Experimental Study on the Effects of Carbonated Steel Slag Fine Aggregate on the Expansion Rate, Mechanical Properties and Carbonation Depth of Mortar
Abstract
:1. Introduction
2. Experimental Program
2.1. Raw Materials
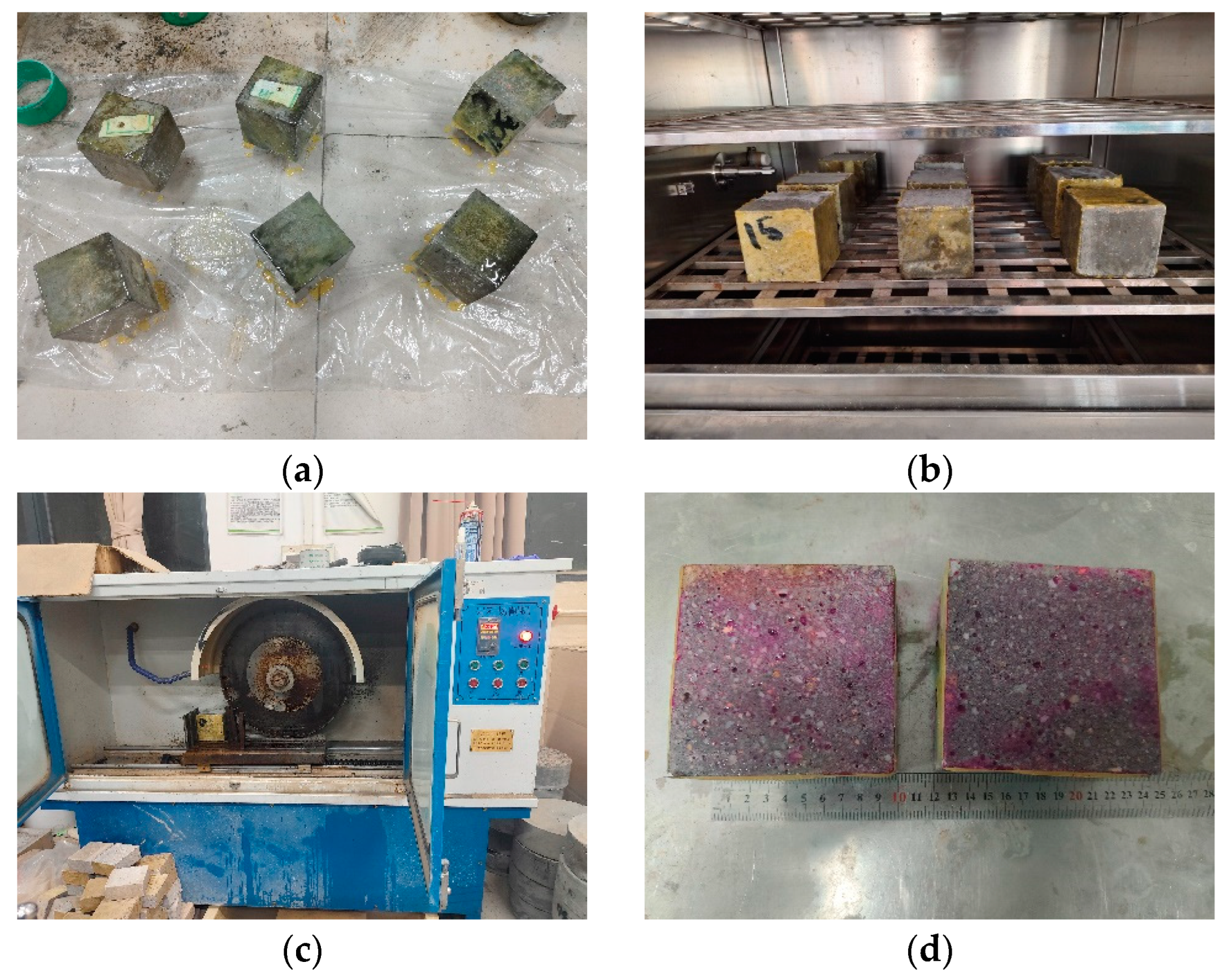
2.2. Carbonation of SSFA
2.3. Mortar Specimens
2.4. Determination of f-CaO Content in SSFA
2.5. Expansion Test of Mortar Specimens
2.6. Compression Test of Mortar Specimens
2.7. Carbonation Test of Mortar Specimens
3. Results and Discussion
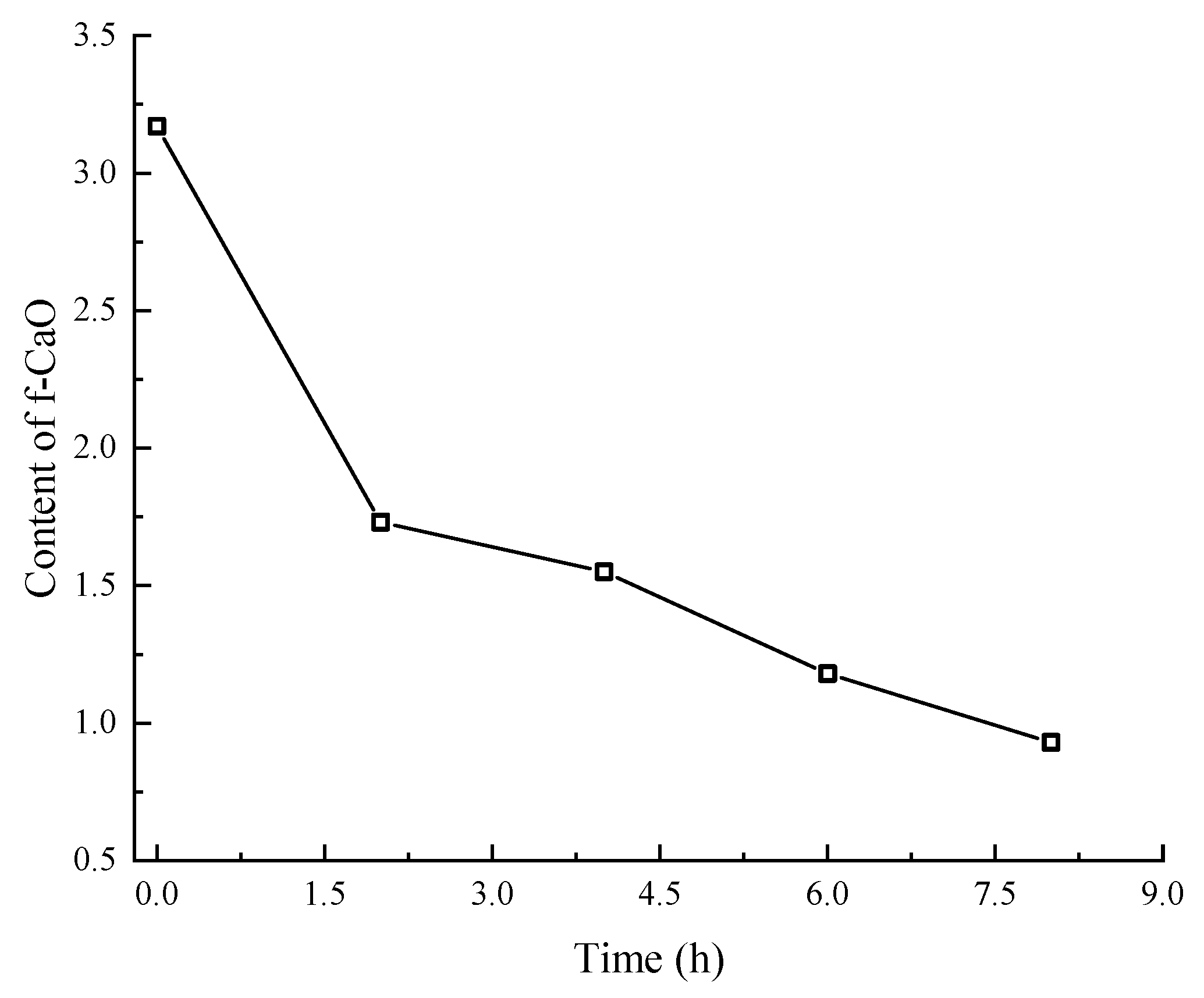
