Synthesis of Up-Conversion CaTiO3: Er3+ Films on Titanium by Anodization and Hydrothermal Method for Biomedical Applications
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of CaTiO3: x%Er3+
2.2. Characterization
2.2.1. Physicochemical Analysis Methods
2.2.2. Biocompatibility Assessment Methods
3. Results and Discussion
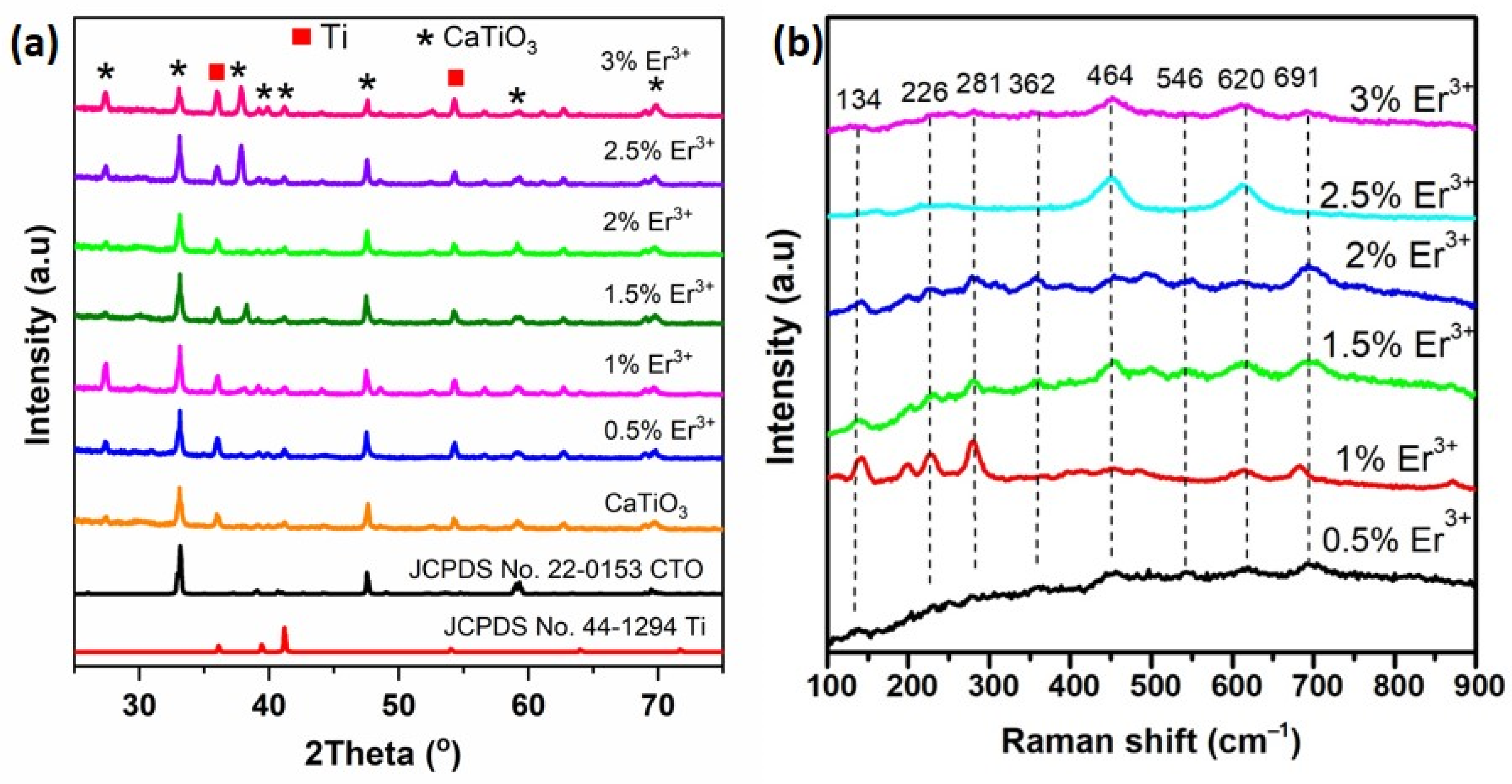
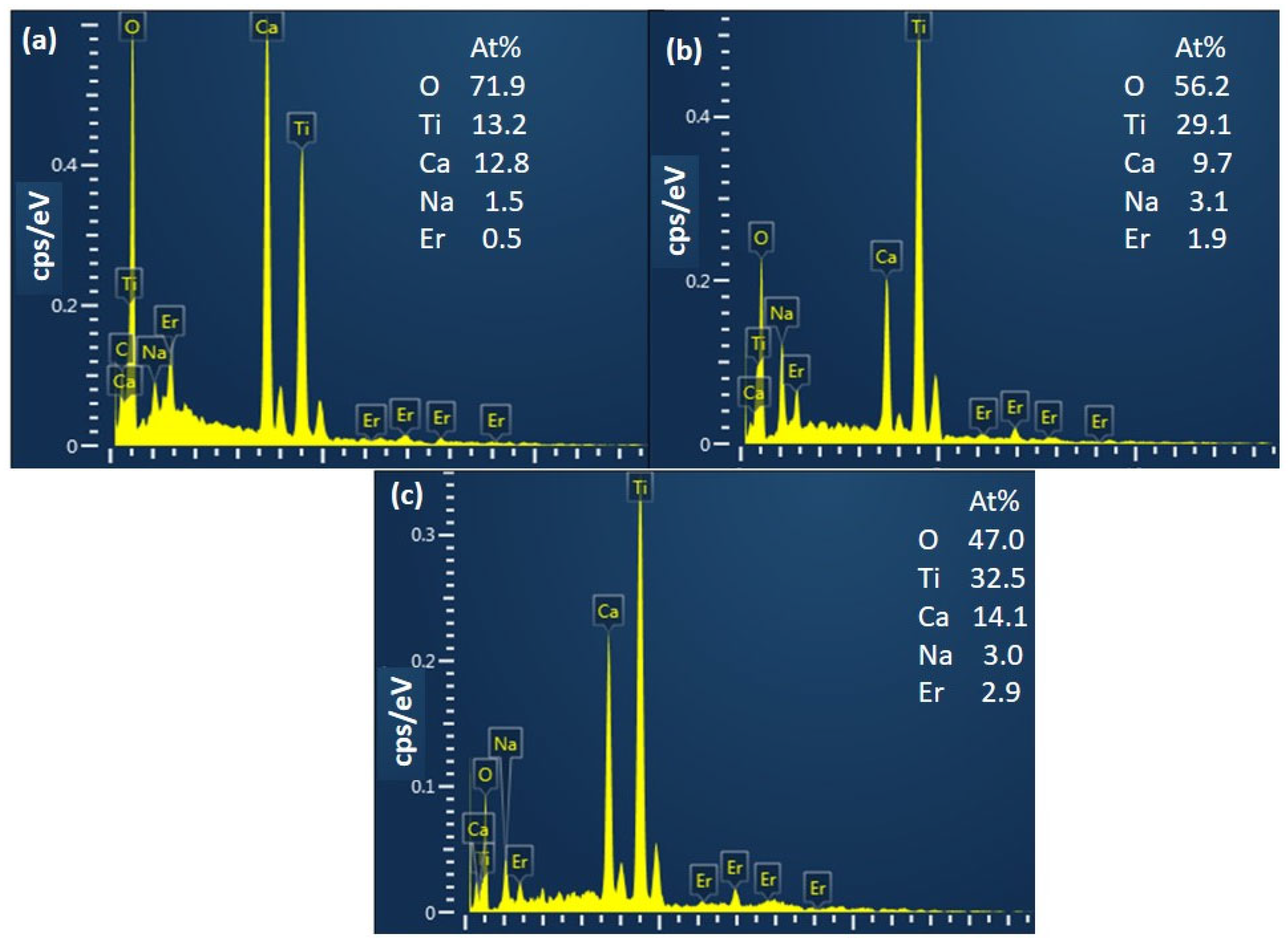
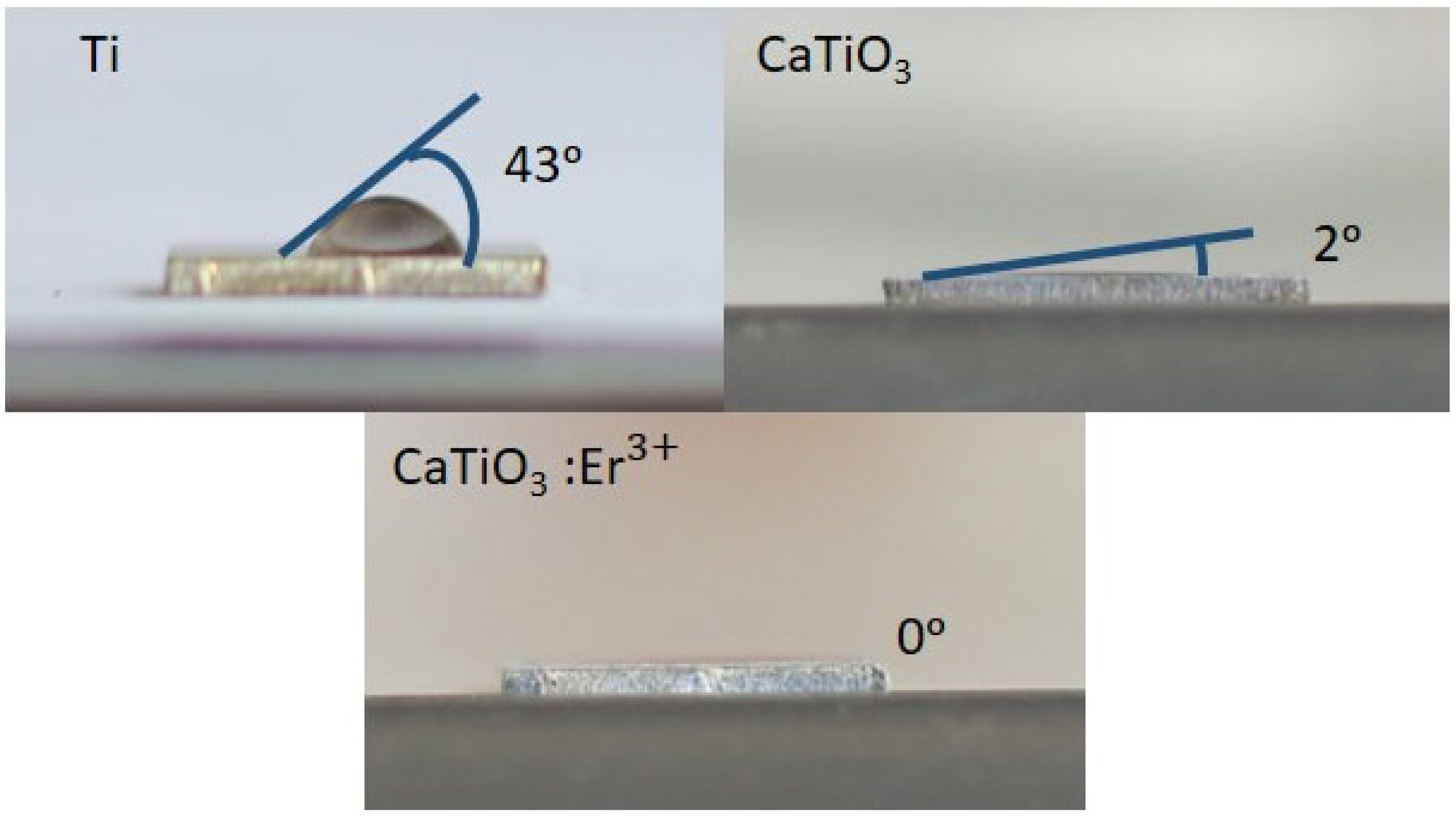
3.1. Results of Material Synthesis
3.2. Material Properties Evaluation Findings
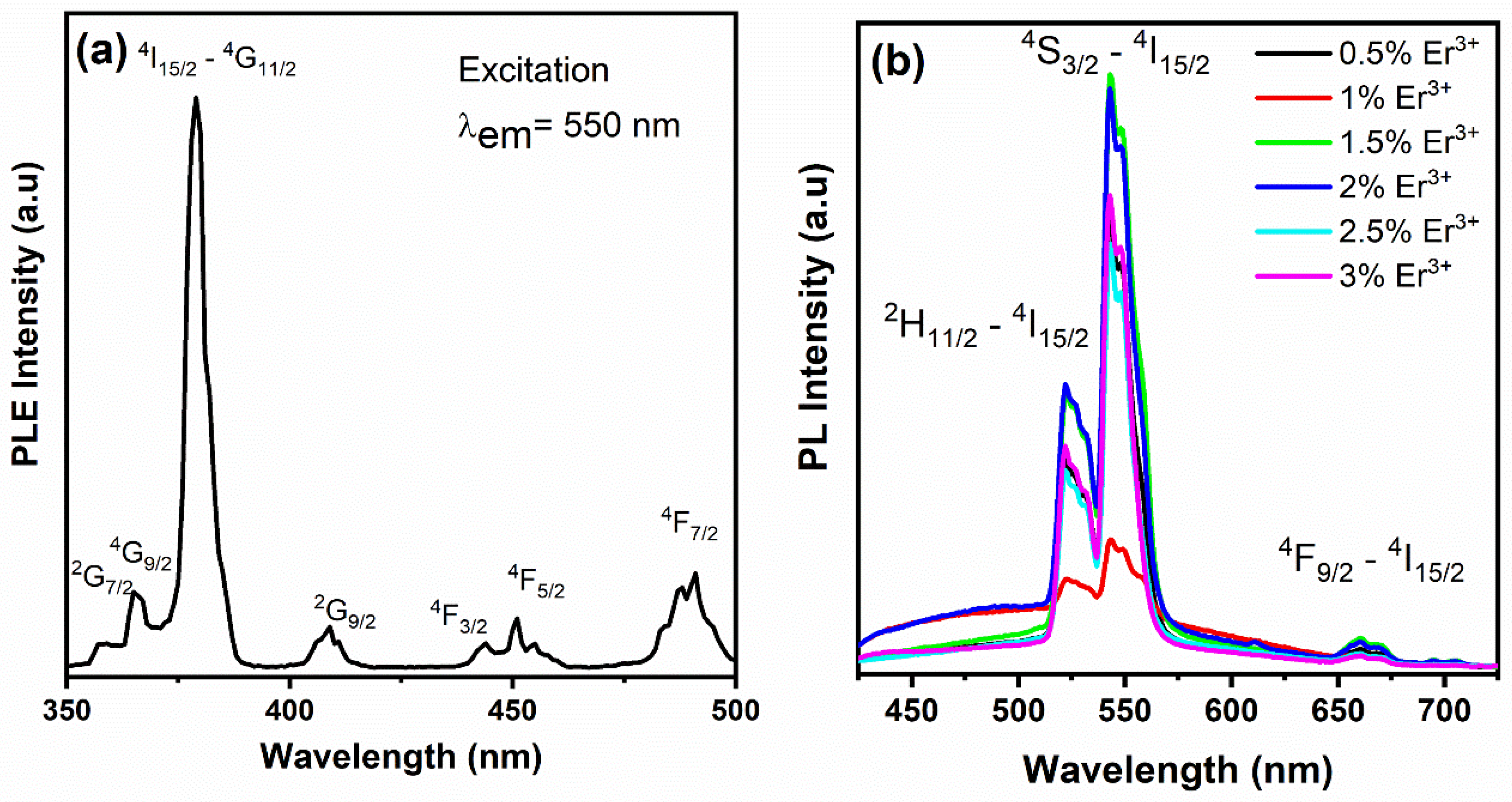
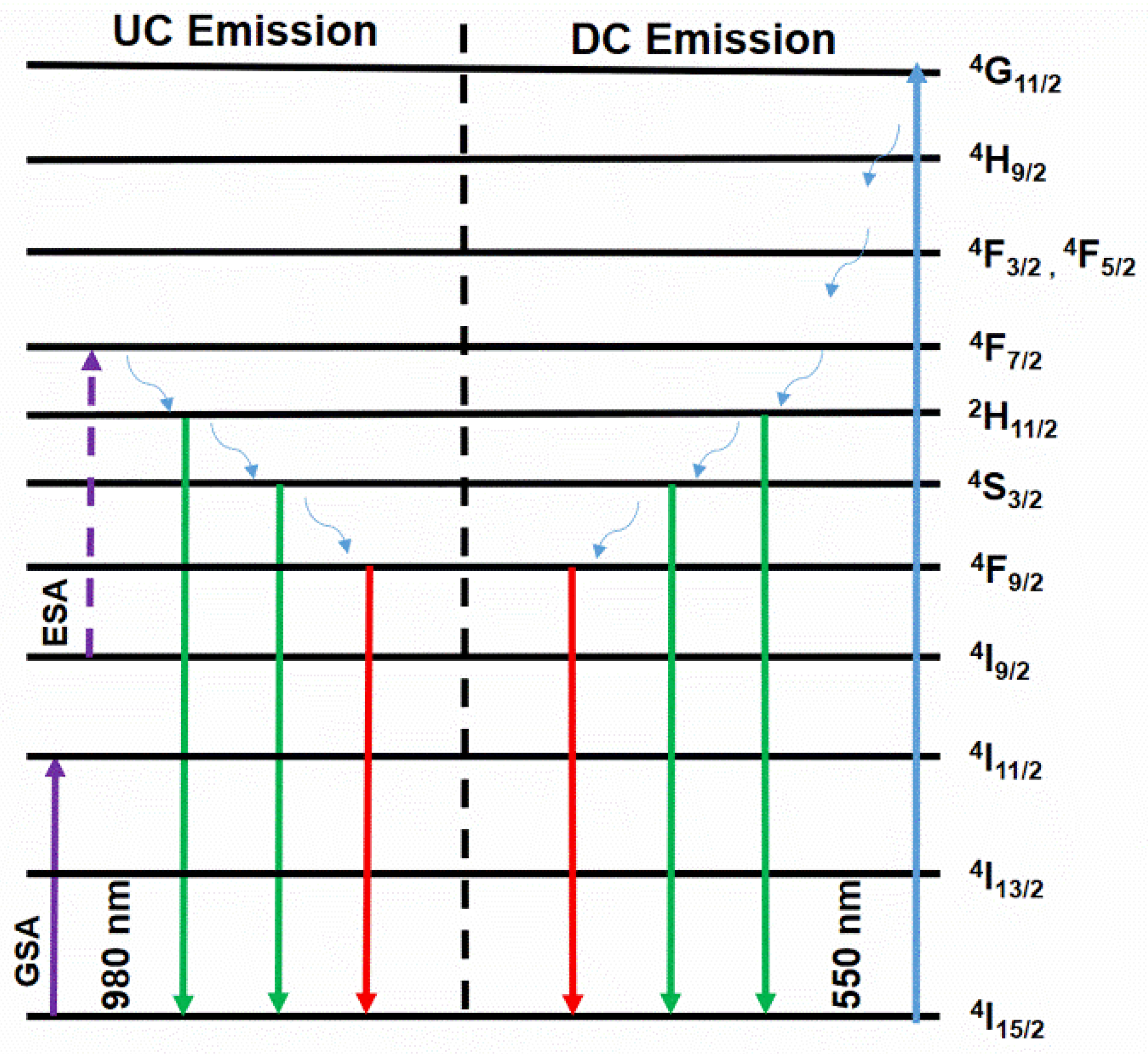
3.2.1. UC Luminescence Properties
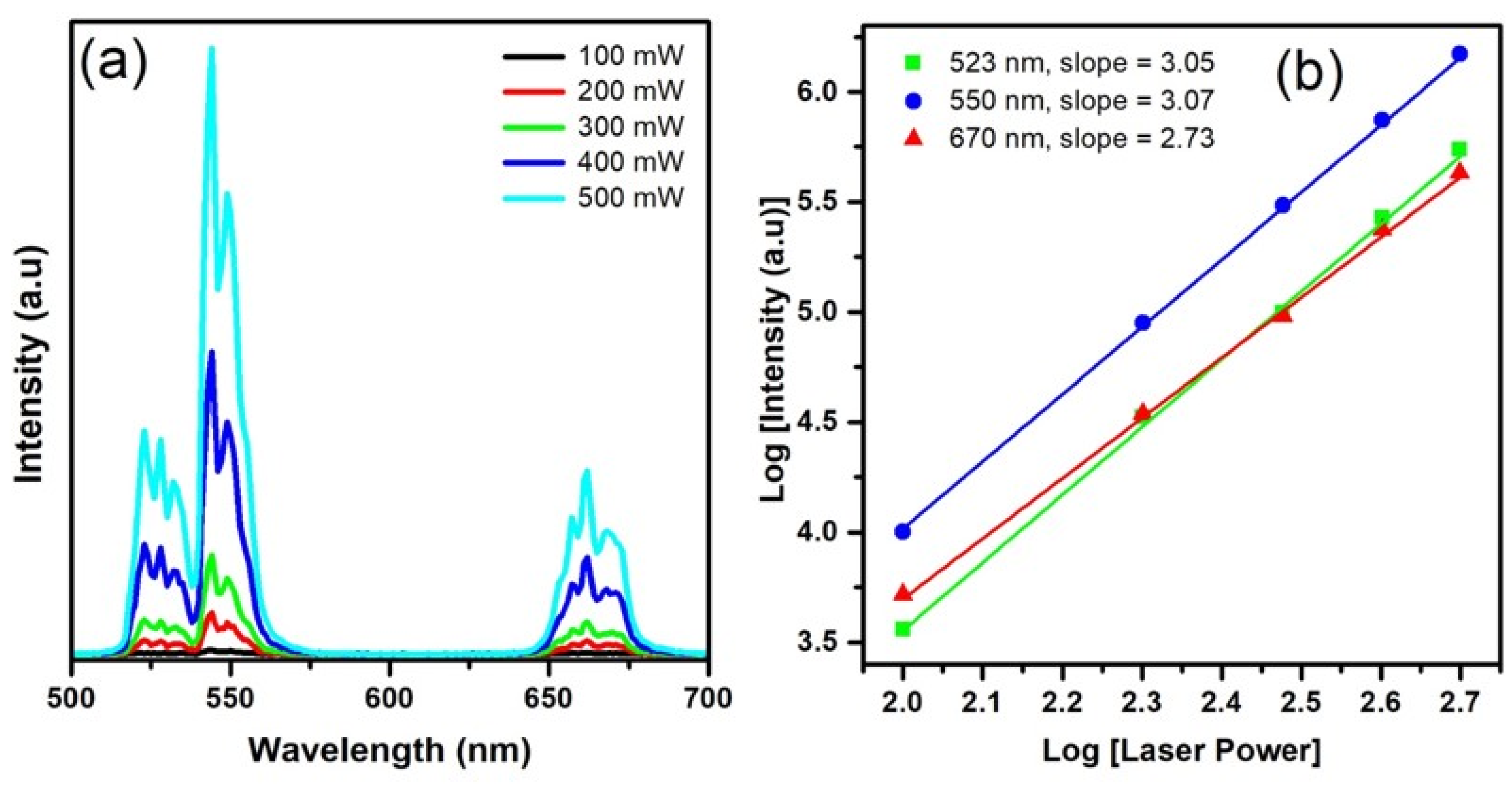
3.2.2. UC Mechanism of Er-Doped Systems
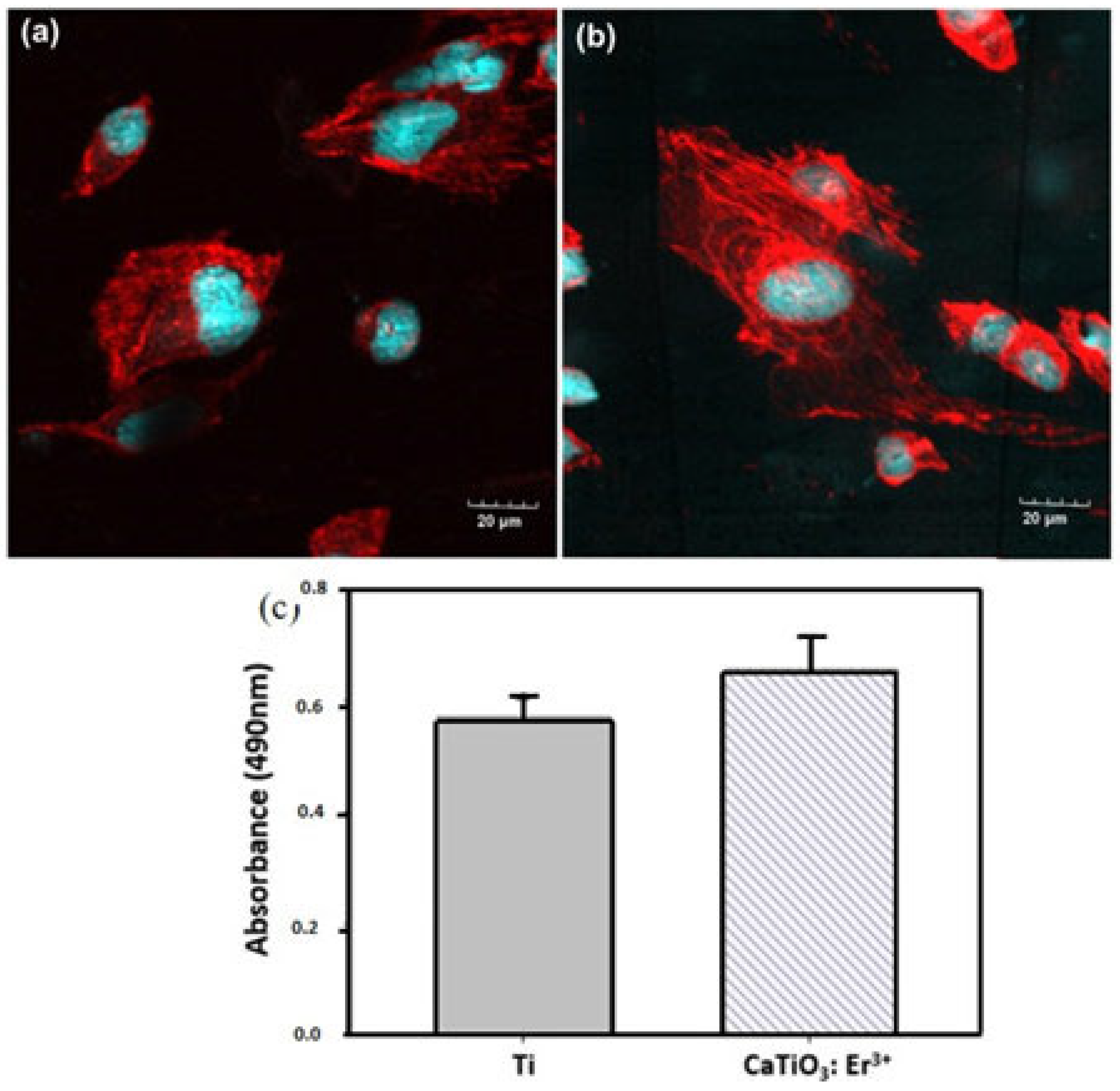
3.3. Biocompatibility of CaTiO3: Er3+
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, L.Q.; Chen, Z.G.; Yu, M.X.; Li, F.Y.; Liu, C.; Huang, C.H. Synthesis, characterization, and in vivo targeted imaging of amine-functionalized rare-earth up-converting nanophosphors. Biomaterials 2009, 30, 5592–5600. [Google Scholar] [CrossRef]
