Aliphatic Polycarbonate-Based Binders for High-Loading Cathodes by Solvent-Free Method Used in High Performance LiFePO4|Li Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chain Extension Binder
2.3. Preparation of Cathode Electrodes
2.4. Characterizations
3. Results and Discussion
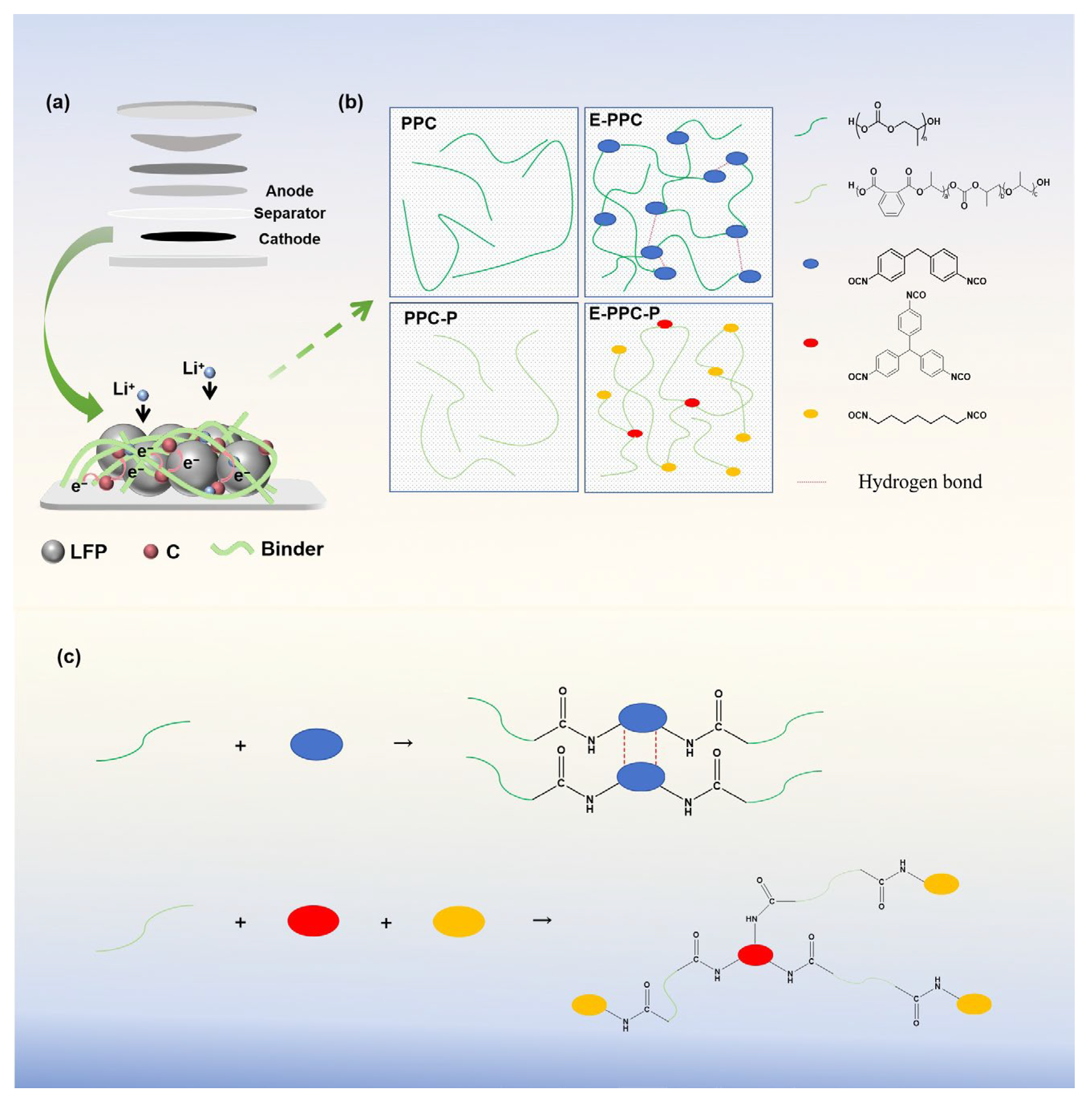
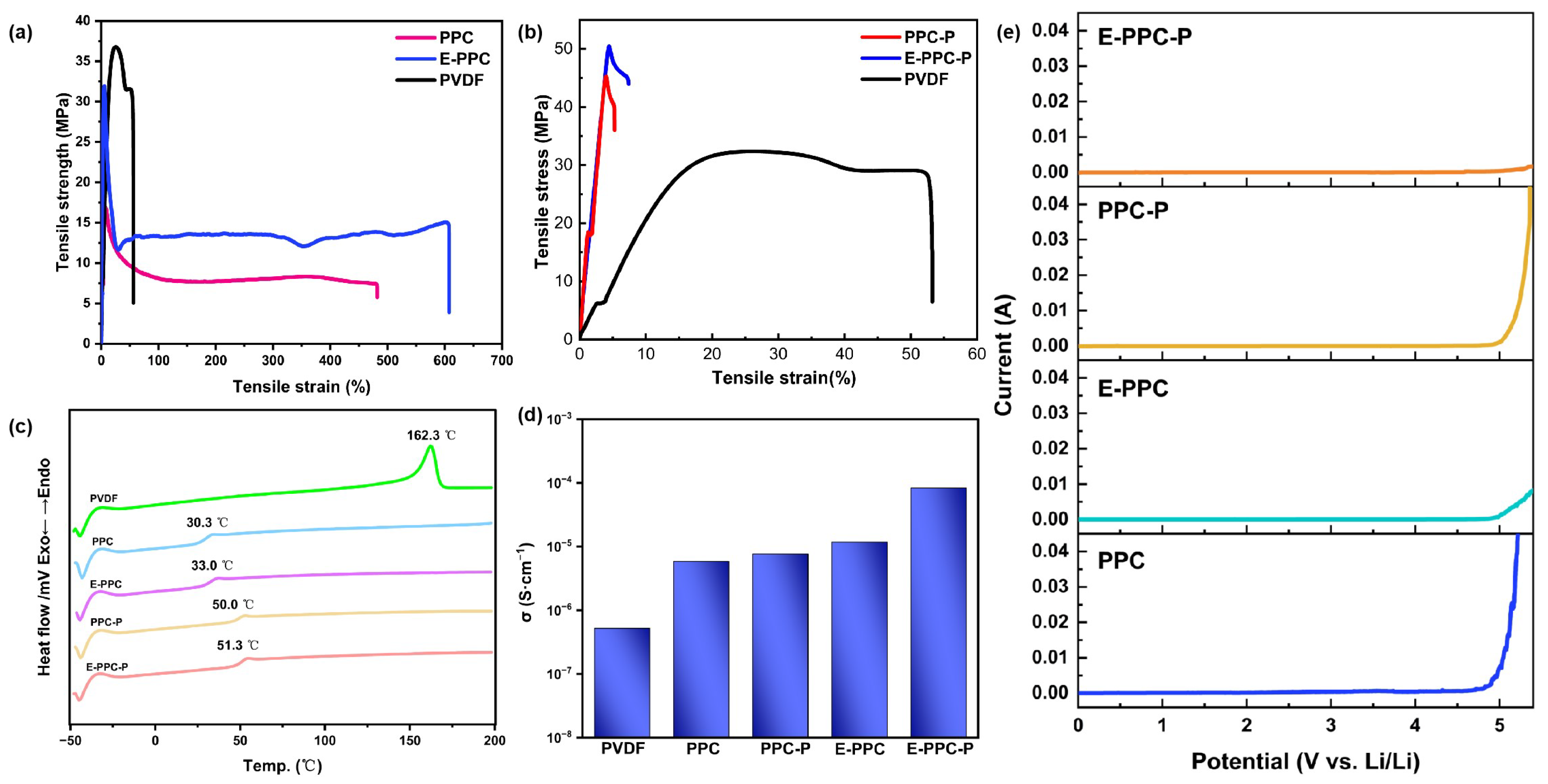
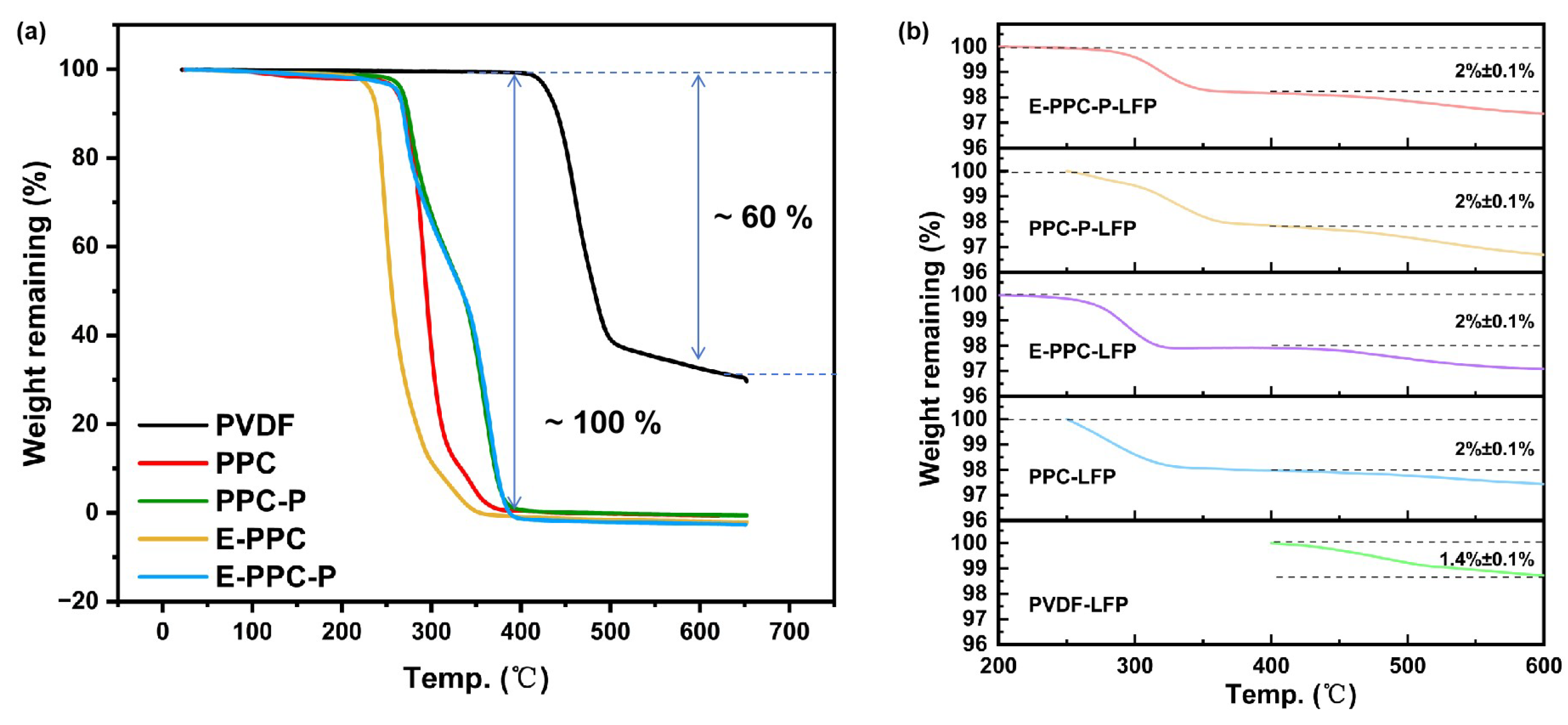
3.1. Analysis of the Physical/Chemical Properties of the Binders
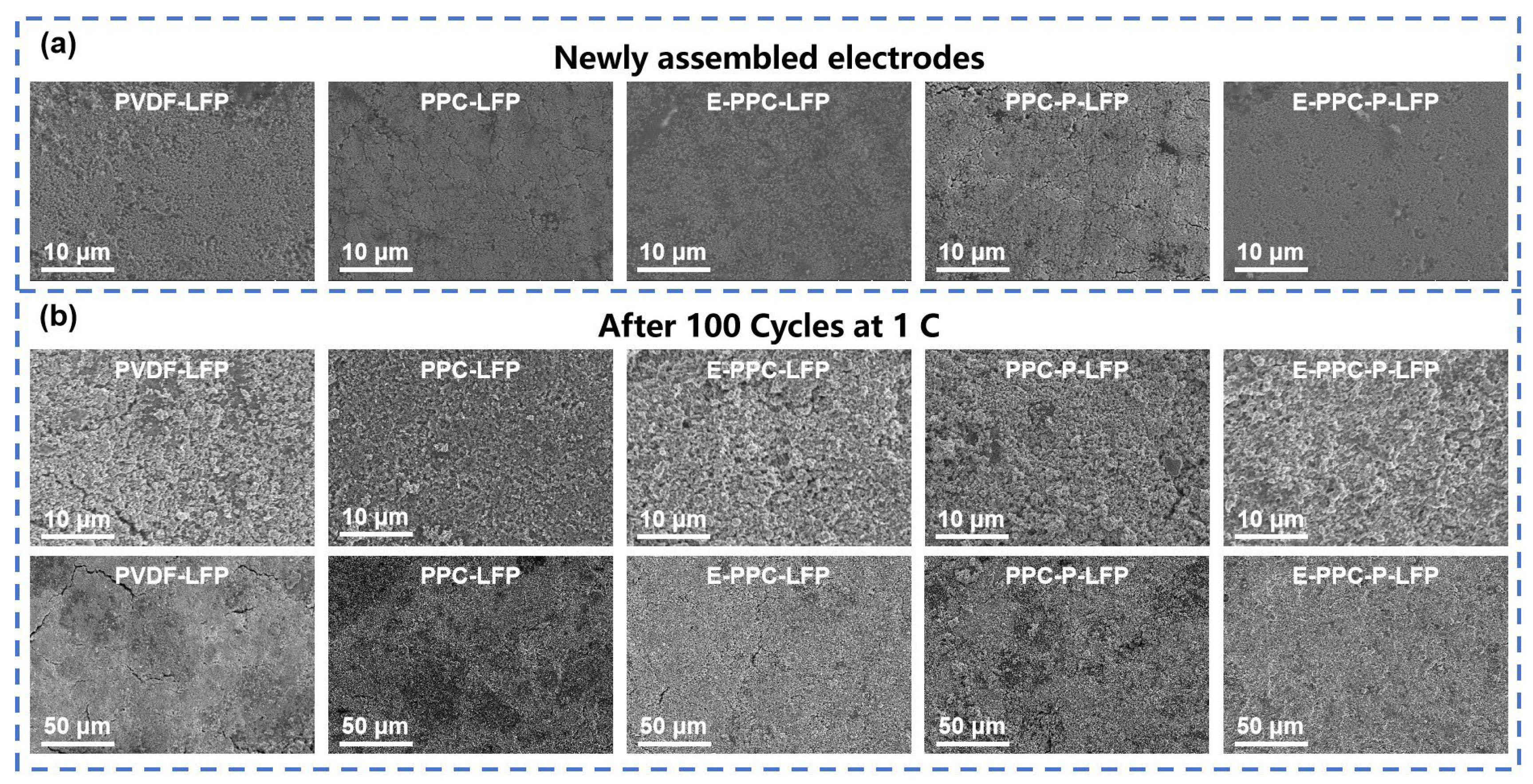
3.2. Electrode Powder Test
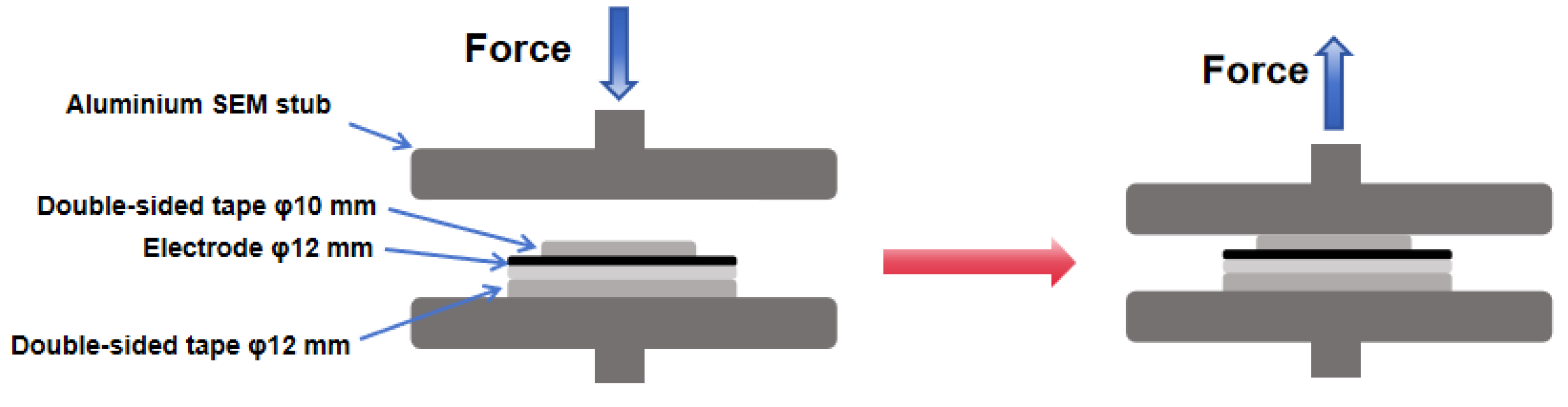
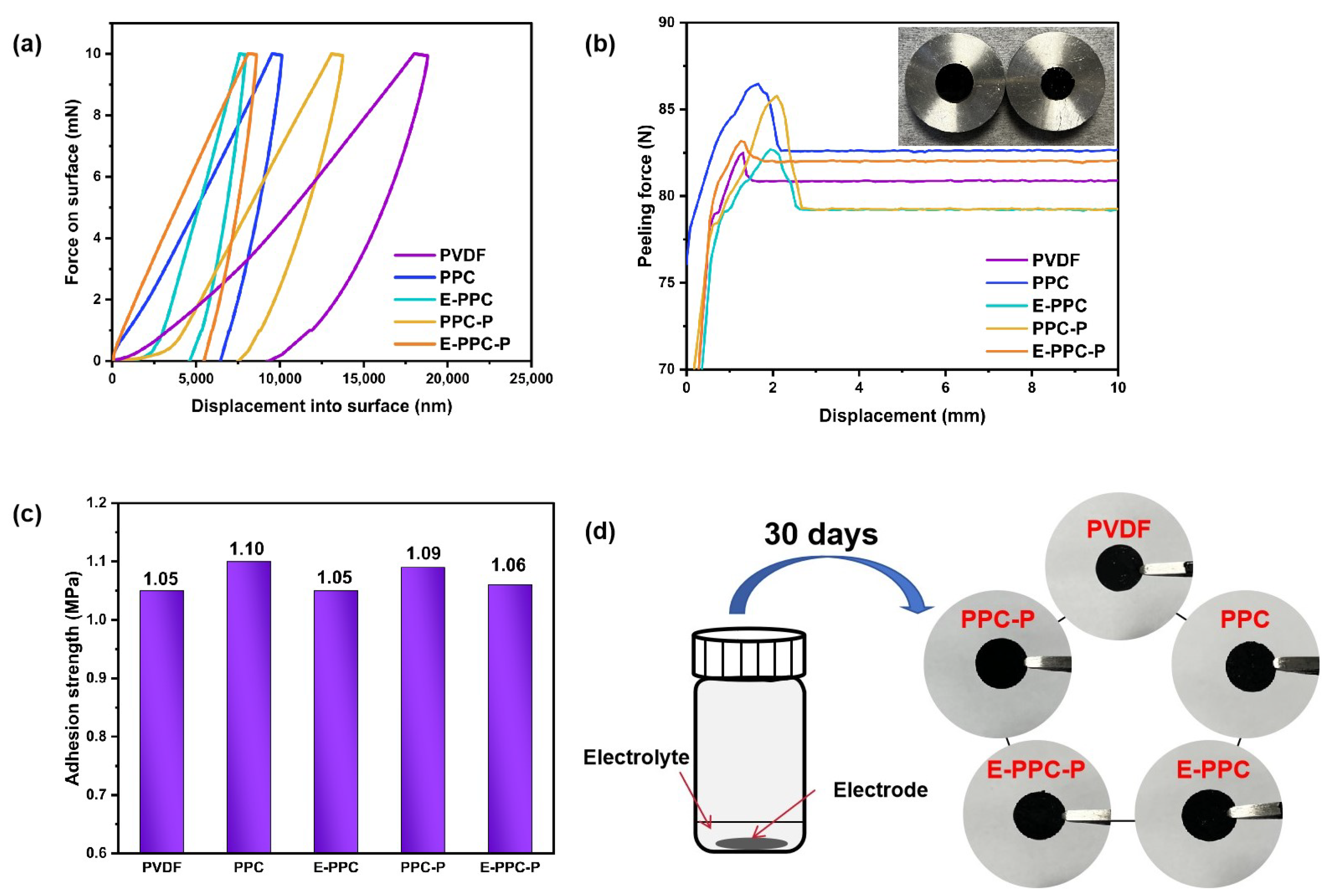
3.3. Adhesion Force Test
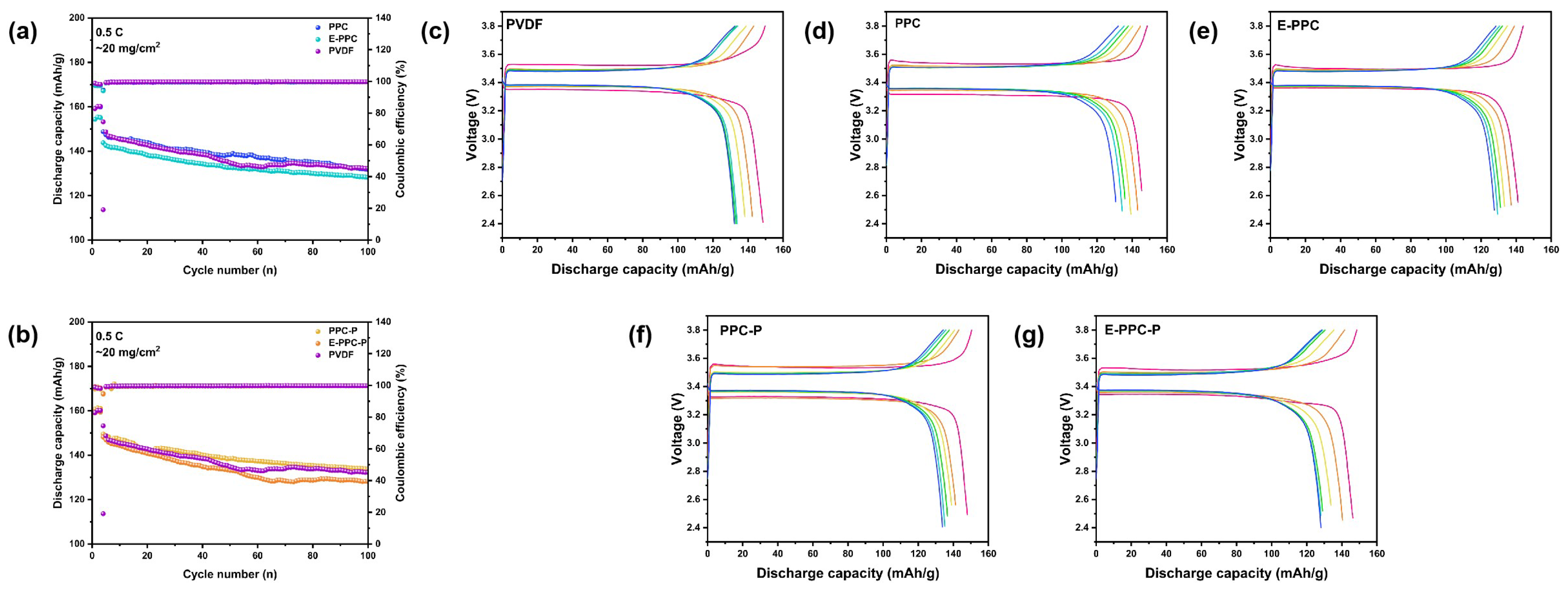
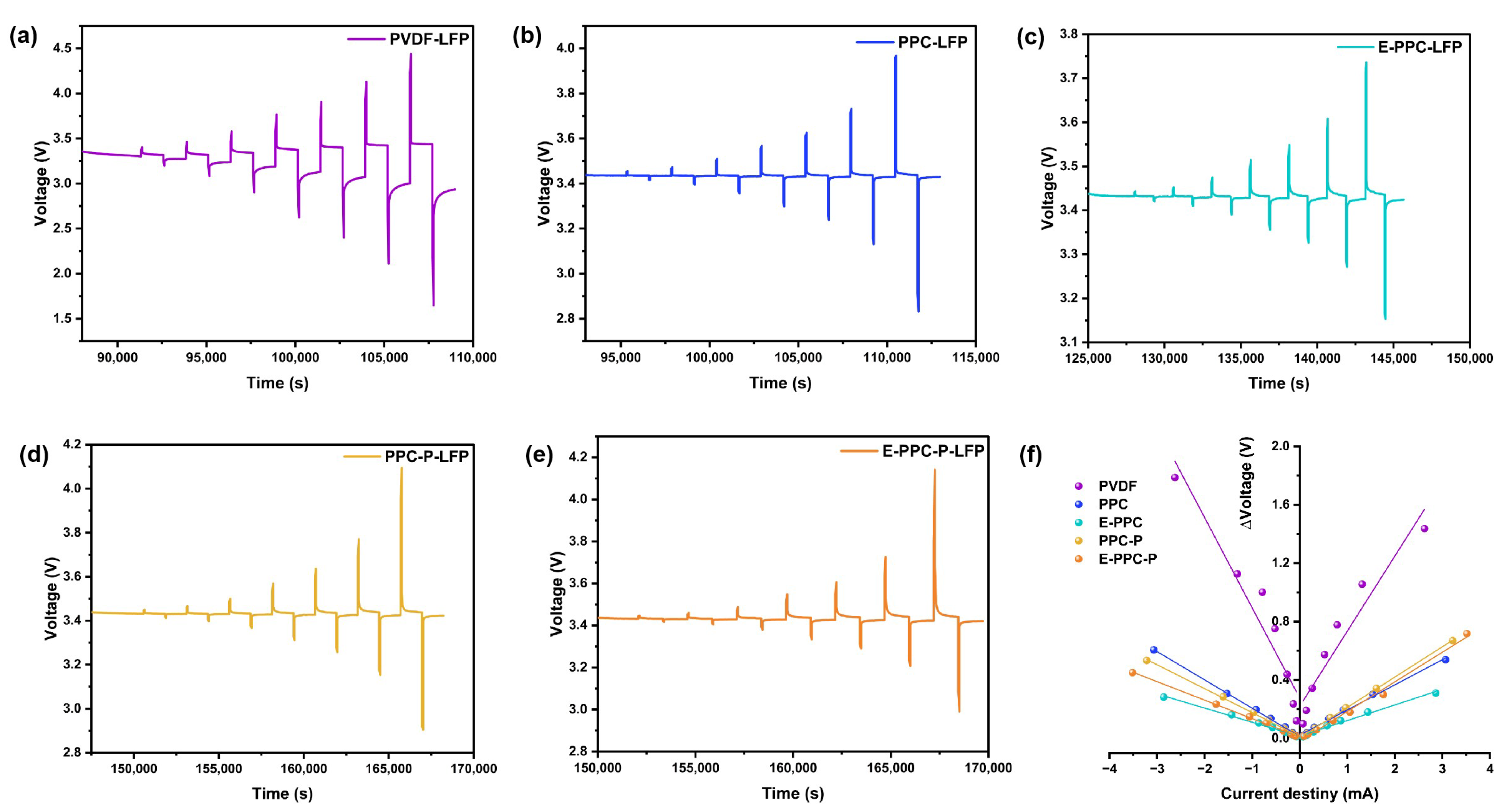
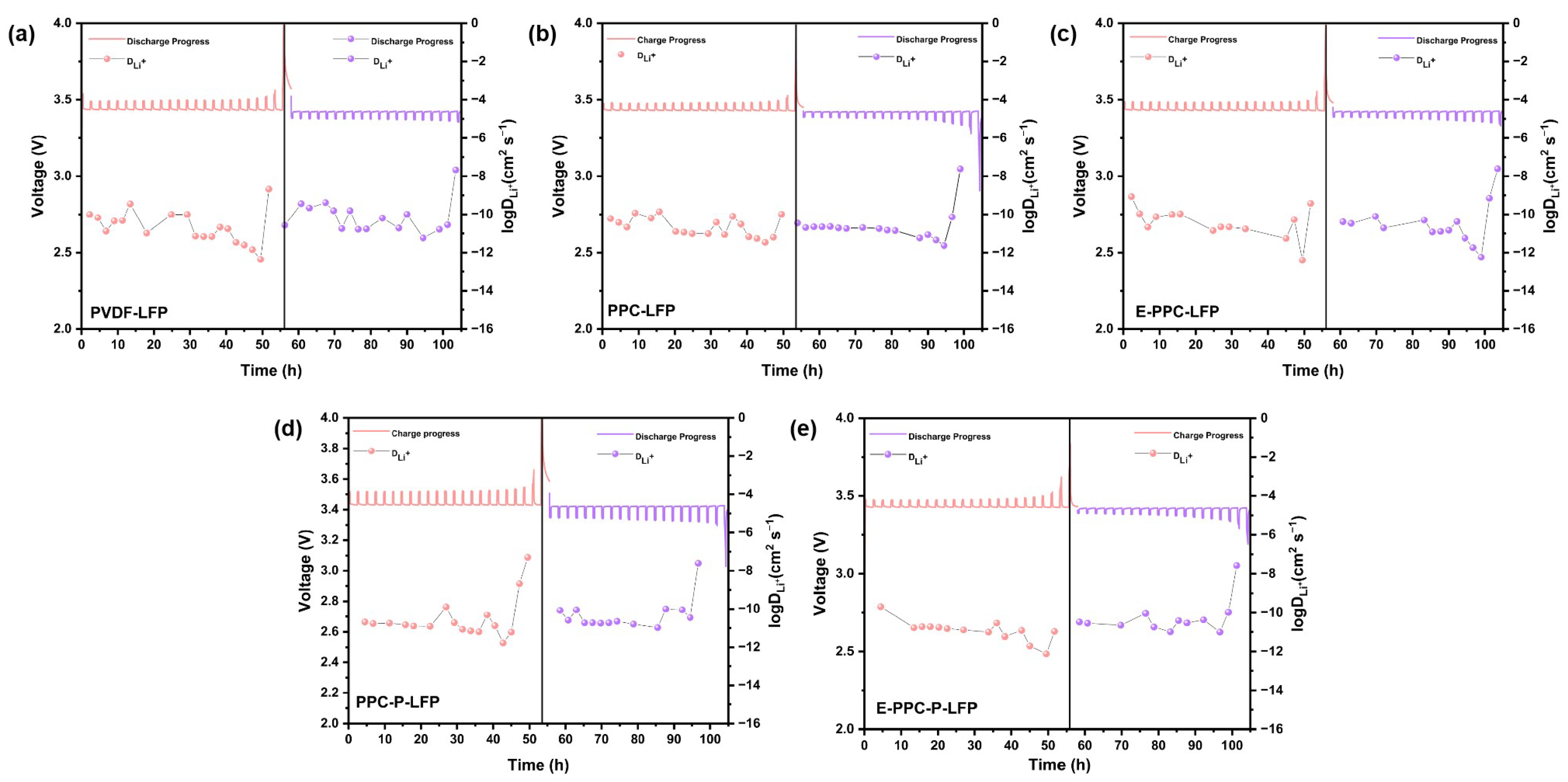
3.4. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Liu, P.; Zhong, L.; Wang, S.; Xiao, M.; Han, D.; Huang, S.; Meng, Y. Covalent Organic Frameworks with Low Surface Work Function Enabled Stable Lithium Anode. Small 2021, 17, 2101496. