Design and Dynamic In Vivo Validation of a Multi-Channel Stretchable Liquid Metal Coil Array
Abstract
:1. Introduction
2. Materials and Methods
2.1. Simulations
2.2. Array Fabrication
2.3. In Vitro Imaging
2.4. In Vivo Imaging
3. Results
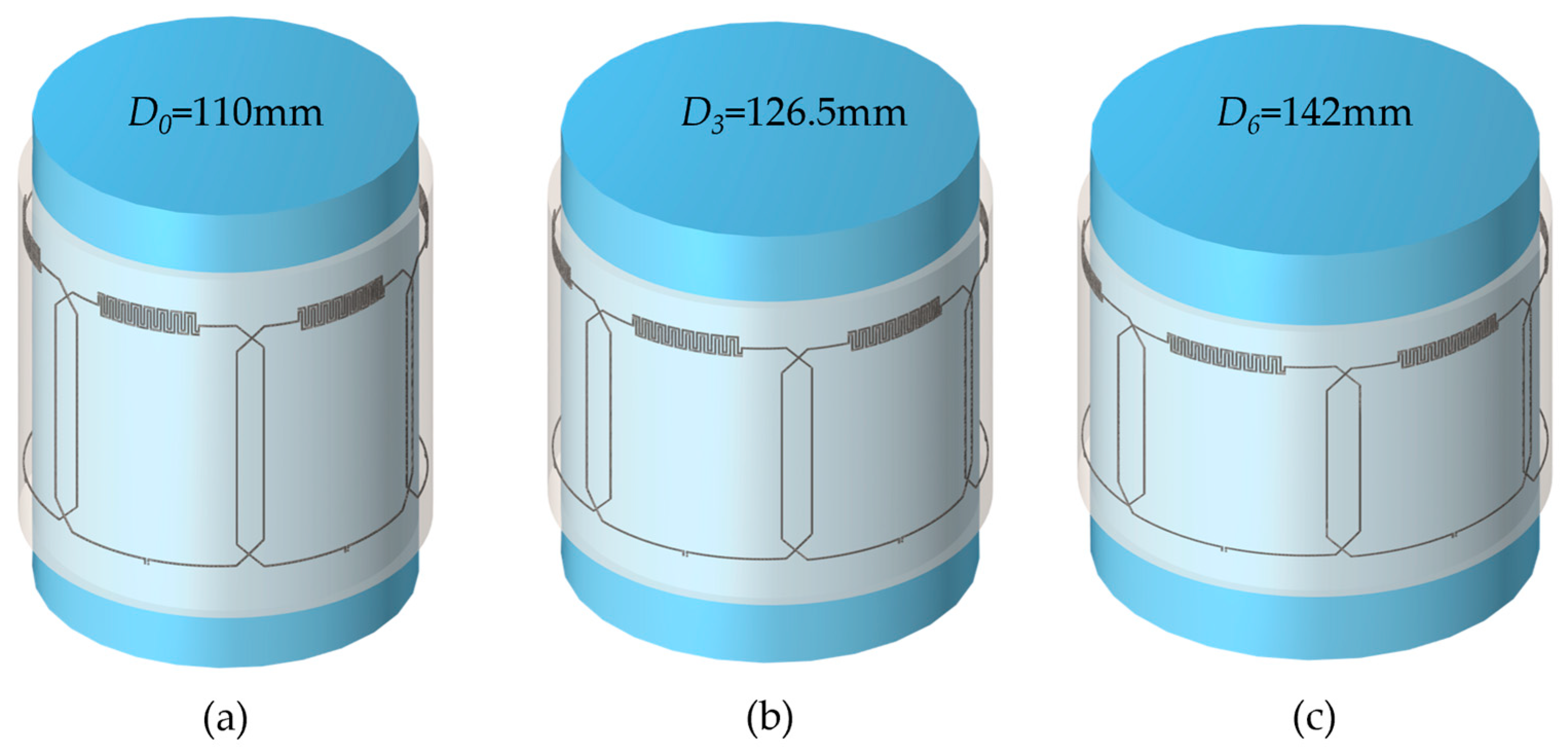
3.1. Simulations
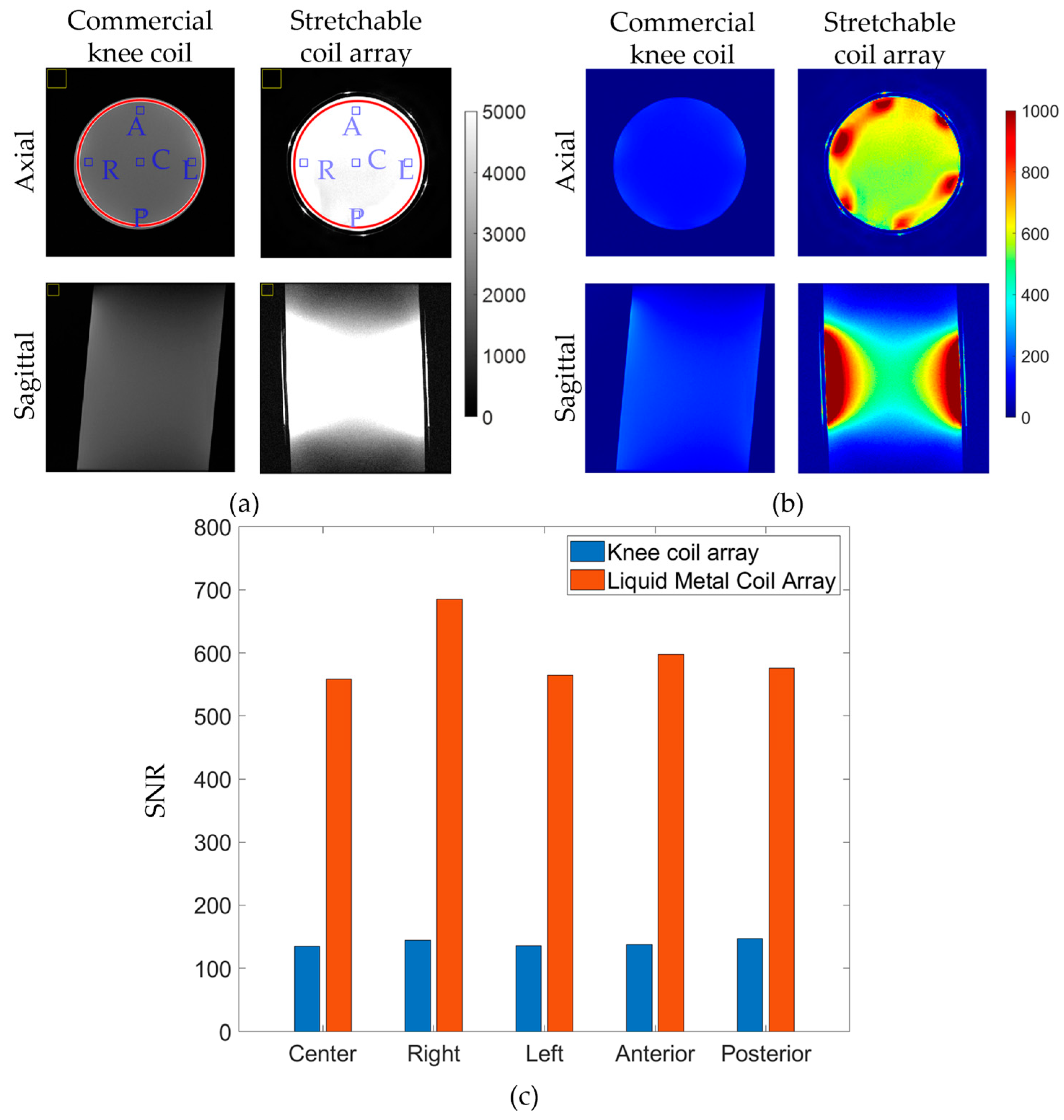
3.2. In Vitro Imaging
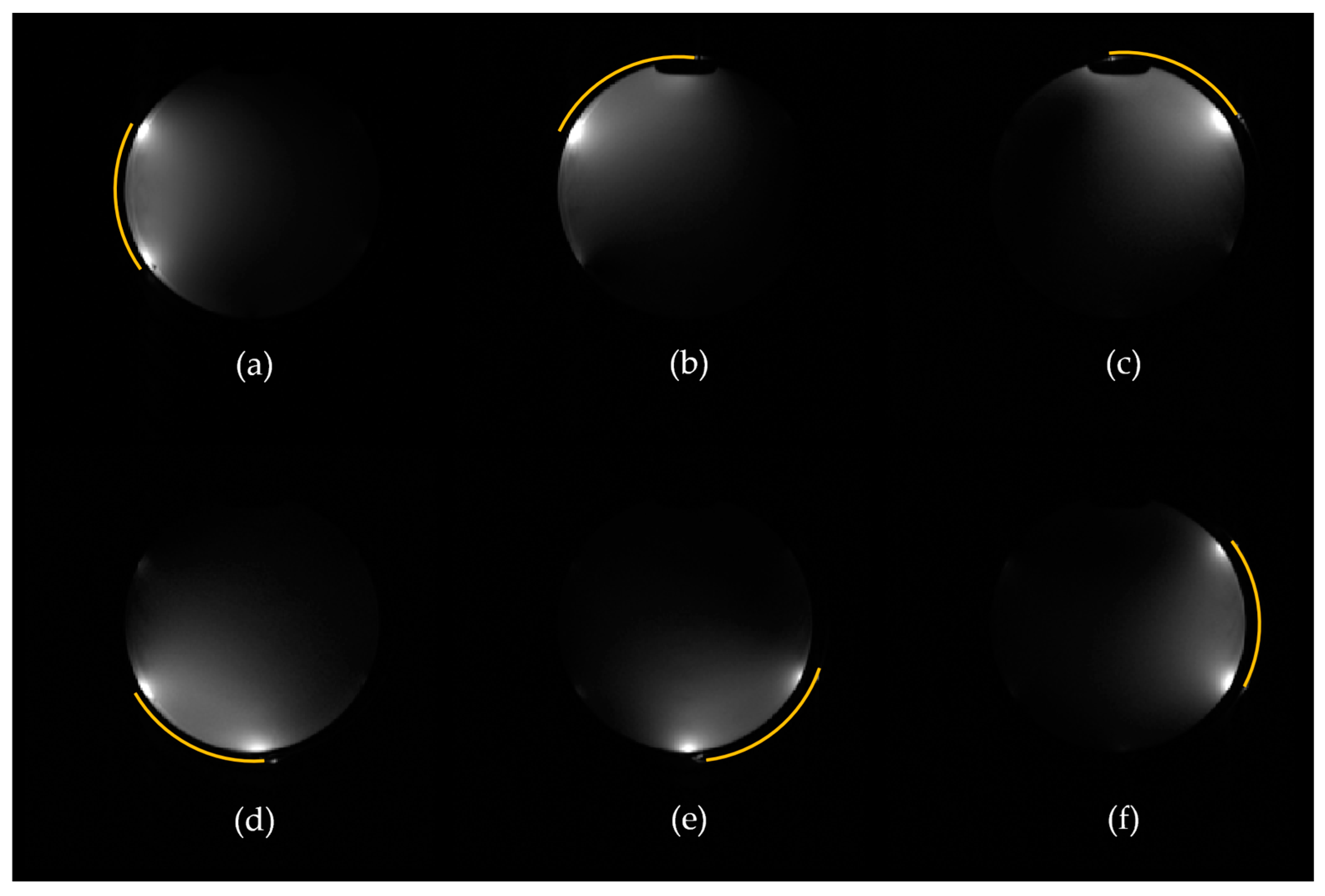
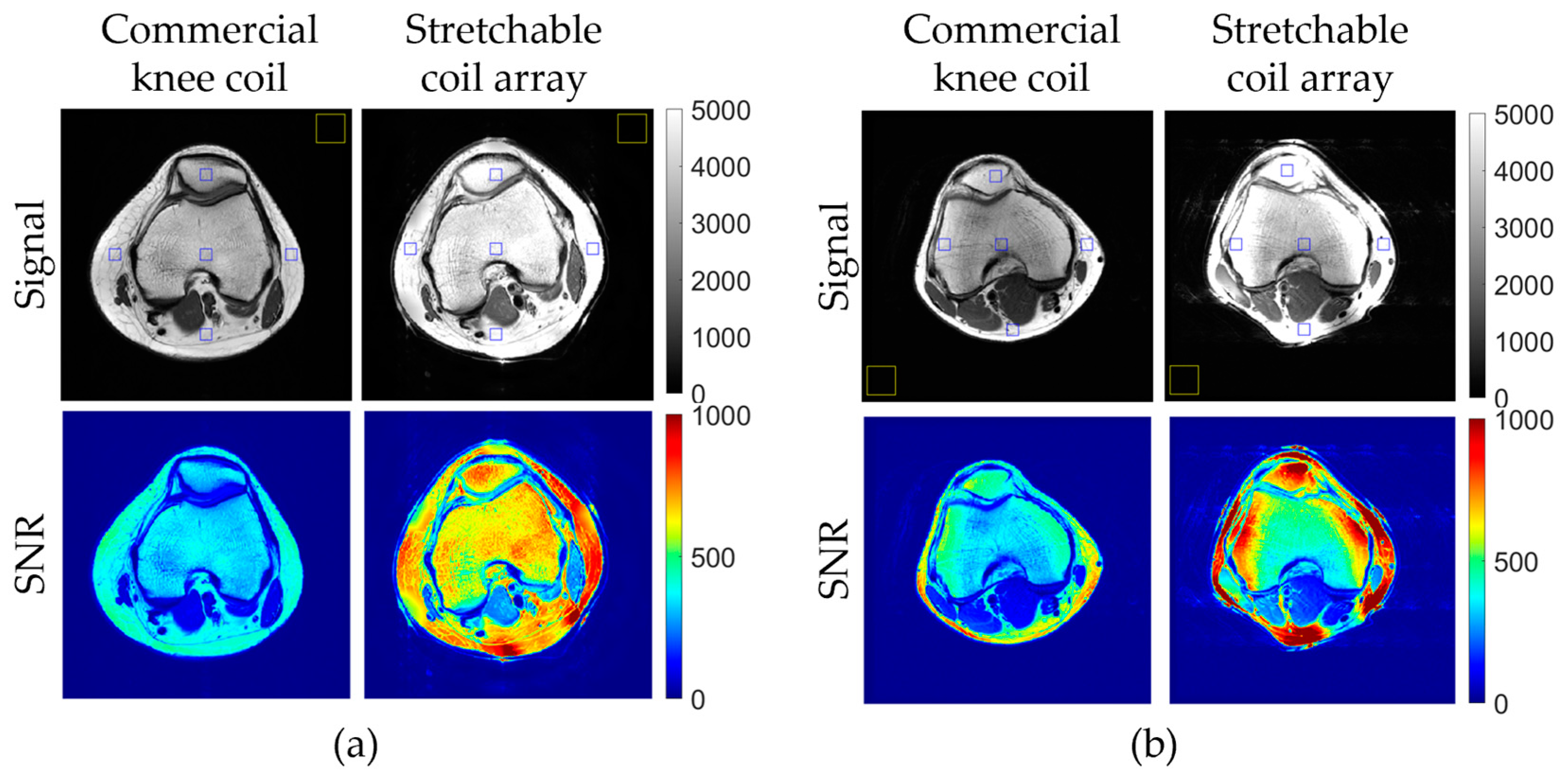
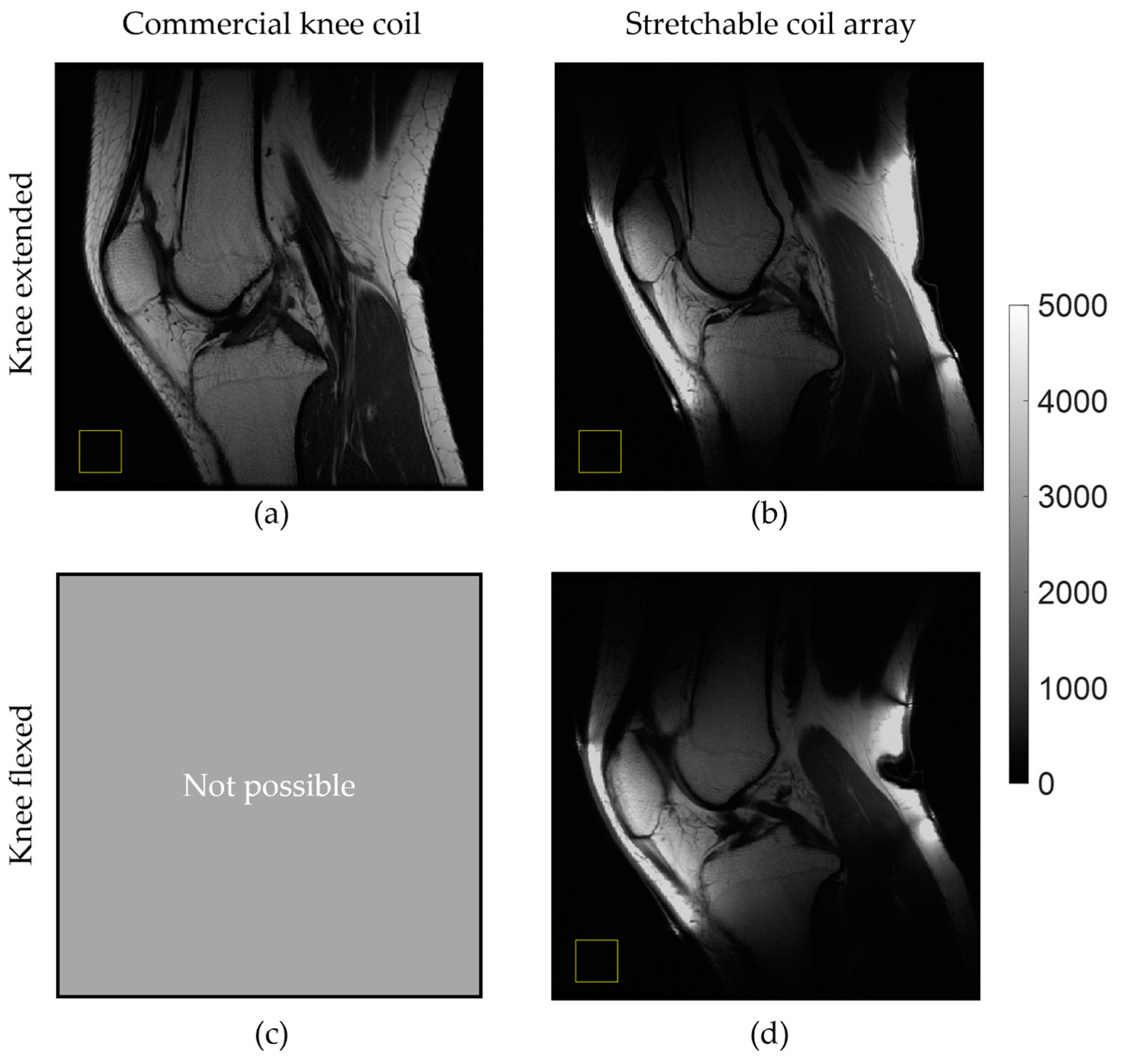
3.3. In Vivo Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webb, A. Introduction to Biomedical Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Kwok, W.E. Basic principles of and practical guide to clinical MRI radiofrequency coils. RadioGraphics 2022, 42, 898–918. [Google Scholar] [CrossRef]
- Hernandez, D.; Kim, K.-N. A review on the RF coil designs and trends for ultra high field magnetic resonance imaging. Investig. Magn. Reson. Imaging 2020, 24, 95–122. [Google Scholar] [CrossRef]
- Darnell, D.; Truong, T.K.; Song, A.W. Recent Advances in Radio-Frequency Coil Technologies: Flexible, Wireless, and Integrated Coil Arrays. J. Magn. Reson. Imaging 2022, 55, 1026–1042. [Google Scholar] [CrossRef]
- Vaughan, J.T.; Griffiths, J.R. RF Coils for MRI; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Corea, J.R.; Flynn, A.M.; Lechêne, B.; Scott, G.; Reed, G.D.; Shin, P.J.; Lustig, M.; Arias, A.C. Screen-printed flexible MRI receive coils. Nat. Commun. 2016, 7, 10839. [Google Scholar] [CrossRef]
- Corea, J.R.; Lechene, P.B.; Lustig, M.; Arias, A.C. Materials and methods for higher performance screen-printed flexible MRI receive coils. Magn. Reson. Med. 2017, 78, 775–783. [Google Scholar] [CrossRef]
