Effect of AlN on the Mechanical and Electrochemical Properties of Aluminum Metal Matrix Composites
Abstract
1. Introduction
2. Materials and Experimental Details
2.1. Materials
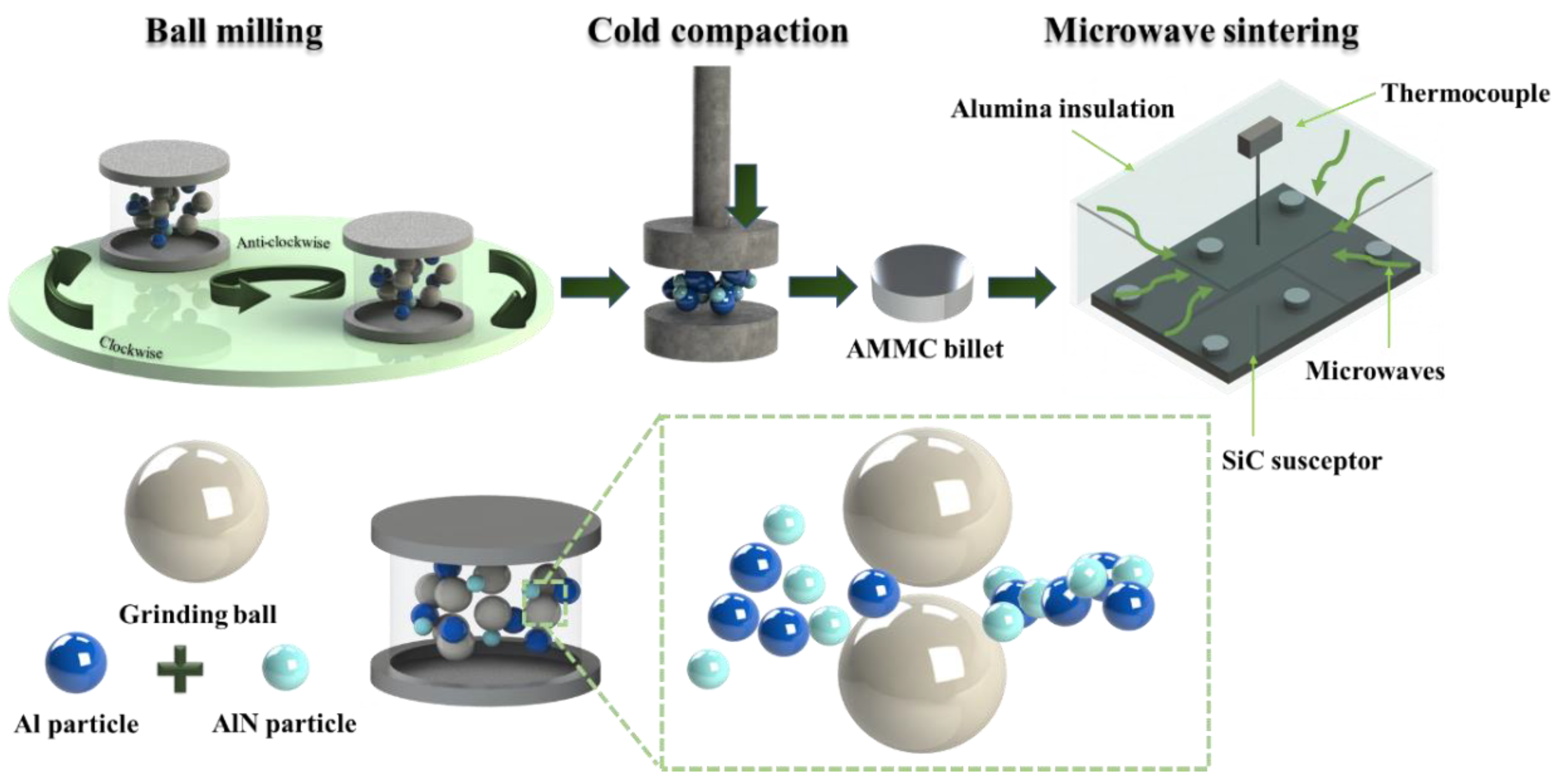
2.2. Composite Processing
2.3. Characterization of Composites
3. Results and Discussion
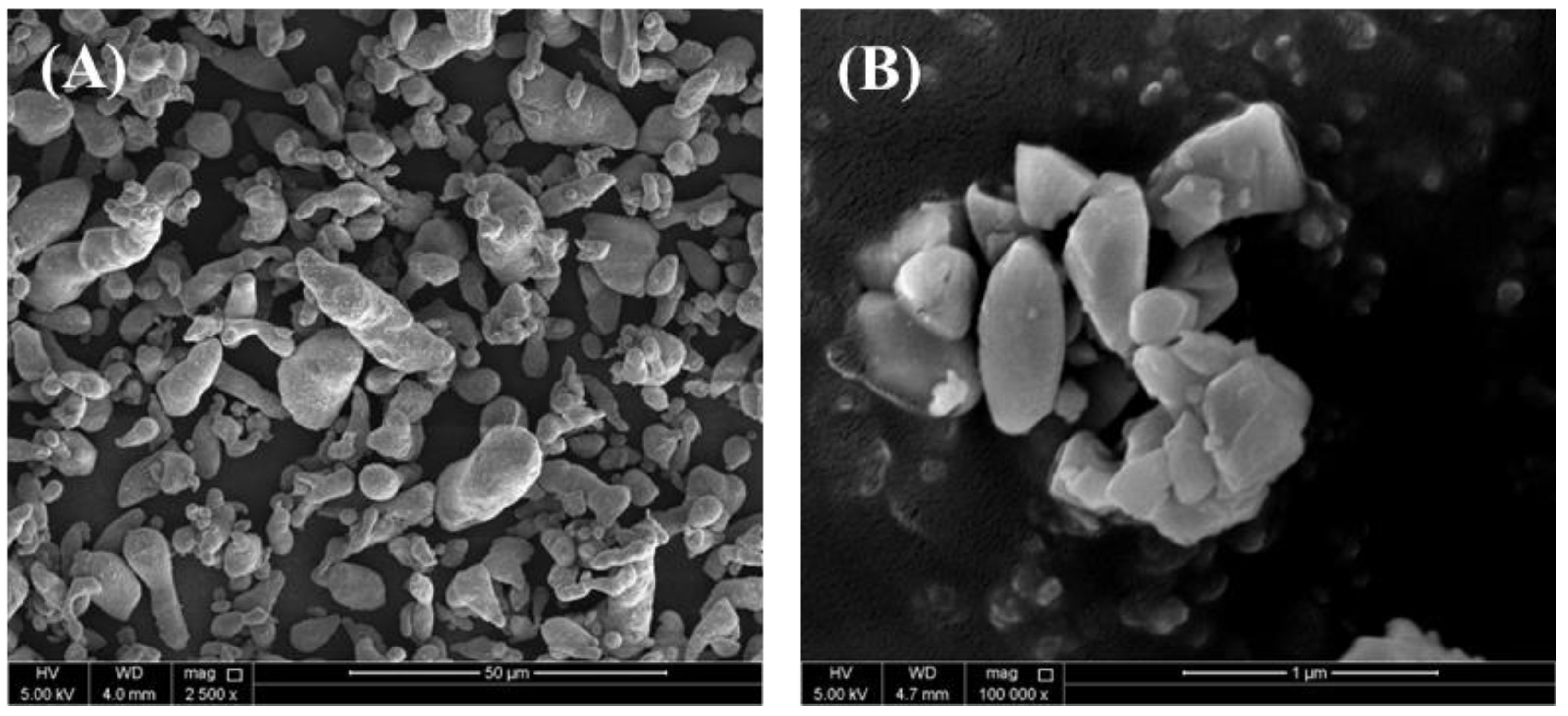
3.1. Morphological Analysis
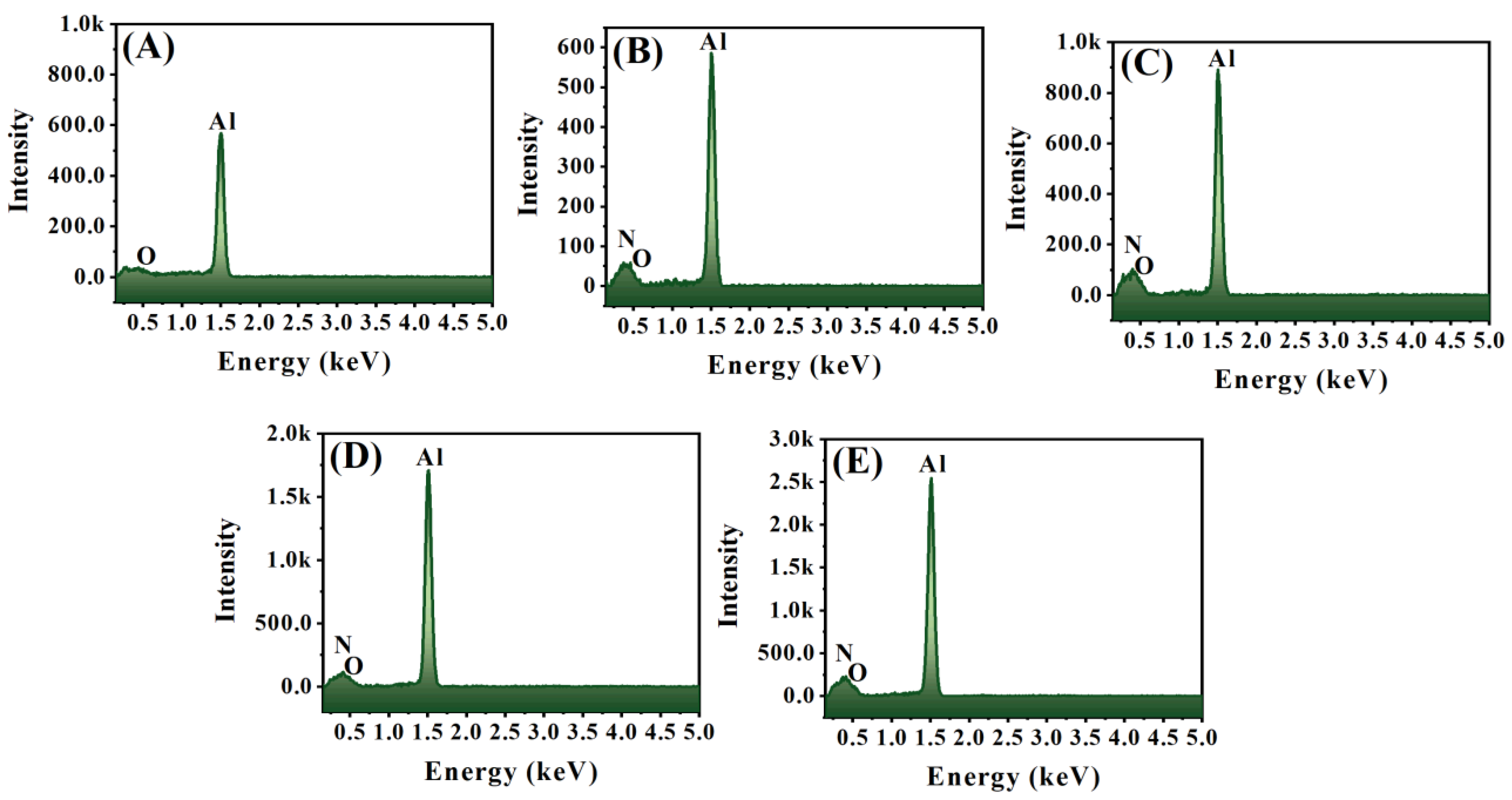
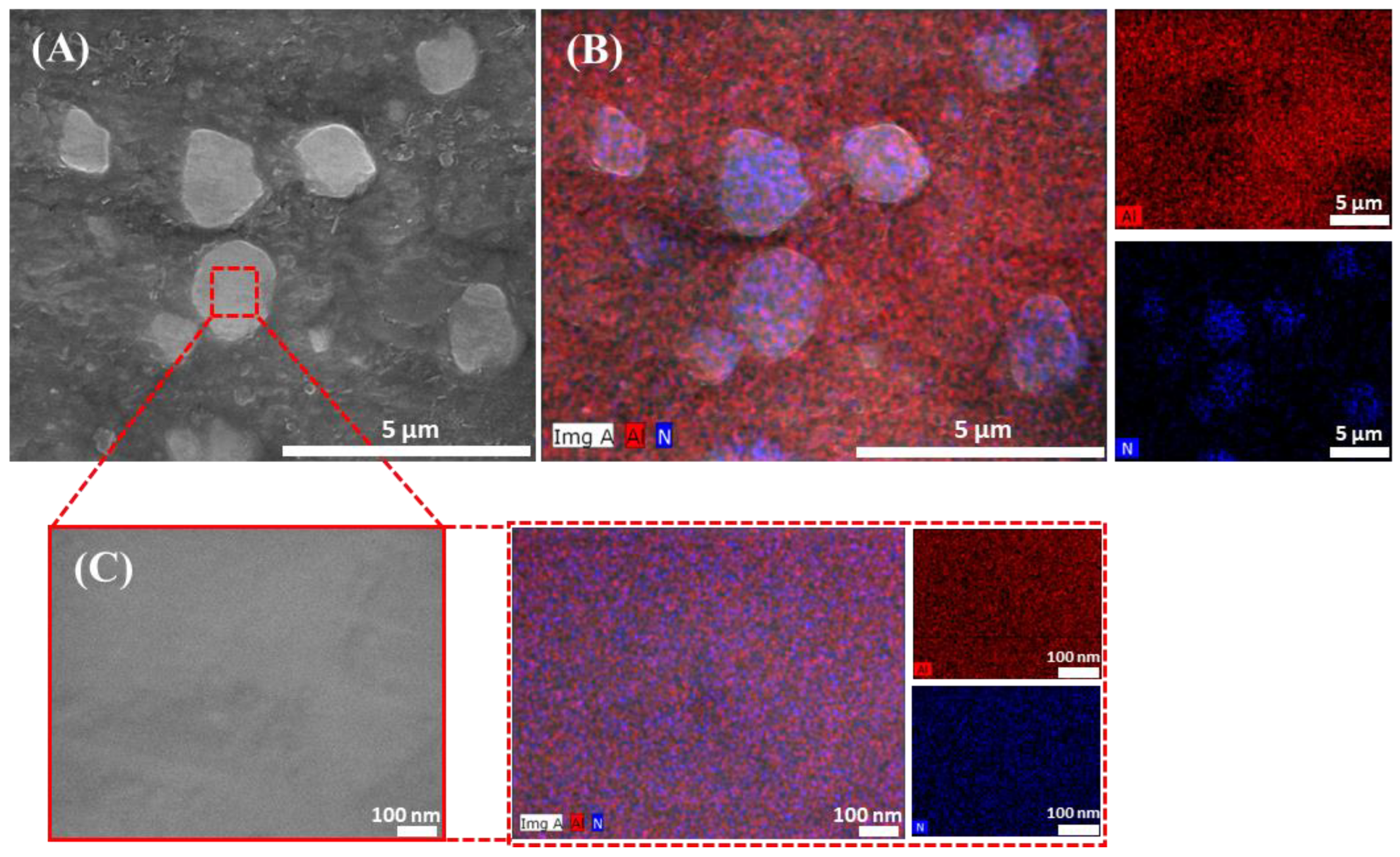
3.2. Microstructural Analysis
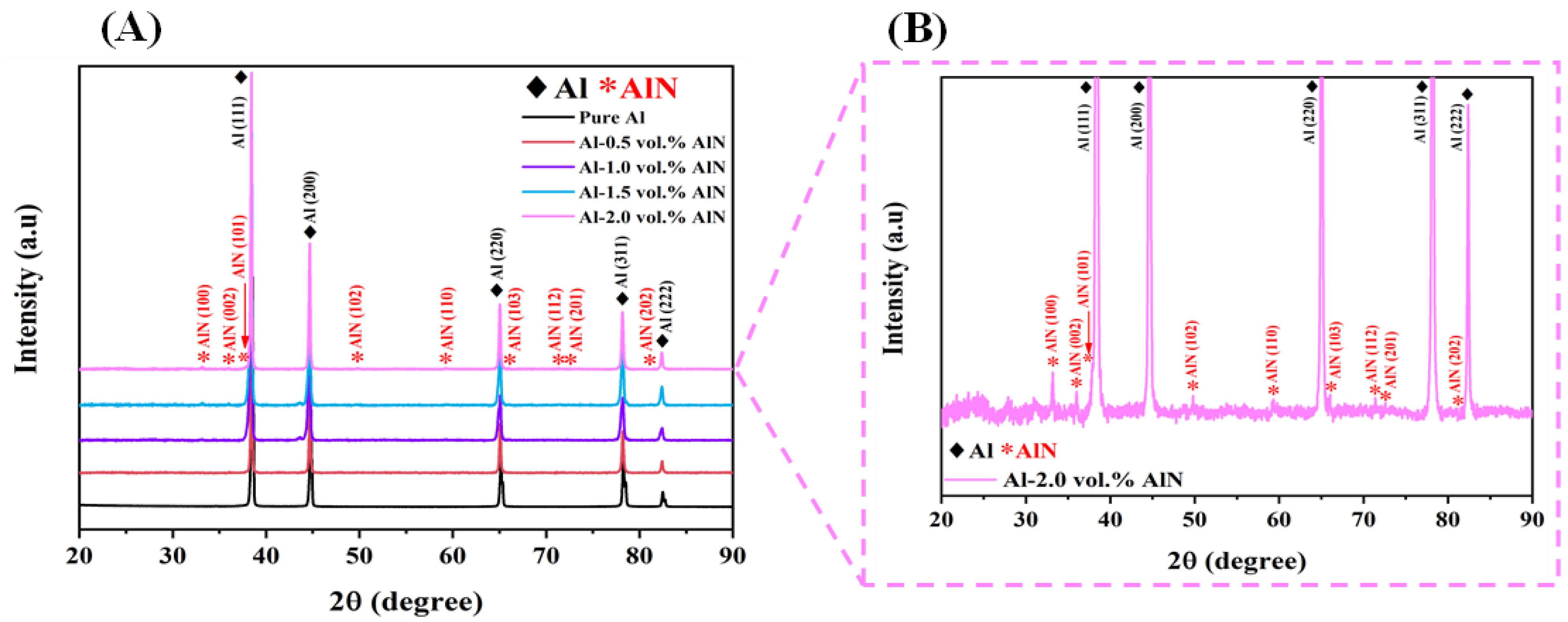
3.3. Structural Analysis Using XRD
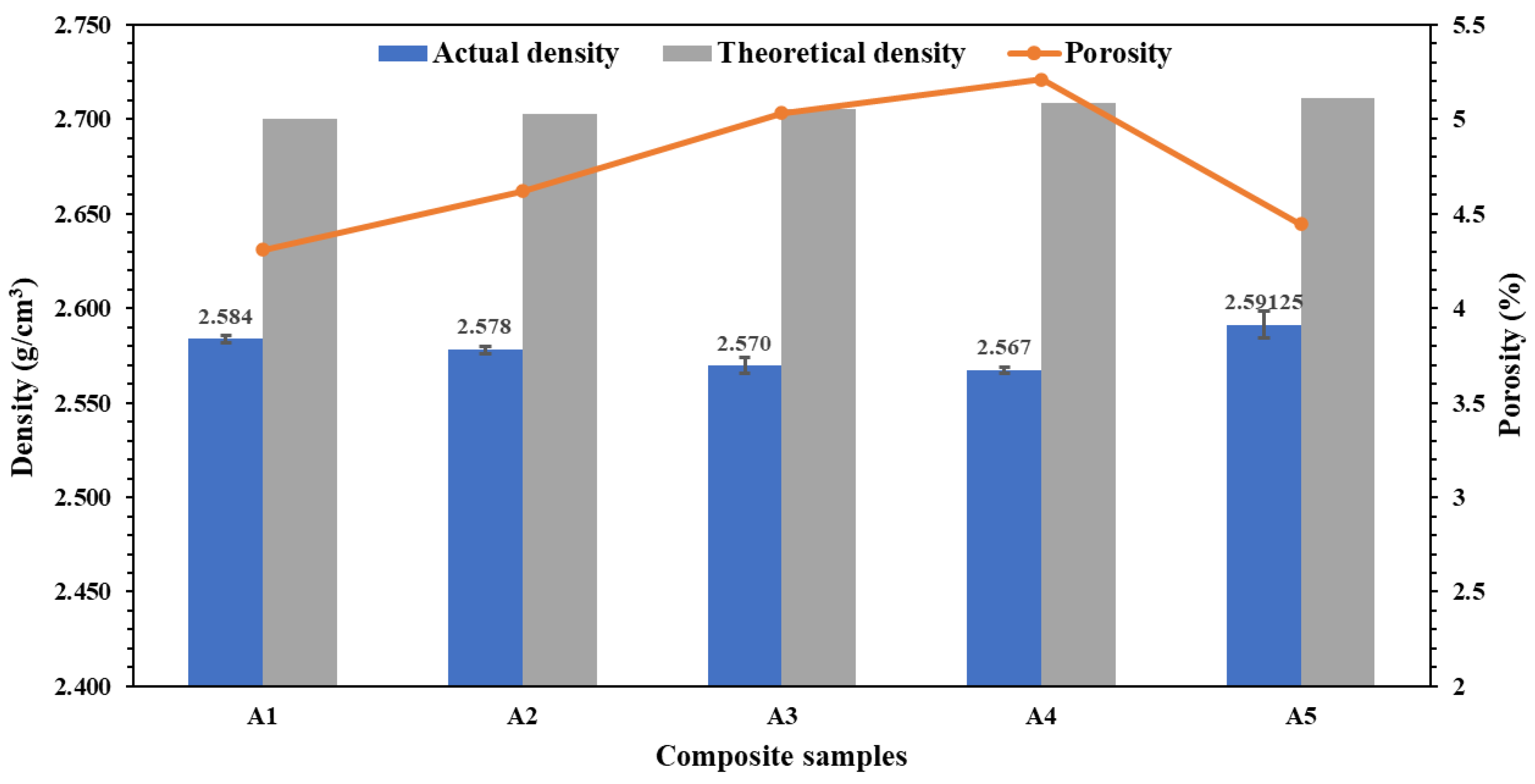
3.4. Density and Porosity Analysis
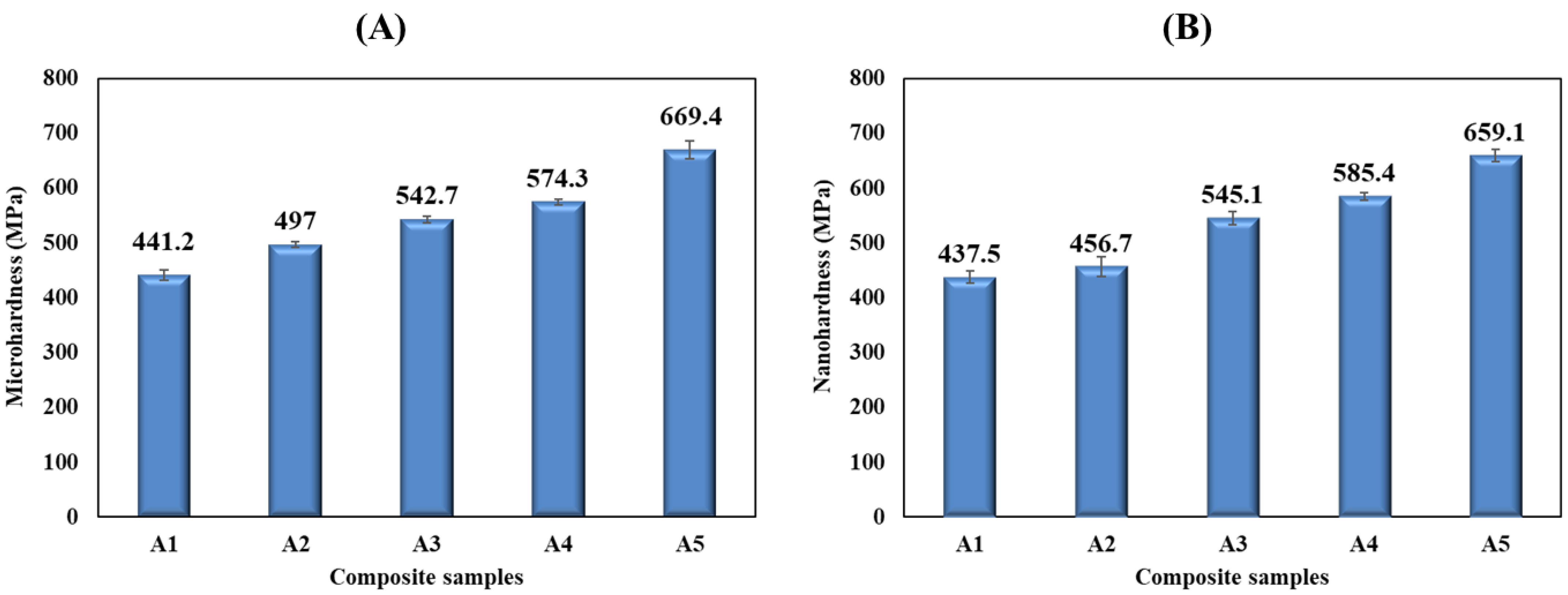
3.5. Microhardness and Nanoindentation Analyses
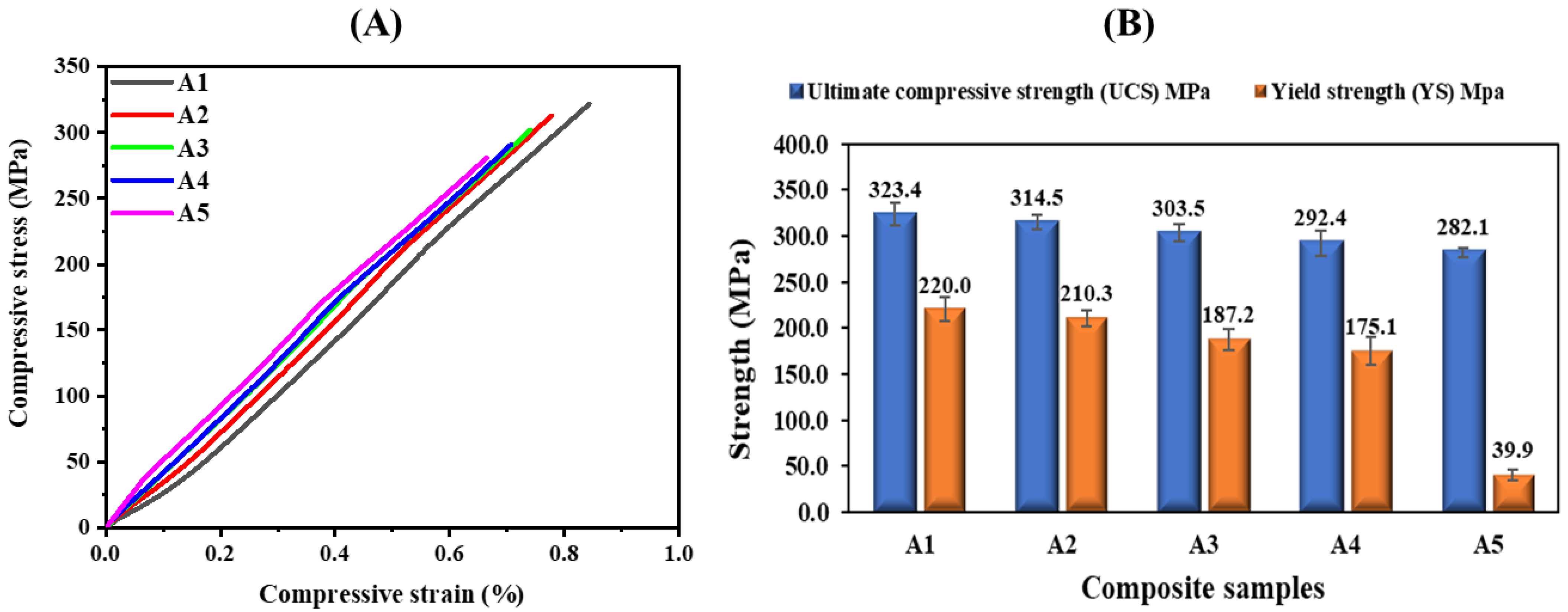
3.6. Compressive Analysis
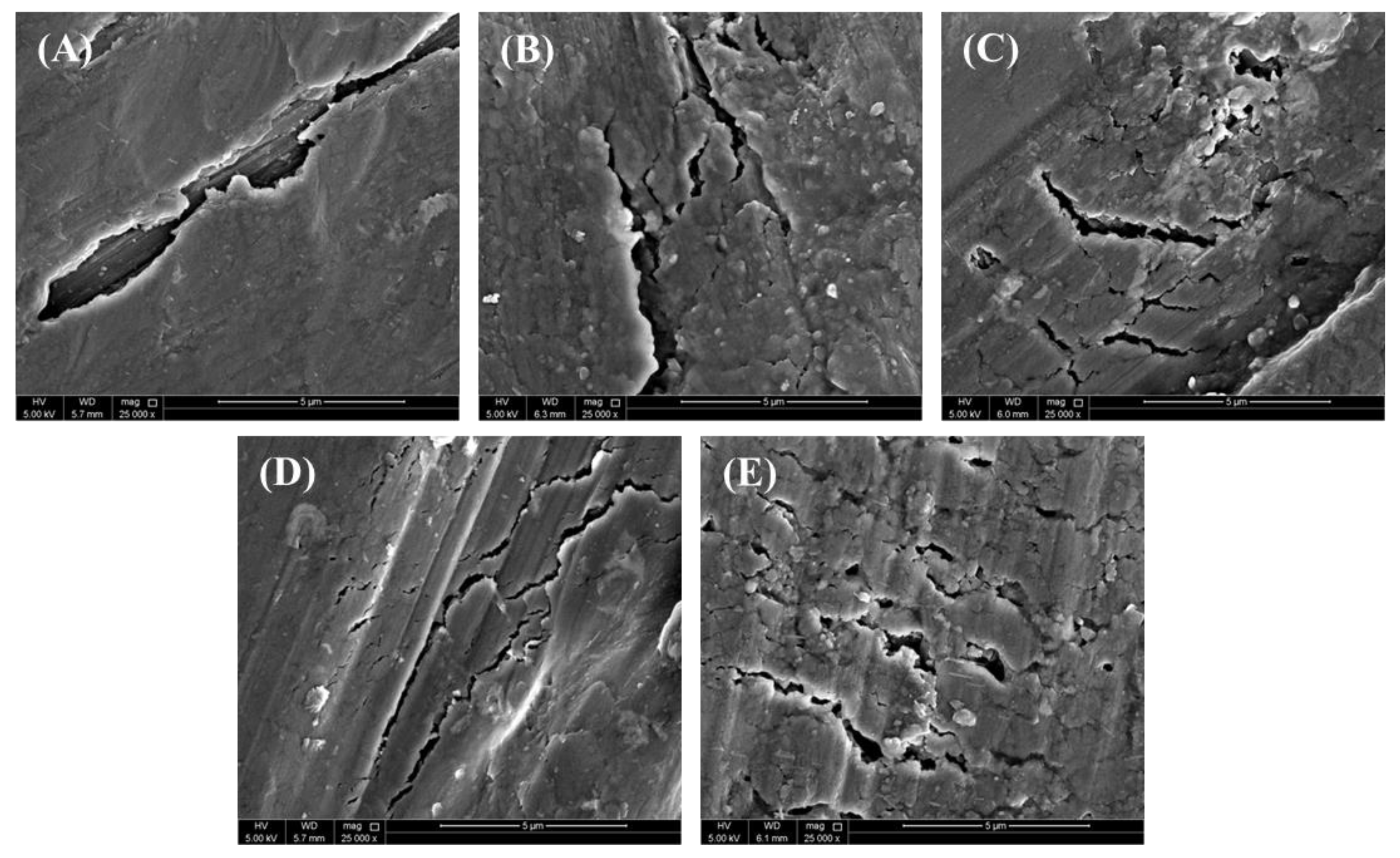
3.7. Fractography Analysis
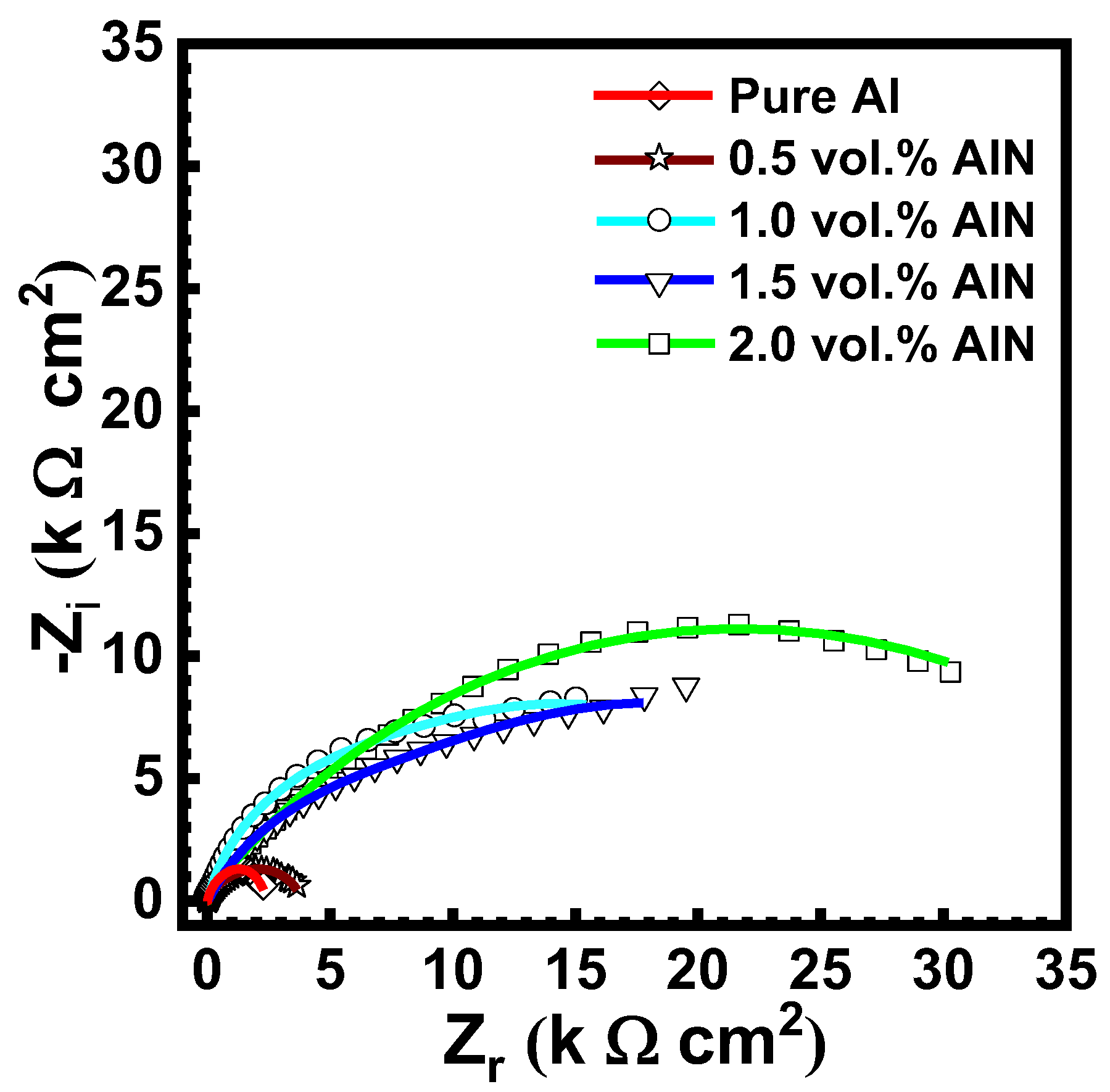
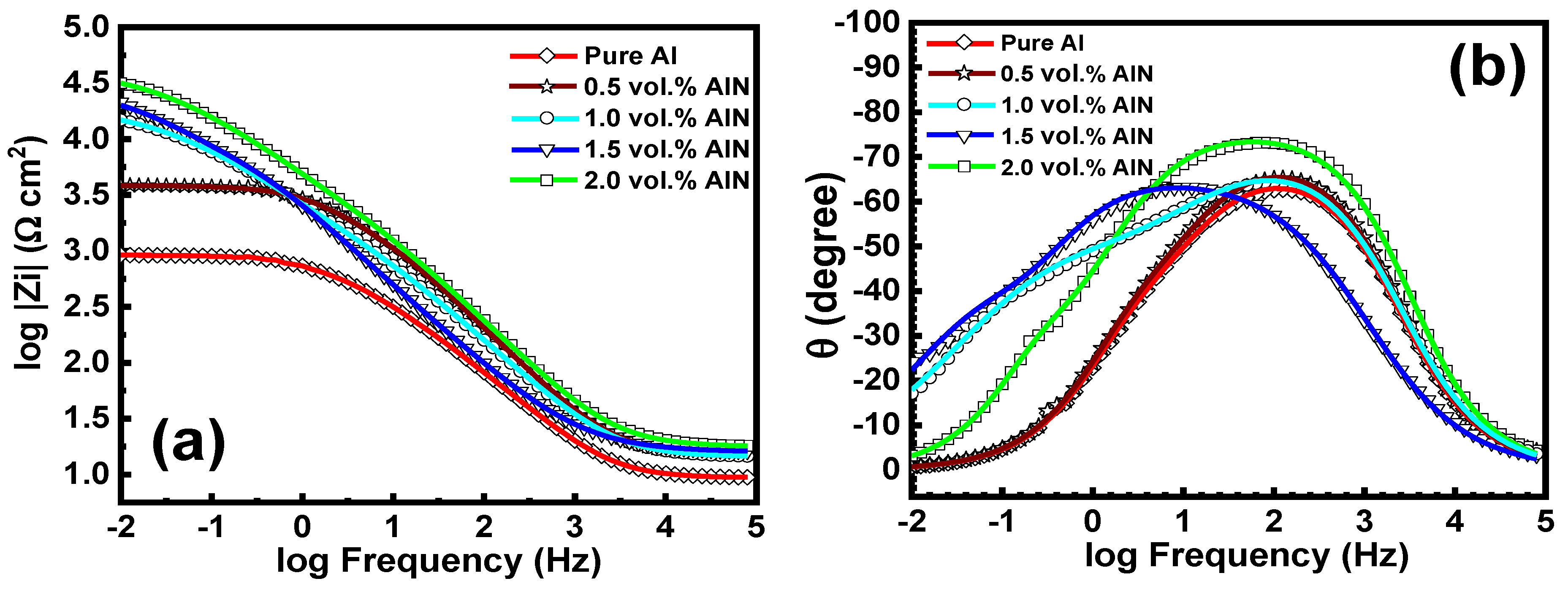
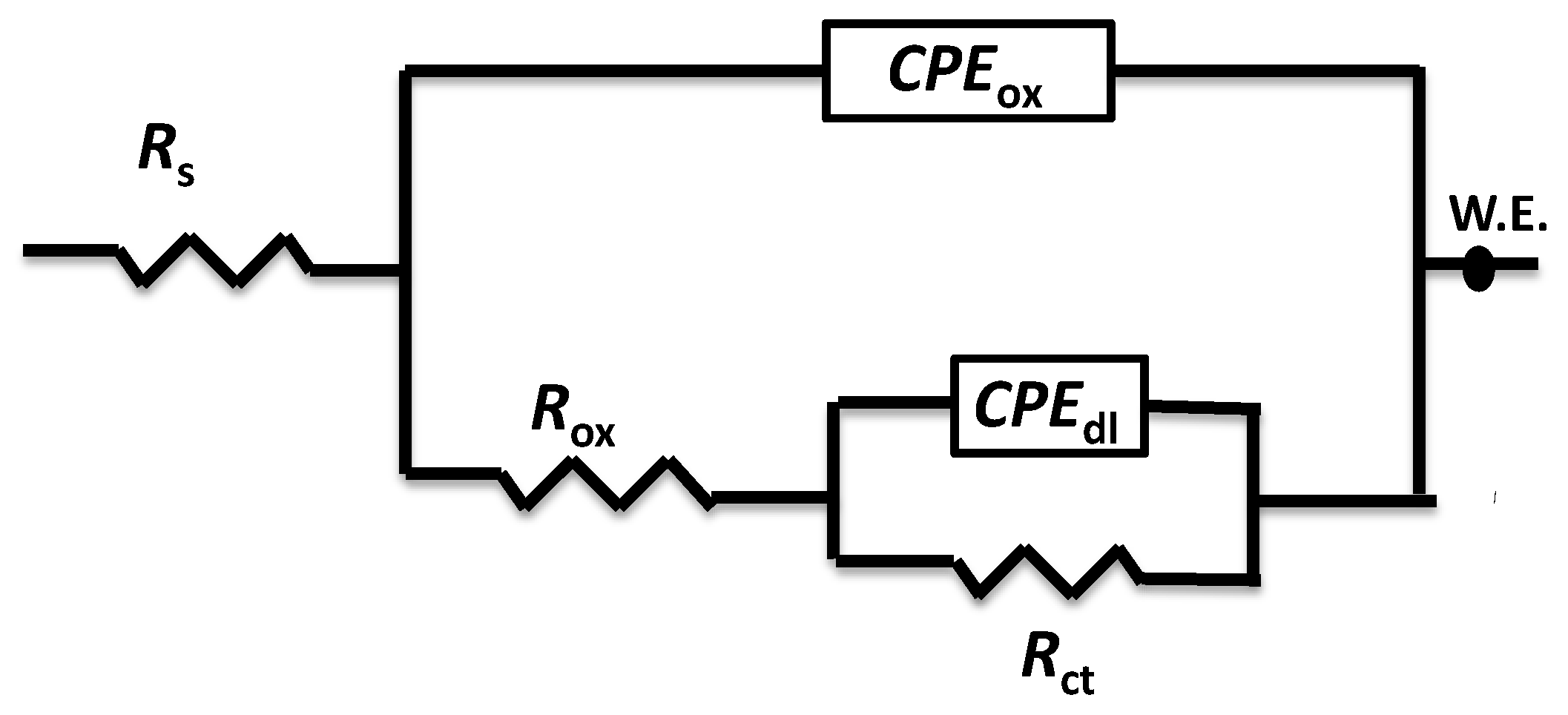
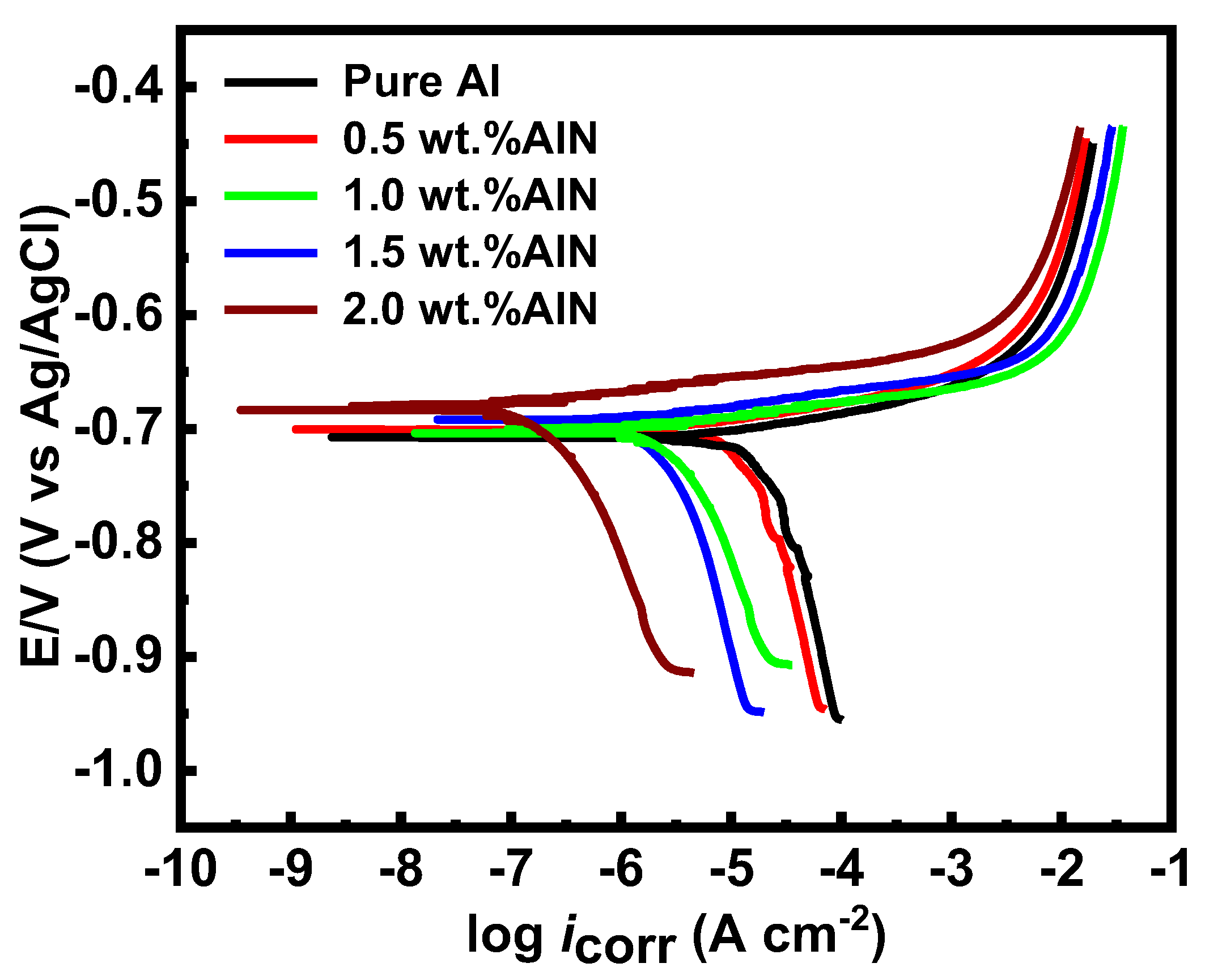
3.8. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shalaby, E.A.; Churyumov, A.Y. Development and characterization of A359/AlN composites for automotive applications. J. Alloys Compd. 2017, 727, 540–548. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, D. High temperature ceramic matrix composites. J. Am. Ceram. Soc. 2014, 8, 1–698. [Google Scholar] [CrossRef]
- Clyne, T.W.; Withers, P.J. An Introduction to Metal Matrix Composites; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar] [CrossRef]