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Sheng, H.; Meng, X.; Zhang, X.; Lei, D.; Sun, X.; Pan, H.; Wang, J.; Yu, X.; Wang, C.; et al. Size controllable single-crystalline Ni-rich cathodes for high-energy lithium-ion batteries. Natl. Sci. Rev. 2023, 10, nwac226. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, Q.; Zhang, Q.; Li, C.; Xu, C.; Ma, Y.; Shi, X.; Zhang, H.; Song, D.; Zhang, L. Li-Ion Transfer Mechanism of Ambient-Temperature Solid Polymer Electrolyte toward Lithium Metal Battery. Adv. Energy Mater. 2023, 13, 2204036. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, R.; Zhang, J.; Song, X.; Liu, J.; Xu, N.; Zhang, H.; Wan, X.; Ji, X.; Ma, Y.; et al. Long-cycling and High-voltage Solid State Lithium Metal Batteries Enabled by Fluorinated and Crosslinked Polyether Electrolytes. Angew. Chem. Int. Ed. 2024, 63, e202400303. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Fan, M.; Chang, X.; Li, H.; Wang, W.; Zhu, Y.; Wan, J.; Zhao, Y.; Wang, F.; Wen, R.; et al. A Functional Prelithiation Separator Promises Sustainable High-Energy Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300507. [Google Scholar] [CrossRef]
- Son, H.B.; Cho, S.; Baek, K.; Jung, J.; Nam, S.; Han, D.Y.; Kang, S.J.; Moon, H.R.; Park, S. All-Impurities Scavenging, Safe Separators with Functional Metal-Organic-Frameworks for High-Energy-Density Li-Ion Battery. Adv. Funct. Mater. 2023, 33, 2302563. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Bondarev, D.; Amatucci, G.G.; Yushin, G. Battery materials for low-cost electric transportation. Mater. Today 2021, 42, 57–72. [Google Scholar] [CrossRef]
- Verdier, N.; Foran, G.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. Challenges in Solvent-Free Methods for Manufacturing Electrodes and Electrolytes for Lithium-Based Batteries. Polymers 2021, 13, 323. [Google Scholar] [CrossRef]
