Preparation of High-Performance Barium Titanate Composite Hydrogels by Deep Eutectic Solvent-Assisted Frontal Polymerization
Abstract
:1. Introduction
2. Experimentation
2.1. Materials
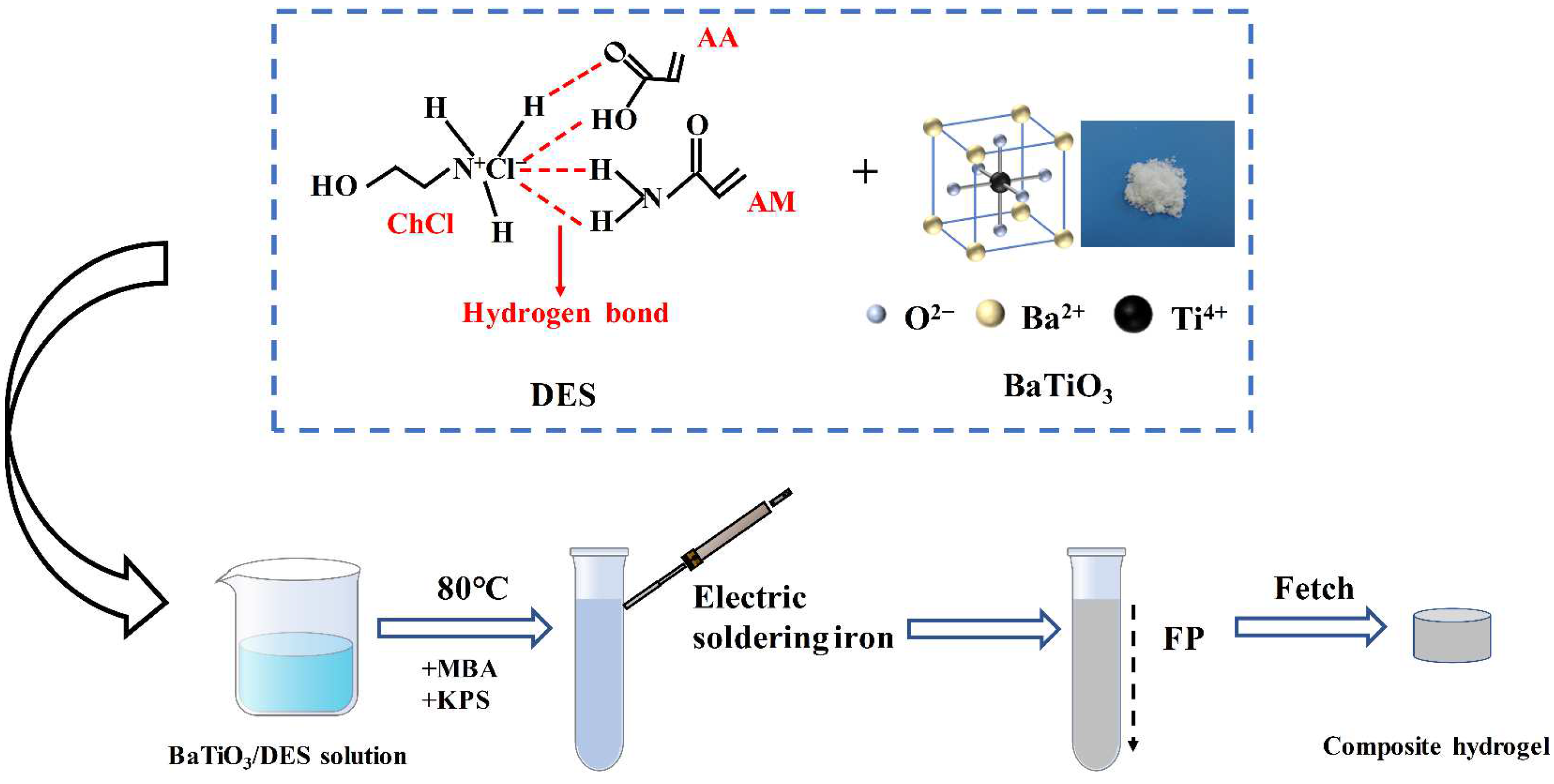
2.2. DES Preparation
2.3. Preparation of Barium Titanate Composite Hydrogel
