Doping Engineering in Manganese Oxides for Aqueous Zinc-Ion Batteries
Abstract
:1. Introduction
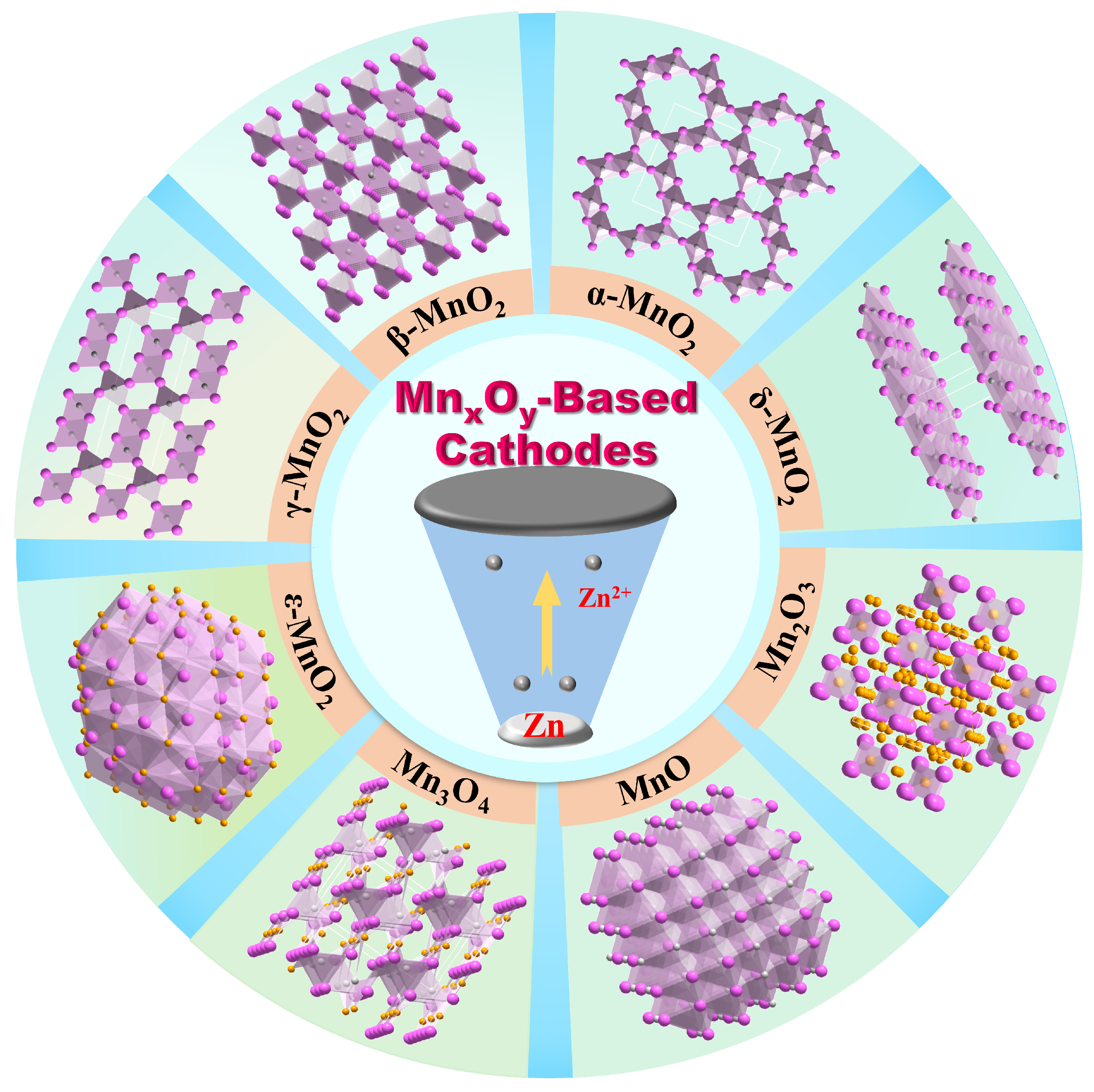
2. Classification of MnxOy-Based Cathodes
2.1. α-MnO2
2.2. β-MnO2
2.3. γ-MnO2
2.4. δ-MnO2
2.5. ε-MnO2
2.6. MnO
2.7. Mn2O3
2.8. Mn3O4
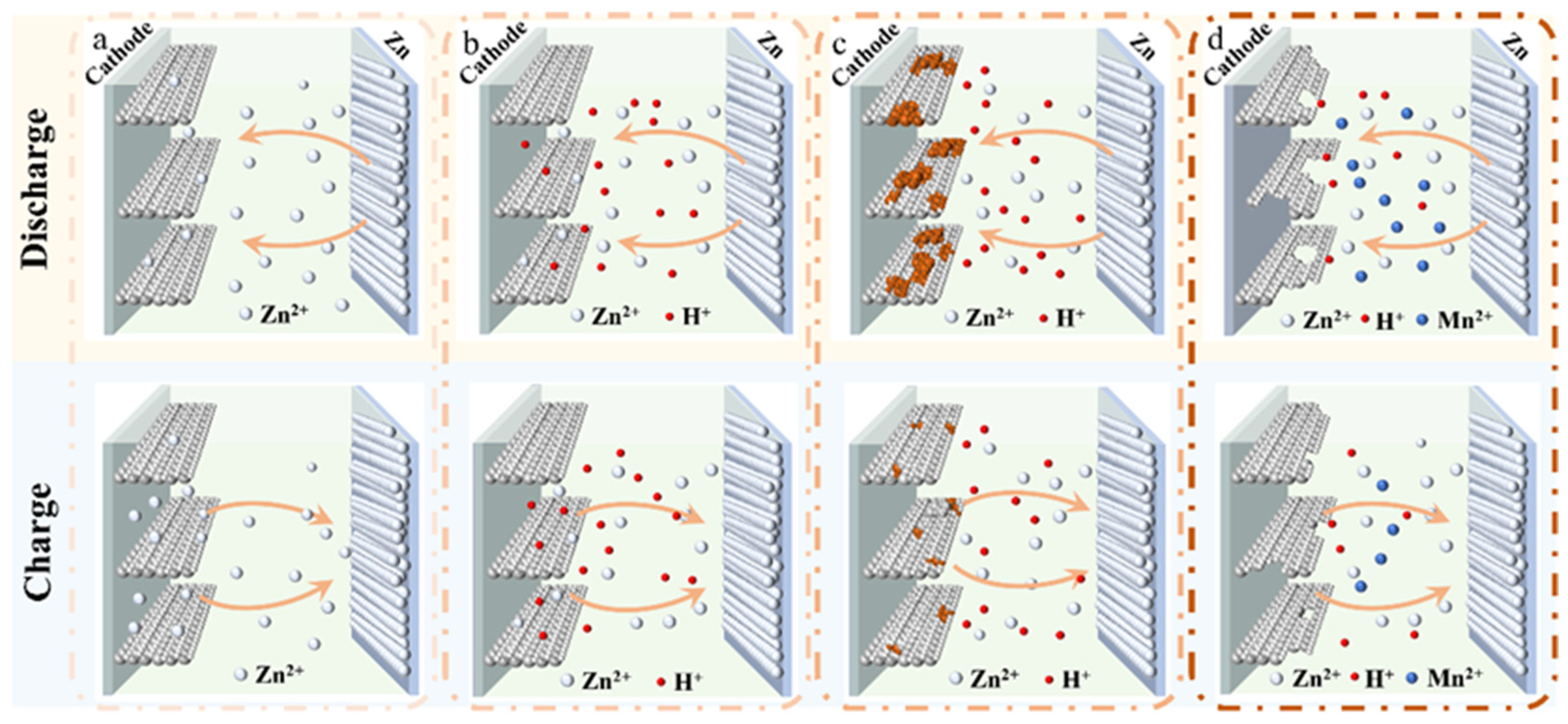
3. Energy Storage Mechanisms of MnxOy-Based AZIBs
3.1. Zn2+ Insertion Mechanism
3.2. Zn2+/H+ Co-Insertion Mechanism
3.3. Conversion Reaction Mechanism
3.4. Dissolution/Deposition Mechanism
4. Doping Engineering of MnxOy-Based Cathodes
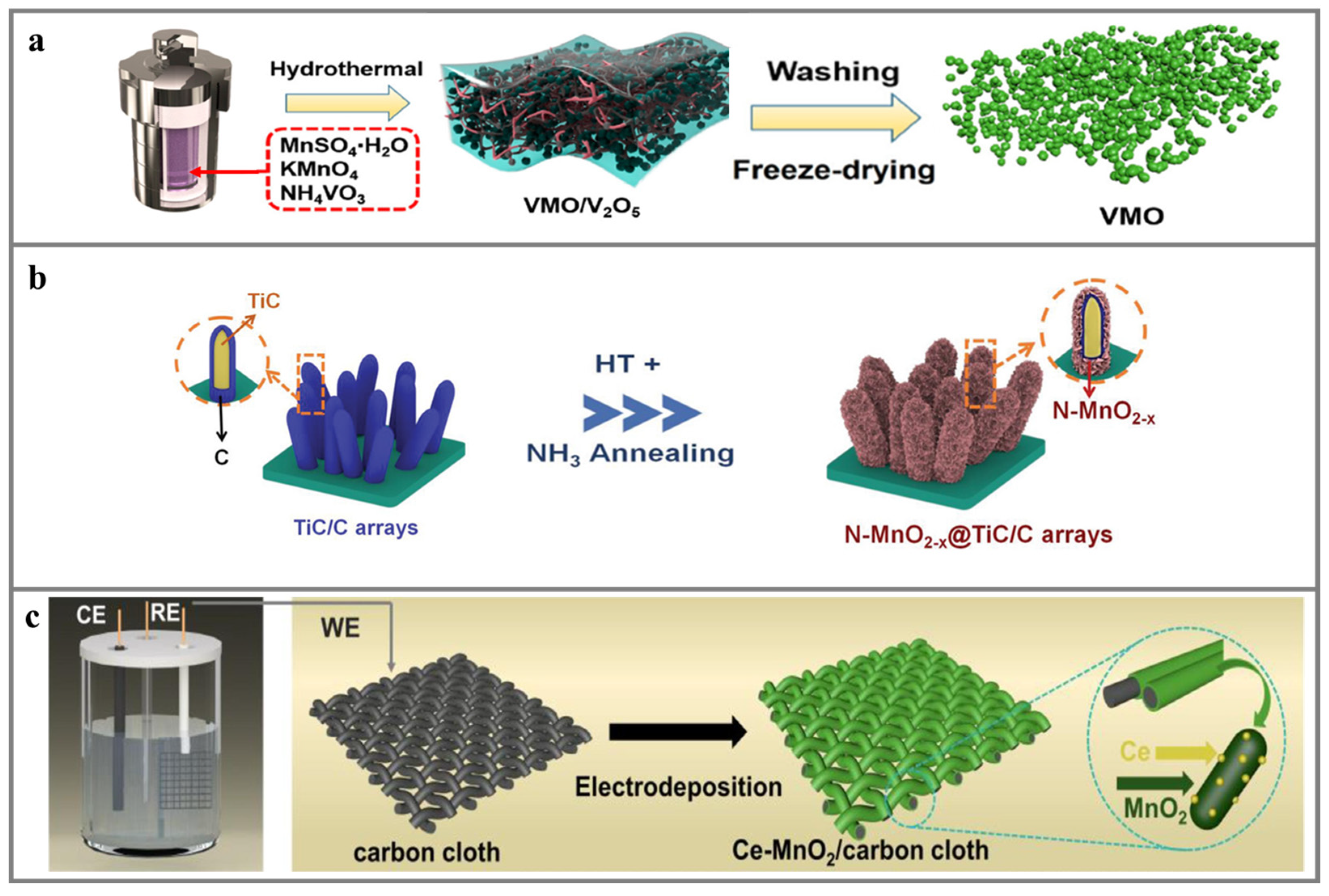
4.1. Synthesis Route of Heteroatom-Doped MnxOy-Based Cathodes
4.1.1. Hydrothermal Method
4.1.2. Co-Precipitation Method
4.1.3. Ball Milling
4.1.4. Calcination Treatment
4.1.5. Sol–Gel Process
4.1.6. Other Methods
4.2. The Role of Doping Engineering
4.2.1. Enhancing Intrinsic Electron/Ion Conductivity
4.2.2. Increasing Electrochemical Active Sites
4.2.3. Promoting Diffusion Kinetics
4.2.4. Maintaining Structural Stability
5. Doped MnxOy-Based Cathodes for AZIBs
5.1. MnO2
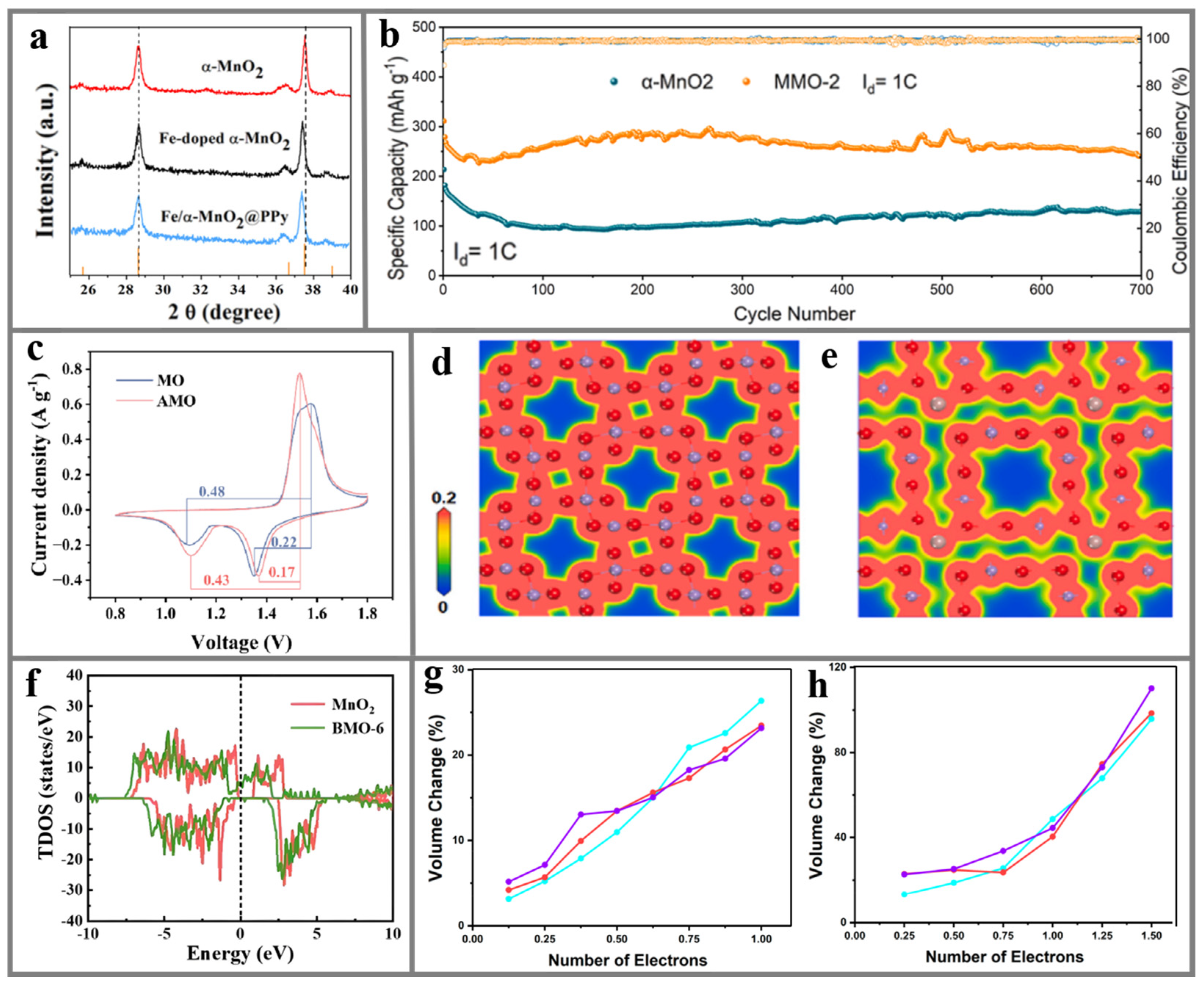
5.1.1. α-MnO2
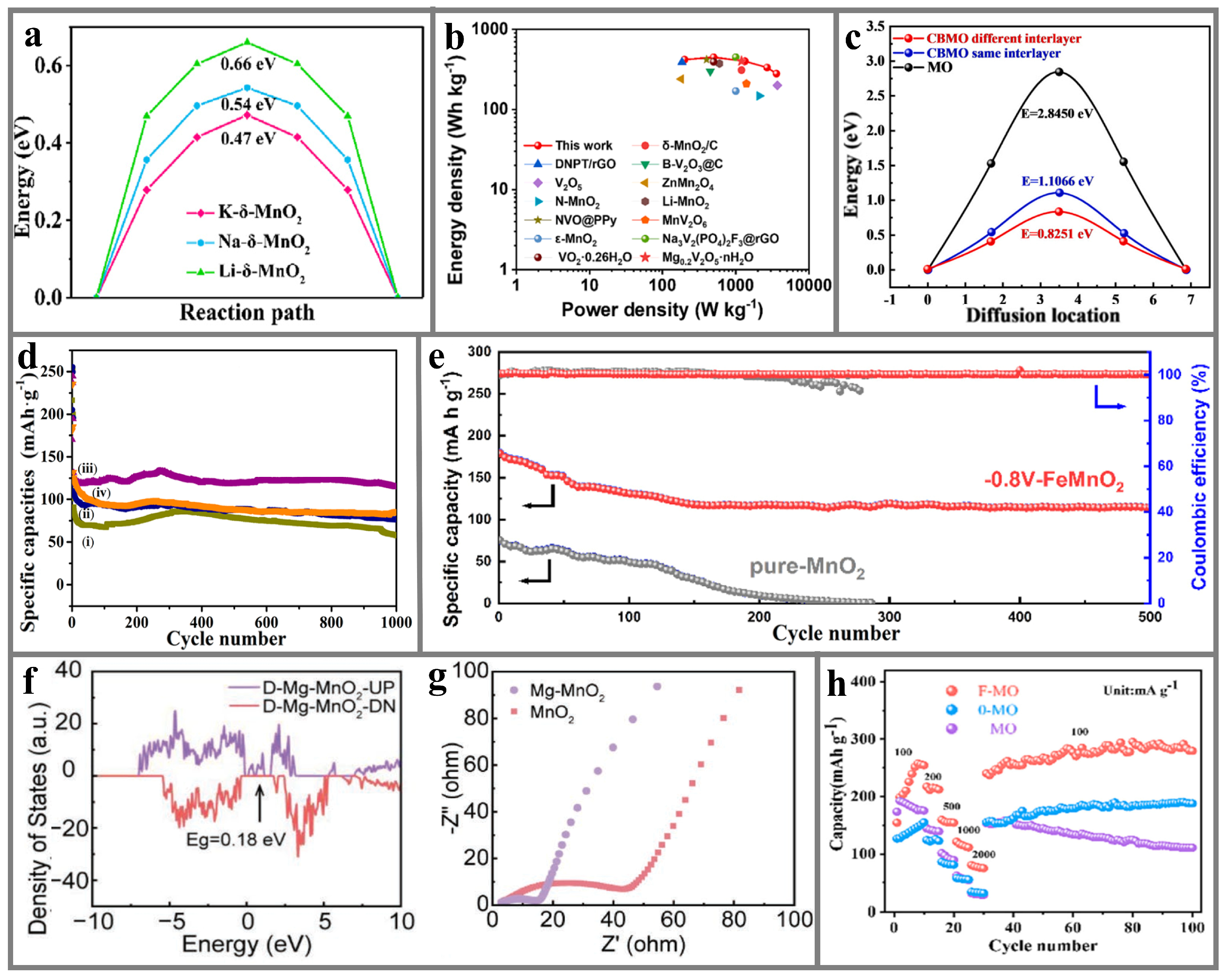
5.1.2. δ-MnO2
5.1.3. β-MnO2, ε-MnO2, and γ-MnO2
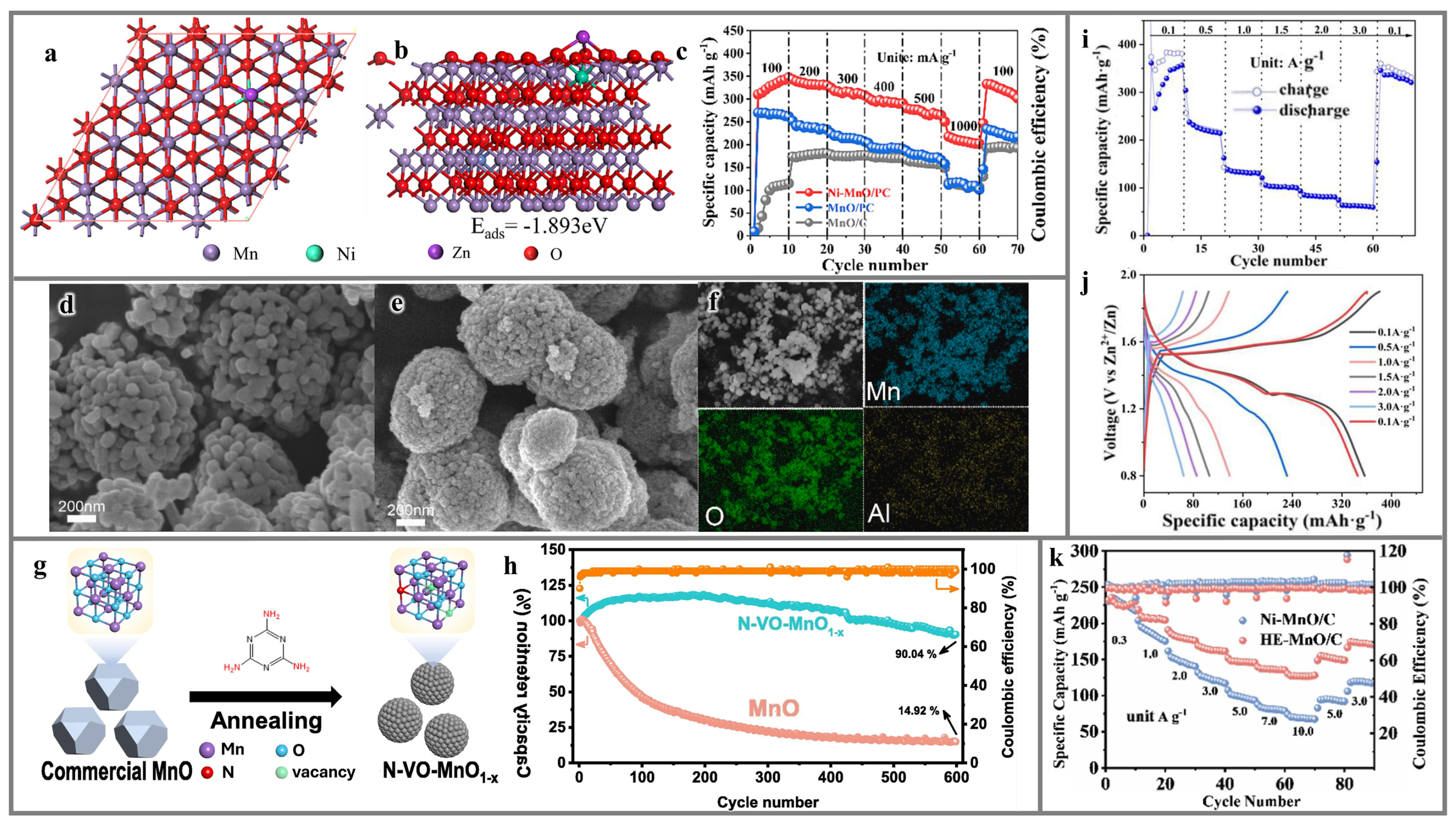
5.2. MnO
5.3. Mn2O3
5.4. Mn3O4
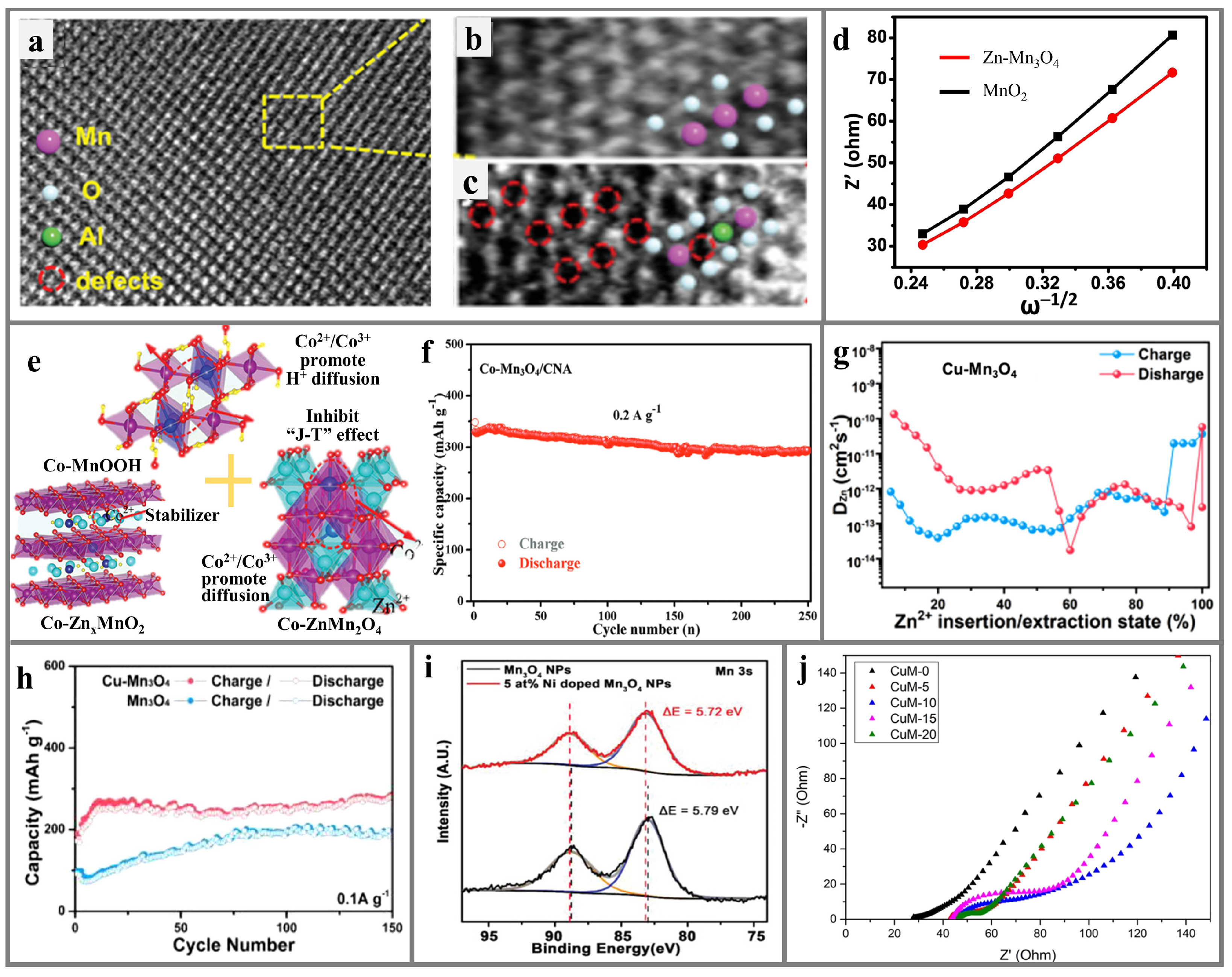
6. Conclusions and Perspective
6.1. Study of Energy Storage Mechanisms
6.2. Construction of Nanostructured MnxOy
6.3. Optimization of Doping Engineering of MnxOy
6.4. Practical Challenges and Limitations of Doping MnxOy for AZIBs
6.5. Application of Doped MnxOy-Based ZIBs in Flexible Storage Field
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasapis, D.; Savvakis, N.; Tsoutsos, T.; Kalaitzakis, K.; Psychis, S.; Nikolaidis, N.P. Design of large scale prosuming in Universities: The solar energy vision of the TUC campus. Energy Build. 2017, 141, 39–55. [Google Scholar] [CrossRef]
- Li, S.; Tian, Q.; Chen, J.; Chen, Y.; Guo, P.; Wei, C.; Cui, P.; Jiang, J.; Li, X.; Xu, Q. An intrinsically non-flammable organic electrolyte for wide temperature range supercapacitors. Chem. Eng. J. 2023, 457, 141265. [Google Scholar] [CrossRef]
- Li, A.; Wei, Z.; Wang, Y.; Zhang, Y.; Wang, M.; Zhang, H.; Ma, Y.; Liu, C.; Zou, J.; Ge, B. Flexible quasi-3D zinc ion microcapacitor based on V2O5-PANI cathode and MXene anode. Chem. Eng. J. 2023, 457, 141339. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, C.; Gao, L.; Lin, M.-F.; Eh, A.L.-S.; Chen, J.; Li, S. Recent advances in electrospun nanostructured electrodes in zinc-ion batteries. Batteries 2024, 10, 22. [Google Scholar] [CrossRef]
- Barzin, R.; Chen, J.J.; Young, B.R.; Farid, M.M. Peak load shifting with energy storage and price-based control system. Energy 2015, 92, 505–514. [Google Scholar] [CrossRef]
- Li, X.; Ma, Y.; Yue, Y.; Li, G.; Zhang, C.; Cao, M.; Xiong, Y.; Zou, J.; Zhou, Y.; Gao, Y. A flexible Zn-ion hybrid micro-supercapacitor based on MXene anode and V2O5 cathode with high capacitance. Chem. Eng. J. 2022, 428, 130965. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Chen, J.; Gao, L.; Guo, P.; Wei, C.; Fu, J.; Xu, Q. Sulfur-bridged bonds enabled structure modulation and space confinement of MnS for superior sodium-ion capacitors. J. Colloid Interface Sci. 2024, 664, 360–370. [Google Scholar] [CrossRef]
- Gao, L.; Ma, Y.; Cao, M. Self-supported Se-doped Na2Ti3O7 arrays for high performance sodium ion batteries. Int. J. Hydrogen Energy 2024, 49, 1–10. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, A.; Wang, D.; Velasco, L.; Azmi, R.; Bhattacharya, S.S.; Bergfeldt, T.; Düvel, A.; Heitjans, P.; Brezesinski, T. Multi-anionic and-cationic compounds: New high entropy materials for advanced Li-ion batteries. Energy Environ. Sci. 2019, 12, 2433–2442. [Google Scholar] [CrossRef]
- Zhang, M.; Song, X.; Ou, X.; Tang, Y. Rechargeable batteries based on anion intercalation graphite cathodes. Energy Storage Mater. 2019, 16, 65–84. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Sun, Z.; Shi, Y.; Cheng, H.-M.; Li, F. A highly reversible Co3S4 microsphere cathode material for aluminum-ion batteries. Nano Energy 2019, 56, 100–108. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-ion battery supply chain considerations: Analysis of potential bottlenecks in critical metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Alias, N.; Mohamad, A.A. Advances of aqueous rechargeable lithium-ion battery: A review. J. Power Sources 2015, 274, 237–251. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.-Y.; Kim, H.; Kim, S.-W.; Kang, K. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; McDonagh, A.; Qiao, S.-Z.; Wang, G. High-capacity aqueous potassium-ion batteries for large-scale energy storage. Adv. Mater. 2017, 29, 1604007. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Sun, W.; Fan, X.; Yang, C.; Wang, F.; Gao, T.; Ma, Z.; Schroeder, M.; von Cresce, A. Advanced high-voltage aqueous lithium-ion battery enabled by “water-in-bisalt” electrolyte. Angew. Chem. 2016, 128, 7252–7257. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.-G.; You, Z.; Wei, C.; Kang, F.; Wei, B. Rechargeable aqueous zinc-ion batteries: Mechanism, design strategies and future perspectives. Mater. Today 2021, 42, 73–98. [Google Scholar] [CrossRef]
