Optimal Limestone Content on Hydration Properties of Ordinary Portland Cement with 5% Ground Granulated Blast-Furnace Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
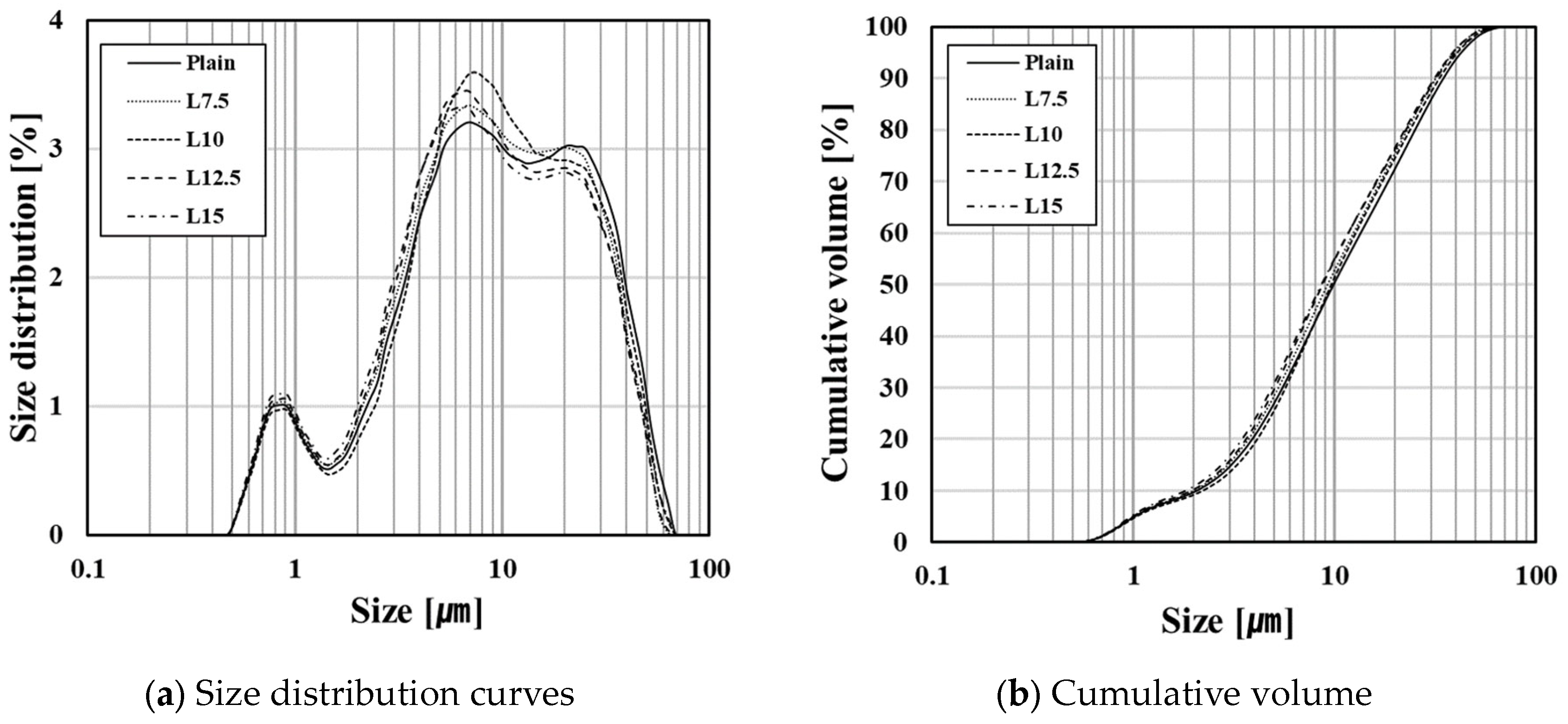
2.2. Materials
2.3. Methods
3. Results
3.1. Flow
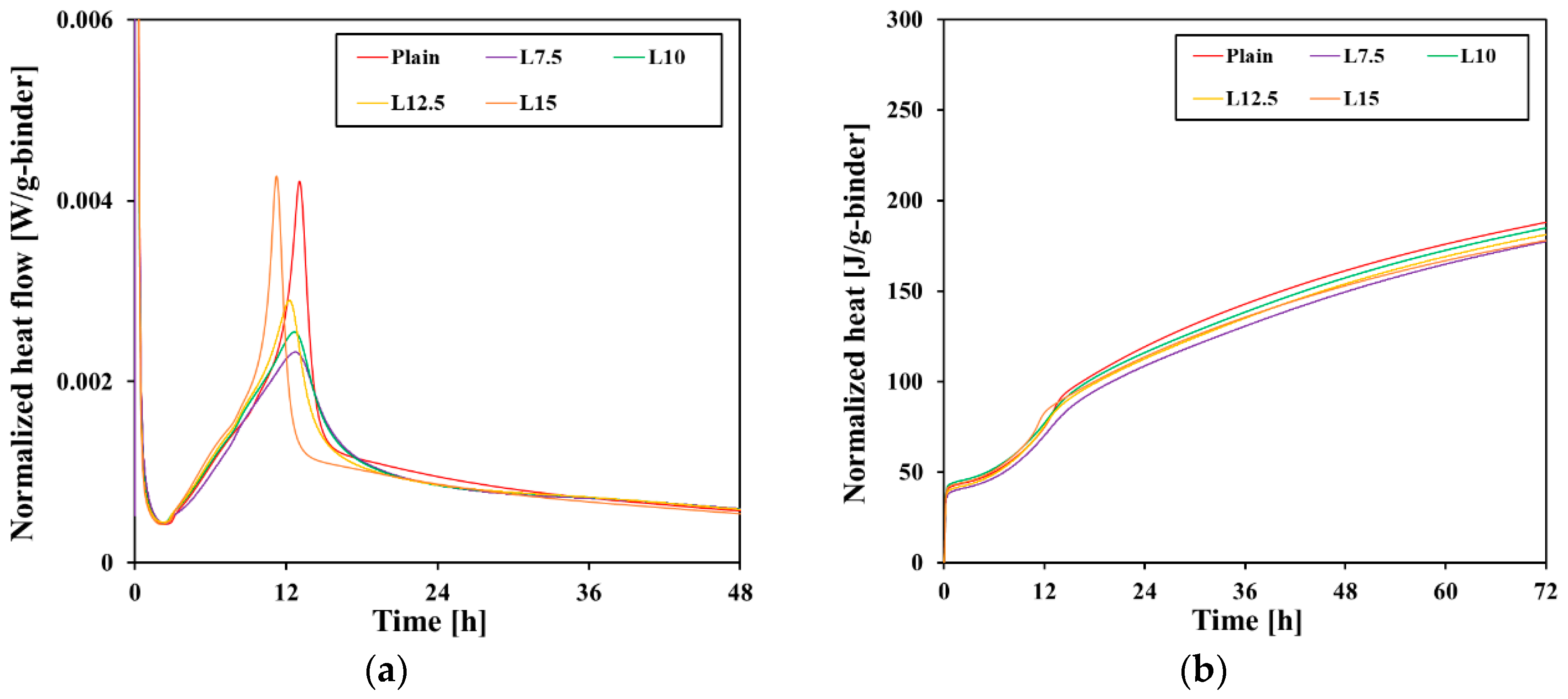
