On the Use of Nanoparticles in Dental Implants
Abstract
:1. Introduction
2. Micro-Scale Modifications of Dental Implant Surfaces
3. Nanoscale Modifications and Coatings of Dental Implant Surfaces by Nanoparticles
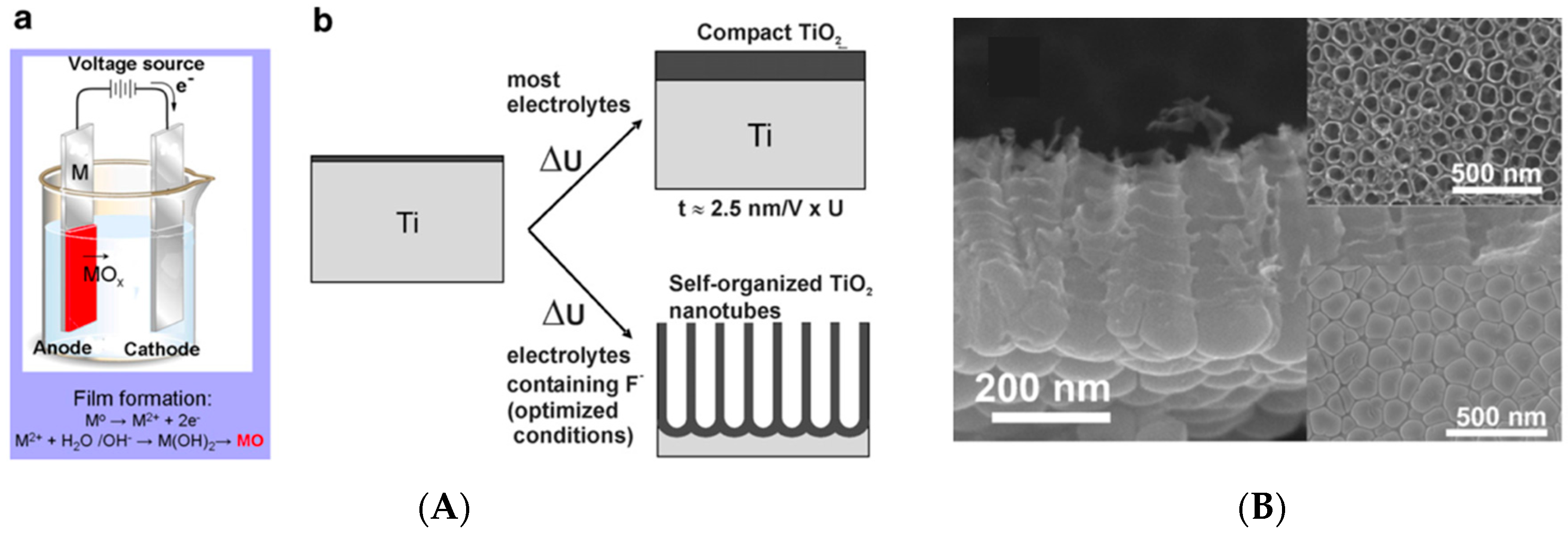
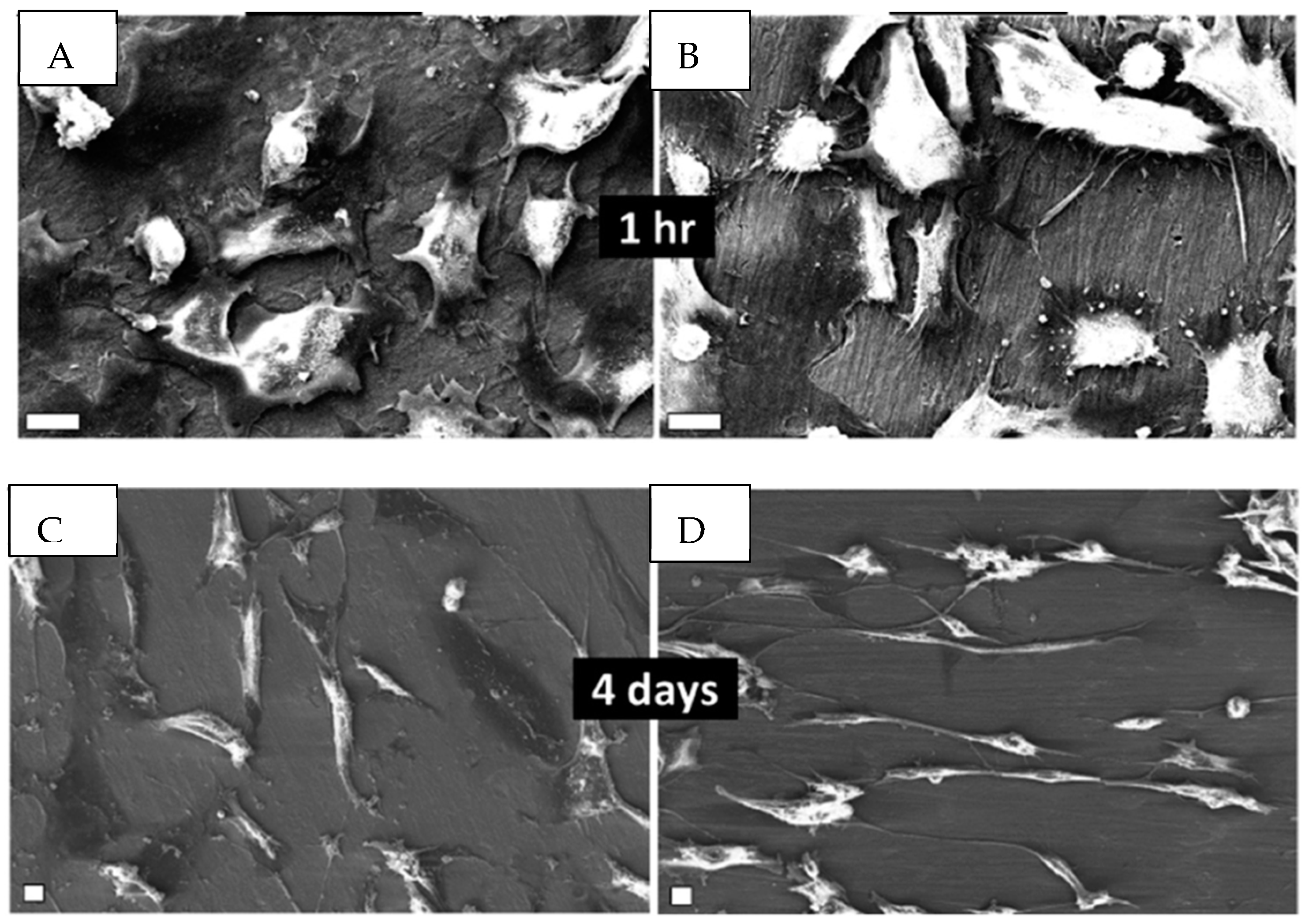
3.1. Titanium Dioxide (TiO2) Nanocoatings
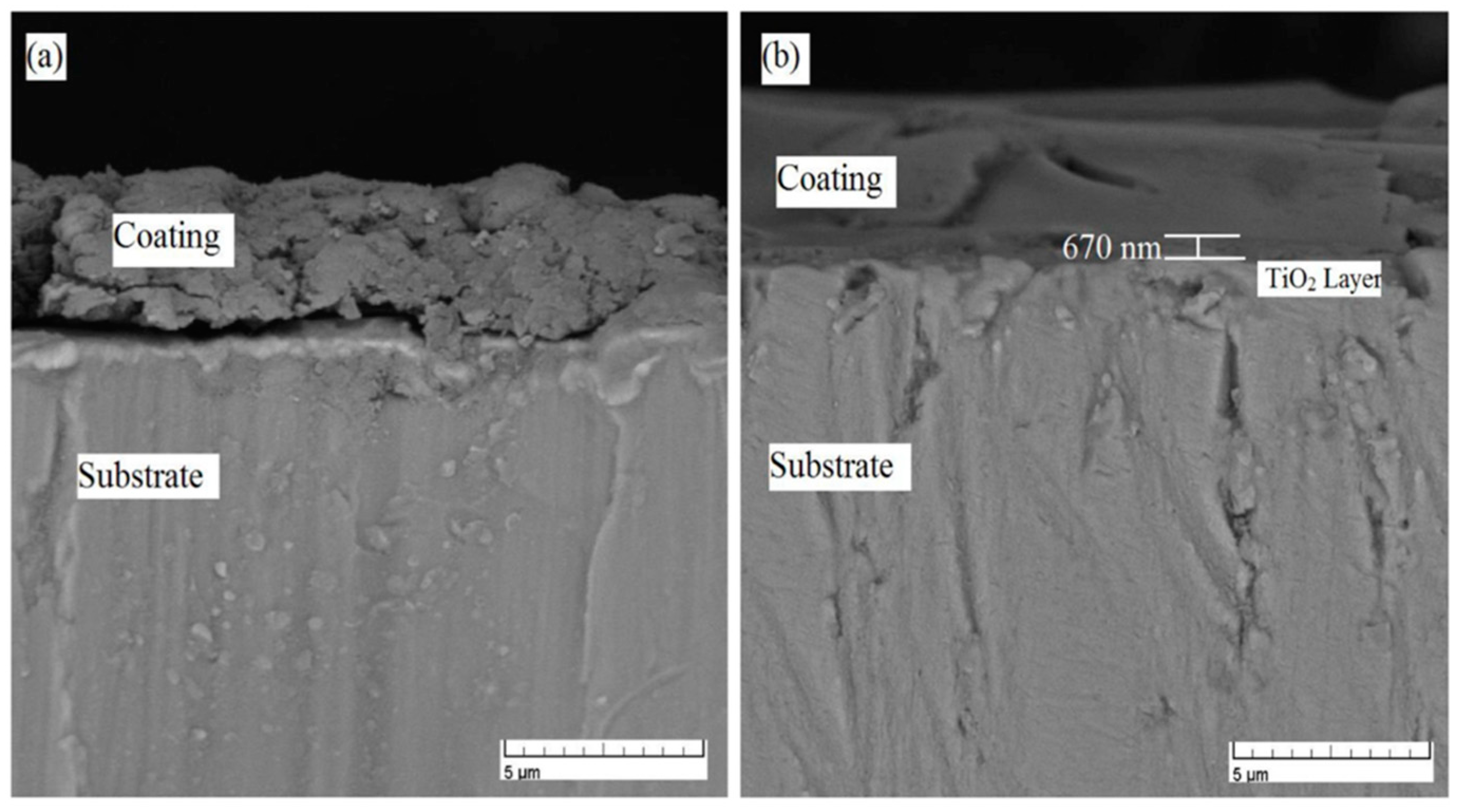
3.2. Hydroxyapatite (HA) Nanocoatings
3.3. Carbon Nanotube (CNT) Nanocoatings
3.4. Nanocoatings of Graphene-Based Materials
4. Potential Toxicity of the Implant Materials and Nanoparticle Coatings
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponnama, D.; Maria, H.J.; Chandra, A.K.; Thomas, S. Rubber nanocomposites: Latest trends and concepts. In Advances in elastomers II, Composites and Nanocomposites; Visakh, P.M., Thomas, S., Chandra, A.K., Mathew, A.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 69–107. [Google Scholar]
- Galimberti, M.; Cipoletti, V.; Musto, S.; Cioppa, S.; Peli, G.; Mauro, M.; Gaetano, G.; Agnelli, S.; Theonis, R.; Kumar, V. Recent advancements in rubber nanocomposites. Rubber Chem. Technol. 2014, 87, 417–442. [Google Scholar] [CrossRef]
- Shah, P.S.; Parekh, M.H.; Nair, K.G. A review of graphene as fillers in rubber nanocomposites. Int. J. Eng. Res. Technol. (IJERT) 2019, 8, 362–366. [Google Scholar]
- Bokobza, L. Elastomer nanocomposites: Effect of filler-matrix and filler-filler interactions. Polymers 2023, 15, 2900. [Google Scholar] [CrossRef] [PubMed]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, R.; Veerapandian, M.; Yun, K.S. Nanoparticles: Functionalization and multifunctional applications in biomedical sciences. Curr. Med. Chem. 2010, 17, 4559–4577. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, G.; Afreen, S. Application of nanotechnology in dental implants. IOSR J. Dent. Med. Sci. 2017, 16, 77–81. [Google Scholar]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles used in dentistry: A review. J. Oral Biol. Cranofacial Res. 2018, 8, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gulati, K.; Li, Z.; Di, P.; Liu, Y. Dental implant nano-engineering: Advances, limitations and future directions. Nanomaterials 2021, 11, 2489. [Google Scholar] [CrossRef]
- Vijay, R.; Mendhi, J.; Prasad, K.; Xiao, Y.; MacLeod, J.; Ostrikov, K.; Zhou, Y. Carbon nanomaterials modified biomimetic dental implants for diabetic patients. Nanomaterials 2021, 11, 2977. [Google Scholar] [CrossRef]
- Hossain, N.; Islam, M.A.; Chowdhury, M.A.; Alam, A. Advances of nanoparticles employment in dental implant applications. Appl. Surf. Sci. Adv. 2022, 12, 100341. [Google Scholar] [CrossRef]
- Gulati, K. Nano-engineering solutions for dental implant applications. Nanomaterials 2022, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasalu, P.K.P.; Dora, C.P.; Swami, R.; Jasthi, V.C.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Anwer, M.K. Nanomaterials in dentistry: Current applications and future scope. Nanomaterials 2022, 12, 1676. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, A.; Khurshid, Z.; Khan, M.T.; Mansoor, E.; Butt, F.A.; Jamal, A.; Palma, P.J. Medical and dental applications of titania nanoparticles: An overview. Nanomaterials 2022, 12, 3670. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, E.; Przybylski, J.; Witkowska-Zimny, M. Biological mechanisms of implant osseointegration. Ortop. Traumatol. Rehabil. 2010, 12, 401–409. [Google Scholar] [PubMed]
- Pandey, C.; Rokaya, D.S.; Bhattarai, B.P. Contemporary concepts in osseointegration of dental implants: A review. Hindawi. BioMed Res. Int. 2022, 2022, 6170452. [Google Scholar] [CrossRef] [PubMed]
- James, L. Osseointegration: Its mechanism and recent updates. J. Dent. Res. Pract. 2022, 4, 001. [Google Scholar]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Kasemo, B.; Larsson, K.; Lundström, I.; McQueen, D.H.; Skalak, R. The interface zone of inorganic implants in vivo: Titanium implants in bone. Ann. Biomed. Eng. 1983, 11, 1–27. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Johansson, C.; Byon, E.; Albrektsson, T. The bone response of oxidized bioactive and non-bioactive titanium implants. Biomaterials 2005, 26, 6720–6730. [Google Scholar] [CrossRef]
- Parnia, F.; Yazdani, J.; Javaherzadeh, V.; Dizaj, S.M. Overview of nanoparticle coating of dental implants for enhanced osseointegration and antimicrobial purposes. J. Pharm. Pharm. Sci. 2017, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.; Nanda, A.; Walsh, L.J.; Xu, C. Microbial decontamination and antibacterial activity of nanostructured titanium dental implants: A narrative review. Nanomaterials 2021, 11, 2336. [Google Scholar] [CrossRef]
