Microwave-Assisted Pyrolysis of Carbon Fiber-Reinforced Polymers and Optimization Using the Box–Behnken Response Surface Methodology Tool
Abstract
:1. Introduction
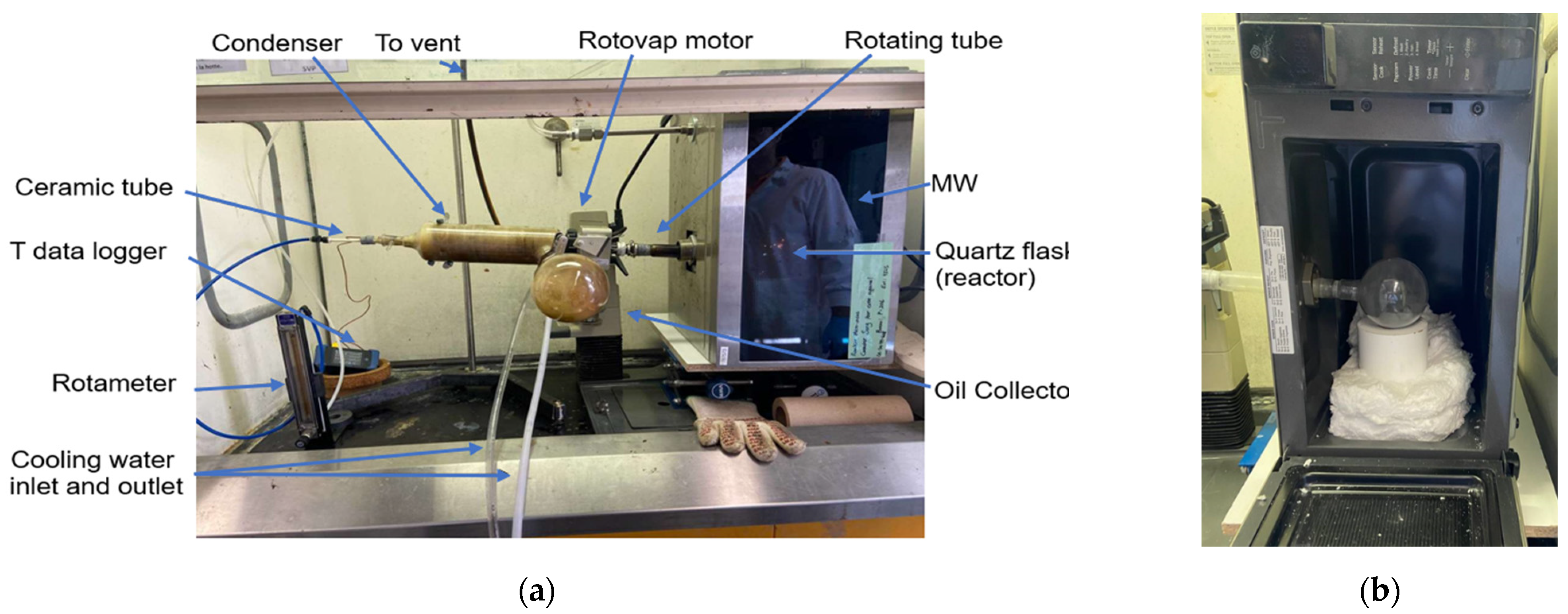
2. Materials and Methods
- Microwave oven (1100 W model);
- Spherical quartz flask (250 mL);
- Two thermocouples;
- Rotary evaporator;
- Data logger;
- Rotameter;
- Bath circulator;
- Ceramic tube.
2.1. Reclamation Procedure
2.2. Design of Experiments via the Three-Level Box–Behnken RSM Tool
2.3. Post-Pyrolysis Treatments
