Investigation of Physical-Mechanical Properties and Microstructure of Mortars with Perlite and Thermal-Treated Materials
Abstract
:1. Introduction
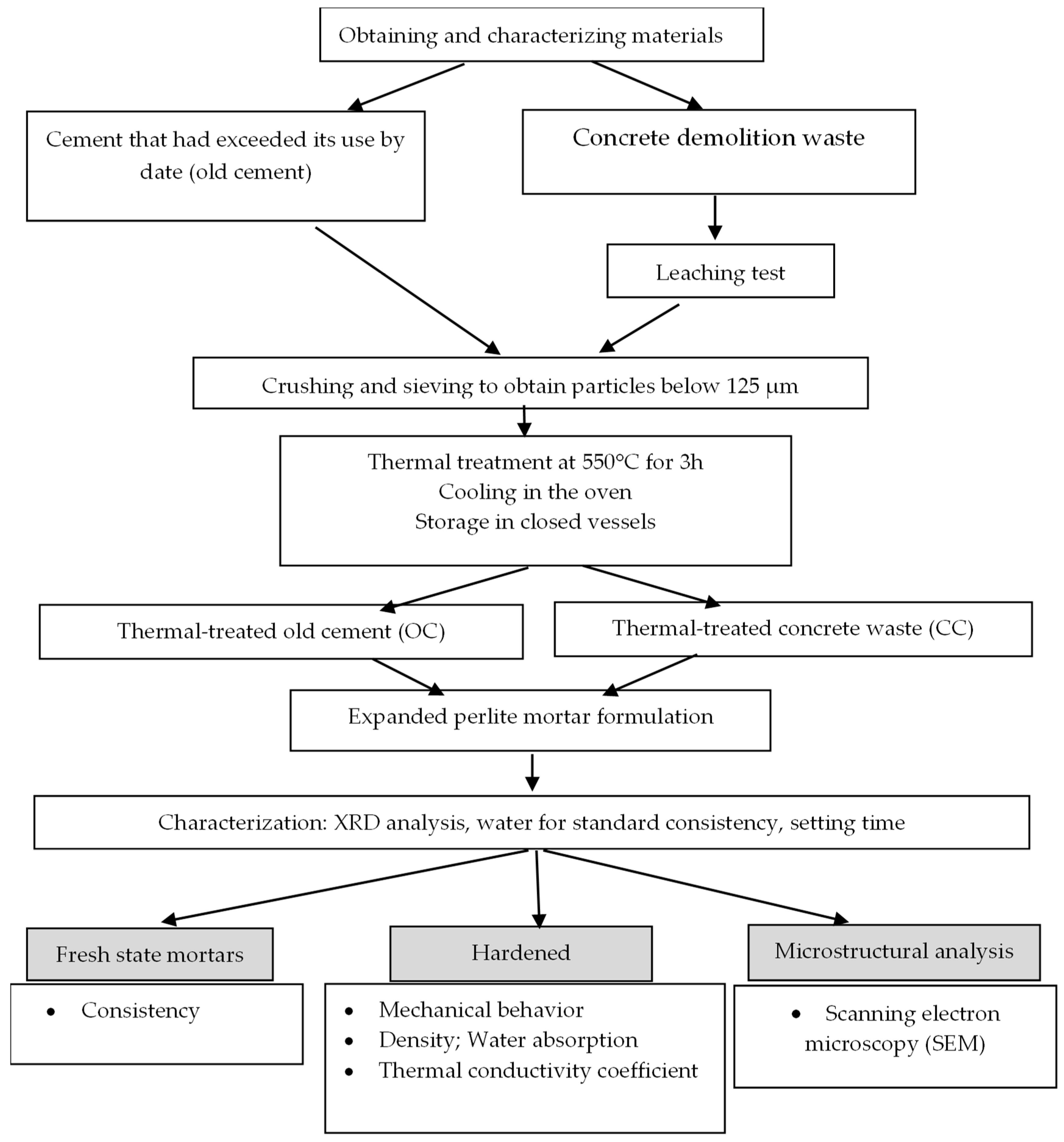
2. Materials and Methods
2.1. Materials
- -
- Evaporable water and some bound water evaporate between 20 and 130 °C.
- -
- Ettringite and gypsum dehydrate between 110 and 200 °C.
- -
- Calcium silicate hydrates and carboaluminate hydrates dehydrate between 140 and 450 °C.
- -
- Portlandite dehydroxilation and α-C2S are formed by calcium silicate depolymerization between 450 and 650 °C.