- Prathap Kumar, M.; Suganya Josephine, G.A.; Tamilarasan, G.; Sivasamy, A.; Sridevi, J. Rare earth doped semiconductor nanomaterials and its photocatalytic and antimicrobial activities. J. Environ. Chem. Eng. 2018, 6, 3907–3917. [Google Scholar]
- Cerrato, E.; Gionco, C.; Berruti, I.; Sordello, F.; Calza, P.; Paganini, M.C. Rare earth ions doped ZnO: Synthesis, characterization and preliminary photoactivity assessment. J. Solid State Chem. 2018, 264, 42–47. [Google Scholar] [CrossRef]
- Pang, X.L.; Jia, C.H.; Li, G.Q.; Zhang, W.F. Bright white upconversion luminescence from Er3+-Tm3+-Yb3+ doped CaSnO3 powders. Opt. Mater. 2011, 34, 234–238. [Google Scholar] [CrossRef]
- Ledemi, Y.; Manzani, D.; Ribeiro, S.J.L.; Messaddeq, Y. Multicolor up conversion emission and color tunability in Yb3+/Tm3+/Ho3+ triply doped heavy metal oxide glasses. Opt. Mater. 2011, 33, 1916–1920. [Google Scholar] [CrossRef]
- Heer, S.; Kömpe, K.; Güdel, H.U.; Haase, M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar] [CrossRef]
- Yun, R.; He, J.; Luo, L.; Liu, X.; Nie, Z.; Zhao, W.; Wen, H. Upconversion luminescence and temperature sensing properties in LiGd(WO4)2: Er3+, Yb3+, Nd3+ microparticles under 785 nm excitation. Ceram. Int. 2021, 47, 16062–16069. [Google Scholar] [CrossRef]