- Bryntesen, S.N.; Strømman, A.H.; Tolstorebrov, I.; Shearing, P.R.; Lamb, J.J.; Stokke Burheim, O. Opportunities for the State-of-the-Art Production of LIB Electrodes—A Review. Energies 2021, 14, 1406. [Google Scholar] [CrossRef]
- Huang, S.; Chen, H.; Chen, M.; Huang, Y.; He, X.; Zhuo, H.; Chen, S. Design of Conductive Binders for LiFePO4 Cathodes with Long-Term Cycle Life. ACS Sustain. Chem. Eng. 2021, 9, 13277–13286. [Google Scholar] [CrossRef]
- Huang, S.; Huang, X.; Huang, Y.; He, X.; Zhuo, H.; Chen, S. Rational Design of Effective Binders for LiFePO4 Cathodes. Polymers 2021, 13, 3146. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Steinhoff, B.; Sun, X.; Sardo, K.; Skelly, B.; Meyer, H.M.; Sawicki, C.; Polizos, G.; Lyu, X.; Du, Z.; et al. High-throughput and high-performance lithium-ion batteries via dry processing. Chem. Eng. J. 2023, 471, 144300. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhang, S.; Liu, T.; Li, D.; Ci, L. Long cycle life all-solid-state batteries enabled by solvent-free approach for sulfide solid electrolyte and cathode films. Chem. Eng. J. 2023, 455, 140605. [Google Scholar] [CrossRef]
