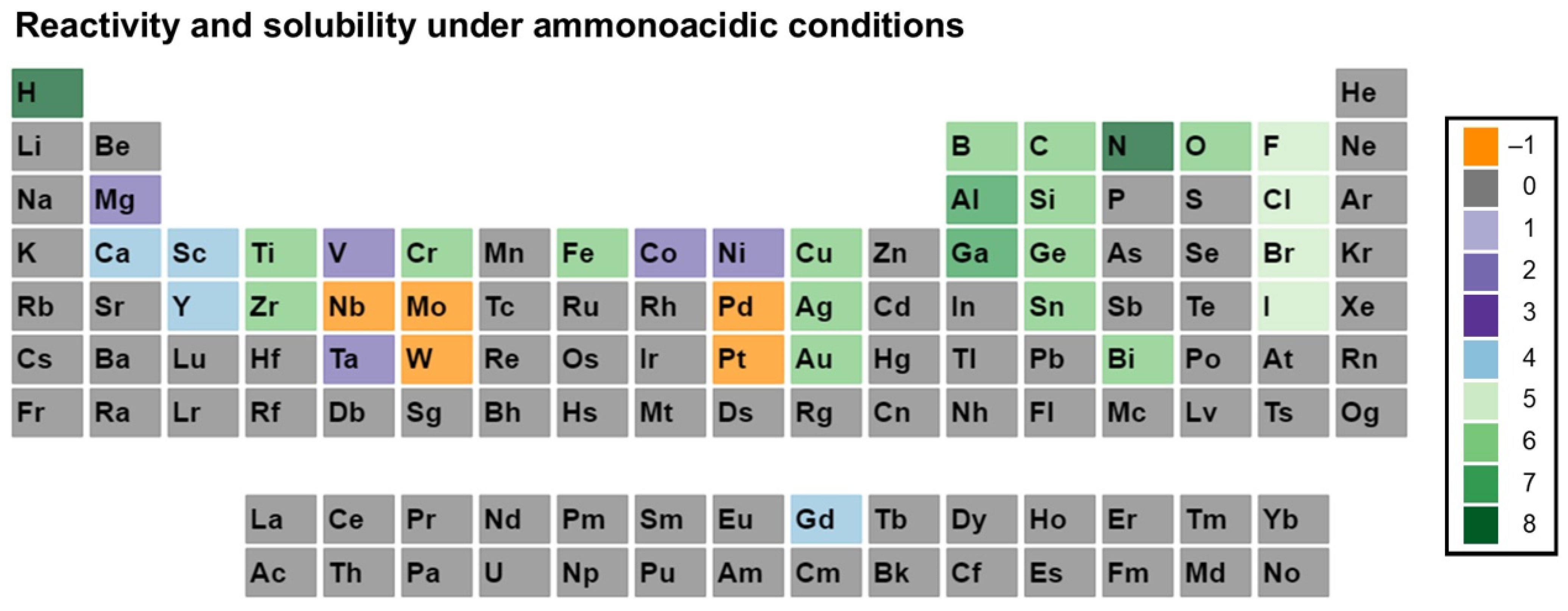
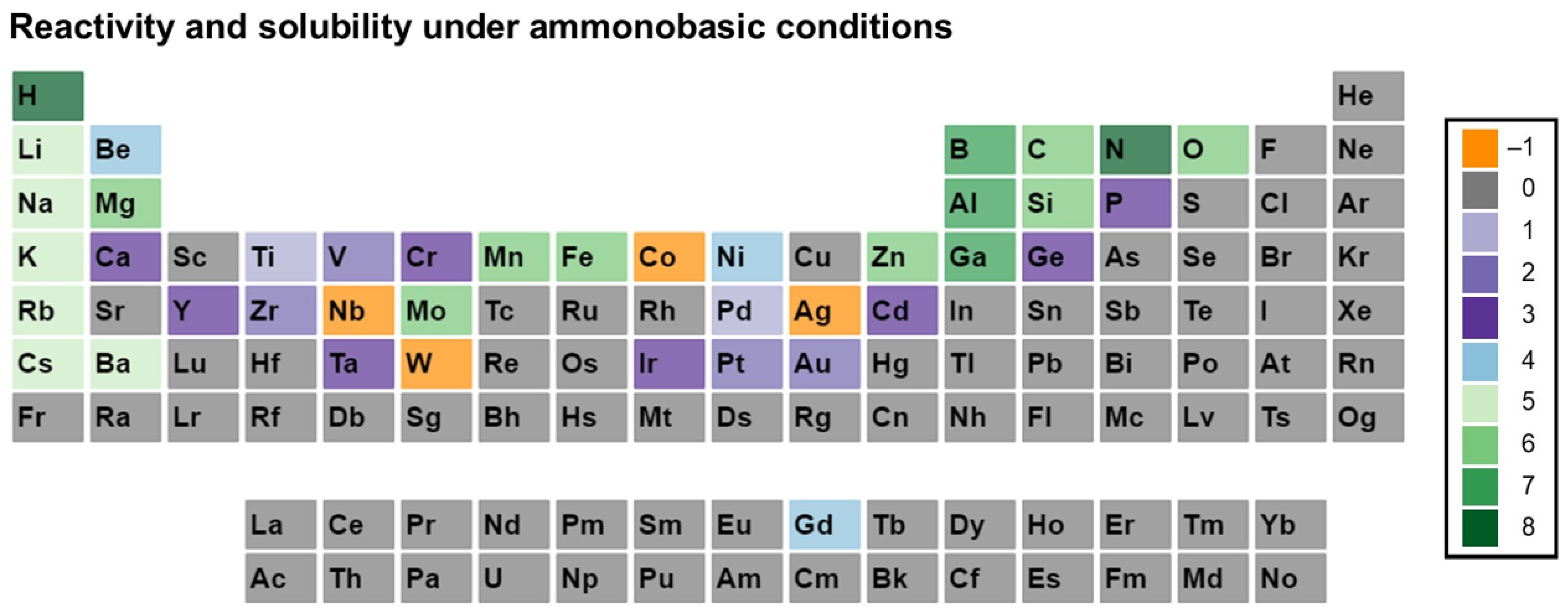
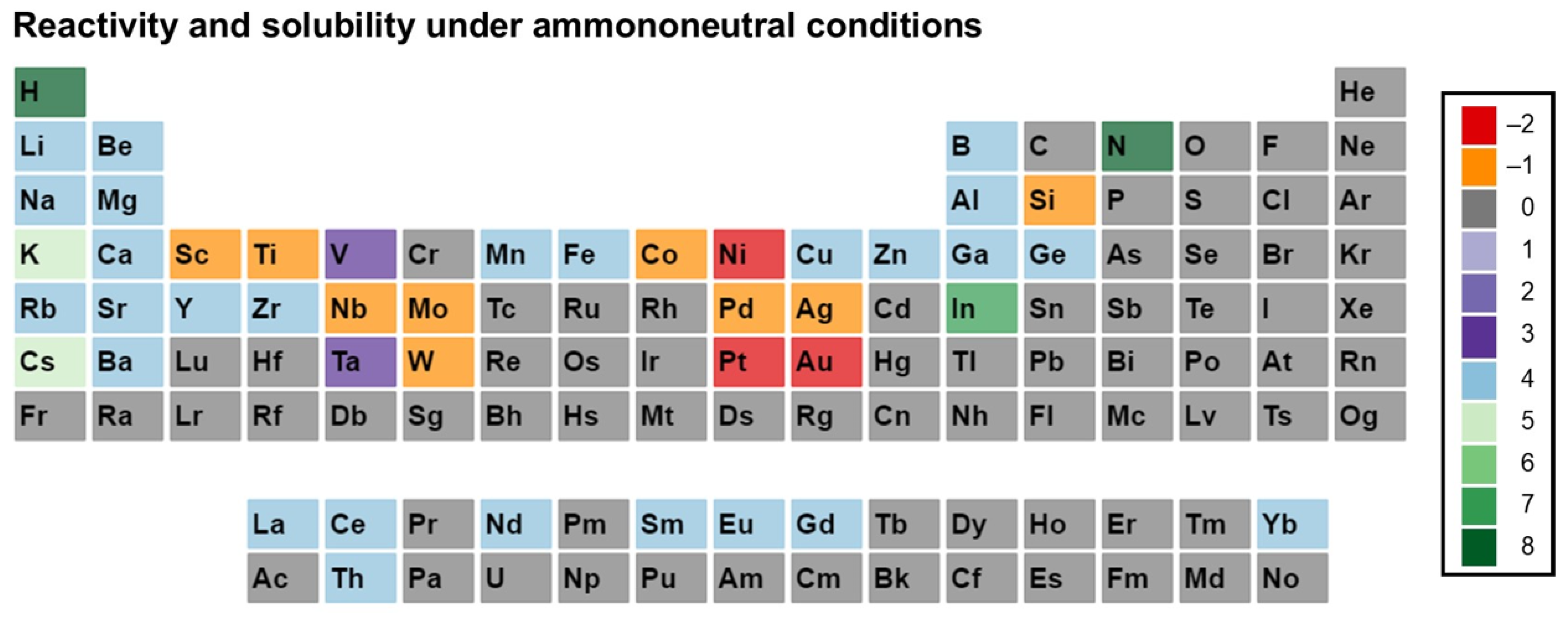

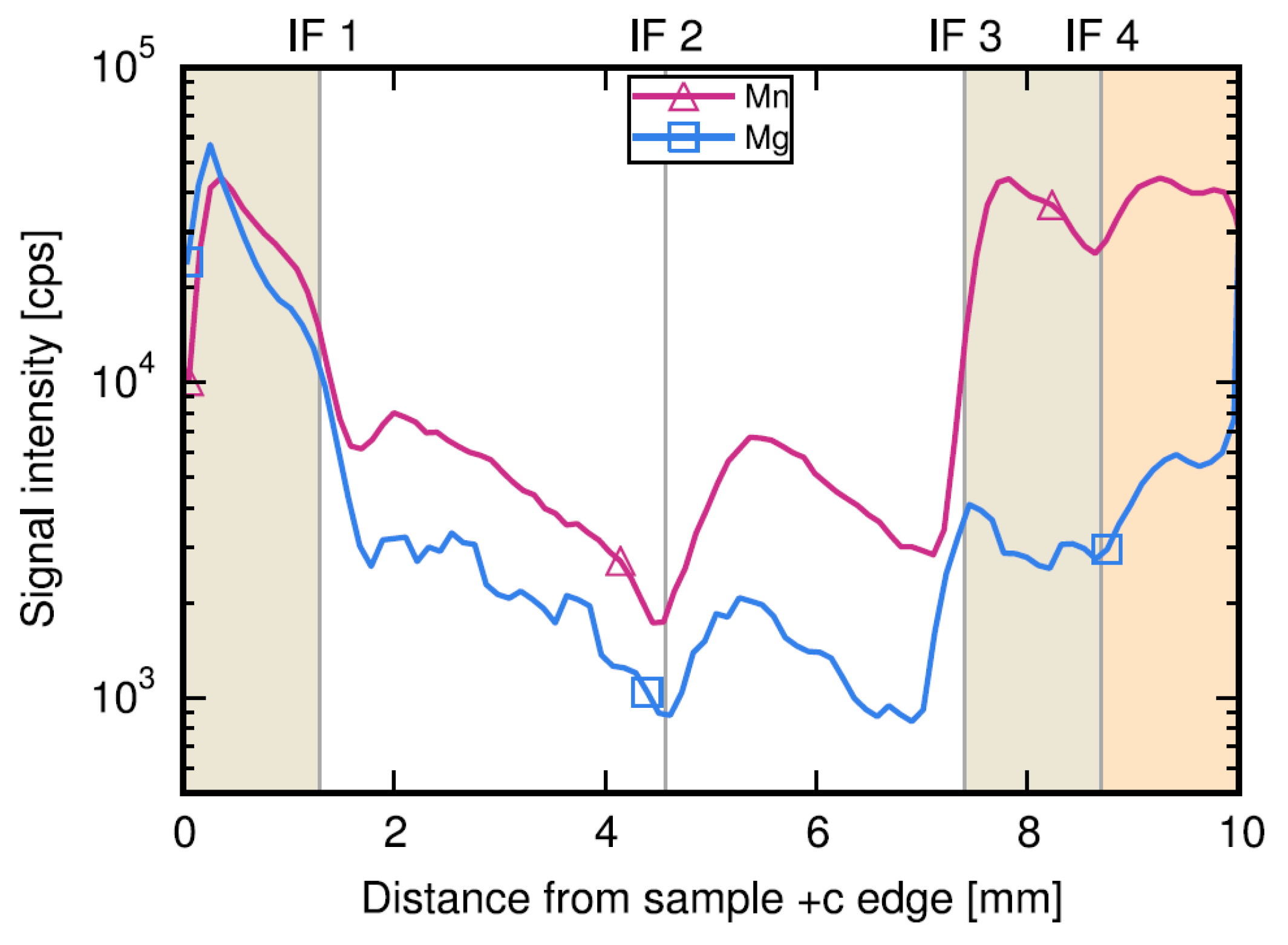


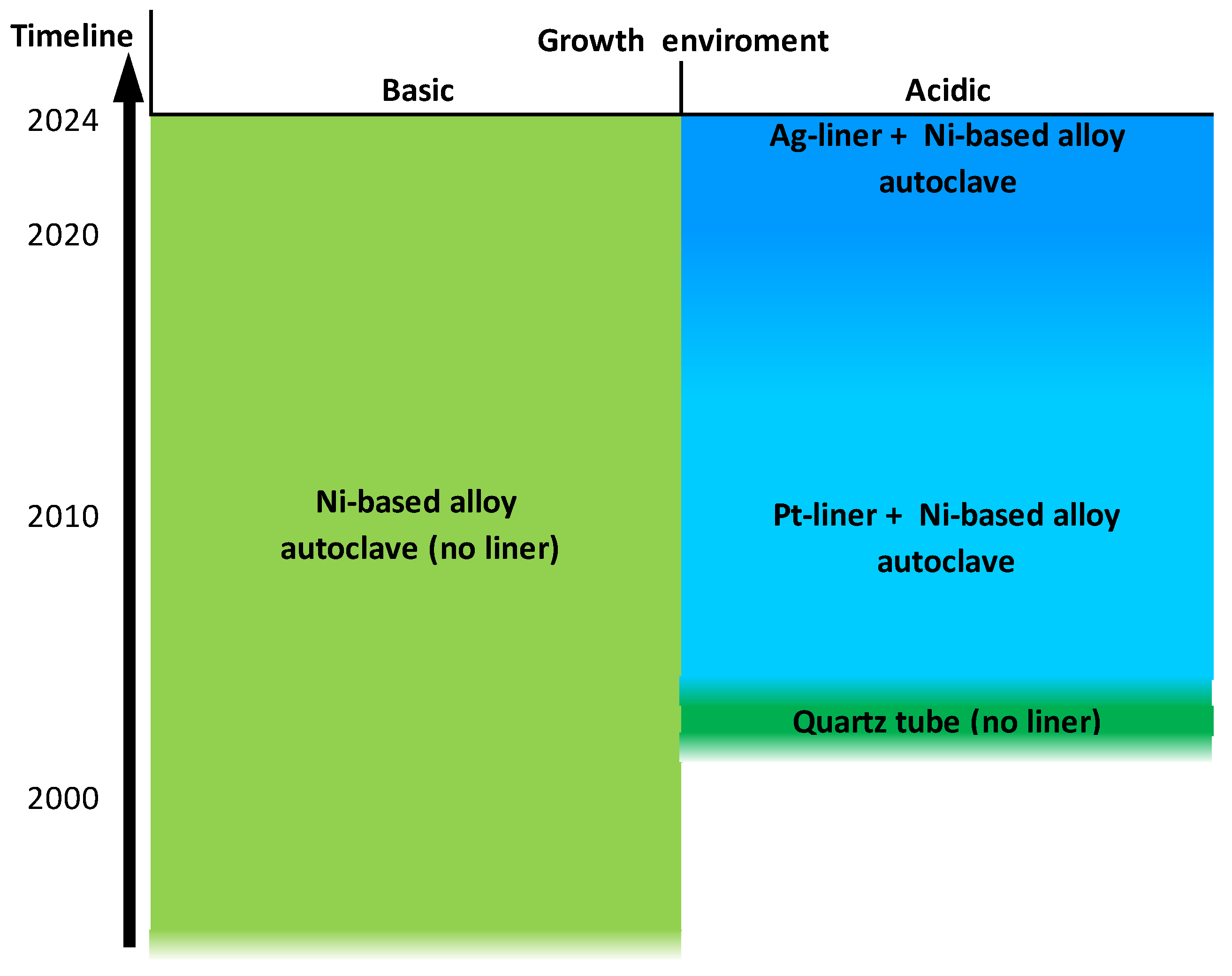
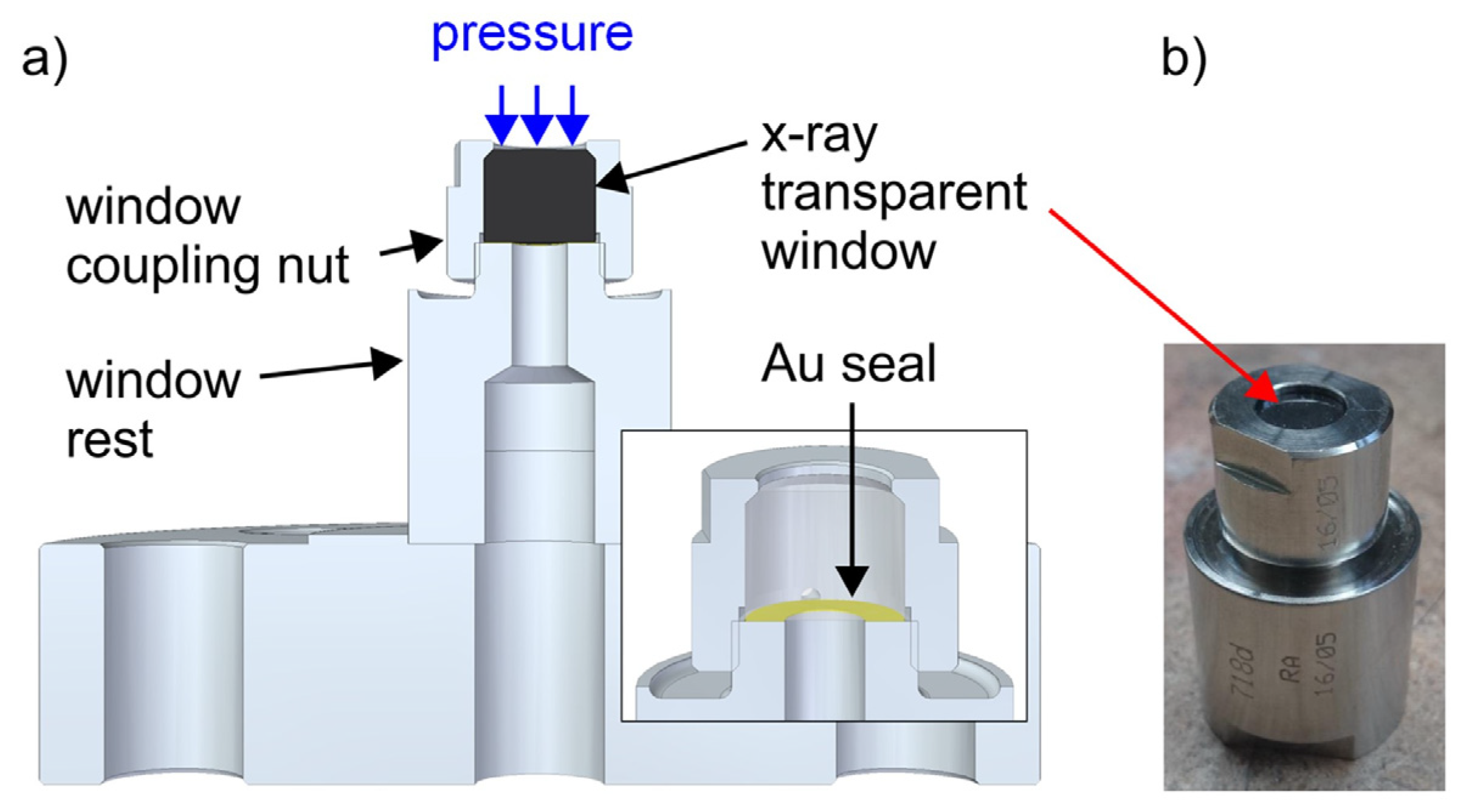
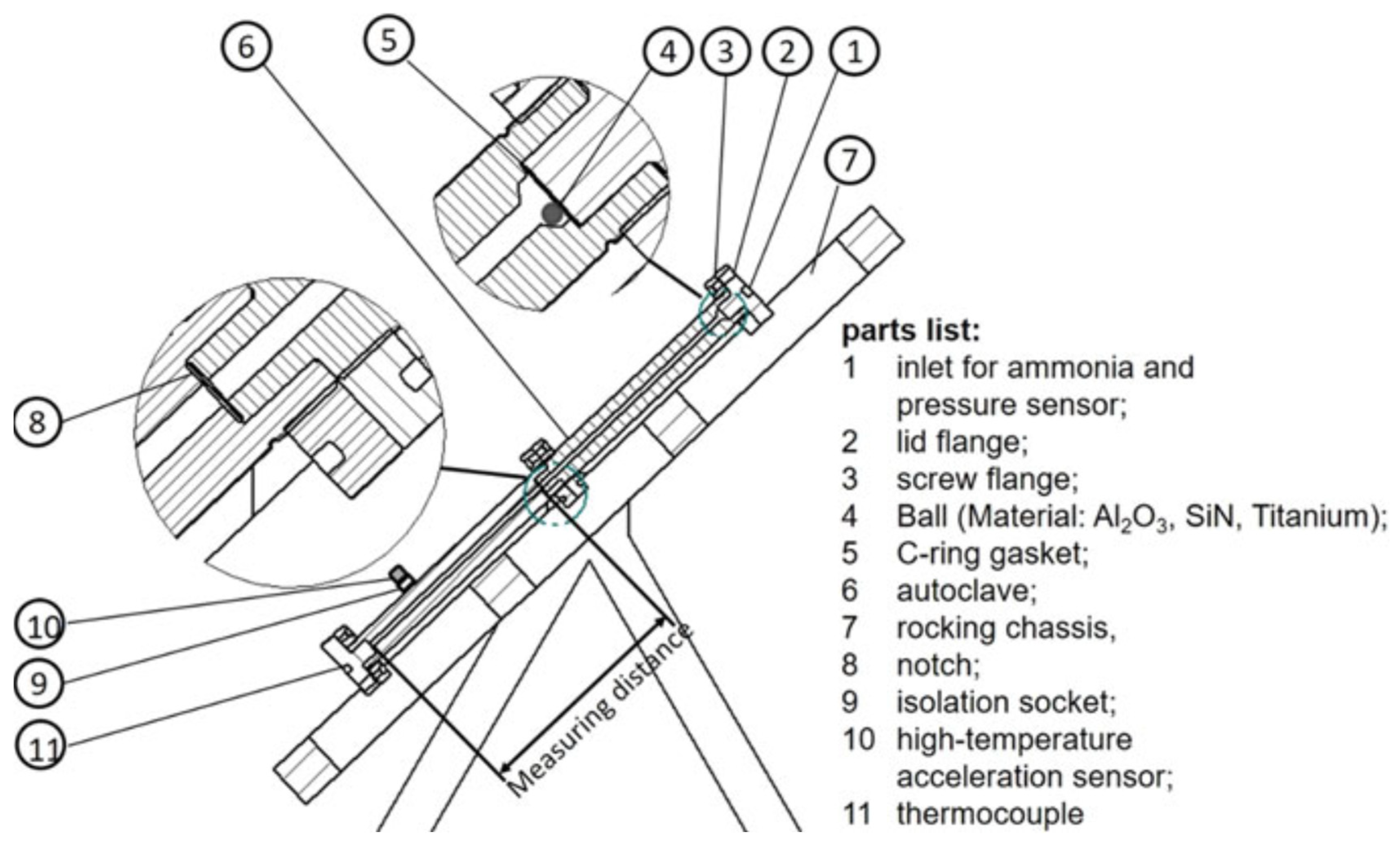

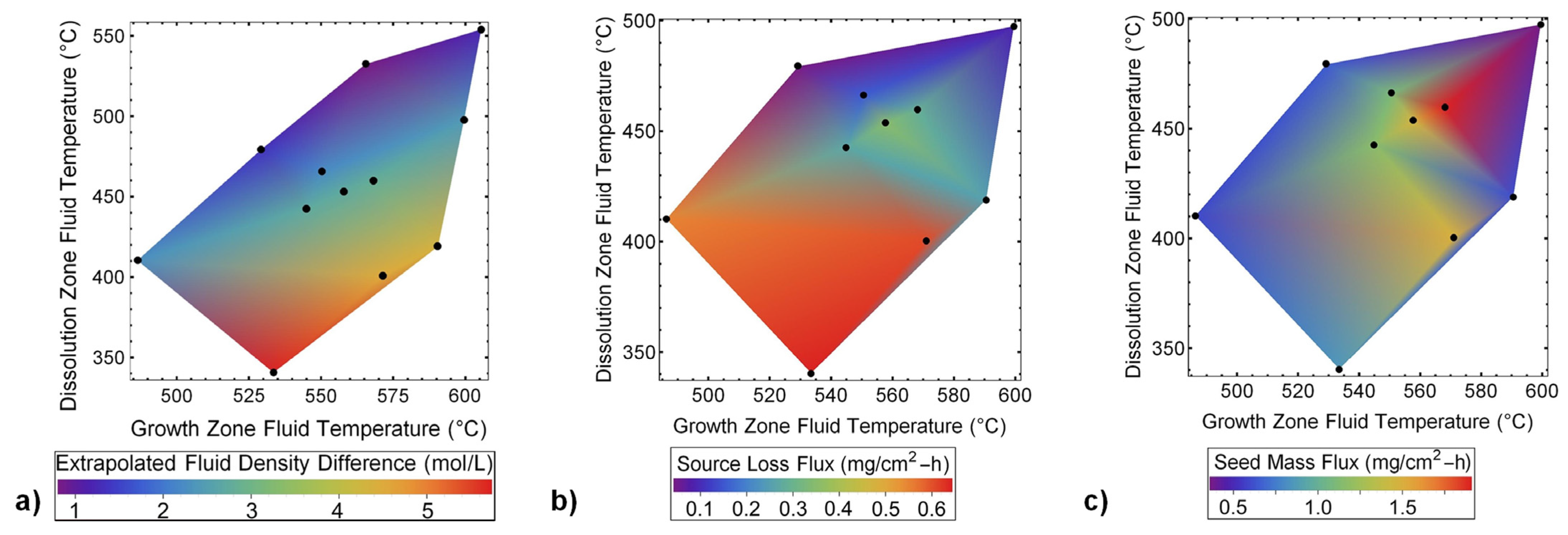
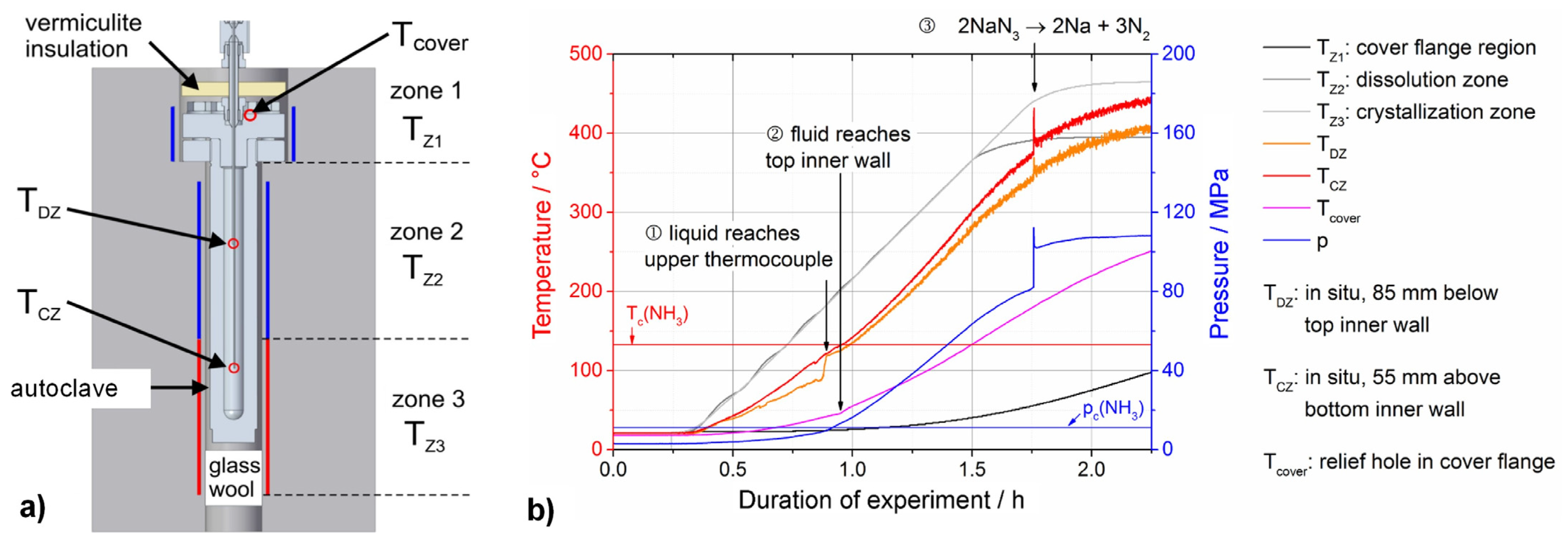
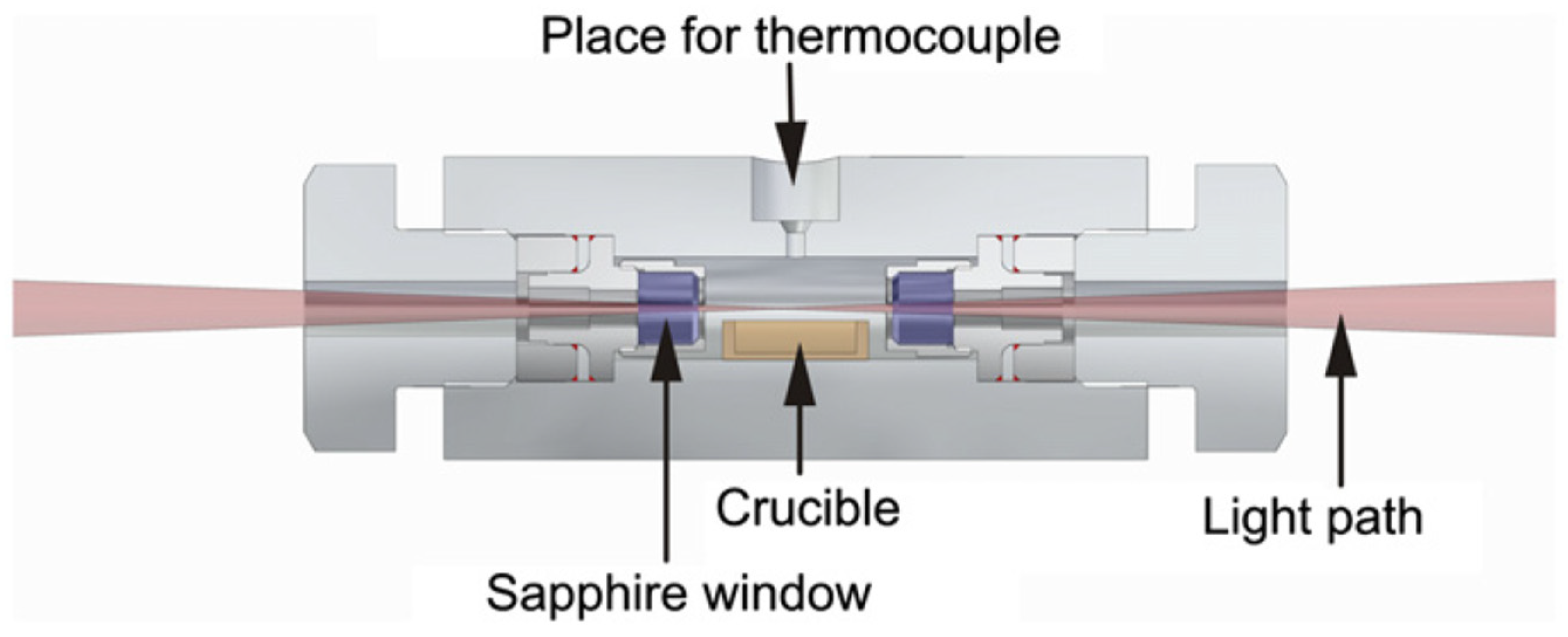
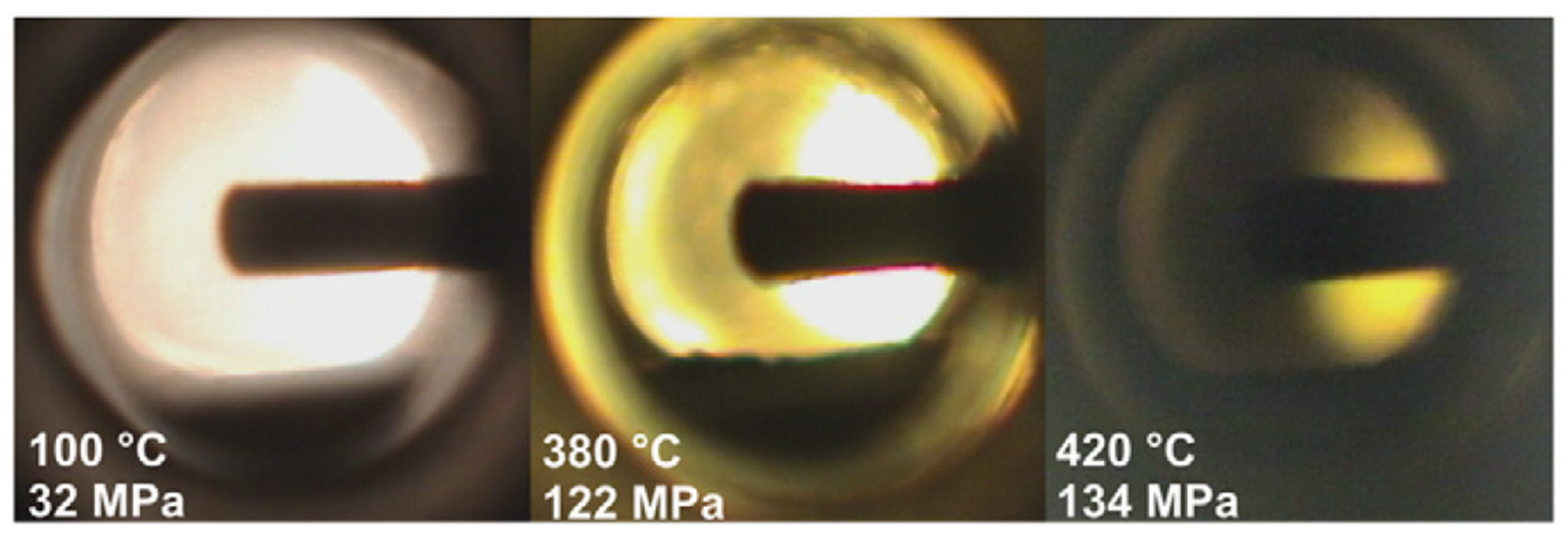

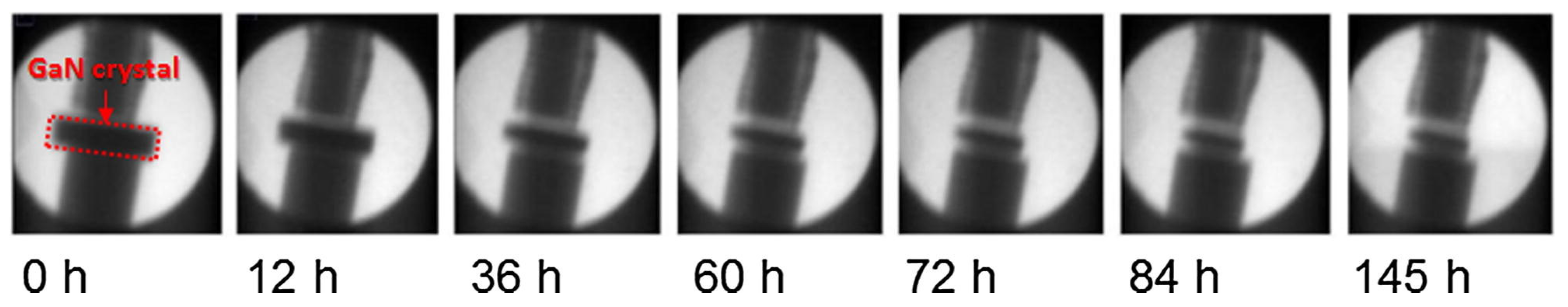
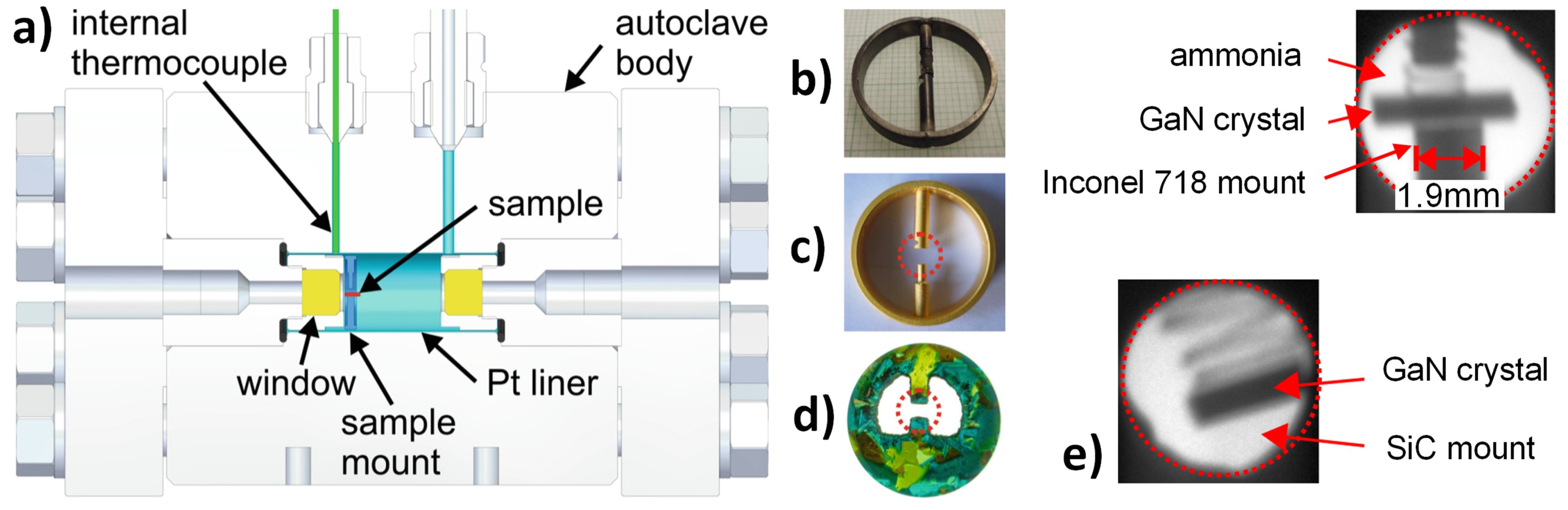
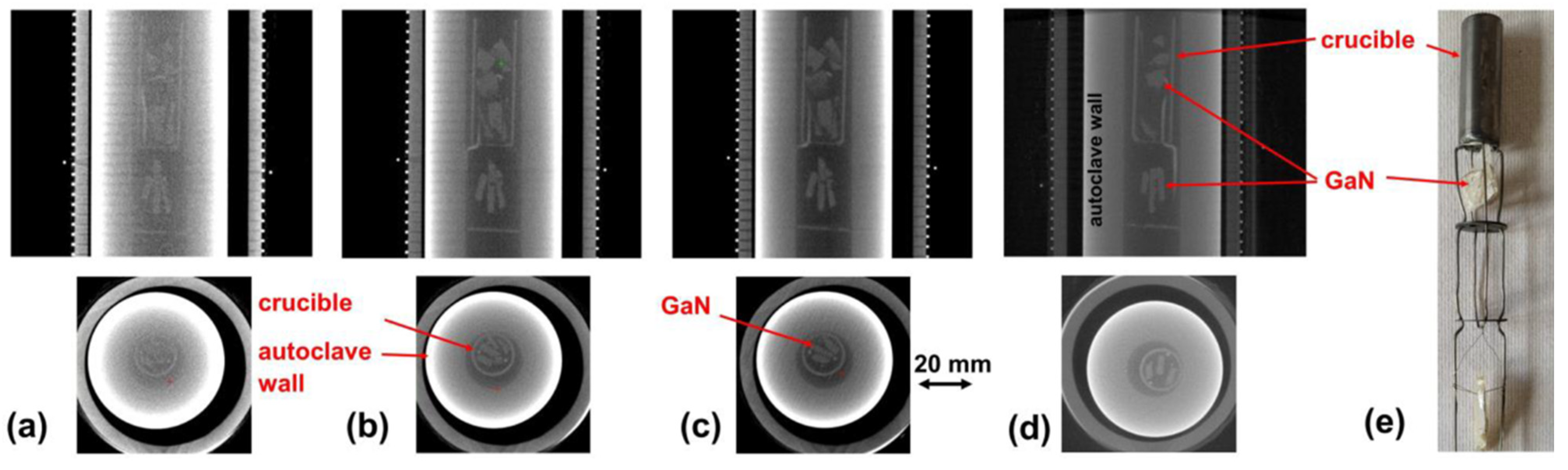

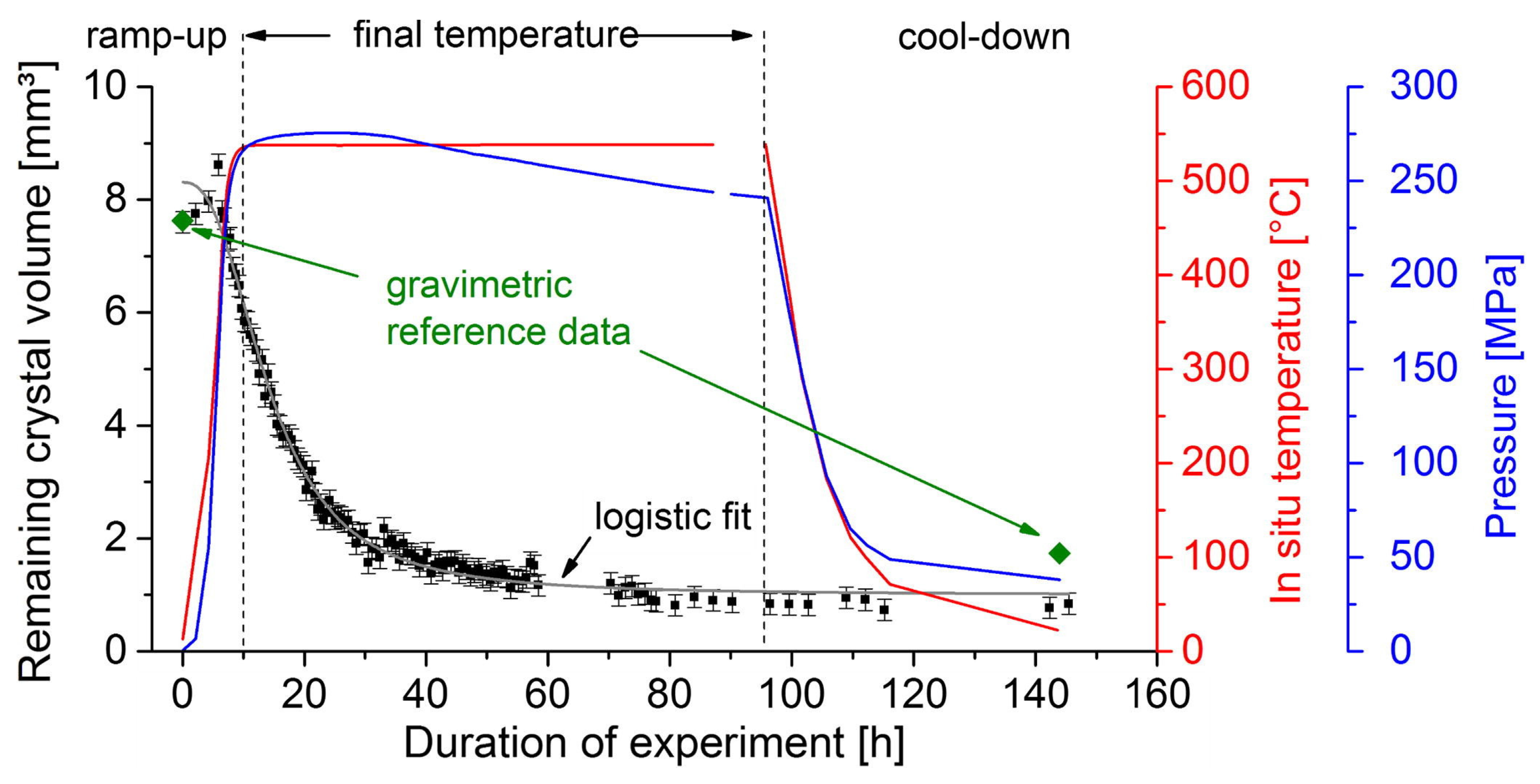

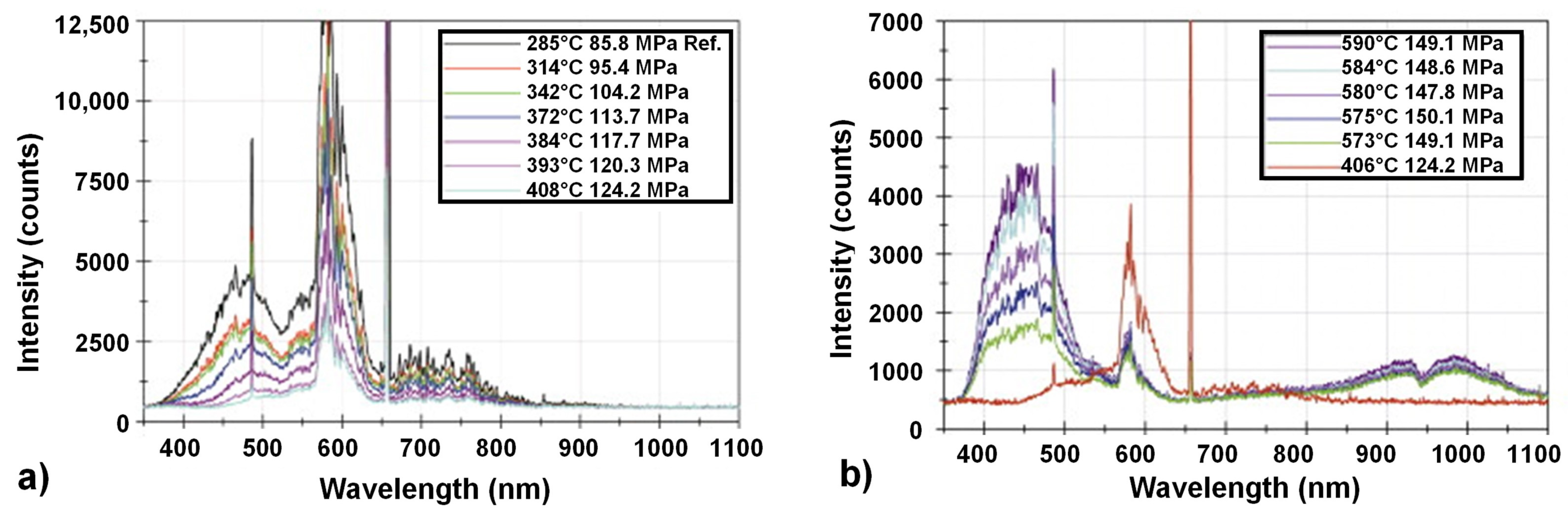

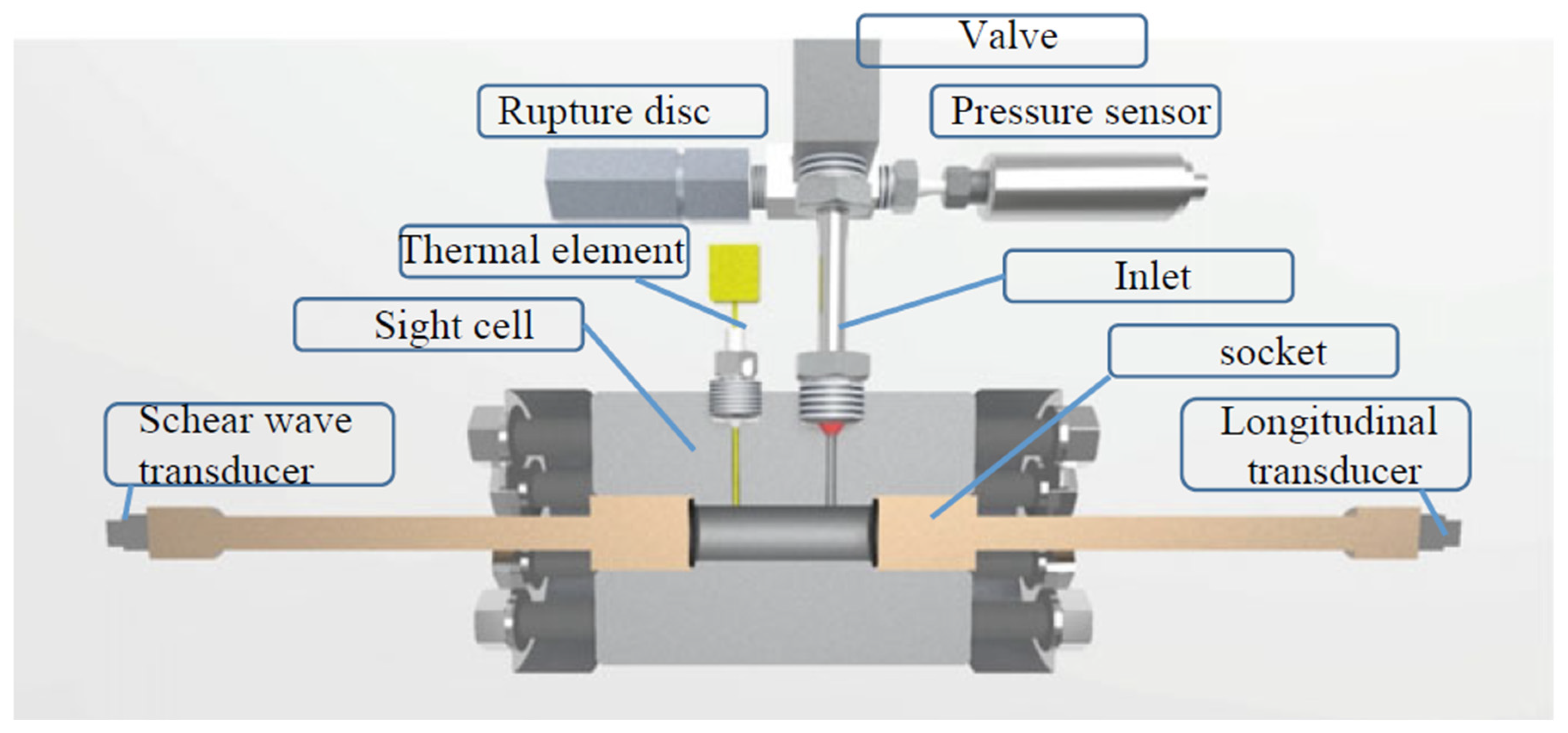
| Fluid Property/Process Characteristic | Hydrothermal (Quartz) | Ammonothermal (GaN) |
|---|---|---|
| Solvent (critical parameters) [132] | Water (374.5 °C, 22.1 MPa) | Ammonia (132.1 °C, 11.3 MPa) |
| Temperature range (°C) | 345–360 [122] | 400–800 [86] |
| Reduced temperature range T/Tc | 0.92–0.96 | 3.03–6.06 |
| Pressure range (MPa) | 70–150 [122] | 100–600 [86] |
| Reduced pressure range p/pc | 3.17–6.79 | 8.85–53.10 |