- Xu, S.; Ma, H.; Fang, D.; Zhang, Z.; Jiang, Z. Tm3+/Er3+/Yb3+-codoped oxyhalide tellurite glasses as materials for three-dimensional display. Mater. Lett. 2005, 59, 3066–3068. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.A.; Imanieh, M.H.; Martín, L.L.; Martín, I.R. Experimental enhancement of the photocurrent in a solar cell using upconversion process in fluoroindate glasses exciting at 1480 nm. Sol. Energy Mater. Sol. Cells 2013, 116, 171–175. [Google Scholar] [CrossRef]
- Yang, H.; Peng, F.; Zhang, Q.; Liu, W.; Sun, D.; Zhao, Y.; Wei, X. Strong upconversion luminescence in LiYMo2O8:Er, Yb towards efficiency enhancement of dye-sensitized solar cells. Opt. Mater. 2013, 35, 2338–2342. [Google Scholar] [CrossRef]
- Tiwari, S.P.; Mahata, M.K.; Kumar, K.; Rai, V.K. Enhanced temperature sensing response of upconversion luminescence in ZnO-CaTiO3: Er3+/Yb3+ nano-composite phosphor. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2015, 150, 623–630. [Google Scholar] [CrossRef]
- Mahata, M.K.; Kumar, K.; Rai, V.K. Structural and optical properties of Er3+/Yb3+ doped barium titanate phosphor prepared by co-precipitation method. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2014, 124, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, L.S.; Marques, V.S.; Sczancoski, J.C.; Escote, M.T.; Joya, M.R.; Varela, J.A.; Santos, M.R.M.C.; Pizani, P.S.; Longo, E. Synthesis, structural refinement and optical behavior of CaTiO3 powders: A comparative study of processing in different furnaces. Chem. Eng. J. 2008, 143, 299–307. [Google Scholar] [CrossRef]
- Naczynski, D.J.; Tan, M.C.; Zevon, M.; Wall, B.; Kohl, J.; Kulesa, A.; Chen, S.; Roth, C.M.; Riman, R.E.; Moghe, P.V. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat. Commun. 2013, 4, 2199. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yang, T.; Yang, Y.; Xu, C.; Li, F. Long-term in vivo biodistribution imaging and toxicity of polyacrylic acid-coated upconversion nanophosphors. Biomaterials 2010, 31, 7078–7085. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Ramesh, R. Point defect chemistry of metal oxide heterostructures. Annu. Rev. Mater. Sci. 1998, 28, 463–499. [Google Scholar] [CrossRef]
- Dereń, P.J.; Mahiou, R.; Pazik, R.; Lemanski, K.; Strek, W.; Boutinaud, P. Upconversion emission in CaTiO3:Er3+ nanocrystals. J. Lumin. 2008, 128, 797–799. [Google Scholar] [CrossRef]
