Experiments on Chloride Binding and Its Release by Sulfates in Cementitious Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
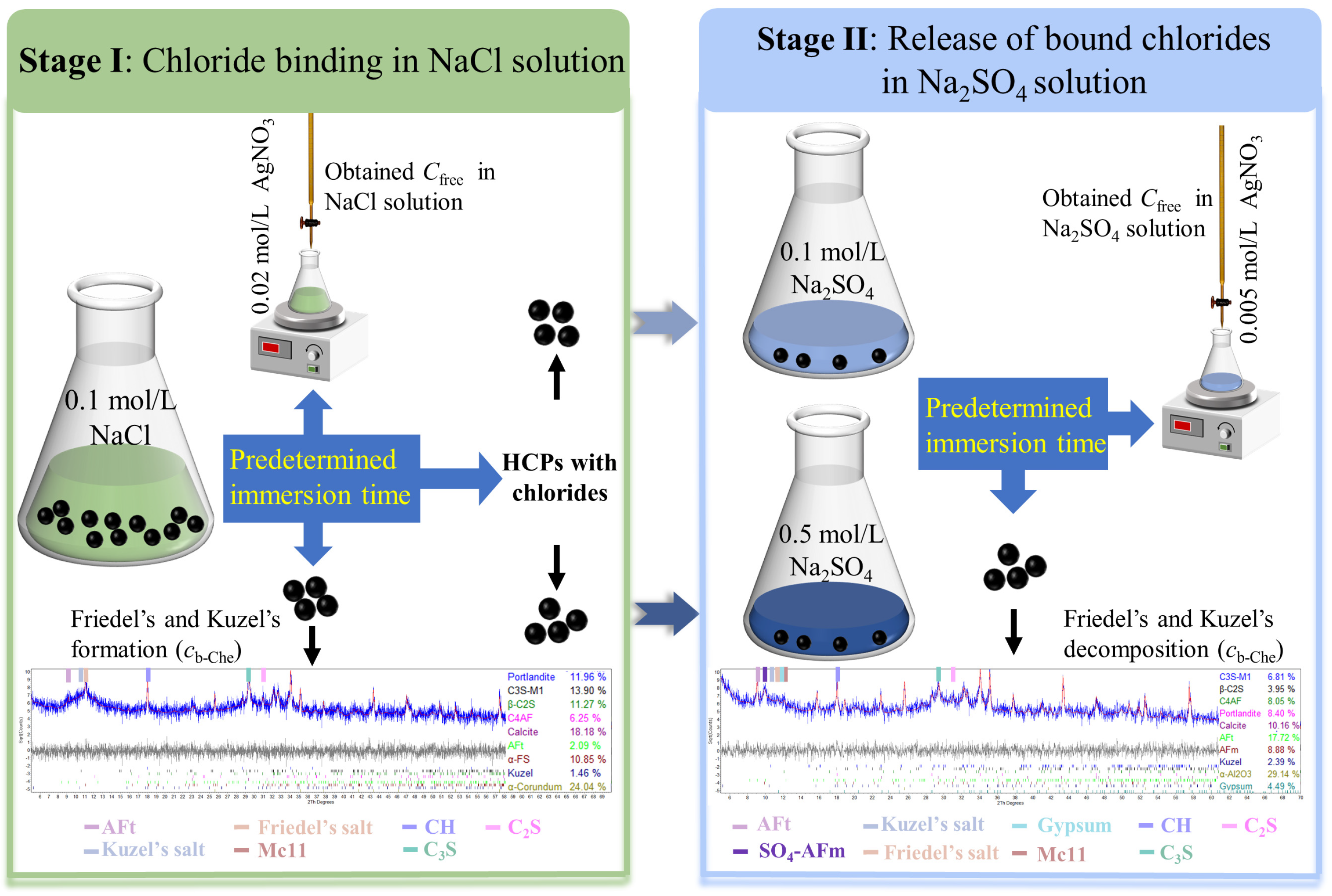
2.3. Immersion Experiment
2.4. Measured Methods
2.4.1. Silver Nitrate Titration
