Abstract
Based on the first principles, the structural stability, mechanical characteristics, electronic structure, and thermodynamic properties of AlCu2M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, Pm) are investigated. The calculated results indicate that the AlCu2Pa crystal structure is more stable and that AlCu2Pa should be easier to form. All of the AlCu2M compounds have structural stability in the ground state. Elastic constants are used to characterize the mechanical stability and elastic modulus, while the B/G values and Poisson ratio demonstrate the brittleness and ductility of AlCu2M compounds. It is demonstrated that all computed AlCu2M compounds are ductile and mechanically stable, with AlCu2Hf having the highest bulk modulus and AlCu2Mn having the highest Young’s modulus. AlCu2Mn has the highest intrinsic hardness among AlCu2M compounds, according to calculations of their intrinsic hardness. The electronic densities of states are discussed in detail; it was discovered that all AlCu2M compounds form Al-Cu and Al-M covalent bonds. Additionally, we observe that the Debye temperature and minimum thermal conductivity of AlCu2Mn and AlCu2Sc are both larger than those of others, indicating stronger chemical bonds and higher thermal conductivities, which is consistent with the elastic modulus results.
1. Introduction
High specific strength and superior thermal conduction have made high-performance Al-Cu-based alloys popular in the microelectronics, new-energy vehicles, and aerospace industries [1,2,3]. However, more research on Al-Cu alloys is needed as the need for lightweight materials with increased heat conductivity and mechanical strength grows. Al-Cu’s strength and heat conductivity can generally be impacted by the addition of alloying elements. As a result, micro-alloying can give Al-Cu alloys a useful means of achieving high conductivity and strong mechanical properties [4,5].
Owing to the exceptional performance of Al-Cu alloys, additional research has concentrated on enhancing their characteristics via theoretical calculations and experimental studies. Based on the excellent performance of Al-Cu alloys, more studies have focused on improving their properties through experimental investigations and theoretical calculations. Song et al. [6] have suggested that the co-addition of rare earth elements Sc and La can significantly increase the ultimate tensile strength, yield strength, and thermal conductivity, which are higher than in Al-4.8Cu alloy. The investigation results show that elements Hf and Si play a role in promoting ordered precipitates, and metastable Al-Cu intermetallic compounds θ″/θ′ have been subjected to quantitative studies [7]. The addition of Mn with Zr/Ti can play a significant role in stabilizing the metastable θ′ precipitates responsible for the alloy’s hardness in high-temperature applications, and density functional theory calculations have shown that the co-precipitate is a key factor that governs microstructural stability beyond 300 °C [8]. Khisamov et al. [9] have used the density functional theory approach to calculate the work function (WF) values of AlCu, Al2Cu, and Al4Cu9 intermetallic phases and compared them to the experimentally measured WF values of the Al-Cu metal matrix composite. Furthermore, ternary intermetallic compounds can be created by adding transition and rare-earth elements, and some investigations based on density-functional theory have been conducted on these compounds [10,11,12,13], which can offer a theoretical foundation for the practical application and quantitative design of Al-Cu alloys.
In this work, we have calculated the structural, mechanical, electronic, and thermodynamic properties of AlCu2M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) ternary intermetallic compounds using first principles based on the density functional theory. Mechanical properties such as elastic constants, elastic modulus, Pugh’s criteria, and Poisson’s ratio have been investigated for AlCu2M compounds. The density of the state is used to illustrate the electronic structures and inter-atomic bonding of AlCu2M compounds. Furthermore, the thermodynamic properties are revealed by estimating their Debye temperatures and minimum thermal conductivity.
2. Computational Methods
The structural, electronic, elastic, and thermodynamic characteristics of AlCu2M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) compounds were assessed using density functional theory (DFT) based on the framework of the Cambridge Sequential Total Energy Package (CASTEP) code [14,15], as implemented in the plane wave pseudo-potential method. The electronic exchange correlation energy of the structural optimization of all AlCu2M compounds was described within Perdew–Burke and Ernzerhof (PBE) using generalized gradient approximation (GGA) [16]. The valence electron configurations for the constituent elements of AlCu2M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) compounds correspond to Al (3s2 3p1), Cu (3d10 4s1), Ti (3s2 3p6 3d2 4s2), Cr (3s2 3p6 3d5 4s1), Zr (4s2 4p6 4d2 5s2), Sc (3s2 3p6 3d1 4s2), Hf (4f14 5s2 5p6 5d2 6s2), Mn (3s2 3p6 3d5 4s2), Pa (5f2 6s2 6p6 6d1 7s2), Lu (4f14 5s2 5p6 5d1 6s2), and Pm (4f5 5s2 5p6 6s2), respectively.