- Duong, H.Q.; Nguyen, V.T.; Ta, Q.T.; Le Thi, T.; Pham, T.M.P.; Cao, X.T.; Dao, X.V.; Nguyen, K.T.; Pham, V.H.; Nguyen, T.N.; et al. Synthesis of TiO2 Nanotubes for Improving the Corrosion Resistance Performance of the Titanium Implants. VNU J. Sci. Math. Phys. 2022, 38, 61–68. [Google Scholar]
- Mazzo, T.M.; da Rocha Oliveira, L.M.; Macario, L.R.; Avansi, W., Jr.; da Silveira André, R.; Rosa, I.L.V.; Varela, J.A.; Longo, E. Photoluminescence properties of CaTiO3:Eu3+ nanophosphor obtained by the polymeric precursor method. Mater. Chem. Phys. 2014, 145, 141–150. [Google Scholar] [CrossRef]
- Anselme, K.; Ploux, L.; Ponche, A. Cell/material interfaces: Influence of surface chemistry and surface topography on cell adhesion. J. Adhes. Sci. Technol. 2010, 24, 831–852. [Google Scholar] [CrossRef]
- Sutthiwanjampa, C.; Hong, S.; Kim, W.J.; Kang, S.H.; Park, H. Hydrophilic Modification Strategies to Enhance the Surface Biocompatibility of Poly(dimethylsiloxane)-Based Biomaterials for Medical Applications. Adv. Mater. Interfaces 2023, 10, 2202333. [Google Scholar] [CrossRef]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1551–1573. [Google Scholar] [CrossRef] [PubMed]
- Huong, D.T.M.; Nam, N.H.; Van Vu, L.; Long, N.N. Preparation and optical characterization of Eu3+-doped CaTiO3 perovskite powders. J. Alloys Compd. 2012, 537, 54–59. [Google Scholar] [CrossRef]