- Utilizing quartz sand abrasion in MAP
- b.
- Chemical dissolution using sulfuric acid and hydrogen peroxide mixture
- c.
- Thermal oxidation
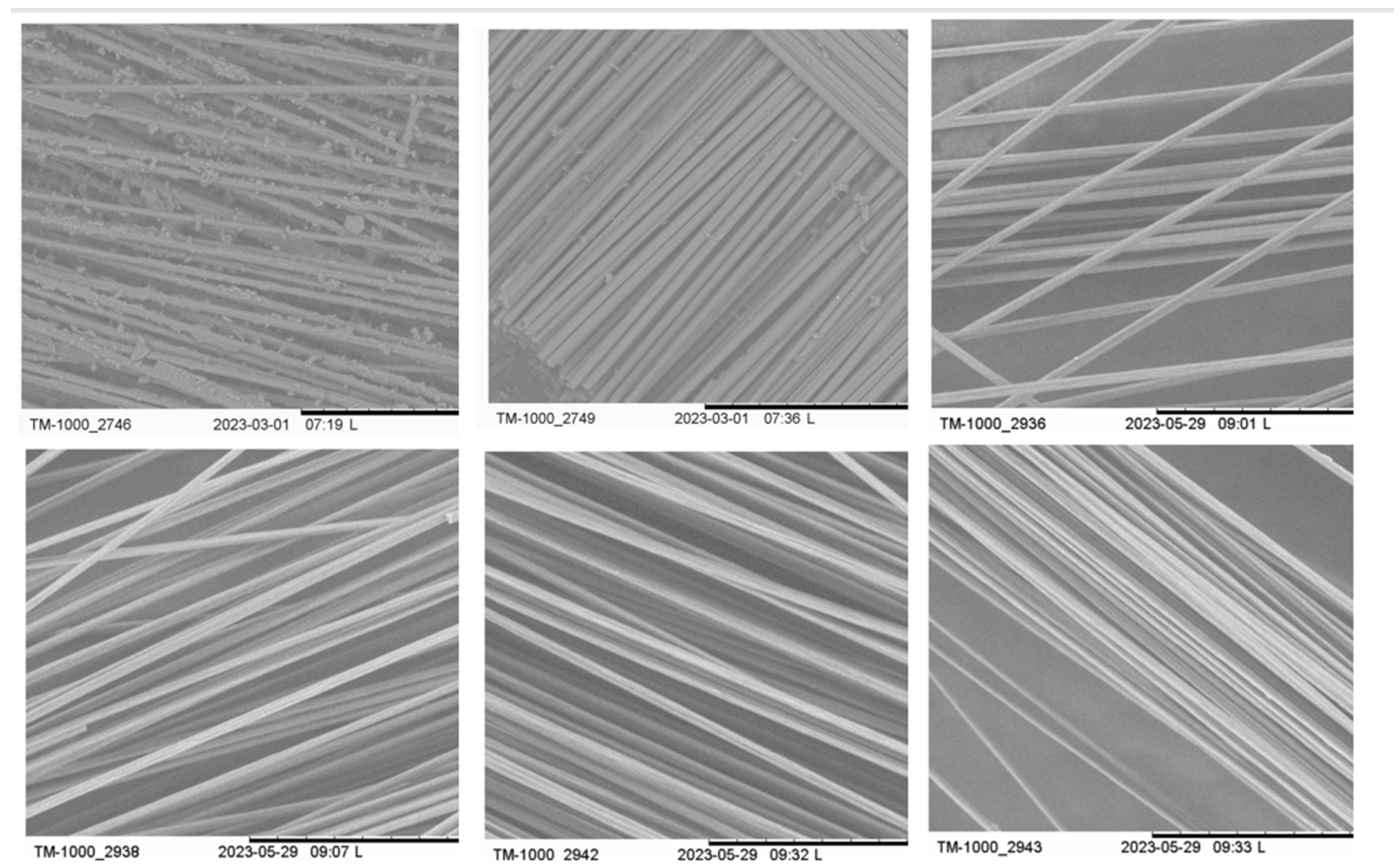
2.4. Characterization of Recycled Carbon Fiber (TGA, SEM, and XPS)
3. Results and Discussion
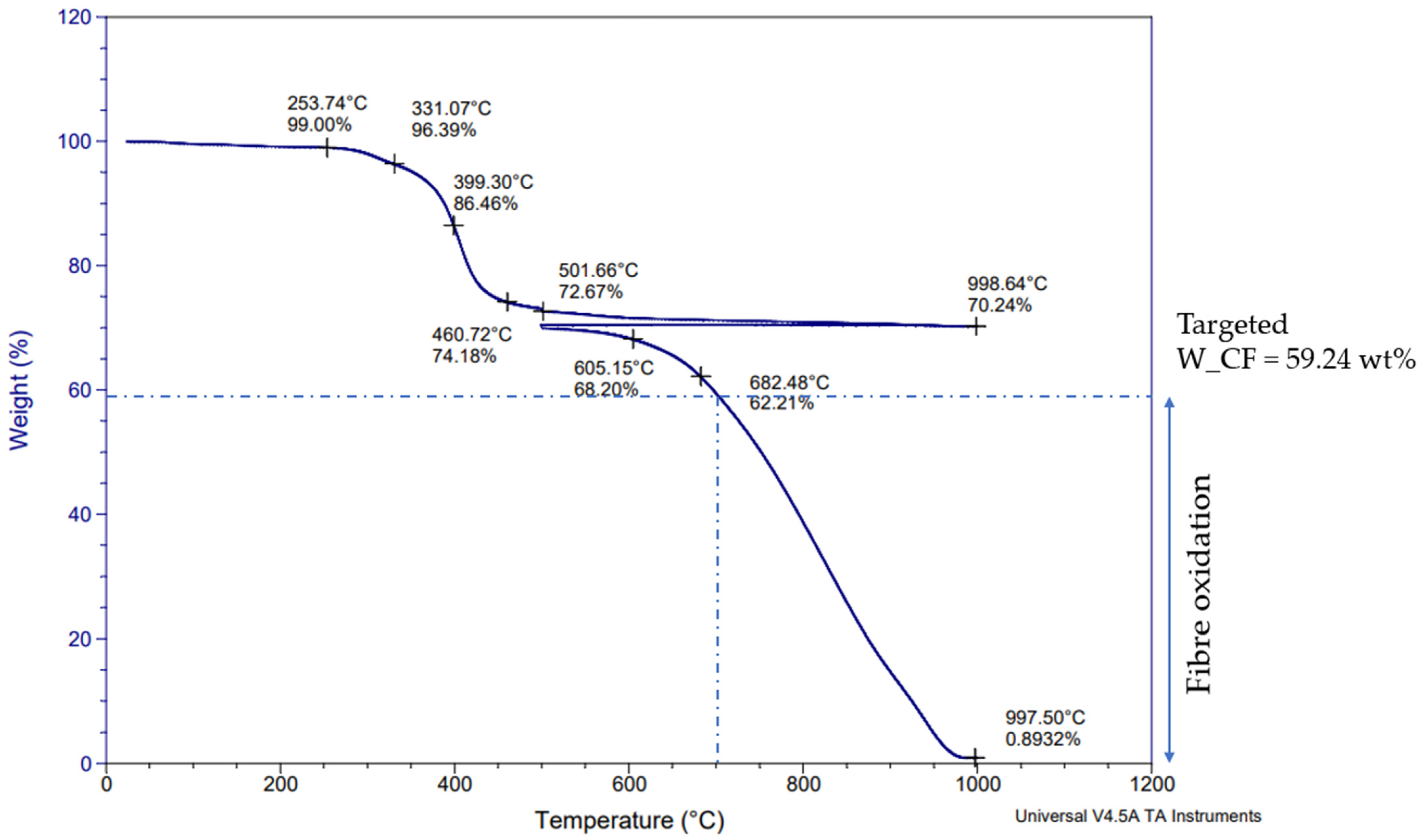
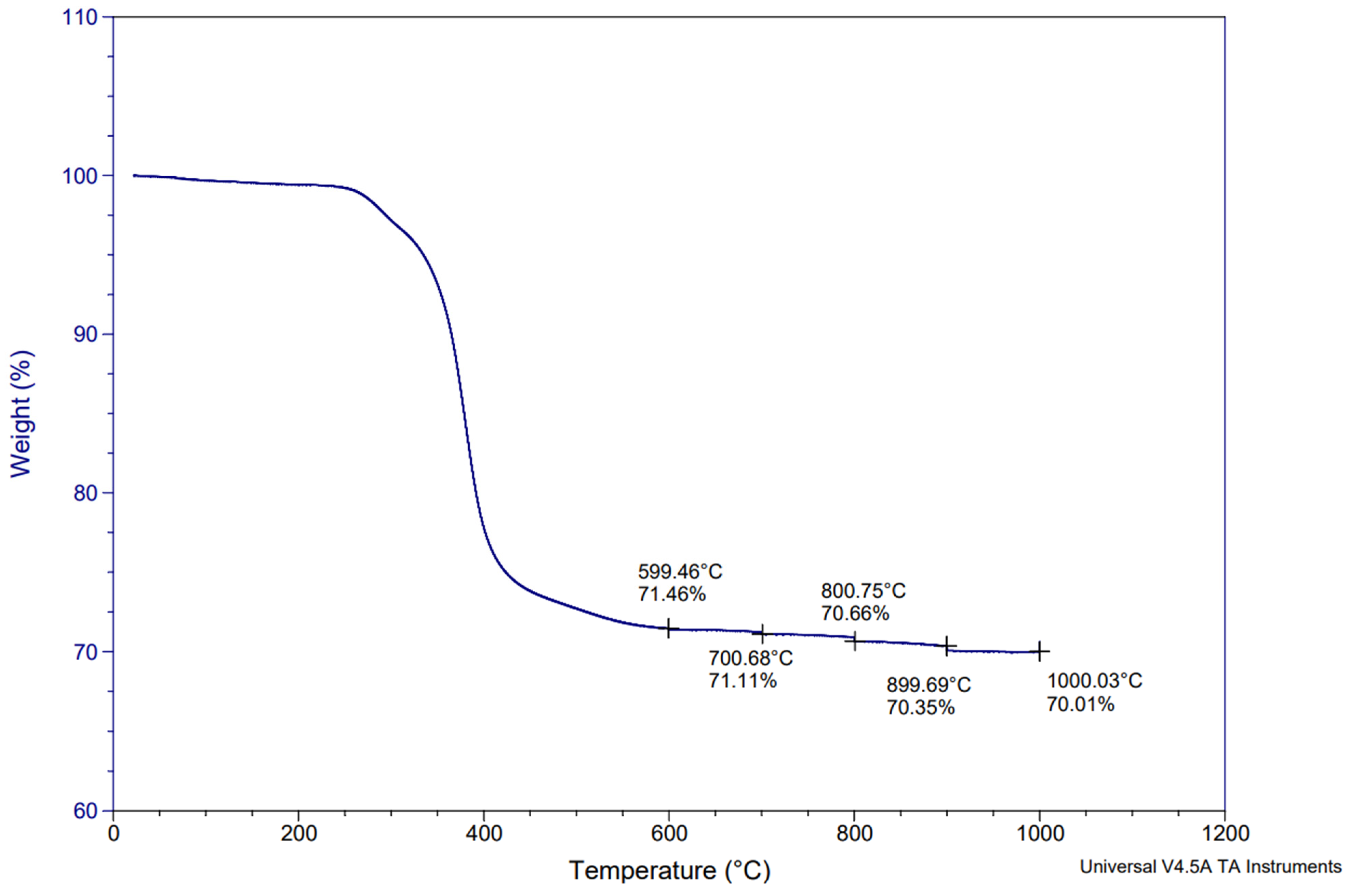
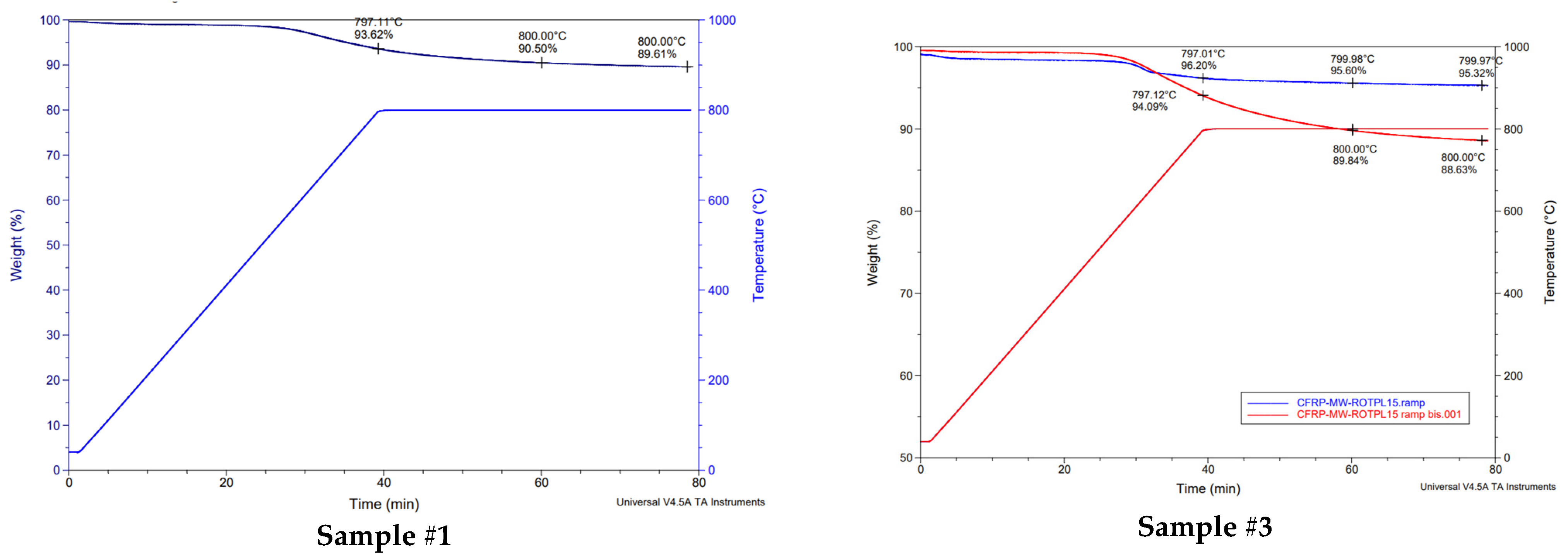
3.1. Initial Characterization of CFRP Samples: TGA (Thermogravimetric Analysis) and Chemical Digestion
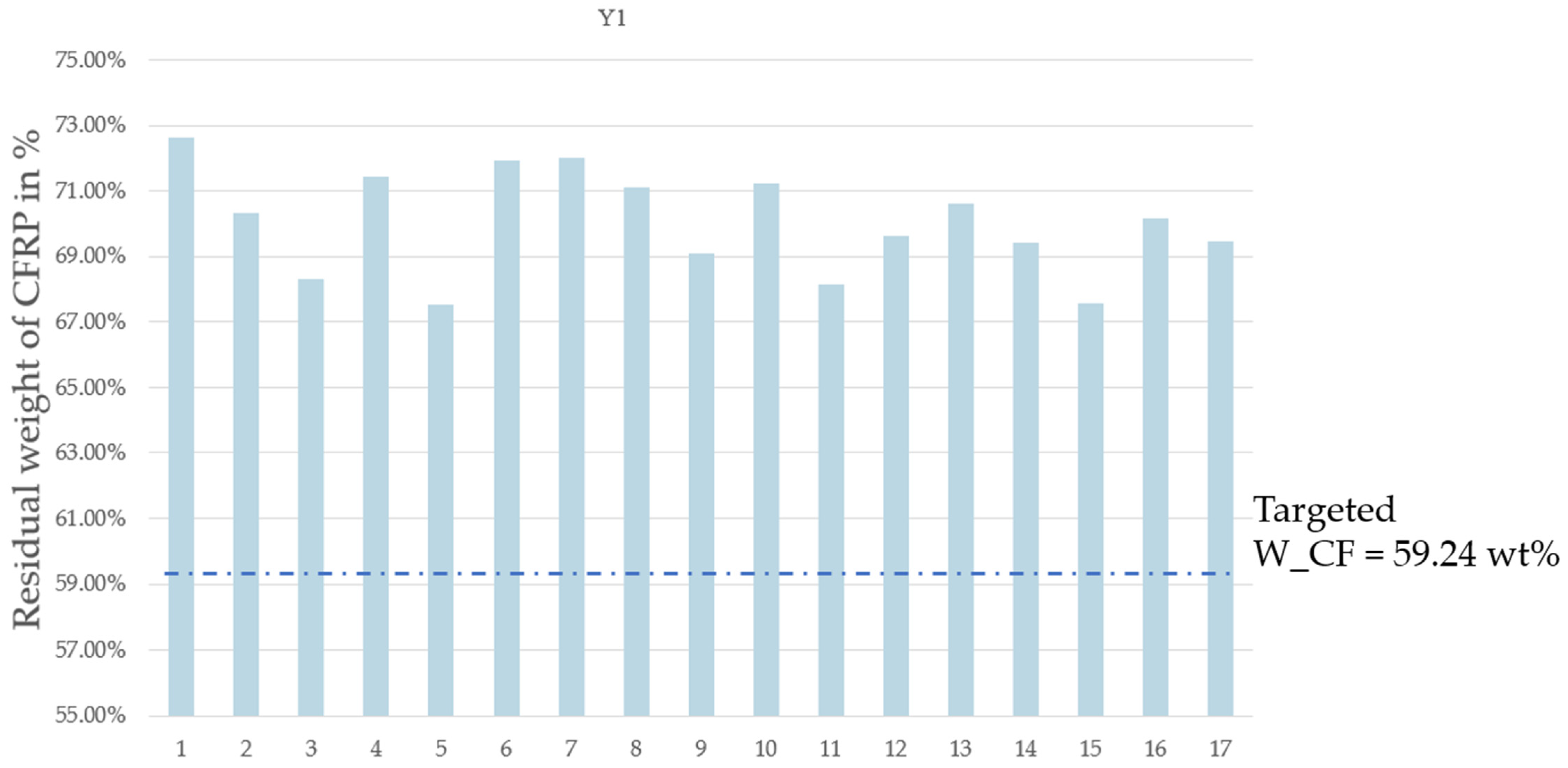