| Solvent density (mol/L) in p-, T-range | 40.7–44.2 | 18.2–36.2 |
| Solvent density (kg/m3) in p-, T-range | 733.8–795.8 | 310.2–616.6 |
| Dynamic viscosity (µPa·s) | 90.3–103.2 | 49.6–284.3 |
| Kinematic viscosity (m2/s) | 1.230 × 10−7–1.296 × 10−7 | 1.597 × 10−7–6.228 × 10−7 |
| Year | 2023 | 2023 | 2022 | 2021 | 2021 | 2020 | 2020 | 2020 | 2018 | 2018 | 2017 | 2015 | 2015 | |
| Reference | [213] | [86] | [98] | [128] | [214] | [81] | [215] | [99] | [105] | [100] | [87] | [142] | [216] | |
| Publication type | R | R | R | R | BC | P | BC | R | R | BC | R | BC | R | |
| Ammonothermal scope of the article (pages) | 4 | 14 | 2 | 25 | 7 | 4 | 5 | 3 | 11 | 13 | 15 | 19 | 3 | |
| Miscellaneous | Process | X | X | F | X | F | X | F | X | X | X | X | F | |
| Historical perspective | F | X | X | X | F | X | ||||||||
| Research group listing | X | X | X | X | X | X | ||||||||
| Perspective view | X | X | ||||||||||||
| Process | X | X | X | X | X | X | X | |||||||
| Crystal properties | General | X | X | X | X | X | X | X | X | X | X | |||
| Defects | X | F | X | X | X | X | F | X | ||||||
| Purity/doping | X | X | X | F | X | F | X | |||||||
