Chemical Stability of High-Entropy Spinel in a High-Pressure Pure Hydrogen Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Mechanochemical Synthesis of Spinel HEOx
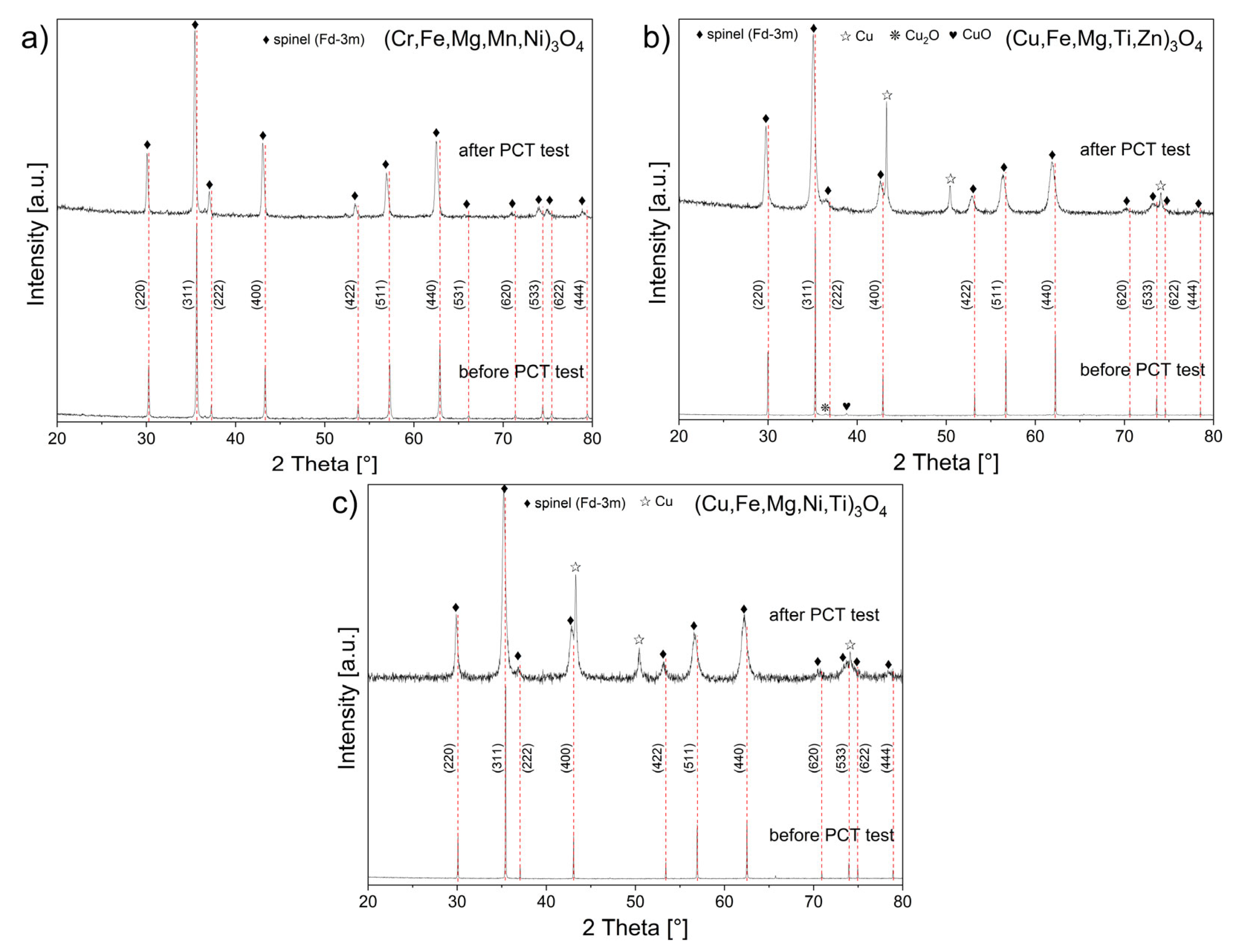
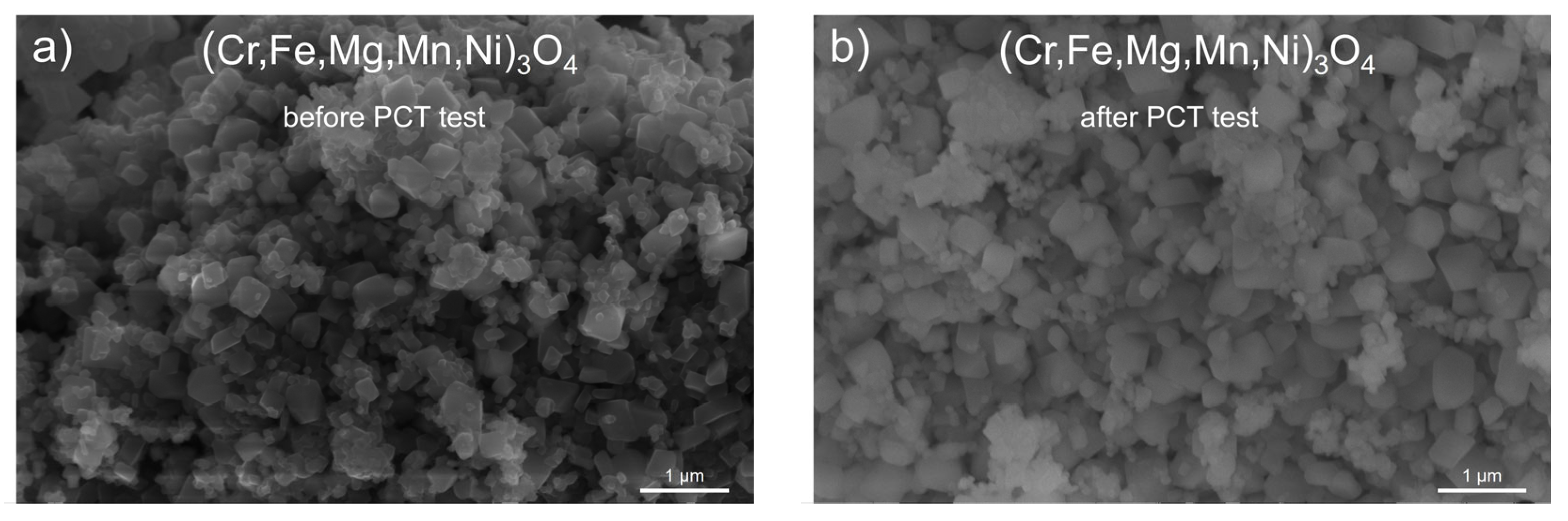
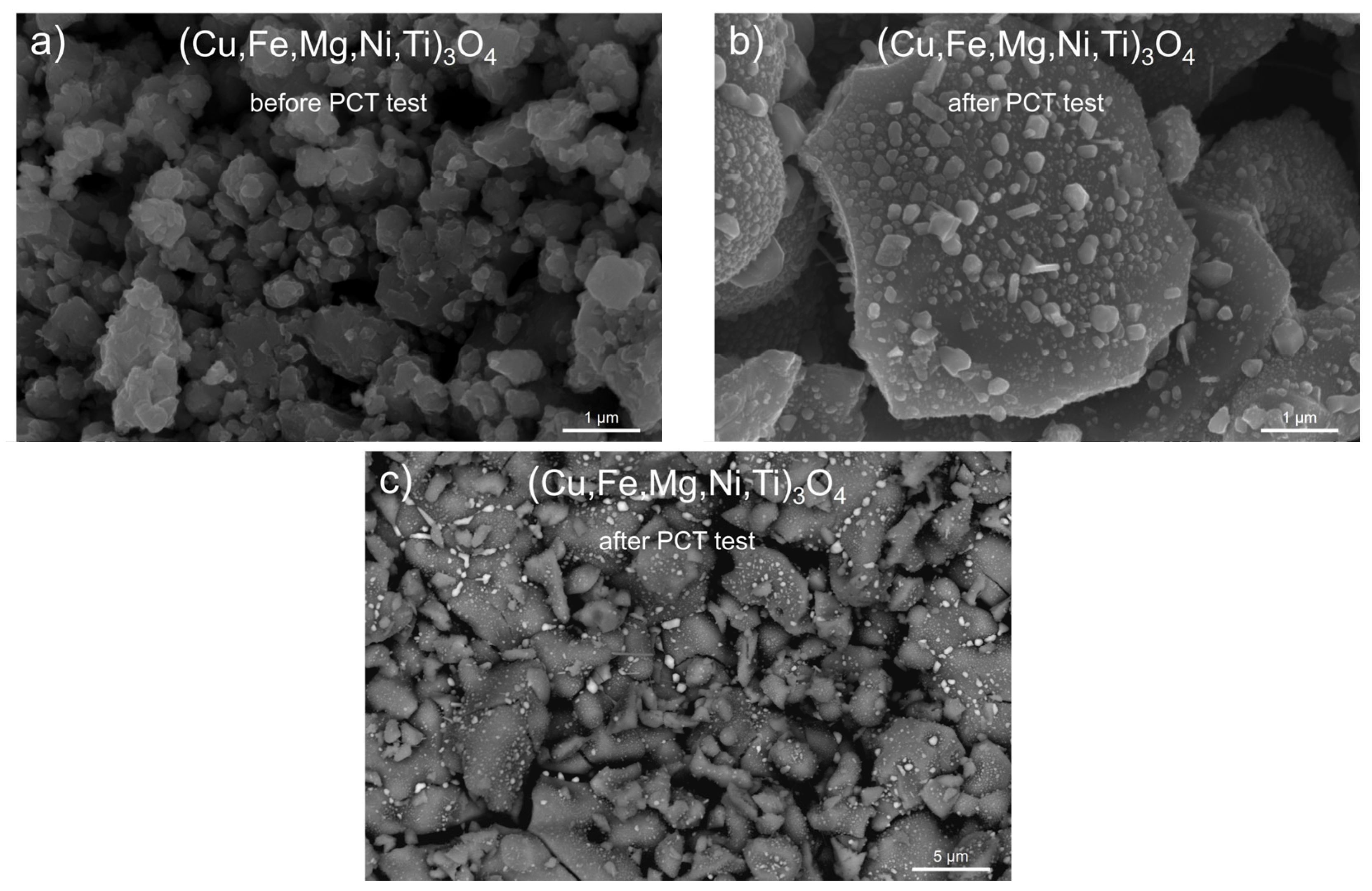
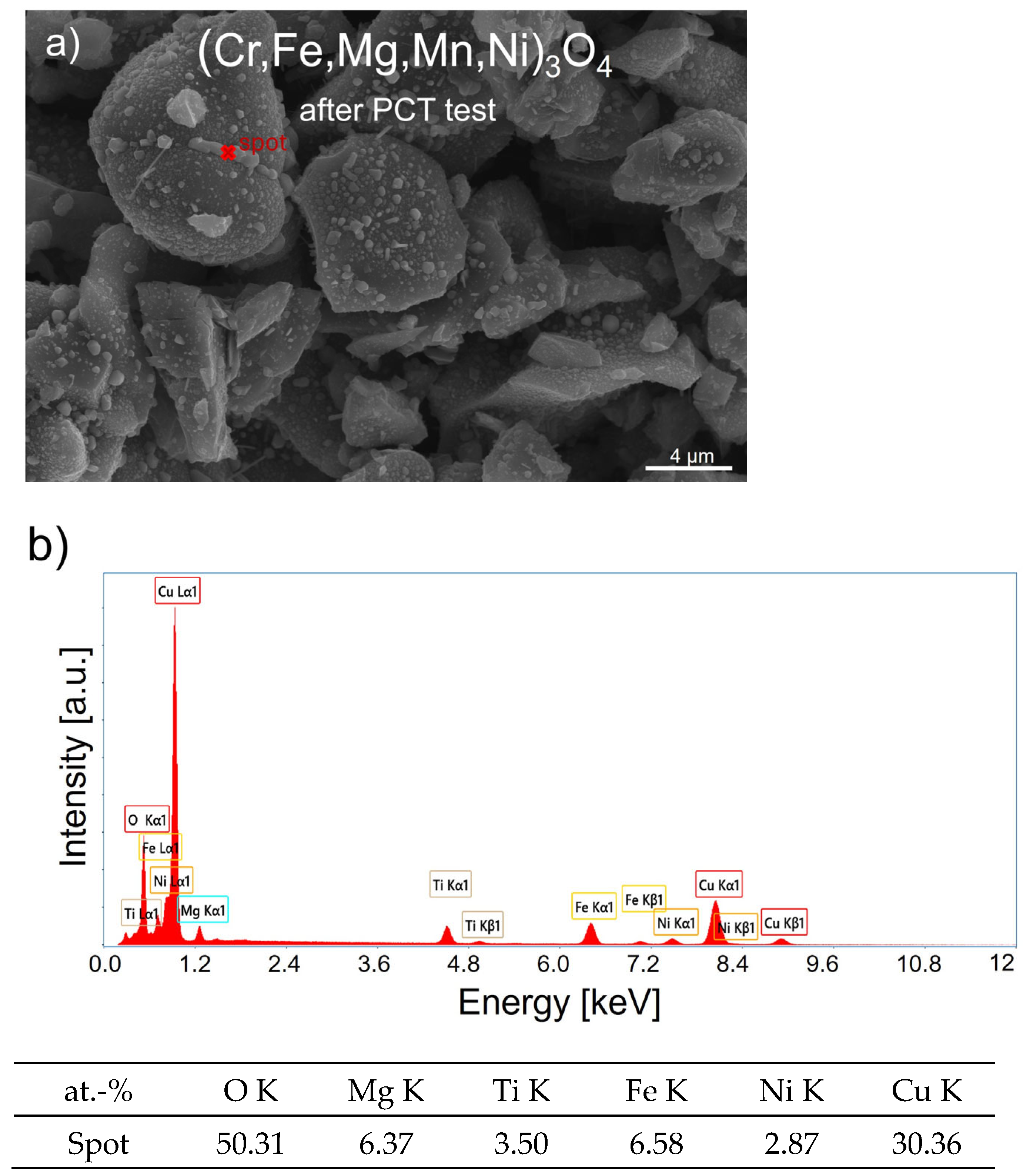
3.2. Spinel HEOx after Hydrogenation
4. Discussion
