Unlocking the Use of LiCl as an Inexpensive Salt for Lithium-Ion Batteries with a Novel Anion Receptor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Materials
2.2. Electrolyte Characterization
2.3. Electrochemical Measurements
3. Results
3.1. Ionic Conductivity Measurements
3.2. Quantification of Li Concentration in the Electrolyte by Elemental Analysis (ICP-OES)
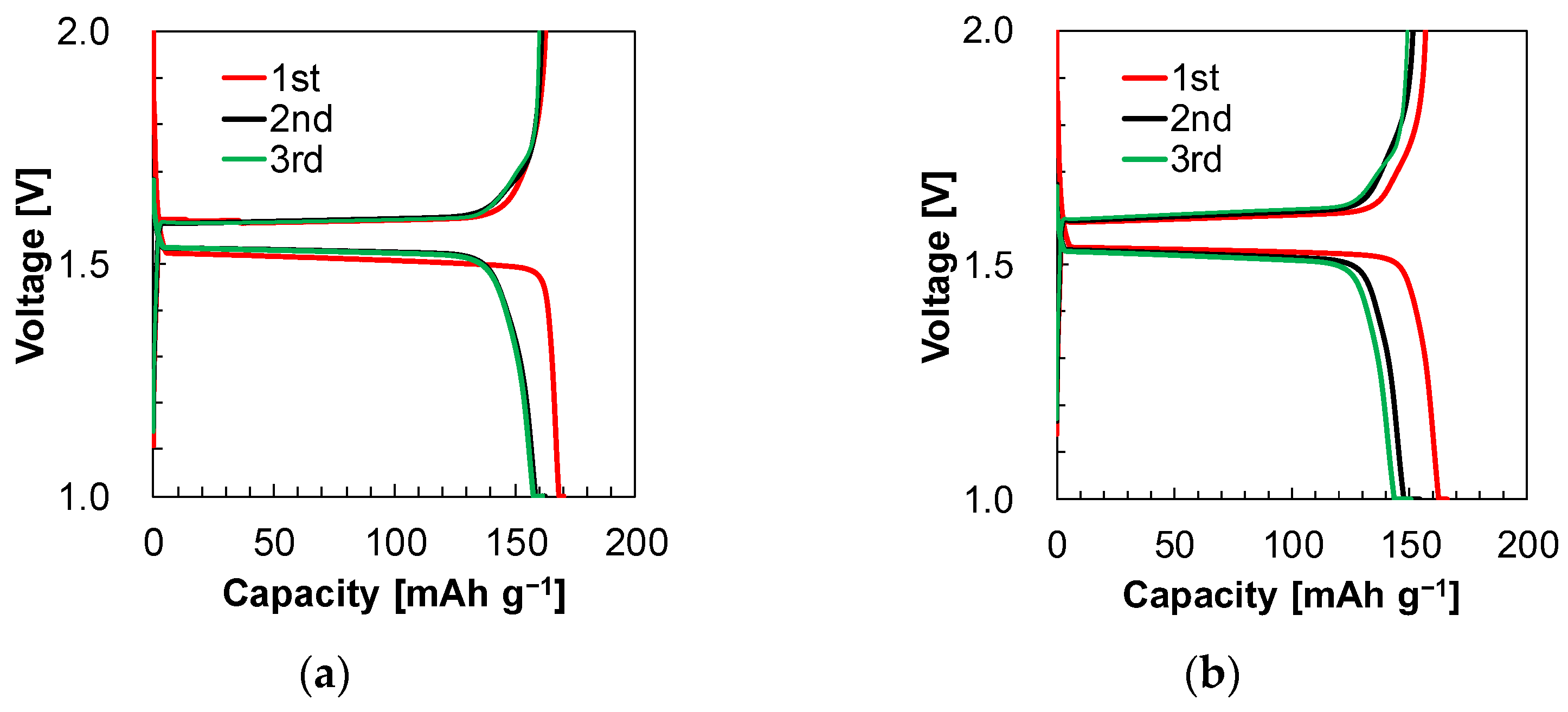
3.3. Initial Charge/Discharge Battery Property and Rate Capability
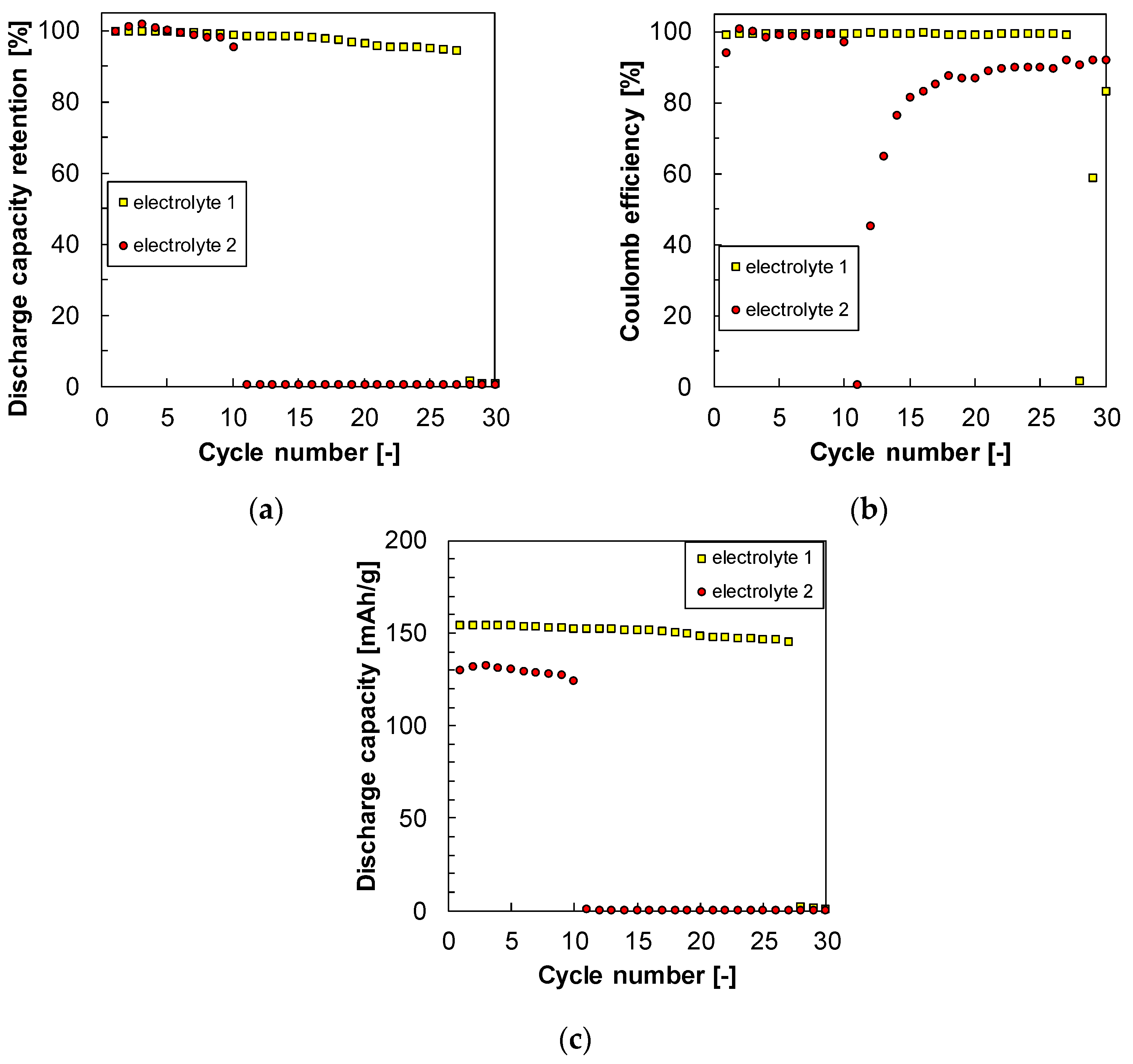
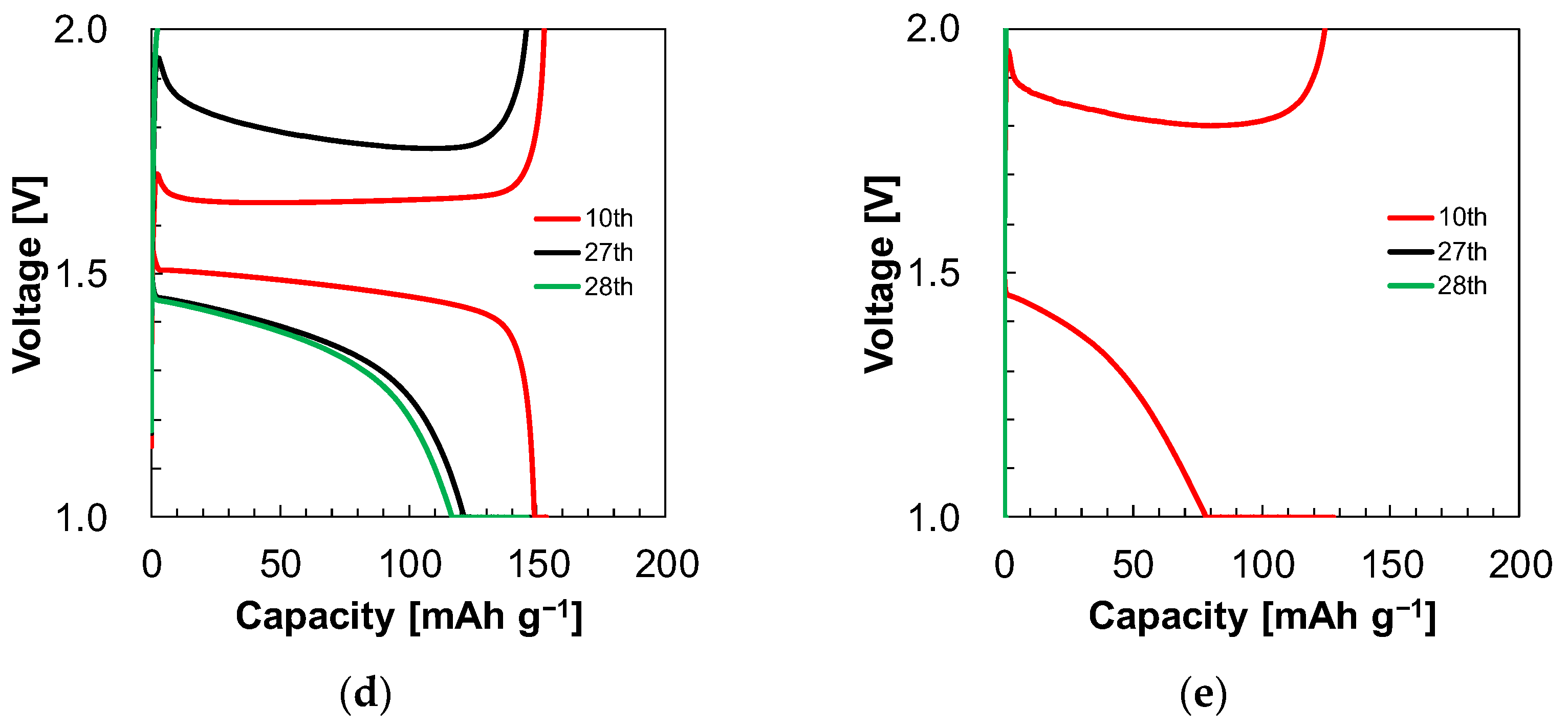
3.4. Cyclability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellis, L.D.; Hill, I.G.; Gering, K.L.; Dahn, J.R. Synergistic Effect of LiPF6and LiBF4as Electrolyte Salts in Lithium-Ion Cells. J. Electrochem. Soc. 2017, 164, A2426–A2433. [Google Scholar] [CrossRef]
- Hofmann, A.; Merklein, L.; Schulz, M.; Hanemann, T. Anodic Aluminum Dissolution of LiTFSA Containing Electrolytes for Li-Ion-Batteries. Electrochim. Acta 2014, 116, 388–395. [Google Scholar] [CrossRef]
- Du, Z.; Wood, D.L.; Belharouak, I. Enabling fast charging of high energy density Li-ion cells with high lithium ion transport electrolytes. Electrochem. Commun. 2019, 103, 109–113. [Google Scholar] [CrossRef]
- Kartha, T.R.; Mallik, B.S. Revisiting LiClO4 as an electrolyte for Li-ion battery: Effect of aggregation behavior on ion-pairing dynamics and conductance. J. Mol. Liq. 2020, 302, 112536. [Google Scholar] [CrossRef]