| Device Applications | F | X | F | X | ||||||||||
| Growth conditions | Environment | b & a | b & a | b | b & a | b | b & a | b & a | b | b | b & a | b | b & a | b & a |
| Growth rates | X | X | X | X | X | X | ||||||||
| Morphology | X | X | X | X | X | |||||||||
| Chemistry | X | X | X | |||||||||||
| Solubility | X | F | X | |||||||||||
| Growth technology | General | F | F | F | F | |||||||||
| Autoclave | X | X | X | X | X | X | ||||||||
| SCoRA | X | X | X | |||||||||||
| SCAAT/LPAAT | X | X | X | |||||||||||
| NEAT | X | |||||||||||||
| 2-stage growth | X | X | X | X | X | F | ||||||||
| Tiled seeds | X | X | ||||||||||||
| Circular-cross section | X | X | ||||||||||||
| Annealing | X | |||||||||||||
| Simulations | F | X | ||||||||||||
| In situ measurements | F | F | ||||||||||||
| Temperature Nutrient-Zone (°C) | Temperature Growth-Zone (°C) | Pressure (MPa) | Mineralizer | Solubility | Mol Ratio Al:mineralizer:NH3 | Growth Time (days) | Product | Layer Thickness-/ Grainsize | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 500 | 600 | 200 | KNH2 | retrograde | 1:0.09:4.5 | 2.7–4.6 | Dense crystalline layers | Some mm | [173] |