2.4. Performance Testing and Characterization of Composite Hydrogels
2.4.1. SEM Characterization
2.4.2. FTIR Characterization
2.4.3. Frontal Velocity and Frontal Temperature of Hydrogels
2.4.4. Piezoelectric Properties Testing of Hydrogels
2.4.5. Mechanical Testing of Hydrogels
2.4.6. Hydrogel Swelling Property Test
2.4.7. Pressure Sensing Testing of Hydrogels
3. Results and Analysis
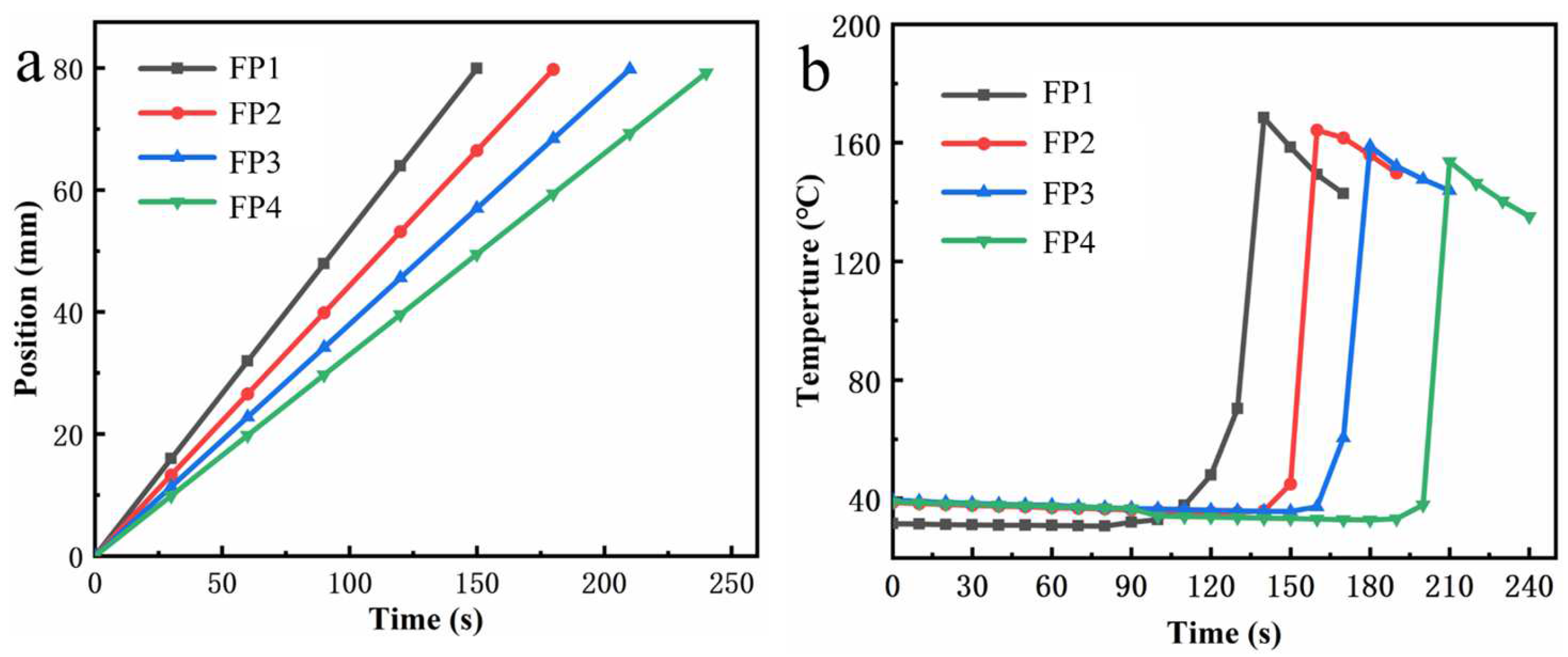
3.1. Frontal Speed and Temperature Measurements
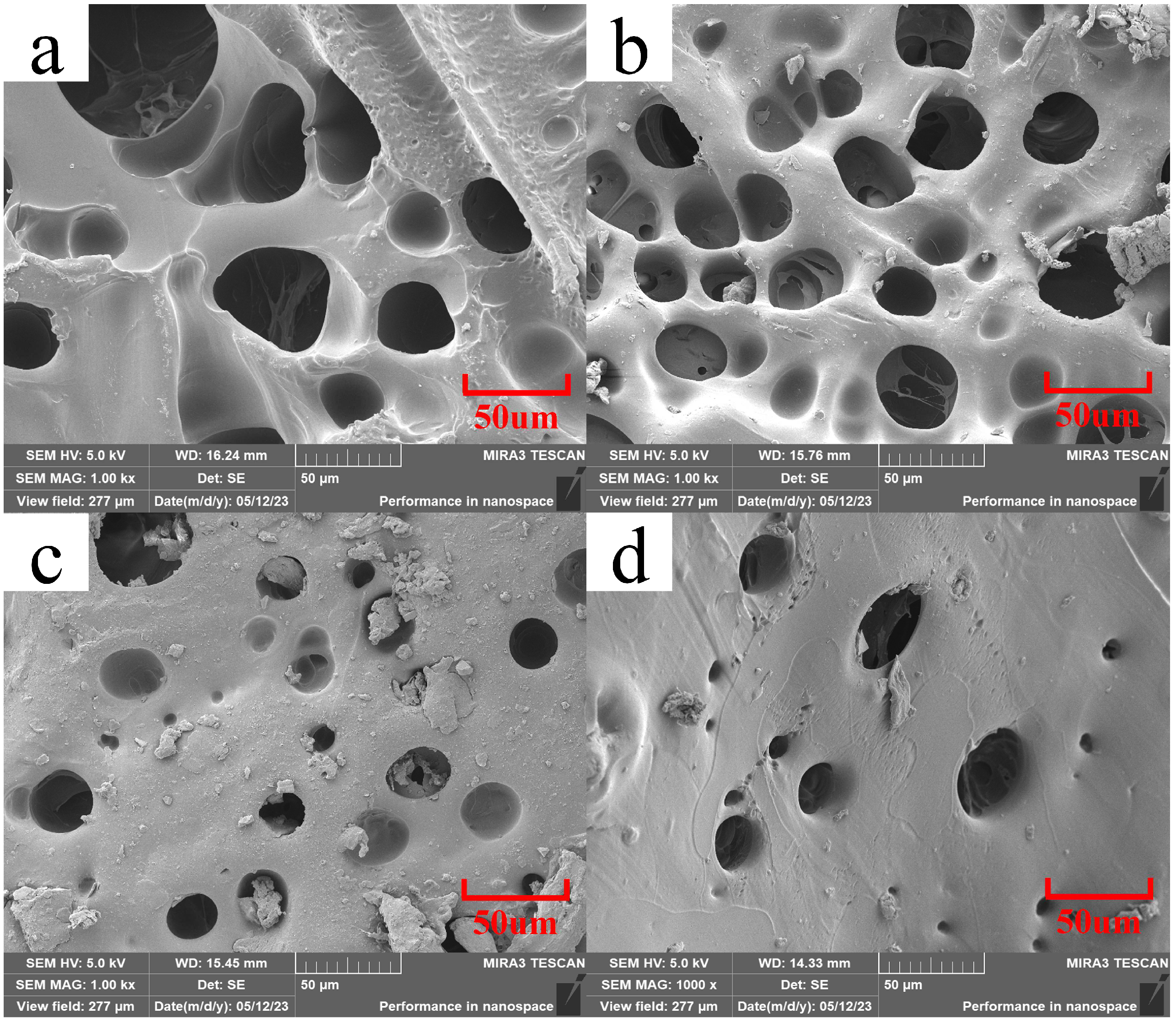
3.2. Microscopic Morphology Analysis of Composite Hydrogels
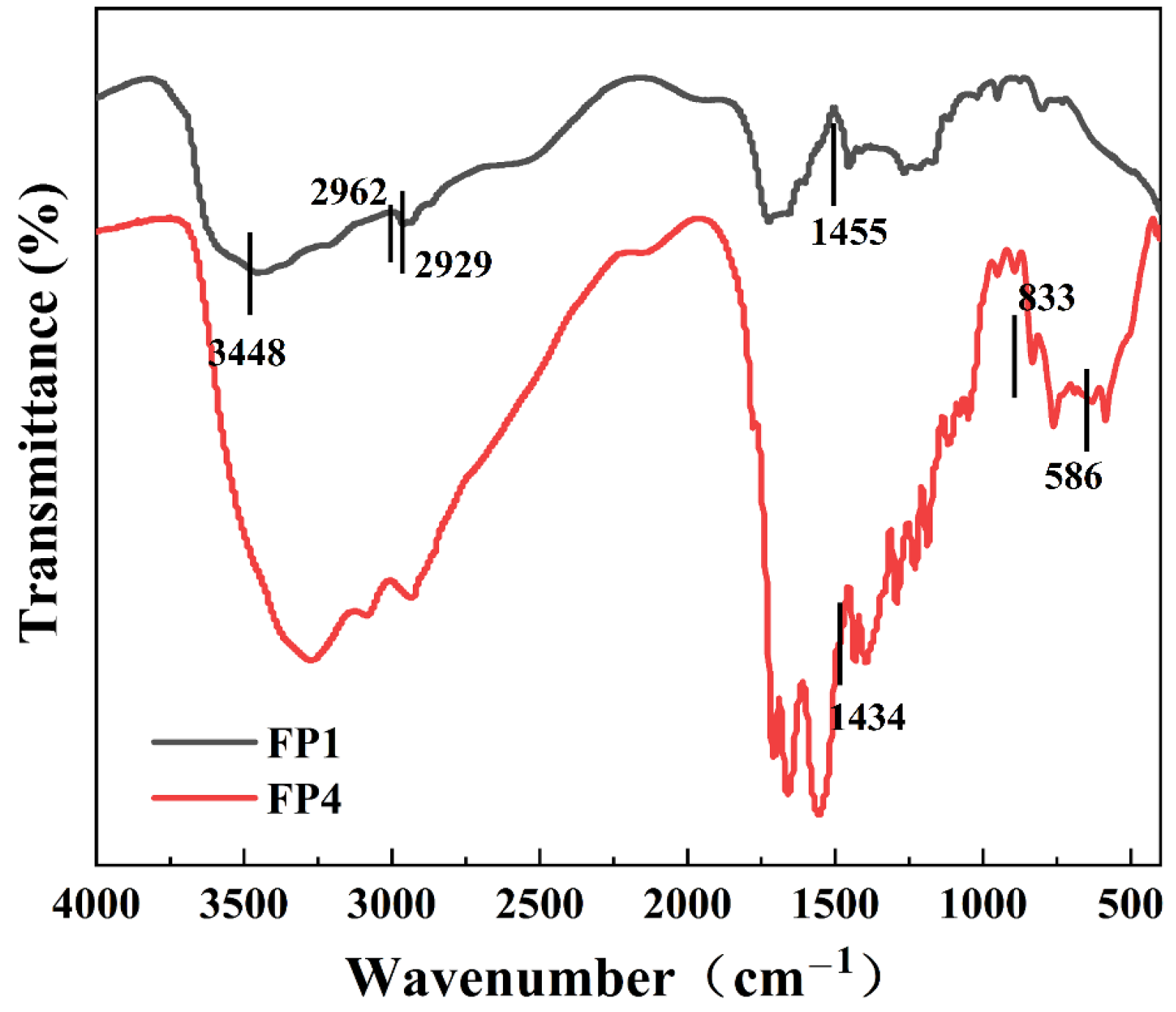