- Winkler, S.A.; Corea, J.; Lechêne, B.; O’brien, K.; Bonanni, J.R.; Chaudhari, A.; Alley, M.; Taviani, V.; Grafendorfer, T.; Robb, F.; et al. Evaluation of a flexible 12-channel screen-printed pediatric MRI coil. Radiology 2019, 291, 180–185. [Google Scholar] [CrossRef]
- Jia, F.; Yuan, H.; Zhou, D.; Zhang, J.; Wang, X.; Fang, J. Knee MRI under varying flexion angles utilizing a flexible flat cable antenna. NMR Biomed. 2015, 28, 460–467. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, X.; Wang, C.; Li, Y.; Pang, Y.; Lu, J.; Xu, D.; Majumdar, S.; Nelson, S.J.; Vigneron, D.B. Flexible transceiver array for ultrahigh field human MR imaging. Magn. Reson. Med. 2012, 68, 1332–1338. [Google Scholar] [CrossRef]
- Zhang, T.; Grafendorfer, T.; Cheng, J.Y.; Ning, P.; Rainey, B.; Giancola, M.; Ortman, S.; Robb, F.J.; Calderon, P.D.; Hargreaves, B.A.; et al. A semiflexible 64-channel receive-only phased array for pediatric body MRI at 3T. Magn. Reson. Med. 2016, 76, 1015–1021. [Google Scholar] [CrossRef]
- Hosseinnezhadian, S.; Frass-Kriegl, R.; Goluch-Roat, S.; Pichler, M.; Sieg, J.; Vít, M.; Poirier-Quinot, M.; Darrasse, L.; Moser, E.; Ginefri, J.-C.; et al. A flexible 12-channel transceiver array of transmission line resonators for 7 T MRI. J. Magn. Reson. 2018, 296, 47–59. [Google Scholar] [CrossRef]
- Zhang, B.; Brown, R.; Cloos, M.; Lattanzi, R.; Sodickson, D.; Wiggins, G. Size-adaptable “Trellis” structure for tailored MRI coil arrays. Magn. Reson. Med. 2019, 81, 3406–3415. [Google Scholar] [CrossRef]
- Nordmeyer-Massner, J.A.; De Zanche, N.; Pruessmann, K.P. Mechanically adjustable coil array for wrist MRI. Magn. Reson. Med. 2009, 61, 429–438. [Google Scholar] [CrossRef]
- Lopez Rios, N.; Foias, A.; Lodygensky, G.; Dehaes, M.; Cohen-Adad, J. Size-adaptable 13-channel receive array for brain MRI in human neonates at 3 T. NMR Biomed. 2018, 31, e3944. [Google Scholar] [CrossRef]
