Analyzing Key Factors Influencing Water Transport in Open Air-Cooled PEM Fuel Cells
Abstract
:1. Introduction
2. Numerical Model
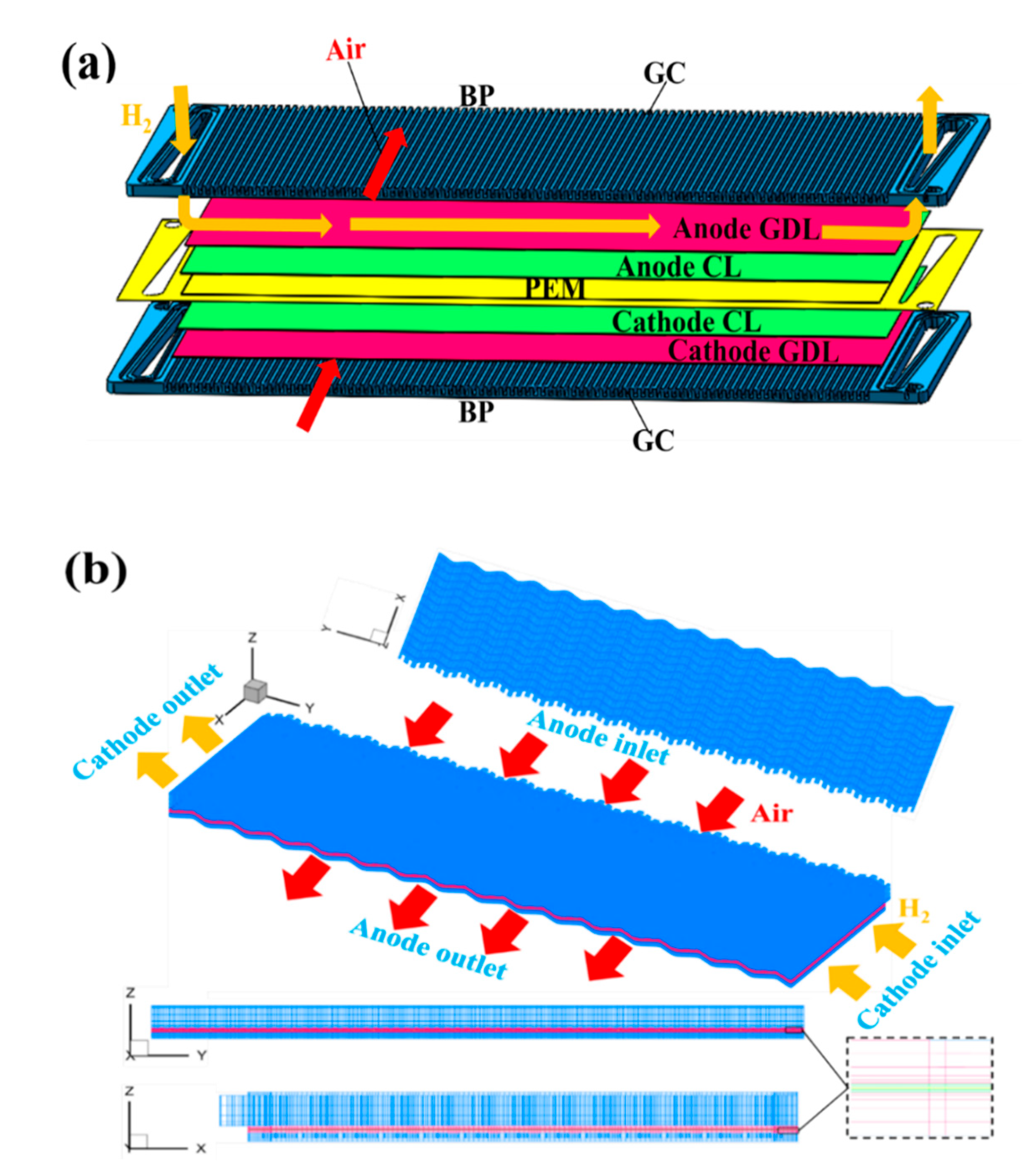
2.1. Computational Domain
2.2. Governing Equations
- (1)
- It is assumed that the model is operated under dry conditions, ignoring the gas–liquid phase transition. There is no need to consider the presence of liquid