- McBrayer, J.D.; Rodrigues, M.-T.F.; Schulze, M.C.; Abraham, D.P.; Apblett, C.A.; Bloom, I.; Carroll, G.M.; Colclasure, A.M.; Fang, C.; Harrison, K.L.; et al. Calendar aging of silicon-containing batteries. Nature Energy 2021, 6, 866–872. [Google Scholar] [CrossRef]
- Bianchi, A.; Bowman-James, K.; Garcia-España, E. Supramolecular Chemistry of Anions; Wiley-VCH: New York, NY, USA, 1997. [Google Scholar]
- Stibor, I. (Ed.) Anion Sensing; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005; Volume 255. [Google Scholar]
- Vilar, R. Recognition of Anions; Springer: Berlin/Heidelberg, Germany, 2008; Volume 129. [Google Scholar]
- Gale, P.A.; Dehaen, W. (Eds.) Anion Recognition in Supramolecular Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; Volume 24. [Google Scholar]
- Gale, P.A.; Howe, E.N.W.; Wu, X. Anion receptor chemistry. Chem 2016, 1, 351–422. [Google Scholar] [CrossRef]
- Molina, P.; Zapata, F.; Caballero, A. Anion recognition strategies based on combined noncovalent ineractions. Chem. Rev. 2017, 117, 9907–9972. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Endo, K.; Iioka, J.; Sato, K.; Matsuta, Y. UV–vis and fluorescence detection by receptors based on an isophthalamide bearing a phenylethynyl group. Tetrahedron Lett. 2017, 58, 4115–4118. [Google Scholar] [CrossRef]
- Kondo, S.; Nagamine, M.; Karasawa, S.; Ishihara, M.; Unno, M.; Yano, Y. Anion recognition by 2,2′-binaphthalene derivatives bearing urea and thiourea groups at 8- and 8′-positions by UV–vis and fluorescence spectroscopies. Tetrahedron 2011, 67, 943–950. [Google Scholar] [CrossRef]