- Gruber, B.; Zink, S. Anatomically adaptive local coils for MRI Imaging–Evaluation of stretchable antennas at 1.5 T. In Proceedings of the 24th Annual Meeting of the ISMRM, Singapore, 7–13 May 2016; p. 543. [Google Scholar]
- Adriany, G.; Van De Moortele, P.-F.; Ritter, J.; Moeller, S.; Auerbach, E.J.; Akgün, C.; Snyder, C.J.; Vaughan, T.; Uğurbil, K. A geometrically adjustable 16-channel transmit/receive transmission line array for improved RF efficiency and parallel imaging performance at 7 Tesla. Magn. Reson. Med. 2008, 59, 590–597. [Google Scholar] [CrossRef]
- Zhang, B.; Sodickson, D.K.; Cloos, M.A. A high-impedance detector-array glove for magnetic resonance imaging of the hand. Nat. Biomed. Eng. 2018, 2, 570–577. [Google Scholar] [CrossRef]
- Abel, F.; Tan, E.T.; Lunenburg, M.; van Leeuwen, C.; van Hooren, T.; van Uden, M.; Arteaga, C.; Vincent, J.; Robb, F.; Sneag, D. Flexible array coil for cervical and extraspinal (FACE) MRI at 3.0 Tesla. Phys. Med. Biol. 2023, 68, 215011. [Google Scholar] [CrossRef]
- Ruytenberg, T.; Webb, A.; Zivkovic, I. Shielded-coaxial-cable coils as receive and transceive array elements for 7T human MRI. Magn. Reson. Med. 2020, 83, 1135–1146. [Google Scholar] [CrossRef]
- Ruytenberg, T.; Webb, A.; Zivkovic, I. A flexible five-channel shielded-coaxial-cable (SCC) transceive neck coil for high-resolution carotid imaging at 7T. Magn. Reson. Med. 2020, 84, 1672–1677. [Google Scholar] [CrossRef]
- Wang, B.; Siddiq, S.S.; Walczyk, J.; Bruno, M.; Khodarahmi, I.; Brinkmann, I.M.; Rehner, R.; Lakshmanan, K.; Fritz, J.; Brown, R. A flexible MRI coil based on a cable conductor and applied to knee imaging. Sci. Rep. 2022, 12, 15010. [Google Scholar] [CrossRef]