3.2. MAP of CFRP
- -
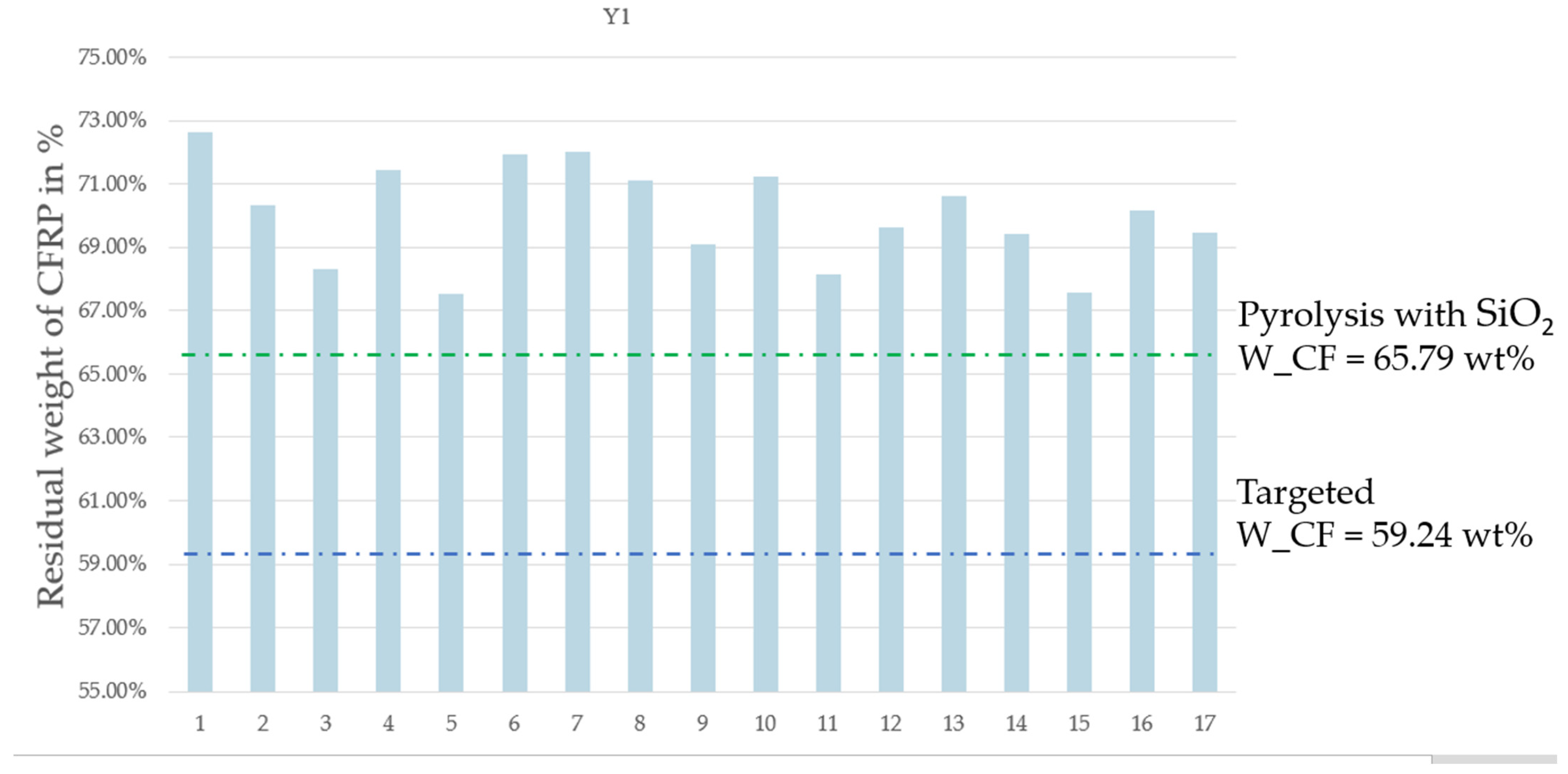
- Y1: the residual weight of CFRP;
- -
- Y2: the weight lost from CFRP;
- -
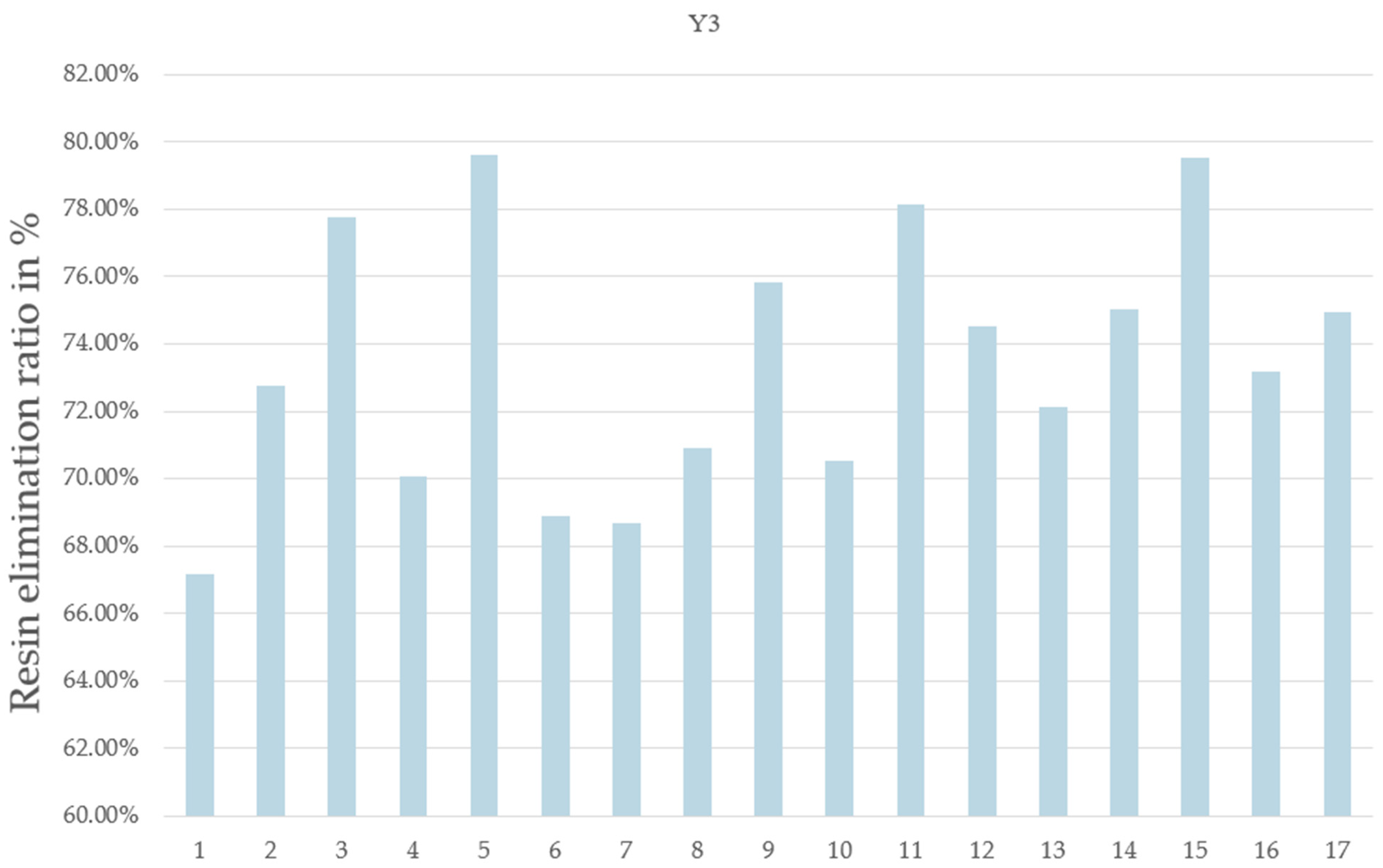
- Y3: the resin elimination percentage.
| Pyrolysis Time | N2 Flow Rate | MW Power | On/Off Frequency | Residual Weight of CFRP | Weight Lost from CFRP | Resin Elimination Percentage | |
|---|---|---|---|---|---|---|---|
| Units | min | L/min | % | Number/run | % | % | % |
| Run | X1 | X2 | X3 | X4 | Y1 | Y2 | Y3 |