water within the model, at which point the fluid motion is presented as a mist flow. According to this premise, a mild super-saturation of gas-phase moisture can be tolerated, because the initial dry gas flow will effectively carry away most of the moisture generated in PEMFC [35].
- (2)
- Various processes within the cell will not vary over time or be affected by gravity.
- (3)
- Gases are incompressible fluids, and this assumption is based on the ideal gas equation of state.
- (4)
- Due to the small pressure gradient and Reynolds number, the state of fluid flow in the cell channels and porous media is considered laminar; i.e., the flow is smooth and without significant turbulence.
- (5)
- A homogeneous medium model is used for describing material transport, and the properties of the medium are the same in all directions.
2.3. Boundary Conditions
2.4. Numerical Method
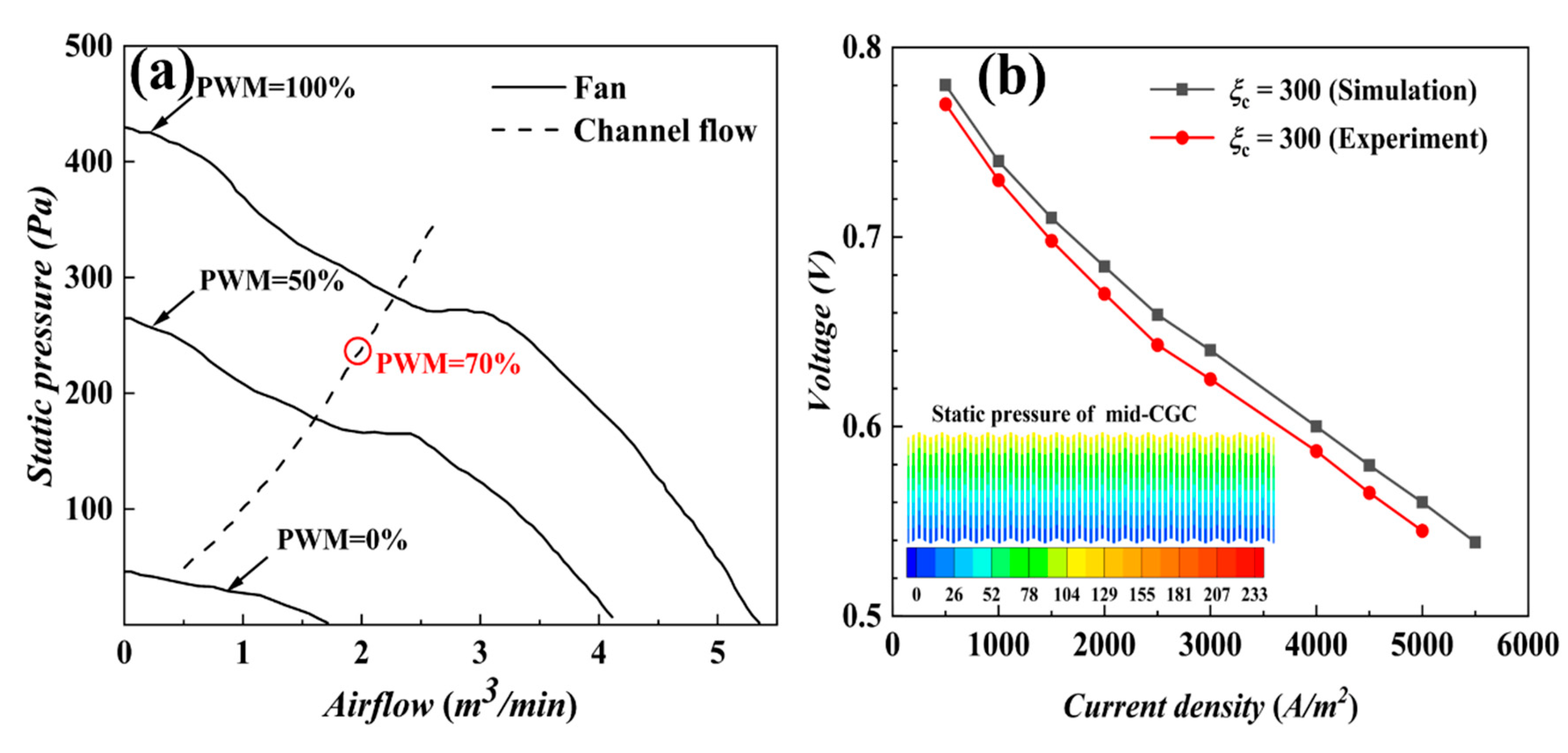
3. Experimental Verification
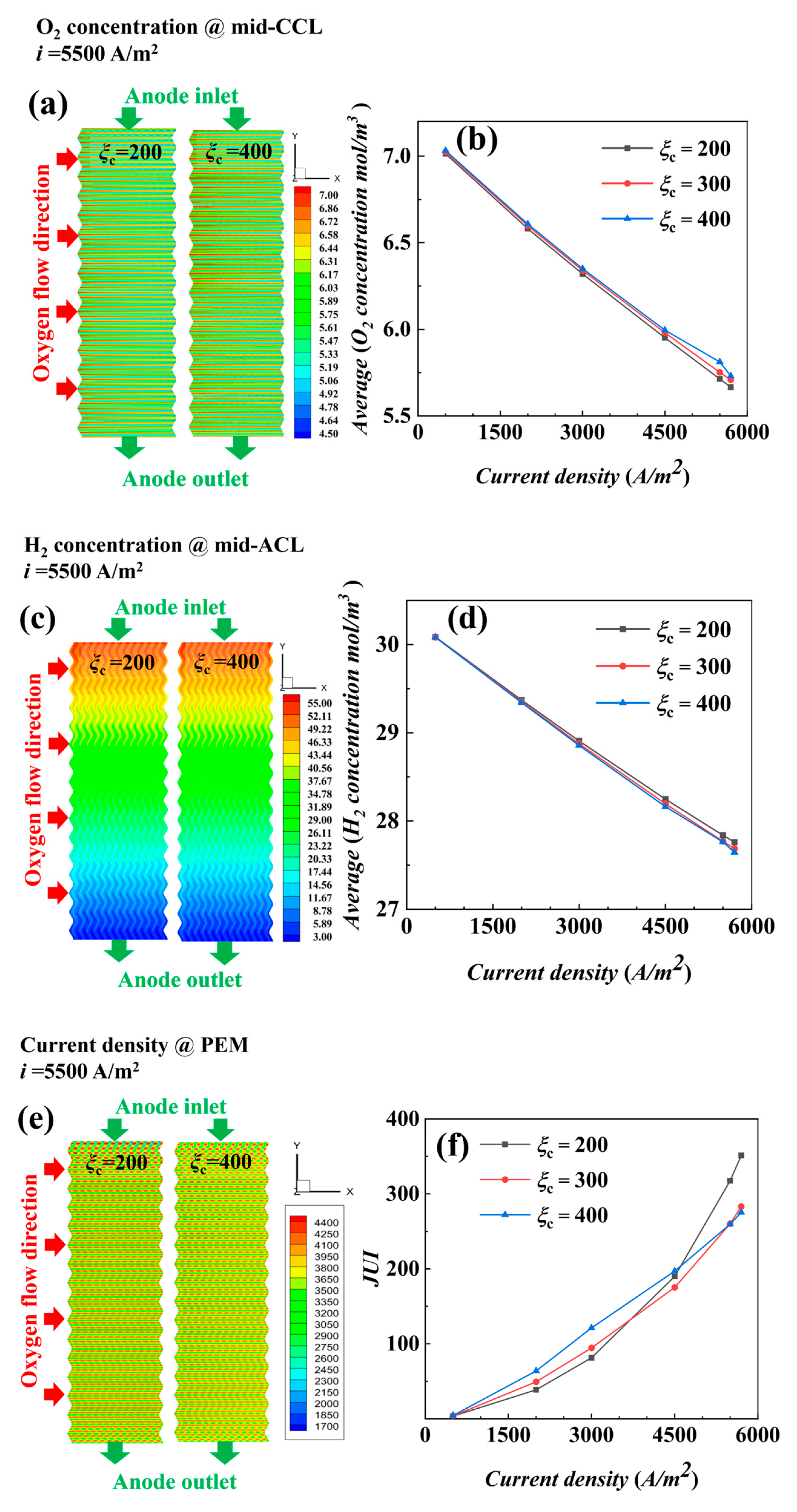
4. Results and Discussion
4.1. Isothermal Calculation
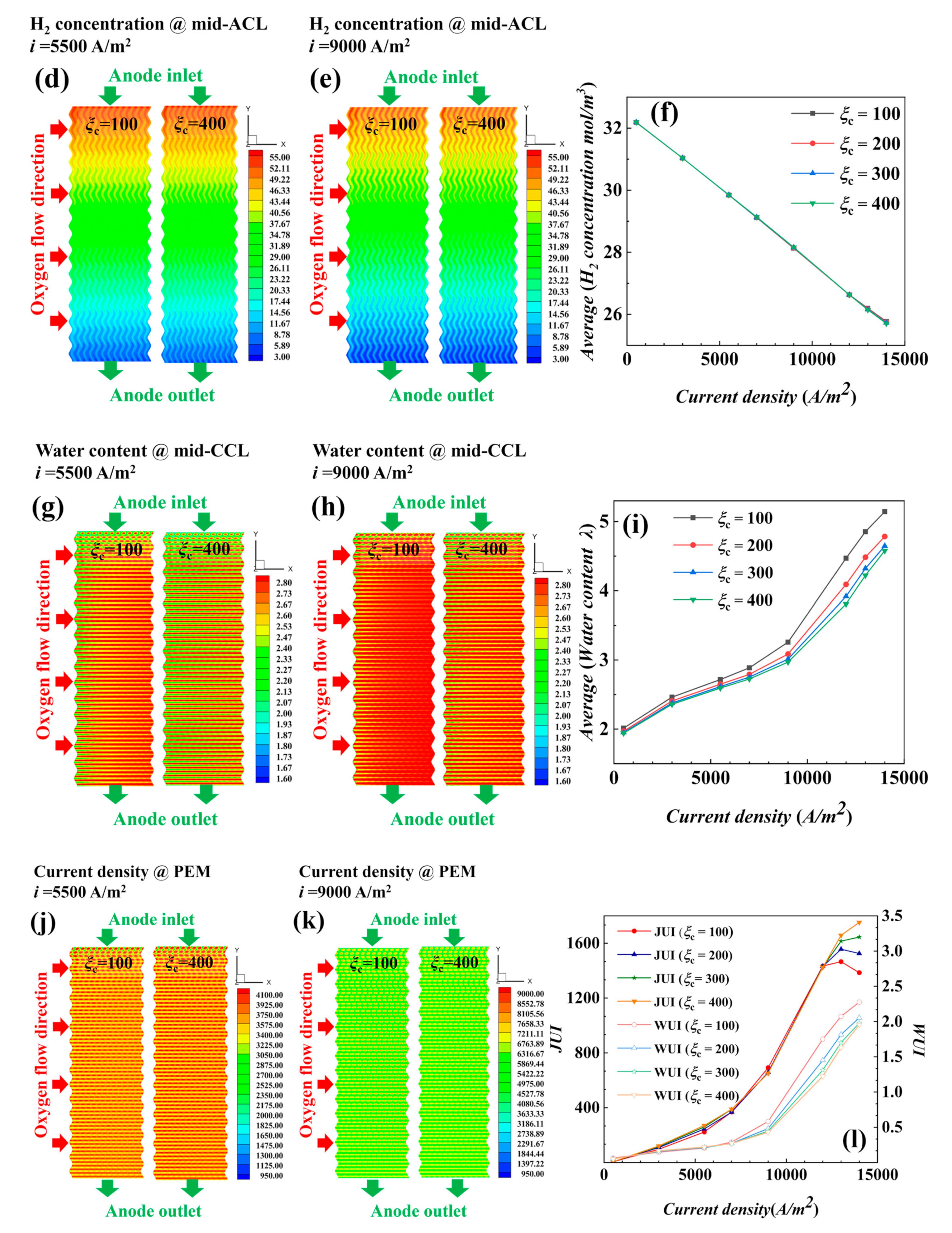
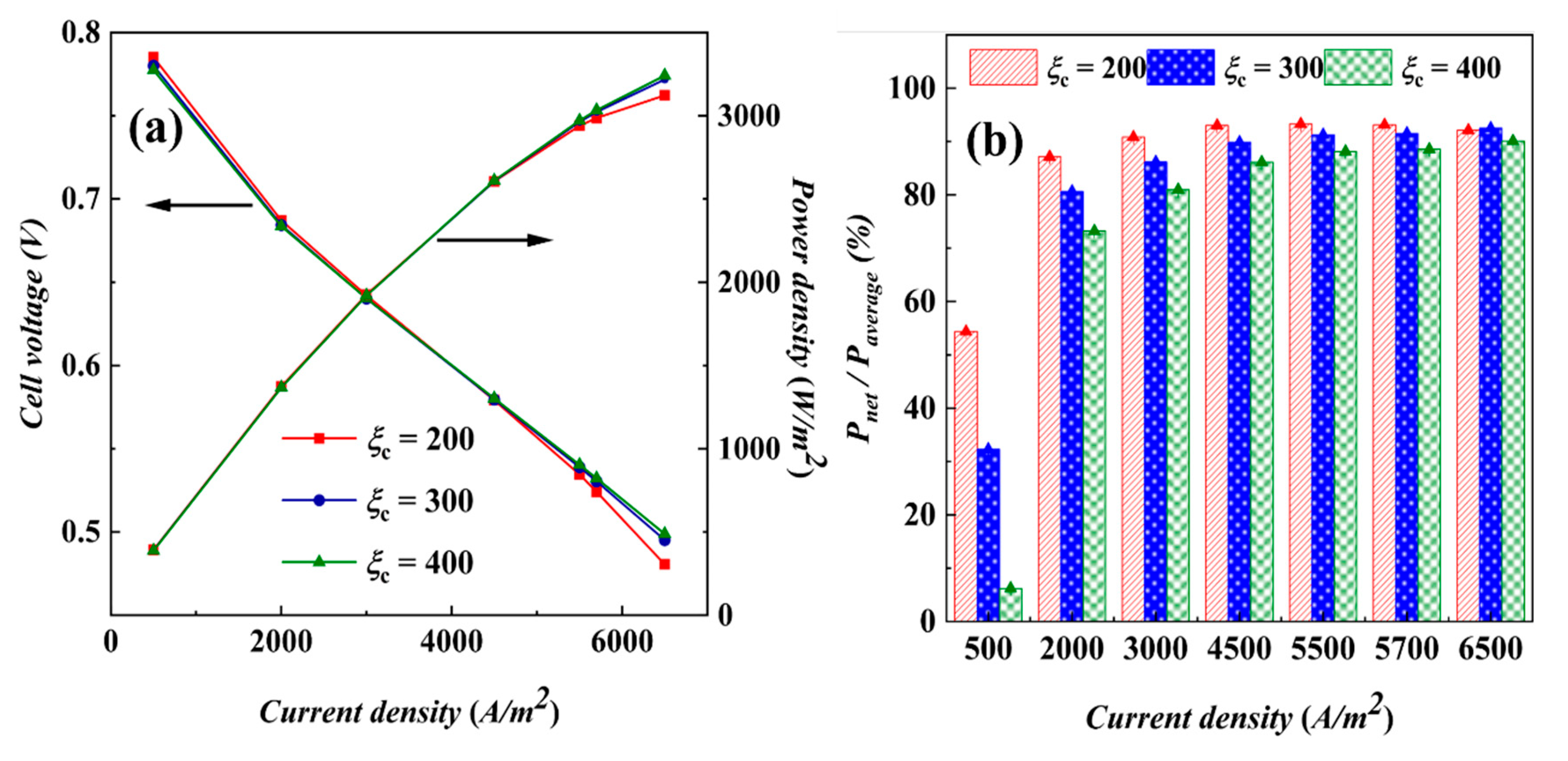
