Synthesis of Low-Silicon X-Type Zeolite from Lithium Slag and Its Fast Exchange Performance of Calcium and Magnesium Ions
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials and Main Reagents
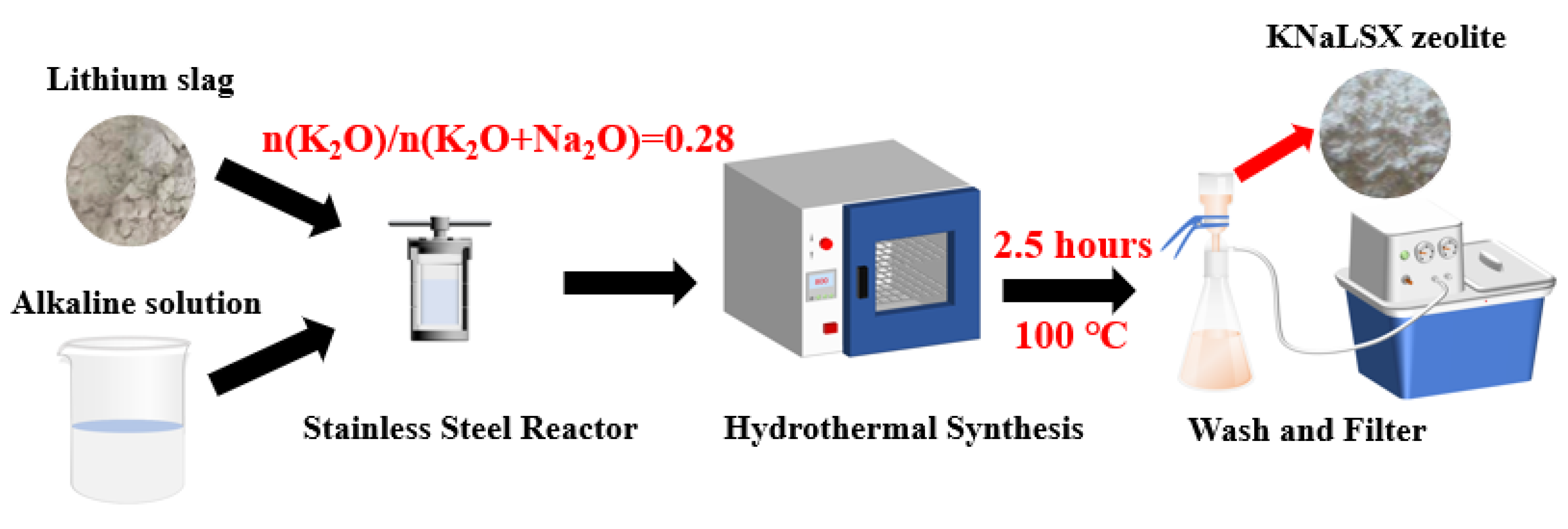
2.2. Synthesis of KNaLSX Zeolite from Lithium Slag
2.3. Characterization of Synthetic Sample
2.4. Determination of Ion Exchange Capacity and Ion Exchange Rate Curves of Calcium and Magnesium Ions
2.4.1. Ion Exchange Capacity of Calcium and Magnesium Ions
2.4.2. Ion Exchange Rate Curve of Calcium and Magnesium Ions
3. Results and Discussions
3.1. Preparation of KNaLSX Zeolite from Lithium Slag
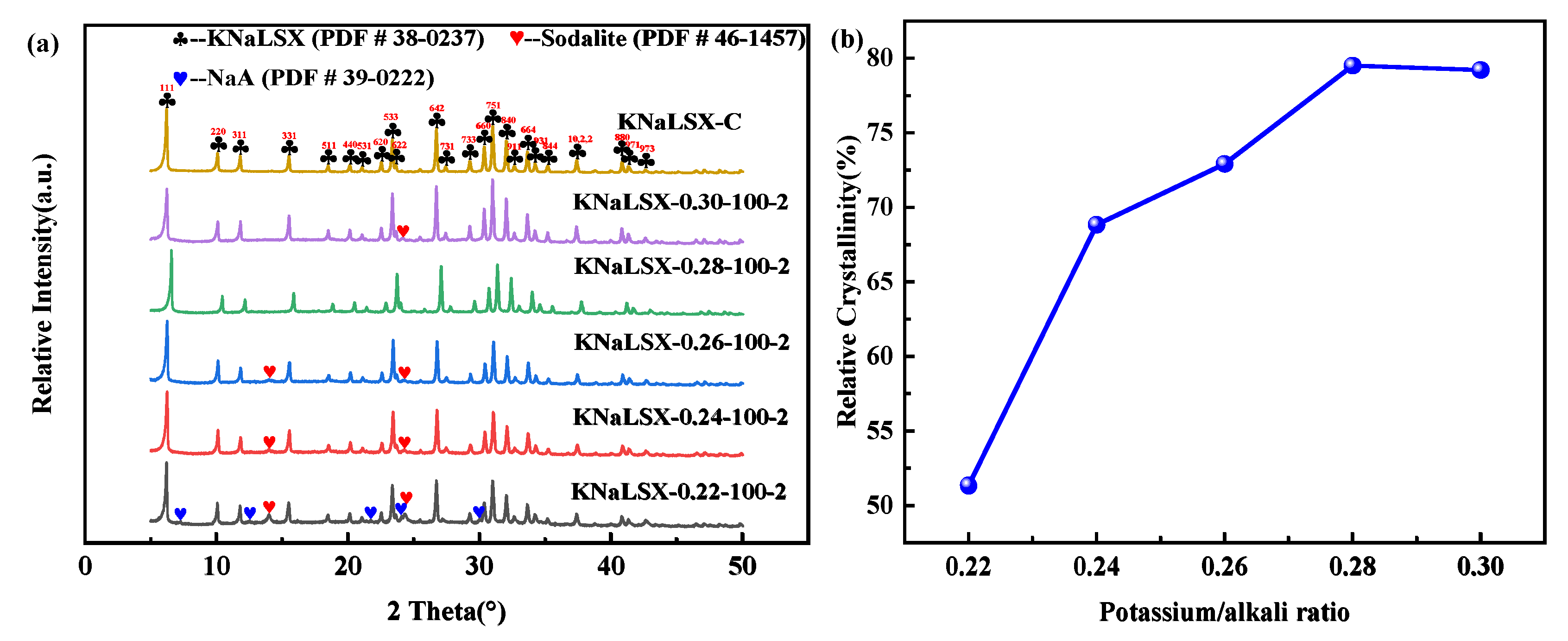
3.1.1. Potassium to Alkali Ratio
3.1.2. Crystallization Temperature
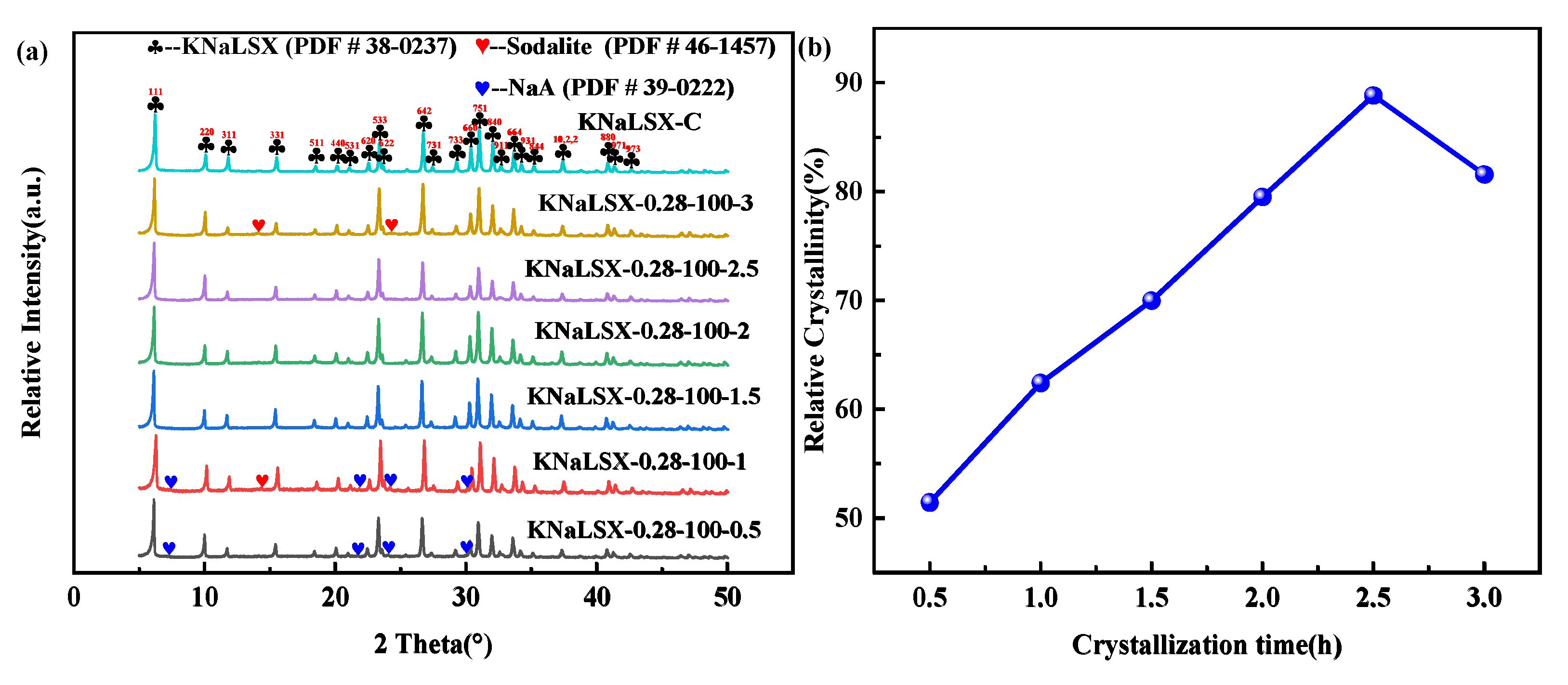
3.1.3. Crystallization Time
3.2. Characterization of the Synthesized KNaLSX
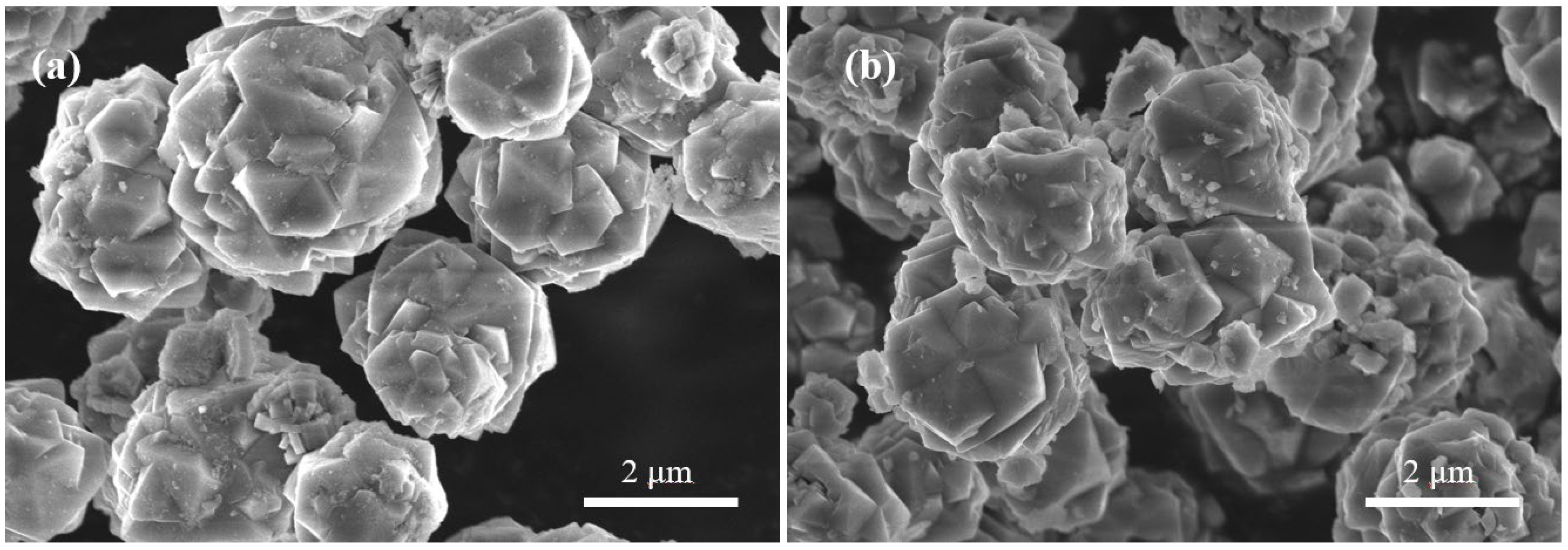
3.2.1. Crystal Morphology
3.2.2. Chemical Composition
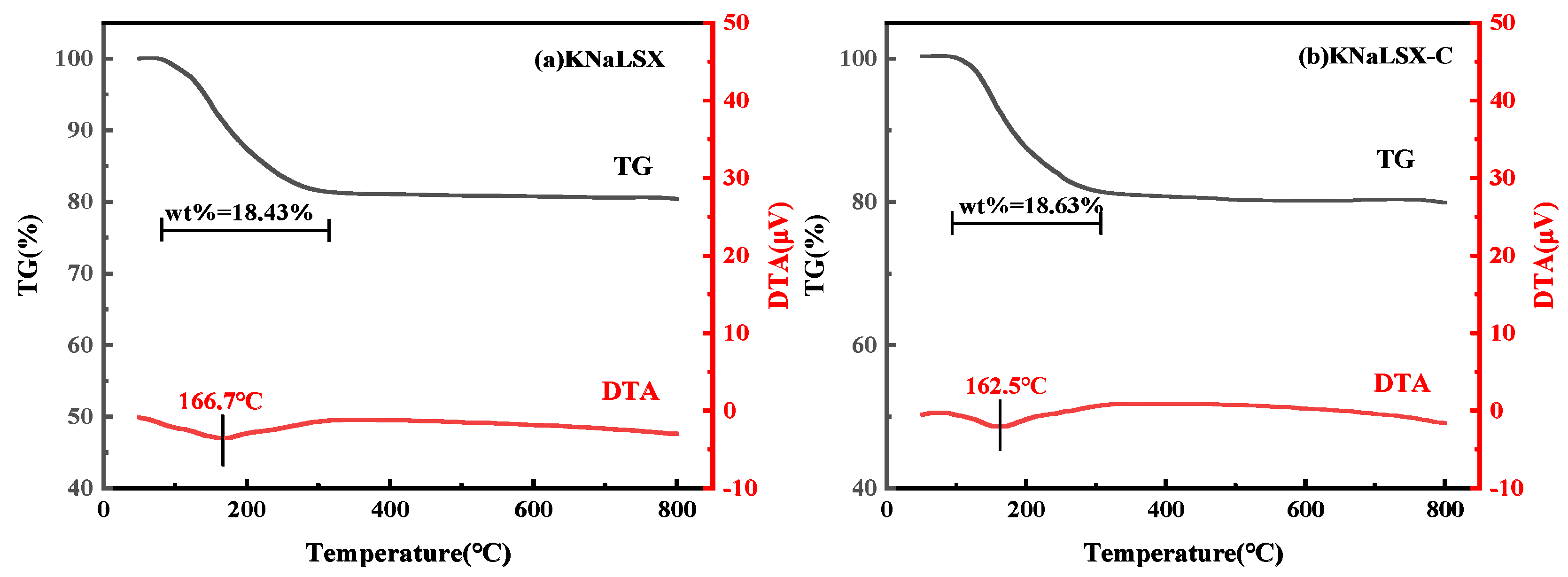
3.2.3. Thermal Stability
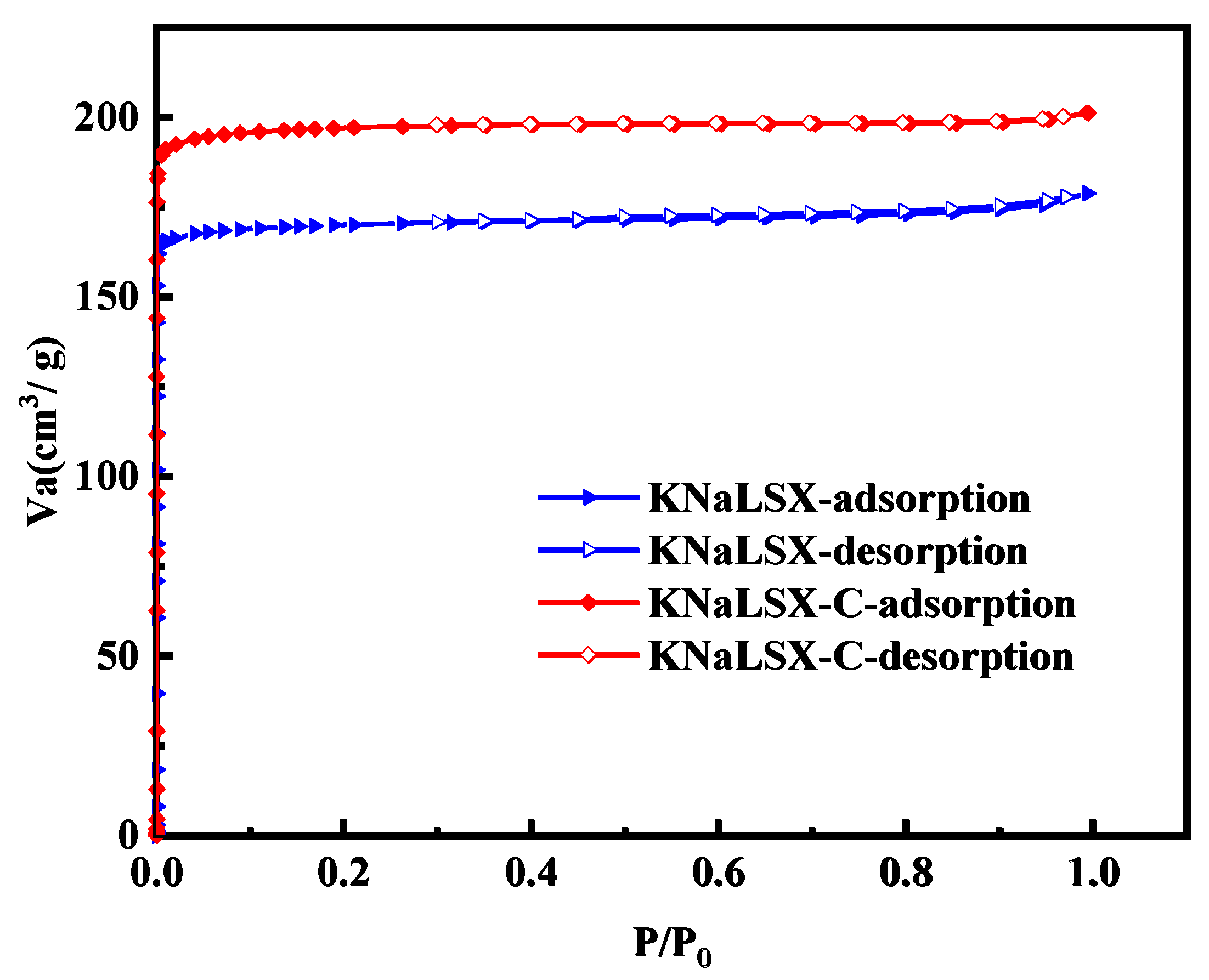
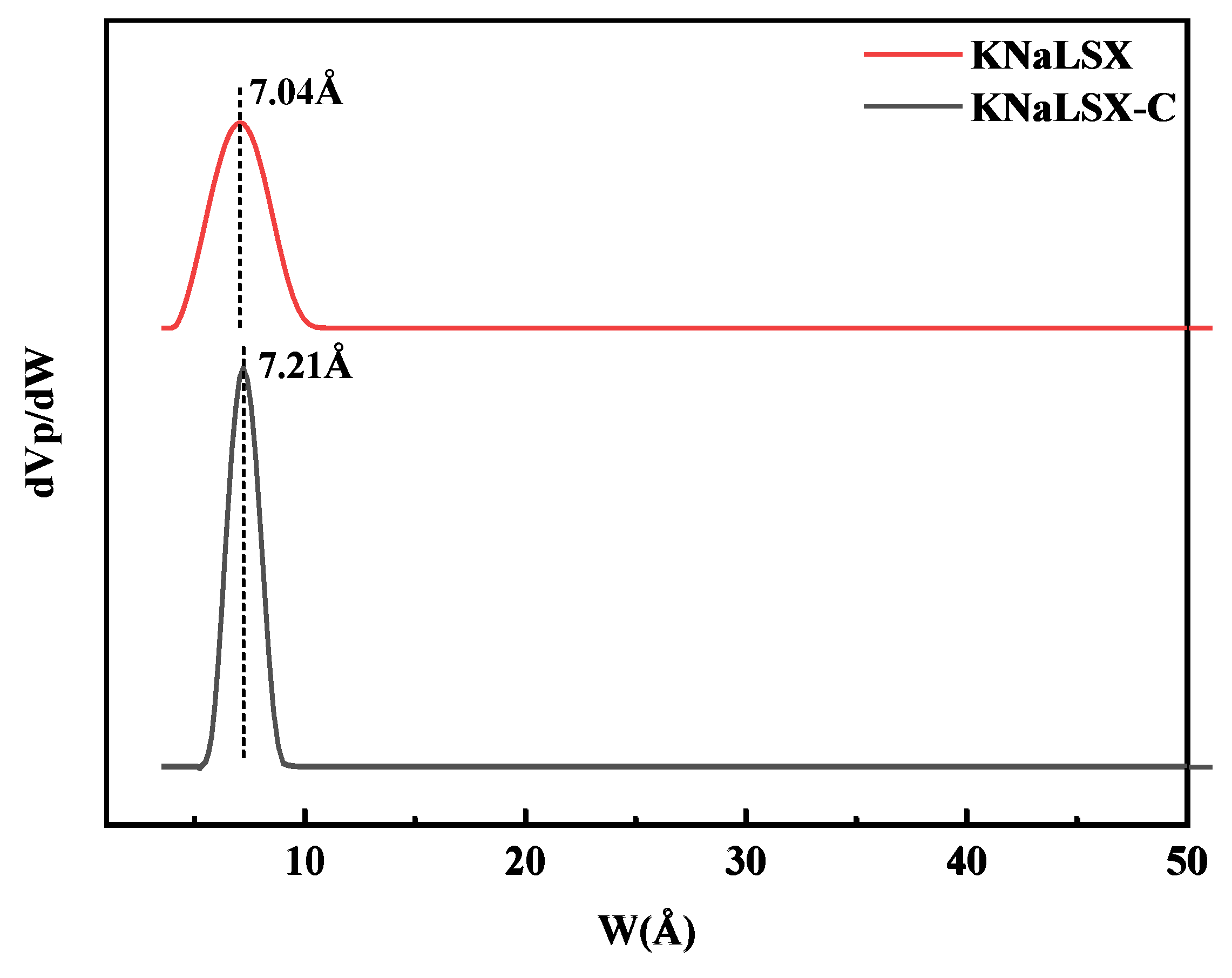
3.2.4. Specific Surface Area and Pore Structure
3.3. Ion Exchange Capacity and Ion Exchange Rate Performance of KNaLSX Zeolite for Calcium and Magnesium Ions
3.3.1. Ion Exchange Capacity for Calcium and Magnesium Ions
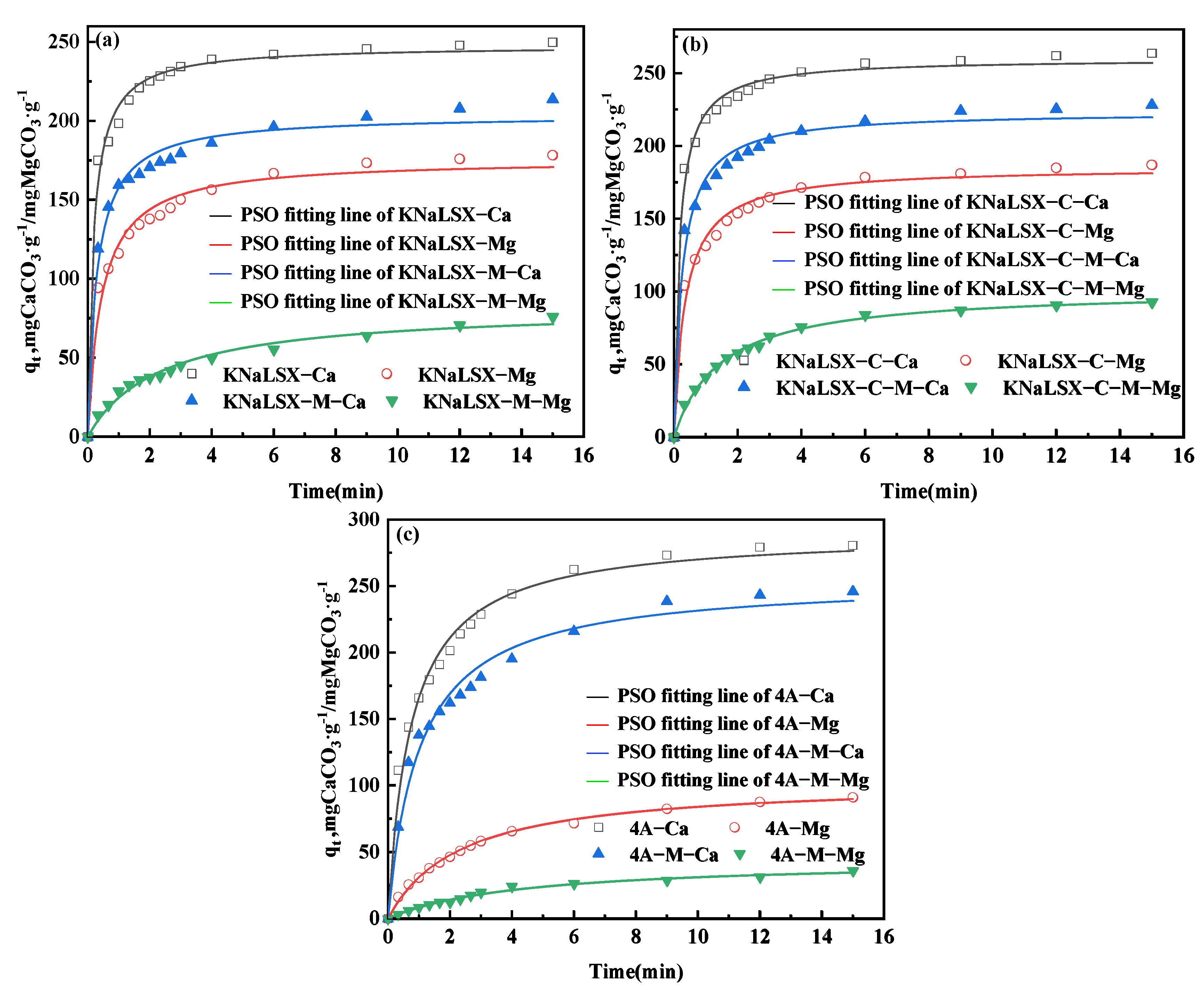
3.3.2. Ion Exchange Rate for Calcium and Magnesium Ions of KNaLSX
3.3.3. Competitive Ion Exchange Relationship of Calcium and Magnesium Ions on Zeolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, T.; Ma, B.; Tan, H.; Liu, X.; Chen, P.; Luo, Z. Effect of TIPA on mechanical properties and hydration properties of cement-lithium slag system. J. Environ. Manag. 2020, 276, 111274. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, S. Recycling of lithium slag as a green admixture for white reactive powder concrete. J. Mater. Cycles. Waste 2020, 22, 1818–1827. [Google Scholar] [CrossRef]