2.4.2. Quantitative X-ray Diffraction (QXRD)
2.4.3. Chloride Content
3. Results and Discussion
3.1. Chloride-Binding Capacity
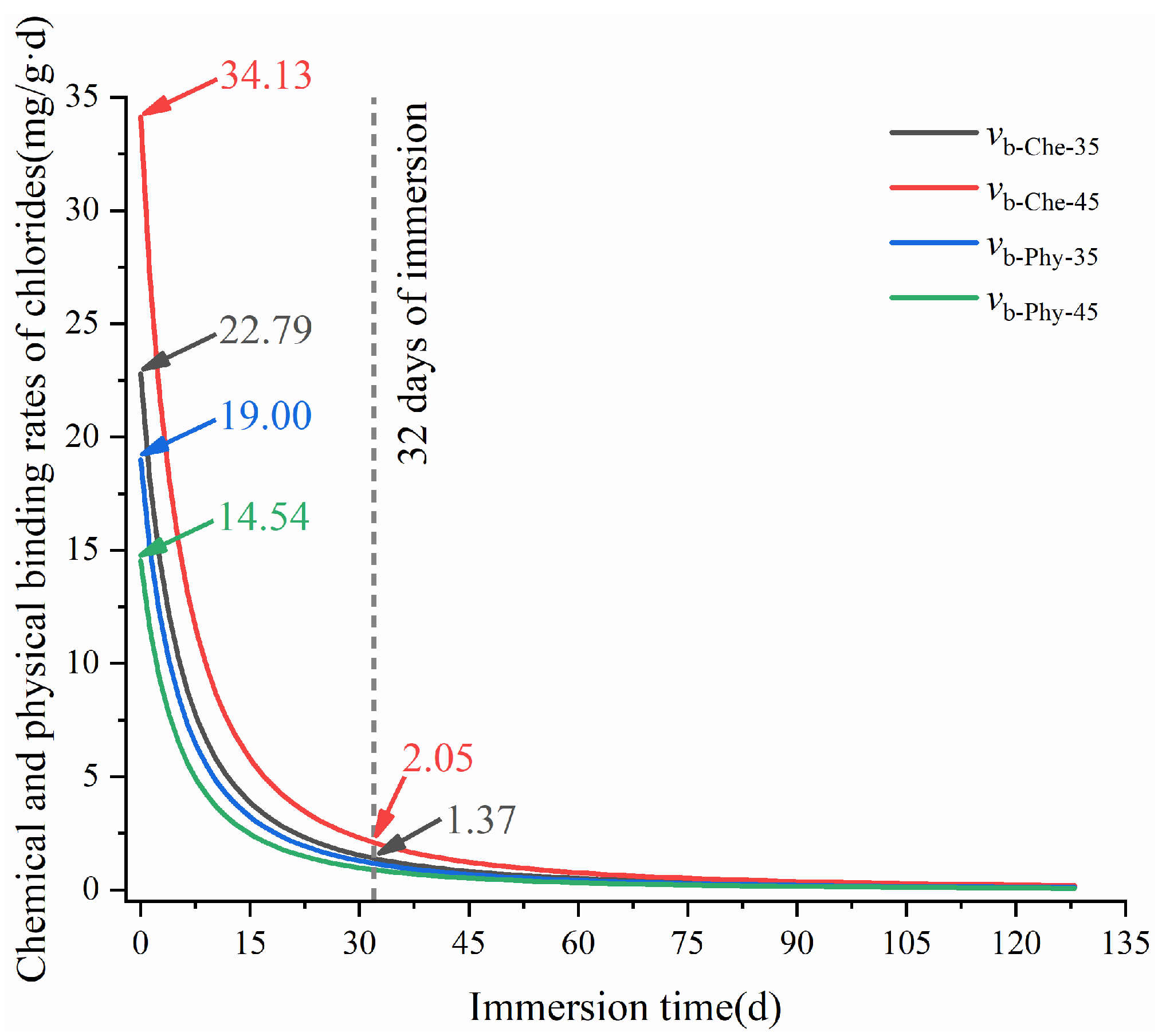
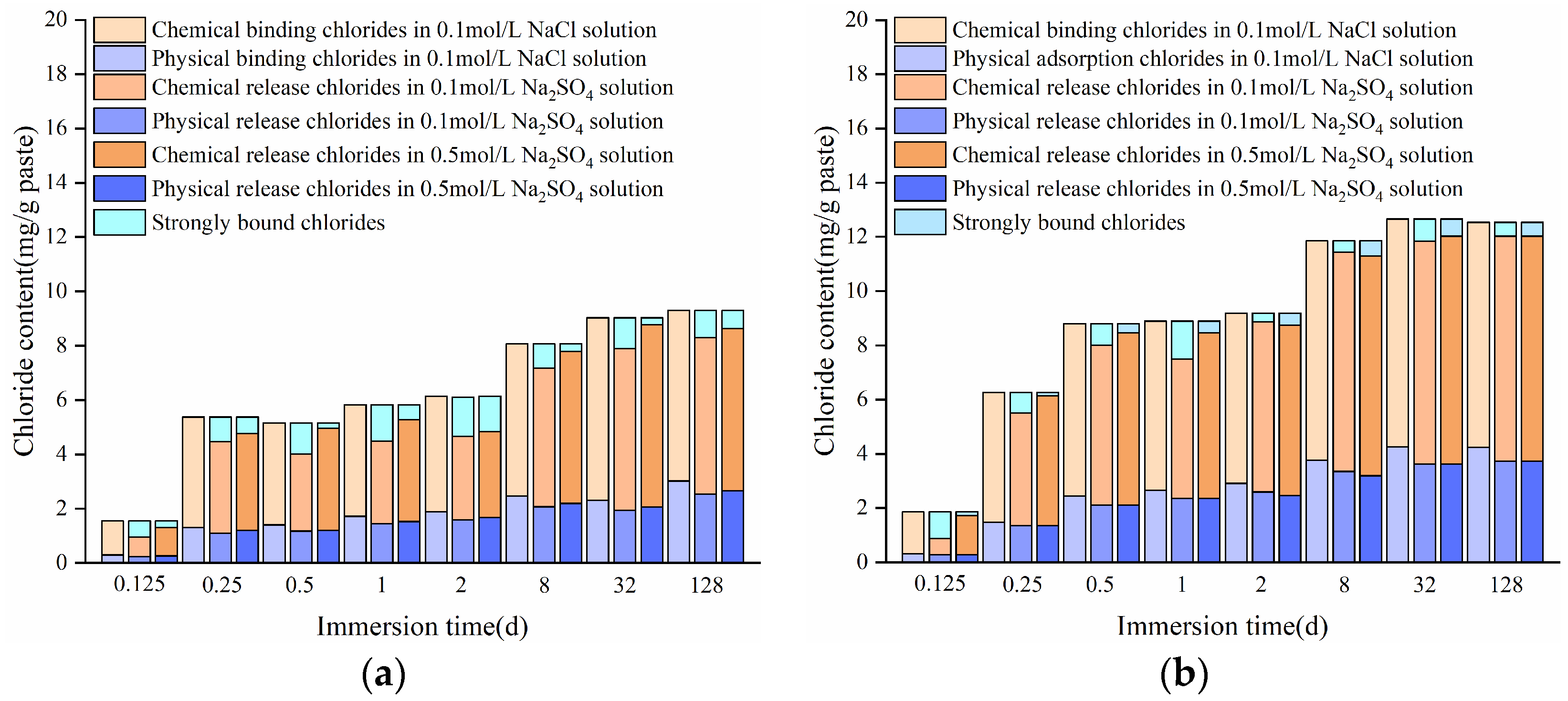
3.1.1. Chloride-Binding Process