4.1.1. Performance of AC-PEMFCs at Different Stoichiometric Ratios under Isothermal Conditions
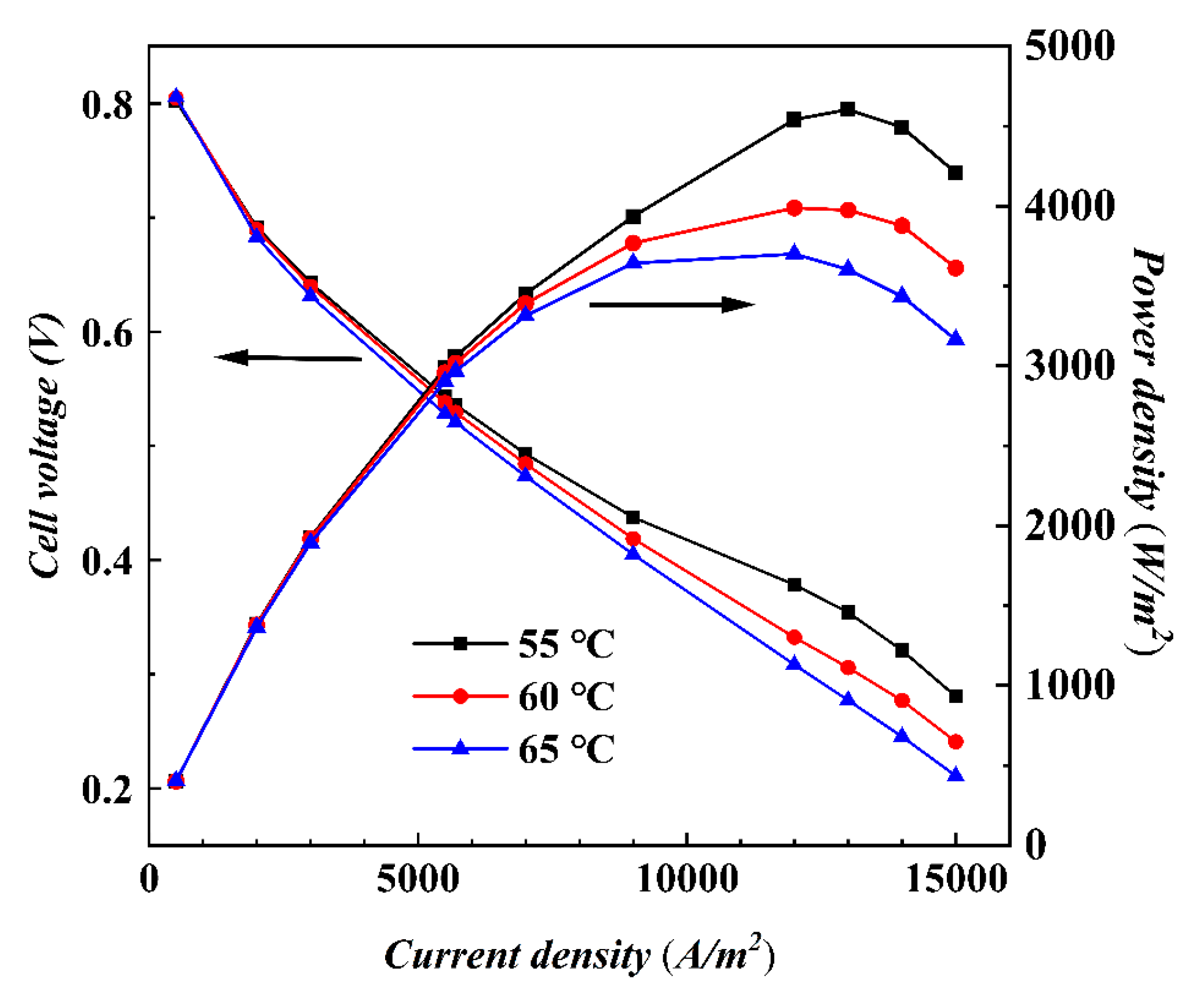
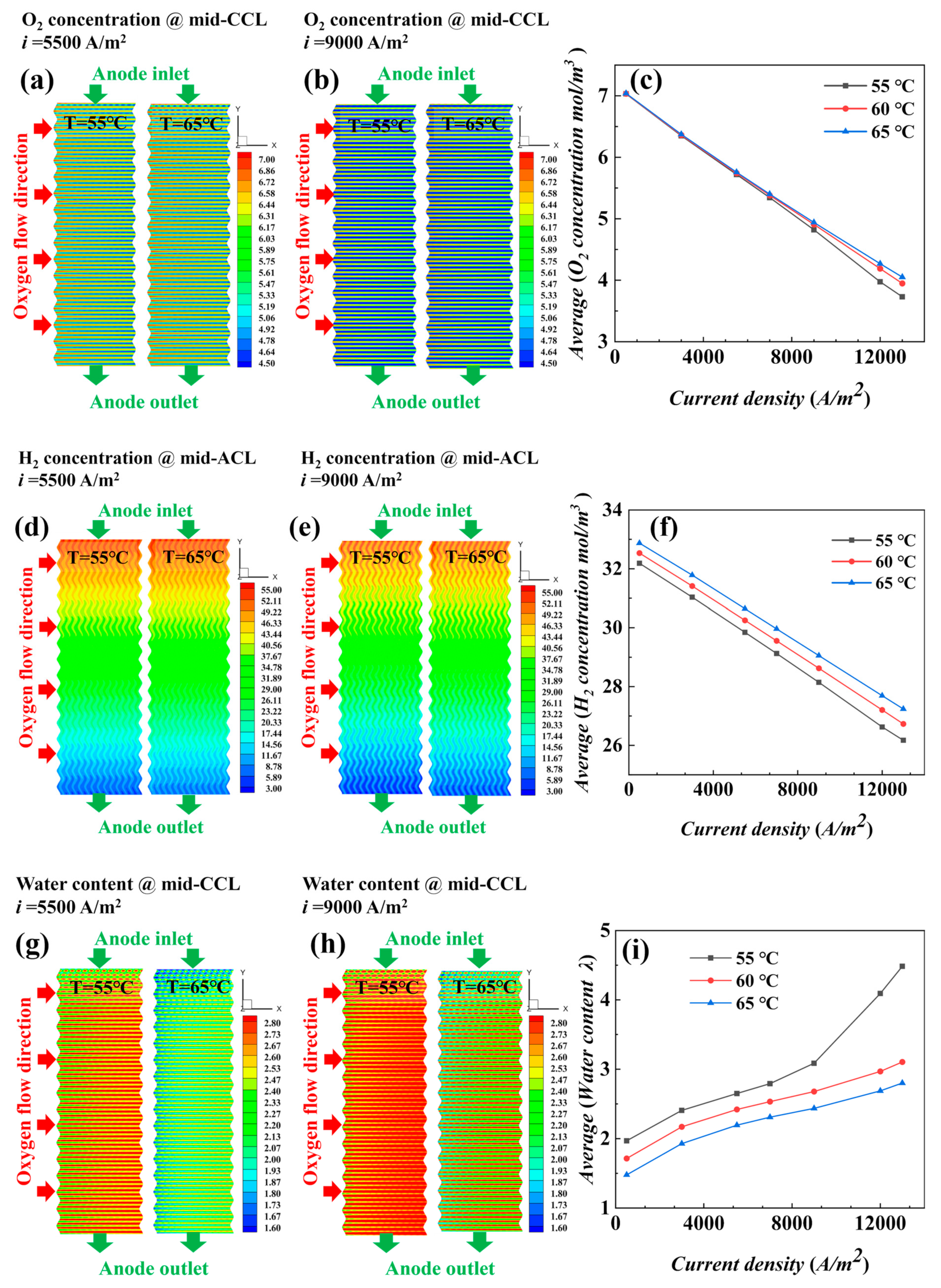
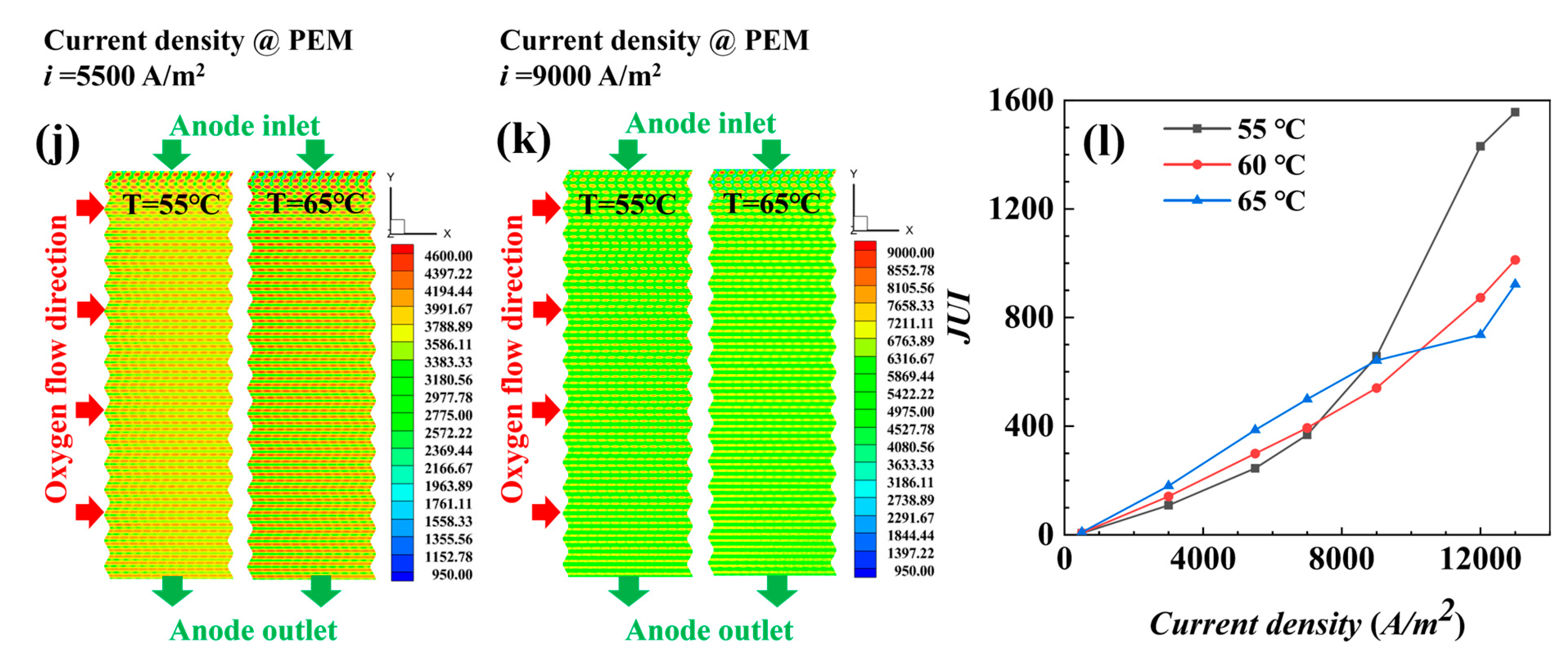
4.1.2. Performance of AC-PEMFCs at Different Temperatures under Isothermal Conditions
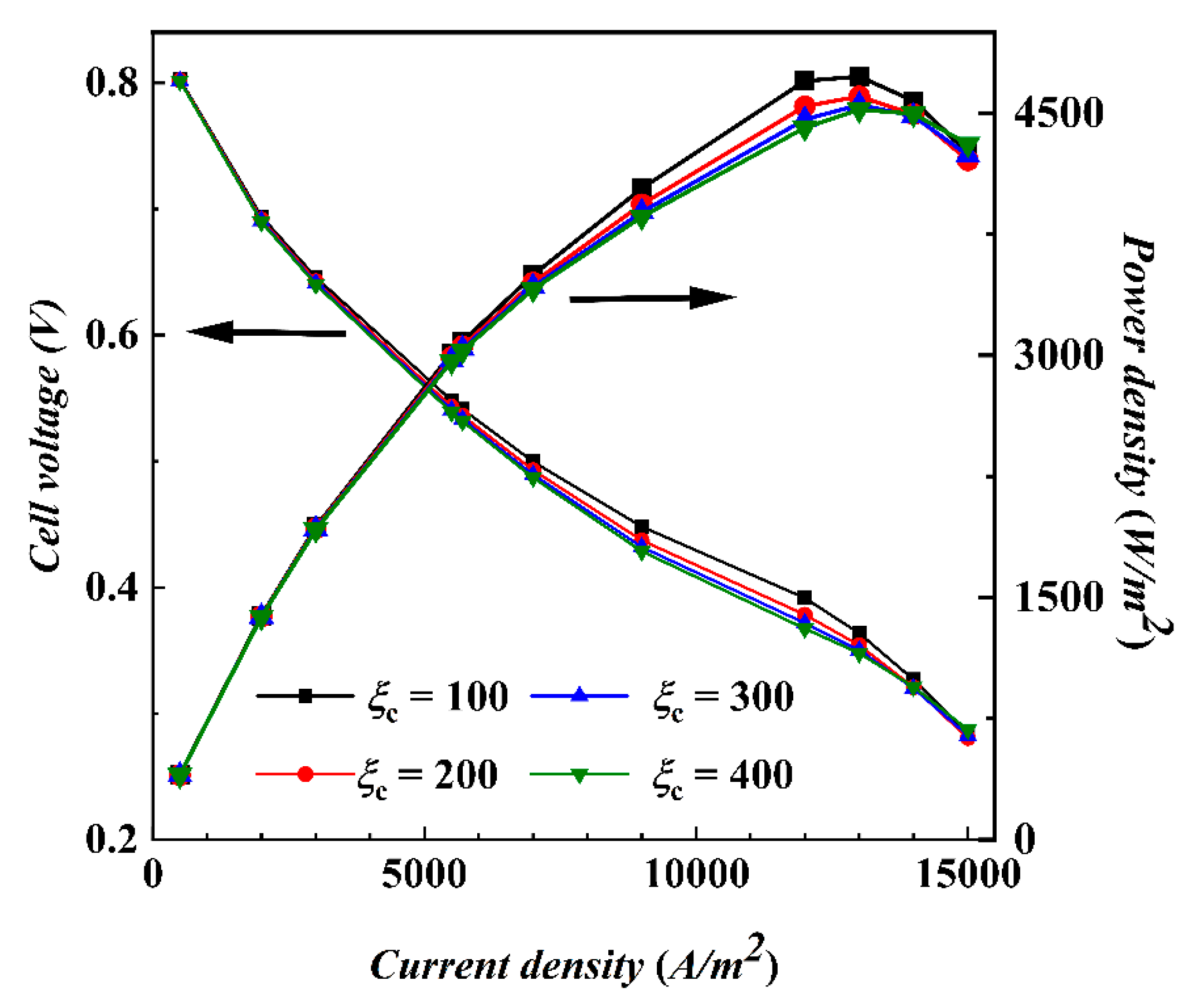
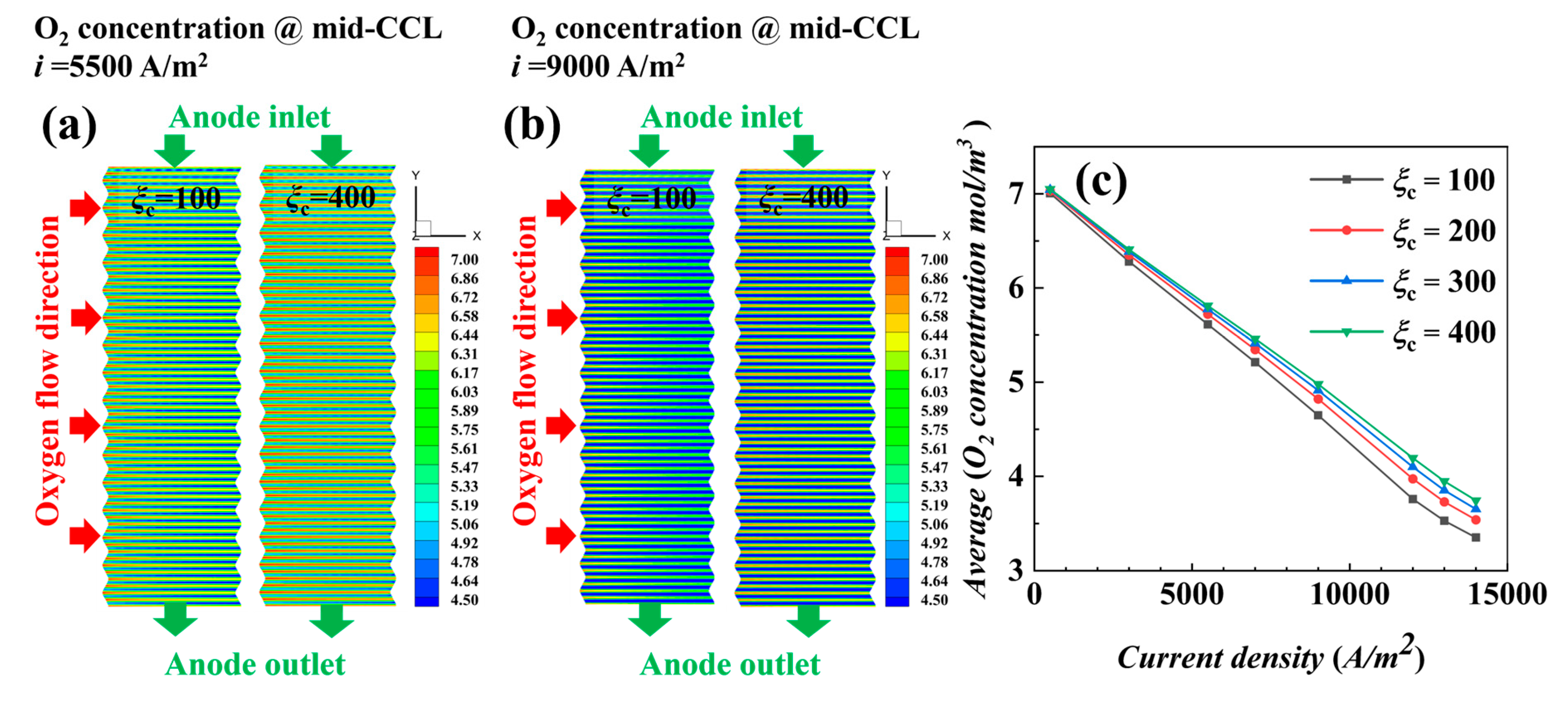