- Kao-ian, W.; Mohamad, A.A.; Liu, W.R.; Pornprasertsuk, R.; Siwamogsatham, S.; Kheawhom, S. Stability enhancement of zinc-ion batteries using non-aqueous electrolytes. Batter. Supercaps 2022, 5, e202100361. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Jiang, Z.; Chen, J.; Wei, C.; Wu, W.; Li, S.; Xu, Q. Cation-anion redox active organic complex for high performance aqueous zinc ion battery. Energy Environ. Mater. 2024, 7, e12507. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Pan, W.; Wang, M.; Zhang, H.; Zhang, D.; Zhang, D. Application of expanded graphite-based materials for rechargeable batteries beyond lithium-ions. Nanoscale 2021, 13, 19291–19305. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Tahir, M.U.; Xiang, B. Conductive copper glue constructs a reversible and stable zinc metal anode interface for advanced aqueous zinc ion battery. J. Colloid Interface Sci. 2022, 608, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Fan, H.J. From aqueous Zn-ion battery to Zn-MnO2 flow battery: A brief story. J. Energy Chem. 2021, 54, 194–201. [Google Scholar] [CrossRef]
- Bai, M.-X.; Gao, J.-F.; He, Z.-H.; Hou, J.-F.; Kong, L.-B. Brookite phase vanadium dioxide (B) with nanosheet structure for superior rate capability aqueous Zn-ion batteries. J. Electroanal. Chem. 2022, 907, 116039. [Google Scholar] [CrossRef]
- Zampardi, G.; La Mantia, F. Prussian blue analogues as aqueous Zn-ion batteries electrodes: Current challenges and future perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, H.; Zhou, L.; Gao, X.; Lu, X. Structural regulation strategies towards high performance organic materials for next generation aqueous Zn-based batteries. ChemPhysMater 2022, 1, 86–101. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Xiong, T.; Wang, X.; Xue, J. Unraveling MoS2 and transition metal dichalcogenides as functional zinc-ion battery cathode: A perspective. Small Methods 2021, 5, 2000815. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Zhou, W.; Ye, C.; Zhang, Q.; Chen, Y.; Gu, L.; Davey, K.; Qiao, S.Z. An electrolytic Zn–MnO2 battery for high-voltage and scalable energy storage. Angew. Chem. 2019, 131, 7905–7910. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, J.; Zhang, X.; Insin, N.; Wang, S.; Han, J.; Zhao, Y.; Qin, J.; Huang, Y. Inhibition of manganese dissolution in Mn2O3 cathode with controllable Ni2+ incorporation for high-performance zinc ion battery. Adv. Funct. Mater. 2021, 31, 2009412. [Google Scholar] [CrossRef]
- Tan, Q.; Li, X.; Zhang, B.; Chen, X.; Tian, Y.; Wan, H.; Zhang, L.; Miao, L.; Wang, C.; Gan, Y. Valence engineering via in situ carbon reduction on octahedron sites Mn3O4 for ultra-long cycle life aqueous Zn-ion battery. Adv. Energy Mater. 2020, 10, 2001050. [Google Scholar] [CrossRef]
- Wang, D.; Lv, H.; Hussain, T.; Yang, Q.; Liang, G.; Zhao, Y.; Ma, L.; Li, Q.; Li, H.; Dong, B. A manganese hexacyanoferrate framework with enlarged ion tunnels and two-species redox reaction for aqueous Al-ion batteries. Nano Energy 2021, 84, 105945. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, W.; Cui, Y.; Chu, H.; Qi, S.; Wang, J.; Xin, B. Utilizing waste Zn-Mn batteries in combination with waste SCR catalyst to construct a magnetically recoverable and highly photocatalytic materials. Chem. Phys. Lett. 2022, 796, 139530. [Google Scholar] [CrossRef]
- Li, X.; Cheng, H.; Hu, H.; Pan, K.; Yuan, T.; Xia, W. Recent advances of vanadium-based cathode materials for zinc-ion batteries. Chin. Chem. Lett. 2021, 32, 3753–3761. [Google Scholar] [CrossRef]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active materials for aqueous zinc ion batteries: Synthesis, crystal structure, morphology, and electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- Lim, M.B.; Lambert, T.N.; Chalamala, B.R. Rechargeable alkaline zinc–manganese oxide batteries for grid storage: Mechanisms, challenges and developments. Mater. Sci. Eng. R Rep. 2021, 143, 100593. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Liang, J.; Xia, X.; Sun, X. Uncovering Sulfur Doping Effect in MnO2 Nanosheets as an Efficient Cathode for Aqueous Zinc Ion Battery. Energy Storage Mater. 2022, 47, 424–433. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Jo, J.; Kim, S.; Mathew, V.; Kim, J. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode. J. Power Sources 2015, 288, 320–327. [Google Scholar]
- Shi, W.; Lee, W.S.V.; Xue, J. Recent development of Mn-based oxides as zinc-ion battery cathode. ChemSusChem 2021, 14, 1634–1658. [Google Scholar] [CrossRef]
- Jia, S.; Li, L.; Shi, Y.; Wang, C.; Cao, M.; Ji, Y.; Zhang, D. Recent development of manganese dioxide-based materials as zinc-ion battery cathode. Nanoscale 2024, 16, 1539–1576. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, G.; Liu, J.; Zhang, J.; Wang, X.; Pu, X.; Wang, J.; Yan, C.; Cao, Y.; Yang, H. Recent advances on challenges and strategies of manganese dioxide cathodes for aqueous zinc-ion batteries. Energy Environ. Mater. 2023, 6, e12575. [Google Scholar]
- Luan, J.; Yuan, H.; Liu, J.; Zhong, C. Recent advances on charge storage mechanisms and optimization strategies of Mn-based cathode in zinc–manganese oxides batteries. Energy Storage Mater. 2024, 13, 103206. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Wang, Y.; Khasraw, A.K.; Zhao, Y.; Sun, X. Rational design of nanostructured MnO2 cathode for high-performance aqueous zinc ion batteries. Chem. Res. Chin. Univ. 2023, 39, 599–611. [Google Scholar] [CrossRef]