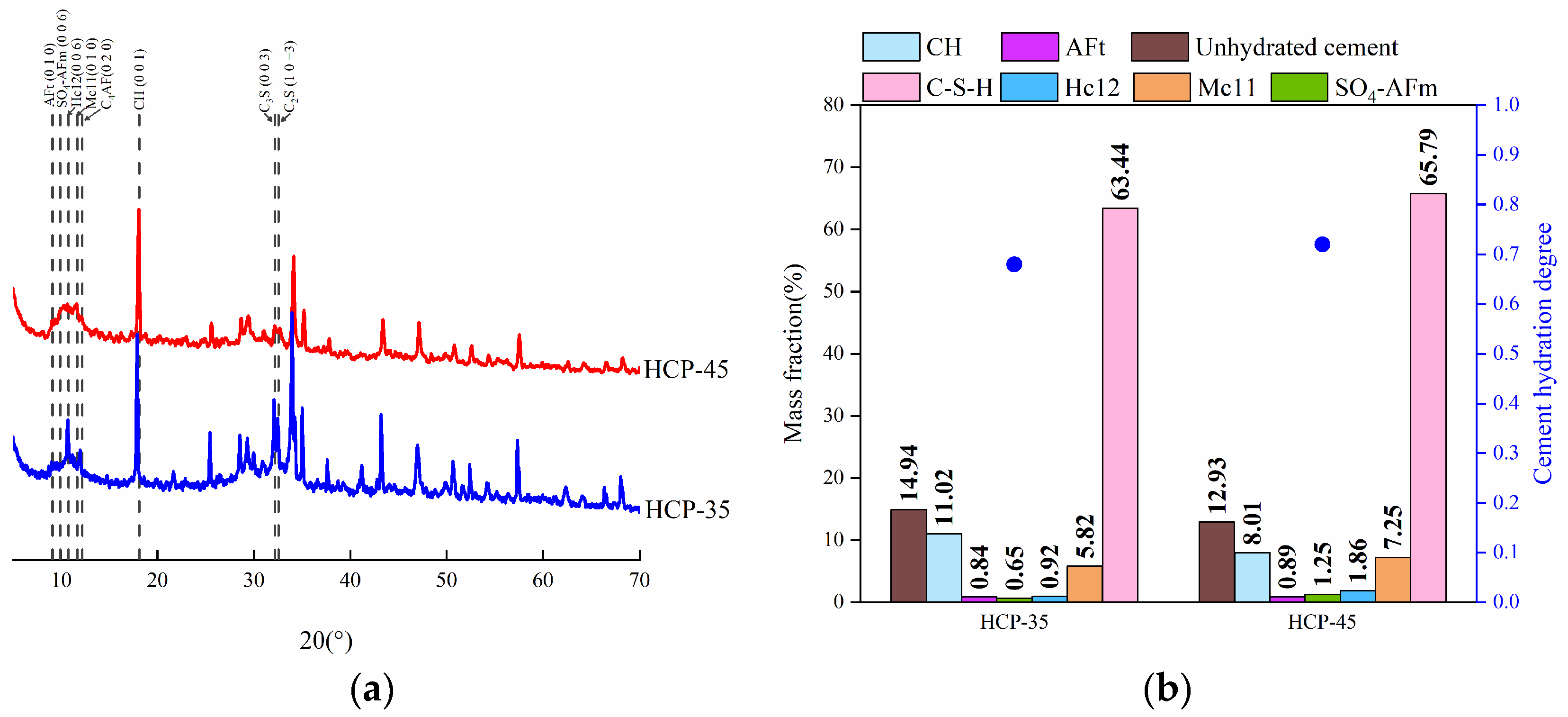
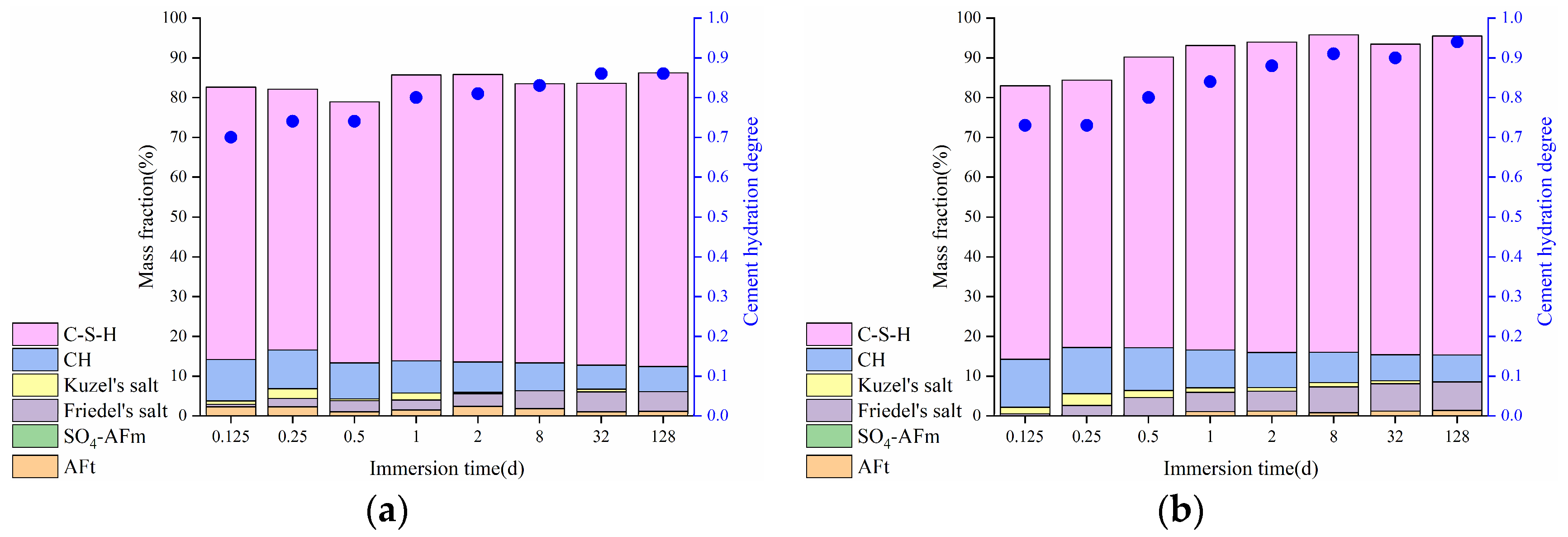
3.1.2. Phase Composition
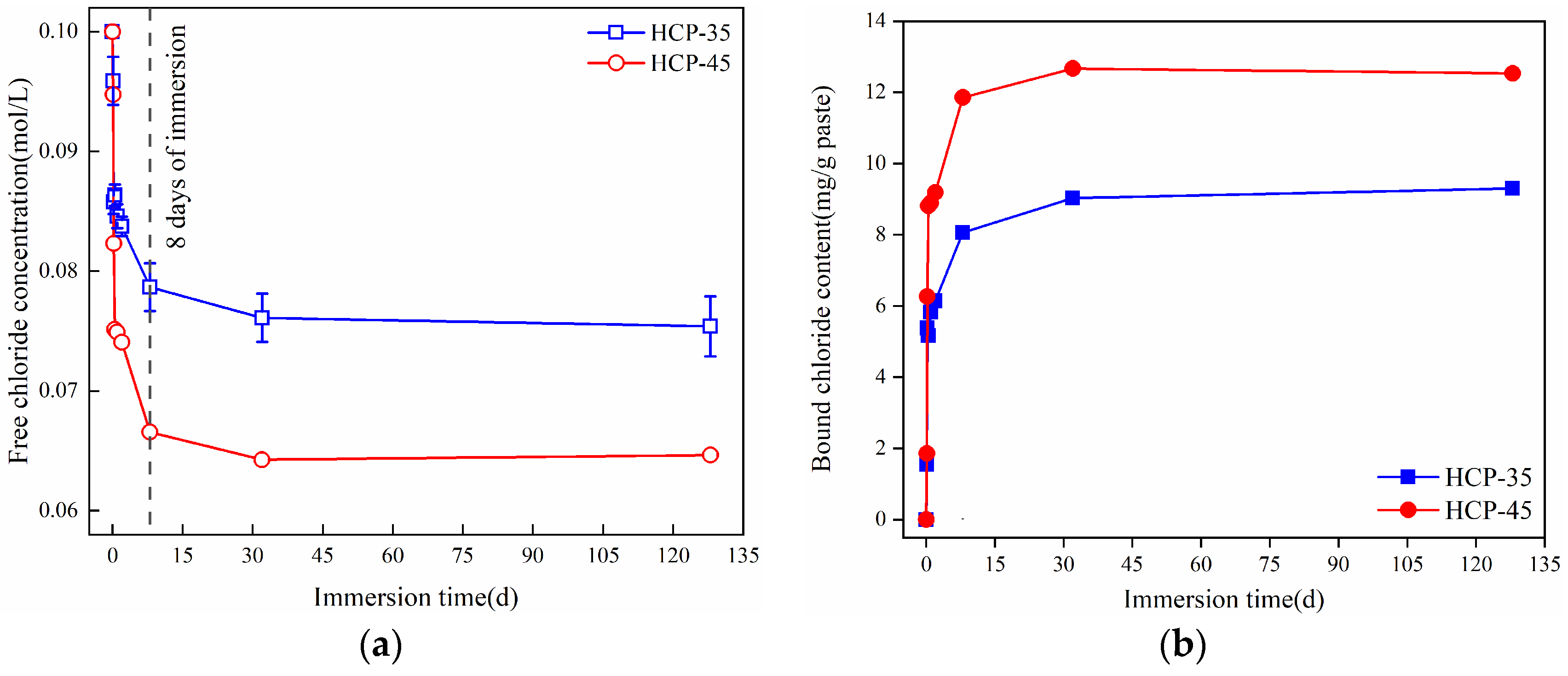
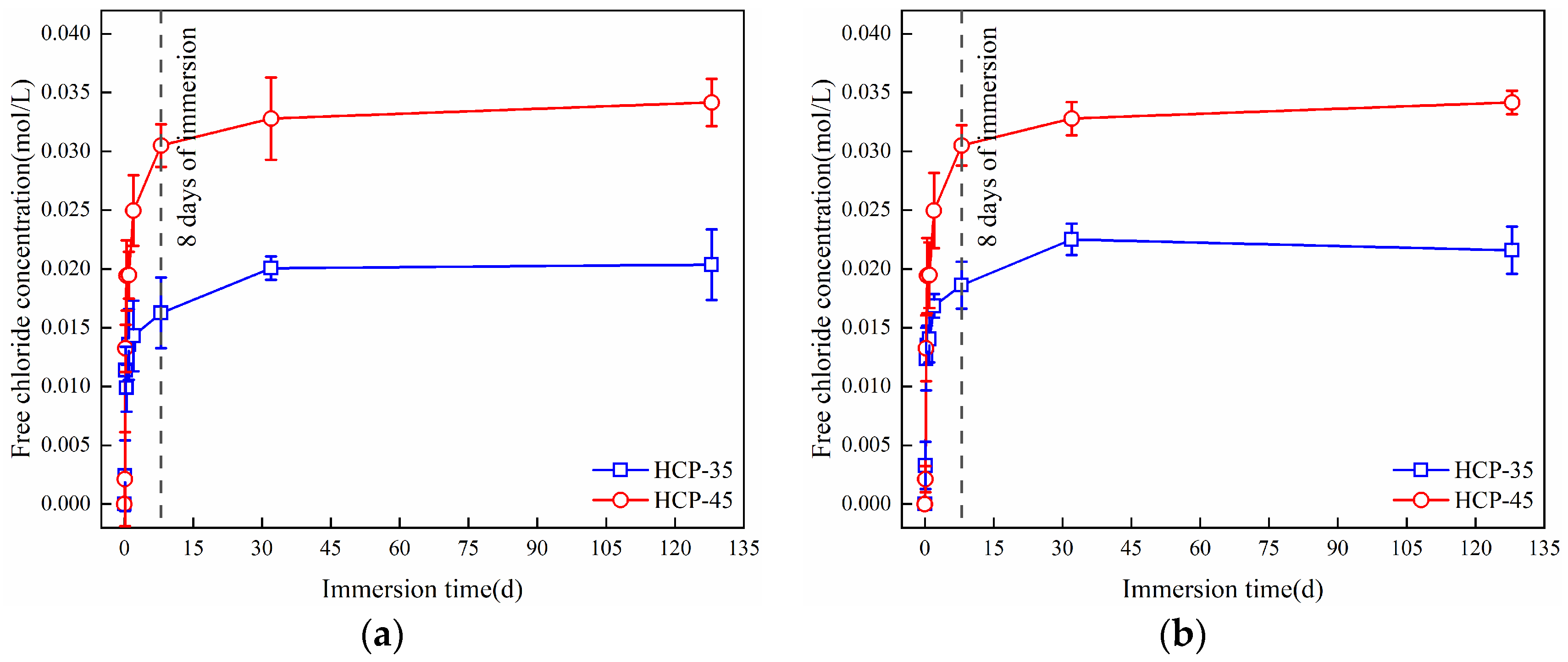
3.1.3. Binding of Free Chlorides
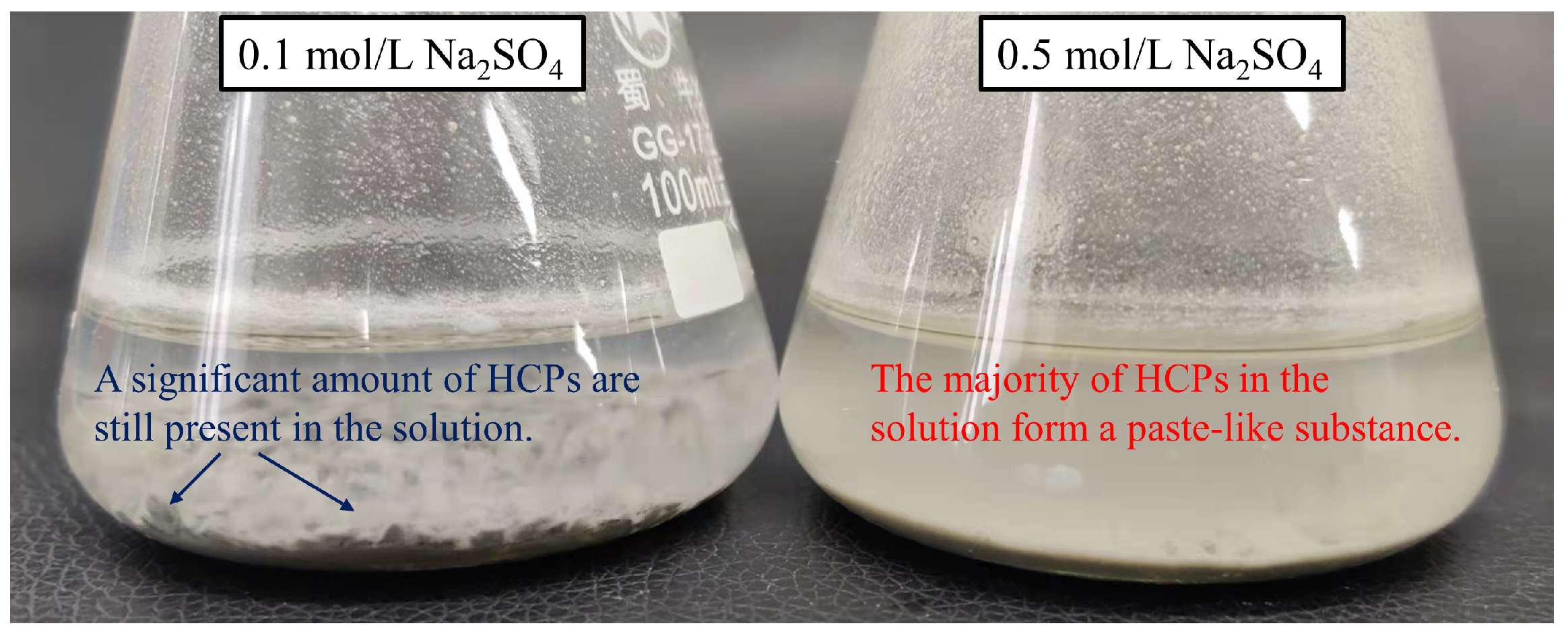