5. Conclusions
- ➢
- Single-phase spinel-structured powders were not obtained via the mechanochemical synthesis (MS) of samples with the nominal compositions (Cr0.2Fe0.2Mg0.2Mn0.2Ni0.2)3O4, (Cu0.2Fe0.2Mg0.2Ti0.2Zn0.2)3O4, and (Cu0.2Fe0.2Mg0.2Ni0.2Ti0.2)3O4.
- ➢
- Performing the free sintering procedure on powders after MS at 1000 °C for 3 h in air achieved single-phase (Cr,Fe,Mg,Mn,Ni)3O4 and (Cu,Fe,Mg,Ni,Ti)3O4 powders with a spinel structure, and in the case of (Cu,Fe,Mg,Ti,Zn)3O4, a spinel phase in the amount of 95 wt.% was achieved.
- ➢
- An increase in spinel unit cell lattice parameters was determined for (Cr,Fe,Mg,Mn,Ni)3O4, (Cu,Fe,Mg,Ti,Zn)3O4, and (Cu,Fe,Mg,Ni,Ti)3O4 powders after high-pressure hydrogenation at 250 °C and with a pressure of up to 40 bar.
- ➢
- A decrease in the spinel phase crystallite size and an increase in lattice strains were established in the above-mentioned spinel powders.
- ➢
- Spinels containing copper in their structure, i.e., (Cu,Fe,Mg,Ti,Zn)3O4 and (Cu,Fe,Mg,Ni,Ti)3O4, were chemically unstable during high-pressure hydrogenation, due to a partial reduction process accompanied by the release of metallic copper.