- Ryu, M.; Hong, Y.; Lee, S.; Park, J.H. Ultrahigh loading dry-process for solvent-free lithium-ion battery electrode fabrication. Nat. Commun. 2023, 14, 1316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Han, D.; Xiao, M.; Wang, S.; Feng, Y.; Huang, S.; Meng, Y. New potential substitute of PVDF binder: Poly(propylene carbonate) for solvent-free manufacturing high-loading cathodes of LiFePO4|Li batteries. Ionics 2023, 29, 3895–3906. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Wang, Z.; Xu, J.; Ma, T.; Chen, L.; Li, H.; Wu, F. Progress in solvent-free dry-film technology for batteries and supercapacitors. Mater. Today 2022, 55, 92–109. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.; Yuan, H.; Hu, J.; Huang, J.; Zhang, Q. Dry electrode technology, the rising star in solid-state battery industrialization. Matter 2022, 5, 876–898. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, L.; Xiao, M.; Wang, S.; Smith, A.T.; Sun, L.; Meng, Y. Synthesis and properties of CO2-based plastics: Environmentally-friendly, energy-saving and biomedical polymeric materials. Prog. Polym. Sci. 2018, 80, 163–182. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Xiao, M.; Wang, S.; Meng, Y. Biodegradable PPC/(PVA-TPU) ternary blend blown films with enhanced mechanical properties. J. Polym. Res. 2016, 23, 80. [Google Scholar] [CrossRef]
- Hong, S.; Lee, Y.; Kim, U.; Bak, C.; Lee, Y.M.; Cho, W.; Hah, H.J.; Sun, Y.; Kim, D. All-Solid-State Lithium Batteries: Li+-Conducting Ionomer Binder for Dry-Processed Composite Cathodes. ACS Energy Lett. 2022, 7, 1092–1100. [Google Scholar] [CrossRef]
- Li, Z.; Wu, G.; Yang, Y.; Wan, Z.; Zeng, X.; Yan, L.; Wu, S.; Ling, M.; Liang, C.; Hui, K.N.; et al. An Ion-Conductive Grafted Polymeric Binder with Practical Loading for Silicon Anode with High Interfacial Stability in Lithium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2201197. [Google Scholar] [CrossRef]