3.2. Sulfate-Induced Release of Bound Chlorides
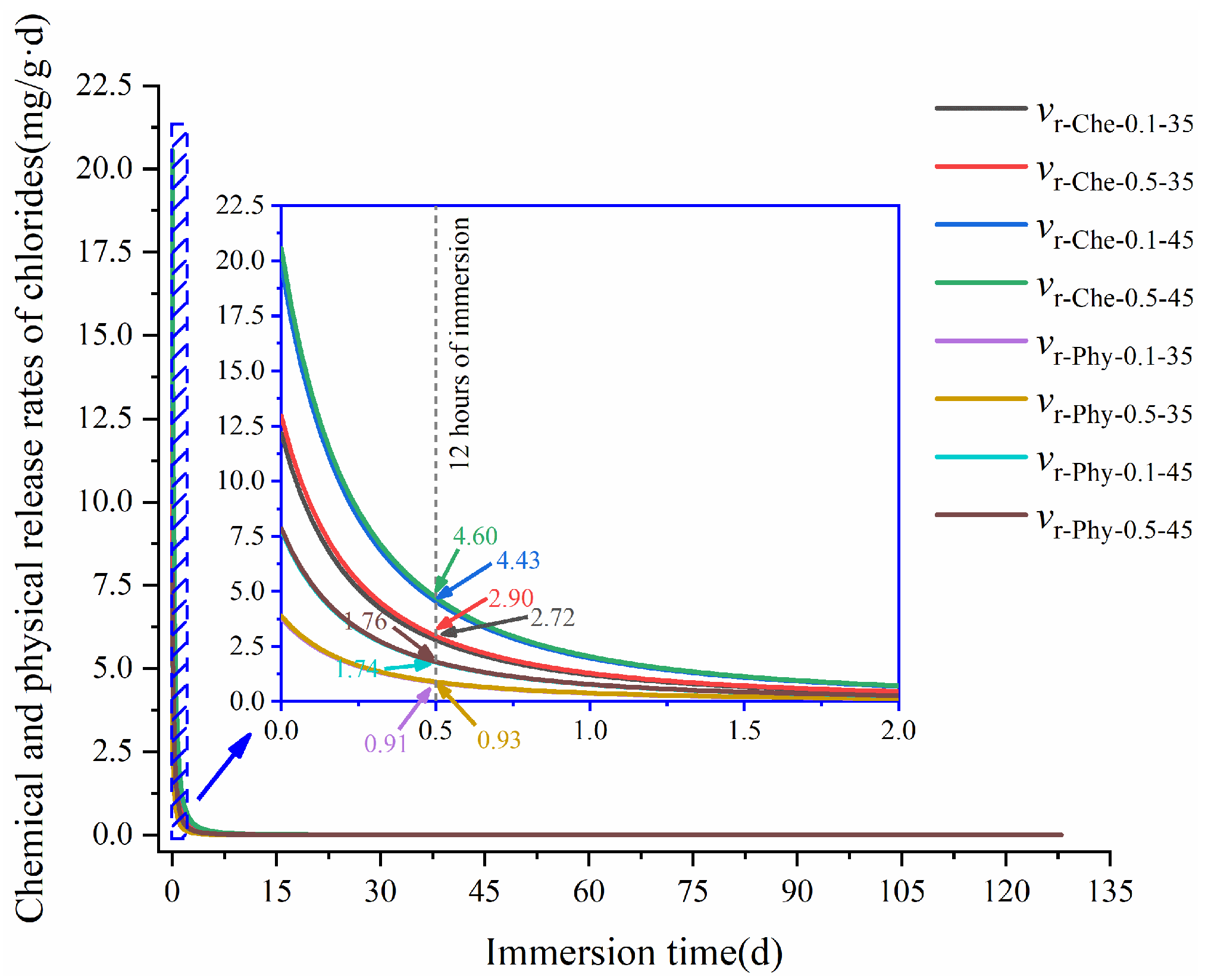
3.2.1. Release Process of Bound Chlorides
3.2.2. Phase Composition
3.2.3. Release of Bound Chlorides
4. Conclusions
- The content of chemically bound chlorides in the HCPs accounts for more than 70% of the total bound chloride content. This is mainly determined by the content of chlorides bound in Friedel’s salt. HPCs with a high w/c exhibit better chloride-binding capacity (CBC) than those with a low w/c.