- Nohava, L.; Czerny, R.; Roat, S.; Obermann, M.; Kuehne, A.; Frass-Kriegl, R.; Felblinger, J.; Ginefri, J.-C.; Laistler, E. Flexible multi-turn multi-gap coaxial RF coils: Design concept and implementation for Magnetic Resonance Imaging at 3 and 7 Tesla. IEEE Trans. Med. Imaging 2021, 40, 1267–1278. [Google Scholar] [CrossRef]
- Nohava, L.; Obermann, M.; Frass-Kriegl, R.; Soanca, O.; Laistler, E. A modular system of flexible receive-only coil arrays for 3 T Magnetic Resonance Imaging. Z. Für Med. Phys. 2023; in press. [Google Scholar]
- Stickle, Y.-J.; Follante, C.; Giancola, M.; Anderson, D.; Robb, F.; Taracila, V.; Stormont, R.; Blahnik, H.; Winkler, S.; Sneag, D. A Novel Ultra-Flexible High-Resolution AIR (Adaptive imaging receive) 64-Channel Bilateral Phased Array for 3T Brachial Plexus MRI. In Proceedings of the ISMRM & SMRT Virtual Conference, Virtual, 8–14 August 2020. [Google Scholar]
- Cogswell, P.M.; Trzasko, J.D.; Gray, E.M.; Campeau, N.G.; Rossman, P.J.; Kang, D.; Robb, F.; Stormont, R.S.; Lindsay, S.A.; Bernstein, M.A.; et al. Application of adaptive image receive coil technology for whole-brain imaging. AJR Am. J. Roentgenol. 2021, 216, 552. [Google Scholar] [CrossRef]
- Nordmeyer-Massner, J.A.; De Zanche, N.; Pruessmann, K.P. Stretchable coil arrays: Application to knee imaging under varying flexion angles. Magn. Reson. Med. 2012, 67, 872–879. [Google Scholar] [CrossRef]
- Vincent, J.M.; Rispoli, J.V. Conductive thread-based stretchable and flexible radiofrequency coils for magnetic resonance imaging. IEEE Trans. Biomed. Eng. 2019, 67, 2187–2193. [Google Scholar] [CrossRef]
- Malko, J.A.; McClees, E.C.; Braun, I.F.; Davis, P.C.; Hoffman, J.C. A flexible mercury-filled surface coil for MR imaging. AJNR Am. J. Neuroradiol. 1986, 7, 246–247. [Google Scholar]