| 525 | 550 | 246–286 | KN3 | retrograde | 1:[1.9–2.6]:[23.5–24.5] | 21 | Crystalline layers | 0.1–1.5 mm | [174] |
| 500 | 600 | 100–200 | KNH2 | retrograde | 1:0.1:[>4] | n/a | Dense crystalline layers | ~1 mm | [160] |
| <500 | 400–500 | Li, K and LiNH2 | n/a | n/a | n/a | Crystalline compact grains | ≤25 µm | [169] | |
| <550 | 500 | KNH2 and LiNH2 | n/a | 1:[n/a]:10 | 14 | Crystalline compact grains as well as small and big needles | Few µm | [220] | |
| 400 | 600 | 50–200 | NH4I | retrograde | 1:0.05:[>2] | n/a | Crystalline powder | 100 nm | [160] |
| 450 | ca. 197 | NH4Cl | n/a | n/a | n/a | Crystalline powder | Ø 32 nm | [159] | |
| 350, 400, 450, 500, 550 | 80–120 | NH4Cl | n/a | 1:1:[n/a] | 5-7 | Crystalline powder | 20–30 nm | [223] | |
| Material | Space Group | p/MPa | T/°C | Mineralizer | Year | Reference |
|---|---|---|---|---|---|---|
| NaTaN2 | Rm | 100 | 600 | NaNH2 | 1988 | [235] |
| KTaN2 | Pbca | 100 | 600 | KNH2 | 1988 | [235] |
| RbTaN2 | Pbca | 100 | 600 | RbNH2 | 1988 | [235] |
| CsTaN2 | Fd3m | 100 | 600 | CsNH2 | 1988 | [235] |
| Li2Ta3N5 | C2/m | 600 | 550 | Li/Li3N/LiNH2 | 1991 | [236] |
| NaSi2N3 | Cmc21 | 600 | 575 | NaNH2 | 1993 | [237] |