3.1. Effect of Carbonation Time on Content of f-CaO in SSFA
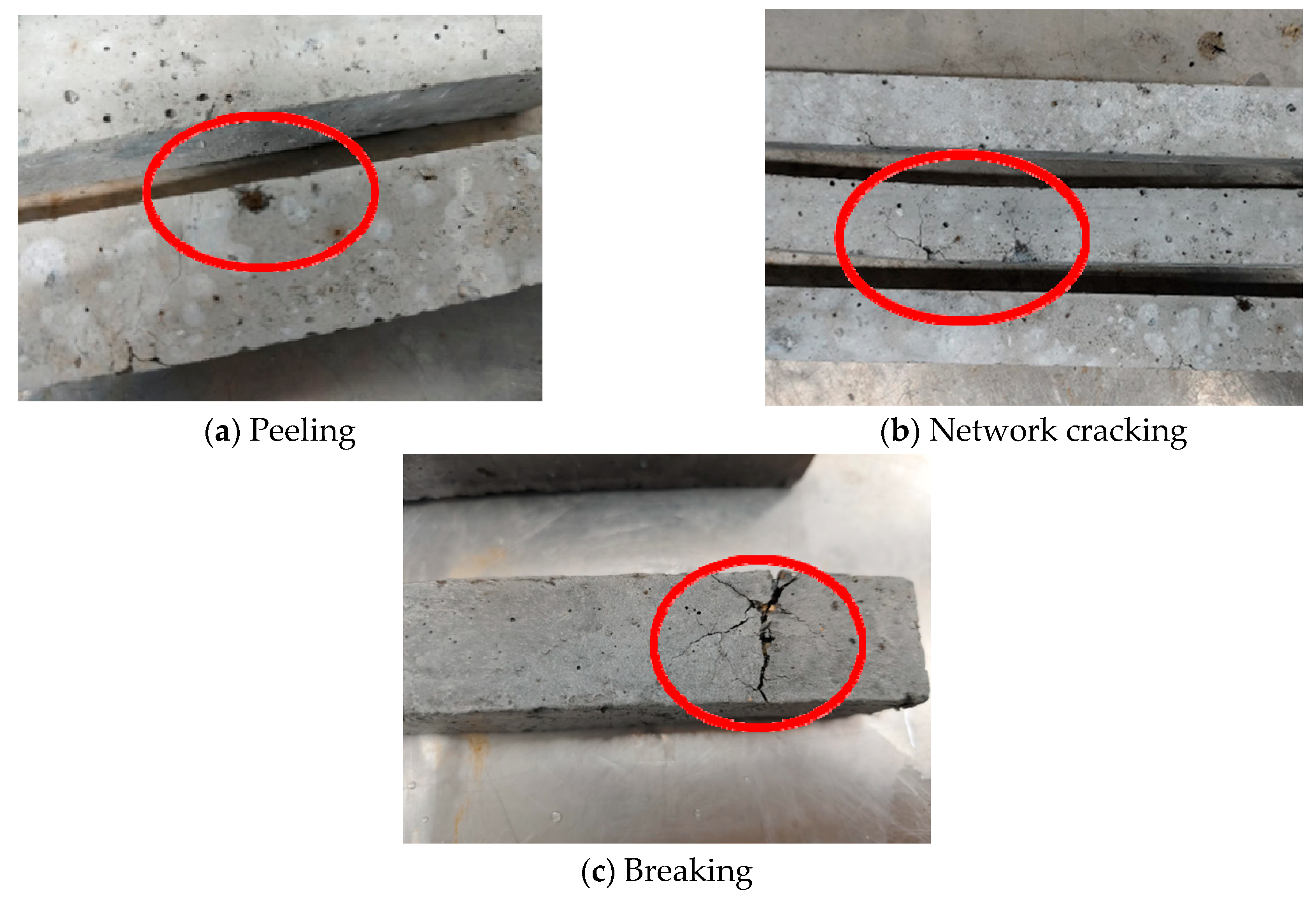
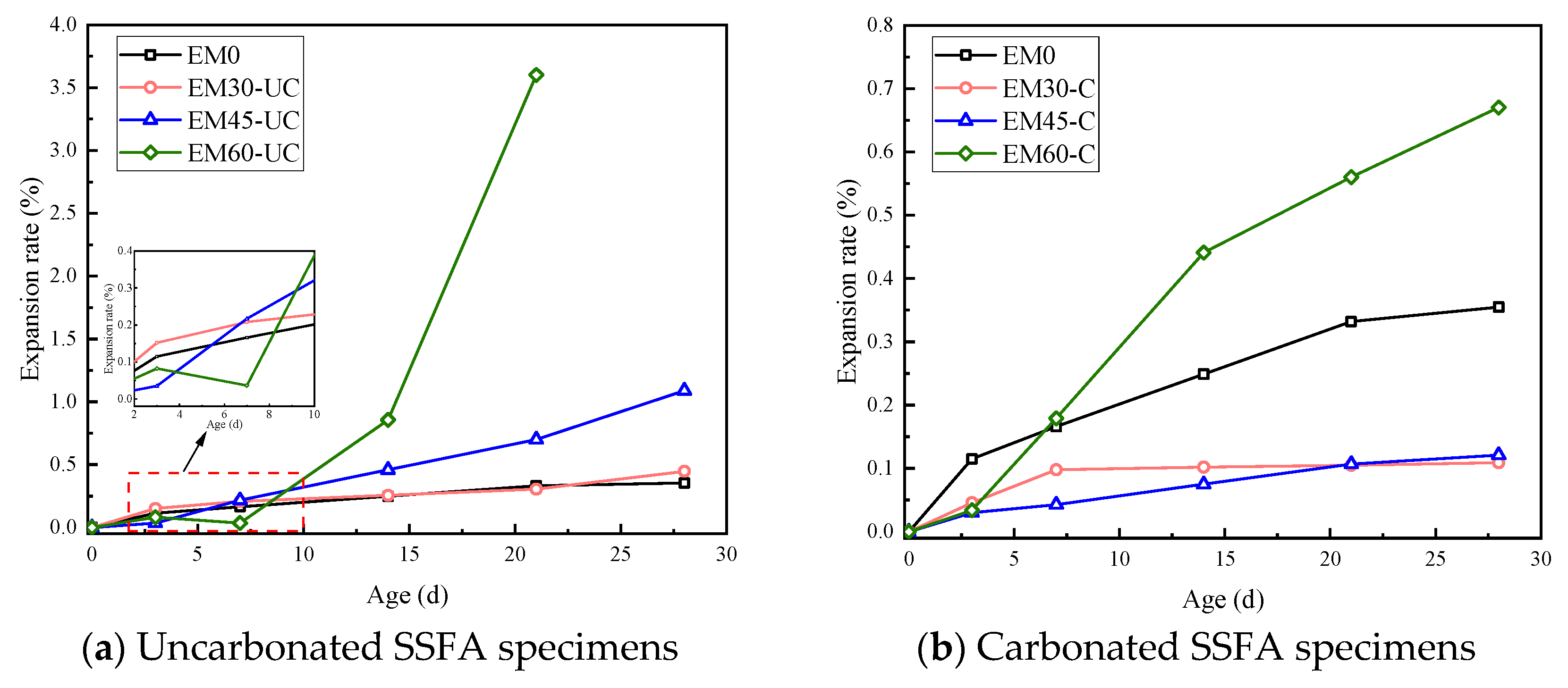
3.2. Expansion Rate of Mortar Specimens
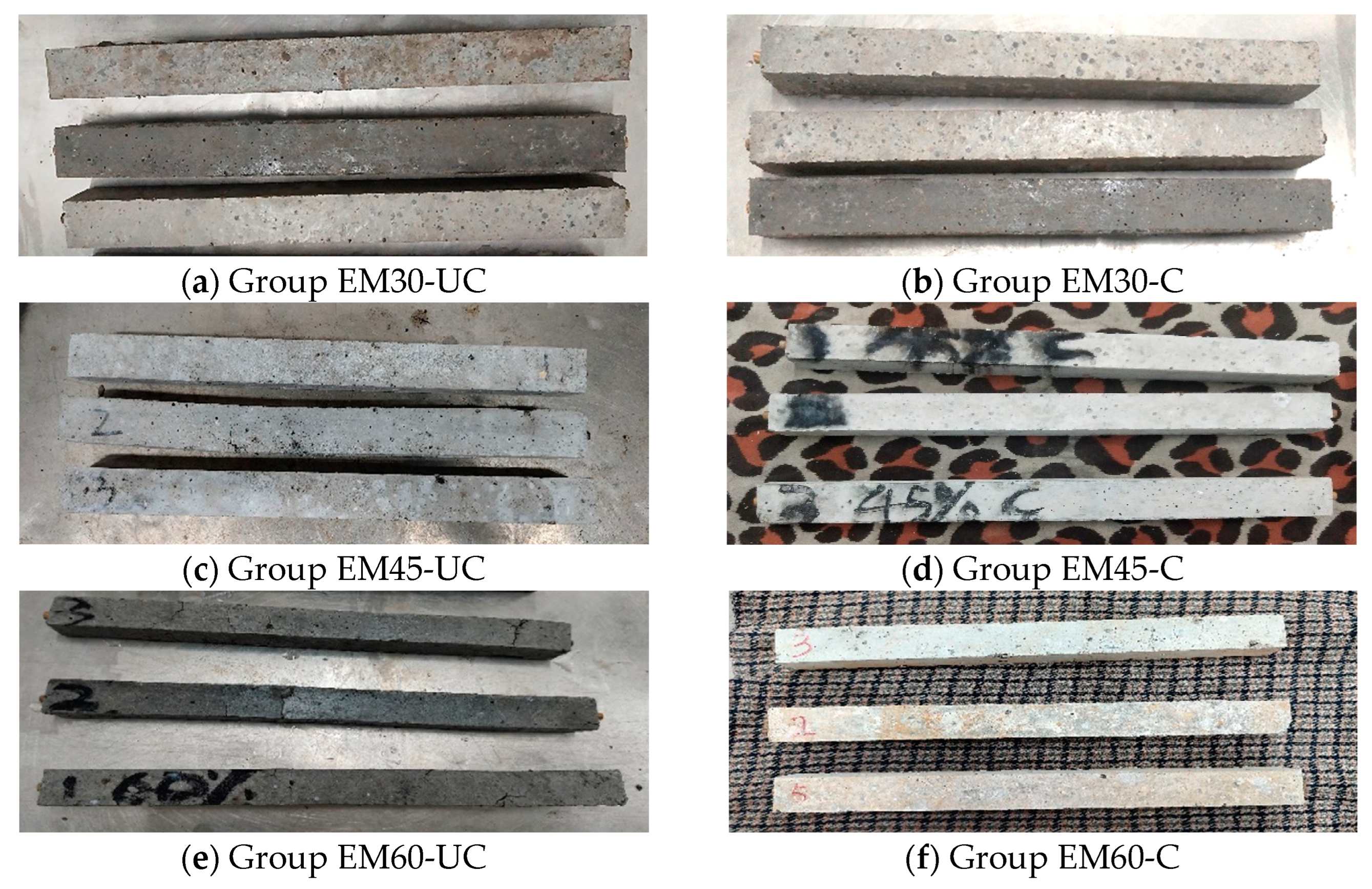
3.3. Compressive Strength of Mortar Specimens
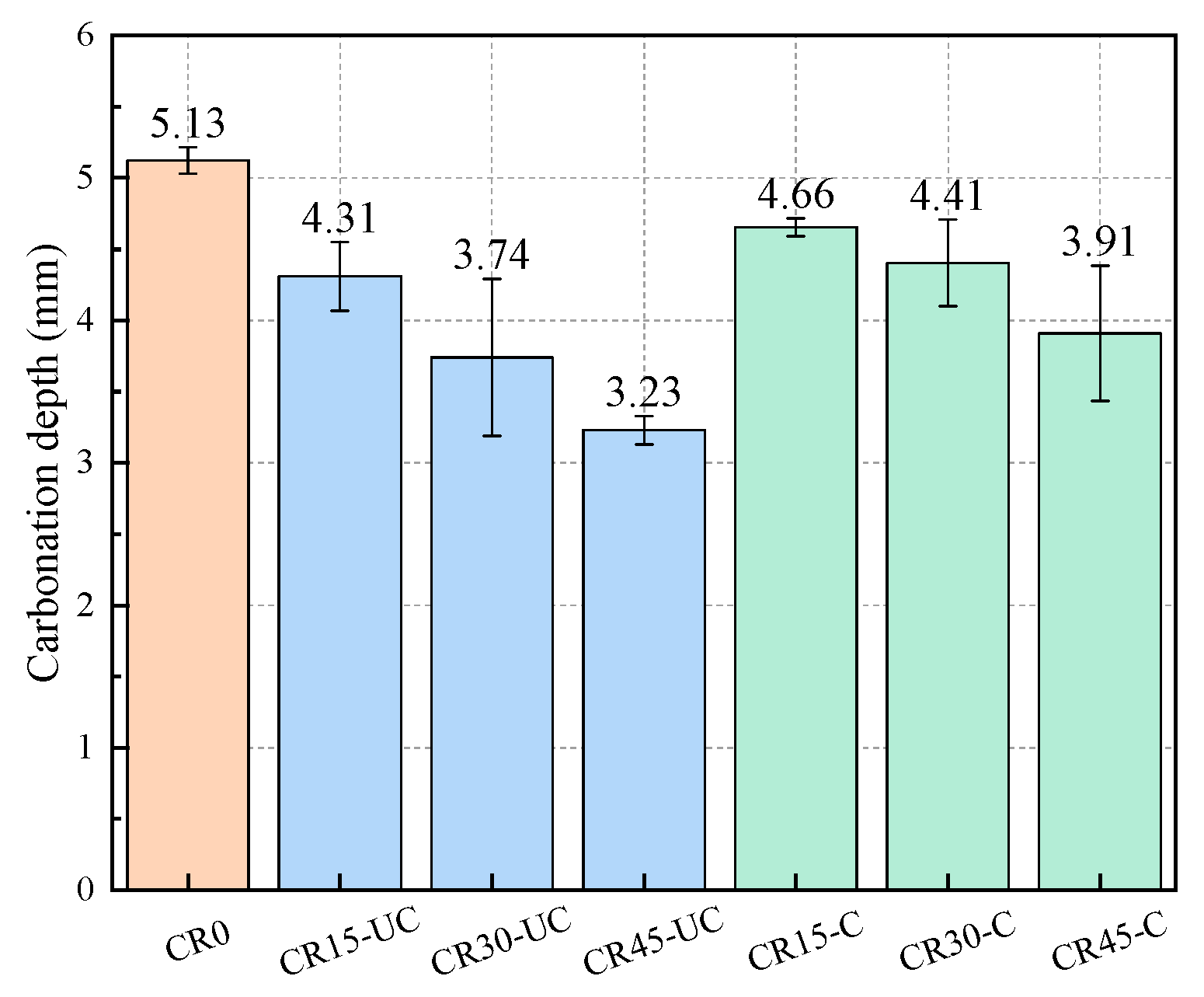
3.4. Carbonation Depth of Mortar Specimens
4. Conclusions
- (1)
- The carbonation treatment of steel slag can effectively reduce the f-CaO content. After 8 h of carbonation, the content of f-CaO in SSFA drops from 3.17% to 0.93%, only 29.34% of the initial content.