- Li, J.; Ruan, P.; Chen, X.; Lei, S.; Lu, B.; Chen, Z.; Zhou, J. Aqueous batteries for human body electronic devices: Focus Review. ACS Energy Lett. 2023, 8, 2904–2918. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.-C.; Guo, Y.-F.; Wang, P.-F.; Zhu, Y.-R.; Yi, T.-F. Insights on rational design and energy storage mechanism of Mn-based cathode materials towards high performance aqueous zinc-ion batteries. Coord. Chem. Rev. 2023, 479, 215009. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, T. Review on recent developments, challenges, and perspectives of Mn-based oxide cathode materials for aqueous zinc-ion batteries and the status of Mn resources in China. Energy Fuels 2023, 37, 4198–4221. [Google Scholar] [CrossRef]
- Wang, J.; Szabo, L.; Madhav, D.; Ferreira, I.; Vandeginste, V. Recent progress in interfacial engineering strategies for Mn-based oxide cathodes in aqueous zinc-ion batteries: Mechanisms, modifications, and performance enhancement. Energy Storage Mater. 2023, 1, 103015. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. InfoMat 2020, 2, 237–260. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, Y.; Lee, W.S.V.; Xue, J. Defect engineering in manganese-based oxides for aqueous rechargeable zinc-ion batteries: A review. Adv. Energy Mater. 2020, 10, 2001769. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Liu, X.; Lu, X. Flexible Zn-ion batteries based on manganese oxides: Progress and prospect. Carbon Energy 2020, 2, 387–407. [Google Scholar] [CrossRef]
- Heng, Y.; Gu, Z.; Guo, J.; Wu, X. Research progresses on vanadium-based cathode materials for aqueous zinc-ion batteries. Acta Phys. Chim. Sin. 2021, 37, 2005013. [Google Scholar] [CrossRef]
- Lin, M.; Shao, F.; Tang, Y.; Lin, H.; Xu, Y.; Jiao, Y.; Chen, J. Layered Co doped MnO2 with abundant oxygen defects to boost aqueous zinc-ion storage. J. Colloid Interface Sci. 2022, 611, 662–669. [Google Scholar] [CrossRef]
- Yu, B.; Lu, L.; He, Y.; Dai, X.; Wang, Y.; Wang, T.; Chong, S.; Liu, L.; Liu, Y.; Tan, Q. Hierarchical porous CS@ Ce-MnO2 as cathode for energy-dense and long-cycling flexible aqueous zinc-ion batteries. J. Colloid Interface Sci. 2024, 654, 56–65. [Google Scholar] [CrossRef]
- Ko, W.Y.; Lubis, A.L.; Wang, H.Y.; Wu, T.C.; Lin, S.T.; Lin, K.J. Facile construction of Zn-doped Mn3O4− MnO2 vertical nanosheets for aqueous zinc-ion battery cathodes. ChemElectroChem 2022, 9, e202200750. [Google Scholar] [CrossRef]
- He, B.; Huang, J.; Ji, P.; Hoang, T.K.; Han, M.; Li, L.; Zhang, L.; Gao, Z.; Ma, J.; Zhi, J. Al doped manganous oxide for high-performance aqueous Zn-ion batteries. J. Power Sources 2023, 554, 232353. [Google Scholar] [CrossRef]
- Kim, H.; Min, K.; Shim, S.E.; Lim, D.; Baeck, S.-H. Ni-doped Mn2O3 microspheres as highly efficient electrocatalyst for oxygen reduction reaction and Zn-air battery. Int. J. Hydrogen Energy 2022, 47, 2378–2388. [Google Scholar] [CrossRef]
- Chen, X.; Ruan, P.; Wu, X.; Liang, S.; Zhou, J. Crystal structures, reaction mechanisms, and optimization strategies of MnO2 cathode for aqueous rechargeable zinc batteries. Acta Phys. Chim. Sin. 2022, 38, 2111003. [Google Scholar]
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion chemistry: The rechargeable zinc ion battery. Angew. Chem. Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Gim, J.; Kim, S.; Song, J.; Baboo, J.P.; Choi, S.H.; Kim, J. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem. Mater. 2015, 27, 3609–3620. [Google Scholar] [CrossRef]
- Liang, W.; Che, Y.; Cai, Z.; Tang, R.; Ma, Z.; Zheng, X.; Wu, X.; Li, J.; Jin, H.; Zhu, C. Surface decoration manipulating Zn2+/H+ carrier ratios for hyperstable aqueous zinc ion battery cathode. Adv. Funct. Mater. 2024, 34, 2304798. [Google Scholar] [CrossRef]
- Li, C.; Yuan, H.; Liu, T.; Zhang, R.; Zhu, J.; Cui, H.; Wang, Y.; Cao, D.; Wang, D.; Zhi, C. Distinguish MnO2/Mn2+ conversion/Zn2+ intercalation/H+ conversion chemistries at different potentials in aqueous Zn||MnO2 batteries. Angew. Chem. 2024, 136, e202403504. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Jin, Y.; Zou, L.; Liu, L.; Engelhard, M.H.; Patel, R.L.; Nie, Z.; Han, K.S.; Shao, Y.; Wang, C.; Zhu, J. Joint charge storage for high-rate aqueous zinc–manganese dioxide batteries. Adv. Mater. 2019, 31, 1900567. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Salvador, J.R.; Wu, J.; Liu, B.; Yang, W.; Yang, J.; Zhang, W.; Liu, J.; Yang, J. Reaction mechanisms for long-life rechargeable Zn/MnO2 batteries. Chem. Mater. 2019, 31, 2036–2047. [Google Scholar] [CrossRef]
- Gao, X.; Wu, H.; Li, W.; Tian, Y.; Zhang, Y.; Wu, H.; Yang, L.; Zou, G.; Hou, H.; Ji, X. H+-insertion boosted α-MnO2 for an aqueous Zn-ion battery. Small 2020, 16, 1905842. [Google Scholar] [CrossRef]
- Fang, G.; Zhu, C.; Chen, M.; Zhou, J.; Tang, B.; Cao, X.; Zheng, X.; Pan, A.; Liang, S. Suppressing manganese dissolution in potassium manganate with rich oxygen defects engaged high-energy-density and durable aqueous zinc-ion battery. Adv. Funct. Mater. 2019, 29, 1808375. [Google Scholar] [CrossRef]
- Xie, S.; Li, X.; Li, Y.; Liang, Q.; Dong, L. Material design and energy storage mechanism of Mn-based cathodes for aqueous zinc-ion batteries. Chem. Rec. 2022, 22, e202200201. [Google Scholar] [CrossRef]
- Hao, J.; Yuan, L.; Johannessen, B.; Zhu, Y.; Jiao, Y.; Ye, C.; Xie, F.; Qiao, S.Z. Studying the conversion mechanism to broaden cathode options in aqueous zinc-ion batteries. Angew. Chem. 2021, 133, 25318–25325. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Huang, Y.; Jiang, B.; Chang, Z.; Xu, C.; Kang, F. β-MnO2 with proton conversion mechanism in rechargeable zinc ion battery. J. Energy Chem. 2021, 56, 365–373. [Google Scholar] [CrossRef]
- Kang, J.; Zhao, Z.; Li, H.; Meng, Y.; Hu, B.; Lu, H. An overview of aqueous zinc-ion batteries based on conversion-type cathodes. Energy Mater. 2022, 2, 200009. [Google Scholar] [CrossRef]