- Mohanty, R.; Balasubramanian, K.; Seshadri, S. Boron carbide-reinforced alumnium 1100 matrix composites: Fabrication and properties. Mater. Sci. Eng. A 2008, 498, 42–52. [Google Scholar] [CrossRef]
- Lloyd, D.J. Particle reinforced aluminium and magnesium matrix composites. Int. Mater. Rev. 1994, 39, 1–23. [Google Scholar] [CrossRef]
- Lee, J.-M.; Lee, S.-K.; Hong, S.-J.; Kwon, Y.-N. Microstructures and thermal properties of A356/SiCp composites fabricated by liquid pressing method. Mater. Des. 2012, 37, 313–316. [Google Scholar] [CrossRef]
- Tiwari, A.; Alenezi, M.R.; Jun, S.C. Advanced Composite Materials; Scrivener Publishing: Beverly, MA, USA, 2016; pp. 1–462. [Google Scholar] [CrossRef]
- Backman, D.G.; Williams, J.C. Advanced Materials for Aircraft Engine Applications. Science 1992, 255, 1082–1087. [Google Scholar] [CrossRef]
- Skoczylas, J.; Samborski, S.; Kłonica, M. The application of composite materials in the aerospace industry. J. Technol. Exploit. Mech. Eng. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Bhoi, N.K.; Singh, H.; Pratap, S. Developments in the aluminum metal matrix composites reinforced by micro/nano particles—A review. J. Compos. Mater. 2020, 54, 813–833. [Google Scholar] [CrossRef]
- Mullen, L. Applications Of. Chic. Rev. 2000, 46, 67. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef]
- Tanzi, M.C.; Farè, S.; Candiani, G. Organization, Structure, and Properties of Materials; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Haghshenas, M. Metal–Matrix Composites. Ref. Modul. Mater. Sci. Mater. Eng. 2016, 57–66. [Google Scholar] [CrossRef]