- (2)
- Compared to the mortar specimens with carbonated SSFA, the specimens with uncarbonated SSFA shows faster and more severe damage and a higher expansion rate. When the replacement ratio of carbonated SSFA is less than 45%, the carbonated SSFA has an inhibitory effect on the expansion development of the specimens. The carbonation treatment of SSFA can improve the replacement ratio of SSFA while ensuring the same volume stability of the mortar specimens.
- (3)
- The compressive strength of mortar specimens with uncarbonated SSFA reduces with the increase in the replacement ratio of SSFA. The compressive strengths of the specimens with carbonated SSFA replacement ratios of 60% and 45% are 1.29% and 6.81% higher than those of the specimens with uncarbonated SSFA replacement ratios of 60% and 45%, which indicates that the carbonation of SSFA can have a positive effect in terms of the improvement in the compressive strength of the specimens. Carbonation treatment can improve the replacement ratio of SSFA while ensuring the compressive strength of specimens.
- (4)
- Mortar specimens with SSFA have better carbonation resistance. When the replacement ratio of SSFA is less than 45%, the carbonation depth of the specimens significantly decreases with the increase in the replacement ratio. Compared with mortar specimens with uncarbonated SSFA, the carbonation depth of mortar specimens with carbonated SSFA is slightly larger, and their anti-carbonation performance is reduced.