The structures were optimized by geometry optimization with suitable convergence parameters. The plane wave energy cut-off was employed in all PBE calculations via the Kohn–Sham function, which was set to 440 eV for AlCu2M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, and Lu) and 480 eV for AlCu2Pm, respectively. The k-point meshes for the Brillouin zone (BZ) were set within the Monkhorst–Pack scheme [17] with 4 × 4 × 4 grids for AlCu2M compounds. The Broyden–Fletcher–Goldfarb–Shanno (BFGS) optimization algorithm was utilized to obtain a structure with the lowest energy [18]. The interactions of ionic core and valence electrons were described by ultrasoft pseudo-potentials in DFT. The following parameters were displayed in all relaxation calculations: the ionic Hellmann–Feynman force [19] acting on each atom was less than 0.01 eV/Å, the self-consistent convergence of total energy was reached at 5 × 10−6 eV, the maximum stress in the unit cell was less than 0.02 GPa, and the maximum ionic displacement was set at 5 × 10−4 Å. During geometry optimization, collinear spin polarization parameters determined the ferromagnetism of AlCu2Cr and AlCu2Mn. Furthermore, the quasi-harmonic Debye model was employed to study the thermodynamic properties of the AlCu2M compounds.
3. Results and Discussion
3.1. Structural Properties
The space group of AlCu2M intermetallic compounds is Fm-3m (space group number: 225), and M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) is bonded in a body-centered cubic geometry to eight equivalent Cu atoms. Cu is bonded in a body-centered cubic geometry to four equivalent M and four equivalent Al atoms. Al is bonded in a body-centered cubic geometry to eight equivalent Cu atoms. The crystal structure of AlCu2M (M = Ti, Cr, Zr, Sc, Hf, Mn) is illustrated in Figure 1a, in which Al atoms occupy the 4a Wyckoff position, Cu atoms occupy the 8c Wyckoff position, and M atoms occupy the 4b Wyckoff position in the unit cell, respectively. The crystal structure of AlCu2M (M = Pa, Lu, Pm) is shown in Figure 1b, where M atoms occupy the 4a Wyckoff position, Cu atoms occupy the 8c Wyckoff position, and Al atoms occupy the 4b Wyckoff position in the unit cell, respectively.
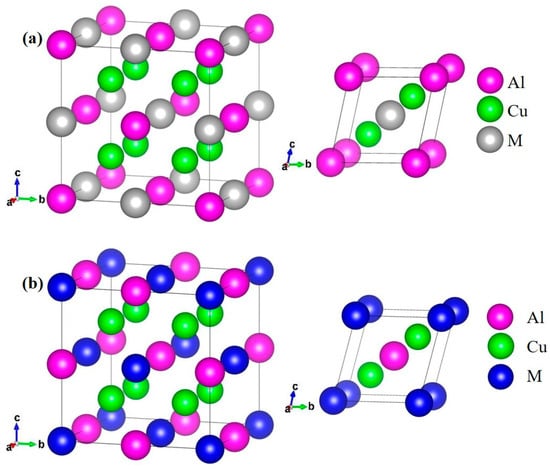
Figure 1.
The crystal structure of the AlCu2M unit cell and primitive cell: the pink balls are Al atoms, green balls are Cu atoms, gray balls are M (M = Ti, Cr, Zr, Sc, Hf, Mn), (a) and blue balls are M (M = Pa, Lu, Pm) (b), respectively.
The lattice constants (α), equilibrium volume (V0), and formation energy ΔH for AlCu2M compounds were calculated and are listed in Table 1. The calculated results of AlCu2M compounds with complete geometry optimization were compared with other experimental and theoretical results from Refs. [10,11,20,21,22,23,24,25]. We observed that the calculated values of α and V0 in this work are reasonably close to the experimental and theoretical values in the references.

Table 1.
Calculated and experimental lattice parameters α (Å), equilibrium volume V0 (Å3), and formation energy ΔH (eV/atom) for AlCu2M compounds.
The formation energy can represent the alloying abilities of AlCu2M compounds and can also indicate the crystal structure’s stability. When the value of formation energy is less than zero, the smaller the value of formation energy is, the more stable the crystal structure is [26]. The formation energy of AlCu2M compounds at zero temperature can be calculated by the following equation [27]:
where Etot is the total energy of AlCu2M compounds; , and are the energies per atom of Al, Cu, and M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, Pm) in solid states; and NAl, NCu, and NM denote the number of Al, Cu, and M atoms, respectively. These calculated results are also listed in Table 1, which demonstrates that all AlCu2M compounds have a negative ΔH and structural stability. It also indicates that elements M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm), Al, and Cu have a strong alloying ability, and all AlCu2M compounds have stable crystal structures. More negative formation energy implies higher stability; thus, the stability of AlCu2M compounds can be sequenced as AlCu2Pa < AlCu2Cr < AlCu2Zr < AlCu2Mn < AlCu2Hf < AlCu2Ti < AlCu2Lu < AlCu2Sc < AlCu2Pm. This suggests that AlCu2Pa has far stronger chemical bonds than the other compounds, implying that AlCu2Pa will form more readily from the lowest formation energy.