- A high concentration of sulfates can accelerate the release of bound chlorides in the HCPs. The chemically bound chlorides can be released due to the sulfate-induced decomposition of Friedel’s and Kuzel’s salts, and the bound chlorides in Kuzel’s salt are not easier to release by sulfates compared to those in Friedel’s salt.
- Both the w/c and sulfate concentration significantly influence the chloride-binding process in cementitious materials, and a high w/c results in high binding rates of chlorides, while increased w/c and sulfate concentration can increase the release rates of chlorides.
- The influence of sulfates on the CBC of HCPs in cementitious materials has been investigated, and it can be quantitatively characterized by the contents of chemically or physically bound chlorides and their release by sulfates. The results of this study provide insights into the interaction between chlorides and sulfates, and further model the coupled chloride and sulfate diffusion in concrete and structures exposed to chloride–sulfate environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, S.W.; Yao, Y.; Andrade, C.; Li, Z.J. Recent durability studies on concrete structure. Cem. Concr. Res. 2015, 78, 143–154. [Google Scholar] [CrossRef]
- Heikal, M.; Ali, M.A.; Ghernaout, D.; Elboughdiri, N.; Ghernaout, B.; Bendary, H.I. Prolonging the durability of maritime constructions through a sustainable and salt-resistant cement composite. Materials 2023, 16, 6876. [Google Scholar] [CrossRef] [PubMed]
- Bonfil, D.; Veleva, L.; Escalante-Garcia, J.I. Effect of a hybrid pumice–Portland cement extract on corrosion activity of stainless steel SS304 and carbon mild steel A36. Materials 2024, 17, 2255. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ou, G.F.; Qiu, Q.W.; Chen, X.C.; Hong, J.; Xing, F. Chloride transport and microstructure of concrete with/without fly ash under atmospheric chloride condition. Constr. Build. Mater. 2017, 146, 493–501. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.J.; De Schutter, G.; Audenaert, K.; Deng, D.H. Chloride binding of cementitious materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Konečný, P.; Ghosh, P.; Hrabová, K.; Lehner, P.; Teplý, B. Effective methodology of sustainability assessment of concrete mixtures. Mater. Struct. 2020, 53, 1–15. [Google Scholar] [CrossRef]
- ASCE. Report Card for America’s Infrastructure; American Society of Civil Engineers: Reston, VA, USA, 2014; Volume 23. [Google Scholar]
- Ming, X.; Liu, Q.; Wang, M.M.; Cai, Y.Q.; Chen, B.M.; Li, Z.J. Improved chloride binding capacity and corrosion protection of cementitious materials by incorporating alumina nano particles. Cem. Concr. Compos. 2023, 136, 104898. [Google Scholar] [CrossRef]
- Chang, H.L.; Wang, X.L.; Wang, Y.F.; Li, C.C.; Guo, Z.K.; Fan, S.Y.; Zhang, H.Z.; Feng, P. Chloride binding behavior of cement paste influenced by metakaolin dosage and chloride concentration. Cem. Concr. Compos. 2023, 135, 104821. [Google Scholar] [CrossRef]
- Lehner, P.; Hrabová, K. Evaluation of degradation and mechanical parameters and sustainability indicators of zeolite concretes. Constr. Build. Mater. 2023, 371, 130791. [Google Scholar] [CrossRef]
- Yin, G.J.; Zuo, X.B.; Li, X.N.; Zou, Y.X. An integrated macro-microscopic model for concrete deterioration under external sulfate attack. Eng. Fract. Mech. 2020, 240, 107345. [Google Scholar] [CrossRef]
- Zhang, C.L.; Chen, W.K.; Mu, S.; Šavija, B.; Liu, Q.F. Numerical investigation of external sulfate attack and its effect on chloride binding and diffusion in concrete. Constr. Build. Mater. 2021, 285, 122806. [Google Scholar] [CrossRef]
- Wilson, W.; Gonthier, J.N.; Georget, F.; Scrivener, K.L. Insights on chemical and physical chloride binding in blended cement pastes. Cem. Concr. Res. 2022, 156, 106747. [Google Scholar] [CrossRef]