- Moreira, M.L.; Paris, E.C.; do Nascimento, G.S.; Longo, V.M.; Sambrano, J.R.; Mastelaro, V.R.; Bernardi, M.I.; Andrés, J.; Varela, J.A.; Longo, E. Structural and optical properties of CaTiO3 perovskite-based materials obtained by microwave-assisted hydrothermal synthesis: An experimental and theoretical insight. Acta Mater. 2009, 57, 5174–5185. [Google Scholar] [CrossRef]
- Zheng, H.; Reaney, I.M.; De Györgyfalva, G.C.; Ubic, R.; Yarwood, J.; Seabra, M.P.; Ferreira, V.M. Raman spectroscopy of CaTiO3-based perovskite solid solutions. J. Mater. Res. 2004, 19, 488–495. [Google Scholar] [CrossRef]
- Li, Y.; Qin, S.; Seifert, F. Phase transitions in A-site substituted perovskite compounds: The (Ca1-2xNaxLax)TiO3 (0 ≤ x ≤ 0.5) solid solution. J. Solid State Chem. 2007, 180, 824–833. [Google Scholar] [CrossRef]
- McMillan, P.; Ross, N. The Raman spectra of several orthorhombic calcium oxide perovskites. Phys. Chem. Miner. 1988, 16, 21–28. [Google Scholar] [CrossRef]
- Pattnaik, S.; Rai, V.K. NIR to green light upconversion emission in Er3+ doped CaTiO3 phosphors. In Proceedings of the 2018 3rd International Conference on Microwave and Photonics (ICMAP), Dhanbad, India, 9–11 February 2018; pp. 1–4. [Google Scholar]