3.2. Mechanical Properties
To investigate the mechanical properties of the AlCu2M compounds, the elastic constants Cij were calculated via a strain–stress function based on Hooke’s law [28]. The elastic constants directly reflect mechanical stability. The mechanical stability of AlCu2M compounds can be judged by independent elastic constants. For the cubic crystal structure, there are three independent elastic constants (C11, C12, and C44), and the mechanical stability requirements for cubic crystals are given as follows [29]:
The calculated elastic constants of AlCu2M compounds in the ground state are listed in Table 2. The results are consistent with the other calculated report, with a difference of about 10% to 30%. It is shown that the calculated elastic constants C12 and C44 of AlCu2Hf calculated in this work are significantly larger than the theoretical values in Ref. [10] from the Vienna ab initio Simulation Package (VASP), whereas Ref. [10] determined the total energies by imposing appropriate strains up to ±2% at 0.5% intervals for each structure. Based on the mechanical stability criteria, the calculated elastic constants of all AlCu2M compounds (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) meet the above criteria, showing mechanical stability. The mechanical stability of AlCu2M compounds (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) is consistent with the corresponding formation energy calculations. Upon further analysis, the mechanical stability results agree well with the theoretical results reported in the literature [10,11,30].

Table 2.
Calculated elastic constants (Cij, in GPa) for the AlCu2M compounds.
According to the Voigt–Reuss–Hill (VRH) approximation method, the bulk modulus B and shear modulus G of the AlCu2M compounds can be estimated from the results of elastic constants [31,32,33]. Furthermore, the Young’s modulus E and Poisson’s ratio ν for each compound are calculated based on B and G, which can be obtained by the following formulas [34]:
Table 3 shows the calculated results of the elastic modulus, Poisson’s ratio, B/G value, and hardness for AlCu2M compounds. To verify the correctness of the calculations, the elastic moduli, Poisson ratios, and B/G of AlCu2M compounds are calculated and compared with the references [10,11,35]. The average error of the data is significant because our calculation results are compared with the other theoretical values with different calculation parameters and methods. The elastic modulus plays an important role in the mechanical properties of solids. The bulk modulus B is used to measure the resistance to volume deformation resulting from applied pressure [36]. From Table 3, it can be seen that the bulk modulus B of AlCu2Hf has the maximum value, and the B of AlCu2Pm decreases quickly. AlCu2Hf is stated to have the strongest resistance to deformation. In addition, it can be concluded that AlCu2Hf, AlCu2T, AlCu2Zr, AlCu2Sc, and AlCu2Mn are more incompressible than other AlCu2M compounds. The Young’s modulus E is used to represent the ability to resist tension and compression in the region of elastic deformation [37]. According to the calculated values in Table 3, the larger the Young’s modulus of AlCu2Mn, the more difficult it is to deform in the alloy; AlCu2Mn can be regarded as a strengthening phase [34].

Table 3.
The calculated bulk, shear, and Young’s modulus (GPa), Poisson ratio ν, B/G, and hardness HVT (GPa) for AlCu2M compounds at zero pressure.
Based on Pugh’s criterion, the ratio of B/G can be used to analyze the brittleness and ductility of AlCu2M compounds [38]. The B/G results for AlCu2M compounds are displayed in Table 3. When the B/G ratio is greater than 1.75, it indicates that the materials exhibit ductile behavior, and a material with B/G < 1.75 will be brittle. It is shown that all the calculated AlCu2M compounds in this work are ductile, with values greater than 1.75. Concurrently, AlCu2Mn has the lowest B/G value and the weakest ductility of any compound. Poisson’s ratios reflect crystals’ resistance to shear stress [39], and the calculated results are also listed in Table 3. All Poisson’s ratios of AlCu2M compounds are greater than 0.26. The greater the Poisson’s ratio, the softer the material, and it is demonstrated that AlCu2Pm is the softest of them all.
The intrinsic hardness is an important index with which to characterize the mechanical performance of AlCu2M compounds. Based on Tian’s theoretical hardness model (HVT), the hardness of AlCu2M compounds can be calculated via the following equation [40]:
where k is the value of B/G, B represents the bulk modulus, and G represents the shear modulus. The calculated results are also summarized in Table 3, which indicates that AlCu2Mn shows high intrinsic hardness; meanwhile, AlCu2Hf and AlCu2Pm have the lowest values according to Tian’s model.