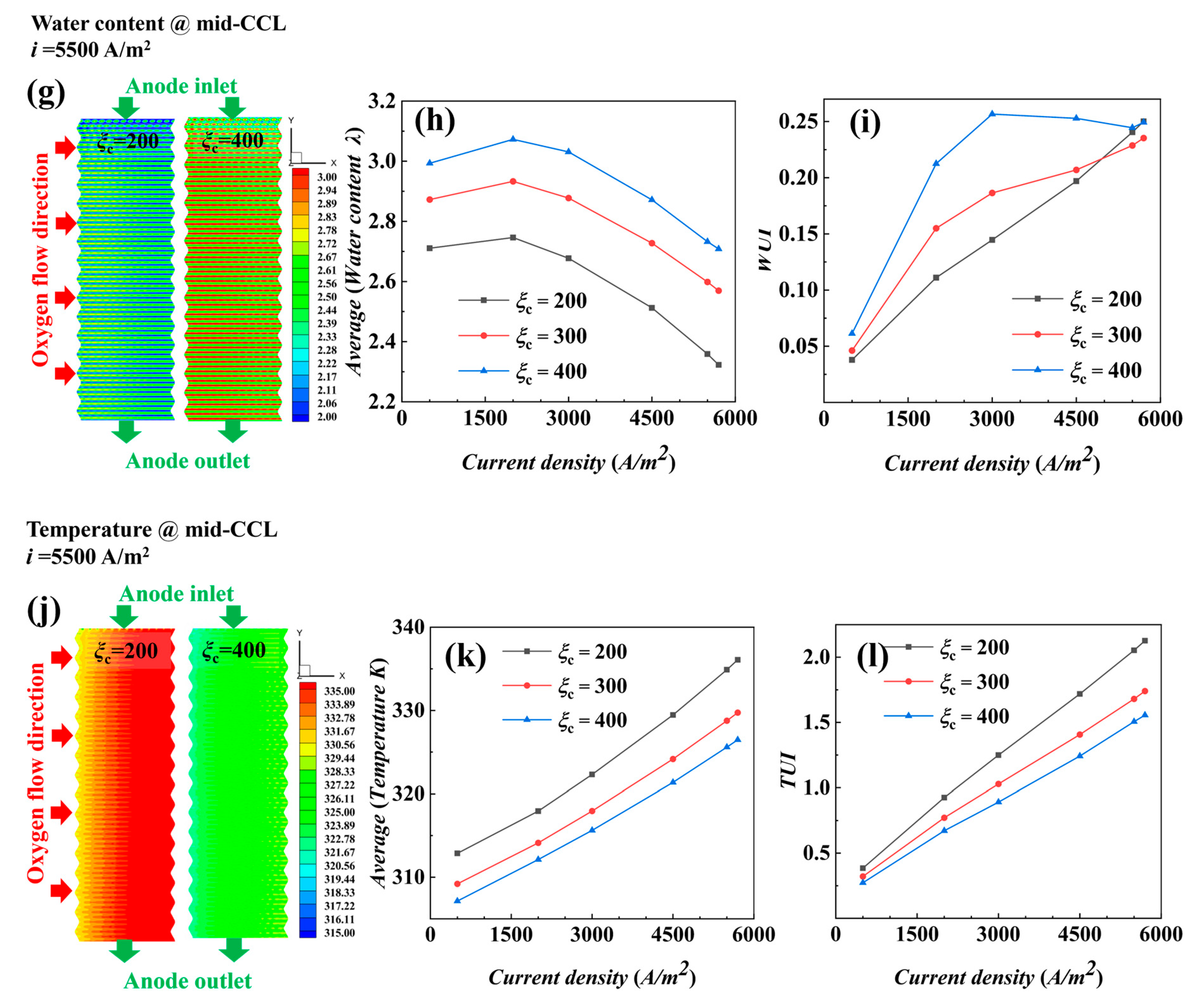
4.2. Non-Isothermal Calculation
5. Conclusions
- (1)
- The air supplied by fans is sufficient, and the cathode stoichiometric ratio has little effect on the concentration of reactants and water vapor. Low stoichiometric ratios at high current densities show slightly better water content and distribution uniformity, leading to better output performance.