- Richard, S.; Rajadurai, J.S.; Manikandan, V. Effects of Particle Loading and Particle Size on Tribological Properties of Biochar Particulate Reinforced Polymer Composites. J. Tribol. 2016, 139, 12202. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal Matrix Composites Reinforced by Nano-Particles—A Review. Metals 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Hassanzadeh-Aghdam, M.; Mahmoodi, M.; Ansari, R. A comprehensive predicting model for thermomechanical properties of particulate metal matrix nanocomposites. J. Alloys Compd. 2018, 739, 164–177. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2013, 47, 013001. [Google Scholar] [CrossRef]
- Dadkhah, M.; Saboori, A.; Fino, P. An Overview of the Recent Developments in Metal Matrix Nanocomposites Reinforced by Graphene. Materials 2019, 12, 2823. [Google Scholar] [CrossRef]
- Pang, W.; Fan, X.; Wang, K.; Chao, Y.; Xu, H.; Qin, Z. Al-Based Nano-Sized Composite Energetic Materials and Performance. Nanomaterials 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.A.; Mohamed, F.A.; Lavernia, E.J. Particulate reinforced metal matrix composites—A review. J. Mater. Sci. 1991, 26, 1137–1156. [Google Scholar] [CrossRef]
- Giannopoulou, D.; Dieringa, H.; Bohlen, J. Influence of AlN Nanoparticle Addition on Microstructure and Mechanical Properties of Extruded Pure Magnesium and an Aluminum-Free Mg-Zn-Y Alloy. Metals 2019, 9, 667. [Google Scholar] [CrossRef]