3.3. Electronic Properties
As presented in Figure 2, the total density of states (TDOS) and partial density of states (PDOS) for the AlCu2M compounds have been studied. There are no band gaps at the Fermi level; it can be seen that all AlCu2M compounds have metallic characteristics. The Fermi energy (EF) results of AlCu2M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) compounds are 1.853 eV, 5.966 eV, 1.510 eV, 1.647 eV, 1.535 eV, 6.094 eV, 4.060 eV, 1.691 eV, and 21.083 eV, respectively. The crystal structures of AlCu2M are very similar, but their TDOSs differ greatly from one another. This variation is mostly due to the electronic configuration of the M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) elements. The highest peak of TDOS for all AlCu2M is roughly at −4.5 eV, except for AlCu2Pm. AlCu2Hf has the broadest pseudo-gap or quasi-gap close to the Fermi energy, indicating that it has the strongest covalent bond, which is consistent with the bulk modulus analysis.
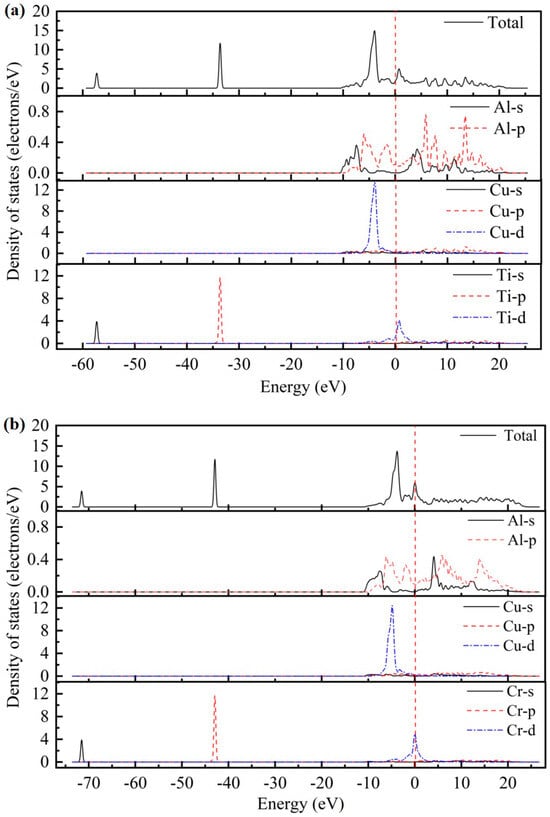
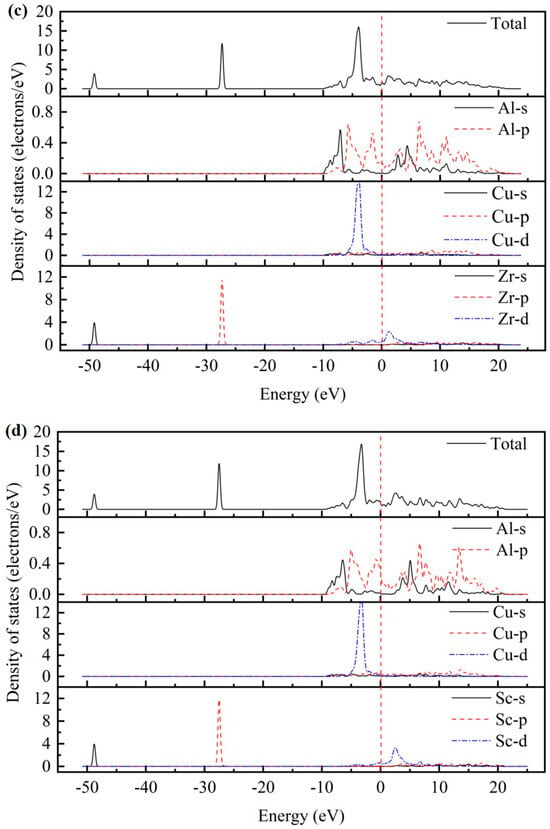
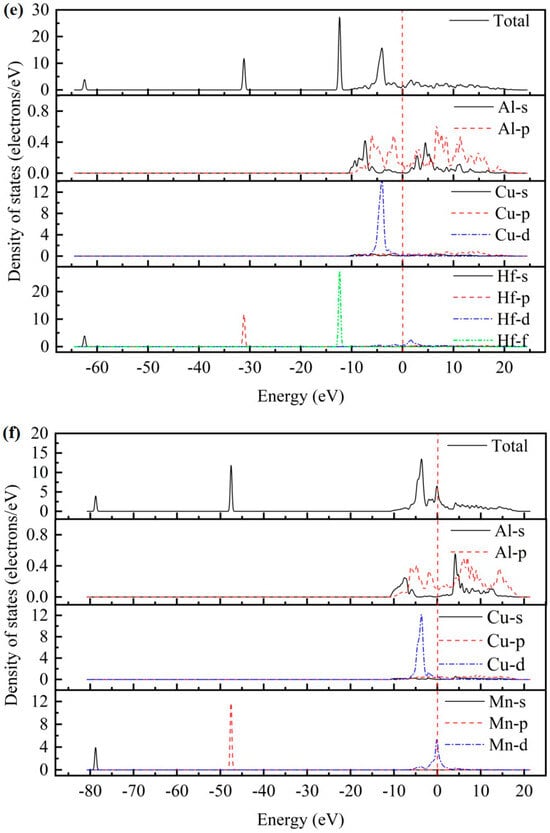
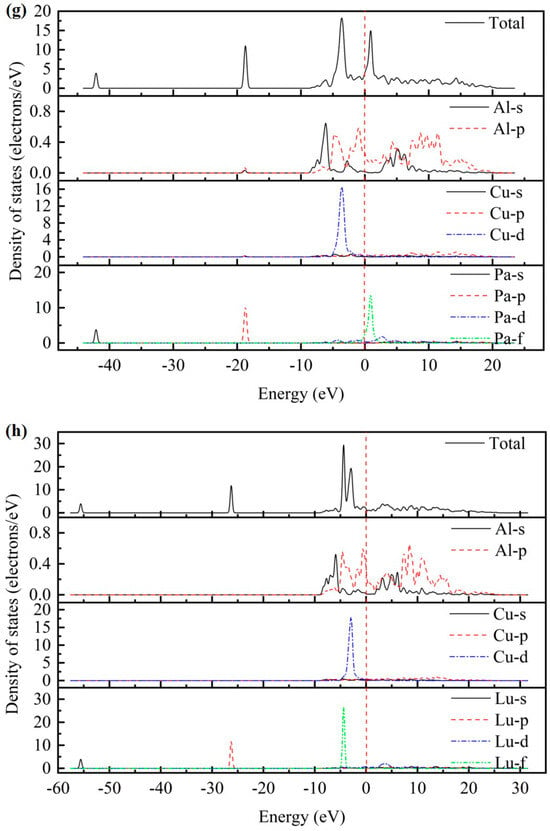
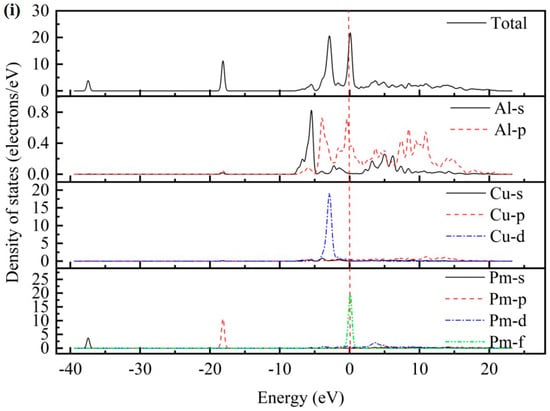
Figure 2.
The total density of states (TDOS) and partial density of states (PDOS) of AlCu2M compounds. (a) AlCu2Ti, (b) AlCu2Cr, (c) AlCu2Zr, (d) AlCu2Sc, (e) AlCu2Hf, (f) AlCu2Mn, (g) AlCu2Pa, (h) AlCu2Lu and (i) AlCu2Pm.