- Al-Hassani, E.; Al-Hassani, F.; Najim, M. Effect of polymer coating on the osseointegration of CP-Ti implant. AIP Conf. Proc. 2018, 1968, 030022. [Google Scholar] [CrossRef]
- López-Valverde, N.; Aragoneses, J.; López-Valverde, B.; Rodriguez, C.; Macedo de Sousa, B.; Aragoneses, J.M. Role of chitosan in titanium coatings. Trends and new generations of coatings. Front. Bioeng. Biotechnol. 2022, 10, 907589. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Kaliaraj, G.S.; Amirtharaj Mosas, K.K. Multifunctional coatings on implant materials—A systematic review of the current scenario. Coatings 2023, 13, 69. [Google Scholar] [CrossRef]
- Ozkan, A.; Çakır, D.A.; Tezel, H.; Sanajou, S.; Yirün, A.; Baydar, T.; Erkekoglu, P. Dental implants and implant coatings: A focus on their toxicity and safety. J. Environ. Pathol. Toxicol. Oncol. 2023, 42, 31–48. [Google Scholar] [CrossRef]
- Butler, J.; Handy, R.D.; Upton, M.; Besinis, A. Review of antimicrobial nanocoatings in medicine and dentistry: Mechanisms of action, biocompatibility performance, safety, and benefits compared to antibiotics. ACS Nano 2023, 17, 7064–7092. [Google Scholar] [CrossRef] [PubMed]
- Alla, R.K.; Ginjupalli, K.; Upadhya, N.; Shammas, M.; Ravi, R.K.; Sekhar, R. Surface roughness of implants: A review. Trends Biomater. Artif. Organs 2011, 25, 112–118. [Google Scholar]
- Al-Radha, A.S.D. The influence of different acids etch on dental implants titanium surface. IOSR J. Dent. Med. Sci. 2016, 15, 87–91. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Ortiz-Garcia, I.; Jiménez-Guerra, A.; Monsalve-Guil, L.; Muñoz-Guzón, F.; Perez, R.A.; Gil, F.J. Comparison between sandblasted acid-etched and oxidized titanium dental implants: In vivo study. Int. J. Mol. Sci. 2019, 20, 3267. [Google Scholar] [CrossRef]
- Louarn, G.; Salou, L.; Hoornaert, A.; Layrolle, P. Nanostructured surface coatings for titanium alloy implants. J. Mater. Res. 2019, 34, 1892–1899. [Google Scholar] [CrossRef]
- Gehrke, S.A.; de Lima, J.H.C.; Rodriguez, F.; Calvo-Guirado, J.L.; Júnior, J.A.; Pérez-Díaz, L.; Mazón, P.; Aragoneses, J.M.; De Aza, P.N. Microgrooves and microrugosities in titanium implant surfaces: An in vitro and in vivo evaluation. Materials 2019, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Giner, L.; Mercadé, M.; Torrent, S.; Punset, M.; Pérez, R.A.; Delgado, L.M.; Gil, F.J. Double acid etching treatment of dental implants for enhanced biological properties. J. Appl. Biomater. Funct. Mater. 2018, 16, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierova, H. Cold atmospheric plasma: A powerful tool for modern medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Hartjen, P.; Gosau, M.; Vollkommer, T.; Grust, A.L.C.; Fuest, S.; Kluwe, L.; Burg, S.; Smeets, R.; Henningsen, A. Effects of a novel cold atmospheric plasma treatment of titanium on the proliferation and adhesion behavior of fibroblasts. Int. J. Mol. Sci. 2022, 23, 420. [Google Scholar] [CrossRef] [PubMed]
- Guastaldi, F.; Yoo, D.; Marin, C.; Jimbo, R.; Tovar, N.; Zanetti-Barbosa, D.; Coelho, P.G. Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int. J. Biomater. 2013, 2013, 354125. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.-S.L. Modifications of dental implants surfaces at the micro- and nano-level for enhanced osseointegration. Materials 2020, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Park, Y.-S. Plasma in dentistry. Clin. Plasma Med. 2014, 2, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Arora, V.; Nikhil, V.; Suri, N.K.; Arora, P. Cold atmospheric plasma (CAP) in dentistry. Dentistry 2014, 4, 1. [Google Scholar] [CrossRef]
- Nair, R.S.; Babu, B.; Mushtaq, E. Cold atmospheric plasma in dentistry. J. Oper. Dent. Endod. 2016, 1, 82. [Google Scholar] [CrossRef]
- Hui, W.L.; Perrotti, V.; Iaculli, F.; Piattelli, A.; Quaranta, A. The emerging role of cold atmospheric plasma in implantology: A review of the literature. Nanomaterials 2020, 10, 1505. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Brambilla, E.; Azzola, F.; Ottobelli, M.; Pellegrini, G.; Francetti, L.A. Laser microtextured titanium implant surfaces reduce in vitro and in situ oral biofilm formation. PLoS ONE 2018, 13, e0202262. [Google Scholar] [CrossRef] [PubMed]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.-A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The impact of dental implant surface modification on osseointegration and biofilm formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Sachin, P.G.; Uppoor, A.S.; Nayak, S.U. Nano-scale surface modification of dental implants- An emerging boon for osseointegration and biofilm control. Acta Marisiensis—Ser. Medica 2022, 68, 154–158. [Google Scholar] [CrossRef]