- He, Q.; Qin, M.; Huang, M.; Wu, H.; Lu, H.; Wang, H.; Mu, X.; Wang, Y.; Qu, X. Synthesis of highly sinterable AlN nanopowders through sol-gel route by reduction-nitridation in ammonia. Ceram. Int. 2019, 45, 14568–14575. [Google Scholar] [CrossRef]
- Franco, A.F., Jr.; Shanafield, D.J. Thermal conductivity of polycrystalline aluminum nitride (AlN) ceramics. Ceramica 2004, 50, 247–253. [Google Scholar] [CrossRef]
- Valente, M.; Rossitti, I.; Sambucci, M. Different Production Processes for Thermoplastic Composite Materials: Sustainability versus Mechanical Properties and Processes Parameter. Polymers 2023, 15, 242. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Bhandari, R.; Pinca-Bretotean, C. A systematic overview on fabrication aspects and methods of aluminum metal matrix composites. Mater. Today Proc. 2020, 45, 4133–4138. [Google Scholar] [CrossRef]
- Rahimian, M.; Ehsani, N.; Parvin, N.; Baharvandi, H.R. The effect of particle size, sintering temperature and sintering time on the properties of Al–Al2O3 composites, made by powder metallurgy. J. Mater. Process. Technol. 2009, 209, 5387–5393. [Google Scholar] [CrossRef]
- Almomani, M.A.; Shatnawi, A.M.; Alrashdan, M.K. Effect of Sintering Time on the Density, Porosity Content and Microstructure of Copper—1 wt.% Silicon Carbide Composites. Adv. Mater. Res. 2014, 1064, 32–37. [Google Scholar] [CrossRef]
- Matli, P.R.; Shakoor, R.A.; Mohamed, A.M.A.; Gupta, M. Microwave Rapid Sintering of Al-Metal Matrix Composites: A Review on the Effect of Reinforcements, Microstructure and Mechanical Properties. Metals 2016, 6, 143. [Google Scholar] [CrossRef]