3.3. Fourier Infrared Spectral Analysis of Composite Hydrogels
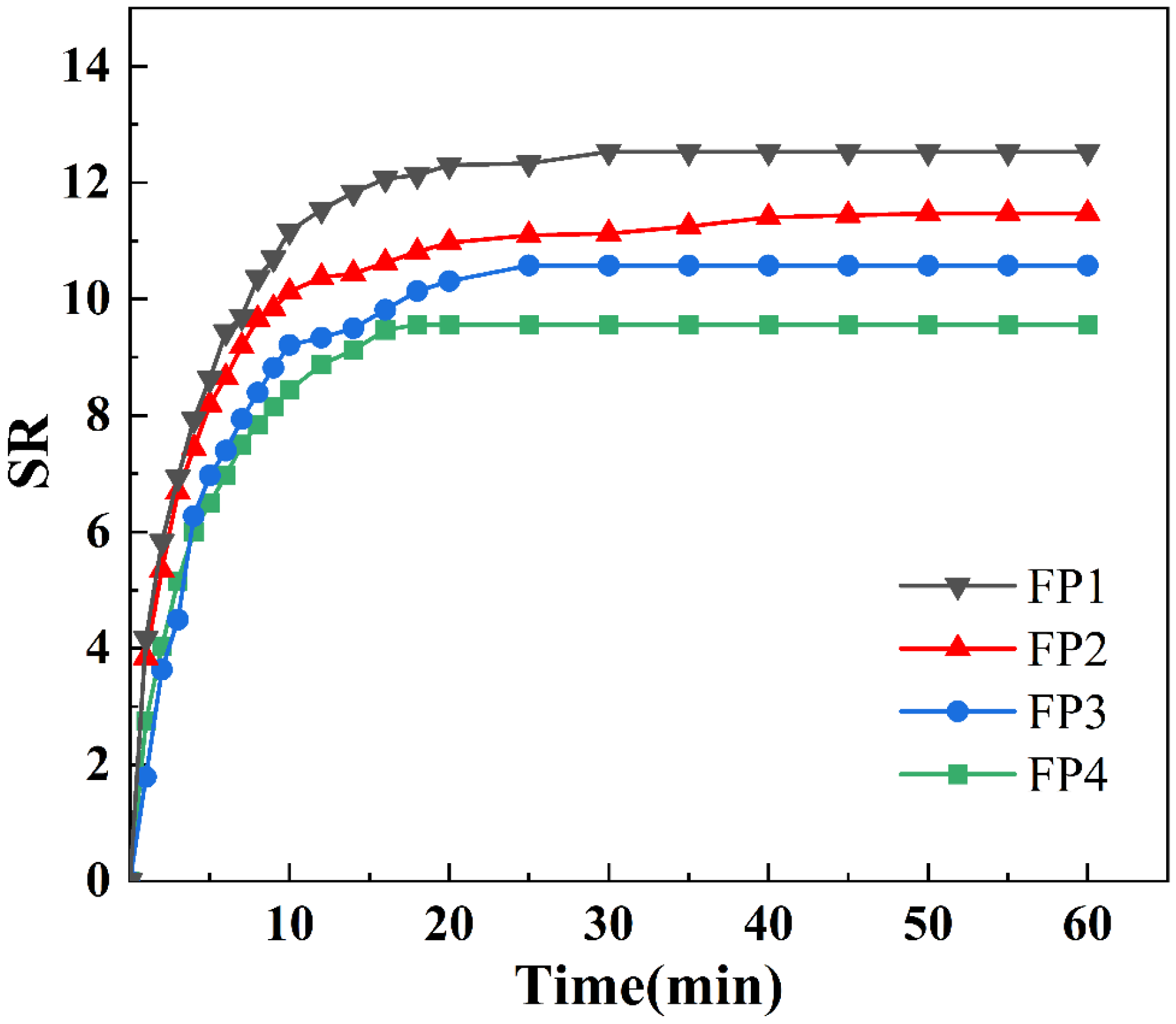
3.4. Swelling Properties of Composite Hydrogels
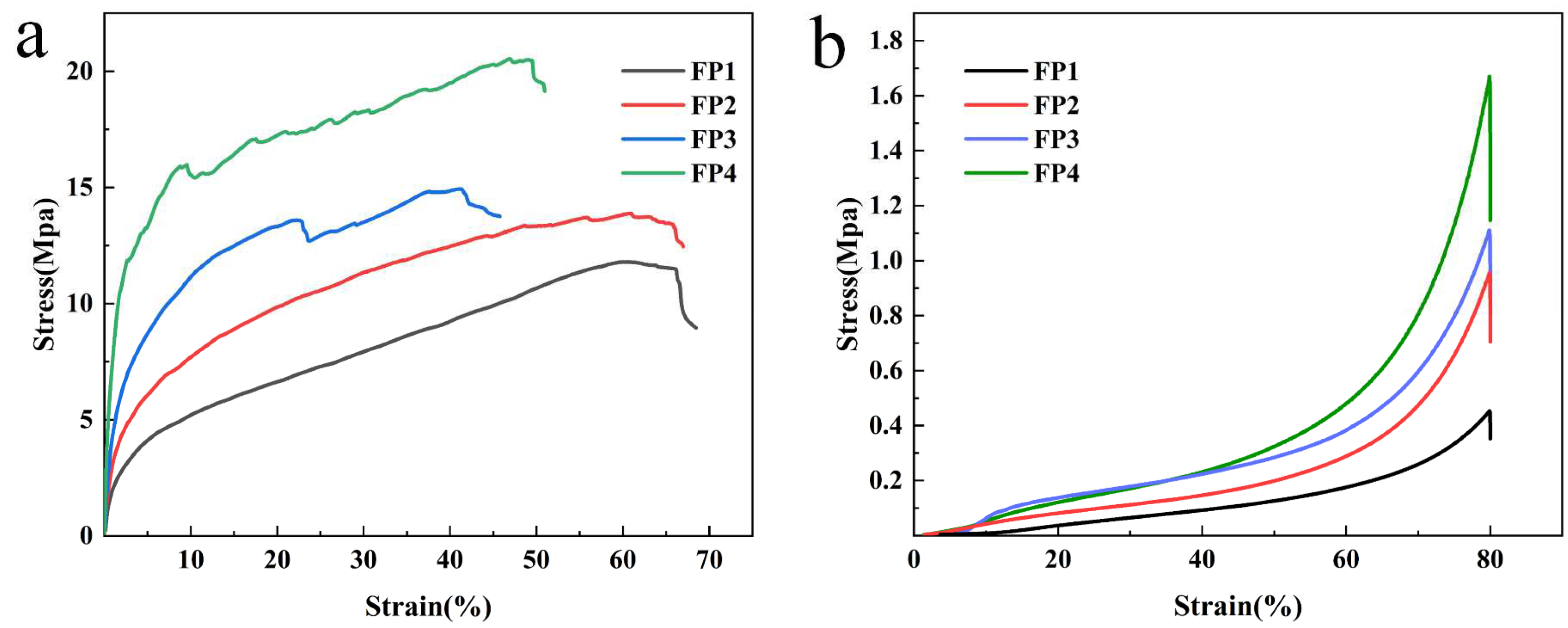
3.5. Mechanical Properties of Composite Hydrogels
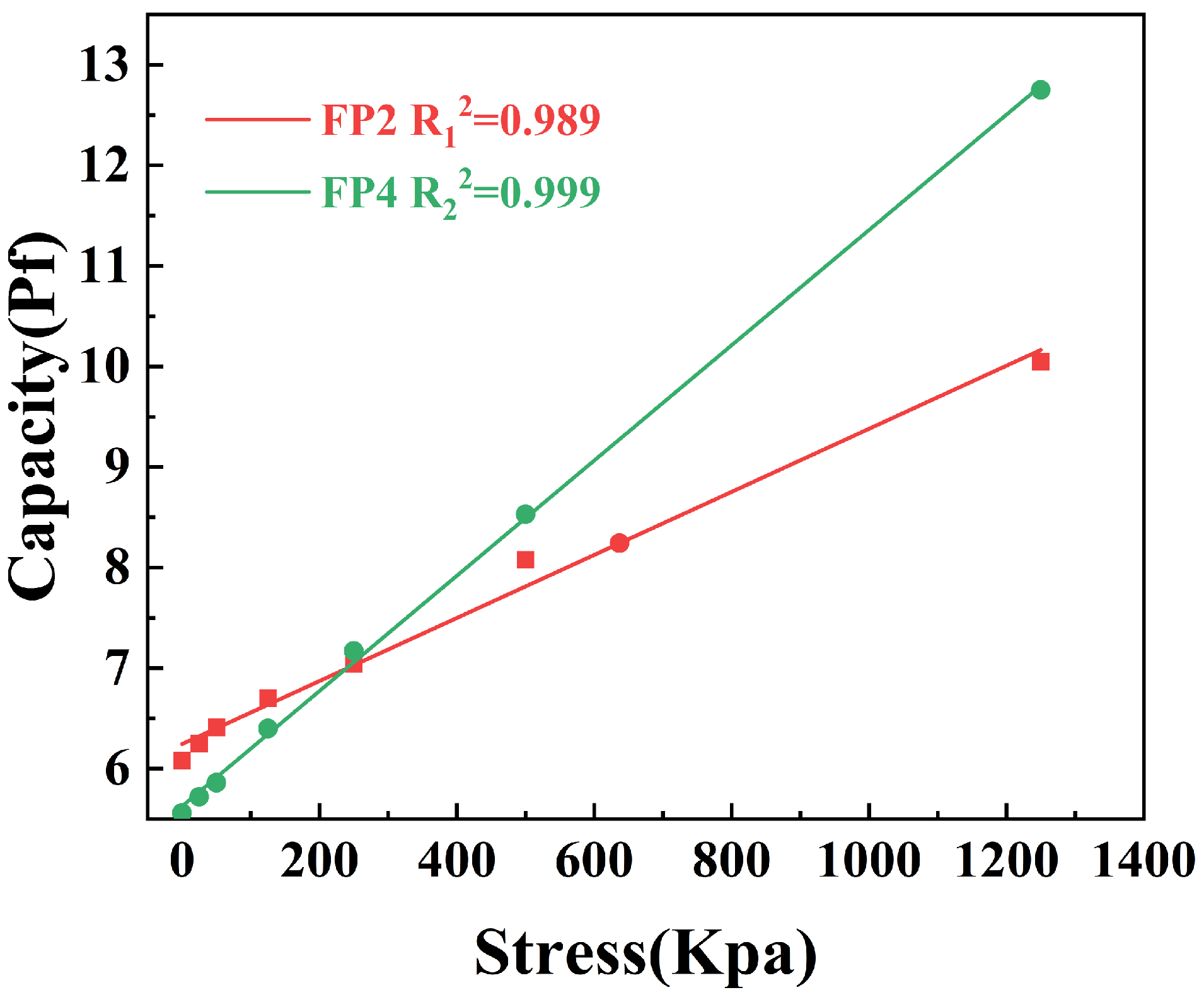
3.6. Pressure-Sensitive Features of Composite Hydrogels