- Kondo, S.; Nagamine, M.; Yano, Y. Synthesis and anion recognition properties of 8,8′-dithioureido-2,2′-binaphthalene. Tetrahedron Lett. 2003, 44, 8801–8804. [Google Scholar] [CrossRef]
- Kondo, S.; Taguchi, Y.; Bie, Y. Solvent dependent intramolecular excimer emission of di(1-pyrenyl)silane and di(1-pyrenyl)methane derivatives. RSC Adv. 2015, 5, 5846–5849. [Google Scholar] [CrossRef]
- Kondo, S.; Watanabe, N.; Takahashi, F.; Takeda, N.; Unno, M. Synthesis and photophysical properties of 2,2′-binaphthalene-based receptor bearing trimethylsilyl groups to improve the solubility. J. Incl. Phenom. Macrocycl. Chem. 2012, 75, 31–38. [Google Scholar] [CrossRef]
- Reddy, V.P.; Blanco, M.; Bugga, R. Boron-based anion receptors in lithium-ion and metal-air batteries. J. Power Sources 2014, 247, 813–820. [Google Scholar] [CrossRef]
- Blazejczyk, A.; Szczupak, M.; Wiezorek, W.; Cmoch, P.; Appetecchi, G.B.; Scrosati, B.; Kovarsky, R.; Golodnitsky, D.; Peled, E. Anion-binding calixarene receptors: Synthesis, microstructure, and effect on properties of polyether electrolytes. Chem. Mater. 2005, 17, 1535–1547. [Google Scholar] [CrossRef]
- Qiao, B.; Leverick, G.M.; Zhao, W.; Flood, A.H.; Johnson, J.A.; Shao-Horn, Y. Supramolecular regulation of anions enhaces conductivity and transference number of lithium in liquid electrolytes. J. Am. Chem. Soc. 2018, 140, 10932–10936. [Google Scholar] [CrossRef]