- Singhal, C.; Murtaza, Q. Parvej Microwave Sintering of Advanced Composites Materials: A Review. Mater. Today Proc. 2018, 5, 24287–24298. [Google Scholar] [CrossRef]
- Mohanavel, V.; Rajan, K.; Ravichandran, M. Synthesis, characterization and properties of stir cast AA6351-aluminium nitride (AlN) composites. J. Mater. Res. 2016, 31, 3824–3831. [Google Scholar] [CrossRef]
- Riquelme, A.; Rodrigo, P. Influence of the Feed Powder Composition in Aluminium Composites Coatings Deposited. Metals 2020, 10, 926. [Google Scholar] [CrossRef]
- Dhawan, P.A.; Sharma, V.; Parmar, D.; Dhawan, A.; Sharma, V.; Parmar, D. Nanomaterials: A challenge for toxicologists. Nanotoxicology 2009, 3, 5390. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, R.; Pendry, J.B. Van der Waals interactions at the nanoscale: The effects of nonlocality. Proc. Natl. Acad. Sci. USA 2014, 111, 18422–18427. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Huang, Z.; Wang, W.; Davé, R.N. Investigation of nanoparticle agglomerates properties using Monte Carlo simulations. Adv. Powder Technol. 2016, 27, 1971–1979. [Google Scholar] [CrossRef]
- Gajewska, M.; Dutkiewicz, J.; Morgiel, J. Microstructure and mechanical properties of AA7475/AlN, compacts with varied reinforcing particles size. Compos. Theory Pract. 2012, 12, 177–181. [Google Scholar]
- Liu, Y.; Cong, H.; Wang, W.; Sun, C.; Cheng, H. AlN nanoparticle-reinforced nanocrystalline Al matrix composites: Fabrication and mechanical properties. Mater. Sci. Eng. A 2009, 505, 151–156. [Google Scholar] [CrossRef]