- (5)
- Carbonation treatment is a beneficial method to improve the stability of SSFA and provide guidance for the future application of mortar. Further study regarding the effect of carbonated steel slag coarse aggregate on the mechanical and expansion properties of concrete is recommended.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Ling, T.C. Production of artificial aggregates from steel-making slag: Influences of accelerated carbonation during granulation and/or post-curing. J. CO2 Util. 2020, 36, 135–144. [Google Scholar] [CrossRef]
- Guo, J.L.; Bao, Y.P.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Wu, Y.D.; Peng, B.; Wu, L.; Lu, W.; Zhang, G.H. Review on global development of treatment and utilization of steel slag. Environ. Eng. 2021, 39, 161–165. [Google Scholar]
- Han, S.W.; Wang, L.Y.; Zhang, X.Y.; Wang, Z.Q.; Cheng, X.X.; Sun, R.Y.; Geng, W.G.; Yuan, D.L.; Zhao, G.J. Synergetic resource utilization of steel slag and sludge and its research progress. Chemistry 2023, 86, 83–90. [Google Scholar]
- Domínguez, M.I.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A. Physicochemical characterization and use of wastes from stainless steel mill. Environ. Prog. Sustain. 2010, 29, 471–480. [Google Scholar] [CrossRef]
- Guo, Y.C.; Xie, J.H.; Zheng, W.Y.; Li, J.L. Effects of steel slag as fine aggregate on static and impact behaviours of concrete. Constr. Build. Mater. 2018, 192, 194–201. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yu, L.H.; Mao, H.B.; Cui, K.K. Microstructure and mechanical properties of high strength porous ceramics with high sewage sludge content. J. Clean. Prod. 2022, 380, 13508. [Google Scholar] [CrossRef]
- Pan, S.Y.; Adhikari, R.; Chen, Y.H.; Li, P.; Chiang, P.C. Integrated and innovative steel slag utilization for iron reclamation, green material production and CO2 fixation via accelerated carbonation. J. Clean. Prod. 2016, 137, 617–631. [Google Scholar] [CrossRef]
- Tsai, T.T.; Kao, C.M.; Wang, J.Y. Remediation of TCE-contaminated groundwater using acid/BOF slag enhanced chemical oxidation. Chemosphere 2011, 83, 687–692. [Google Scholar] [CrossRef]
- Bowden, L.I.; Jarvis, A.P.; Younger, P.L.; Johnson, K. Phosphorus removal from waste waters using basic oxygen steel slag. Environ. Sci. Technol. 2009, 43, 2476–2481. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ni, J.; Fu, C.H.; Gao, S.J.; Li, Y. Preparation of green artificial reefs concrete using steel slag and blast furnace slag. Multipurp. Util. Miner. Resour. 2012, 5, 39–43. [Google Scholar]
- Dhoble, Y.N.; Ahmed, S. Review on the innovative uses of steel slag for waste minimization. J. Mater. Cycles Waste Manag. 2018, 20, 1373–1382. [Google Scholar] [CrossRef]
- Wang, A.G.; He, M.C.; Mo, L.W.; Liu, K.W.; Li, Y.; Zhou, Y.; Sun, D.S. Research progress of building materials prepared from the carbonized curing steel slag. Mater. Rep. 2019, 33, 2939–2948. [Google Scholar]
- Wang, G. Determination of the expansion force of coarse steel slag aggregate. Constr. Build. Mater. 2010, 24, 1961–1966. [Google Scholar] [CrossRef]
- Qasrawi, H. The use of steel slag aggregate to enhance the mechanical properties of recycled aggregate concrete and retain the environment. Constr. Build. Mater. 2014, 54, 298–304. [Google Scholar] [CrossRef]
- Faraone, N.; Tonello, G.; Furlani, E.; Maschio, S. Steelmaking slag as aggregate for mortars: Effects of particle dimension on compression strength. Chemosphere 2009, 77, 1152–1156. [Google Scholar] [CrossRef]