- Taskin, O.S.; Yuca, N.; Papavasiliou, J.; Avgouropoulos, G. Interconnected conductive gel binder for high capacity silicon anode for Li-ion batteries. Mater. Lett. 2020, 273, 127918. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, B.; Shi, Z.; Chen, W.; Zhang, Z.; Zhang, L. Re-Engineering Poly(Acrylic Acid) Binder toward Optimized Electrochemical Performance for Silicon Lithium-Ion Batteries: Branching Architecture Leads to Balanced Properties of Polymeric Binders. Adv. Funct. Mater. 2020, 30, 1908558. [Google Scholar] [CrossRef]
- Li, G. Regulating Mass Transport Behavior for High-Performance Lithium Metal Batteries and Fast-Charging Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2002891. [Google Scholar] [CrossRef]
- Jenkins, C.A.; Coles, S.R.; Loveridge, M.J. Investigation into Durable Polymers with Enhanced Toughness and Elasticity for Application in Flexible Li-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 12494–12505. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Zhang, Z.; Wu, C.; Huang, S.; Xiao, M.; Wang, S.; Guo, H.; Han, D.; Meng, Y. Aliphatic Polycarbonate-Based Binders for High-Loading Cathodes by Solvent-Free Method Used in High Performance LiFePO4|Li Batteries. Materials 2024, 17, 3153. https://doi.org/10.3390/ma17133153
Chen B, Zhang Z, Wu C, Huang S, Xiao M, Wang S, Guo H, Han D, Meng Y. Aliphatic Polycarbonate-Based Binders for High-Loading Cathodes by Solvent-Free Method Used in High Performance LiFePO4|Li Batteries. Materials. 2024; 17(13):3153. https://doi.org/10.3390/ma17133153
Chicago/Turabian StyleChen, Bin, Zhe Zhang, Change Wu, Sheng Huang, Min Xiao, Shuanjin Wang, Hui Guo, Dongmei Han, and Yuezhong Meng. 2024. "Aliphatic Polycarbonate-Based Binders for High-Loading Cathodes by Solvent-Free Method Used in High Performance LiFePO4|Li Batteries" Materials 17, no. 13: 3153. https://doi.org/10.3390/ma17133153