2.2. Mixing, Molding, and Curing Conditions
2.3. Methods
2.3.1. Mortars in Fresh State
2.3.2. Mortars in Hardened State
Water Absorption
Mechanical Strengths
Thermal Conductivity
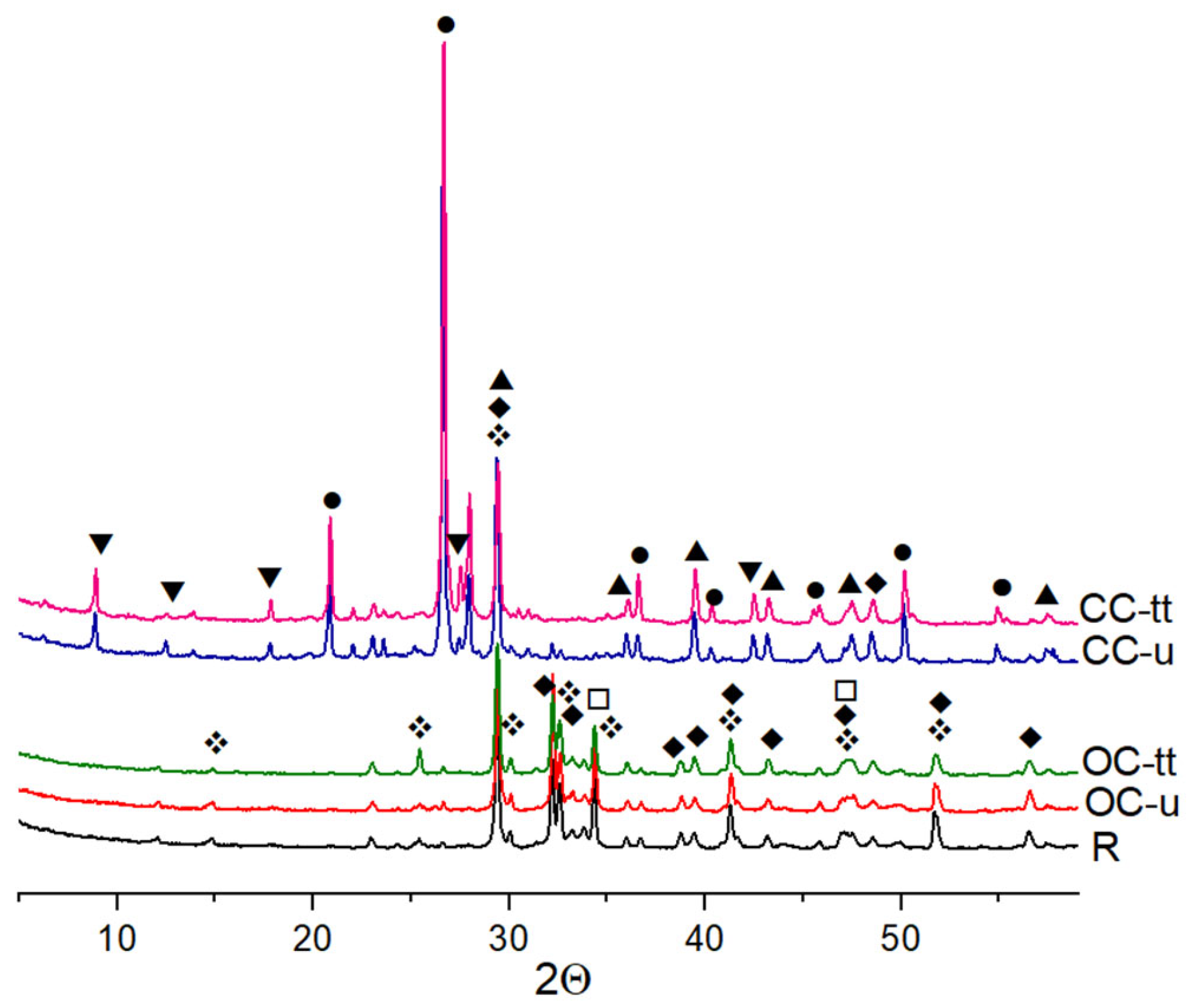
X-ray Diffraction
Microstructure of Mortars
3. Results and Discussion
3.1. Mortars in Fresh State
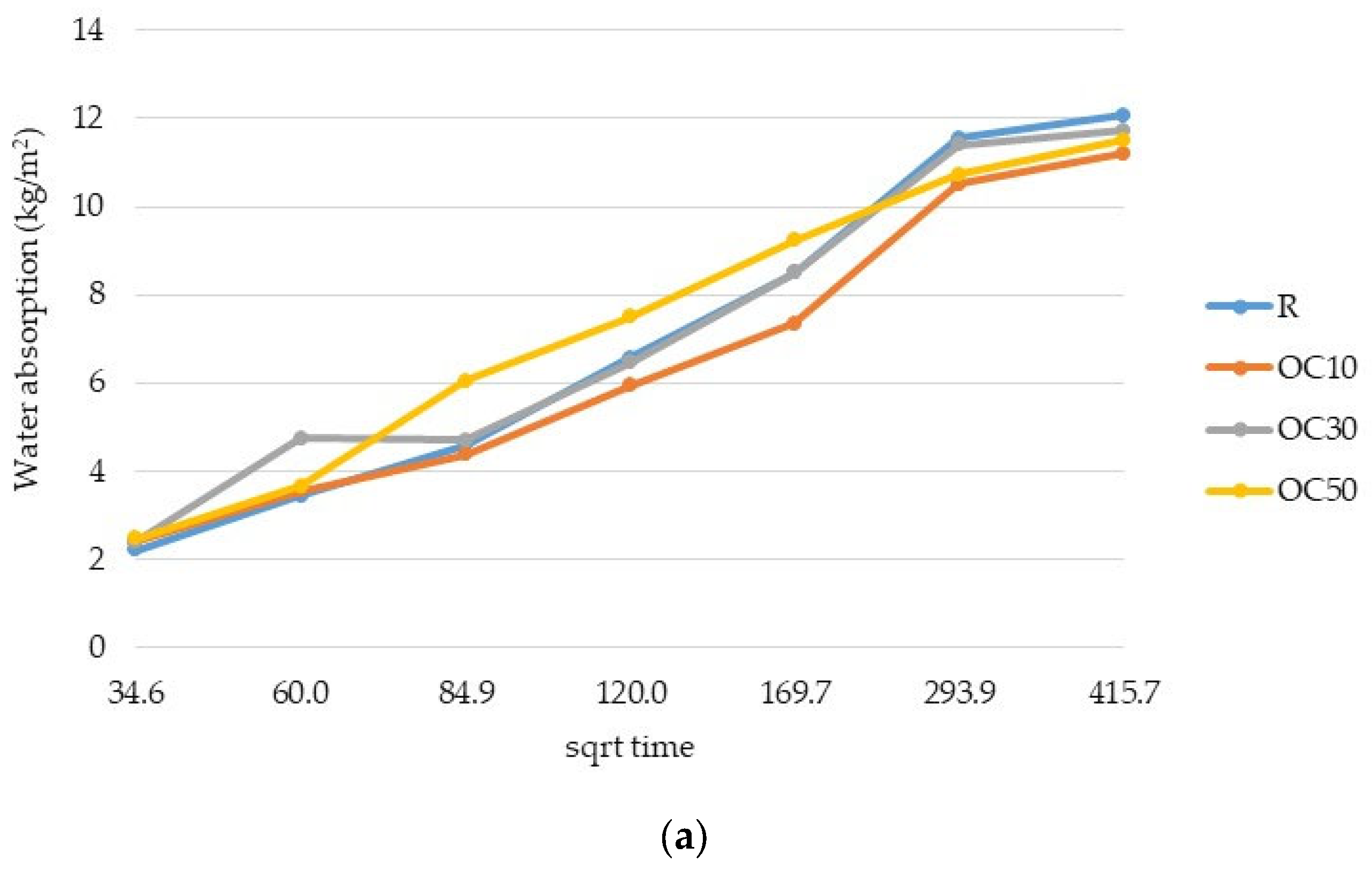
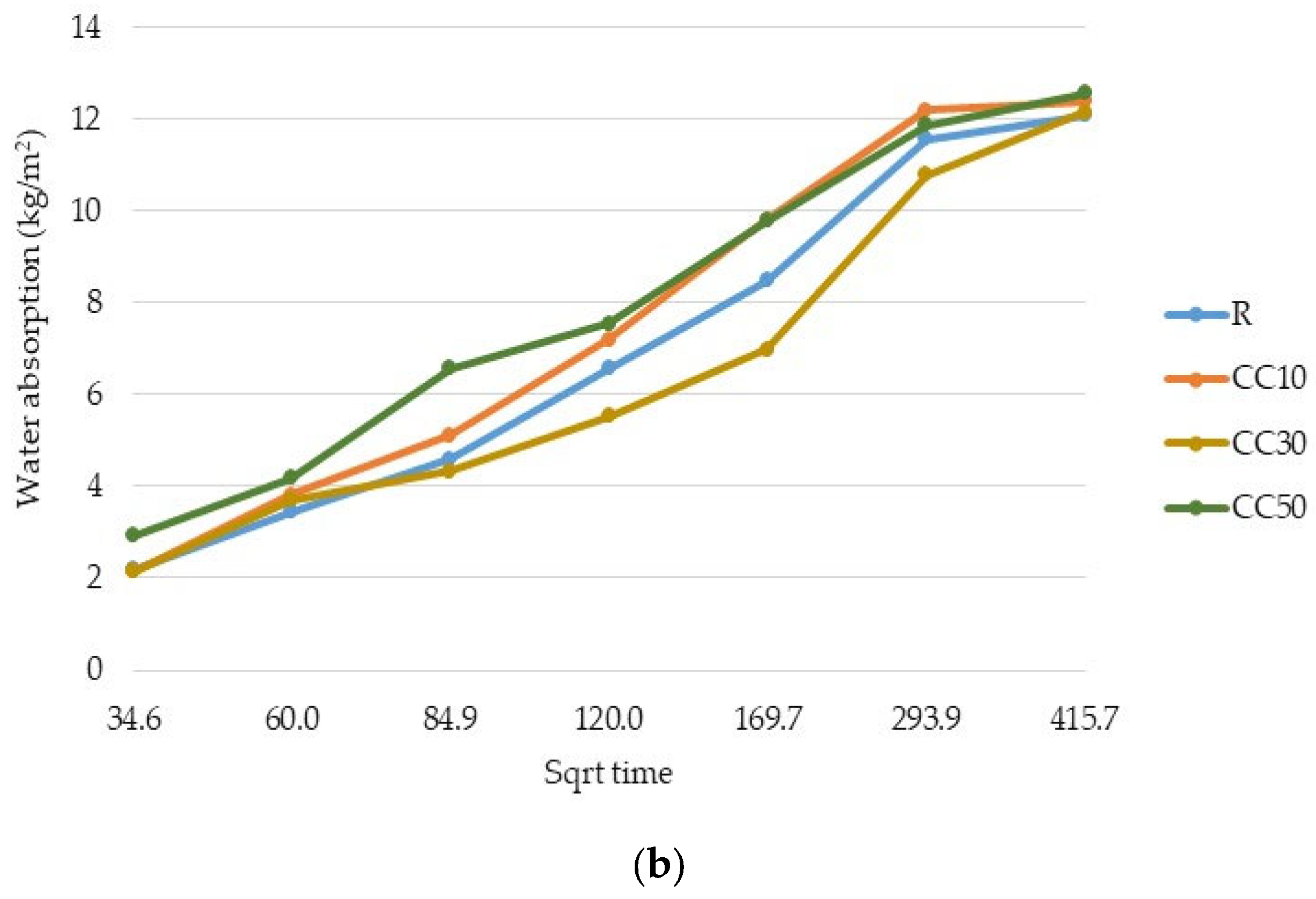
3.2. Water Absorption
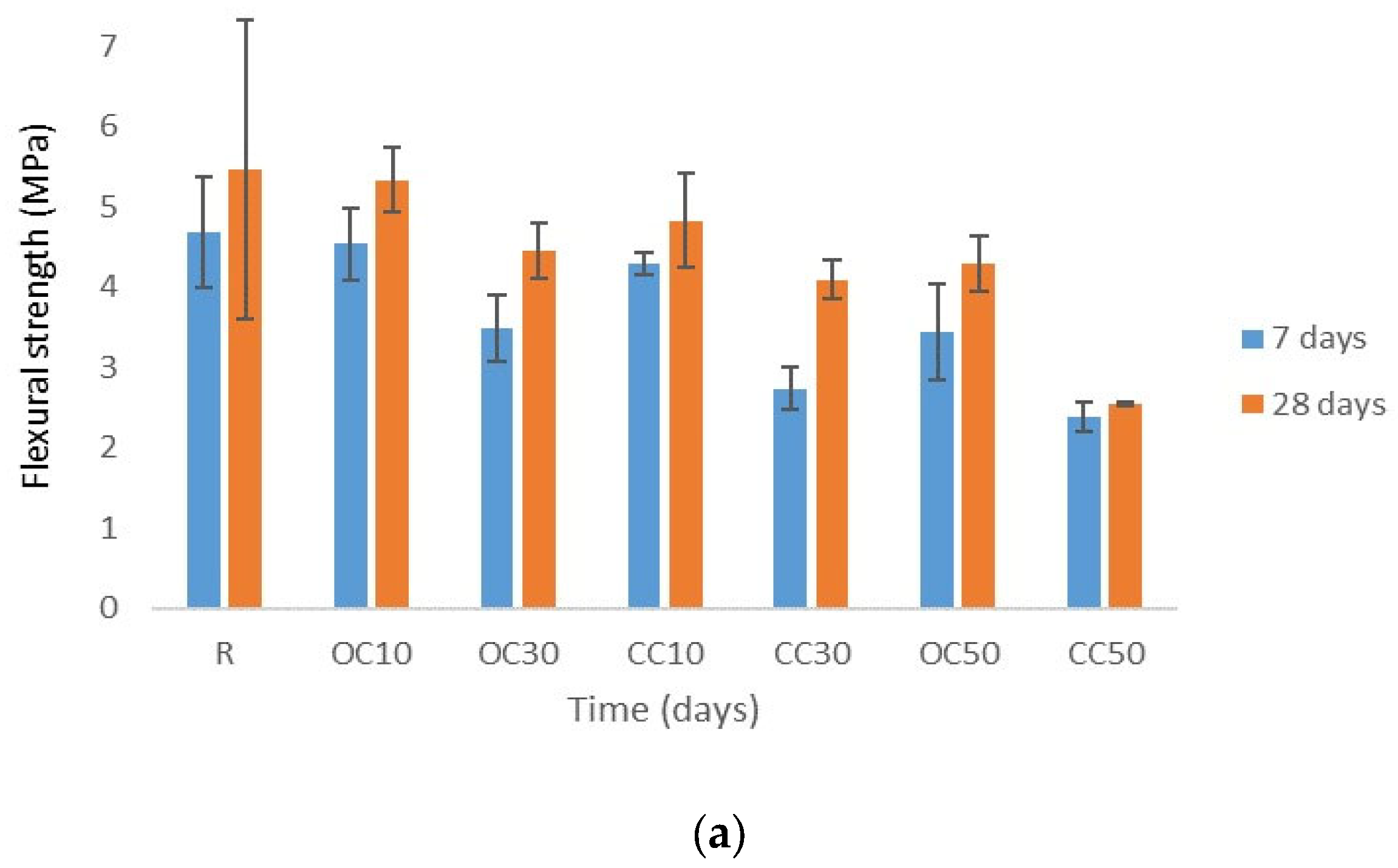
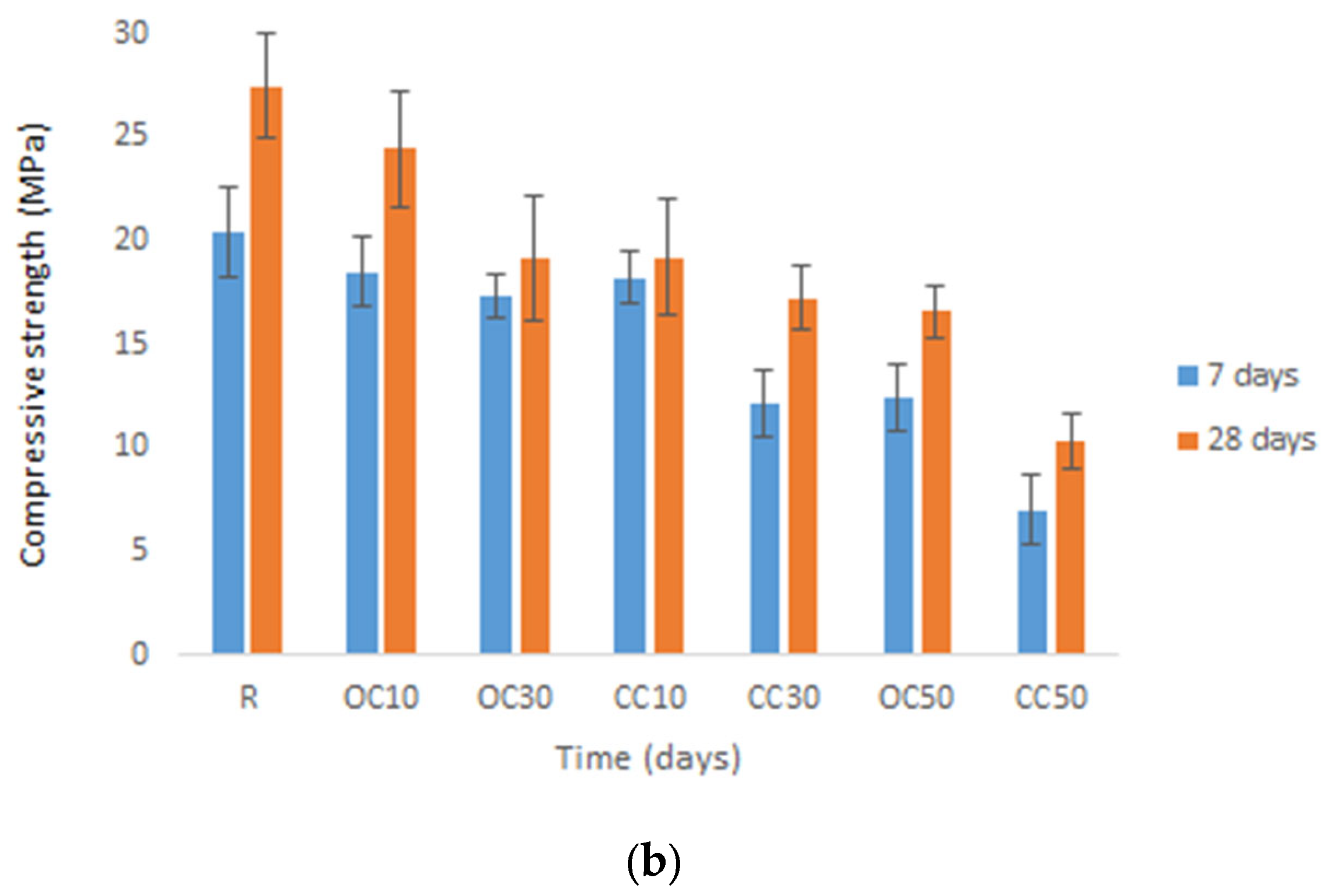