| K3P6N11 | P4132 | 600 | 500 | KNH2 | 1997 | [238] |
| SrAlSiN3 | Cmc21 | 100 | 500 | NaNH2 | 2012 | [94] |
| MgSiN2 | Pna21 | 170 | 797 | KN3 | 2017 | [36] |
| MgGeN2 | Pna21 | 230 | 597 | NaN3 | 2017 | [36] |
| MnSiN2 | Pna21 | 170 | 797 | KN3 | 2017 | [36] |
| MnGeN2 | Pna21 | 170 | 597 | NaN3 | 2017 | [36] |
| LiSi2N3 | Cmc21 | 170 | 697 | LiN3 | 2017 | [36] |
| LiGe2N3 | Cmc21 | 230 | 627 | LiN3 | 2017 | [36] |
| ZnSiN2 | Pna21 | 230 | 797 | KN3 | 2017 | [42] |
| ZnGeN2 | Pna21 | 230 | 797 | KN3 | 2017 | [42] |
| Mg2PN3 | Cmc21 | 140 | 797 | NaN3 | 2018 | [58] |
| Zn2PN3 | Cmc21 | 200 | 527 | KN3 | 2018 | [58] |
| Mg0.5Mn0.5SiN2 | Pna21 | 150 | 797 | KN3 | 2019 | [9] |
| Mg0.5Zn0.5SiN2 | Pna21 | 150 | 797 | KN3 | 2019 | [9] |
| Mn0.5Zn0.5SiN2 | Pna21 | 150 | 797 | KN3 | 2019 | [9] |
| Mg0.5Mn0.5GeN2 | Pna21 | 200 | 597 | NaN3 | 2019 | [9] |
| Mg0.5Zn0.5GeN2 | Pna21 | 200 | 597 | NaN3 | 2019 | [9] |
| Mn0.5Zn0.5GeN2 | Pna21 | 200 | 597 | NaN3 | 2019 | [9] |
| NaTaN2 | m | 170 | 800 | NaN3/KN3/RbN3/CsN3 | 2019 | [186] |
| KTaN2 | Pbca | 170 | 800 | NaN3/KN3/RbN3/CsN3 | 2019 | [186] |