Meanwhile, the partial densities of states (PDOS) of AlCu2M compounds are calculated to study the electronic structure, as shown in Figure 2. In the case of AlCu2M (M = Ti, Cr, Zr, Sc, Mn, Pa, and Pm), in the energy region below −10 eV, the bands are primarily provided by M-s and M-p states. For AlCu2M (M = Hf and Lu) compounds, the M-s, M-p, and M-f states are primarily responsible for the TDOS below −10eV. Strong hybridization is observed in the energy range of −10ev to 0 eV, and the Al-Cu covalent bond is attributed to hybridization between Al-p and Cu-d states The Al-M covalent bond is attributed to hybridization between Al-s and M-f states, which produced two sharp peaks in TDOS. This is consistent with the results of Dong et al. [10] and Pang et al. [11]. Atomic orbitals of Al-s, Al-p, and M-d states hybridize near the Fermi energy for AlCu2M (M = Ti, Cr, Zr, Sc, and Mn) compounds, and the Al-s and Al-p states hybridize with M-f states for the AlCu2M (M = Hf and Lu) compounds. It is shown that atoms of Al and M form Al-M covalent bonds and produce a second peak in the TDOS. Above Fermi energy, the Al-p states hybridize with M-d states for AlCu2M compounds, with the exception of the Al-p states, which hybridize with M-f states for AlCu2Pa. Moreover, the Al-M covalent bonds in AlCu2M (M = Pa, Lu, and Pm) are connected to the Al-s, Al-p, and M-f hybridization states and are stronger than those in other AlCu2M (M = Ti, Cr, Zr, Sc, Hf, and Mn) compounds.