- Li, J.; Lian, P.; Huang, S.; Huang, L. Recycling of lithium slag extracted from lithium mica by preparing white Portland cement. J. Environ. Manag. 2020, 265, 110551. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Tu, S.; Chen, L.; Wu, F.; Xie, L.; Zhuo, Q.; Yu, S. Investigation of the performance of cement mortar incorporating lithium slag as a super-fine aggregate. Front. Mater. 2023, 10, 1134622. [Google Scholar] [CrossRef]
- Gu, X.; Wang, H.; Zhu, Z.; Liu, J.; Xu, X.; Wang, Q. Synergistic effect and mechanism of lithium slag on mechanical properties and microstructure of steel slag-cement system. Constr. Build. Mater. 2023, 396, 131768. [Google Scholar] [CrossRef]
- Xiong, H.; Xian, W.; Deng, W.; Sun, N.; Wang, W.; Sun, J.; Liu, C.; Li, S.; Luo, L. Preparation of lithium slag based foamed ceramics and high temperature phase transformation. Non-Metallic Mines 2023, 46, 99–102+106. [Google Scholar]
- Xiong, H.; Sun, J.; Liu, C.; Xian, W.; Wang, W.; Luo, L.; Li, S. Study on sintering behavior and properties of lithium slag-based foamed ceramics. J. Non-Cryst. Solids 2023, 617, 122499. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Duan, P.; Ruan, S.; Zhang, Z.; Ge, W. The effects of lithium slag on microstructure and mechanical performance of metakaolin-based geopolymers designed by response surface method (RSM). Constr. Build. Mater. 2021, 299, 123950. [Google Scholar] [CrossRef]
- Karrech, A.; Dong, M.; Skut, J.; Elchalakani, M.; Shahin, M. Delithiated beta-spodumene as a geopolymer precursor. Constr. Build. Mater. 2021, 309, 124974. [Google Scholar] [CrossRef]
- Cortez, D.M.; Bekke, M.T.; Liang, Z.; Stamminger, R. The impact of detergent performance on sustainable consumer laundry behavior; a socio-technical challenge. Tenside Surfact. Det. 2024, 61, 203–215. [Google Scholar] [CrossRef]