- Mimuro, T.; Yoshida, A.; Kamo, K.; Hirasawa, M.; Kondo, S. Highly soluble bisurea derivatives for anion recognition. Org. Biomol. Chem. 2023, 21, 5281–5287. [Google Scholar] [CrossRef]
- Seki, H.; Yoshima, K.; Yamashita, Y.; Matsuno, S.; Takami, N. Aqueous lithium-ion battery of Li4Ti5O12/LiMn2O4 using a lithium-ion conductive solid electrolytes separator. J. Power Sources 2021, 482, 228950. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Wang, Y.; Wu, L.; Hu, Y. Experimental and Modeling of Conductivity for Electrolyte Solution Systems. ACS Omega 2020, 5, 22465–22474. [Google Scholar] [CrossRef]
- Robinson, R.A.; Stokes, R.H. Electrolyte Solutions. Butterworth & Co. Ltd.: Washington, DC, USA, 1959. [Google Scholar]
- Takami, N.; Hoshina, K.; Inagaki, H. Lithium Diffusion in Li4/3Ti5/3O4 Particles during Insertion and Extraction. J. Electrochem. Soc. 2011, 158, A725–A730. [Google Scholar] [CrossRef]
- Mahdavi, F.; Abdul, R.; Khanif, Y. Intercalation of urea into kaolinite for preparation of controlled release fertilizer. Chem. Ind. Chem. Eng. Q. 2014, 20, 207–213. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Gao, F.; Li, X.; Liu, C.; Li, H.; Boyd, S.A.; Johnston, C.T.; Teppen, B.J. Mechanism Associated with Kaolinite Intercalation with Urea: Combination of Infrared Spectroscopy and Molecular Dynamics Simulation Studies. J. Phys. Chem. C 2017, 121, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; Paula, J.d. Atkins’ Physical Chemistry; W. H. Freeman and Company: New York, NY, USA, 2006. [Google Scholar]