- McCarthy, D.W.; Mark, J.E.; Clarson, S.J.; Schaffer, D.W. Synthesis, structure, and properties of hybrid organic-inorganic composites based on polysiloxanes. II. Comparisons between poly(methylphenylsiloxane) and poly(dimethylsiloxane), and between titania and silica. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1191–1200. [Google Scholar] [CrossRef]
- Sharma, R.; Sarkar, A.; Jha, R.; Sharma, A.K.; Sharma, D. Sol-gel-mediated synthesis of TiO2 nanocrystals: Structural, optical, and electrochemical properties. Int. J. Appl. Ceram. Technol. 2020, 17, 1400–1409. [Google Scholar] [CrossRef]
- Bokobza, L.; Diop, A.L. Reinforcement of poly(dimethylsiloxane) by sol-gel in situ generated silica and titania particles. Express Polym. Lett. 2010, 4, 355–363. [Google Scholar] [CrossRef]
- Latifi, A.; Afshar, A.; Behnamghader, A.; Joughehdoust, S. Sol-gel derived titania coating on titanium substrate. Iran. J. Pharm. Sci. 2008, 4, 17–32. [Google Scholar]
- Choi, A.; Cazalbou, S.; Ben-Nissan, B. Nanobiomaterial coatings in dentistry. Biomater. Oral Craniomaxillofacial Appl. 2015, 17, 49–61. [Google Scholar]
- Tarala, V.A.; Dolgalev, A.A.; Kravtsov, A.A.; Chikulina, I.S.; Bukhalov, B.V. Modification of the titanium implants surface with TiO2 coatings obtained by sol-gel method via dip-coating. MATEC Web Conf. 2018, 226, 03014. [Google Scholar] [CrossRef]
- Kumar, P.S.; Kumar, S.; Grandhi, V.V.; Gupta, V. The effect of titanium implant surface topography on osseointegration: Litterature review. JMIR Biomed. Eng. 2019, 4, e13237. [Google Scholar] [CrossRef]
- Zwilling, V.; Darque-Ceretti, E.; Boutry-Forveille, A.; David, D.; Perrin, M.Y.; Aucouturier, M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf. Interface Anal. 1999, 27, 629–637. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Sathish, S. Nanotubes: A step further in implants. Int. J. Oral Health Dent. 2016, 2, 213–216. [Google Scholar]
- Monetta, T.; Acquesta, A.; Carangelo, A.; Bellucci, F. TiO2 nanotubes on Ti dental implant. Part 1: Formation and aging in Hank’s solution. Metals 2017, 7, 167. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Carangelo, A.; Bellucci, F. TiO2 nanotubes on Ti dental implant. Part 2: EIS characterization in Hank’s solution. Metals 2017, 7, 220. [Google Scholar] [CrossRef]
- Acquesta, A.; Carangelo, A.; Monetta, T. TiO2 nanotubes on Ti dental implant. Part 3: Electrochemical behavior in Hank’s solution of titania nanotubes formed in ethylene glycol. Metals 2018, 8, 489. [Google Scholar] [CrossRef]
- Li, T.; Gulati, K.; Wang, N.; Zhang, Z.; Ivanovski, S. Understanding and augmenting the stability of therapeutic nanotubes on anodized titanium implants. Mater. Sci. Eng. C 2018, 88, 182–195. [Google Scholar] [CrossRef]
- Zakir, O.; Idouhli, R.; Elyaagoubi, M.; Khadiri, M.; Aityoub, A.; Koumya, Y.; Rafqah, S.; Abouelfida, A.; Outzourhit, A. Fabrication of TiO2 nanotube by electrochemical anodization:Toward photocatalytic application. Hindawi J. Nanomater. 2020, 2020, 4745726. [Google Scholar]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef]
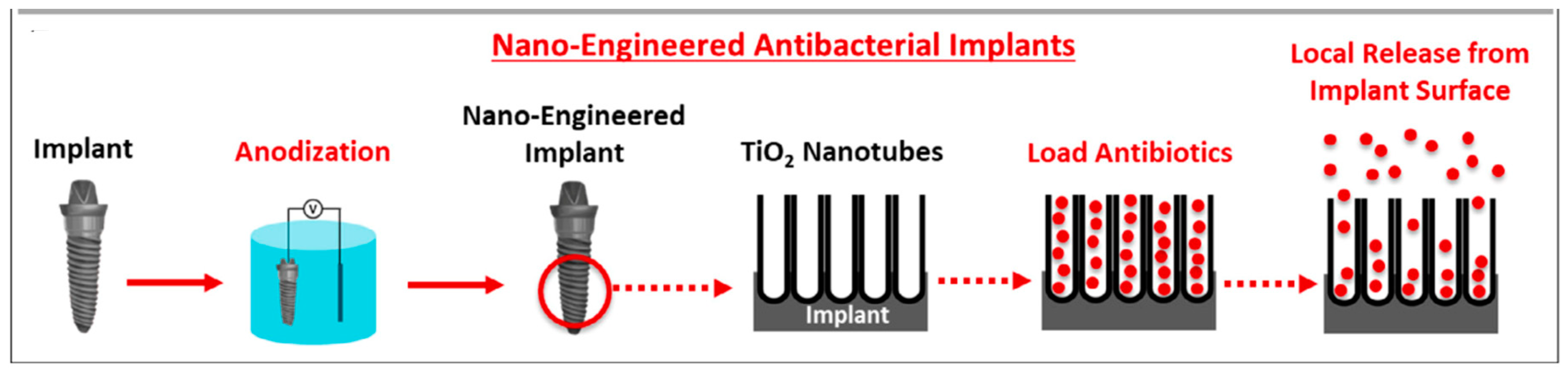
- Gulati, K.; Kogawa, M.; Maher, S.; Atkins, G.; Findlay, D.; Losic, D. Titania nanotubes for local drug delivery from implant surfaces. In Electrochemically Engineered Nanoporous Materials, Methods, Properties and Applications; Losic, D., Santos, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Chapter 10; pp. 307–355. [Google Scholar]
- Gulati, K.; Ivanovski, S. Dental implants with drug releasing titania nanotubes: Therapeutic potential and developmental challenges. Expert Opin. Drug Deliv. 2017, 14, 1009–1024. [Google Scholar] [CrossRef]
- Chopra, D.; Gulati, K.; Ivanovski, S. Understanding and optimizing the antibacterial functions of anodized nano-engineered titanium implants. Acta Biomater. 2021, 127, 80–101. [Google Scholar] [CrossRef]
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and hot responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef]
- Venugopal, A.; Muthuchamy, N.; Tejani, H.; Gopalan, A.-I.; Lee, K.-P.; Lee, H.-J.; Kyung, H.M. Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties. Korean J. Orthod. 2017, 47, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of silver nanoparticles in dentistry: Advances and Technological innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef] [PubMed]