- Varga, M.; Mehmann, A.; Marjanovic, J.; Reber, J.; Vogt, C.; Pruessmann, K.P.; Tröster, G. Adsorbed eutectic GaIn structures on a neoprene foam for stretchable MRI coils. Adv. Mater. 2017, 29, 1703744. [Google Scholar] [CrossRef]
- Mehmann, A.; Varga, M.; Vogt, C.; Port, A.; Reber, J.; Marjanovic, J.; Pruessmann, K.P.; Troester, G. On the bending and stretching of liquid metal receive coils for magnetic resonance imaging. IEEE Trans. Biomed. Eng. 2018, 66, 1542–1548. [Google Scholar] [CrossRef]
- Mehmann, A.; Vogt, C.; Varga, M.; Port, A.; Reber, J.; Marjanovic, J.; Pruessmann, K.P.; Sporrer, B.; Huang, Q.; Troster, G. Automatic resonance frequency retuning of stretchable liquid metal receive coil for magnetic resonance imaging. IEEE Trans. Med. Imaging 2018, 38, 1420–1426. [Google Scholar] [CrossRef]
- Port, A.; Luechinger, R.; Albisetti, L.; Varga, M.; Marjanovic, J.; Reber, J.; Brunner, D.O.; Pruessmann, K.P. Detector clothes for MRI: A wearable array receiver based on liquid metal in elastic tubes. Sci. Rep. 2020, 10, 8844. [Google Scholar] [CrossRef]
- Port, A.; Luechinger, R.; Brunner, D.O.; Pruessmann, K.P. Elastomer coils for wearable MR detection. Magn. Reson. Med. 2021, 85, 2882–2891. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and Soft Electronics using Liquid Metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef]
- Hallfors, N.; Khan, A.; Dickey, M.D.; Taylor, A.M. Integration of pre-aligned liquid metal electrodes for neural stimulation within a user-friendly microfluidic platform. Lab Chip 2013, 13, 522–526. [Google Scholar] [CrossRef]