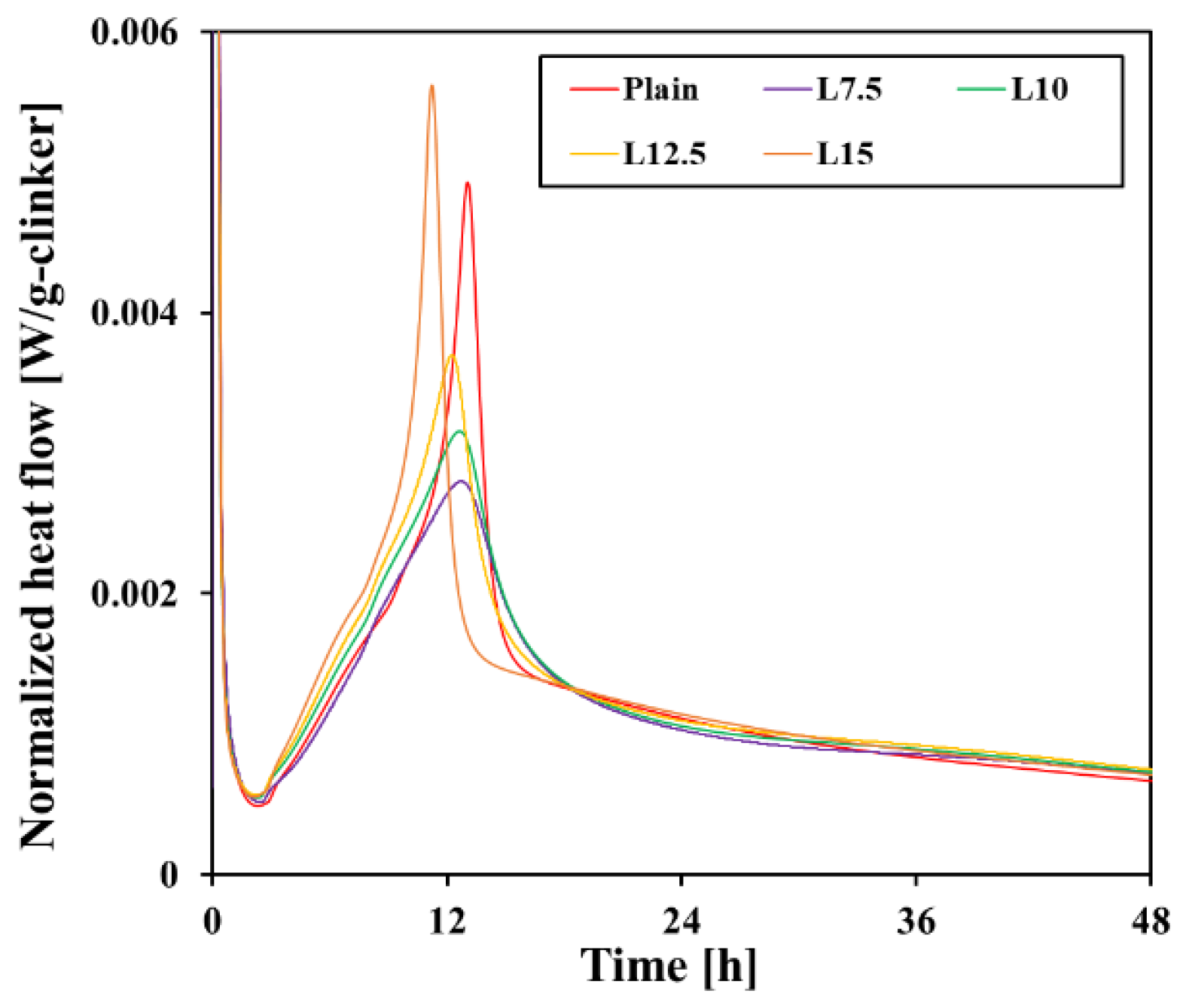
3.2. Hydration Kinetics
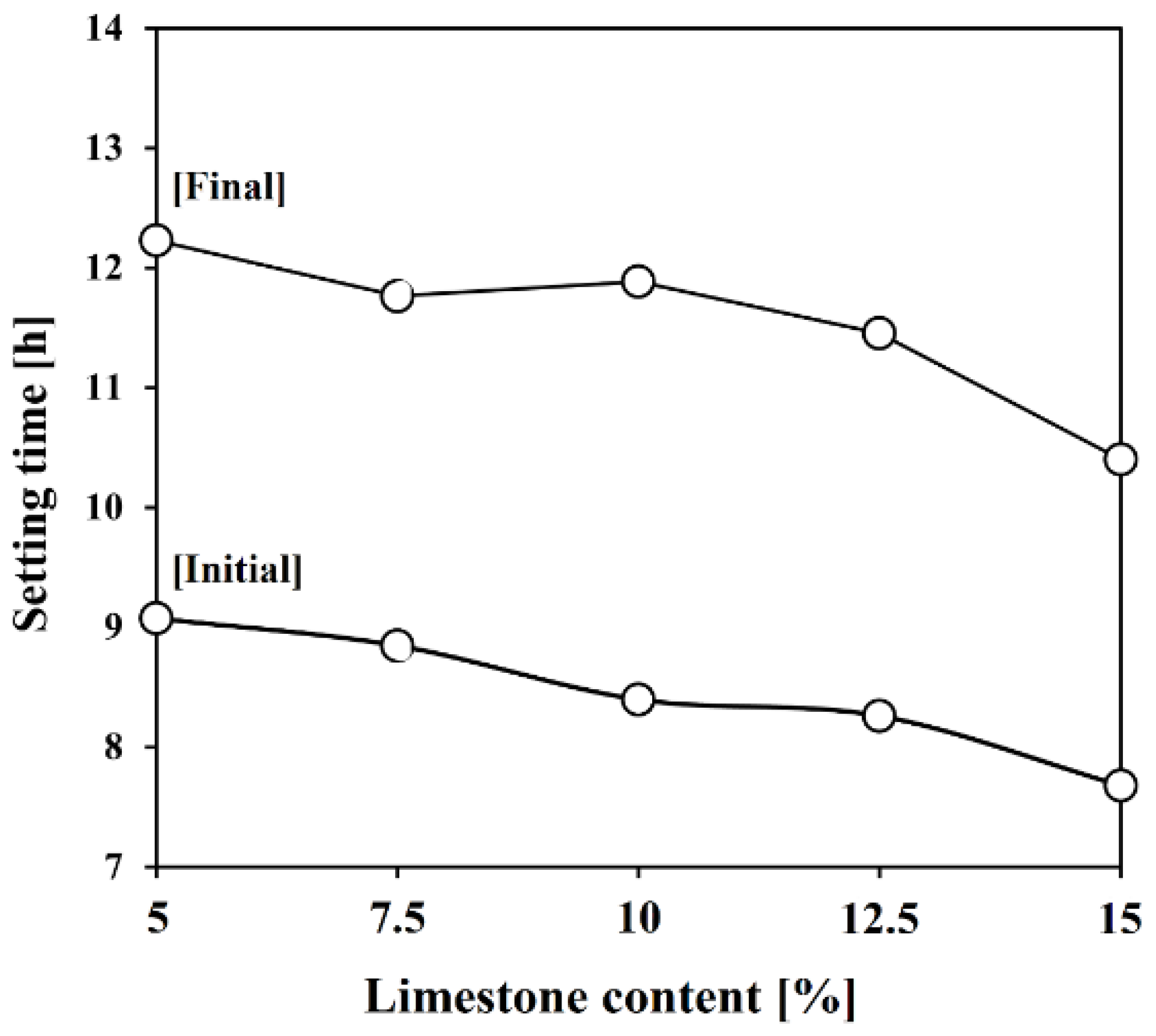
3.3. Setting Time
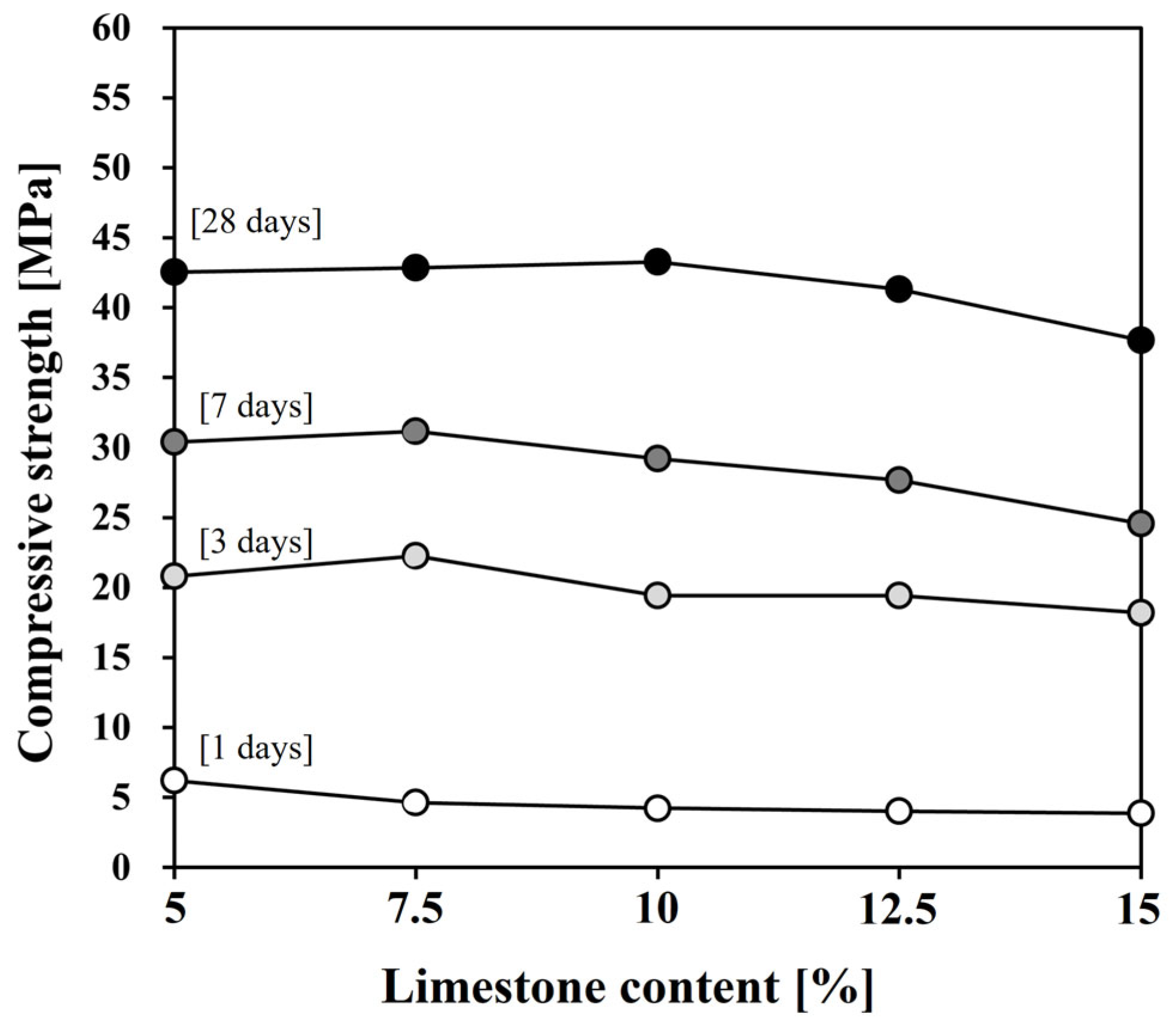
3.4. Compressive Strength Development
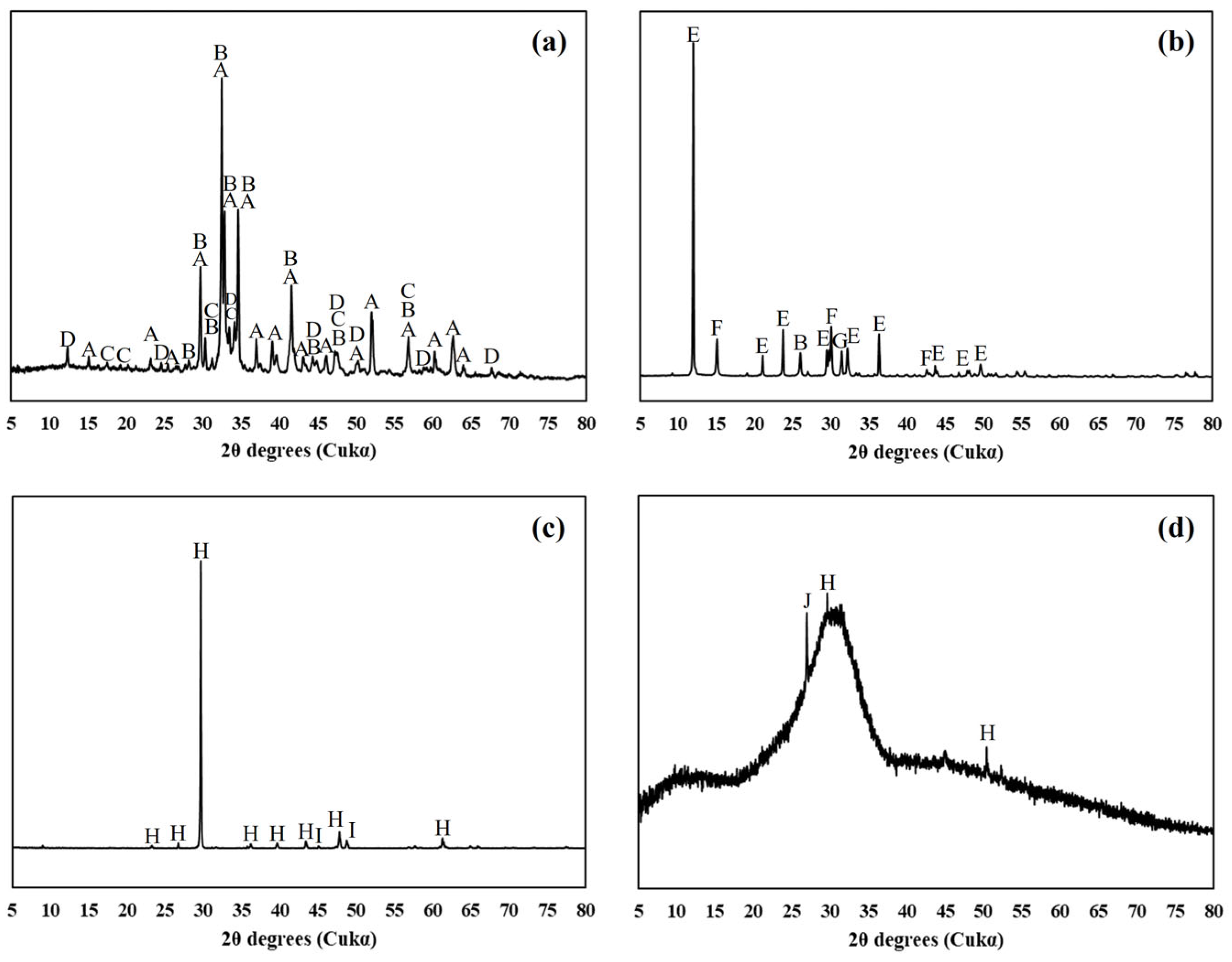
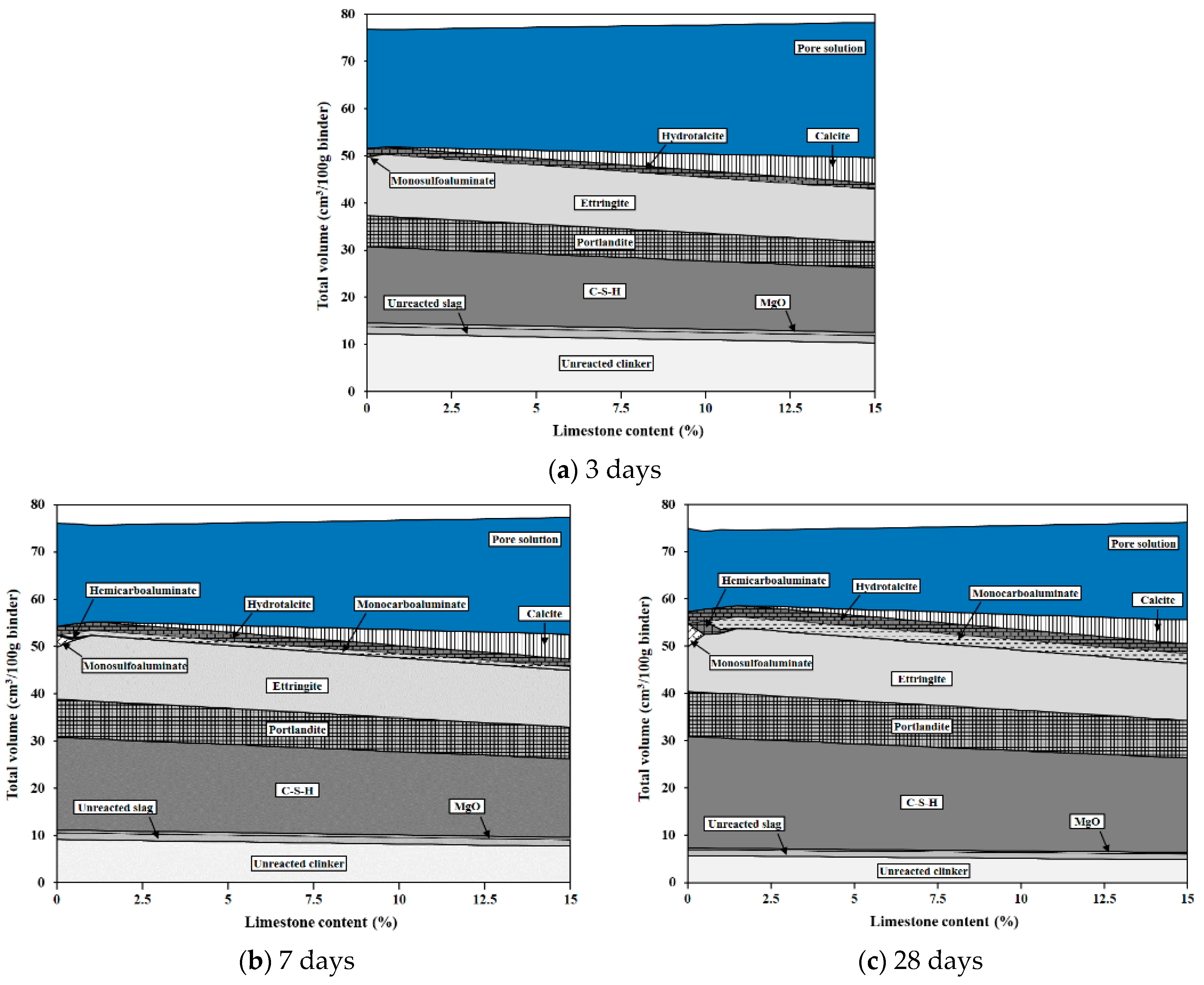
3.5. Phase Assemblages
3.5.1. X-ray Powder Diffraction
3.5.2. Thermodynamic Modeling
4. Discussion
4.1. Flow
4.2. Effect of Hydration Kinetics on Setting Time and Compressive Strength
4.3. Phase Assemblages
4.4. Determination of the Optimal Limestone Content in OPC
5. Conclusions
- With increasing limestone content, the flow decreased slightly; however, there was no significant effect on workability. Furthermore, the flow was unaffected by limestone’s particle size distribution due to co-grinding. Additional research is required to explore how flow is influenced by limestone quality, including particle size distribution.
- The filler effect of limestone accelerated the hydration of C3S after the induction period and shortened the time until the main peak. However, after 12–13 h, the dilution effect became more dominant, resulting in less heat emission than the plain cement. The clear C3A peak that occurs after gypsum consumption was not observed due to the decrease in clinker content.