3.4. Thermodynamic Properties
To investigate the influence of M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) element doping of Al-Cu alloys on their heat-conducting properties, it is necessary to study the thermodynamic properties of AlCu2M compounds. In this section, the Debye temperature (ΘD) and the minimum thermal conductivity (κmin) are calculated and discussed at length. Debye temperature can establish the relationship between the elastic modulus and thermophysical properties of AlCu2M compounds, and that is related to characterizing the strength of chemical bonds, which can be expressed as follows [41]:
where h refers to Planck’s constant, kB is Boltzmann’s constant, NA is Avogadro’s number, ρ is the density of AlCu2M compounds, M is the molecular weight, n is the number of atoms per formula unit, and Vm refers to the average wave velocity, which can be calculated via the following equation [42].
where Vt represents the traverse elastic wave velocity, and Vl is the longitudinal elastic wave velocity. The values of Vt and Vl can be obtained by Navier’s equation [42]:
The results of shear modulus G and the bulk modulus B are shown in Table 3. The calculated values of ρ, Vt, Vl, Vm, and ΘD for the AlCu2M compounds are listed in Table 4. AlCu2Mn exhibits the highest Debye temperatures, and AlCu2Pm is the lowest at zero pressure and zero Kelvin. It is shown that AlCu2Mn has a stronger strength of chemical bonds than that of other AlCu2M compounds, which is consistent with the behavior of the elastic modulus mentioned above.

Table 4.
The density ρ (g/cm3), elastic wave velocity (m/s), Debye temperature ΘD (K), and the minimum thermal conductivity κmin (W/m·K) of AlCu2M compounds.
In general, the thermal conductivity reduces as the temperature increases, and the minimum thermal conductivity means that it reaches a minimum state in which the lattice vibration is weakest [43]. The minimum thermal conductivity (κmin) is another important thermal property that reflects the ability of materials to transfer heat. Study of the thermal conductivity of the doping elements M is required to determine whether or not they are advantageous for conducting heat. The κmin for Clarke’s model at high temperatures (higher than the Debye temperature) is found through the following expressions [43]:
where the symbols of kB, M, n, NA, and ρ have the same meaning as used in Equation (6), and E is the Young’s modulus, calculated above in Section 3.2. Based on the Cahill–Pohl model [44], the equation of κmin is as follows:
where kB is the Boltzmann constant, n’ is the density of atoms per volume, and Vt and Vl are as above in Equations (8) and (9). The calculated results of the minimum thermal conductivity of AlCu2M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) are also listed in Table 4. The results from Clarke’s model are marginally higher than those of the Cahill–Pohl model. Table 4 shows that AlCu2Mn has a higher Debye temperature than the other phases and thus has the greatest thermal conductivity for both Clarke’s model and the Cahill–Pohl model. Furthermore, it can also be observed that AlCu2Pm has a lower κmin value than that of the others, indicating a poor heat transfer capability. The results of the above Debye temperature agree well with the calculations from the two models of κmin in this paper.
4. Conclusions
In summary, the structural, elastic, electronic, and thermodynamic properties of AlCu2M (M = Ti, Cr, Zr, Sc, Hf, Mn, Pa, Lu, and Pm) compounds have been systematically investigated using first-principles calculations based on density functional theory. Due to the achievement of the negative formation energy and the fulfillment of mechanical stability requirements, it is demonstrated that all AlCu2M compounds have both structural and mechanical stability. The calculated Young’s modulus E and hardness of AlCu2Mn are higher than those of the others. The Poisson’s ratio and B/G results show that all AlCu2M compounds are ductile. The DOS of AlCu2M compounds is mainly contributed by Al-s states, Al-p states, Cu-d states, M-d states (M = Ti, Cr, Zr, Sc, and Mn), or M-f states (M = Hf, Pa, Lu, and Pm), and strong bonding forms between Al atoms and Cu atoms. Meanwhile, the Debye temperature is determined by the average wave velocity; AlCu2Mn and AlCu2Sc have higher Debye temperatures than the others. The minimum thermal conductivity is also calculated from the average elastic wave velocity; AlCu2Mn and AlCu2Sc have larger minimum thermal conductivities, which are consistent with the Young’s modulus values. This work may be expanded to other intermetallic compounds and offers some alloying recommendations for enhancing the ductility, hardness, and thermal conductivity of AlCu2M compounds.