- Lee, B.; Seo, H.R.; Lee, H.R.; Yoon, C.S.; Kim, J.H.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Critical role of pH evolution of electrolyte in the reaction mechanism for rechargeable zinc batteries. ChemSusChem 2016, 9, 2948–2956. [Google Scholar] [CrossRef]
- Yang, J.; Cao, J.; Peng, Y.; Yang, W.; Barg, S.; Liu, Z.; Kinloch, I.A.; Bissett, M.A.; Dryfe, R.A. Unravelling the mechanism of rechargeable aqueous Zn–MnO2 batteries: Implementation of charging process by electrodeposition of MnO2. ChemSusChem 2020, 13, 4103–4110. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Fu, J.; Deng, Y.-P.; Pei, Y.; Zhang, M.; Yu, A.; Chen, Z. Parasitic electrodeposition in Zn-MnO2 batteries and its suppression for prolonged cyclability. Energy Storage Mater. 2021, 36, 478–484. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Liang, S.; Lu, B.; Zhou, J. pH-buffer contained electrolyte for self-adjusted cathode-free Zn–MnO2 batteries with coexistence of dual mechanisms. Small Struct. 2021, 2, 2100119. [Google Scholar] [CrossRef]
- Moon, H.; Ha, K.H.; Park, Y.; Lee, J.; Kwon, M.S.; Lim, J.; Lee, M.H.; Kim, D.H.; Choi, J.H.; Choi, J.H. Direct proof of the reversible dissolution/deposition of Mn2+/Mn4+ for mild-acid Zn-MnO2 batteries with porous carbon interlayers. Adv. Sci. 2021, 8, 2003714. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, P.; Tervoort, E.; Bouzid, A.; Pasquarello, A.; Kundu, D. Oxide versus nonoxide cathode materials for aqueous Zn batteries: An insight into the charge storage mechanism and consequences thereof. ACS Appl. Mater. Interfaces 2018, 11, 674–682. [Google Scholar] [CrossRef]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Islam, S.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.-k.; Kim, J. The dominant role of Mn2+ additive on the electrochemical reaction in ZnMn2O4 cathode for aqueous zinc-ion batteries. Energy Storage Mater. 2020, 28, 407–417. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, J.; Bai, C.; Li, X.; Fang, G.; Liang, S. Zn/MnO2 battery chemistry with dissolution-deposition mechanism. Mater. Today Energy 2020, 16, 100396. [Google Scholar] [CrossRef]
- Ni, Z.; Liang, X.; Zhao, L.; Zhao, H.; Ge, B.; Li, W. Tin doping manganese dioxide cathode materials with the improved stability for aqueous zinc-ion batteries. Mater. Chem. Phys. 2022, 287, 126238. [Google Scholar] [CrossRef]
- Chen, C.; Shi, M.; Zhao, Y.; Yang, C.; Zhao, L.; Yan, C. Al-Intercalated MnO2 cathode with reversible phase transition for aqueous Zn-Ion batteries. Chem. Eng. J. 2021, 422, 130375. [Google Scholar] [CrossRef]
- Ren, L.; Yu, G.; Xu, H.; Wang, W.; Jiang, Y.; Ji, M.; Li, S. Doping-induced static activation of MnO2 cathodes for aqueous Zn-ion batteries. ACS Sustain. Chem. Eng. 2021, 9, 12223–12232. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Duan, H.; Xie, S.; Dai, R.; Rong, J.; Kang, F.; Dong, L. Aerogel-structured MnO2 cathode assembled by defect-rich ultrathin nanosheets for zinc-ion batteries. Chem. Eng. J. 2022, 441, 136008. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L.; Luo, M.; Cui, Y.; Wang, J.; Zhao, D.; Yang, C.; Wang, X.; Cao, B. Synergistic combination of a Co-doped σ-MnO2 cathode with an electrolyte additive for a high-performance aqueous zinc-ion battery. ChemPhysMater 2023, 2, 77–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, S.; Luo, M.; Pan, G.; Zeng, Y.; Lu, X.; Ai, C.; Liu, Q.; Xiong, Q.; Wang, X. Defect promoted capacity and durability of N-MnO2–x branch arrays via low-temperature NH3 treatment for advanced aqueous zinc ion batteries. Small 2019, 15, 1905452. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, J.; Qiao, R.; Dai, X.; Jing, W.; Song, J.; Chen, Y.; Guo, S.; Sun, J.; Tan, Q.; et al. Binder-free flexible zinc-ion batteries: One-step potentiostatic electrodeposition strategy derived Ce doped-MnO2 cathode. Chem. Eng. J. 2022, 431, 133387. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, W.; Luo, J.; Zhang, H.; Liu, J.; Liu, X.; Yang, Y.; Lu, X. A high-energy-density aqueous zinc–manganese battery with a La–Ca co-doped ε-MnO2 cathode. J. Mater. Chem. A 2020, 8, 11642–11648. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Zhao, B.; Zhang, X.; Wang, X.; Wen, Z.; Ji, S.; Sun, J. Joint influence of nitrogen doping and oxygen vacancy on manganese dioxide as a high-capacity cathode for zinc-ion batteries. J. Phys. Chem. C 2021, 125, 20195–20203. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, S.; Li, Y.; Yang, S.; Xiang, Y.; Jiang, J.; Liu, Z.; Fan, D.; Zhang, H.; Zhu, L. Na-containing manganese-based cathode materials synthesized by sol-gel method for zinc-based rechargeable aqueous battery. J. Alloys Compd. Interdiscip. J. Mater. Sci. Solid-State Chem. Phys. 2021, 858, 157744. [Google Scholar] [CrossRef]
- Jiao, Y.; Kang, L.; Berry-Gair, J.; McColl, K.; Li, J.; Dong, H.; Jiang, H.; Wang, R.; Corà, F.; Brett, D.J. Enabling stable MnO2 matrix for aqueous zinc-ion battery cathodes. J. Mater. Chem. A 2020, 8, 22075–22082. [Google Scholar] [CrossRef]
- Xiong, T.; Yu, Z.G.; Wu, H.; Du, Y.; Xie, Q.; Chen, J.; Zhang, Y.W.; Pennycook, S.J.; Lee, W.S.V.; Xue, J. Defect engineering of oxygen-deficient manganese oxide to achieve high-performing aqueous zinc ion battery. Adv. Energy Mater. 2019, 9, 1803815. [Google Scholar] [CrossRef]
- Du, M.; Miao, Z.; Li, H.; Sang, Y.; Liu, H.; Wang, S. Strategies of structural and defect engineering for high-performance rechargeable aqueous zinc-ion batteries. J. Mater. Chem. A 2021, 9, 19245–19281. [Google Scholar] [CrossRef]