| RbTaN2 | Pbca | 170 | 627 | NaN3/KN3/RbN3/CsN3 | 2019 | [186] |
| CsTaN2 | m | 170 | 350 | NaN3/KN3/RbN3/CsN3 | 2019 | [186] |
| Sr3P3N7 | P2/c | 140 | 797 | NaN3 | 2020 | [239] |
| Impurity | Growth Environment | Impurity Type | Concentration | Potential Impurity Sources | ||
|---|---|---|---|---|---|---|
| Min. | Max. | |||||
| Si, Al, Mg | Basic | Minor | High 1016 | Low 1017 | GaN nutrient (impurities) | [100] |
| Mineralizer (impurities) | [100] | |||||
| Autoclave | [146] | |||||
| Mn, Fe, Zn | Basic | Minor | High 1016 | Low 1017 | Autoclave | [140,146] |
| C | Basic | Minor | High 1016 | Low 1017 | Surface contamination | [87,100] |
| Na | Basic | Minor | low 1016 | High 1018 | Mineralizer (solvent) | [87,100] |
| H | Basic | Major | Low 1018 | Mid 1020 | Ammonia (solvent) | [87,100] |
| Acidic | Major | Mid 1017 | High 1019 | Mineralizer (solvent) | [103,262,263] | |
| O | Basic Acidic | Major Major | Low 1018 High 1017 | Mid 1020 High 1019 | Surface contamination | [87,100] |
| Mineralizer (impurities) | [103,262,263] | |||||
| GaN nutrient (impurities) | [100,140,252,262] | |||||