- As limestone content increased, setting times were shortened, with the initial limestone accelerating the hydration of C3S. The setting times were reduced significantly by 2 h.
- After 1 day, the development of compressive strength was relatively low, despite the initial acceleration by the limestone’s filler effect. However, starting at 3 days, limestone chemically participated in the hydration reaction, forming CO3–AFm phases and stabilizing the ettringite, partially compensating for the strength reduction caused by the dilution effect. Notably, when 7.5% limestone was used, the test sample exhibited higher strength than the plain cement on all days after 3 days; at 28 days, even L10 developed higher strength than the plain cement.
- By utilizing limestone and increasing limestone content, drying shrinkage was not negatively impacted. The greatest shrinkage occurred in the plain cement, and the shrinkage reduction effect increased with higher limestone content.
- From the comparative analysis of limestone’s chemical effect through modeling and XRD patterns conducted in this study, both analytical methods confirmed that limestone participated in the chemical reaction through the formation of CO3–AFm phases and stabilization of ettringite. However, some differences in the types of phases formed depending on the age and limestone content were observed according to the analytical method.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andres, J.; Marland, G.; Boden, T.; Bischof, S. Global and National CO2 Emissions from Fossil Fuel Burning, Cement Manufacture, and Gas Flaring: 1751–2014; US Department of Energy: Washington, DC, USA, 2017.
- Favier, A.; De Wolf, C.; Scrivener, K.; Habert, G. A Sustainable Future for the European Cement and Concrete Industry-Technology Assessment for Full Decarbonisation of the Industry by 2050; ETH Zurich: Zürich, Switzerland, 2018. [Google Scholar] [CrossRef]
- EN 197-1; Cement—Part 1 Composition, Specifications and Uniformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2011.
- CSA A3001; Cementitious Materials for Use in Concrete. Canadian Standards Association: Toronto, ON, Canada, 2018.
- ASTM C595; Standard Specification for Blended Hydraulic Cements. American Society of Testing and Materials: West Conshohocken, PA, USA, 2021.
- Arora, A.; Sant, G.; Neithalath, N. Ternary blends containing slag and interground/blended limestone: Hydration, strength, and pore structure. Constr. Build. Mater. 2016, 102, 113–124. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H.; Ou, Z. A review on use of limestone powder in cement-based materials: Mechanism, hydration and microstructures. Constr. Build. Mater. 2018, 181, 659–672. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.W.; Pu, X.C.; Ye, J.X. Characteristics and mechanisms for autogenous shrinkage of cement concrete with very low water-binder ratio. Jianzhu Cailiao Xuebao/J. Build. Mater. 2010, 13, 75–79. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, W.K.; Kang, S.H. Hydration Mechanism of Ground Granulated Blast Furnace Slag. Mag. Korea Concr. Inst. 2012, 24, 31–34. [Google Scholar] [CrossRef]
- Zajac, M.; Rossberg, A.; Le Saout, G.; Lothenbach, B. Influence of limestone and anhydrite on the hydration of Portland cements. Cem. Concr. Compos. 2014, 46, 99–108. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Zaitri, R.; Bederina, M.; Bouziani, T.; Makhloufi, Z.; Hadjoudja, M. Development of high performances concrete based on the addition of grinded dune sand and limestone rock using the mixture design modelling approach. Constr. Build. Mater. 2014, 60, 8–16. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the filler effect on the nucleation and growth of C-S-H. J. Am. Ceram. Soc. 2014, 37, 64–73. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Haha, M.B.; Zajac, M.; Ludwig, H.M. Early hydration of SCM-blended Portland cements: A pore solution and isothermal calorimetry study. Cem. Concr. Res. 2017, 93, 71–82. [Google Scholar] [CrossRef]
- Adu-Amankwah, S.; Zajac, M.; Stabler, C.; Lothenbach, B.; Black, L. Influence of limestone on the hydration of ternary slag cements. Cem. Concr. Res. 2017, 100, 96–109. [Google Scholar] [CrossRef]
- Sprung, S. Assessment of the suitability of limestone for producing Portland limestone cement (PKZ). ZLG Int. 1991, 1, 43–49. [Google Scholar]
- KS L 5201; Portland Cement. Korea Standard Committee: Seoul, Republic of Korea, 2021.
- KS L ISO 679; Cement-Test Methods-Determination of Strength. Korea Standard Committee: Seoul, Republic of Korea, 2022.
- KS L 5105; Test Method for Compressive Strength of Hydraulic Cement Mortar. Korea Standard Committee: Seoul, Republic of Korea, 2022.
- KS F 2436; Standard Test Method for Setting Times of Concrete Mixture by Penetration Resistance. Korea Standard Committee: Seoul, Republic of Korea, 2022.