- Jia, H.; Li, Y.; Fu, L.; Ali, U.; Liu, B.; Zhang, L.; Wang, H.; Li, L.; Wang, H.G.; Wang, C. Ion pre-embedding engineering of δ-MnO2 for chemically self-charging aqueous zinc ions batteries. Small 2023, 19, 2303593. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, B.; Sun, B.; Jia, D.; Gao, Z.; Gao, S. A mini review: Applications of pre-embedding active ion strategies in electrochemical energy storage systems. J. Electroanal. Chem. 2024, 3, 118076. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Cheng, Q.; Liao, F.; Yang, G.; Lu, X.; Chen, L. Interlayer engineering of preintercalated layered oxides as cathode for emerging multivalent metal-ion batteries: Zinc and beyond. Energy Storage Mater. 2021, 38, 397–437. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Li, J.; Yang, Z.; Huang, C.; Yu, H.; Zhang, L.; Shu, J. Pre-intercalation chemistry of electrode materials in aqueous energy storage systems. Coord. Chem. Rev. 2022, 460, 214477. [Google Scholar] [CrossRef]
- Li, G.; Sun, L.; Zhang, S.; Zhang, C.; Jin, H.; Davey, K.; Liang, G.; Liu, S.; Mao, J.; Guo, Z. Developing cathode materials for aqueous zinc ion batteries: Challenges and practical prospects. Adv. Funct. Mater. 2024, 34, 2301291. [Google Scholar] [CrossRef]
- Shi, X.; Liu, X.; Wang, E.; Cao, X.; Yu, Y.; Cheng, X.; Lu, X. Boosting the Zn ion storage ability of amorphous MnO2 via surface engineering and valence modulation. Carbon Neutralization 2023, 2, 28–36. [Google Scholar] [CrossRef]
- Panda, M.R.; El Meragawi, S.; Mirshekarloo, M.S.; Chen, W.; Shaibani, M.; Majumder, M. Acidity-aided surface modification strategy to enhance in situ MnO2 deposition for high performance Zn-MnO2 battery prototypes. Small 2024, 4, 2311933. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Pan, C.; Yan, H.; Zhu, Y.; Pan, Y.; Yin, C. Polyaniline-functionalized graphene composite cathode with enhanced Zn2+ storage performance for aqueous zinc-ion battery. Chem. Eng. J. 2022, 440, 135930. [Google Scholar] [CrossRef]
- Yang, Q.; Li, X.; Chen, Z.; Huang, Z.; Zhi, C. Cathode engineering for high energy density aqueous Zn batteries. Acc. Mater. Res. 2021, 3, 78–88. [Google Scholar] [CrossRef]
- Galos, J.; Pattarakunnan, K.; Best, A.S.; Kyratzis, I.L.; Wang, C.H.; Mouritz, A.P. Energy storage structural composites with integrated lithium-ion batteries: A review. Adv. Mater. Technol. 2021, 6, 2001059. [Google Scholar] [CrossRef]
- Kamenskii, M.A.; Volkov, F.S.; Eliseeva, S.N.; Tolstopyatova, E.G.; Kondratiev, V.V. Enhancement of electrochemical performance of aqueous zinc ion batteries by structural and interfacial design of MnO2 cathodes: The metal ion doping and introduction of conducting polymers. Energies 2023, 16, 3221. [Google Scholar] [CrossRef]
- Qian, H.; Ren, H.; Zhang, Y.; He, X.; Li, W.; Wang, J.; Hu, J.; Yang, H.; Sari, H.M.K.; Chen, Y. Surface doping vs. bulk doping of cathode materials for lithium-ion batteries: A review. Electrochem. Energy Rev. 2022, 5, 2. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Z.; Yu, H.; Zhang, X.; Shu, J. Heteroatom-doped carbon-based materials for lithium and sodium ion batteries. Energy Storage Mater. 2020, 32, 65–90. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Liu, B.; Zhang, Y.; Liang, X.; Xia, X. Heteroatom doping: An effective way to boost sodium ion storage. Adv. Energy Mater. 2020, 10, 2000927. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Sun, W.; Shao, Y.; Zhang, L.; Zhao, K. Challenges and perspectives for doping strategy for manganese-based zinc-ion battery cathode. Energies 2022, 15, 4698. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, A.; Ding, S.; Qin, R.; Cui, Y.; Li, S.; Pan, F. Preintercalation strategy in manganese oxides for electrochemical energy storage: Review and prospects. Adv. Mater. 2020, 32, 2002450. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; Hwang, C.; Jang, H.; Kane, N.; Ahn, Y.; Kwak, M.J.; Luo, Z.; Li, T.; Kim, M.G.; Liu, N. Tuning proton insertion chemistry for sustainable aqueous zinc-ion batteries. Small 2024, 20, 2306919. [Google Scholar] [CrossRef]
- Young, M.J.; Holder, A.M.; George, S.M.; Musgrave, C.B. Charge storage in cation incorporated α-MnO2. Chem. Mater. 2015, 27, 1172–1180. [Google Scholar] [CrossRef]
- Hu, Z.; Xiao, X.; Huang, L.; Chen, C.; Li, T.; Su, T.; Cheng, X.; Miao, L.; Zhang, Y.; Zhou, J. 2D vanadium doped manganese dioxides nanosheets for pseudocapacitive energy storage. Nanoscale 2015, 7, 16094–16099. [Google Scholar] [CrossRef]
- Radhamani, A.; Surendra, M.K.; Rao, M.R. Zn doped δ-MnO2 nano flakes: An efficient electrode material for aqueous and solid state asymmetric supercapacitors. Appl. Surf. Sci. 2018, 450, 209–218. [Google Scholar] [CrossRef]
- Nasser, R.; Zhang, G.-F.; Song, J.-M. Facile and low-cost synthesis of cobalt-doped MnO2 decorated with graphene oxide for high performance 2.3 V aqueous asymmetric supercapacitors. Electrochim. Acta 2020, 345, 136198. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, K.; Huang, X.; Wang, X.; Cheng, K.; Xiao, X.; Wang, G.; Cao, D. Preparation of Mg1.1Mn6O12· 4.5H2O with nanobelt structure and its application in aqueous magnesium-ion battery. J. Power Sources 2017, 338, 136–144. [Google Scholar] [CrossRef]
- Wu, J.; Raza, W.; Wang, P.; Hussain, A.; Ding, Y.; Yu, J.; Wu, Y.; Zhao, J. Zn-doped MnO2 ultrathin nanosheets with rich defects for high performance aqueous supercapacitors. Electrochim. Acta 2022, 418, 140339. [Google Scholar] [CrossRef]