Author Contributions
Conceptualization, Y.G.; methodology, Y.G. and B.J.; investigation, Y.G. and X.Z.; validation, S.L. and Y.G.; resources, Y.G. and B.J.; data curation, Y.G., S.L. and X.Z.; writing-original draft preparation, Y.G., B.J. and X.Z.; writing-review and editing, Y.G., B.J. and X.Z.; visualization, Y.G., B.J., X.Z. and S.L.; supervision, B.J.; project administration, Y.G.; funding acquisition, Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Huzhou Science and Technology Project (No.2022YZ34) and the Huzhou College Research Project (No.2021HXKJ02).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wen, M.C.; Hsu, Y.D.; Chen, M.C.; Yang, W.C.; Lee, S.L. Effects of heterogenization treatment on the hot-working temperature and mechanical properties of Al-Cu-Mg-Mn-(Zr) alloys. Materials 2023, 16, 4256. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.D.; Liu, W.S.; Xiao, D.H.; Huang, L.P. Influence of thermal exposure on the microstructure evolution and mechanical behaviors of an Al-Cu-Li alloy. Mater. Des. 2023, 227, 111767. [Google Scholar] [CrossRef]
- Belov, N.A.; Cherkasov, S.O.; Korotkova, N.O.; Motkov, M.M. The impact of thermomechanical treatment on structure, electrical resistance and hardness of Al-4%Cu-3%Mn alloy casted in an electromagnetic crystallizer. Phys. Met. Metallogr. 2024, 125, 203–210. [Google Scholar] [CrossRef]
- Bayram, U.; Marasli, N. Thermal conductivity and electrical resistivity dependences on growth rate in the directionally solidified Al-Cu-Ni eutectic alloy. J. Alloy Compd. 2018, 753, 695–702. [Google Scholar] [CrossRef]
- Deng, S.X.; Li, J.F.; Ning, H.; Lu, D.D.; Zeng, G.J.; Liu, Z.Z.; Xiang, H.; Que, J.R.; Liu, D.Y. Effect of Zr addition on the microstructure evolution and mechanical properties of extruded Al-Cu-Li-Mn alloys. Mater. Charact. 2023, 202, 113011. [Google Scholar] [CrossRef]
- Song, Z.X.; Li, Y.D.; Liu, W.J.; Yang, H.K.; Cao, Y.J.; Bi, G.L. Effect of La and Sc co-addition on the mechanical properties and thermal conductivity of as-cast Al-4.8%Cu alloys. Metals 2021, 11, 1866. [Google Scholar] [CrossRef]
- Ujjval, B.; Mahander, P.S.; Sukla, M.; Shyam, K.S.; Surendra, K.M.; Aloke, P.; Kamanio, C. The interplay of precipitation of ordered compounds and interfacial segregation in Al-Cu-Hf-Si alloys for high-temperature strength. Acta Mater. 2022, 240, 118355. [Google Scholar] [CrossRef]
- Jonathan, D.P.; Brian, K.M.; Lawrence, F.A.; Dongwon, S.; Patrick, S.; Matthew, F.C.; Amit, S. The synergistic role of Mn and Zr/Ti in producing θ’/L12 co-precipitates in Al-Cu alloys. Acta Mater. 2020, 194, 577–586. [Google Scholar] [CrossRef]
- Khisamov, R.K.; Khalikova, G.R.; Kistanov, A.A.; Korznikova, G.F.; Korznikova, E.A.; Nazarov, K.S.; Sergeev, S.N.; Shayakhmetov, R.U.; Timiryaev, R.R.; Yumaguzin, Y.M.; et al. Microstructure, microhardness and work function of in-situ Al-Cu composite processed by mechanical alloying by means of high-pressure torsion. Continuum Mech. Thermodyn. 2023, 35, 1433–1444. [Google Scholar] [CrossRef]
- Dong, L.M.; Han, Z.D.; Guan, Y.Z.; Li, W.; Zhang, X.Y. Mechanical properties and work function of L21 structure AlCu2X (X=Ti, Mn, Zr, or Hf) intermetallics. Mat. Sci. Eng. A 2012, 545, 13–19. [Google Scholar] [CrossRef]
- Pang, M.J.; Zhan, Y.Z.; Wang, H.Z.; Jiang, W.P.; Du, Y. Ab initio study of AlCu2M (M=Sc, Ti and Cr) ternary compounds under pressures. Comput. Mater. Sci. 2011, 50, 2930–2937. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Chen, H.T.; Xu, H.Y.; Hu, M.L.; Ji, Z.S. First-principles study on stability, electronic, mechanical and thermodynamic properties of Al-Cu-RE ternary compounds. Solid State Commun. 2019, 287, 63–67. [Google Scholar] [CrossRef]
- Huang, G.; Liu, L.; Zhang, L.; Jin, Z. Thermodynamic description of the Al-Cu-Yb ternary system supported by first-principles calculations. J. Min. Metall. B 2016, 52, 177–183. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter. 2002, 14, 2717. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of crystals with the Quasi-Newton method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Feynman, R.P. Forces in molecules. Phys. Rev. 1939, 56, 340–343. [Google Scholar] [CrossRef]