| 1 | 10 | 0.5 | 50 | 4 | 72.62 | 27.38 | 67.17 |
| 2 | 10 | 2.9 | 50 | 0 | 70.34 | 29.66 | 72.78 |
| 3 | 10 | 2.9 | 100 | 0 | 68.30 | 31.70 | 77.78 |
| 4 | 10 | 0.5 | 80 | 0 | 71.43 | 28.57 | 70.09 |
| 5 | 6 | 2.9 | 80 | 0 | 67.55 | 32.45 | 79.62 |
| 6 | 6 | 0.5 | 50 | 0 | 71.93 | 28.07 | 68.87 |
| 7 | 6 | 2.9 | 50 | 4 | 72.00 | 28.00 | 68.69 |
| 8 | 6 | 0.5 | 80 | 4 | 71.11 | 28.89 | 70.89 |
| 9 | 8 | 1.5 | 80 | 2 | 69.09 | 30.91 | 75.82 |
| 10 | 8 | 1.5 | 80 | 2 | 71.25 | 28.75 | 70.53 |
| 11 | 6 | 2.9 | 100 | 4 | 68.15 | 31.85 | 78.15 |
| 12 | 10 | 2.9 | 80 | 4 | 69.62 | 30.38 | 74.54 |
| 13 | 8 | 1.5 | 80 | 2 | 70.60 | 29.40 | 72.12 |
| 14 | 10 | 0.5 | 100 | 4 | 69.41 | 30.59 | 75.04 |
| 15 | 6 | 2.9 | 80 | 1 | 67.58 | 32.42 | 79.54 |
| 16 | 8 | 1.5 | 80 | 2 | 70.18 | 29.82 | 73.16 |
| 17 | 10 | 0.5 | 50 | 4 | 69.46 | 30.54 | 74.93 |
3.3. Post-Pyrolysis Treatments
3.3.1. Utilizing Quartz Sand Abrasion in MAP
3.3.2. Chemical Digestion Using Sulfuric Acid and Hydrogen Peroxide Mixture
3.3.3. Thermal Oxidation
- Thermal oxidation using microwave applicator oven
- b.