- Bai, H.; Liang, S.; Wei, T.; Zhou, Q.; Shi, M.; Jiang, Z.; Feng, J.; Zhang, M.; Fan, Z. Enhanced pseudo-capacitance and rate performance of amorphous MnO2 for supercapacitor by high Na doping and structural water content. J. Power Sources 2022, 523, 231032. [Google Scholar] [CrossRef]
- Li, W.; Qin, L.; Liu, Z.; Li, L.; Li, W.; Fang, G. Potassium-ion-doped manganese oxides and kaolinite electrolyte additives for aqueous zinc-ion batteries. ACS Appl. Nano Mater. 2024, 7, 9720–9729. [Google Scholar] [CrossRef]
- Li, H.; Huang, Z.; Chen, B.; Jiang, Y.; Li, C.; Xiao, W.; Yan, X. A high-performance MnO2 cathode doped with group Ⅷ metal for aqueous Zn-ion batteries: In-situ X-Ray diffraction study on Zn2+ storage mechanism. J. Power Sources 2022, 527, 231198. [Google Scholar] [CrossRef]
- Hou, X.; Li, C.; Li, M.; Liu, Y.; Zhu, W.; Li, Z.; Xu, Y. Synthesis of Cu-doped layered transition metal oxide cathode materials directly from metal-organic Frameworks for Sodium-Ion Batteries. Chin. J. Chem. 2023, 41, 2597–2603. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Ji, S.; Hui, K.S.; Wang, S.; Xu, H.; Wang, K.; Dinh, D.A.; Zha, C.; Shao, Z. Zinc-doping strategy on P2-type Mn-based layered oxide cathode for high-performance potassium-ion batteries. Small 2023, 19, 2302160. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, H.-C.; Yang, C.; Liu, R.; Peng, Z.; Cao, H.; Wang, K. Unveiling performance evolution mechanisms of MnO2 polymorphs for durable aqueous zinc-ion batteries. Energy Storage Mater. 2022, 44, 508–516. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, G.; Yan, M.; Xiong, T.; He, P.; He, L.; Xu, X.; Mai, L. Graphene scroll-coated α-MnO2 nanowires as high-performance cathode materials for aqueous Zn-ion battery. Small 2018, 14, 1703850. [Google Scholar] [CrossRef]
- Juran, T.R.; Young, J.; Smeu, M. Density functional theory modeling of MnO2 polymorphs as cathodes for multivalent ion batteries. J. Phys. Chem. C 2018, 122, 8788–8795. [Google Scholar] [CrossRef]
- Islam, S.; Alfaruqi, M.H.; Mathew, V.; Song, J.; Kim, S.; Kim, S.; Jo, J.; Baboo, J.P.; Pham, D.T.; Putro, D.Y. Facile synthesis and the exploration of the zinc storage mechanism of β-MnO2 nanorods with exposed (101) planes as a novel cathode material for high performance eco-friendly zinc-ion batteries. J. Mater. Chem. A 2017, 5, 23299–23309. [Google Scholar] [CrossRef]
- Kwon, N.H.; Lee, K.-G.; Kim, H.K.; Hwang, S.-J. MnO2-based nanostructured materials for various energy applications. Mater. Chem. Front. 2021, 5, 3549–3575. [Google Scholar] [CrossRef]
- Worku, A.K.; Ayele, D.W.; Habtu, N.G.; Teshager, M.A.; Workineh, Z.G. Recent progress in MnO2-based oxygen electrocatalysts for rechargeable zinc-air batteries. Mater. Today Sustain. 2021, 13, 100072. [Google Scholar] [CrossRef]
- Guo, X.; Yang, S.; Wang, D.; Chen, A.; Wang, Y.; Li, P.; Liang, G.; Zhi, C. The energy storage mechanisms of MnO2 in batteries. Curr. Opin. Electrochem. 2021, 30, 100769. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, W.; Pan, F.; Liu, G. Block copolymer-derived porous carbon fibers enable high MnO2 loading and fast charging in aqueous zinc-ion battery. Batter. Supercaps 2022, 5, e202100380. [Google Scholar] [CrossRef]
- Wei, C.; Xu, C.; Li, B.; Du, H.; Kang, F. Preparation and characterization of manganese dioxides with nano-sized tunnel structures for zinc ion storage. J. Phys. Chem. Solids 2012, 73, 1487–1491. [Google Scholar] [CrossRef]
- Xu, J.-W.; Gao, Q.-L.; Xia, Y.-M.; Lin, X.-S.; Liu, W.-L.; Ren, M.-M.; Kong, F.-G.; Wang, S.-J.; Lin, C. High-performance reversible aqueous zinc-ion battery based on iron-doped alpha-manganese dioxide coated by polypyrrole. J. Colloid Interface Sci. 2021, 598, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Zhu, Y.; Du, W.; Liu, W.; Yao, M.; Wang, Y.; Qian, Y.; Feng, S. Unlocking the critical role of Mg doping in α-MnO2 cathode for aqueous zinc ion batteries. Chem. Eng. J. 2024, 11, 150077. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Y.; Ye, M.; Cai, W.; Xiao, M.; Hu, C.; Zhong, B.; Wan, F.; Guo, X. Regeneration of spent lithium manganate batteries into Al-doped MnO2 cathodes toward aqueous Zn batteries. ACS Appl. Mater. Interfaces 2023, 15, 59475–59481. [Google Scholar] [CrossRef]
- Gao, L.; Hu, H.; Zhang, C.; Cao, M. Gallium regulated MnO2 toward high performance Zn ion batteries. Vacuum 2024, 219, 112671. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, M.; Liu, R.; Xiao, H.; Liu, Y.; Wang, X.; Huang, Y.; Yuan, G. Molecular tailoring of MnO2 by bismuth doping to achieve aqueous zinc-ion battery with capacitor-level durability. Energy Storage Mater. 2022, 48, 212–222. [Google Scholar] [CrossRef]
- Le, T.; Takeuchi, E.S.; Takeuchi, K.J.; Marschilok, A.C.; Liu, P. Tuning discharge behavior of hollandite α-MnO2 in hydrated zinc ion battery by transition metal substitution. J. Phys. Chem. C 2023, 127, 907–918. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Ji, T.; Zhang, M.; Yan, X.; Yao, M.; Sheng, D.; Li, S.; Ren, P.; Shen, Z. Potassium ion doped manganese oxide nanoscrolls enhanced the performance of aqueous zinc-ion batteries. Chin. Chem. Lett. 2024, 5, 109551. [Google Scholar] [CrossRef]
- Lan, R.; Gkanas, E.; Sahib, A.J.S.; Greszta, A.; Bhagat, R.; Roberts, A. The effect of copper doping in α-MnO2 as cathode material for aqueous Zinc-ion batteries. J. Alloys Compd. 2024, 16, 174528. [Google Scholar] [CrossRef]