- Pellegrino, C.; Cavagnis, P.; Faleschini, F.; Brunelli, K. Properties of concretes with Black/Oxidizing Electric Arc Furnace slag aggregate. Cem. Concr. Compos. 2013, 37, 232–240. [Google Scholar] [CrossRef]
- Subathra Devi, V.; Gnanavel, B.K. Properties of concrete manufactured using steel slag. Procedia Eng. 2014, 97, 95–104. [Google Scholar] [CrossRef]
- Zhang, T.S.; Liu, F.T.; Wang, J.W.; Li, Z.H.; Cheng, X. Recent development of steel slag stability and activating activity. Bull. Chin. Ceram. Soc. 2007, 5, 980–984. [Google Scholar]
- Juckes, L.M. The volume stability of modern steelmaking slags. Miner. Process. Extr. Metall. 2003, 112, 177–197. [Google Scholar] [CrossRef]
- Wang, Q. Be considerate when using steel slag as concrete aggregate. China Concr. 2016, 2, 90–91. [Google Scholar]
- Fronek, B.; Bosela, P.; Delatte, N. Steel slag aggregate used in Portland cement concrete: US and international perspectives. Transport. Res. Rec. 2012, 2267, 37–42. [Google Scholar] [CrossRef]
- Lun, Y.X.; Zhou, M.K.; Chen, M.Z. Volume stability and application prospect of steel slag aggregates in engineering. Mod. Min. 2006, 4, 37–40. [Google Scholar]
- Qian, G.R.; Sun, D.D.; Tay, J.H.; Lai, Z.Y. Hydrothermal reaction and autoclave stability of Mg bearing RO phase in steel slag. Br. Ceram. Trans. 2002, 101, 159–164. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Zhuang, S. The soundness of steel slag with different free CaO and MgO contents. Constr. Build. Mater. 2017, 151, 138–146. [Google Scholar] [CrossRef]
- Reddy, K.R.; Gopakumar, A.; Chetri, J.K. Critical review of applications of iron and steel slags for carbon sequestration and environmental remediation. Rev. Environ. Sci. Bio/Technol. 2019, 18, 127–152. [Google Scholar] [CrossRef]
- Ghouleh, Z.; Guthrie, R.I.L.; Shao, Y. Production of carbonate aggregates using steel slag and carbon dioxide for carbon-negative concrete. J. CO2 Util. 2017, 18, 125–138. [Google Scholar] [CrossRef]
- Yang, S.; Mo, L.W.; Deng, M. Effects of ethylenediamine tetra-acetic acid (EDTA) on the accelerated carbonation and properties of artificial steel slag aggregates. Cem. Concr. Comp. 2021, 118, 103948. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.H.; Hu, X.; Chang, J.; Zhang, T.T.; Shi, C.J. Utilization of accelerated carbonation to enhance the application of steel slag: A review. J. Sustain. Cem. Based Mater. 2023, 12, 471–486. [Google Scholar] [CrossRef]
- Liu, P.; Zhong, J.K.; Zhang, M.; Mo, L.W.; Deng, M. Effect of CO2 treatment on the microstructure and properties of steel slag supplementary cementitious materials. Constr. Build. Mater. 2021, 309, 125171. [Google Scholar] [CrossRef]
- Chen, Z.M.; Li, R.; Liu, J.Z. Preparation and properties of carbonated steel slag used in cementitious materials. Constr. Build. Mater. 2021, 283, 122667. [Google Scholar] [CrossRef]
- Pang, B.; Zhou, Z.H.; Xu, H.X. Utilization of carbonated and granulated steel slag aggregate in concrete. Constr. Build. Mater. 2015, 84, 454–467. [Google Scholar] [CrossRef]
- Yu, H.D.; Xu, P.Z.; Zhu, Y.G.; Ding, J.; Wan, X.M.; Zhu, H.Y. Effect of carbonated steel slag as substitute for sand on the strength and durability of cement mortar. J. Qingdao Univ. Technol. 2023, 44, 20–28. [Google Scholar]