- Thermal oxidation using a conventional oven
3.4. XPS Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Element | C1s | N1s | O1s | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Functional Group | C=C (sp2) | C-C (sp3) | C-N | C-O, C-O-C | C=O | O-C=O | C=C (sp2) (1) | N (2) | C-N | N (3) | (sp2) (4) | O (5) | C=O, O-C=O | C-O, C-O-C | O-C=O | O (6) |
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| Binding energy (Ev) | 284.6 | 285.0 | 285.7 | 286.5 | 287.9 | 289.2 | 291.2–294.0 | 398.8 | 400.2 | 400.9 | 402.8 | 530.7 | 531.5 | 532.9 | 533.7 | 536.4 |
| Virgin CF | 63.0 | 21.3 | 0.8 | 1.4 | 13.5 | |||||||||||
| CFRP_MW_rot_PL01_15 | 42.6 | 28.7 | 12.4 | 1.9 | 0.9 | 2.8 | 2.4 | 3.6 | 0.6 | 3.1 | 0.9 | |||||
| CFRP_MW_quartz_sand | 56.6 | 21.3 | 7.4 | 1.6 | 0.8 | 2.7 | 1.8 | 2.6 | 1.6 | 3.0 | 0.6 | |||||
| CFRP_ASTMD3171B | 53.7 | 14.6 | 6.6 | 3.6 | 1.3 | 2.4 | 1.4 | 0.7 | 2.5 | 6.1 | 3.5 | 3.6 | ||||
| CFRP_D2584_540C-1h | 64.3 | 6.1 | 3.8 | 1.9 | 0.9 | 1.0 | 4.8 | 3.9 | 0.9 | 3.8 | 5.4 | 2.0 | 1.5 | |||
| CFRP_MW_Pyr_Oxy10 min | 67.1 | 8.5 | 1.3 | 1.7 | 1.3 | 3.0 | 4.6 | 4.3 | 1.1 | 1.9 | 1.1 | 1.4 | 1.5 | 1.1 | ||
References
- Chen, P.-Y.; Feng, R.; Xu, Y.; Zhu, J.-H. Recycling and Reutilization of Waste Carbon Fiber Reinforced Plastics: Current Status and Prospects. Polymers 2023, 15, 3508. [Google Scholar] [CrossRef]
- Zhu, J.H.; Chen, P.Y.; Su, M.N.; Pei, C.; Xing, F. Recycling of carbon fibre reinforced plastics by electrically driven heterogeneous catalytic degradation of epoxy resin. Green Chem. 2019, 21, 1635–1647. [Google Scholar] [CrossRef]
- Sauer, M.; Kuhnel, M. Composites market report 2019. Carbon Compos. 2019, 2, 1–11. [Google Scholar]
- Carberry, W. Airplane Recycling Efforts benefit boeing operators. Boeing Aero Mag. QRT 2008, 4, 6–13. [Google Scholar]
- Mallick, P.K. Fiber-Reinforced Composites: Materials, Manufacturing, and Design, 3rd ed.; Mechanical Engineering; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kuruc, M.; Vopat, T.; Šimna, V.; Necpal, M. Influence of Ultrasonic Assistance on Delamination During Machining of Different Composite Materials. In Proceedings of the 28th DAAAM International Symposium on Intelligentmanufacturing and Automation, Zadar, Croatia, 8–11 November 2017. [Google Scholar] [CrossRef]
- Lefeuvre, A.; Garnier, S.; Jacquemin, L.; Pillain, B.; Sonnemann, G. Anticipating in-use stocks of carbon fiber reinforced polymers and related waste flows generated by the commercial aeronautical sector until 2050. Resour. Conserv. Recycl. 2017, 125, 264–272. [Google Scholar] [CrossRef]
- Stoeffler, K.; Andjelic, S.; Legros, N.; Roberge, J.; Schougaard, S. Polyphenylene sulfide (PPS) composites reinforced with recycled carbon. Compos. Sci. Technol. 2013, 84, 65–71. [Google Scholar] [CrossRef]
- Scheirs, J. Polymer Recycling: Science, Technology and Applications; John Wiley & Sons Ltd.: Chichester, UK, 1998. [Google Scholar]
- Feng, H.; Xu, X.; Wang, B.; Su, Y.; Liu, Y.; Zhang, C.; Zhu, J.; Ma, S. Facile preparation, closed-loop recycling of multifunctional carbon fiber reinforced polymer composites. Compos. Part B Eng. 2023, 257, 110677. [Google Scholar] [CrossRef]
- Palmer, J.; Savage, L.; Ghita, O.R.; Evans, K.E. Sheet moulding compound (SMC) from carbon fibre recyclate. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1232–1237. [Google Scholar] [CrossRef]
- Palmer, J.; Ghita, O.R.; Savage, L.; Evans, K.E. Successful closed-loop recycling of thermoset composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 490–498. [Google Scholar] [CrossRef]
- Torres, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Legarreta, J.A.; Cabrero, M.A.; González, A.; Chomón, M.J.; Gondra, K. Recycling by pyrolysis of thermoset composites: Characteristics of the liquid and gaseous fuels obtained. Fuel 2000, 79, 897–902, ISSN 0016-2361. [Google Scholar] [CrossRef]
- López, F.A.; Rodríguez, O.; Alguacil, F.J.; García-Díaz, I.; Centeno, T.A.; García-Fierro, J.L.; González, C. Recovery of carbon fibres by the thermolysis and gasification of waste prepreg. J. Anal. Appl. Pyrolysis 2013, 104, 675–683. [Google Scholar] [CrossRef]
- Iwaya, T.; Tokuno, S.; Sasaki, M.; Goto, M.; Shibata, K. Recycling of fiber reinforced plastics using depolymerization by solvothermal reaction with catalyst. J. Mater. Sci. 2007, 43, 2452–2456. [Google Scholar] [CrossRef]
- Lam, K.; Oyedun, A.; Cheung, K.; Lee, K.; Hui, C.-H. pyrolysis with dynamic heating. Chem. Eng. Sci. 2011, 66, 6505–6514, ISSN 0009-2509. [Google Scholar] [CrossRef]
- Giorgini, L.; Benelli, T.; Mazzocchetti, L.; Leonardi, C.; Zattini, G.; Minak, G.; Dolcini, E.; Tosi, C.; Montanari, I. Pyrolysis as a way to close a CFRC life cycle: Carbon fibers recovery and their use as feedstock for a new composite production. AIP Conf. Proc. 2014, 1599, 354–357. [Google Scholar]
- Lee, H.S.; Jung, S.; Lin, K.-Y.A.; Kwon, E.E.; Lee, J. Upcycling textile waste using pyrolysis process. Sci. Total Environ. 2023, 859, 160393. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qi, R.; Xiong, M.; Zhang, D.; Gu, H.; Chen, W. Conversion of cotton textile waste to clean solid fuel via surfactant-assisted hydrothermal carbonization: Mechanisms and combustion behaviors. Bioresour. Technol. 2021, 321, 124450–124460. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, J.A.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave heating processes involving carbon materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Juárez-Pérez, E.J.; Ruisánchez, E.; Bermúdez, J.M.; Arenillas, A. Ball lightning plasma and plasma arc formation during the microwave heating of carbons. Carbon 2011, 49, 346–349. [Google Scholar] [CrossRef]
- Kamble, Z.; Behera, B.K. Upcycling textile wastes: Challenges and innovations. Text. Prog. 2021, 53, 65–122. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Jiang, Z.; Tang, T. Chemical recycling of carbon fibre reinforced epoxy resin composites in subcritical water: Synergistic effect of phenol and KOH on the decomposition efficiency. Polym. Degrad. Stab. 2012, 97, 214–220. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, L.; Zhou, J. Curing multidirectional carbon fiber reinforced polymer composites with indirect microwave heating. Int. J. Adv. Manuf. Technol. 2018, 97, 1137–1147. [Google Scholar] [CrossRef]
- Leclerc, P. Dépolymérisation du Polystyrène par Pyrolyse Micro-Onde [Thèse de doctorat, École Polytechnique de Montréal]. PolyPublie. Ph.D. Thesis, Polytechnique Montréal, Montréal, QC, Canada, 2018. Available online: https://publications.polymtl.ca/2982/ (accessed on 24 June 2024).