- Chen, D.; Hu, X.; Shi, L.; Cui, Q.; Wang, H.; Yao, H. Synthesis and characterization of zeolite X from lithium slag. Appl. Clay Sci. 2012, 59–60, 148–151. [Google Scholar] [CrossRef]
- Zhuang, Q.; Lin, G.; Cui, Q.; Wang, H. Characterization and performance of FAU/LTA co-crystalline zeolite synthesized by lithium slag. Acta. Petrol. Sin. 2014, 30, 348–352. [Google Scholar]
- Lin, G.; Zhuang, Q.; Cui, Q.; Wang, H.; Yao, H. Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid. Chin. J. Chem. Eng. 2015, 23, 1768–1773. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Liu, Z.; Wang, D. Insight into the Growth Mechanism of Low-Temperature Synthesis of High-Purity Lithium Slag-Based Zeolite, A. Materials 2024, 17, 568. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Ma, B.; Liu, Y.; Chen, Y. Adsorption behavior and mechanism of mixed heavy metal ions by zeolite adsorbent prepared from lithium leach residue. Micropor. Mesopor. Mat. 2022, 329, 111553. [Google Scholar] [CrossRef]
- Hu, X.; Zhuang, Q.; Chen, D.; Cui, Q.; Wang, H. The characterization and adsorption properties of NaX zeolites synthesized from lithium sludge. J. Chem. Eng. Chin. Univ. 2013, 27, 708–713. [Google Scholar]
- Huang, S.; Wang, Y.; Zhang, L.; Zhu, S.; Ma, Z.; Cui, Q.; Wang, H. Insight into the kinetic behavior of microwave-assisted synthesis of NaX zeolite from lithium slag. New J. Chem. 2023, 47, 14335. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Papynov, E.K.; Ivanov, N.P.; Balanov, M.I.; Dran’kov, A.N.; Shkuratov, A.L.; Zarubina, N.V.; Fedorets, A.N.; Mayorov, V.Y.; Lembikov, A.O.; et al. Study of adsorption and immobilization of Cs+, Sr2+, Co2+, Pb2+, La3+ ions on Na-Faujasite zeolite transformed in solid state matrices. Sep. Purif. Technol. 2024, 332, 125662. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Yarusova, S.B.; Ivanov, N.P.; Papynov, E.K.; Belov, A.A.; Azon, S.A.; Buravlev, I.Y.; Myagchilov, A.V.; Fedorets, A.N.; Rastorguev, V.L.; et al. Calcium silicate solid-state matrices from boric acid production waste for 60Co removal and immobilization by spark plasma sintering. J. Water Process Eng. 2024, 59, 105042. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M.; Maghsoodlu, M. Application of zeolites as non-phosphate detergent builders: A review. J. Environ. Chem. Eng. 2020, 8, 104287. [Google Scholar] [CrossRef]
- Xue, Z.; Li, Z.; Ma, J.; Bai, X.; Kang, Y.; Hao, W.; Li, R. Effective removal of Mg2+ and Ca2+ ions by mesoporous LTA zeolite. Desalination 2014, 341, 10–18. [Google Scholar] [CrossRef]
- Le Van Mao, R.; Vu, N.T.; Xiao, S.; Ramsaran, A. Modified zeolites for the removal of calcium and magnesium from hard water. J. Mater. Chem. 1994, 4, 1143. [Google Scholar] [CrossRef]
- Ibsaine, F.; Azizi, D.; Dionne, J.; Tran, L.H.; Coudert, L.; Pasquier, L.C.; Blais, J.F. Conversion of aluminosilicate residue generated from lithium extraction process to NaX zeolite. Minerals 2023, 13, 1467. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Q.; Yan, K.; Wang, L.; Xiao, F. Solvent-free synthesis of FAU zeolite from coal fly ash. Dalton Trans. 2023, 52, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Ma, W.; Tao, L. Study on softening of hard water by 13X-zeolite. China Min. Mag. 2003, 11, 60–62. [Google Scholar]
- Chandrasekhar, S.; Pramada, P.N. Investigation on the synthesis of zeolite NaX from Kerala kaolin. J. Porous Mat. 1999, 6, 283–297. [Google Scholar] [CrossRef]
- Kuhl, G.H.; Sherry, H.S. Low-silica type X zeolite as a potential component of laundry detergents. In Proceedings of the Fifth International Conference on Zeolites, Naples, Italy, 2–6 June 1980; pp. 813–822. [Google Scholar]
- De Lucas, A.; Uguina, M.A.; Covian, I.; Rodriguez, L. Use of Spanish natural clays as additional silica sources to synthesize 13X zeolite from kaolin. Ind. Eng. Chem. Res. 2002, 32, 1645–1650. [Google Scholar] [CrossRef]