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnesa, B.; Miron, G.D.; Myers, R.J. Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef]
- Helgeson, H.C.; Kirkham, D.H.; Flowers, G.C. Theoretical prediction of the thermodynamic behavior of aqueous electrolytes by high pressures and temperatures; IV, Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 600 degrees C and 5kb. Am. J. Sci. 1981, 281, 1249–1516. [Google Scholar] [CrossRef]
- Parrot, L.J.; Killoh, D.C. Prediction of cement hydration. Br. Ceram. Proc. 1984, 35, 41–53. [Google Scholar]
- Fernández, Á.; Lothenbach, B.; Alonso, M.C.; Calvo, J.L.G. Thermodynamic modelling of short and long term hydration of ternary binders. Influence of Portland cement composition and blast furnace slag content. Const. Buil. Mat. 2018, 166, 510–521. [Google Scholar] [CrossRef]
- Lothenbach, B.; Winnefeld, F. Thermodynamic modelling of the hydration of Portland cement. Cem. Concr. Res. 2006, 36, 209–226. [Google Scholar] [CrossRef]
- Lothenbach, B. Thermodynamic equilibrium calculations in cementitious systems. Mater. Struct. 2010, 43, 1413–1433. [Google Scholar] [CrossRef]
- Lothenbach, B.; Wieland, E. A thermodynamic approach to the hydration of sulphate-resisting Portland cement. Waste Manag. 2006, 26, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Neuville, N.; Lécolier, E.; Aouad, G.; Rivereau, A.; Damidot, D. Effect of curing conditions on oilwell cement paste behaviour during leaching: Experimental and modelling approaches. Comptes Rendus Chim. 2009, 12, 511–520. [Google Scholar] [CrossRef]
- Briki, Y.; Haha, M.Z.M.B.; Scrivener, K. Impact of limestone fineness on cement hydration at early age. Cem. Concr. Res. 2021, 147, 106515. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Ghiasvand, E.; Nickseresht, I.; Mahdikhani, M.; Moodi, F. Influence of various amounts of limestone powder on performance of Portland limestone cement concretes. Cem. Concr. Compos. 2009, 31, 715–720. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Hallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Tsivilis, S.; Voglis, N.; Photu, J. A study of the intergrinding of clinker and limestone. Miner. Eng. 1999, 12, 837–840. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.J.; Sakai, E.; Daimon, M. Effect of particle size distribution of fly ash–cement system on the fluidity of cement pastes. Cem. Concr. Res. 2003, 33, 763–768. [Google Scholar] [CrossRef]
- Sakai, H.; Hoshimo, S.; Ohba, Y.; Daimon, M. The Fluidity of Cement Paste with Various Types of Inorganic Powders. In Proceedings of the 10th International Congress on the Chemistry of Cement, Gothenburg, Sweden, 2–6 June 1997; pp. 19–26. [Google Scholar]
- Abdulgader, M.; Khalid, H.R.; Ibrahim, M.; Adekunle, S.K.; Al-Osta, M.A.; Ahmad, S.; Sajid, M. Physicochemical properties of limestone calcined clay cement (LC3) concrete made using Saudi clays. J. Mater. Res. Technol. 2023, 25, 2769–2783. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.S.; Lee, J.I.; Cho, J.W. Effects of Limestone Powder on the Fluidity of Ordinary Portland Cement Paste. J. Korean Ceram. Soc. 2013, 50, 149–156. [Google Scholar] [CrossRef]
- Ouyang, X.; Koleva, D.A.; Ye, G.; Breugel, K.C. Understanding the adhesion mechanisms between CSH and fillers. Cem. Concr. Res. 2017, 100, 275–283. [Google Scholar] [CrossRef]
- John, E.; Matschei, T.; Stephan, D. Nucleation seeding with calcium silicate hydrate—A review. Cem. Concr. Res. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- Barbarulo, R.; Peycelon, H.; Prene, S. Experimental study and modelling of sulfate sorption on calcium silicate hydrates. Ann. Chim. Sci. Mater. 2003, 28, S5–S10. [Google Scholar]
- Kadri, E.H.; Aggoun, S.; Schutter, G.; Ezziane, K. Combined effect of chemical natureand fineness of mineral powders on Portland cement hydration. Mater. Struct. 2010, 43, 665–673. [Google Scholar] [CrossRef]
- Bergold, S.T.; Goetz-Neunhoeffer, F.; Neubauer, J. Interaction of silicate and aluminate reaction in a synthetic cement system: Implications for the process of alite hydration. Cem. Concr. Res. 2017, 93, 32–44. [Google Scholar] [CrossRef]
- Quennoz, A.; Scrivener, K.L. Interactions between alite and C3A-gypsum hydrations in model cements. Cem. Concr. Res. 2013, 44, 46–54. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. The reaction between metakaolin and limestone and its effect in porosity refinement and mechanical properties. Cem. Concr. Res. 2021, 140, 106307. [Google Scholar] [CrossRef]
- Bonavetti, V.L.; Rahhal, V.F.; Irassar, E.F. Studies on the carboaluminate formation in limestone filler-blended cements. Cem. Concr. Res. 2001, 31, 853–859. [Google Scholar] [CrossRef]
- Scrivener, K.; Ouzia, A.; Juilland, P.; Mohamed, A.K. Advances in understanding cement hydration mechanisms. Cem. Concr. Res. 2019, 124, 105823. [Google Scholar] [CrossRef]