- Zhang, G.; Li, M.; Zhu, Z. Effect of aluminium substitution on physical adsorption of chloride and sulphate ions in cement-based materials. Materials 2023, 16, 6029. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Peng, T.J.; Sun, H.J.; Ding, W.J.; Luo, L.M.; You, H.; Yao, X.M. Influences of Friedel’s salt produced by CaO-activated titanium-extracted tailing slag on chloride binding. Materials 2023, 16, 2843. [Google Scholar] [CrossRef] [PubMed]
- Balonis, M.; Lothenbach, B.; Saout, G.L.; Glasser, F.P. Impact of chloride on the mineralogy of hydrated Portland cement systems. Cem. Concr. Res. 2010, 40, 1009–1022. [Google Scholar] [CrossRef]
- Mesbah, A.; François, M.; Cau-dit-Coumes, C.; Frizon, F.; Filinchuk, Y.; Leroux, F.; Ravaux, J.; Renaudin, G. Crystal structure of Kuzel’s salt 3CaO·Al2O3·1/2CaSO4·1/2CaCl2·11H2O determined by synchrotron powder diffraction. Cem. Concr. Res. 2011, 41, 504–509. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Hirao, H.; Yamada, K.; Takahashi, H.; Zibara, H. Chloride binding of cement estimated by binding isotherms of hydrates. J. Adv. Concr. Technol. 2005, 3, 77–84. [Google Scholar] [CrossRef]
- Florea, M.V.A.; Brouwers, H.J.H. Chloride binding related to hydration products: Part I: Ordinary Portland cement. Cem. Concr. Res. 2012, 42, 282–290. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Nawa, T.; Kurumisawa, K. Electrokinetic potential of hydrated cement in relation to adsorption of chlorides. Cem. Concr. Res. 2009, 39, 340–344. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Liu, J.; Feng, Z.X.; Kong, S. Comparative study on chloride binding capacity of cement-fly ash system and cement-ground granulated blast furnace slag system with diethanol-isopropanolamine. Materials 2020, 13, 4103. [Google Scholar] [CrossRef] [PubMed]
- Zibara, H.; Hooton, R.D.; Thomas, M.D.A.; Stanish, K. Influence of the C/S and C/A ratios of hydration products on the chloride ion binding capacity of lime-SF and lime-MK mixtures. Cem. Concr. Res. 2008, 38, 422–426. [Google Scholar] [CrossRef]
- Gou, M.F.; Guan, X.M.; Sun, Q. Adsorption of chloride ion by calcium silicate hydrate. J. Build. Mater. 2015, 18, 363–368. [Google Scholar]
- Cao, Y.Z.; Guo, L.P.; Chen, B. Influence of sulfate on the chloride diffusion mechanism in mortar. Constr. Build. Mater. 2019, 197, 398–405. [Google Scholar] [CrossRef]
- Cheng, S.K.; Shui, Z.H.; Sun, T.; Gao, X.; Guo, C. Effects of sulfate and magnesium ion on the chloride transportation behavior and binding capacity of Portland cement mortar. Constr. Build. Mater. 2019, 204, 265–275. [Google Scholar]
- Guerrero, A.; Goñi, S.; Allegro, V.R. Effect of temperature on the durability of class C fly ash belite cement in simulated radioactive liquid waste: Synergy of chloride and sulphate ions. J. Hazard. Mater. 2009, 165, 903–908. [Google Scholar] [PubMed]
- Shi, C.J.; Hu, X.; Wang, X.G.; Wu, Z.M. Effects of chloride ion binding on microstructure of cement pastes. J. Mater. Civ. Eng. 2017, 29, 04016183. [Google Scholar] [CrossRef]
- Jin, Z.Q.; Zhao, X.; Zhao, T.J.; Li, J.Q. Chloride ions transportation behavior and binding capacity of concrete exposed to different marine corrosion zones. Constr. Build. Mater. 2018, 177, 170–183. [Google Scholar]
- Lee, Y.; Lim, S.; Lee, H. Chloride resistance of Portland cement-based mortar incorporating high aluminate cement and calcium carbonate. Materials 2020, 13, 359. [Google Scholar] [CrossRef]
- GB/T 175-2007; Common Portland Cement. China Standard Press: Beijing, China, 2007.
- GB/T 17671-1999; Method of Testing Cements-Determination of Strength. China Standard Press: Beijing, China, 1999.
- JTS/T 236-2019; Technical Specification for Concrete Testing of Port and Waterway Engineering. China Standard Press: Beijing, China, 2019.
- Rapin, J.P.; Renaudin, G.; Elkaim, E.; Francois, M. Structural transition of Friedel’s salt 3CaO·Al2O3·CaCl2·10H2O studied by synchrotron powder diffraction. Cem. Concr. Res. 2002, 32, 513–519. [Google Scholar] [CrossRef]