- Suttasattakrit, K.; Khamkeaw, A.; Tangwongsan, C.; Pavasant, P.; Phisalaphong, M. Ionic silver and electrical treatment for susceptibility and disinfection of Escherichia coli biofilm-contaminated titanium surface. Molecules 2022, 27, 180. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, A.-P.; Vega-Jiménez, A.L.; Vázquez-Olmos, A.R.; Ortega-Maldonado, M.; Ximenez-Fyvie, L.-A. Antibacterial properties in vitro of magnesium oxide nanoparticles for dental applications. Nanomaterials 2023, 13, 502. [Google Scholar] [CrossRef]
- Gulati, K.; Moon, H.-J.; Li, T.; Kumar, P.T.S.; Ivanovski, S. Titania nanopores with dual micro-/nano-topography for selective cellular bioactivity. Mater. Sci. Eng. C 2018, 91, 624–630. [Google Scholar] [CrossRef]
- Rattan, P.V.; Sidhu, T.S.; Mittal, M. An overview of hydroxyapatite coated titanium implants. Asian J. Eng. Appl. Technol. 2012, 1, 40–43. [Google Scholar] [CrossRef]
- Jung, J.-H.; Kim, S.-Y.; Yi, Y.-J.; Lee, B.-K.; Kim, Y.-K. Hydroxyapatite-coated implant: Clinical prognosis assessment via a retrospective follow-up study for the average of 3 years. J. Adv. Prosthodont. 2018, 10, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Ali, S.; Kumar, B.; Zafar, M.S.; Khurshid, Z. Hydroxyapatite and nanocomposite implant coatings. In Dental Implants; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 5; pp. 69–92. [Google Scholar]
- Hashimoto, K.; Toda, Y.; Miura, K.; Udagawa, S.; Kanazawa, T. Synthesis of hydroxyapatite by sol-gel method. Phosphorus Res. Bull. 1995, 5, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jillavenkatesa, A.; Condrate SR, R.A. Sol-gel processing of hydroxyapatite. J. Mater. Sci. 1998, 33, 4111–4119. [Google Scholar] [CrossRef]
- Liu, D.-M.; Troczynsky, T.; Tseng, W.J. Water-based sol-gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Saranya, K.; Kowshik, M.; Ramanan, S.R. Synthesis of hydroxyapatite nanopowders by sol-gel emulsion technique. Bull. Mater. Sci. 2011, 34, 1749–1753. [Google Scholar] [CrossRef]
- Jafari, H.; Hessam, H.; Shahri, S.M.G.; Assadian, M.; Shairazifard, S.H.P.; Idris, M.H. Characterizing sintered nano-hydroxyapatite sol-gel coating deposited on a biomedical Ti-Zr-Nb alloy. J. Mater. Eng. Perform. 2016, 25, 901–909. [Google Scholar] [CrossRef]
- Suwanprateeb, J.; Suvannapruk, W.; Chokevivat, W.; Kiertkrittikhoon, S.; Jaruwangsanti, N.; Tienboon, P. Bioactivity of a sol-gel-derived hydroxyapatite coating on titanium implants in vitro and in vivo. Asian Biomed. 2018, 12, 35–44. [Google Scholar] [CrossRef]
- Azis, Y.; Adrian, M.; Alfarisi, C.D.; Sri, K.R.M. Synthesis of hydroxyapatite from egg shells by sol-gel method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012040. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-gel derived hydroxyapatite coatings for titanium implants: A review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Ishikawa, K.; Garskaite, E.; Kareiva, A. Sol-gel synthesis of calcium phosphate-based biomaterials-A review of environmentally benign, simple, and effective synthesis routes. J. Sol-Gel Sci. Technol. 2020, 94, 551–572. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Blanco, I.; Piccolella, S.; Pacifico, S. Coatings of titanium substrates with hydroxyapatite for biomedical application. Coatings 2020, 10, 203. [Google Scholar] [CrossRef]
- Kim, H.-W.; Koh, Y.-H.; Li, L.-H.; Lee, S.; Kim, H.-E. Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol-gel method. Biomaterials 2004, 25, 2533–2538. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Kim, H.-E.; Salih, V.; Knowles, J.C. Hydroxyapatite and titania sol-gel composite coatings on titanium for hard tissue implants; mechanical and in vitro biological performance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72B, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Azari, R.; Rezaie, H.R.; Khavandi, A. Investigation of functionally graded HA-TiO2 coating on Ti-6Al-4V substrate fabricated by sol-gel method. Ceram. Int. 2019, 45, 17545–17555. [Google Scholar] [CrossRef]
- Anavadya, K.K.; Vijayalakshmi, U. A comprehensive review of fabrication techniques and their impact on mechanical behaviour and osteoregenerative applications of bioactive inorganic substituents. Mater. Res. Lett. 2023, 11, 821–855. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Bokobza, L. Multiwall carbon nanotube elastomeric composites: A review. Polymer 2007, 48, 4907–4920. [Google Scholar] [CrossRef]