- El-Makaty, F.M.; Ahmed, H.K.; Youssef, K.M. Review: The effect of different nanofiller materials on the thermoelectric behavior of bismuth telluride. Mater. Des. 2021, 209, 109974. [Google Scholar] [CrossRef]
- Oveisi, H.; Geramipour, T. High mechanical performance alumina-reinforced aluminum nanocomposite metal foam produced by powder metallurgy: Fabrication, microstructure characterization, and mechanical properties. Mater. Res. Express 2020, 6, 1250c2. [Google Scholar] [CrossRef]
- Paupler, P.G.E. Dieter. Mechanical Metallurgy, 3rd ed.; Mc Graw-Hill Book Co.: New York, NY, USA, 1986; p. 194. ISBN 0–07–016893–8. [Google Scholar]
- Bisht, A.; Srivastava, M.; Kumar, R.M.; Lahiri, I.; Lahiri, D. Strengthening mechanism in graphene nanoplatelets reinforced aluminum composite fabricated through spark plasma sintering. Mater. Sci. Eng. A 2017, 695, 20–28. [Google Scholar] [CrossRef]
- Fale, S.; Likhite, A.; Bhatt, J. Compressive, tensile and wear behavior of ex situ Al/AlN metal matrix nanocomposites. J. Compos. Mater. 2015, 49, 1917–1928. [Google Scholar] [CrossRef]
- Şenel, M.C.; Gürbüz, M.; Koç, E. Fabrication and characterization of aluminum hybrid composites reinforced with silicon nitride/graphene nanoplatelet binary particles. J. Compos. Mater. 2019, 53, 4043–4054. [Google Scholar] [CrossRef]
- Abdelatty, R.; Khan, A.; Yusuf, M.; Alashraf, A.; Shakoor, R.A. Effect of Silicon Nitride and Graphene Nanoplatelets on the Properties of Aluminum Metal Matrix Composites. Materials 2021, 14, 1898. [Google Scholar] [CrossRef]