- Tontisirin, S. Synthesis and characterization of co-crystalline zeolite composite of LSX/A. Micropor. Mesopor. Mat. 2017, 239, 123–129. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Cempa, M.; Białecka, B. Synthesis of Na-LSX type zeolite from Polish fly ash. Gospod. Surowcami. Min. 2020, 36, 145–166. [Google Scholar]
- Han, Z.; Gou, M.; Chen, Z.; Duan, Y.; Liu, Z. Sy2016d its ion exchange property. J. Chin. Ceram. Soc. 2023, 51, 2978–2985. [Google Scholar]
- Liu, C.; Rao, F.; Guo, Y.; Lu, Z.; Deng, W.; Li, G.; Zhang, H.; Qi, T.; Hu, G. CO2 capture using low silica X zeolite synthesized from low-grade coal gangue via a two-step activation method. J. Environ. Chem. Eng. 2024, 12, 112074. [Google Scholar] [CrossRef]
- Basaldella, E.I.; Tara, J.C. Synthesis of LSX zeolite in the NaK system: Influence of the NaK ratio. Zeolites 1995, 15, 243–246. [Google Scholar] [CrossRef]
- Outram, J.G.; Collins, F.J.; Millar, G.J.; Couperthwaite, S.J.; Beer, G. Process optimisation of low silica zeolite synthesis from spodumene leachate residue. Chem. Eng. Res. Des. 2023, 189, 358–370. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Zang, Y.; Zhang, R.; Qi, L.; Wang, X. Preparation of Coal Gangue Based LSX Zeolite and Its Application for Zn2+ and Ni2+ Adsorption. Sci. Adv. Mater. 2023, 15, 1311–1317. [Google Scholar]
- Panezai, H.; Sun, J.H.; Ullah, M.; Ullah, R.; Sarwar, A. Effectiveness of Bi-metallic LSX zeolites in O2 purification: Examining the impact of extra-framework cations. J. Phys. Chem. Solids 2024, 189, 111877. [Google Scholar] [CrossRef]
- Chinese Standard QB/T 1768:2003. 4A Zeolite for Detergents. Light Industry Standard of the People’s Republic of China: Beijing, China, 2003.
- Chinese Standard QB/T 19421:2003. Test Methods of Crystalline Layered Sodium Disilicate. Light Industry Standard of the People’s Republic of China: Beijing, China, 2003.