- Bokobza, L. Natural rubber nanocomposites: A review. Nanomaterials 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Avilès, F.; Cauich-Rodriguez, J.V.; Toro-Estay, P.; Yazdani-Pedram, M.; Aguilar-Bolados, H. Improving carbon nanotube/polymer interactions in nanocomposites. In Carbon Nanotube-Reinforced Polymers: From Nanoscale to Macroscale; Rafiee, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 5; pp. 83–115. [Google Scholar]
- Speranza, G. The role of functionalization in the applications of carbon materials: An overview. C J. Carbon Res. 2019, 5, 84. [Google Scholar] [CrossRef]
- Bokobza, L. Enhanced electrical and mechanical properties of multiwall carbon nanotube rubber composites. Polym. Adv. Technol. 2012, 23, 1543–1549. [Google Scholar] [CrossRef]
- Bhavikatti, S.K.; Bhardwaj, S.; Prabhuji, M.L.V. Current applications of nanotechnology in dentistry: A review. Gen. Dent. 2014, 62, 72–77. [Google Scholar] [PubMed]
- Castro-Rojas, M.A.; Vega-Cantu, Y.I.; Cordell, G.A.; Rodriguez-Garcia, A. Dental applications of carbon nanotubes. Molecules 2021, 26, 4423. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Yousefi, K.; Hashemi, S.A.; Afsa, M.; Bahrani, S.; Gholami, A.; Ghahramani, Y.; Alizadeh, A.; Chiang, W.-H. Renewable carbon nanomaterials: Novel resources for dental tissue engineering. Nanomaterials 2021, 11, 2800. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Lee, J.H.; Hong, S.W.; Lee, J.H.; Han, D.-W. Nanocomposites for enhanced osseointegration of dental and orthopedic implants revisited: Surface functionalization by nanomaterial coating. J. Compos. Sci. 2021, 5, 23. [Google Scholar] [CrossRef]
- Balani, K.; Anderson, R.; Laha, T.; Andara, M.; Tercero, J.; Crumpler, E.; Agarwal, A. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials 2007, 28, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Facca, S.; Lahiri, D.; Fioretti, F.; Messadeq, N.; Mainard, D.; Benkirane-Jessel, N.; Argawal, A. In vivo osseointegration of nano-designed composite coatings on titanium implants. ACS Nano 2011, 5, 4790–4799. [Google Scholar] [CrossRef] [PubMed]
- Abrishamchian, A.; Hooshmand, T.; Mohammadi, M.; Najafi, F. Preparation and characterization of multi-walled carbon nanotube/hydroxyapatite nanocomposite film dip coated on Ti-6Al-4V by sol-gel method for biomedical applications: An in vitro study. Mater. Sci. Eng. C 2013, 33, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Noor, A.-F.M. Hydroxyapatite reinforced with multi-walled carbon nanotubes and bovine serum albumin for bone substitute applications. AIP Conf. Proc. 2016, 1791, 020015. [Google Scholar]
- Terada, M.; Abe, S.; Akasaka, T.; Uo, M.; Kitagawa, Y.; Watari, F. Multiwalled carbon nanotube coating on titanium. Bio-Med. Eng. 2009, 19, 45–52. [Google Scholar] [CrossRef]
- Park, J.-E.; Jang, Y.-S.; Bae, T.-S.; Lee, M.-H. Multi-walled carbon nanotube coating on alkali treated TiO2 nanotubes surface for improvement of biocompatibility. Coatings 2018, 8, 159. [Google Scholar] [CrossRef]
- Dhand, V.; Rhee, K.Y.; Kim, H.J.; Jung, D.H. A comprehensive review of graphene nanocomposites: Research status and trends. J. Nanomater. 2013, 2013, 763953. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Ibrahim, A.; Klopocinska, A.; Horvat, K.; Hamid, Z.A. Graphene-based nanocomposites: Synthesis, mechanical properties, and characterizations. Polymers 2021, 13, 2869. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, C.; Shi, G. Functional composite materials based on chemically converted graphene. Adv. Mater. 2011, 23, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Development of graphene-based polymeric nanocomposites: A brief overview. Polymers 2021, 13, 2978. [Google Scholar] [CrossRef]
- Al-Harthi, M.A.; Hussain, M. Effect on the surface functionalization of graphene and MWCNT on the thermodynamic, mechanical and electrical properties of the graphene/MWCNT-PVDF nanocomposites. Polymers 2022, 14, 2976. [Google Scholar] [CrossRef]
- Sarath, P.S.; Thomas, S.; Haponiuk, J.T.; George, S.C. Fabrication, characterization and properties of silane functionalized graphene oxide/silicone rubber nanocomposites. J. Appl. Polym. Sci. 2022, 139, e52299. [Google Scholar] [CrossRef]
- Li, X.; Liang, X.; Wang, Y.; Wang, D.; Teng, M.; Xu, H.; Zhao, X.; Han, L. Graphene-based nanomaterials for dental applications: Principles, current advances, and future outlook. Front. Bioeng. Biotechnol. 2022, 10, 804201. [Google Scholar] [CrossRef]