- Shahzad, K.; Radwan, A.B.; Fayyaz, O.; Shakoor, R.; Uzma, M.; Umer, M.A.; Baig, M.; Raza, A. Effect of concentration of TiC on the properties of pulse electrodeposited Ni–P–TiC nanocomposite coatings. Ceram. Int. 2021, 47, 19123–19133. [Google Scholar] [CrossRef]
- Jlassi, K.; Radwan, A.B.; Sadasivuni, K.K.; Mrlik, M.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. Anti-corrosive and oil sensitive coatings based on epoxy/polyaniline/magnetite-clay composites through diazonium interfacial chemistry. Sci. Rep. 2018, 8, 13369. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Radwan, A.B.; Kalambate, P.K.; Laiwattanapaisal, W.; Ubaid, F.; Akbar, H.M.; Shakoor, R.A.; Kahraman, R. Synergistic Behavior of Polyethyleneimine and Epoxy Monomers Loaded in Mesoporous Silica as a Corrosion-Resistant Self-Healing Epoxy Coating. ACS Omega 2022, 7, 31700–31712. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.B.; Moussa, A.M.; Al-Qahtani, N.H.; Case, R.; Castaneda, H.; Abdullah, A.M.; El-Haddad, M.A.M.; Bhadra, J.; Al-Thani, N.J. Evaluation of the Pitting Corrosion of Modified Martensitic Stainless Steel in CO2 Environment Using Point Defect Model. Metals 2022, 12, 233. [Google Scholar] [CrossRef]


| Sample No. | Sample Name | Volume Fraction of AlN (vol.%) |
|---|---|---|
| 1 | A1 | 0 |
| 2 | A2 | 0.5 |
| 3 | A3 | 1 |
| 4 | A4 | 1.5 |
| 5 | A5 | 2 |
| Sample | Rox, kΩ cm2 | Rct, kΩ cm2 | Cdl1 μF | Cdl2 μF |
|---|---|---|---|---|
| Pure Al | 0.645 | 3.6 | 2.2 | 2.4 |
| 0.5 vol.%AlN | 0.712 | 5.1 | 1.8 | 1.6 |