- ➢
- Pure hydrogen did not have a notable effect on the structure and morphology of (Cr,Fe,Mg,Mn,Ni)3O4, which indicates the potential of this ceramic material for future use in hydrogen technologies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Zhang, R.-C.; Reece, M.J. Review of high entropy ceramics: Design, synthesis, structure and properties. J. Mater. Chem. A 2019, 7, 22148. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-entropy metal diborides: A new class of high-entropy materials and a new type of ultra high temperature ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef] [PubMed]
- Braic, V.; Balaceanu, M.; Braic, M.; Vladescu, A.; Panseri, S.; Russo, A. Characterizationof multi-principal-element (TiZrNbHfTa)N and (TiZrNbHfTa)C coatings for biomedical applications. J. Mech. Biomed. 2012, 10, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, J.; Zhang, F.; Niu, B.; Lei, L.; Wang, W. High-entropy carbide: A novel class of multicomponent ceramics. Ceram. Int. 2018, 44, 22014. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Stygar, M.; Dabrowa, J.; Mozdzierz, M.; Zajusz, M.; Skubida, W.; Mroczka, K.; Berent, K.; Swierczek, K.; Danielewski, M. Formation and properties of high entropy oxides in Co-Cr-Fe-Mg-Mn-Ni-O system: Novel (Cr,Fe,Mg,Mn,Ni)3O4 and (Co,Cr,Fe,Mg,Mn)3O4 high entropy spinels. J. Eur. Ceram. Soc. 2019, 40, 1644–1650. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R. Rare earth and transition metal based entropy stabilised perovskite type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Peng, Y.; Luo, Y.; Liu, T.; He, W.; Chen, F.; Ding, M. Novel high-entropy perovskite-type symmetrical electrode for efficient and durable carbon dioxide reduction reaction. Adv. Powder Mater. 2023, 2, 100129. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, H.; Wang, X.; Tian, G.; Du, D.; Shu, C. Strain-rich high-entropy perovskite oxide of (La0.8Sr0.2)(Mn0.2Fe0.2Cr0.2Co0.2Ni0.2)O3 for durable and effective catalysis of oxygen redox reactions in lithium-oxygen battery. Bat. Energy 2024, 3, 20230053. [Google Scholar] [CrossRef]
- Jiang, B.; Bridges, C.A.; Unocic, R.R.; Pitike, K.C.; Cooper, V.R.; Zhang, Y.; Lin, D.-Y.; Page, K. Probing the local site disorder and distortion in pyrochlore high-entropy oxides. J. Am. Chem. Soc. 2021, 143, 4193–4204. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zhu, L.; Tan, Y.; Zeng, S.; Xia, Y.; Wang, Y.; Zhang, H. Synthesis and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc. 2020, 40, 1639–1643. [Google Scholar] [CrossRef]
- Pitike, K.C.; Macias, A.; Eisenbach, M.; Bridges, C.A.; Cooper, V.R. Computationally Accelerated Discovery of High Entropy Pyrochlore Oxides. Chem. Mater. 2022, 34, 1459–1472. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, C.; De, Y.; Zhang, S.; Zhang, C.; Li, X. Phase structure of high-entropy pyrochlore oxides: From powder synthesis to ceramic sintering. J. Eur. Ceram. Soc. 2023, 43, 7613–7622. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, X.; Yue, Y.; Palma, M.; Reece, M.J.; Yan, H. Multi elements substituted Aurivillius phase relaxor ferroelectrics using high entropy design concept. Mater. Des. 2021, 200, 109447. [Google Scholar] [CrossRef]