- Rho, H.; Park, C.; Alam, K.; Kim, D.; Ji, M.-K.; Lim, H.-P.; Cho, H. Biological effects of plasma-based graphene oxide deposition on titanium. Hindawi. J. Nanomater. 2019, 2019, 9124989. [Google Scholar] [CrossRef]
- Di Carlo, R.; Di Crescenzo, A.; Pilato, S.; Ventrella, A.; Piattelli, A.; Recinella, L.; Chiavaroli, A.; Giordani, S.; Baldrighi, M.; Camisasca, A.; et al. Osteoblastic differentiation on graphene oxide-functionalized titanium surfaces: An in vitro study. Nanomaterials 2020, 10, 654. [Google Scholar] [CrossRef]
- Qi, X.; Jiang, F.; Zhou, M.; Zhang, W.; Jiang, X. Graphene oxide as a promising material in dentistry and tissue regeneration: A review. Smart Mater. Med. 2021, 2, 280–291. [Google Scholar] [CrossRef]
- Jang, W.-H.; Kim, H.-S.; Alam, K.; Ji, M.-K.; Cho, H.-S.; Lim, H.-P. Direct-deposited graphene oxide on dental implants for antimicrobial activities and osteogenesis. Int. J. Nanomed. 2021, 16, 5745–5754. [Google Scholar] [CrossRef] [PubMed]
- Nartita, R.; Andrei, M.; Ionita, D.; Didilescu, A.C.; Demetrescu, I. Can graphene oxide help to prevent peri-implantitis in the case of metallic implants? Coatings 2022, 12, 1202. [Google Scholar] [CrossRef]
- Shin, Y.C.; Bae, J.-H.; Lee, J.H.; Raja, I.S.; Kang, M.S.; Kim, B.; Hong, S.W.; Huh, J.-B.; Han, D.-W. Enhanced osseointegration of dental implants with reduced graphene oxide coating. Biomater. Res. 2022, 26, 11. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Sebastin, A.X.S.; Uthirapathy, V. In vitro electrochemical behavior of sol-gel derived hydroxyapatite/graphene oxide composite coatings on 316L SS for biomedical applications. ChemistrySelect 2020, 5, 12140–12147. [Google Scholar] [CrossRef]
- Tan, J.; Li, L.; Li, B.; Tian, X.; Song, P.; Wang, X. Titanium surfaces modified with graphene oxide/gelatin composite coatings for enhanced antibacterial properties and biological activities. ACS Omega 2022, 7, 27359–27368. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Jiang, W.; Via, B.K. Facile synthesis of soluble graphene quantum dots and its improved property in detecting heavy metal ions. Colloids Surf. B Biointerfaces 2014, 118, 72–76. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. Photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Wang, J.; Wan, H.; Zhang, Y.; Ning, Y. Photoluminescence mechanism in graphene quantum dots: Quantum confinement effect and surface/edge state. Nano Today 2017, 13, 10–14. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and applications of graphene quantum dots: A review. Nanotechnol. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Li, L.; Wu, G.; Yang, G.; Peng, J.; Zhao, J.; Zhu, J.-J. Focusing on luminescent graphene dots: Current status and future perspectives. Nanoscale 2013, 5, 4015–4039. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Jahdaly, B.A.; Elsadek, M.F.; Ahmed, B.M.; Farahat, F.F.; Taher, M.M.; Khalil, A.M. Outstanding graphene quantum dots from carbon source for biomedical and corrosion inhibition applications: A review. Sustainability 2021, 13, 2127. [Google Scholar] [CrossRef]
- Ozkan, M. Quantum dots and other nanoparticles: What can they other to drug discovery. Drug Discov. Today 2004, 9, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Jia, Q.; Liu, W.; Lan, M.; Zhou, B.; Guo, L.; Zhou, H.; Zhang, H.; Wang, Y.; Gu, Y.; et al. Carbon dots with intrinsic theranostic properties for bioimaging, red-light-triggered photodynamic/photothermal simultaneous therapy in vitro and in vivo. Adv. Healthc. Mater. 2016, 5, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, D.; Mou, X.; Li, J.; Guo, W.; Wang, S.; Yu, X.; Ma, B.; Zhang, S.; Tang, W.; et al. Effects of graphene quantum dots on the self-renewal and differentiation of mesenchymal stem cells. Adv. Healthc. Mater. 2016, 5, 702–710. [Google Scholar] [CrossRef]
- Chen, F.; Gao, W.; Qiu, X.; Zhang, H.; Liu, L.; Liao, P.; Fu, W.; Luo, Y. Graphene quantum dots in biomedical applications: Recent advances and future challenges. Front. Lab. Med. 2017, 1, 192–199. [Google Scholar] [CrossRef]
- Younis, M.R.; He, G.; Lin, J.; Huang, P. Recent advances on graphene quantum dots for bioimaging applications. Front. Chem. 2020, 8, 424. [Google Scholar] [CrossRef]
- Zhao, C.; Song, X.; Liu, Y.; Fu, Y.; Ye, L.; Wang, N.; Wang, F.; Li, L.; Mohammadniael, M.; Zhang, M.; et al. Synthesis of graphene quantum dots and their applications in drug delivery. J. Nanobiotechnol. 2020, 18, 142. [Google Scholar] [CrossRef]
- Ghahramani, Y.; Javanmardi, N. Graphene quantum dots and their applications via stem cells: A mini-review. Adv. Appl. NanoBio-Technol. 2021, 2, 54–56. [Google Scholar]