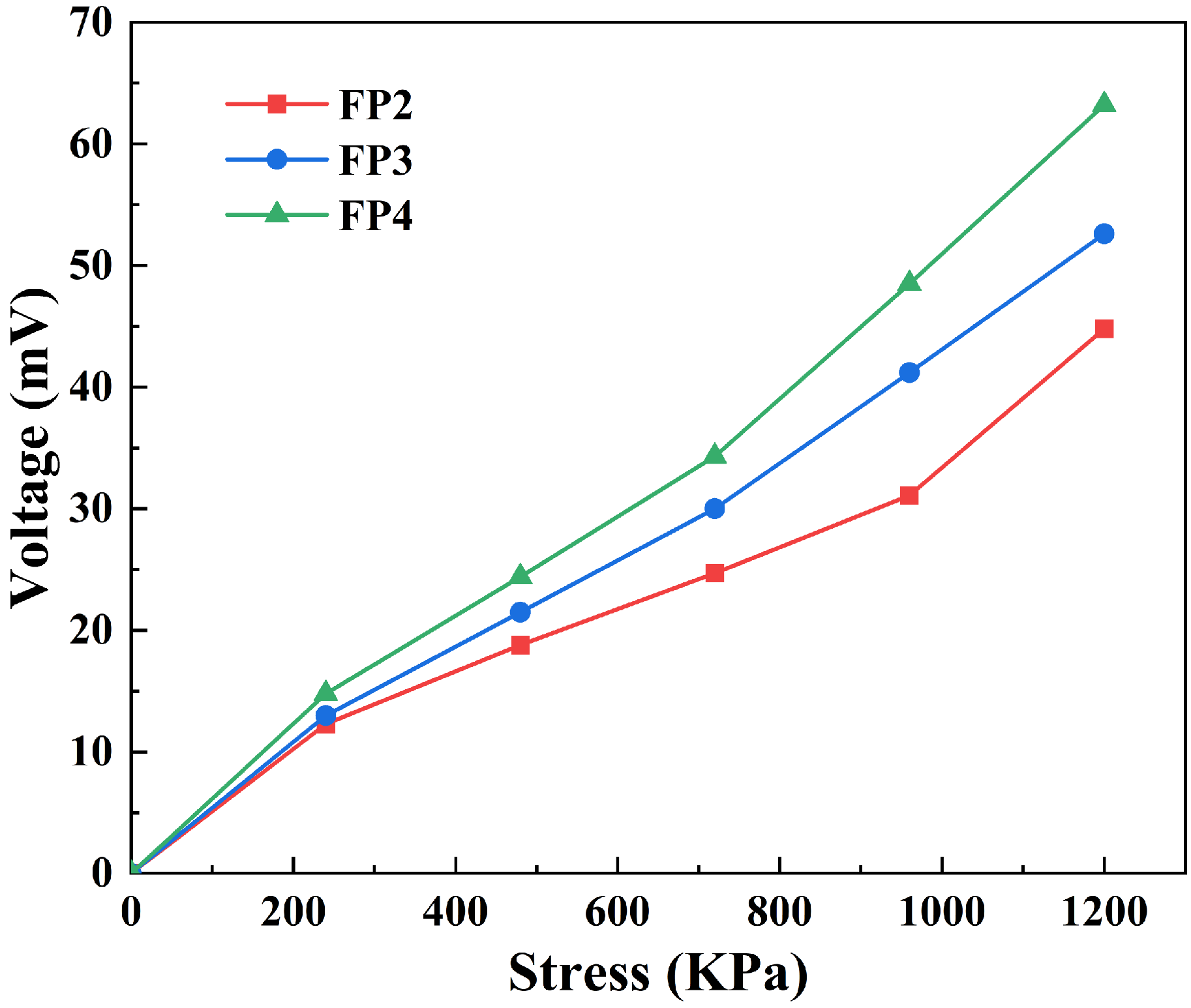
3.7. Piezoelectric Properties of Composite Hydrogels
4. Conclusions
- (1)
- The mechanical characteristics of the composite hydrogels were improved as the content of BTNPs increased, as BTNPs served as cross-linking sites in the hydrogels. Upon reaching a content of 0.6 wt% of BTNPs, the hydrogel exhibited a significant increase in its maximum tensile strength, approximately 1.8 times higher than that of the hydrogel without additional BTNPs. Similarly, the hydrogel’s maximum compressive strength rose by a factor of 3.68 compared with the hydrogel without BTNPs.