- Ren, X.; Tian, Z.; Zhang, J.; Wang, J. Equiatomic quaternary (Y1/4Ho1/4Er1/4Yb1/4)2SiO5 silicate: A perspective multifunctional thermal and environmental barrier coating material. Scr. Mater. 2019, 168, 47–50. [Google Scholar] [CrossRef]
- Pianassola, M.; Loveday, M.; McMurray, J.W.; Koschan, M.; Melcher, C.L.; Zhuravleva, M. Solid-state synthesis of multicomponent equiatomic rare-earth oxides. J. Am. Ceram. Soc. 2020, 103, 2908–2918. [Google Scholar] [CrossRef]
- Pianassola, M.; Loveday, M.; Chakoumakos, B.C.; Koschan, M.; Melcher, C.L.; Zhuravleva, M. Crystal growth and elemental homogeneity of the multicomponent rare-earth garnet (Lu1/6Y1/6Ho1/6Dy1/6Tb1/6Gd1/6)3Al5O12. Cryst. Growth Des. 2020, 20, 6769–6776. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Duan, C.; Mao, J.; Dong, Y.; Dong, K.; Wang, Z.; Luo, S.; Liu, Y.; Qi, X. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. J. Alloys Compd. 2020, 844, 156158. [Google Scholar] [CrossRef]
- Tian, K.-H.; Duan, C.-Q.; Ma, Q.; Li, X.-L.; Wang, Z.-Y.; Sun, H.-Y.; Luo, S.-H.; Wang, D.; Liu, Y.-G. High-entropy chemistry stabilizing spinel oxide (CoNiZnXMnLi)3O4 (X=Fe,Cr) for high-performance anode of Li-ion batteries. Rare Met. 2021, 41, 1265–1275. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X.; Huang, Y.; Du, F.; Zeng, Y. Entropy stabilization effect and oxygen vacancies enabling spinel oxide highly reversible lithium-ion storage. ACS Appl. Mater. Interfaces 2021, 13, 58674–58681. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.Y.; Lou, S.F.; Zheng, W.; Qian, Z.Y.; Cui, C.; Zuo, P.J.; Du, C.Y.; Xie, J.Y.; Wang, J.J.; Yin, G.P. Synergistic engineering of defects and architecture in Co3O4@C nanosheets toward Li/Na ion batteries with enhanced pseudocapacitances. Nano Energy 2020, 78, 105366. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Y.; Mo, L.; Liu, C.F.; Hsu, K.; Ding, Y.C.; Zhang, X.X.; Li, X.L.; Hu, L.H.; Ji, D.H.; et al. Impacts of oxygen vacancies on zinc ion intercalation in VO2. ACS Nano 2020, 14, 5581–5589. [Google Scholar] [CrossRef]
- Biesuz, M.; Spiridigliozzi, L.; Dell’Agli, G.; Bortlott, M.; Sglavo, V.M. Synthesis and sintering of (Mg,Co,Ni,Cu,Zn)O entropy-stabilized oxides obtained by wet chemical methods. J. Mater. Sci. 2018, 53, 8074–8085. [Google Scholar] [CrossRef]
- Okejiri, F.; Zhang, Z.; Liu, J.; Liu, M.; Yang, S.; Dai, S. Room-temperature synthesis of high-entropy perovskite oxide nanoparticle catalysts through ultrasonication based method. ChemSusChem 2020, 13, 111–115. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Usharani, N.J.; Sanghvi, K.P.; Chakravadhanula, V.S.K.; Gandhi, A.S.; Hahn, H.; Bhattacharya, S.S. Nanocrystalline multicomponent entropy stabilised transition metal oxides. J. Eur. Ceram. Soc. 2017, 37, 747–754. [Google Scholar] [CrossRef]
- Sushil, J.; Kumar, A.; Gautam, A.; Ahmad, M.I. High entropy phase evolution and fine structure of five component oxide (Mg,Co,Ni,Cu,Zn)O by citrate gel method. Mater. Chem. Phys. 2021, 259, 124014. [Google Scholar] [CrossRef]