- Fallahinezhad, F.; Afsa, M.; Ghahramani, Y. Graphene quantum dots and their application in regenerative medicine: A mini-review. Adv. Appl. NanoBio-Technol. 2021, 2, 59–72. [Google Scholar]
- Krukiewicz, K.; Kazek-Kęsik, A.; Brzychczy-Włoch, M.; Łos, M.J.; Ateba, C.N.; Mehrbod, P.; Ghavami, S.; Shyntum, D.Y. Recent advances of clinically important biofilms. Int. J. Mol. Sci. 2022, 23, 9526. [Google Scholar] [CrossRef]
- Moradlou, O.; Rabiei, Z.; Delavari, N. Antibacterial effects of carbon quantum dots@hematite nanostructures deposited on titanium against Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. A Chem. 2019, 379, 144–149. [Google Scholar] [CrossRef]
- Wang, Y.; Kadiyala, U.; Qu, Z.; Elvati, P.; Altheim, C.; Kotov, N.A.; Violi, A.; VanEpps, J.S. Anti-biofilm activity of graphene quantum dots via self-assembly with bacterial amyloid proteins. ACS Nano 2019, 13, 4278–4289. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Shi, H.; Qi, Y.; Li, J.; Jing, A.; Liu, Q.; Feng, W.; Li, G.; Gao, S. Specific anti-biofilm activity of carbon quantum dots by destroying P. gingivalis biofilm related genes. Int. J. Nanomed. 2020, 15, 5473–5489. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ruiz, R.; Romanos, G. Potential causes of titanium particle and ion release in implant dentistry: A systematic review. Int. J. Mol. Sci. 2018, 19, 3585. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implant. Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Raghavan, R.V.; Melath, A.; Subair, K.; Feroz, T.P.M. Titanium toxicity-A review. J. Multidiscip. Dent. Res. 2020, 6, 81–85. [Google Scholar] [CrossRef]
- Romanos, G.E.; Fischer, G.A.; Delgado-Ruiz, R. Titanium wear of dental implants from placement, under loading and maintenance protocols. Int. J. Mol. Sci. 2021, 22, 1067. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Shi, Q.; Wang, J.; Chen, X.; Hao, Y.; Zhang, Y.; Wang, X. The unfavorable role of titanium particles released from dental implants. Nanotheranostics 2021, 5, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Callejas, J.A.; Gil, J.; Brizuela, A.; Pérez, R.A.; Bosch, B.M. Effect of the size of titanium particles released from dental implants on immunological response. Int. Mol. Sci. 2022, 23, 7333. [Google Scholar] [CrossRef] [PubMed]
- Shelly, S.; Zaltsman, S.L.; Ben-Gal, O.; Dayan, A.; Ganmore, I.; Shemesh, C.; Atrakchi, D.; Garra, S.; Ravid, O.; Rand, D.; et al. Potential neurotoxicity of titanium implants: Prospective, in-vivo and in-vitro study. Biomaterials 2021, 276, 121039. [Google Scholar] [CrossRef] [PubMed]
- Czajka, M.; Sawicki, K.; Sikorska, K.; Popek, S.; Kruzewski, M.; Kapka-Skrzypczak, L. Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol. Vitr. 2015, 29, 1042–1052. [Google Scholar] [CrossRef]
- Song, B.; Liu, J.; Feng, X.; Wei, L.; Shao, L. A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res. Lett. 2015, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Dhein, J.; Haller, C.; Reichl, F.-X.; Milz, S.; Hickel, R.; Kollmuss, M.; Högg, C. Intranuclear cell uptake and toxicity of titanium dioxide and zirconia particles as well as bacterial adhesion on dental titanium- and zirconia-implants. Dent. Mater. 2022, 38, 517–528. [Google Scholar] [CrossRef]
- Abbasi, R.; Shiney, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanoparticle Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Fubini, B.; Fenoglio, I.; Tomatis, M.; Turci, F. Effect of chemical composotion and state of the surface on the toxic response to high aspect ratio nanomaterials. Nanomedicine 2011, 6, 899–920. [Google Scholar] [CrossRef]
- Fenoglio, I.; Greco, G.; Tomatis, M.; Muller, J.; Raymundo-Piñero, E.; Béguin, F.; Fonseca, A.; Nagy, J.B.; Lison, D.; Fubini, B. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes/Physicochemical aspects. Chem. Res. Toxicol. 2008, 21, 1690–1697. [Google Scholar] [CrossRef]
- Williams, A.G.; Moore, E.; Thomas, A.; Johnson, J.A. Graphene-based materials in dental applications: Antibacterial, biocompatible, and bone regenerative properties. Hindawi Int. J. Biomater. 2023, 2023, 8803283. [Google Scholar] [CrossRef] [PubMed]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piatteli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-based nanomaterials for tissue engineering in the dental field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Nasirzadeh, N.; Azari, M.R.; Rasoulzadeh, Y.; Mohammadian, Y. An assessment of the cytotoxic effects of graphene nanoparticles on the epithelial cells of the human lung. Toxicol. Ind. Health 2019, 35, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Xiaoli, F.; Qiyue, C.; Weihong, G.; Yaqing, Z.; Chen, H.; Junrong, W.; Longquan, S. Toxicology data of graphene-family nanomaterials: An update. Arch. Toxicol. 2020, 94, 1915–1939. [Google Scholar] [CrossRef] [PubMed]