3.3. Mechanical Strengths
3.4. Thermal Conductivity of Mortars
3.5. The Microstructure of Mortars
4. Conclusions
- -
- The water absorption and mechanical strengths of the mortars were influenced by the type and content of calcinated materials.
- -
- The replacement of cement type CEM II/A-LL 42.5R with thermal-treated old cement decreased the 28-day compressive strength of expanded perlite mortar from 11% for 10% OC-tt up to 40% for 50% OC-tt. After 48 h, the water absorption of mortars with 30% and 50% OC-tt was 11.72 kg/m2 and 11.50 kg/m2, respectively, in comparison with 12.3 kg/m2 for the reference mortar.
- -
- The results showed that mortars with CC-tt presented lower efficiency than those with OC-tt and CEM II/A-LL 42.5R. This observation is related to the lower quantity of cement in CC-tt, emphasizing how crucial it is to separate the aggregate from cement paste in concrete demolition waste. The decrease in 28-day compressive strength was in the range of 30–62.5%, the higher diminishment corresponding to 50% CC-tt. At 48 h, the water absorption was comparable to the reference mortar (12.14–12.55 kg/m2).
- -
- The thermal conductivity coefficient of the mortars with thermal-treated materials was between 0.37 and 0.48 W/m·K. The reference had the highest λ-value.
- -
- The SEM images of the mortars revealed the presence of ettringite crystals, calcium silicate hydrates as crumped foils, and hexagonal portlandite crystals. The expanded perlite pores were unbroken.