- Wang, C.; Yang, H.; Wang, B.; Ding, P.; Wan, Y.; Bao, W.; Li, Y.; Ma, S.; Liu, Y.; Lu, Y. Dual cation doping enabling simultaneously boosted capacity and rate capability of MnO2 cathodes for Zn//MnO2 batteries. Nano Res. 2023, 16, 9488–9495. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Mathew, V.; Song, J.; Kim, S.; Tung, D.P.; Jo, J.; Kim, S.; Baboo, J.P.; Xiu, Z. Ambient redox synthesis of vanadium-doped manganese dioxide nanoparticles and their enhanced zinc storage properties. Appl. Surf. Sci. 2017, 404, 435–442. [Google Scholar] [CrossRef]
- Zhang, R.; Liang, P.; Yang, H.; Min, H.; Niu, M.; Jin, S.; Jiang, Y.; Pan, Z.; Yan, J.; Shen, X. Manipulating intercalation-extraction mechanisms in structurally modulated δ-MnO2 nanowires for high-performance aqueous zinc-ion batteries. Chem. Eng. J. 2022, 433, 133687. [Google Scholar] [CrossRef]
- Wang, H.; Liang, M.; Gao, J.; Ma, C.; He, Z.; Zhao, Y.; Miao, Z. Robust structural stability of flower-like δ-MnO2 as cathode for aqueous zinc ion battery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128804. [Google Scholar] [CrossRef]
- Xie, Q.; Cheng, G.; Xue, T.; Huang, L.; Chen, S.; Sun, Y.; Sun, M.; Wang, H.; Yu, L. Alkali ions pre-intercalation of δ-MnO2 nanosheets for high-capacity and stable Zn-ion battery. Mater. Today Energy 2022, 24, 100934. [Google Scholar] [CrossRef]
- Yan, L.; Liu, B.; Hao, J.; Han, Y.; Zhu, C.; Liu, F.; Zou, X.; Zhou, Y.; Xiang, B. In–situ cation–inserted MnO2 with selective accelerated intercalation of individual H+ or Zn2+ ions in aqueous zinc ion batteries. J. Energy Chem. 2023, 82, 88–102. [Google Scholar] [CrossRef]
- Xia, J.; Zhou, Y.; Zhang, J.; Lu, T.; Gong, W.; Zhang, D.; Wang, X.; Di, J. Triggering high capacity and superior reversibility of manganese oxides cathode via magnesium modulation for Zn//MnO2 batteries. Small 2023, 19, 2301906. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Xiang, Y.; Yang, S.; Li, Y.; Du, H.; Liu, Y.; Wu, X.; Wu, X. Layered manganese dioxide nanoflowers with Cu2+ and Bi3+ intercalation as high-performance cathode for aqueous zinc-ion battery. J. Colloid Interface Sci. 2022, 616, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fee, J.; Khanna, H.; March, S.; Nisly, N.; Rubio, S.J.B.; Cui, C.; Li, Z.; Suib, S.L. A two-electron transfer mechanism of the Zn-doped δ-MnO2 cathode toward aqueous Zn-ion batteries with ultrahigh capacity. J. Mater. Chem. A 2022, 10, 6762–6771. [Google Scholar] [CrossRef]
- Xia, X.; Zhao, Y.; Zhao, Y.; Xu, M.; Liu, W.; Sun, X. Mo doping provokes two electron reaction in MnO2 with ultrahigh capacity for aqueous zinc ion batteries. Nano Res. 2023, 16, 2511–2518. [Google Scholar] [CrossRef]
- Pu, X.; Li, X.; Wang, L.; Maleki Kheimeh Sari, H.; Li, J.; Xi, Y.; Shan, H.; Wang, J.; Li, W.; Liu, X. Enriching oxygen vacancy defects via Ag–O–Mn bonds for enhanced diffusion kinetics of δ-MnO2 in zinc-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 21159–21172. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Z.; Gao, X.-W.; Gu, Q.; Zhao, L.; Luo, W.-B. Massive anionic fluorine substitution two-dimensional δ-MnO2 nanosheets for high-performance aqueous zinc-ion battery. J. Energy Storage 2023, 72, 108740. [Google Scholar] [CrossRef]
- Sun, Y.; Zhuang, S.; Ren, Y.; Jiang, S.; Pan, X.; Sun, G.; Zhu, B.; Wen, Y.; Li, X.; Tu, F. Promoting cycle stability and rate performance of birnessite-type MnO2 cathode via cupper and bismuth dual ions pre-intercalation for aqueous zinc-ion batteries. J. Energy Storage 2023, 74, 109589. [Google Scholar] [CrossRef]
- Ding, X.; Wen, Y.; Qing, C.; Wei, Y.; Wang, P.; Liu, J.; Peng, Z.; Song, Y.; Chen, H.; Rong, Q. Cr-induced enhancement of structural stability in δ-MnO2 for aqueous Zn-ion batteries. J. Alloys Compd. 2024, 11, 174041. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, X.; Li, Q.; Wang, Y.; Fan, Y.; Zhao, Y.; Liu, W.; Sun, X. Activating the redox chemistry of MnO2/Mn2+ in aqueous Zn batteries based on multi-ions doping regulation. Energy Storage Mater. 2024, 67, 115–123. [Google Scholar] [CrossRef]
- Han, M.; Huang, J.; Liang, S.; Shan, L.; Xie, X.; Yi, Z.; Wang, Y.; Guo, S.; Zhou, J. Oxygen defects in β-MnO2 enabling high-performance rechargeable aqueous zinc/manganese dioxide battery. iScience 2020, 23, 100797. [Google Scholar] [CrossRef]
- Deng, Z.; Huang, J.; Liu, J.; Ren, L.; Zhu, L.; Xiao, X.; Tan, M. β-MnO2 nanolayer coated on carbon cloth as a high-activity aqueous zinc-ion battery cathode with high-capacity and long-cycle-life. Mater. Lett. 2019, 248, 207–210. [Google Scholar] [CrossRef]
- Han, R.; Pan, Y.; Du, C.; Xiang, Y.; Wang, Y.; Zhu, H.; Yin, C. Eu doping β-MnO2 as cathode materials for high specific capacity aqueous zinc ion batteries. J. Energy Storage 2024, 80, 110250. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, Z.; Wu, X.; Wen, Y.; Chen, H.; Ni, X.; Liu, G.; Huang, J.; Peng, S. MnO2 cathode materials with the improved stability via nitrogen doping for aqueous zinc-ion batteries. J. Energy Chem. 2022, 64, 23–32. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, B.C.; Li, W.P. Synthesis and electrochemical properties of Cu2+-doped MnO2 as cathode materials for aqueous zinc ion batteries. CIESC J. 2021, 72, 5402–5408. [Google Scholar]
- Ji, J.; Yao, J.; Xu, Y.; Wan, H.; Zhang, B.; Lv, L.; Li, J.; Wang, N.; Zheng, Z.; Zhang, J. Promoting proton migration kinetics by Ni2+ regulating enables improved aqueous Zn-MnO2 batteries. Energy Environ. Mater. 2023, 6, e12340. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, X.; Chen, X.; Liu, J.-H.; Huang, Y.; Cao, D. Trimetallic-organic framework-derived Ni-doped MnO/PC as cathodes for high-performance aqueous zinc-ion batteries. Chem. Eng. J. 2023, 478, 147411. [Google Scholar] [CrossRef]