| Process Variant | Dopant | Doping Concentration (cm−3) | Conductivity Type | Carrier Concentration (cm−3) | Doping Efficiency (%) | Resistivity (Ωcm) | Carrier Mobility (cm2/(V·s)) | Ref. |
|---|---|---|---|---|---|---|---|---|
| basic | O | 50–100 × 1018 | n | 6–1018 | 12–10 | n/a | 68 | [282] |
| basic | O | 3.5–10 × 1018 | n | 2.8–7.5 × 1018 | 80–75 | 1–10 × 10−3 | ca. 250–200 | [105] |
| acidic | O | 1.5–27 × 1018 | n | 0.66–15 × 1018 | 44–56 | n/a | 565–155 | [252] |
| acidic | O | 1–10 × 1018 | n | 0.9–20 × 1018 | 90–50 | 2–20 × 10−3 | 300–100 | [283] |
| basic | Mg | 7–8 × 1018 | p | n/a | -- | 106 | n/a | [105] |
| basic (+an.) | Mg | 7–8 × 1018 | p | 3.8 × 1016 | 0.54–0.47 | 30 | 6 | [105] |
| basic | Mg | 1–2 × 1018 | SI | <1012–1013 | -- | ≥1010 | n/a | [105] |
| basic | Mn | 10 × 1019 | SI | n/a | -- | >1012 | n/a | [105] |
| Applicable Measurement Techniques | Required Information |
|---|---|
| Thermometry | Continuous temperature measurement |
| X-ray absorption imaging | Crystal dissolution kinetics, solubility, solute concentrations and solvent density changes, potentially crystal growth rates |
| Computed tomography | Time-dependent etch-back and crystal growth rates, crystal geometry, parasitic deposition, nutrient morphology changes (tracking of mass transport in 3D) |
| Optical spectroscopy (UV/Vis, Raman) | Monitoring reaction kinetics via composition of the formed compounds Prospectively contribute to the identification of solutes such as intermediates |
| Viscometry | Viscosity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wostatek, T.; Chirala, V.Y.M.R.; Stoddard, N.; Civas, E.N.; Pimputkar, S.; Schimmel, S. Ammonothermal Crystal Growth of Functional Nitrides for Semiconductor Devices: Status and Potential. Materials 2024, 17, 3104. https://doi.org/10.3390/ma17133104
Wostatek T, Chirala VYMR, Stoddard N, Civas EN, Pimputkar S, Schimmel S. Ammonothermal Crystal Growth of Functional Nitrides for Semiconductor Devices: Status and Potential. Materials. 2024; 17(13):3104. https://doi.org/10.3390/ma17133104
Chicago/Turabian StyleWostatek, Thomas, V. Y. M. Rajesh Chirala, Nathan Stoddard, Ege N. Civas, Siddha Pimputkar, and Saskia Schimmel. 2024. "Ammonothermal Crystal Growth of Functional Nitrides for Semiconductor Devices: Status and Potential" Materials 17, no. 13: 3104. https://doi.org/10.3390/ma17133104