- (2)
- The temperature is the key factor affecting water content and output power. As current density increases, lower temperature conditions help maintain MEA humidity and improve the uniformity of current density distribution, positively impacting cell performance.
- (3)
- Under actual operating conditions, the performance of AC-PEMFC is affected by multiple factors. An increase in temperature above the operating range usually leads to performance deterioration. The difficulty of heat dissipation in the channel region further aggravates performance degradation. However, increasing the stoichiometric ratio can significantly enhance heat dissipation and improve water content and temperature uniformity, resulting in a more homogeneous electric density distribution. This study found that the optimal fuel cell performance is achieved with a stoichiometric ratio of 300. The proper selection of a stoichiometric ratio is essential in designing and operating AC-PEMFC to ensure optimal performance under various operating conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Huang, W.; Niu, T.; Liu, Z.; Li, G.; Cao, D. Review of Clustering Technology and Its Application in Coordinating Vehicle Subsystems. Automot. Innov. 2023, 6, 89–115. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, J.; Wei, Z.D. Strategies for tuning porous structures of air electrode in fuel cells. CIESC J. 2020, 71, 4553–4574. [Google Scholar]
- Bo, D.; Wentao, H.; Qifei, J. An open-cathode PEMFC efficiency optimization strategy based on exergy analysis and data-driven modeling. Energy 2023, 264, 126148. [Google Scholar]
- Zhang, C.; Qiu, Y.; Chen, J.; Li, Y.; Liu, Z.; Liu, Y.; Zhang, J.; Hwa, C.S. A comprehensive review of electrochemical hybrid power supply systems and intelligent energy management for unmanned aerial vehicles in public services. Energy AI 2022, 9, 100175. [Google Scholar] [CrossRef]
- Xing, S.; Zhao, C.; Zou, J.; Zaman, S.; Yu, Y.; Gong, H.; Wang, Y.; Chen, M.; Wang, M.; Lin, M.; et al. Recent advances in heat and water management of forced-convection open-cathode proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2022, 165, 112558. [Google Scholar] [CrossRef]
- Yu, X.; Chang, H.; Zhao, J.; Tu, Z.; Chan, S.H. Application of self-adaptive temperature recognition in cold-start of an air-cooled proton exchange membrane fuel cell stack. Energy AI 2022, 9, 100155. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, F.; Wu, X. Analysis and review on air-cooled open cathode proton exchange membrane fuel cells: Bibliometric, environmental adaptation and prospect. Renew. Sustain. Energy Rev. 2024, 197, 114408. [Google Scholar] [CrossRef]
- Huaxin, Y.; Wei, Z.; Zhiyuan, Q.; Naichao, C. Flow field plate of polymer electrolyte membrane fuel cells: A review. J Renew Sustain Energy 2023, 15, 012701. [Google Scholar]
- Felseghi, R.-A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen fuel cell technology for the sustainable future of stationary applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Peng, M.; Chen, L.; Gong, K.; Tao, W. Effects of cathode gas channel design on air-cooled proton exchange membrane fuel cell performance. Appl. Therm. Eng. 2024, 236, 121614. [Google Scholar] [CrossRef]
- Pang, J.; Ma, C.; Dou, Y.; Hou, M. Design of collaborative control system for low-temperature operation of air-cooled proton exchange membrane fuel cell. J. Phys. Conf. Ser. 2023, 2418, 012010. [Google Scholar] [CrossRef]
- Shakhshir, A.S.; Gao, X.; Berning, T. An Experimental Study of the Effect of a Turbulence Grid on the Stack Performance of an Air-Cooled Proton Exchange Membrane Fuel Cell. J. Electrochem. Energy Convers. Storage 2019, 17, 1. [Google Scholar] [CrossRef]
- Luo, L.; Jian, Q.; Huang, B.; Huang, Z.; Zhao, J.; Cao, S. Experimental study on temperature characteristics of an air-cooled proton exchange membrane fuel cell stack. Renew. Energy 2019, 143, 1067–1078. [Google Scholar] [CrossRef]
- Chang, H.; Cai, F.; Yu, X.; Duan, C.; Chan, S.H.; Tu, Z. Experimental study on the thermal management of an open-cathode air-cooled proton exchange membrane fuel cell stack with ultra-thin metal bipolar plates. Energy 2023, 263, 125724. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Wan, Z.; Chen, X.; Chan, S.H.; Tu, Z. Progress and perspectives of integrated thermal management systems in PEM fuel cell vehicles: A review. Renew. Sustain. Energy Rev. 2022, 155, 111908. [Google Scholar] [CrossRef]
- Wang, Q.; Li, B.; Yang, D.; Dai, H.; Zheng, J.P.; Ming, P.; Zhang, C. Research progress of heat transfer inside proton exchange membrane fuel cells. J. Power Sources 2021, 492, 229613. [Google Scholar] [CrossRef]
- Yang, L.; Nik-Ghazali, N.N.; Ali, M.A.; Chong, W.T.; Yang, Z.; Liu, H. A review on thermal management in proton exchange membrane fuel cells: Temperature distribution and control. Renew. Sustain. Energy Rev. 2023, 187, 113737. [Google Scholar] [CrossRef]
- Berning, T.; Kær, K.S. A Thermodynamic Analysis of an Air-Cooled Proton Exchange Membrane Fuel Cell Operated in Different Climate Regions. Energies 2020, 13, 2611. [Google Scholar] [CrossRef]
- Ming, P.; Qiangfeng, X.; Jiang, L.C.L.; Wenquan, T. Numerical simulation of the effects of cathode excess coefficient and flow field arrangement on the performance of air-cooled fuel cells. J. Chem. Ind. 2023, 74, 4267–4276. [Google Scholar]
- Zhang, Y.; He, S.; Jiang, X.; Yang, X.; Wang, Z.; Zhang, S.; Cao, J.; Fang, H.; Li, Q. Full-scale three-dimensional simulation of air-cooling metal bipolar plate proton exchange membrane fuel cell stack considering a non-isothermal multiphase model. Appl. Energy 2024, 357, 122507. [Google Scholar] [CrossRef]
- Lin, W.; Jian, G.; Zihao, L.; Mohmed, F.J.D.A. Study on the effect of air flow rate on the performance of air-cooled fuel cell stacks. J. Chem. Ind. 2022, 73, 3222–3231. [Google Scholar]
- Zhao, C.; Xing, S.; Liu, W.; Chen, M.; Wang, H. Performance and thermal optimization of different length-width ratios for air-cooled open-cathode fuel cell. Renew. Energy 2021, 178, 1250–1260. [Google Scholar] [CrossRef]
- Guk, E.; Venkatesan, V.; Babar, S.; Jackson, L.; Kim, J.S. Parameters and their impacts on the temperature distribution and thermal gradient of solid oxide fuel cell. Appl. Energy 2019, 241, 164–173. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, R.; Pan, R.; Cao, G. Disclosure of the internal transport phenomena in an air-cooled proton exchange membrane fuel cell— part II: Parameter sensitivity analysis. Int. J. Hydrog. Energy 2021, 46, 18589–18603. [Google Scholar] [CrossRef]