- Iwama, M.; Suzuki, Y.; Plévert, J.; Itabashi, K.; Ogura, M.; Okubo, T. Location of alkali ions and their relevance to crystallization of low silica X zeolite. Cryst. Growth Des. 2010, 10, 3471–3479. [Google Scholar] [CrossRef]
- Kuronen, M.; Harjula, R.; Jenström, J.; Vestenius, M.; Lehto, J. Effect of the framework charge density on zeolite ion exchange selectivities. Phys. Chem. Chem. Phys. 2000, 2, 2655–2659. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Yan, C.; Chen, G. Solvent-free preparation of hierarchical 4A zeolite monoliths: Role of experimental conditions. J. Cryst. Growth 2019, 528, 125286. [Google Scholar] [CrossRef]
- Coker, E.N.; Rees, L.V. Kinetics of ion exchange in quasi-crystalline aluminosilicate zeolite precursors. Micropor. Mesopor. Mat. 2005, 84, 171–178. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao, C.Y.H. Pure, single phase, high crystalline, chamfered-edge zeolite 4A synthesized from coal fly ash for use as a builder in detergents. J. Hazard. Mater. 2006, 137, 401–409. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Li, Z. Synthesis of a loess-based 4A molecular sieve and its application performance in detergents. Inorg. Chim. Acta 2024, 559, 121798. [Google Scholar] [CrossRef]
- Ayele, L.; Pérez-Pariente, J.; Chebude, Y.; Diaz, I. Synthesis of zeolite A using kaolin from Ethiopia and its application in detergents. New J. Chem. 2016, 40, 3440. [Google Scholar] [CrossRef]
- Meshram, S.U.; Khandekar, U.R.; Mane, S.M.; Mohan, A. Novel route of producing zeolite a resin for quality-improved detergents. J. Surfactants Deterg. 2015, 18, 259–266. [Google Scholar] [CrossRef]
- Zang, Y.; Wang, X.L.; Wang, S.Y. Preparation of LSX zeolite from coal gangue in Wuhai area. China Powder Sci. Technol. 2019, 25, 118613. [Google Scholar]