- Barta, R.; Volotovskyy, V.; Wachowicz, K.; Fallone, B.G.; De Zanche, N. How thin can you go? Performance of thin copper and aluminum RF coil conductors. Magn. Reson. Med. 2021, 85, 2327–2333. [Google Scholar] [CrossRef]
- Lin, W.; Wang, S. Elastic Radio Frequency Coil. Google Patents US2019219648A1, 18 July 2019. [Google Scholar]
- Motovilova, E.; Tan, E.T.; Taracila, V.; Vincent, J.M.; Grafendorfer, T.; Shin, J.; Potter, H.G.; Robb, F.J.L.; Sneag, D.B.; Winkler, S.A. Stretchable self-tuning MRI receive coils based on liquid metal technology (LiquiTune). Sci. Rep. 2021, 11, 16228. [Google Scholar] [CrossRef]
- Motovilova, E.; Winkler, S.A. Self-Tuning Liquid Metal RF Coil and Coil Array for MRI. U.S. Patent App. Provisional Patent Application 63/181,664, 29 April 2021. [Google Scholar]
- Larkman, D.J.; Nunes, R.G. Parallel magnetic resonance imaging. Phys. Med. Biol. 2007, 52, R15. [Google Scholar] [CrossRef]
- Motovilova, E.; Ching, T.; Vincent, J.; Shin, J.; Tan, E.T.; Taracila, V.; Robb, F.; Hashimoto, M.; Sneag, D.B.; Winkler, S.A. Dual-Channel Stretchable, Self-Tuning, Liquid Metal Coils and Their Fabrication Techniques. Sensors 2023, 23, 7588. [Google Scholar] [CrossRef]
- Motovilova, E.; Ching, T.; Vincent, J.; Shin, J.; Tan, E.T.; Taracila, V.; Winkler, S.A. Fabrication Methods for Stretchable, Self-tuning Multi-element Liquid Metal Coil Arrays (LiquiTune). In Proceedings of the Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting, London, UK, 7–12 May 2022. [Google Scholar]
- Motovilova, E.; Vincent, J.; Taracila, V.; Tan, E.T.; Robb, F.; Sneag, D.; Winkler, S.A. Bi-Directional Stretchable Capacitors for Self-Tuning MRI Receive Coils. In Proceedings of the 2023 IEEE International Symposium on Antennas and Propagation and USNC-URSI Radio Science Meeting (USNC-URSI), Portland, OR, USA, 23–28 July 2023; pp. 775–776. [Google Scholar]