- (2)
- Due to BTNPs’ high dielectric constant, the BTNPs/P(AM-co-AA) composite hydrogel exhibits enhanced pressure sensitivity when the capacitor is fabricated as a dielectric layer. As the concentration of BTNPs in the composite hydrogel grows incrementally, the capacitor’s pressure sensitivity likewise increases progressively.
- (3)
- BTNPs are a common piezoelectric substance that confers piezoelectric capabilities to the composite hydrogel when applied as a filler. With an increase in the quantity of BTNPs in the composite hydrogel from 0.2 wt% to 0.6 wt%, the voltage produced by the composite hydrogel also rose from 44.8 mV to 63.2 mV when exposed to a pressure of 1200 Kpa.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Gancheva, T.; Virgilio, N. Enhancing and Tuning the Response of Environmentally Sensitive Hydrogels With Embedded and Interconnected Pore Networks. Macromolecules 2016, 49, 5866–5876. [Google Scholar] [CrossRef]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Sun, X.; Yao, F.; Li, J. Nanocomposite hydrogel-based strain and pressure sensors: A review. J. Mater. Chem. A 2020, 8, 18605–18623. [Google Scholar] [CrossRef]
- Li, J.; Jia, X.; Yin, L. Hydrogel: Diversity of Structures and Applications in Food Science. Food Rev. Int. 2021, 37, 313–372. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Gutiérrez, M.C.; Sanchez, I.C.; Luna-Bárcenas, G.; del Monte, F. Frontal polymerizations carried out in deep-eutectic mixtures providing both the monomers and the polymerization medium. Chem. Commun. 2011, 47, 5328–5330. [Google Scholar] [CrossRef] [PubMed]
- Mota-Morales, J.D.; Gutiérrez, M.C.; Ferrer, M.L.; Sanchez, I.C.; Elizalde-Peña, E.A.; Pojman, J.A.; Monte, F.D.; Luna-Bárcenas, G. Deep eutectic solvents as both active fillers and monomers for frontal polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1767–1773. [Google Scholar] [CrossRef]
- Li, B.; Xu, X.J.; Hu, Z.G.; Li, Y.J.; Zhou, M.J.; Liu, J.Z.; Jiang, Y.J.; Wang, P. Rapid preparation of N-CNTs/P(AA-AM) composite hydrogel frontal polymerization and its mechanical and conductive properties. RSC Adv. 2022, 12, 19022–19028. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Ehterami, A.; Kazemi, M.; Nazari, B.; Saraeian, P.; Azami, M. Fabrication and characterization of highly porous barium titanate based scaffold coated by Gel/HA nanocomposite with high piezoelectric coefficient for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2018, 79, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wang, X.; Wen, H.; Chen, L.; Chen, L.; Li, L. Ferroelectric properties of nanocrystalline barium titanate ceramics. Appl. Phys. Lett. 2006, 88, 252905. [Google Scholar] [CrossRef]
- Zhao, T.; Lu, H.; Chen, F.; Yang, G.; Chen, Z. Stress-induced enhancement of second-order nonlinear optical susceptibilities of barium titanate films. J. Appl. Phys. 2000, 87, 7448–7451. [Google Scholar] [CrossRef]
- Pham, H.T.; Yang, J.H.; Lee, D.-S.; Lee, B.H.; Jeong, H.-D. Ferroelectric/Dielectric Double Gate Insulator Spin-Coated Using Barium Titanate Nanocrystals for an Indium Oxide Nanocrystal-Based Thin-Film Transistor. ACS Appl. Mater. Interfaces 2016, 8, 7248–7256. [Google Scholar] [CrossRef] [PubMed]
- Cholleti, E.R.; Stringer, J.; Kelly, P.; Bowen, C.; Aw, K. Studying the creep behaviour of strechable capacitive sensor with barium titanate silicone elastomer composite. Sens. Actuators A Phys. 2021, 319, 112560. [Google Scholar] [CrossRef]
- Page, K.; Proffen, T.; Niederberger, M.; Seshadri, R. Probing Local Dipoles and Ligand Structure in BaTiO3 Nanoparticles. Chem. Mater. 2010, 22, 4386–4391. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs. ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Li, B.; Zhou, M.; Cheng, M.; Liu, J.; Xu, X.; Xie, X. Rapid preparation of ZnO nanocomposite hydrogels by frontal polymerization of a ternary DES and performance study. RSC Adv. 2022, 12, 12871–12877. [Google Scholar] [CrossRef]