- Han, Z.Y.; Gou, M.L.; Chen, Z.W. Synthesis of low silicon aluminum ratio X molecular sieve from potassium feldspar and its ion exchange property. J. Chin. Ceram. Soc. 2023, 51, 2978–2985. [Google Scholar]
- Bai, Y.; Zhao, B.; Miao, Q.Y. Activation of the potassic rocks by mixed alkali fusion method and synthesis of low silica X zeolite. Non-Met. Mines 2016, 39, 20–23+53. [Google Scholar]
- Miao, Q.Y.; Zhao, B.; Liu, S.J.; Guo, J.; Tong, Y.; Cao, J. Decomposition of the potassic rocks by sub-molten salt method and synthesis of low silica X zeolite. Asia Pac. J. Chem. Eng. 2018, 57, 8381–8387. [Google Scholar] [CrossRef]
- Luo, H.J.; Zhou, T.; Zhao, W.N. Hydrothermal synthesis of high purity LSX zeolite with coal-measure kaolin. Multipurp. Util. Miner. Resour. 2012, 5, 32–34+49. [Google Scholar]


| Component | SiO2 | Al2O3 | Na2O | K2O | CaO | Fe2O3 | n (SiO2/Al2O3) |
|---|---|---|---|---|---|---|---|
| KNaLSX | 41.55 | 35.12 | 13.82 | 8.46 | 0.55 | 0.13 | 2.01 |
| KNaLSX-C | 41.19 | 35.10 | 14.48 | 9.11 | 0.03 | 0.02 | 2.00 |
| Sample | BET Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) |
|---|---|---|---|
| KNaLSX | 715 | 0.238 | 0.235 |
| KNaLSX-C | 827 | 0.264 | 0.261 |
| Sample | KNaLSX | KNaLSX-C | 4A |
|---|---|---|---|
| Calcium exchange capacity (mgCaCO3/g) | 302 | 317 | 325 |
| Magnesium exchange capacity (mgMgCO3/g) | 191 | 202 | 92 |
| Zeolite Type | Raw Material | Calcium Exchange Capacity (mgCaCO3/g) | Magnesium Exchange Capacity (mgMgCO3/g) | Reference |
|---|---|---|---|---|
| 4A | Fly ash | 142.5~188.5 | —— | [43] |
| Loess | 318.5 | —— | [44] | |
| Kaolin | 310 | —— | [45] | |
| Rice husk ash | 272.5 | —— | [46] | |
| NaX | Lithium slag | 150 | —— | [23] |
| Fly ash | 119.5 | 119 | [24] | |
| Gold mine Tailing | 62.78 | 12.56 | [25] | |
| Kaolin | 211 | 115 | [26] | |
| LSX | Lithium slag | 302 | 191 | This work |
| Model | Sample | Constant | KNaLSX | KNaLSX-C | 4A |
|---|---|---|---|---|---|