- Saghir, A.V.; Beidokhti, S.M.; Khaki, J.V.; Salimi, A. One-step synthesis of single-phase (Co, Mg, Ni, Cu, Zn)O high entropy oxide nanoparticles through SCS procedure: Thermodynamics and experimental evaluation. J. Eur. Ceram. Soc. 2021, 41, 563–579. [Google Scholar] [CrossRef]
- Seyedia, M.; Haratiana, S.; Vahdati Khakia, J. Mechanochemical synthesis of Fe2O3 nanoparticles. Procedia Mater. Sci. 2015, 11, 309–313. [Google Scholar] [CrossRef]
- Lu, J.; Ng, K.M.; Yang, S. Efficient, one-step mechanochemical process for the synthesis of ZnO nanoparticles. Ind. Eng. Chem. Res. 2008, 47, 1095–1101. [Google Scholar] [CrossRef]
- Li, Y.X.; Chen, W.F.; Zhou, X.Z.; Gu, Z.Y.; Chen, C.M. Synthesis of CeO2 nanoparticles by mechanochemical processing and the inhibiting action of NaCl on particle agglomeration. Mater. Lett. 2005, 59, 48–52. [Google Scholar] [CrossRef]
- Chen, H.; Lin, W.; Zhang, Z.; Jie, K.; Mullins, D.R.; Sang, X.; Yang, S.-Z.; Jafta, C.J.; Bridges, C.A.; Hu, X.; et al. Mechanochemical synthesis of high entropy oxide materials under ambient conditions: Dispersion of catalysts via entropy maximization. ACS Mater. Lett. 2019, 1, 83–88. [Google Scholar] [CrossRef]
- Domaradzki, K.; Nowak, M.; Sitarz, M.; Łapiński, M.; Brylewski, T.; Jurczyk, M. Influence of hydrogen on a nanocrystalline high-entropy oxide with application potential in hydrogen technologies. Ceram. Int. 2023, 49, 15544–15552. [Google Scholar] [CrossRef]
- Balcerzak, M.; Kawamura, K.; Bobrowski, R.; Rutkowski, P.; Brylewski, T. Mechanochemical synthesis of (Co, Cu, Mg, Ni, Zn)O high-entropy oxide and its physicochemical properties. J. Electron. Mater. 2019, 48, 7105–7113. [Google Scholar] [CrossRef]
- Zavaliy, I.Y.; Denys, R.V.; Cerný, R.; Koval’chuck, I.V.; Wiesinger, G.; Hilscher, G. Hydrogen-induced changes in crystal structure and magnetic properties of the Zr3MOx (M = Fe, Co) phases. J. Alloys Compd. 2005, 386, 26–34. [Google Scholar] [CrossRef]




| Sample | (Cr,Fe,Mg,Mn,Ni)3O4 | (Cu,Fe,Mg,Ti,Zn)3O4 | (Cu,Fe,Mg,Ni,Ti)3O4 | |||
|---|---|---|---|---|---|---|
| Before PCT | After PCT | Before PCT | After PCT | Before PCT | After PCT | |
| Parameter “a” of the spinel cell [Å] | 8.3529 | 8.3974 | 8.4348 | 8.4758 | 8.3995 | 8.4366 |
| Identified secondary phases/mass content [wt.%] | - | - | CuO (2.5%) Cu2O (1.7%) | Cu (8.9%) | - | Cu (11.9%) |
| Crystalline size [nm] | 65(1) | 50(1) | 149(1) | 60(2) | 128(1) | 29(1) |
| Microstresses (×10−3) | 0.4 | 2.1 | 0.2 | 6.2 | 0.07 | 4.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domaradzki, K.; Adamczyk, A.; Pyzalski, M.; Brylewski, T.; Nowak, M.; Jurczyk, M. Chemical Stability of High-Entropy Spinel in a High-Pressure Pure Hydrogen Atmosphere. Materials 2024, 17, 3309. https://doi.org/10.3390/ma17133309
Domaradzki K, Adamczyk A, Pyzalski M, Brylewski T, Nowak M, Jurczyk M. Chemical Stability of High-Entropy Spinel in a High-Pressure Pure Hydrogen Atmosphere. Materials. 2024; 17(13):3309. https://doi.org/10.3390/ma17133309
Chicago/Turabian StyleDomaradzki, Kamil, Anna Adamczyk, Michał Pyzalski, Tomasz Brylewski, Marek Nowak, and Mieczysław Jurczyk. 2024. "Chemical Stability of High-Entropy Spinel in a High-Pressure Pure Hydrogen Atmosphere" Materials 17, no. 13: 3309. https://doi.org/10.3390/ma17133309