- Chiticaru, E.A.; Ionita, M. Graphene toxicity and future perspectives in healthcare and biomedicine. FlatChem 2022, 35, 100417. [Google Scholar] [CrossRef]
- Lazăr, A.-I.; Aghasoleimani, K.; Semertsidou, A.; Vyas, J.; Rosca, A.-L.; Ficai, D.; Ficai, A. Graphene-related nanomaterials for biomedical applications. Nanomaterials 2023, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.V.; Kramberger, C. Cytotoxicity of carbon nanotubes, graphene, fullerenes, and dots. Nanomaterials 2023, 13, 1458. [Google Scholar] [CrossRef]
- Kang, M.S.; Jang, H.J.; Lee, S.H.; Lee, J.E.; Jo, H.J.; Jeong, S.J.; Kim, B.; Han, D.-W. Potential of carbon-based nanocomposites for dental tissue engineering and regeneration. Materials 2021, 14, 5104. [Google Scholar] [CrossRef]

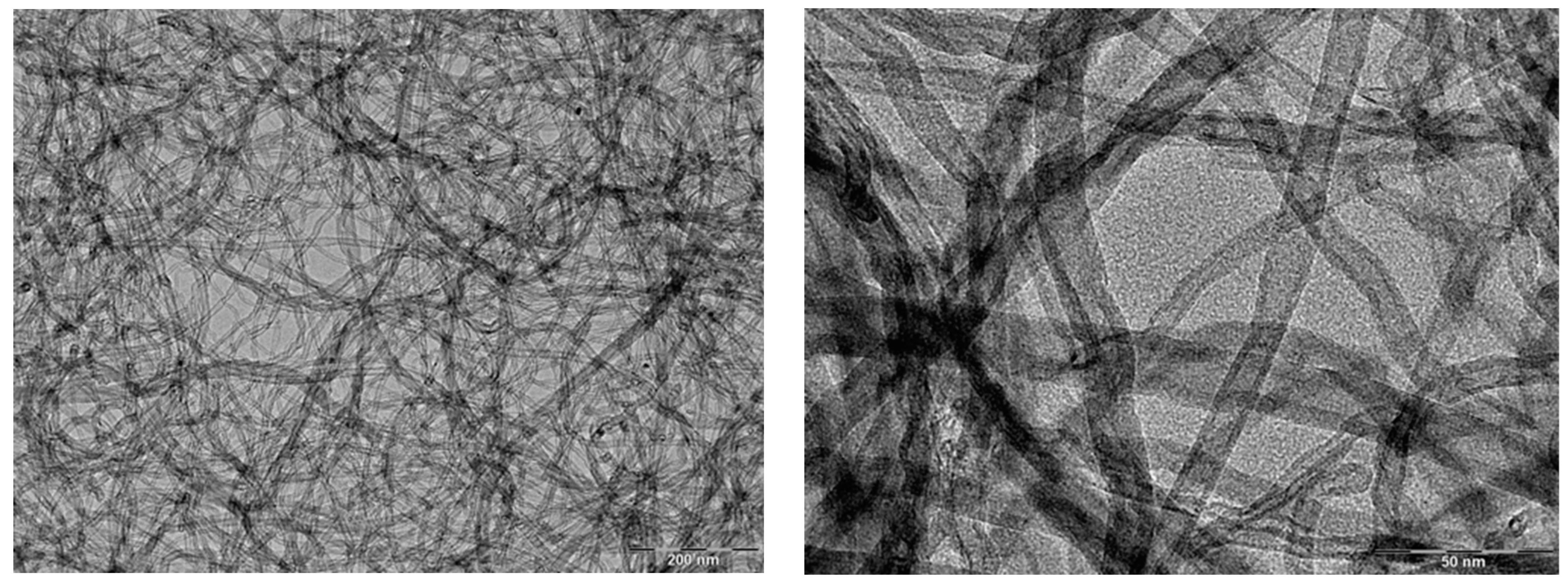
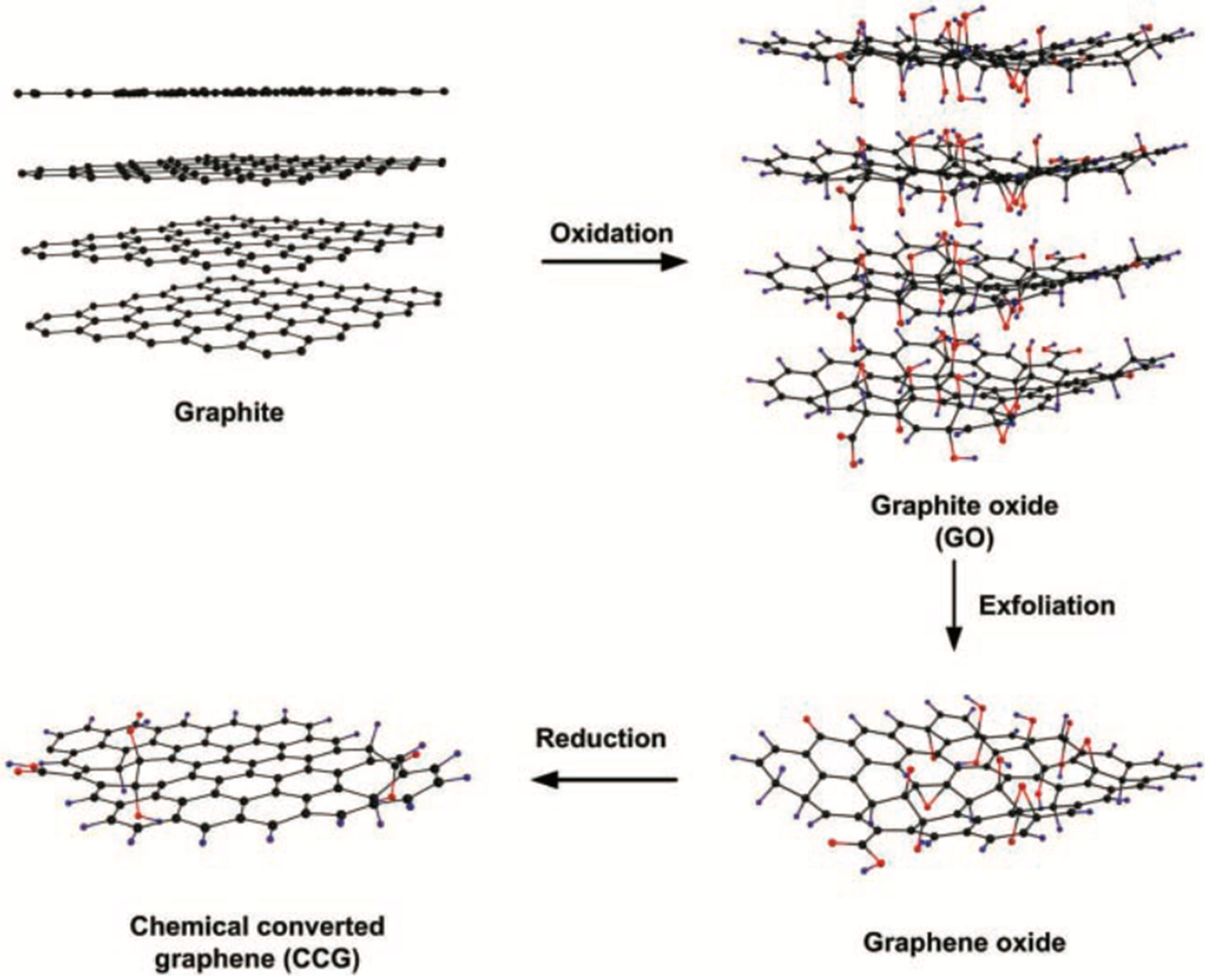
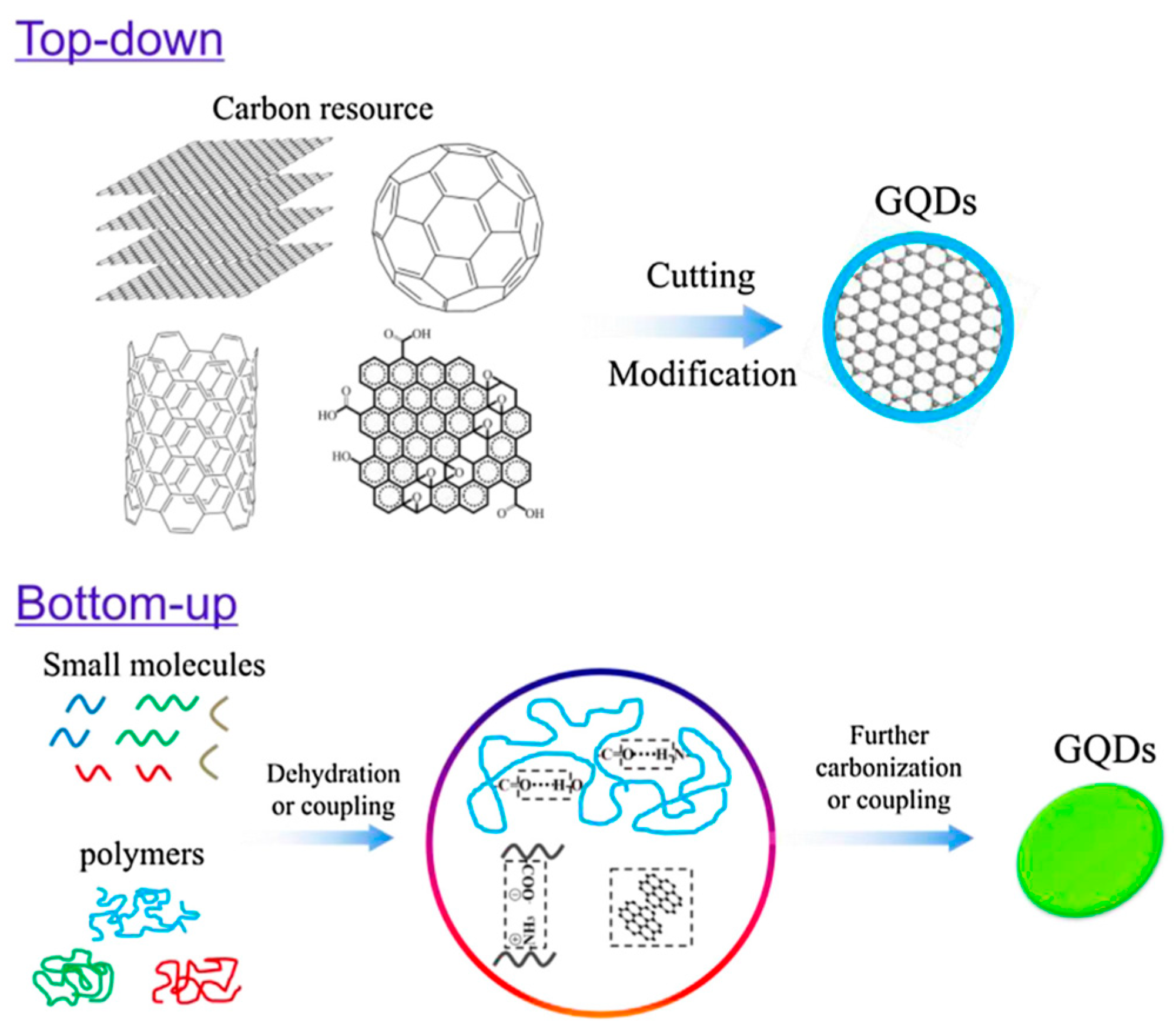
| Nanoparticles | Coating Technique | Properties | References |
|---|---|---|---|
| TiO2 | Sol-gel technique | -Produces homogeneous fine-grained titania coatings with a high surface area and almost absence of defects and fissures. -Improves the corrosion resistance of Ti. -Can be used as a buffer layer between the substrate and another coating. | [49,50,51,52] |
| TiO2 | Electrochemical anodization | -Allows the growth of 10 nm to 40 μm of compact TiO2 oxide layers. -The presence of fluoride ions allows the formation of tubular structures with, a high surface area, a high structural order and a high biocompatibility. -The nanotubes can be used as drug delivery systems. | [10,38,54,56,60] |
| Hydroxyapatite (HA) | Sol-gel technique | -Produces uniform and homogeneous coatings however poor adhesion to the substrate and low mechanical properties. -Optimizing the sol-gel processing parameters is required to achieve ideal HA coatings. -HA coated over a TiO2 layer on the Ti substrate improves the bonding to the Ti substrate as a result of the affinity of the titania layer toward HA and Ti. -HA-based nanocomposites often display better adhesion and improved mechanical properties than HA coating alone. | [82,85,86,87] [74,88] |
| Carbon nanotubes | Dip-coating | -Used as reinforcing agents on account of their exceptional mechanical and electrical properties. -The addition of CNTs in HA composite coatings improves the microhardness and elastic modulus. But they have to be used at low content (below 1 wt%) to avoid the formation of agglomerates. -Surface functionalization of CNTs can provide the desired surface chemistry in order to improve their dispersion and to allow their use at higher contents for enhanced mechanical properties. | [95,96,97] [101] [92,93,96,98] |
| Graphene-based materials | Electrophoretic deposition Sol-gel process | -Most promising materials on account of their outstanding mechanical, electrical and thermal properties. -But GO, is more suitable for dental applications since it brings hydrophilicity required to improve adhesion to the substrate. -GO-functionalization via its reactive oxygen groups increases its biocompatibility, its antibacterial properties and a better integration with the surrounding bone tissues. - Compared with pure HA coating GO/HA composite coatings display enhanced adhesion strength and corrosion resistance and reduce coating cracks. -Incorporation of GO into HA increases the hardness of the material and the corrosion resistance with a superior bonding strength between HA and the substrate. -Graphene quantum dots which are the new comers in the graphene family offer great promise as anti-biofilms and anti-bacterial agents. | [106,107] [114] [119] [120] [138,139,140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokobza, L. On the Use of Nanoparticles in Dental Implants. Materials 2024, 17, 3191. https://doi.org/10.3390/ma17133191
Bokobza L. On the Use of Nanoparticles in Dental Implants. Materials. 2024; 17(13):3191. https://doi.org/10.3390/ma17133191
Chicago/Turabian StyleBokobza, Liliane. 2024. "On the Use of Nanoparticles in Dental Implants" Materials 17, no. 13: 3191. https://doi.org/10.3390/ma17133191
APA StyleBokobza, L. (2024). On the Use of Nanoparticles in Dental Implants. Materials, 17(13), 3191. https://doi.org/10.3390/ma17133191