- Chen, J.; Shih, B.; Adibnazari, I.; Park, Y.-L.; Tolley, M. Open-loop printing of liquid metal for the low-cost rapid fabrication of soft sensors. In Proceedings of the 2022 IEEE 5th International Conference on Soft Robotics (RoboSoft), Edinburgh, UK, 4–8 April 2022; pp. 889–895. [Google Scholar]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Mengüç, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D printing of strain sensors within highly stretchable elastomers. Adv. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef]
- Oh, J.; Bae, J. A direct ink writing based fabric-embedded soft sensor for improved durability and sewability. Smart Mater. Struct. 2022, 31, 065020. [Google Scholar] [CrossRef]
- Handschuh-Wang, S.; Gan, T.; Wang, T.; Stadler, F.J.; Zhou, X. Surface tension of the oxide skin of gallium-based liquid metals. Langmuir 2021, 37, 9017–9025. [Google Scholar] [CrossRef]
- Dickey, M.D.; Chiechi, R.C.; Larsen, R.J.; Weiss, E.A.; Weitz, D.A.; Whitesides, G.M. Eutectic gallium-indium (EGaIn): A liquid metal alloy for the formation of stable structures in microchannels at room temperature. Adv. Funct. Mater. 2008, 18, 1097–1104. [Google Scholar] [CrossRef]
- Boley, J.W.; White, E.L.; Chiu, G.T.-C.; Kramer, R.K. Direct writing of gallium-indium alloy for stretchable electronics. Adv. Funct. Mater. 2014, 24, 3501–3507. [Google Scholar] [CrossRef]
- Dobosz, A.; Novakovic, R.; Gancarz, T. Liquid metals: Thermophysical properties of alloys from the Ga-Sn-Zn system. J. Mol. Liq. 2021, 343, 117646. [Google Scholar] [CrossRef]
- Hoult, D.I.; Richards, R. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J. Magn. Reson. (1969) 1976, 24, 71–85. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Wang, H.; Li, L.; Liu, X.; Wang, W.; Hu, L.; Fan, Y. Gallium-Based Liquid Metal Composites and the Continuous Forming Methods. Adv. Eng. Mater. 2024, 26, 2302029. [Google Scholar] [CrossRef]