- John, E.; Lothenbach, B. Cement hydration mechanisms through time—A review. J. Mater. Sci. 2023, 58, 9805–9833. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Farzadnia, N.; Shi, Z.; Jia, H. A review on effects of linestone powder on the properties of concrete. Constr. Build. Mater. 2018, 192, 153–166. [Google Scholar] [CrossRef]
- Moon, G.D.; Oh, S.W.; Jung, S.H.; Choi, Y.C. Effects of the fineness of limestone powder and cement on the hydration and strength development of PLC concrete. Constr. Build. Mater. 2017, 135, 129–136. [Google Scholar] [CrossRef]
- Bentz, D.P.; Stutzman, P.E.; Zunino, F. Low-temperature curing strength enhancement in cement-based materials containing limestone powder. Mater. Struct. Constr. 2017, 50, 551. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. Microstructural developments of limestone calcined clay cement (LC3) pastes after long-term (3 years) hydration. Cem. Concr. Res. 2022, 153, 106693. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Chou, L.; Garrels, R.M.; Wollast, R. Comparative study of the kinetics and mechanisms of dissolution of carbonate minerals. Chem. Geol. 1989, 78, 269–282. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Bloom, P.R. An evaluation of rate equations for calcite precipitation kinetics at pCO2 less than 0.01atm and pH greater than 8. Geochim. Cosmochim. Acta 1985, 49, 2165–2180. [Google Scholar] [CrossRef]
- Lothenbach, B.; Matschei, T.; Möschner, G.; Glasser, F.P. Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cem. Concr. Res. 2008, 38, 1–18. [Google Scholar] [CrossRef]
- Lee, J.K.; Chu, Y.S.; Song, H. Effect of Limestone Powder on Hydration of C3A-CaSO4. J. Korean Ceram. Soc. 2011, 48, 584–588. [Google Scholar] [CrossRef]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete; Prentice Hall: Upper Saddle River, NJ, USA, 2003; pp. 67–68. [Google Scholar]
- Pan, S.-Y.; Lai, B.; Ren, Y. Mechanistic insight into mineral carbonation and utilization in cement-based materials at solid–liquid interfaces. RSC Adv. 2019, 53, 31052–31061. [Google Scholar] [CrossRef] [PubMed]
- Tsivilis, S.; Chaniotakis, E.; Kakali, G.; Batis, G. An analysis of the properties of Portland limestone cements and concrete. Cem. Concr. Compos. 2002, 24, 371–378. [Google Scholar] [CrossRef]



| Sample | Materials (%) | |||
|---|---|---|---|---|
| Clinker | Gypsum | Limestone | GGBFS | |
| Plain | 85.5 | 4.5 | 5.0 | 5 |
| L7.5 | 83.125 | 4.375 | 7.5 | 5 |
| L10 | 80.75 | 4.25 | 10.0 | 5 |
| L12.5 | 78.375 | 4.125 | 12.5 | 5 |
| L15 | 76.0 | 4.0 | 15.0 | 5 |
| Property | Material (%) | ||||
|---|---|---|---|---|---|
| Clinker | Gypsum | Limestone | GGBFS | ||
| Density (g/cm3) | 3.15 | 2.81 | 2.75 | 2.95 | |
| Chemical composition (%) | CaO | 64.2 | 32.4 | 52.0 | 44.2 |
| SiO2 | 17.1 | 1.7 | 0.3 | 33.2 | |
| Al2O3 | 4.0 | 0.7 | 0.1 | 13.2 | |
| MgO | 2.5 | 0.3 | 0.5 | 3.3 | |
| Fe2O3 | 3.4 | 0.8 | 0.1 | 0.8 | |
| K2O | 1.3 | 0.1 | 0.02 | 0.6 | |
| SO3 | 3.5 | 37.6 | 0.03 | 1.8 | |
| L.O.I | 2.1 | 24.4 | 40.7 | 0.92 | |
| Property | Material (%) | |||||
|---|---|---|---|---|---|---|
| Plain | L7.5 | L10 | L12.5 | L15 | ||
| Physical | Density (g/cm3) | 3.15 | 3.14 | 3.15 | 3.14 | 3.14 |
| Fineness (cm2/g) | 3.693 | 3.825 | 3.823 | 3.940 | 4.001 | |
| Mean (μm) | 14.67 | 13.52 | 14.11 | 13.15 | 13.28 | |
| Chemical (%) | CaO | 62.6 | 63.1 | 62.9 | 63.1 | 63.5 |
| SiO2 | 17.5 | 16.9 | 16.3 | 15.7 | 14.9 | |
| Al2O3 | 4.7 | 4.6 | 4.4 | 4.3 | 4.2 | |
| MgO | 2.3 | 2.1 | 2.1 | 2.0 | 1.9 | |
| Fe2O3 | 3.1 | 3.1 | 2.9 | 2.9 | 2.9 | |
| K2O | 1.2 | 1.2 | 1.0 | 1.0 | 1.0 | |
| SO3 | 3.7 | 3.5 | 3.4 | 3.4 | 3.2 | |
| L.O.I | 3.4 | 4.1 | 5.5 | 6.4 | 7.3 | |
| Sample | Relative Compressive Strength, f/fPlain (%) | |||
|---|---|---|---|---|
| 1 Day | 3 Days | 7 Days | 28 Days | |
| Plain | 100 | 100 | 100 | 100 |
| L7.5 | 74.2 | 107.2 | 102.6 | 100.9 |
| L10 | 67.7 | 93.3 | 96.1 | 101.9 |
| L12.5 | 64.5 | 93.3 | 91.1 | 97.2 |
| L15 | 62.9 | 87.5 | 80.9 | 88.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, I.; Shin, S.; Kim, J. Optimal Limestone Content on Hydration Properties of Ordinary Portland Cement with 5% Ground Granulated Blast-Furnace Slag. Materials 2024, 17, 3255. https://doi.org/10.3390/ma17133255
Kang I, Shin S, Kim J. Optimal Limestone Content on Hydration Properties of Ordinary Portland Cement with 5% Ground Granulated Blast-Furnace Slag. Materials. 2024; 17(13):3255. https://doi.org/10.3390/ma17133255
Chicago/Turabian StyleKang, Ingyu, Sangchul Shin, and Jinman Kim. 2024. "Optimal Limestone Content on Hydration Properties of Ordinary Portland Cement with 5% Ground Granulated Blast-Furnace Slag" Materials 17, no. 13: 3255. https://doi.org/10.3390/ma17133255