- Tossavainen, M.; Engström, F.; Yang, Q.; Menad, N.; Lidström Larsson, M.; Björkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.M. Behavior of steel slag aggregate in mortar and concrete—A comprehensive overview. J. Build. Eng. 2022, 53, 104536. [Google Scholar] [CrossRef]
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Architecture & Building Press: Beijing, China, 2009.
- YB/T 4328-2012; Method for the Determination of Content of Free Calcium Oxide in Steel Slag. Ministry of Industry and Information Technology of China: Beijing, China, 2012.
- JGJ 52-2006; Standard for Technical Requirements and Test Method of Sand and Crushed Stone (or Gravel) for Ordinary Concrete. Ministry of Construction of China: Beijing, China, 2007.
- GB/T 17671-2021; The Method of Cement Mortar Strength (ISO Method). General Administration of Quality Supervision, Inspection and Quarantine of China (AQSIQC): Beijing, China, 2021.
- Zhong, X.Z.; Li, L.F.; Jiang, Y.; Ling, T.C. Elucidating the dominant and interaction effects of temperature, CO2 pressure and carbonation time in carbonating steel slag blocks. Constr. Build. Mater. 2021, 302, 124158. [Google Scholar] [CrossRef]

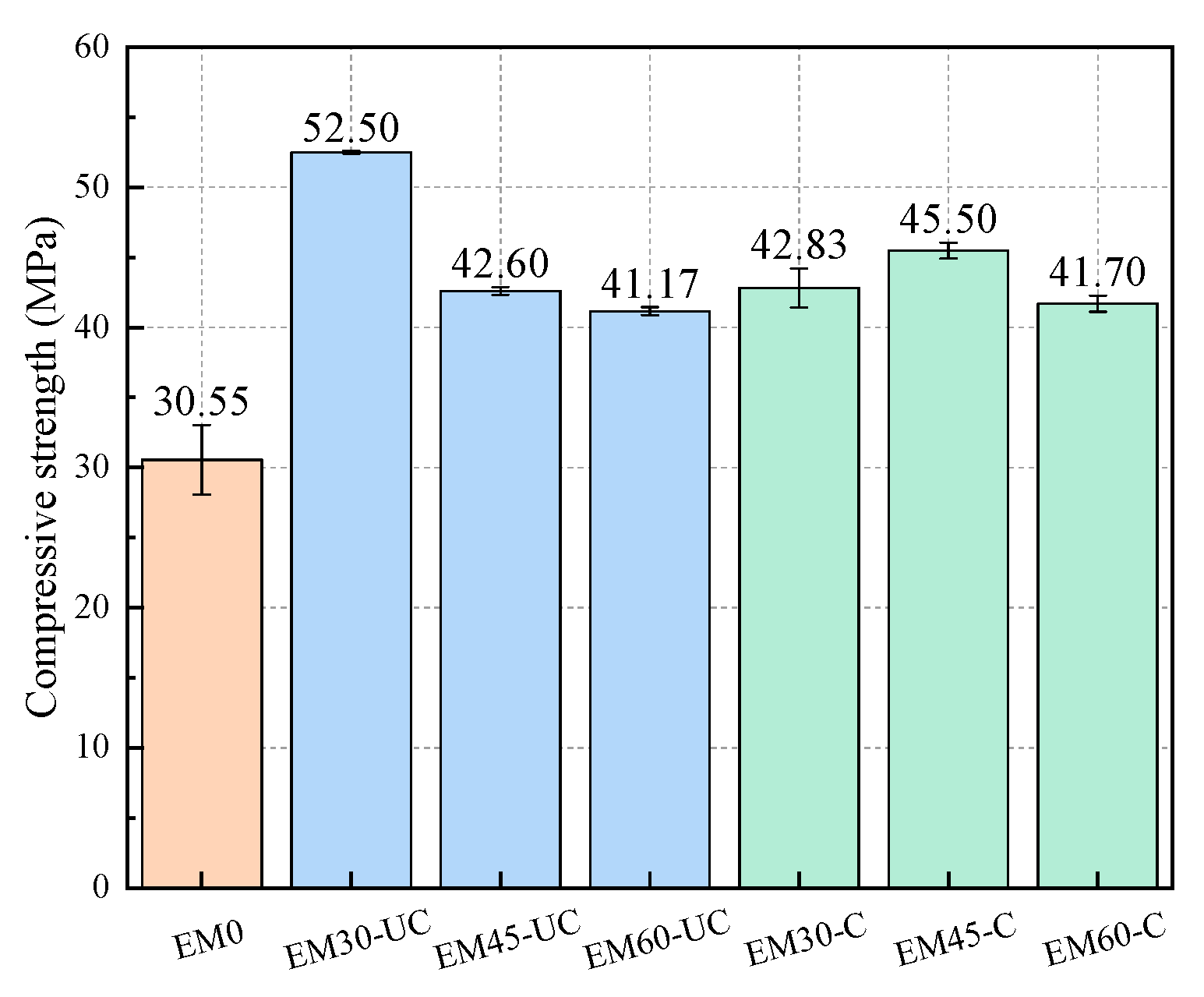
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Loss |
|---|---|---|---|---|---|---|
| 22.25 | 4.98 | 3.47 | 64.84 | 1.51 | 1.83 | 1.12 |
| Group Number | Expansion Test | Compression Test | Replacement Ratio of SSFA (%) | SSFA |
|---|---|---|---|---|
| EM0 | 3 | 3 | 0 | — |
| EM30-UC | 3 | 3 | 30 | Uncarbonated |
| EM30-C | 3 | 3 | Carbonated | |
| EM45-UC | 3 | 3 | 45 | Uncarbonated |
| EM45-C | 3 | 3 | Carbonated | |
| EM60-UC | 3 | 3 | 60 | Uncarbonated |
| EM60-C | 3 | 3 | Carbonated |
| Aperture Size of Test Sieves (mm) | 4.75~2.36 | 2.36~1.18 | 1.18~0.60 | 0.60~0.30 | 0.30~0.15 |
|---|---|---|---|---|---|
| Content (%) | 10 | 25 | 25 | 25 | 15 |
| Group Number | Replacement Ratio of SSFA (%) | SSFA |
|---|---|---|
| CR0 | 0 | — |
| CR15-UC | 15 | Uncarbonated |
| CR15-C | Carbonated | |
| CR30-UC | 30 | Uncarbonated |
| CR30-C | Carbonated | |
| CR45-UC | 45 | Uncarbonated |
| CR45-C | Carbonated |
| Aperture Size of Test Sieves (mm) | >4.75 | 4.75~2.36 | 2.36~1.18 | 1.18~0.60 | 0.60~0.30 | 0.30~0.15 | <0.15 |
|---|---|---|---|---|---|---|---|
| Content (%) | 3.70 | 13.34 | 24.44 | 30.37 | 22.22 | 5.93 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Wang, J.; Cui, J.; Yuan, Y.; Song, Y. Experimental Study on the Effects of Carbonated Steel Slag Fine Aggregate on the Expansion Rate, Mechanical Properties and Carbonation Depth of Mortar. Materials 2024, 17, 3279. https://doi.org/10.3390/ma17133279
Gao P, Wang J, Cui J, Yuan Y, Song Y. Experimental Study on the Effects of Carbonated Steel Slag Fine Aggregate on the Expansion Rate, Mechanical Properties and Carbonation Depth of Mortar. Materials. 2024; 17(13):3279. https://doi.org/10.3390/ma17133279
Chicago/Turabian StyleGao, Pengfei, Jian Wang, Jianjun Cui, Yongyu Yuan, and Yuanyuan Song. 2024. "Experimental Study on the Effects of Carbonated Steel Slag Fine Aggregate on the Expansion Rate, Mechanical Properties and Carbonation Depth of Mortar" Materials 17, no. 13: 3279. https://doi.org/10.3390/ma17133279
APA StyleGao, P., Wang, J., Cui, J., Yuan, Y., & Song, Y. (2024). Experimental Study on the Effects of Carbonated Steel Slag Fine Aggregate on the Expansion Rate, Mechanical Properties and Carbonation Depth of Mortar. Materials, 17(13), 3279. https://doi.org/10.3390/ma17133279