| 1.0 vol.%AlN | 1.5 | 15 | 1.4 | 1.3 |
| 1.5 vol.%AlN | 3.4 | 19 | 1.1 | 0.8 |
| 2.0 vol.%AlN | 4.3 | 30 | 0.9 | 0.6 |
| Sample | βa (V·decade−1) | βc (V·decade−1) | icorr (µ A cm−2) | Corrosion Rate (mm y−1) |
|---|---|---|---|---|
| Pure Al | 0.0141 | 0.151 | 8 | 0.16 |
| 0.5 vol.%AlN | 0.0137 | 0.087 | 4.1 | 0.082 |
| 1.0 vol.%AlN | 0.0131 | 0.066 | 2.5 | 0.05 |
| 1.5 vol.%AlN | 0.0121 | 0.027 | 1.2 | 0.02 |
| 2.0 vol.%AlN | 0.0111 | 0.012 | 0.23 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelatty, R.H.; Radwan, A.B.; Youssef, K.; Ijaz, M.F.; Abdul Shakoor, R. Effect of AlN on the Mechanical and Electrochemical Properties of Aluminum Metal Matrix Composites. Materials 2024, 17, 3258. https://doi.org/10.3390/ma17133258
Abdelatty RH, Radwan AB, Youssef K, Ijaz MF, Abdul Shakoor R. Effect of AlN on the Mechanical and Electrochemical Properties of Aluminum Metal Matrix Composites. Materials. 2024; 17(13):3258. https://doi.org/10.3390/ma17133258
Chicago/Turabian StyleAbdelatty, Rokaya H., Ahmed Bahgat Radwan, Khaled Youssef, Muhammad Farzik Ijaz, and Rana Abdul Shakoor. 2024. "Effect of AlN on the Mechanical and Electrochemical Properties of Aluminum Metal Matrix Composites" Materials 17, no. 13: 3258. https://doi.org/10.3390/ma17133258
APA StyleAbdelatty, R. H., Radwan, A. B., Youssef, K., Ijaz, M. F., & Abdul Shakoor, R. (2024). Effect of AlN on the Mechanical and Electrochemical Properties of Aluminum Metal Matrix Composites. Materials, 17(13), 3258. https://doi.org/10.3390/ma17133258