- Wang, H. Study on the Temperature Characteristics of Air-Cooled Proton Exchange Membrane Fuel Cells; Hunan University of Science and Engineering: Yongzhou, China, 2021. [Google Scholar] [CrossRef]
- Chen, J.; Sun, L.; Zhu, W.; Pei, H.; Xing, L.; Tu, Z. Influence of cathode air supply mode on the performance of an open cathode air-cooled proton exchange membrane fuel cell stack. Appl. Therm. Eng. 2024, 243, 122709. [Google Scholar] [CrossRef]
- Guo, Z.; Tian, C.; Gong, K.; Xu, W.; Chen, L.; Tao, W.-Q. Experimental study on the dynamic response of voltage and temperature of an open-cathode air-cooled proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2024, 57, 601–615. [Google Scholar] [CrossRef]
- Yuan, W.W.; Ou, K.; Kim, Y.B. Thermal Management for an Air Coolant System of a Proton Exchange Membrane Fuel Cell Using Heat Distribution Optimization. Appl. Therm. Eng. 2020, 167, 114715. [Google Scholar] [CrossRef]
- Wang, Y.X.; Chen, Q.; Zhang, J.; He, H. Real-time Power Optimization for an Air-coolant Proton Exchange Membrane Fuel Cell Based on Active Temperature Control. Energy 2020, 220, 119497. [Google Scholar] [CrossRef]
- Mahjoubi, C.; Olivier, J.-C.; Skander-Mustapha, S.; Machmoum, M.; Slama-Belkhodja, I. An Improved Thermal Control of Open Cathode Proton Exchange Membrane Fuel Cell. Int. J. Hydrog. Energy 2019, 44, 11332–11345. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Zhang, G.; Bai, H.; Qu, Z. Enhancing water hydration in air-cooled proton exchange membrane fuel cell using a staggered tapered slotted flow field. Appl. Energy Combust. Sci. 2024, 17, 100239. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Zhang, G.; Bai, H.; Qu, Z. Optimal and modeling study of air-cooled proton exchange membrane fuel cell with various length-width ratios and numbers. Int. Commun. Heat Mass Transfer. 2023, 142, 106668. [Google Scholar]
- Wei, L.; Dafalla, A.M.; Liao, Z.; Guo, J.; Ai, X.; Jiang, F. Improved flow field design for air-cooled proton exchange membrane fuel cells. Int. J. Green Energy 2024, 21, 13–25. [Google Scholar] [CrossRef]
- Atyabi, S.A.; Afshari, E.; Zohravi, E.; Udemu, C.M. Three-dimensional simulation of different flow fields of proton exchange membrane fuel cell using a multi-phase coupled model with cooling channel. Energy 2021, 234, 121247. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, C.F.; Guo, C.Q.; Lu, Z.X.; Shi, Y.; Wang, Z.D. A computational analysis of the operational behavior of open-cathode polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2020, 45, 34125–34138. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X. Oxygen transport in polymer electrolyte membrane fuel cells based on measured electrode pore structure and mass transport properties. Energy Convers. Manag. 2019, 186, 570–585. [Google Scholar] [CrossRef]
- Nima, A.; Hesam, M. Numerical simulation of using DBD plasma air blower for a proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2024, 50, 817–830. [Google Scholar]
- Dong, P.; Xie, G.; Ni, M. The mass transfer characteristics and energy improvement with various partially blocked flow channels in a PEM fuel cell. Energy 2020, 206, 117977. [Google Scholar] [CrossRef]
- Lee, J.; Gundu, M.H.; Lee, N.; Lim, K.; Lee, S.W.; Jang, S.S.; Kim, J.Y.; Ju, H. Innovative cathode flow-field design for passive air-cooled polymer electrolyte membrane (PEM) fuel cell stacks. Int. J. Hydrog. Energy 2020, 45, 11704–11713. [Google Scholar] [CrossRef]
- Zhang, G.; Jiao, K. Three-dimensional multi-phase simulation of PEMFC at high current density utilizing Eulerian-Eulerian model and two-fluid model. Energy Convers. Manag. 2018, 176, 409–421. [Google Scholar] [CrossRef]
- Motupally, S.; Becker, A.J.; Weidner, J.W. Diffusion of water in Nafion 115 membranes. J. Electrochem. Soc. 2000, 147, 3171–3177. [Google Scholar] [CrossRef]
- Ghasemi, M.; Ramiar, A.; Ranjbar, A.; Rahgoshay, S. A numerical study on thermal analysis and cooling flow fields effect on PEMFC performance. Int. J. Hydrog. Energy 2017, 42, 24319–24337. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Cathode channel length (mm) | 60 |
| Anode channel depth (mm) | 0.35 |
| Anode channel width(mm) | 1 |
| Cathode channel depth (mm) | 2 |
| Depth of cathode channel (mm) | 1 |
| Thickness of GDL (mm) | 0.015 |
| Thickness of PEM (mm) | 0.01 |
| Anode/cathode stoichiometric ratio | 1.2/100, 200, 300, 400 |
| Anode/cathode runner inlet temperature (K) | 301/301 |
| Anode/cathode relative humidity (%) | 0/50 |
| Anode/cathode operating pressure (MPa) | 0.15/0.1 (ABS) |
| Parameter | Value |
|---|---|
| Porosity of porous layers | |
| Ionomer volume fraction of ACL/CCL | |
| Volumetric reference current density (A/m3) | |
| Transfer coefficient | |
| Electro-osmotic drag coefficient [33] | |
| GDL/MPL/CL permeability (m2) [33] | |
| BP/GDL/MPL/CL electrical conductivity (S/m) | |
| Ionic conductivity of ionomer (S/m) | |
| Thermal conductivity (W/m·K) [40] | |
| Gas diffusivity | |
| Diffusivity of MEA water (m2/s) [41] | |
| Dry MEA density (kg/m3) | |
| MEA equivalent weight (kg/mol) |
| Number of Grids | Current Density i = 3000 A/m2 | ||
|---|---|---|---|
| Output Voltage (V) | Relative Error | ||
| Grid 1 | 1,537,350 | 0.52310 | 2.2% |
| Grid 2 | 2,835,200 | 0.53461 | 0.13% |
| Grid 3 | 3,758,670 | 0.53531 | / |
| ξc | Static Pressure (Pa) | PWM | Fan Power (W) |
|---|---|---|---|
| 200 | 152 | 45% | 24.34 |
| 300 | 233 | 70% | 35.64 |
| 400 | 363 | 100% | 49.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Wei, L.; Hu, F.; Dafalla, A.M.; Guo, J.; Wang, C.; Jiang, F. Analyzing Key Factors Influencing Water Transport in Open Air-Cooled PEM Fuel Cells. Materials 2024, 17, 3267. https://doi.org/10.3390/ma17133267
He B, Wei L, Hu F, Dafalla AM, Guo J, Wang C, Jiang F. Analyzing Key Factors Influencing Water Transport in Open Air-Cooled PEM Fuel Cells. Materials. 2024; 17(13):3267. https://doi.org/10.3390/ma17133267
Chicago/Turabian StyleHe, Bin, Lin Wei, Fengping Hu, Ahmed Mohmed Dafalla, Jian Guo, Cuihua Wang, and Fangming Jiang. 2024. "Analyzing Key Factors Influencing Water Transport in Open Air-Cooled PEM Fuel Cells" Materials 17, no. 13: 3267. https://doi.org/10.3390/ma17133267