- Dobosz, A.; Plevachuk, Y.; Sklyarchuk, V.; Sokoliuk, B.; Gancarz, T. Thermophysical properties of the liquid Ga–Sn–Zn eutectic alloy. Fluid Phase Equilibria 2018, 465, 1–9. [Google Scholar] [CrossRef]
- Reis Carneiro, M.; Majidi, C.; Tavakoli, M. Gallium-Based Liquid–Solid Biphasic Conductors for Soft Electronics. Adv. Funct. Mater. 2023, 33, 2306453. [Google Scholar] [CrossRef]
- Vaidya, M.V.; Collins, C.M.; Sodickson, D.K.; Brown, R.; Wiggins, G.C.; Lattanzi, R. Dependence of and field patterns of surface coils on the electrical properties of the sample and the mr operating frequency. Concepts Magn. Reson. Part B Magn. Reson. Eng. 2016, 46, 25–40. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motovilova, E.; Ching, T.; Vincent, J.; Tan, E.T.; Taracila, V.; Robb, F.; Hashimoto, M.; Sneag, D.B.; Winkler, S.A. Design and Dynamic In Vivo Validation of a Multi-Channel Stretchable Liquid Metal Coil Array. Materials 2024, 17, 3325. https://doi.org/10.3390/ma17133325
Motovilova E, Ching T, Vincent J, Tan ET, Taracila V, Robb F, Hashimoto M, Sneag DB, Winkler SA. Design and Dynamic In Vivo Validation of a Multi-Channel Stretchable Liquid Metal Coil Array. Materials. 2024; 17(13):3325. https://doi.org/10.3390/ma17133325
Chicago/Turabian StyleMotovilova, Elizaveta, Terry Ching, Jana Vincent, Ek Tsoon Tan, Victor Taracila, Fraser Robb, Michinao Hashimoto, Darryl B. Sneag, and Simone Angela Winkler. 2024. "Design and Dynamic In Vivo Validation of a Multi-Channel Stretchable Liquid Metal Coil Array" Materials 17, no. 13: 3325. https://doi.org/10.3390/ma17133325