- Westphal, T.; Füllmann, T.; Pöllmann, H. Rietveld quantification of amorphous portions with an internal standard—Mathematical consequences of the experimental approach. Powder Diffr. 2009, 24, 239–243. [Google Scholar] [CrossRef]
- Whitfield, P.S.; Mitchell, L.D. Quantitative Rietveld analysis of the amorphous content in cement and clinkers. J. Materi. Sci. 2003, 38, 4415–4421. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Füllmann, T.; Gallucci, E.; Walenta, G.; Bermejo, E. Quantitative study of Portland cement hydration by X-ray diffraction/Rietveld analysis and independent methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Xu, J.X.; Zhang, C.K.; Jiang, L.H.; Tang, L.; Gao, G.F.; Xu, Y.P. Releases of bound chlorides from chloride-admixed plain and blended cement pastes subjected to sulfate attacks. Constr. Build. Mater. 2013, 45, 53–59. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Guo, L.P.; Chen, B.; Wu, J.D. Thermodynamic modelling and experimental investigation on chloride binding in cement exposed to chloride and chloride-sulfate solution. Constr. Build. Mater. 2020, 246, 118398. [Google Scholar] [CrossRef]
- Guo, B.B.; Qiao, G.F.; Han, P.; Li, Z.M.; Fu, Q. Effect of natural carbonation on chloride binding behaviours in OPC paste investigated by a thermodynamic model. J. Build. Eng. 2022, 49, 104021. [Google Scholar]
- Baquerizo, L.G.; Matschei, T.; Scrivener, K.L.; Saeidpour, M.; Wadsöc, L. Hydration states of AFm cement phases. Cem. Concr. Res. 2015, 73, 143–157. [Google Scholar] [CrossRef]
- Zhang, B.L.; Tan, H.B.; Ma, B.G.; Chen, F.J.; Lv, Z.H.; Li, X. Preparation and application of fine-grinded cement in cement-based material. Constr. Build. Mater. 2017, 157, 34–41. [Google Scholar] [CrossRef]
- Dousti, A.; Beaudoin, J.J.; Shekarchi, M. Chloride binding in hydrated MK, SF and natural zeolite-lime mixtures. Constr. Build. Mater. 2017, 154, 1035–1047. [Google Scholar] [CrossRef]
- Chen, F.; Gao, J.M.; Qi, B.; Shen, D.M. Deterioration mechanism of plain and blended cement mortars partially exposed to sulfate attack. Constr. Build. Mater. 2017, 154, 849–856. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olek, J. Mechanism of sulfate attack: A fresh look: Part 1: Summary of experimental results. Cem. Concr. Res. 2002, 32, 915–921. [Google Scholar] [CrossRef]
- Wang, X.G.; Shi, C.J.; He, F.Q.; Yuan, Q.; Wang, D.H.; Huang, Y.; Li, Q.L. Chloride binding and its effects on microstructure of cementitious materials. J. Chin. Chem. Soc. 2013, 41, 187–198. [Google Scholar] [CrossRef]
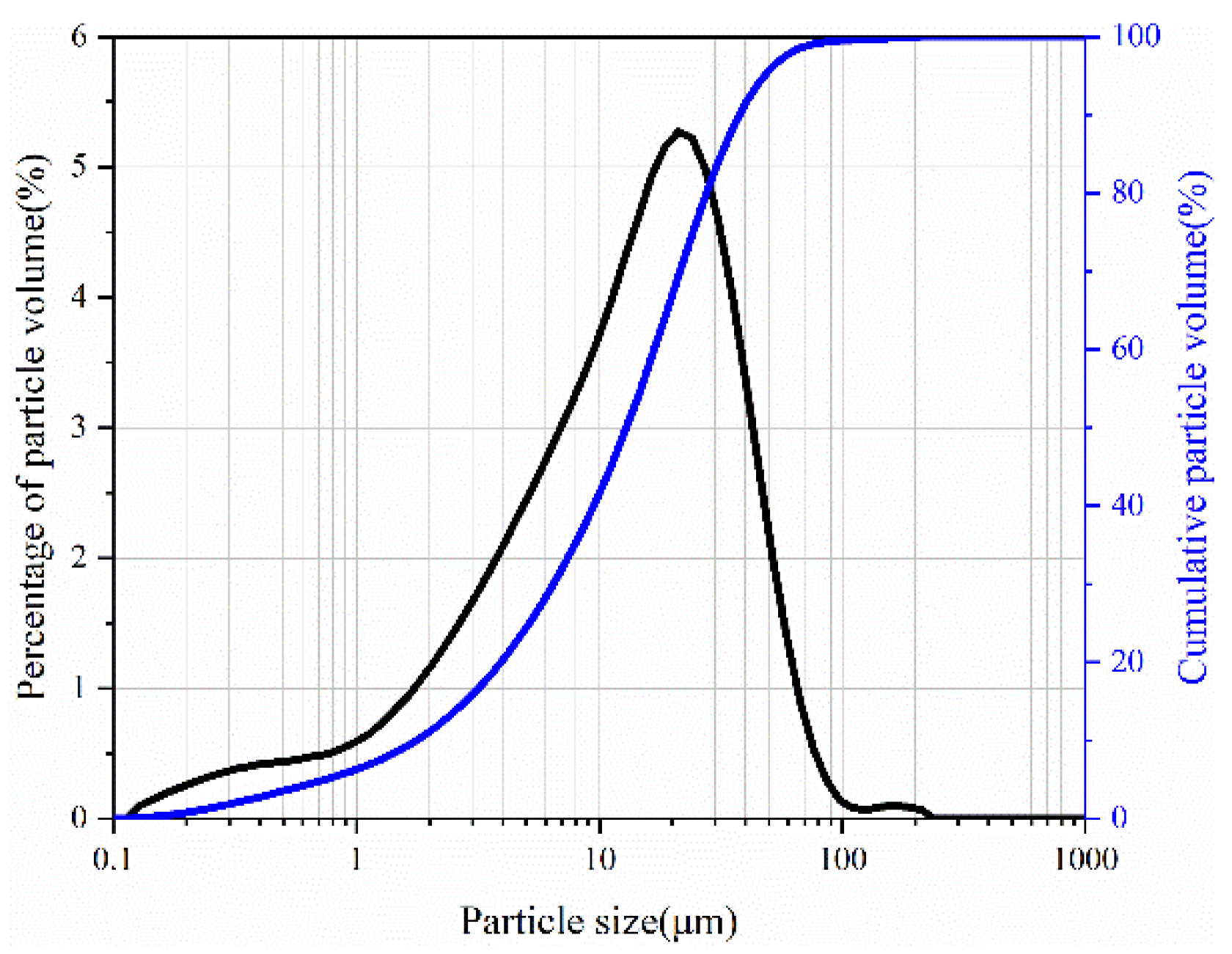




| Chemical compositions | CaO | SiO2 | Fe2O3 | Al2O3 | MgO | K2O | TiO2 | Na2O | P2O5 | SO3 |
| 60.65 | 19.58 | 3.8 | 7.17 | 4.03 | 1.09 | 0.34 | 0.31 | 0.06 | 2.55 | |
| Mineralogical compositions | C3S | C2S | C4AF | C3A | Gypsum | Calcite | Periclase | Anhydrite | Lime | Amorphous |
| 40.60 | 20.37 | 13.37 | 8.64 | 2.46 | 3.77 | 4.71 | 1.36 | 0.52 | 4.21 |
| Sample | Cement (wt. %) | Water (wt. %) | w/c |
|---|---|---|---|
| HCP-35 | 74.07 | 25.93 | 0.35 |
| HCP-45 | 68.97 | 31.03 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.-J.; Zou, Y.-X.; Zuo, X.-B.; Li, L. Experiments on Chloride Binding and Its Release by Sulfates in Cementitious Materials. Materials 2024, 17, 3429. https://doi.org/10.3390/ma17143429
Dong J-J, Zou Y-X, Zuo X-B, Li L. Experiments on Chloride Binding and Its Release by Sulfates in Cementitious Materials. Materials. 2024; 17(14):3429. https://doi.org/10.3390/ma17143429
Chicago/Turabian StyleDong, Jian-Jun, Yu-Xiao Zou, Xiao-Bao Zuo, and Liang Li. 2024. "Experiments on Chloride Binding and Its Release by Sulfates in Cementitious Materials" Materials 17, no. 14: 3429. https://doi.org/10.3390/ma17143429
APA StyleDong, J.-J., Zou, Y.-X., Zuo, X.-B., & Li, L. (2024). Experiments on Chloride Binding and Its Release by Sulfates in Cementitious Materials. Materials, 17(14), 3429. https://doi.org/10.3390/ma17143429