- Liu, W.; Zhang, H.; Wang, H.; Zhang, M.; Guo, M. Titanium mesh supported TiO2 nanowire arrays/upconversion luminescence Er3+-Yb3+ codoped TiO2 nanoparticles novel composites for flexible dye-sensitized solar cells. Appl. Surf. Sci. 2017, 422, 304–315. [Google Scholar] [CrossRef]
- Babu, P.; Seo, H.J.; Kesavulu, C.R.; Jang, K.H.; Jayasankar, C.K. Thermal and optical properties of Er3+-doped oxyfluorotellurite glasses. J. Lumin. 2009, 129, 444–448. [Google Scholar] [CrossRef]
- Ma, Z.; Gou, J.; Zhang, Y.; Man, Y.; Li, G.; Li, C.; Tang, J. Yb3+/Er3+ co-doped Lu2TeO6 nanophosphors: Hydrothermal synthesis, upconversion luminescence and highly sensitive temperature sensing performance. J. Alloys Compd. 2019, 772, 525–531. [Google Scholar] [CrossRef]
- Van, H.N.; Vu, N.H.; Pham, V.H.; Van Huan, P.; Hoan, B.T.; Nguyen, D.H.; Le Manh, T. A novel upconversion emission material based on Er3+-Yb3+-Mo6+ tridoped Hydroxyapatite/Tricalcium phosphate (HA/β-TCP). J. Alloys Compd. 2020, 827, 154288. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Gloushankova, N.A.; Sheveiko, A.N.; Kharitonova, M.A.; Moizhess, T.G.; Levashov, E.A.; Rossi, F. Design, characterization and testing of Ti-based multicomponent coatings for load-bearing medical applications. Biomaterials 2005, 26, 2909–2924. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuyen, N.T.T.; Tuan, T.Q.; Toan, L.V.; Tam, L.T.; Pham, V.-H. Synthesis of Up-Conversion CaTiO3: Er3+ Films on Titanium by Anodization and Hydrothermal Method for Biomedical Applications. Materials 2024, 17, 3376. https://doi.org/10.3390/ma17133376
Tuyen NTT, Tuan TQ, Toan LV, Tam LT, Pham V-H. Synthesis of Up-Conversion CaTiO3: Er3+ Films on Titanium by Anodization and Hydrothermal Method for Biomedical Applications. Materials. 2024; 17(13):3376. https://doi.org/10.3390/ma17133376
Chicago/Turabian StyleTuyen, Nguyen Thi Thanh, Ta Quoc Tuan, Le Van Toan, Le Thi Tam, and Vuong-Hung Pham. 2024. "Synthesis of Up-Conversion CaTiO3: Er3+ Films on Titanium by Anodization and Hydrothermal Method for Biomedical Applications" Materials 17, no. 13: 3376. https://doi.org/10.3390/ma17133376
APA StyleTuyen, N. T. T., Tuan, T. Q., Toan, L. V., Tam, L. T., & Pham, V.-H. (2024). Synthesis of Up-Conversion CaTiO3: Er3+ Films on Titanium by Anodization and Hydrothermal Method for Biomedical Applications. Materials, 17(13), 3376. https://doi.org/10.3390/ma17133376