- -
- A study on the influence of the storage conditions of thermally treated materials on their composition is necessary to identify the maximum storage time in closed vessels, under laboratory conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rashad, A. A synopsis about perlite as a building material—A best practice guide for Civil Engineer. Constr. Build. Mater. 2016, 121, 338–353. [Google Scholar] [CrossRef]
- Petrella, A.; Spasiano, D.; Rizzi, V.; Cosma, P.; Race, M.; De Vietro, N. Lead Ion Sorption by Perlite and Reuse of the Exhausted Material in the Construction Field. Appl. Sci. 2018, 8, 1882. [Google Scholar] [CrossRef]
- Yi, W.; Zhou, X.; Yang, J.; Wang, W.; Tian, T. A comprehensive performance evaluation of the cement-based expanded perlite plastering mortar. Sci. Total Environ. 2023, 858, 159705. [Google Scholar] [CrossRef]
- Gandage, A.S.; Rao, V.R.V.; Sivakumar, M.V.N.; Vasan, A.; Venu, M.; Yaswanth, A.B. Effect of Perlite on Thermal Conductivity of Self Compacting Concrete. Procedia-Soc. Behav. Sci. 2013, 104, 188–197. [Google Scholar] [CrossRef]
- Sengul, O.; Azizi, S.; Karaosmanoğlu, F.; Tasdemir, M. Effect of expanded perlite on the mechanical properties and thermal conductivity of lightweight concrete. Energy Build. 2011, 43, 671–676. [Google Scholar] [CrossRef]
- Tie, T.S.; Mo, K.H.; Alengaram, U.J.; Kaliyavaradhan, S.K.; Ling, T.C. Study on the use of lightweight expanded perlite and vermiculite aggregates in blended cement mortars. Eur. J. Environ. Civ. Eng. 2022, 26, 3612–3631. [Google Scholar] [CrossRef]
- Patthanavarit, J.; Kitiwan, M.; Keawprak, N.; Tunthawiroon, N. Effect of Expanded Perlite on Physical and Mechanical Properties of Cement Mortar. AIP Conf. Proc. 2021, 2397, 070001. [Google Scholar] [CrossRef]
- Lanzon, M.; Lanzon-Garcia, M. Lightweight cement mortars: Advantages and inconveniences of expanded perlite and its influence on fresh and hardened state and durability. Constr. Build. Mater. 2008, 22, 1798–1806. [Google Scholar] [CrossRef]
- Balbuena, J.; Sánchez, M.; Sánchez, L.; Cruz-Yusta, M. Lightweight Mortar Incorporating Expanded Perlite, Vermiculite, and Aerogel: A Study on the Thermal Behavior. Materials 2024, 17, 711. [Google Scholar] [CrossRef] [PubMed]
- Oswaldo Burciaga-Diaz, J.; Gomez-Zamorano, L.Y.; Ivan Escalante-Garcia, J. Transforming construction and demolition waste concrete as a precursor in sustainable cementitious materials: An innovative recycling approach. Resour. Conserv. Recycl. 2024, 204, 107474. [Google Scholar] [CrossRef]
- Popescu, D.; Burlacu, A. Consideration on the benefits of using recyclable materials for construction. Rom. J. Transp. Infrastruct. 2017, 6, 43–53. [Google Scholar] [CrossRef]
- Dacic, A.; Fenyvesi, O. Packing density investigation of blended cement pastes incorporating waste perlite and recycled concrete powder. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Bogas, J.A.; Carriço, A.; Tenza-Abril, A.J. Microstructure of thermoactivated recycled cement pastes. Cem. Concr. Res. 2020, 138, 106226. [Google Scholar] [CrossRef]
- Bogas, J.A.; Real, S.; Carriço, A.; Abrantes, J.C.C.; Guedes, M. Hydration and phase development of recycled cement. Cem. Concr. Compos. 2022, 127, 104405. [Google Scholar] [CrossRef]
- Carriço, A.; Real, S.; Bogas, J.A.; Costa Pereira, M.F. Mortars with thermo activated recycled cement: Fresh and mechanical characterization. Constr. Build. Mater. 2020, 256, 119502. [Google Scholar] [CrossRef]
- Alonso, C.; Fernandez, L. Dehydration and rehydration processes of cement paste exposed to high-temperature environments. J. Mater. Sci. 2004, 39, 3015–3024. [Google Scholar] [CrossRef]
- Shui, Z.; Xuan, D.; Chen, W.; Yu, R.; Zhang, R. Cementitious characteristics of hydrated cement paste subjected to various dehydration temperatures. Constr. Build. Mater. 2009, 23, 531–537. [Google Scholar] [CrossRef]
- Bogas, J.A.; Carriço, A.; Pereira, M.F.C. Mechanical characterization of thermally activated low-carbon recycled cement mortars. J. Clean. Prod. 2019, 218, 377–389. [Google Scholar] [CrossRef]
- Wang, J.; Mu, M.; Liu, Y. Recycled cement. Constr. Build. Mater. 2018, 190, 1124–1132. [Google Scholar] [CrossRef]
- Florea, M.V.A.; Ning, Z.; Brouwers, H.J.H. Activation of liberated concrete fines and their application in mortars. Constr. Build. Mater. 2014, 50, 1–12. [Google Scholar] [CrossRef]
- Florea, M.V.A.; Brouwers, H.J.H. Recycled concrete fines and aggregates—The composition of various size fractions related to crushing history. In Proceedings of the 18th International Conference on Building Materials (IBAUSIL), Weimar, Germany, 12–15 September 2012; pp. 1034–1041. Available online: https://josbrouwers.bwk.tue.nl/publications/Conference92.pdf (accessed on 4 July 2024).