- Dega, C.; Boukhili, R.; Léger, K.; Laviolette, J.-P.; Jadidinia, A. Recycling of carbon fiber reinforced plastics by microwave pyrolysis composites and Advanced Materials Expo, CAMX 2021. In Proceedings of the 8th Annual Composites and Advanced Materials Expo, CAMX 2021, Dallas, TX, USA, 19 October 2021; pp. 513–525. [Google Scholar]
- Doucet, J.; Laviolette, J.-P.; Farag, S.; Chaouki, J. Distributed microwave pyrolysis of domestic waste. Waste Biomass Valorization 2013, 5, 1–10. [Google Scholar] [CrossRef]
- Meyer, L.O.; Schulte, K.; Grove-Nielsen, E. CFRP-Recycling Following a Pyrolysis Route: Process Optimization and Potentials. J. Compos. Mater. 2009, 43, 1121–1132. [Google Scholar] [CrossRef]
- Beneroso, D.; Bermúdez, J.M.; Arenillas, A.; Menéndez, J.A. Influence of carrier gas on microwave-induced pyrolysis. J. Anal. Appl. Pyrolysis 2015, 113, 153–157. [Google Scholar] [CrossRef]
- Srividya, K.; Ravichandran, S.; Kumarasamy, M.; Pattanaik, B.; Pattnaik, M.; Subbiah, R.; Satishkumar, P. Design and analysis of wear parameters for epoxy-based composite using RSM-box behnken optimization tool. Int. J. Interact. Des. Manuf. 2023. [Google Scholar] [CrossRef]
- Rabinowicz, E.; Tanner, R.I. Friction and wear of materials. J. Appl. Mech. 1966, 33, 479. [Google Scholar] [CrossRef]
- Moore, M.A. The effect of particle shape on abrasive wear: Comparison of theory and experiment. Wear Mater. 1983, 249, 201–207. [Google Scholar]
- Hecker, R.L.; Liang, S.Y. Predictive modeling of surface roughness in grinding. Int. J. Mach. Tools Manuf. 2003, 43, 755–761. [Google Scholar] [CrossRef]
- Lortz, W. A model of the cutting mechanism in grinding. Wear 1979, 53, 115–128. [Google Scholar] [CrossRef]
- Jacobson, S.; Wallén, P.; Hogmark, S. Fundamental aspects of abrasive wear studied by a new numerical simulation model. Wear 1988, 123, 207–223. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Sata, T. Simulated grinding process by Monte Carlo method. Ann. CIRP 1968, 16, 297–302. [Google Scholar]
- Mulhearn, T.O.; Samuels, L.E. The abrasion of metals: A model of the process. Wear 1962, 5, 478–498. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, Z.; Dong, C.; Fan, J.; Ren, Y. A closed-loop recycling process for carbon fiber reinforced vinyl ester resin composite. Chem. Eng. J. 2022, 446, 137254. [Google Scholar] [CrossRef]
- Fazeli, M.; Jayaprakash, S.; Baniasadi, H.; Abidnejad, R.; Lipponen, J. Recycled carbon fiber reinforced composites: Enhancing mechanical properties through co-functionalization of carbon nanotube-bonded microfibrillated cellulose. Compos. Part A Appl. Sci. Manuf. 2024, 180, 108097. [Google Scholar] [CrossRef]
- Sarmah, A.; Sarikaya, S.; Thiem, J.; Upama, S.T.; Khalfaoui, A.N.; Dasari, S.S.; Arole, K.; Hawkins, S.A.; Naraghi, M.; Vashisth, A.; et al. Recycle and Reuse of Continuous Carbon Fibers from Thermoset Composites Using Joule Heating. ChemSusChem 2022, 15, e202200989. [Google Scholar] [CrossRef]

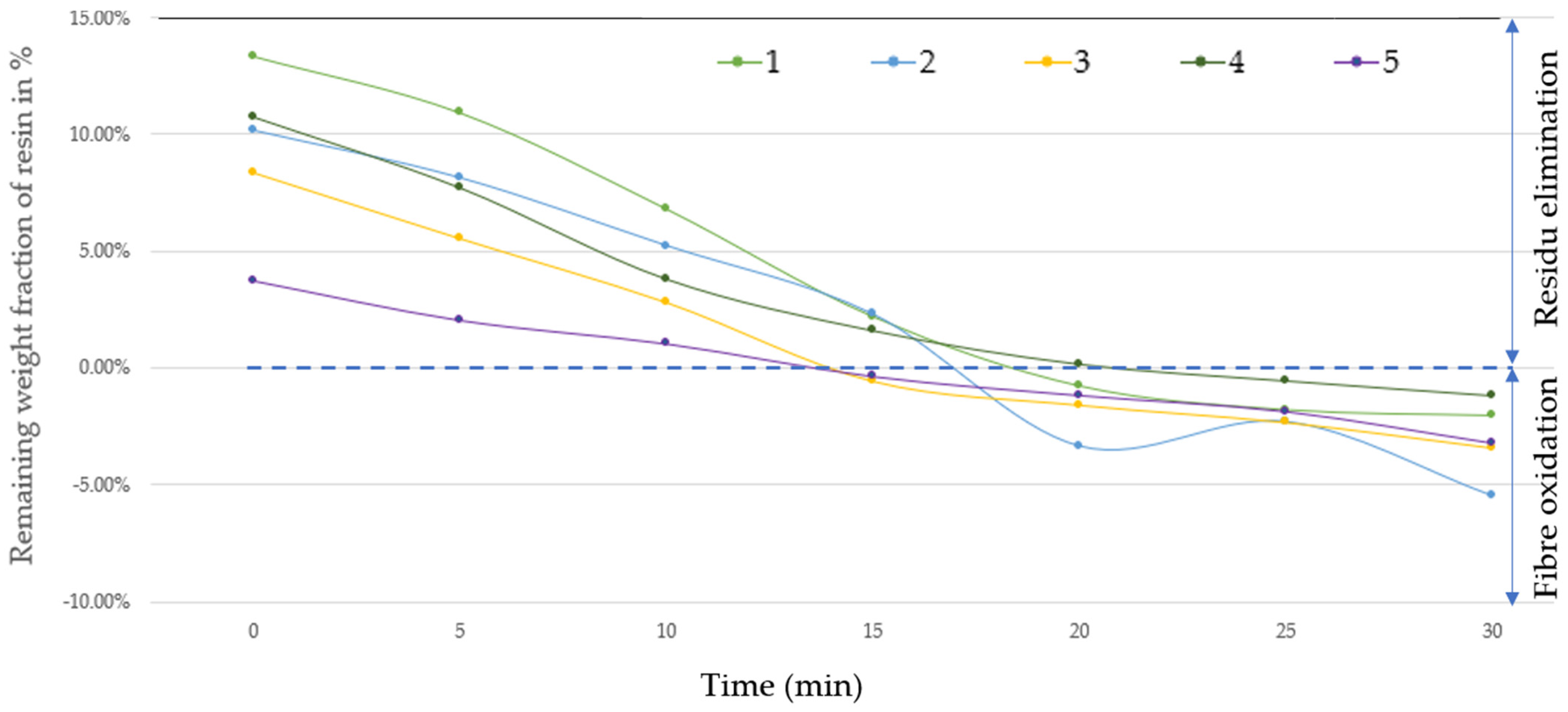
| Fixed Parameter Description | Numerical Value |
|---|---|
| Rotary evaporator speed of rotation | 40 rpm |
| Sample mass | 15 g |
| Feedstock composition | 40.76% weight fraction of resin content (Wm_ini) |
| Feedstock size (square shape) | 1.5 cm × 1.5 cm |
| Flask size | 250 mL |
| Reaction flask angle in connection with the rotovap | 15 degrees |
| Variable and Unit | X1 | X2 | X3 | X4 | |
|---|---|---|---|---|---|
| Pyrolysis Time | N2 Flow Rate | MW Power | On/Off Frequency | ||
| min | L/min | % | Number per Run | ||
| Level | 1 | 6 | 0.5 | 50 | 0 |
| 2 | 8 | 1.5 | 80 | 2 | |
| 3 | 10 | 2.9 | 100 | 4 | |
| Run | X1 | X2 | X3 | X4 |
|---|---|---|---|---|
| 1 | 10 | 0.5 | 50 | 4 |