- Morita, M.; Shibata, T.; Yoshimoto, N.; Ishikawa, M. Anodic behavior of aluminum current collector in LiTFSI solutions with different solvent compositions. J. Power Sources 2003, 119–121, 784–788. [Google Scholar] [CrossRef]
- Zhang, X.; Devine, T.M. Factors That Influence Formation of AlF3 Passive Film on Aluminum in Li-Ion Battery Electrolytes with LiPF6. J. Electrochem. Soc. 2006, 153, B375. [Google Scholar] [CrossRef]
- Zeidabadinejad, L.; Hosseini, S.M.A. Computational study of inhibitory effect of urea, n-allylthiourea, thiosemicarbazide, and phenylhydrazine on the aluminum surfaces. Mater. Corros. 2019, 70, 1715–1725. [Google Scholar] [CrossRef]
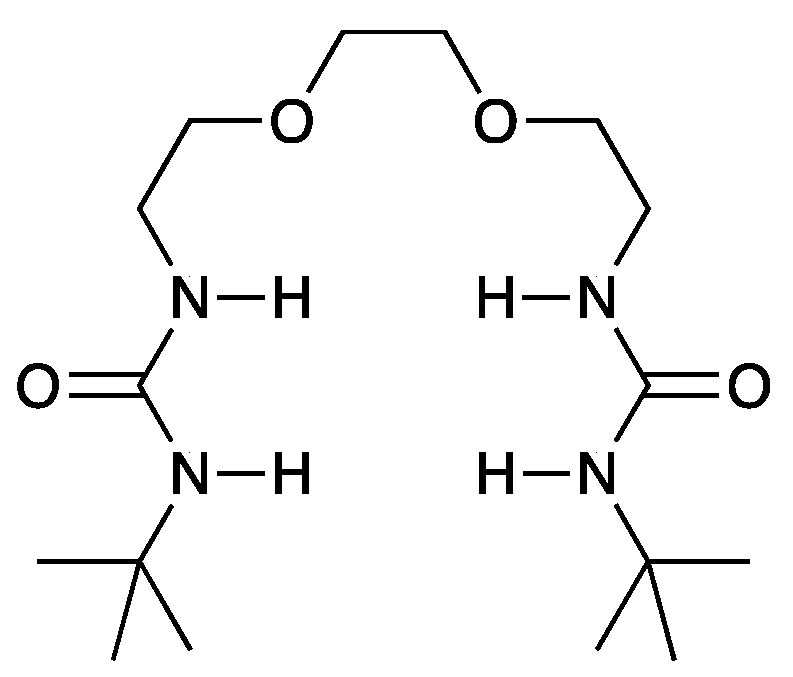

| Item | Salt | Solvent | Anion Receptor | State |
|---|---|---|---|---|
| Electrolyte 1 | LiCl | EC/DMC = 1/1 (v/v) | 0.05 M | LiCl saturated |
| Electrolyte 2 | none | |||
| Electrolyte 3 | LiPF6 | none | 1 M |
| Electrolytes | Ionic Conductivity (mS cm−1) | Ratio of Ionic Conductivity |
|---|---|---|
| Electrolyte 1 | 0.075 | 3.3 |
| Electrolyte 2 | 0.023 | 1 |
| Electrolyte 3 | 11.9 | 517 |
| Electrolytes | ) (ppm) | (M) | Ratio of Li Concentration |
|---|---|---|---|
| Electrolyte 1 | 1100 | 0.17 | 4.2 |
| Electrolyte 2 | 260 | 0.04 | 1 |
| Electrolytes | Coulombic Efficiency (%) | Charge Capacity (mAh g−1) | Discharge Capacity (mAh g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| First | Second | Third | First | Second | Third | First | Second | Third | |