- Kwon, E.; Ahn, J.; Cho, B.; Park, D. A study on the development of recycled cement made from waste cementitious powder. Constr. Build. Mater. 2018, 83, 174–180. [Google Scholar] [CrossRef]
- He, Z.; Zhu, X.; Wang, J.; Mu, M.; Wang, Y. Comparison of CO2 emissions from OPC and recycled cement production. Constr. Build. Mater. 2019, 211, 965–973. [Google Scholar] [CrossRef]
- Gastaldi, D.; Canonico, F.; Capelli, L.; Buzzi, L.; Boccaleri, E.; Irico, S. An investigation on the recycling of hydrated cement from concrete demolition waste. Cem. Concr. Comp. 2015, 61, 29–35. [Google Scholar] [CrossRef]
- Tokareva, A.; Kaassamani, S.; Waldmann, D. Fine demolition wastes as Supplementary cementitious materials for CO2 reduced cement production. Constr. Build. Mater. 2023, 392, 131991. [Google Scholar] [CrossRef]
- Sousa, V.; Bogas, J.A. Comparison of energy consumption and carbon emissions from clinker and recycled cement production. J. Clean. Prod. 2021, 306, 127277. [Google Scholar] [CrossRef]
- Decree no. 95/2005 on Establishing Acceptance Criteria and Preliminary Procedures for the Acceptance of Waste Storage and National List of Waste Accepted in Each Class of Landfill. Official Gazette no. 194, 8 March 2005.
- SR EN 12457-1; Characterisation of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges-Part 1: One Stage Batch Test at a Liquid to Solid Ratio of 2 L/kg for Materials with High Solid Content and with Particle Size below 4 mm (Without or With Size Reduction). Romanian Standard Association: Bucharest, Romania, 2003.
- SR EN 12457-2; Characterisation of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges-Part 2: One Stage Batch Test at a Liquid-to-Solid Ratio of 10 L/kg for Materials with Particle Size below 4 mm (Without or With Size Reduction). Romanian Standard Association: Bucharest, Romania, 2003.
- Xu, L.; Wang, J.; Li, K.; Lin, S.; Li, M.; Hao, T.; Ling, Z.; Xiang, D.; Wang, T. A systematic review of factors affecting properties of thermal-activated recycled cement. Resour. Conserv. Recycl. 2022, 185, 106432. [Google Scholar] [CrossRef]
- Algourdin, N.; Sy Hung, B.; Mesticou, Z.A.; Si, L. Effects of high temperature on mechanical behaviour and physicochemical properties of recycled mortars and its components. Constr. Build. Mater. 2020, 248, 118554. [Google Scholar] [CrossRef]
- SR EN 196-3; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. Romanian Standard Association: Bucharest, Romania, 2017.
- Carriço, A.; Bogas, J.A.; Guedes, M. Thermoactivated cementitious materials—A review. Constr. Build. Mater. 2020, 250, 118873. [Google Scholar] [CrossRef]
- Shui, Z.; Xuan, D.; Wan, H.; Cao, B. Rehydration reactivity of recycled mortar from concrete waste experienced to thermal treatment. Constr. Build. Mater. 2008, 22, 1723–1729. [Google Scholar] [CrossRef]
- SR EN 1015-3; Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table). Romanian Standard Association: Bucharest, Romania, 2001.
- SR EN ISO 15148; Hydrothermal Performance of Building Materials and Products-Determination of Water Absorption Coefficient by Partial Immersion. Romanian Standard Association: Bucharest, Romania, 2004.
- SR EN 1015-11; Methods of Test for Mortar for Masonry Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. Romanian Standard Association: Bucharest, Romania, 2019.
- EN 12667; Thermal Performance of Building Materials and Products—Determination of Thermal Resistance by Means of Guarded Hot Plate and Heat Flow Meter Methods—Products of High and Medium Thermal Resistance. European Committee for Standardization: Brussels, Belgium, 2001.