| 2 | 10 | 2.9 | 50 | 0 |
| 3 | 10 | 2.9 | 100 | 0 |
| 4 | 10 | 0.5 | 80 | 0 |
| 5 | 6 | 2.9 | 80 | 0 |
| 6 | 6 | 0.5 | 50 | 0 |
| 7 | 6 | 2.9 | 50 | 4 |
| 8 | 6 | 0.5 | 80 | 4 |
| 9 | 8 | 1.5 | 80 | 2 |
| 10 | 8 | 1.5 | 80 | 2 |
| 11 | 6 | 2.9 | 100 | 4 |
| 12 | 10 | 2.9 | 80 | 4 |
| 13 | 8 | 1.5 | 80 | 2 |
| 14 | 10 | 0.5 | 100 | 4 |
| 15 | 6 | 2.9 | 80 | 1 |
| 16 | 8 | 1.5 | 80 | 2 |
| 17 | 6 | 0.5 | 100 | 0 |
| Source | Estimation | Standard Error | t Ratio | p-Value | Prob. > |t| |
|---|---|---|---|---|---|
| Flow_rate | 3.363360 | 0.692071 | 4.86 | 0.00039 | 0.0004 |
| Power | 2.800000 | 0.875871 | 3.20 | 0.00768 | 0.0077 |
| Rotation_Freq | −1.880026 | 0.692071 | −2.72 | 0.01873 | 0.0187 |
| Time | −1.080026 | 0.692071 | −1.56 | 0.14460 | 0.1446 |
| #ID | Nomenclature | Time (min) | Y1 % | Y2 % | Y3 % |
|---|---|---|---|---|---|
| 1 | P1_3(X1 = 1) − OXY 5 | 5 | 64.53 | 35.47 | 87.03 |
| 2 | P1_3(X1 = 1) − OXY 10 | 10 | 62.96 | 37.04 | 90.87 |
| 3 | P1_3(X1 = 1) − OXY 15 | 15 | 53.14 | 46.86 | 114.96 |
| 4 | P1_3(X1 = 1) − OXY 15 | 15 | 55.90 | 44.10 | 108.19 |
| Nomenclature | Description | |
|---|---|---|
| 1 | CFRP_MW__P1_1 | MAP sample, Run #1 per Table 3 |
| 2 | CFRP_MW_P1_14 | MAP sample, Run #14 per Table 3 |
| 3 | CFRP_MW_P1_15 | MAP sample, Run #15 per Table 3 |
| 4 | P1_3(X1 = 1) | 1st repetition of optimum MAP corresponding to P1_3(X1 = 1) |
| 5 | CFRP_MW_Pyr_Oxy_10 min | P1_3(X1 = 1) followed by 10 min of oxidation in microwave oven |
| ID | Description |
|---|---|
| Virgin CF | Coated virgin carbon fiber reference, HTS40 Carbon fiber |
| CFRP_MW_PL01_15 | MAP sample, RUN #15 per Table 3 |
| CFRP_MW_quartz_sand | Quartz sand MAP sample as described in Section 2.3. (a) |
| CFRP_D3171B | CFRP digested following ASTM 3171 B as described in Section 2.3. (b) |
| CFRP_D2584_1h_540C | CFRP cleaned in a conventional oven, under air, at 540degC, for 1 h. |
| CFRP_MW_Pyr_Oxy_10 min | MAP sample, P1_3(X1 = 1) followed by 10 min of oxidation in microwave oven. |
| Element | |||||
|---|---|---|---|---|---|
| C(%) | O (%) | N(%) | Other * (%) | O/C(%) | |
| Virgin CF | 85.40 | 12.80 | 1.30 | 0.5 | 14.99 |
| CFRP_MW_rot_PL01_15 | 88.80 | 4.20 | 6.40 | 0.6 | 4.73 |
| CFRP_MW_quartz_sand | 90.00 | 4.40 | 4.50 | 1.1 | 4.89 |
| CFRP_ASTMD3171B | 83.60 | 12.50 | 3.40 | 0.5 | 14.95 |
| CFRP_D2584_540C-1h | 77.40 | 11.60 | 10.00 | 1.0 | 14.99 |
| CFRP_MW_Pyr_Oxy10min | 81.70 | 6.50 | 10.80 | 1.0 | 7.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dega, C.; Boukhili, R.; Esmaeili, B.; Laviolette, J.-P.; Doucet, J.; Decaens, J. Microwave-Assisted Pyrolysis of Carbon Fiber-Reinforced Polymers and Optimization Using the Box–Behnken Response Surface Methodology Tool. Materials 2024, 17, 3256. https://doi.org/10.3390/ma17133256
Dega C, Boukhili R, Esmaeili B, Laviolette J-P, Doucet J, Decaens J. Microwave-Assisted Pyrolysis of Carbon Fiber-Reinforced Polymers and Optimization Using the Box–Behnken Response Surface Methodology Tool. Materials. 2024; 17(13):3256. https://doi.org/10.3390/ma17133256
Chicago/Turabian StyleDega, Cynthie, Rachid Boukhili, Babak Esmaeili, Jean-Philippe Laviolette, Jocelyn Doucet, and Justine Decaens. 2024. "Microwave-Assisted Pyrolysis of Carbon Fiber-Reinforced Polymers and Optimization Using the Box–Behnken Response Surface Methodology Tool" Materials 17, no. 13: 3256. https://doi.org/10.3390/ma17133256
APA StyleDega, C., Boukhili, R., Esmaeili, B., Laviolette, J.-P., Doucet, J., & Decaens, J. (2024). Microwave-Assisted Pyrolysis of Carbon Fiber-Reinforced Polymers and Optimization Using the Box–Behnken Response Surface Methodology Tool. Materials, 17(13), 3256. https://doi.org/10.3390/ma17133256