- Ruediger MZ, R.; Peter, C.S.; Alarich, W. Reaction of Hydrogen with the Heusler-Type Phases Cu2TiAl and Cu2ZrAl*. Z. Für Phys. Chem. 1989, 163, 103–108. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; Engen, P.G.V. Magnetic and magneto-optical properties of heusler alloys based on aluminum and gallium. J. Magn. Magn. Mater. 1981, 25, 90–96. [Google Scholar] [CrossRef]
- Dwight, A.E.; Kimball, C.W. ScT2X and LnT2X compounds with the MnCu2al-type structure. J. Less-Common Metals 1987, 127, 179–182. [Google Scholar] [CrossRef]
- Takeuchi, A.; Yubuta, K.; Yokoyama, Y.; Makino, A.; Inoue, A. Noncrystalline atomic arrangements computationally created from crystalline compound by treating groups of atoms as hypothetical clusters. Intermetallics 2008, 16, 283–292. [Google Scholar] [CrossRef]
- Markiv, V.Y.; Voroshilov, Y.V.; Kripyakevich, P.I.; Cherkashin, E.E. New compounds of the MnCu2Al and MgZn2 types containing aluminum and gallium. Sov. Phys. Crystallogr. 1964, 9, 619–620. [Google Scholar]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Zubov, V.I.; Tretiakov, N.P.; Teixeira Rabelo, J.N.; Sanchez Ortiz, J.F. Calculations of the thermal expansion, cohesive energy and thermodynamic stability of a Van der Waals crystal-fullerene C60. Phys. Lett. A 1994, 194, 223–227. [Google Scholar] [CrossRef]
- Andrey, D.I.; Andrey, A.K.; Artem, A.I.; Elena, A.K. The nitriding effect on the stability and mechanical properties of the iron titan phase: First-principles investigation. Phys. Chem. Chem. Phys. 2023, 25, 24060. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.X. Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Wang, A.J.; Shang, S.L.; Du, Y.; Kong, Y.; Zhang, L.J.; Chen, L. Structural and elastic properties of cubic and hexagonal TiN and AlN from first-principles calculations. Comput. Mater. Sci. 2010, 48, 705–709. [Google Scholar] [CrossRef]
- Guan, Y.Z.; Zhang, H.Y.; Li, W. First-principles study on alloying stability, electronic structure, and mechanical properties of Al-based intermetallics. Phys. B Condens. Matter 2011, 406, 1149–1153. [Google Scholar] [CrossRef]
- Voigt, W. Lehrbuch der Kristallphysik: Teubner-Leipzig; Macmillan: New York, NY, USA, 1928. [Google Scholar]
- Reuss, A. Berechnung del flieβgrenze von mischkristallen auf grund der plastizitätbedingung für einkristalle. J. Appl. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The elastic behavior of crystalline aggregate. Proc. Phys. Soc. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Chen, H.T.; Xu, H.Y.; Hu, M.L.; Ji, Z.S. Evaluation of stabilities and thermophysical properties of Si-Sr intermetallics based on first-principles analysis. Results Phys. 2020, 18, 103207. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, X.D.; Wang, F. Structural, elastic, anisotropic, electronic, thermal properties and tensile strength of AlTM2Ti (TM = Ni, Fe, Cu, Co, Au) studied by first-principles calculations. Chem. Phys. Lett. 2023, 830, 140796. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.; Zheng, Q. Influence of Ir concentration on the structure, elastic modulus and elastic anisotropy of Nb-Ir based compounds from first-principles calculations. J. Alloy Compd. 2019, 789, 860–866. [Google Scholar] [CrossRef]
- Pan, Y. RuAl2: Structure, electronic and elastic properties from first-principles. Mater. Res. Bull. 2017, 93, 56–62. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Ravindran, P.; Fast, L.; Korzhavyi, P.A.; Johansson, B.; Wills, J.; Eriksson, O. Density functional theory for calculation of elastic properties of orthorhombic crystals: Applications to TiSi2. J. Appl. Phys. 1998, 84, 4891–4904. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, B.; Zhao, Z. Microscopic theory of hardness and design of novel superhard crystals. Int. J. Refract. Met. Hard Mater. 2012, 33, 93–106. [Google Scholar] [CrossRef]
- Anderson, O.L. A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Schreiber, E.; Anderson, O.L.; Soga, N. Elastic Constants and Their Measurement; McGraw-Hill Companies: New York, NY, USA, 1974. [Google Scholar]
- Clarke, D.R. Materials selection guidelines for low thermal conductivity thermal barrier coatings. Surf. Coat. Technol. 2003, 163, 67–74. [Google Scholar] [CrossRef]
- Cahill, D.G.; Pohl, R.O. Lattice vibrations and heat transport in crystals and glasses. Ann. Rev. Phys. Chem. 1988, 39, 93–121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).