- Yu, P.; Zhou, J.; Zheng, M.; Li, M.; Hu, H.; Xiao, Y.; Liu, Y.; Liang, Y. Boosting zinc ion energy storage capability of inert MnO cathode by defect engineering. J. Colloid Interface Sci. 2021, 594, 540–549. [Google Scholar] [CrossRef]
- Zou, R.; Tang, Z.; Chen, X.; Li, Z.; Lei, G. Exploration of calcium-doped manganese monoxide cathode for high-performance aqueous zinc-ion batteries. Energy Fuels 2022, 36, 13296–13306. [Google Scholar] [CrossRef]
- Sun, K.; Shen, Y.; Min, J.; Pang, J.; Zheng, Y.; Gu, T.; Wang, G.; Chen, L. MOF-derived Zn/Co co-doped MnO/C microspheres as cathode and Ti3C2@ Zn as anode for aqueous zinc-ion full battery. Chem. Eng. J. 2023, 454, 140394. [Google Scholar] [CrossRef]
- Jia, H.; Li, Y.; Ali, U.; Liu, B.; Jin, Z.; Li, L.; Chen, Y.; Zhang, L.; Wang, T.; Wang, C. High-entropy doping strategy towards reinforced Mn-O bond for durable aqueous zinc ion batteries. Nano Energy 2024, 122, 109348. [Google Scholar] [CrossRef]
- Liu, N.; Wu, X.; Yin, Y.; Chen, A.; Zhao, C.; Guo, Z.; Fan, L.; Zhang, N. Constructing the efficient ion diffusion pathway by introducing oxygen defects in Mn2O3 for high-performance aqueous zinc-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 28199–28205. [Google Scholar] [CrossRef] [PubMed]
- Komolov, A.; Lazneva, E.; Gerasimova, N.; Panina, Y.A.; Sobolev, V.; Koroleva, A.; Pshenichnyuk, S.; Asfandiarov, N.; Modelli, A.; Handke, B. Conduction band electronic states of ultrathin layers of thiophene/phenylene co-oligomers on an oxidized silicon surface. J. Electron Spectrosc. Relat. Phenom. 2019, 235, 40–45. [Google Scholar] [CrossRef]
- Pronin, I.A.; Averin, I.A.; Karmanov, A.A.; Yakushova, N.D.; Komolov, A.S.; Lazneva, E.F.; Sychev, M.M.; Moshnikov, V.A.; Korotcenkov, G. Control over the surface properties of zinc oxide powders via combining mechanical, electron beam, and thermal processing. Nanomaterials 2022, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Saadi-motaallegh, S.; Javanbakht, M.; Omidvar, H.; Habibzadeh, S. A Novel Ni-doped ZnMn2O4/Mn2O3 nanocomposite synthesized by pulsed potential as superior zinc ion battery cathode material. J. Alloys Compd. 2023, 963, 171119. [Google Scholar] [CrossRef]
- Karuppaiah, M.; Sakthivel, P.; Asaithambi, S.; Bharat, L.K.; Nagaraju, G.; Ahamad, T.; Balamurugan, K.; Yuvakkumar, R.; Ravi, G. Elevated energy density and cycle stability of α-Mn2O3 3D-microspheres with addition of neodymium dopant for pouch-type hybrid supercapacitors. Electrochim. Acta 2020, 362, 137169. [Google Scholar] [CrossRef]
- Darjazi, H.; Davarani, S.S.H.; Moazami, H.R.; Yousefi, T.; Tabatabaei, F. Evaluation of charge storage ability of chrome doped Mn2O3 nanostructures derived by cathodic electrodeposition. Prog. Nat. Sci. Mater. Int. 2016, 26, 523–527. [Google Scholar] [CrossRef]
- Zhao, F.F.; Li, C.Y.; Li, S.H.; Wang, B.; Huang, B.K.; Hu, K.; Liu, L.H.; Yu, W.W.; Li, H.Z. Continuous solar energy conversion windows integrating zinc anode-based electrochromic device and IoT system. Adv. Mater. 2024, 2405035. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, S.; Li, C.; Chen, X.; Zhang, W.; Ge, X.; Lin, H.; Shi, Z.; Feng, S. High-performance aqueous zinc-ion battery based on an Al0.35Mn2.52O4 cathode: A design strategy from defect engineering and atomic composition tuning. Small 2022, 18, 2105970. [Google Scholar] [CrossRef]
- Ji, J.; Wan, H.; Zhang, B.; Wang, C.; Gan, Y.; Tan, Q.; Wang, N.; Yao, J.; Zheng, Z.; Liang, P. Co2+/3+/4+-Regulated electron state of Mn-O for superb aqueous zinc-manganese oxide batteries. Adv. Energy Mater. 2021, 11, 2003203. [Google Scholar] [CrossRef]
- Li, D.; Wang, Z.-R.; Xia, Y.-M.; Gao, Q.-L.; Ren, M.-M.; Liu, W.-L.; Kong, F.-G.; Wang, S.-J.; Li, S.-H. Copper-doped manganese tetroxide composites with excellent electrochemical performance for aqueous zinc-ion batteries. J. Electroanal. Chem. 2021, 888, 115214. [Google Scholar] [CrossRef]
- Li, S.; Yao, Y.; Zhang, Y.; Gong, Y.; Wu, M.; Xue, Y.; Yang, J.; Li, L. High-loading cobalt-doped manganese tetroxide on carbon cloth as an electrode material for high-performance zinc ion hybrid capacitors. Electrochim. Acta 2023, 458, 142491. [Google Scholar] [CrossRef]
- Hong, J.S.; Seo, H.; Lee, Y.H.; Cho, K.H.; Ko, C.; Park, S.; Nam, K.T. Nickel-doping effect on Mn3O4 nanoparticles for electrochemical water oxidation under neutral condition. Small Methods 2020, 4, 1900733. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Liu, K.; Yin, J.; Li, Y.; Fu, G.; Zhang, Y.; Tang, Y. Hollow yolk-shell nanoboxes assembled by Fe-doped Mn3O4 nanosheets for high-efficiency electrocatalytic oxygen reduction in Zn-Air battery. Chem. Eng. J. 2022, 427, 131992. [Google Scholar] [CrossRef]
- Adoor, P.; Hegde, S.S.; Bhat, B.R.; Yethadka, S.N.; Yeenduguli, R. Elucidating the role of copper-induced mixed phases on the electrochemical performance of Mn-based thin-film electrodes. ACS Omega 2023, 8, 46640–46652. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, F.; Yu, J.; Hou, S.; Hu, J.; Li, S. Doping Engineering in Manganese Oxides for Aqueous Zinc-Ion Batteries. Materials 2024, 17, 3327. https://doi.org/10.3390/ma17133327
Ji F, Yu J, Hou S, Hu J, Li S. Doping Engineering in Manganese Oxides for Aqueous Zinc-Ion Batteries. Materials. 2024; 17(13):3327. https://doi.org/10.3390/ma17133327
Chicago/Turabian StyleJi, Fanjie, Jiamin Yu, Sen Hou, Jinzhao Hu, and Shaohui Li. 2024. "Doping Engineering in Manganese Oxides for Aqueous Zinc-Ion Batteries" Materials 17, no. 13: 3327. https://doi.org/10.3390/ma17133327