- Xiong, L.; Jin, S.; Zhang, F.; Li, K.; Li, J.; Mei, C.; Han, J.; Xiao, H.; Seidi, F. Bioinspired fabrication of self-recovery, adhesive, and flexible conductive hydrogel sensor driven by dynamic borate ester bonds and tannic acid-mediated noncovalent network. Eur. Polym. J. 2022, 180, 111636. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, X.; Zhang, Y. Study on Hydrolysis Properties and Mechanism of Poly(3-Methacrylamido Propyl Trimethyl Ammonium Chloride) Solution. Polymers 2022, 14, 2811. [Google Scholar] [CrossRef]
- Orozco-Guareño, E.; Santiago-Gutiérrez, F.; Morán-Quiroz, J.L.; Hernandez-Olmos, S.L.; Soto, V.; Cruz, W.d.l.; Manríquez, R.; Gomez-Salazar, S. Removal of Cu(II) ions from aqueous streams using poly(acrylic acid-co-acrylamide) hydrogels. J. Colloid Interface Sci. 2010, 349, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hao, W.; Wu, A.; Zhou, M.; Yan, Q.; Zhang, H.; Su, L. Preparation and characterization of PA/P(AA-co-AM) composite hydrogels via photopolymerization. RSC Adv. 2023, 13, 22831–22837. [Google Scholar] [CrossRef] [PubMed]
- GhaedRahmati, H.; Frounchi, M.; Dadbin, S. Piezoelectric behavior of Gamma-radiated nanocomposite hydrogel based on PVP-PEG-BaTiO3. Mater. Sci. Eng. B 2022, 276, 115535. [Google Scholar] [CrossRef]
- Serra-Gómez, R.; Dreiss, C.A.; González-Benito, J.; González-Gaitano, G. Structure and Rheology of Poloxamine T1107 and Its Nanocomposite Hydrogels with Cyclodextrin-Modified Barium Titanate Nanoparticles. Langmuir 2016, 32, 6398–6408. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Yang, Y.; Fan, A.; Chi, R.; Shi, J.; Zhang, X. Poly (vinyl alcohol) (PVA) hydrogel incorporated with Ag/TiO2 for rapid sterilization by photoinspired radical oxygen species and promotion of wound healing. Appl. Surf. Sci. 2019, 494, 708–720. [Google Scholar] [CrossRef]
- Zaragoza, J.; Fukuoka, S.; Kraus, M.; Thomin, J.; Asuri, P. Exploring the Role of Nanoparticles in Enhancing Mechanical Properties of Hydrogel Nanocomposites. Nanomaterials 2018, 8, 882. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Deng, X.; Wen, H.; Li, L. Phase transition and high dielectric constant of bulk dense nanograin barium titanate ceramics. Appl. Phys. Lett. 2006, 89, 162902. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Qing, X. A flexible capacitive sensor based on the electrospun PVDF nanofiber membrane with carbon nanotubes. Sens. Actuators A Phys. 2019, 299, 111579. [Google Scholar] [CrossRef]
- Fu, R.; Zhong, X.; Xiao, C.; Lin, J.; Guan, Y.; Tian, Y.; Zhou, Z.; Tan, G.; Hu, H.; Zhou, L.; et al. A stretchable, biocompatible, and self-powered hydrogel multichannel wireless sensor system based on piezoelectric barium titanate nanoparticles for health monitoring. Nano Energy 2023, 14, 108617. [Google Scholar]

| Sample | AA:AM:ChCl (Molar Ratio) | BTNPs (wt%) | KPS(g) (wt%) | MBA(g) (wt%) |
|---|---|---|---|---|
| FP1 | 1:1:1 | 0 | 0.15 | 0.5 |
| FP2 | 1:1:1 | 0.2 | 0.15 | 0.5 |
| FP3 | 1:1:1 | 0.4 | 0.15 | 0.5 |
| FP4 | 1:1:1 | 0.6 | 0.15 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Wu, A.; Zhou, M.; Wang, Y.; Hu, Z.; Su, L. Preparation of High-Performance Barium Titanate Composite Hydrogels by Deep Eutectic Solvent-Assisted Frontal Polymerization. Materials 2024, 17, 3262. https://doi.org/10.3390/ma17133262
Li B, Wu A, Zhou M, Wang Y, Hu Z, Su L. Preparation of High-Performance Barium Titanate Composite Hydrogels by Deep Eutectic Solvent-Assisted Frontal Polymerization. Materials. 2024; 17(13):3262. https://doi.org/10.3390/ma17133262
Chicago/Turabian StyleLi, Bin, Aolin Wu, Mengjing Zhou, Ying Wang, Zhigang Hu, and Lihua Su. 2024. "Preparation of High-Performance Barium Titanate Composite Hydrogels by Deep Eutectic Solvent-Assisted Frontal Polymerization" Materials 17, no. 13: 3262. https://doi.org/10.3390/ma17133262
APA StyleLi, B., Wu, A., Zhou, M., Wang, Y., Hu, Z., & Su, L. (2024). Preparation of High-Performance Barium Titanate Composite Hydrogels by Deep Eutectic Solvent-Assisted Frontal Polymerization. Materials, 17(13), 3262. https://doi.org/10.3390/ma17133262