| Electrolyte 1 | 95.6 | 99.2 | 99.2 | 170.2 | 162.4 | 161.4 | 162.6 | 161.1 | 160.0 |
| Electrolyte 2 | 94.5 | 98.1 | 99.1 | 166.1 | 154.4 | 150.6 | 156.9 | 151.5 | 149.2 |
| Electrolytes | Charge Capacity (mAh g−1) | Discharge Capacity (mAh g−1) | ||||
|---|---|---|---|---|---|---|
| 0.2 C | 0.5 C | 1 C | 0.2 C | 0.5 C | 1 C | |
| Electrolyte 1 | 154 | 148 | 67 | 156 | 155 | 152 |
| Electrolyte 2 | 140 | 85 | 8 | 142 | 138 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirasawa, M.; Orita, A.; Mimuro, T.; Kondo, S.-i. Unlocking the Use of LiCl as an Inexpensive Salt for Lithium-Ion Batteries with a Novel Anion Receptor. Materials 2024, 17, 3244. https://doi.org/10.3390/ma17133244
Hirasawa M, Orita A, Mimuro T, Kondo S-i. Unlocking the Use of LiCl as an Inexpensive Salt for Lithium-Ion Batteries with a Novel Anion Receptor. Materials. 2024; 17(13):3244. https://doi.org/10.3390/ma17133244
Chicago/Turabian StyleHirasawa, Manabu, Akihiro Orita, Tsubasa Mimuro, and Shin-ichi Kondo. 2024. "Unlocking the Use of LiCl as an Inexpensive Salt for Lithium-Ion Batteries with a Novel Anion Receptor" Materials 17, no. 13: 3244. https://doi.org/10.3390/ma17133244
APA StyleHirasawa, M., Orita, A., Mimuro, T., & Kondo, S.-i. (2024). Unlocking the Use of LiCl as an Inexpensive Salt for Lithium-Ion Batteries with a Novel Anion Receptor. Materials, 17(13), 3244. https://doi.org/10.3390/ma17133244