| PFO | Calcium ion | qe, mg/g | 232.9 | 244.8 | 261.5 |
| k1, 1/min | 3.0978 | 3.3477 | 0.9022 | ||
| R2 | 0.95 | 0.96 | 0.94 | ||
| Magnesium ion | qe, mg/g | 159.9 | 170.1 | 86.1 | |
| k1, 1/min | 1.456 | 1.7752 | 0.3934 | ||
| R2 | 0.90 | 0.92 | 0.95 | ||
| PSO | Calcium ion | qe, mg/g | 247.7 | 256.0 | 290.3 |
| k2, g/(mg·min) | 0.0221 | 0.0227 | 0.0046 | ||
| R2 | 0.99 | 0.99 | 0.99 | ||
| Magnesium ion | qe, mg/g | 175.8 | 185.5 | 103.6 | |
| k2, g/(mg·min) | 0.0128 | 0.0152 | 0.0041 | ||
| R2 | 0.98 | 0.99 | 0.99 |
| Model | Sample | Constant | KNaLSX-M | KNaLSX-C-M | 4A-M |
|---|---|---|---|---|---|
| PSO | Calcium ion | qe, mg/g | 203.7 | 223.5 | 255.1 |
| k2, g/(mg·min) | 0.0168 | 0.0173 | 0.0039 | ||
| R2 | 0.98 | 0.99 | 0.98 | ||
| Magnesium ion | qe, mg/g | 82.3 | 101.7 | 44.5 | |
| k2, g/(mg·min) | 0.0052 | 0.0067 | 0.0052 | ||
| R2 | 0.98 | 0.99 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Deng, L.; Zhang, L.; Cui, Q.; Wang, H. Synthesis of Low-Silicon X-Type Zeolite from Lithium Slag and Its Fast Exchange Performance of Calcium and Magnesium Ions. Materials 2024, 17, 3181. https://doi.org/10.3390/ma17133181
Wang Y, Deng L, Zhang L, Cui Q, Wang H. Synthesis of Low-Silicon X-Type Zeolite from Lithium Slag and Its Fast Exchange Performance of Calcium and Magnesium Ions. Materials. 2024; 17(13):3181. https://doi.org/10.3390/ma17133181
Chicago/Turabian StyleWang, Yu, Longbin Deng, Lin Zhang, Qun Cui, and Haiyan Wang. 2024. "Synthesis of Low-Silicon X-Type Zeolite from Lithium Slag and Its Fast Exchange Performance of Calcium and Magnesium Ions" Materials 17, no. 13: 3181. https://doi.org/10.3390/ma17133181
APA StyleWang, Y., Deng, L., Zhang, L., Cui, Q., & Wang, H. (2024). Synthesis of Low-Silicon X-Type Zeolite from Lithium Slag and Its Fast Exchange Performance of Calcium and Magnesium Ions. Materials, 17(13), 3181. https://doi.org/10.3390/ma17133181