- Silva, L.M.; Ribeiro, R.A.; Labrincha, J.A.; Ferreira, V.M. Role of lightweight fillers on the properties of a mixed-binder mortar. Cem. Concr. Compos. 2010, 32, 19–24. [Google Scholar] [CrossRef]
- Xiong, H.; Yuan, K.; Xu, J.; Wen, J. Pore structure, adsorption, and water absorption of expanded perlite mortar in external thermal insulation composite system during aging. Cem. Concr. Compos. 2021, 116, 103900. [Google Scholar] [CrossRef]
- Xiong, H.R.; Yuan, K.L.; Wen, M.J.; Yu, A.N.; Xu, J.M. Influence of pore structure on the moisture transport property of external thermal insulation composite system as studied by NMR. Const. Build. Mater. 2019, 228, 116815. [Google Scholar] [CrossRef]
- Martys, N.S.; Ferraris, C.F. Capillary transport in mortars and concrete. Cem. Concr. Res. 1997, 27, 747–760. [Google Scholar] [CrossRef]
- Yang, L.; Gao, D.; Zhang, Y.; Tang, J.; Li, Y. Relationship between sorptivity and capillary coefficient for water absorption of cement-based materials: Theory analysis and experiment. R. Soc. Open Sci. 2019, 6, 190112. [Google Scholar] [CrossRef]
- Maaloufa, Y.; Mounir, S.; Khabbazi, A.; Kettar, J.; Khaldoun, A. Thermal Characterization of Materials based on Clay and Granular: Cork or Expanded Perlite. Energy Procedia 2015, 74, 1150–1161. [Google Scholar] [CrossRef]






| Colour | Light Grey |
|---|---|
| Bulk density (SR EN 1097-3/2002) | 40–65 kg/m3 |
| Size distribution (SR EN 933/2002) | 0–2 mm, max 10% 0.5 mm |
| Thermal conductivity coefficient (SR EN 12667/2002) | max 0.042 W/m K |
| Compaction resistance (SR EN 13055-1/2003) | Min. 0.07 N/mm2 |
| Fire reaction class | A1 |
| L/S Ratio | ||||
|---|---|---|---|---|
| 2 | 10 | |||
| Concentration (mg/kg) | Reference Value (mg/kg) | Concentration (mg/kg) | Reference Value (mg/kg) | |
| As | 0.012 | 0.1 | 0.05 | 0.5 |
| Ba | 0.064 | 7 | 0.17 | 20 |
| Cd | <0.002 | 0.03 | <0.01 | 0.04 |
| Cr total | 0.004 | 0.2 | 0.04 | 0.5 |
| Cu | 0.13 | 0.9 | 0.69 | 2 |
| Hg | <0.0001 | 0.003 | <0.0005 | 0.01 |
| Mo | 0.036 | 0.3 | 0.05 | 0.5 |
| Ni | 0.008 | 0.2 | 0.03 | 0.4 |
| Pb | 0.006 | 0.2 | 0.02 | 0.5 |
| Sb | 0.01 | 0.02 | 0.02 | 0.06 |
| Se | <0.002 | 0.06 | <0.01 | 0.1 |
| Zn | 0.052 | 2 | 0.34 | 4 |
| Fluorides | 2.001 | 4 | 5.998 | 10 |
| Phenol index | <0.2 | 0.5 | <1 | 1 |
| Old Cement Thermal Treated (OC-tt) | Concrete Waste Thermal Treated (CC-tt) | CEM II/A-LL 42.5R (R) | |
|---|---|---|---|
| Bulk density (kg/m3) | 883 | 991 | 1113 |
| Water for standard consistency (%) SR EN 196-3 [32] | 0.38 | 0.34 | 0.31 |
| Setting time (min.) | |||
| SR EN 196-3 [32] | |||
| initial | 190 | >24 ore | 135 |
| final | 295 | >100 ore | 195 |
| Compressive strength (MPa) on paste, 7 days | 31.4 | 0.16 | 47.2 |
| Mortar Code | CEM II/A-LL 42.5R (kg) | OC-tt (kg) | CC-tt (kg) | Water/Cement | Consistency (mm) | Density of Dry Mortars (kg/m3) |
|---|---|---|---|---|---|---|
| R | 100 | 0.53 | 142 | 1332 | ||
| OC10 | 90 | 10 | 0.55 | 141 | 1317 | |
| OC30 | 70 | 30 | 0.56 | 144 | 1260 | |
| OC50 | 50 | 50 | 0.61 | 152 | 1171 | |
| CC10 | 90 | 10 | 0.56 | 147 | 1315 | |
| CC30 | 70 | 30 | 0.61 | 141 | 1254 | |
| CC50 | 50 | 50 | 0.69 | 159.5 | 1027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saca, N.; Radu, L.; Stoleriu, S.; Dobre, D.; Calotă, R.; Truşcă, R. Investigation of Physical-Mechanical Properties and Microstructure of Mortars with Perlite and Thermal-Treated Materials. Materials 2024, 17, 3412. https://doi.org/10.3390/ma17143412
Saca N, Radu L, Stoleriu S, Dobre D, Calotă R, Truşcă R. Investigation of Physical-Mechanical Properties and Microstructure of Mortars with Perlite and Thermal-Treated Materials. Materials. 2024; 17(14):3412. https://doi.org/10.3390/ma17143412
Chicago/Turabian StyleSaca, Nastasia, Lidia Radu, Stefania Stoleriu, Daniela Dobre, Răzvan Calotă, and Roxana Truşcă. 2024. "Investigation of Physical-Mechanical Properties and Microstructure of Mortars with Perlite and Thermal-Treated Materials" Materials 17, no. 14: 3412. https://doi.org/10.3390/ma17143412
APA StyleSaca, N., Radu, L., Stoleriu, S., Dobre, D., Calotă, R., & Truşcă, R. (2024). Investigation of Physical-Mechanical Properties and Microstructure of Mortars with Perlite and Thermal-Treated Materials. Materials, 17(14), 3412. https://doi.org/10.3390/ma17143412








