Zinc and Copper Oxide Nanoparticles: Pioneering Antibacterial and Antibiofilm Strategies for Environmental Restoration against Antibiotic-Resistant Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
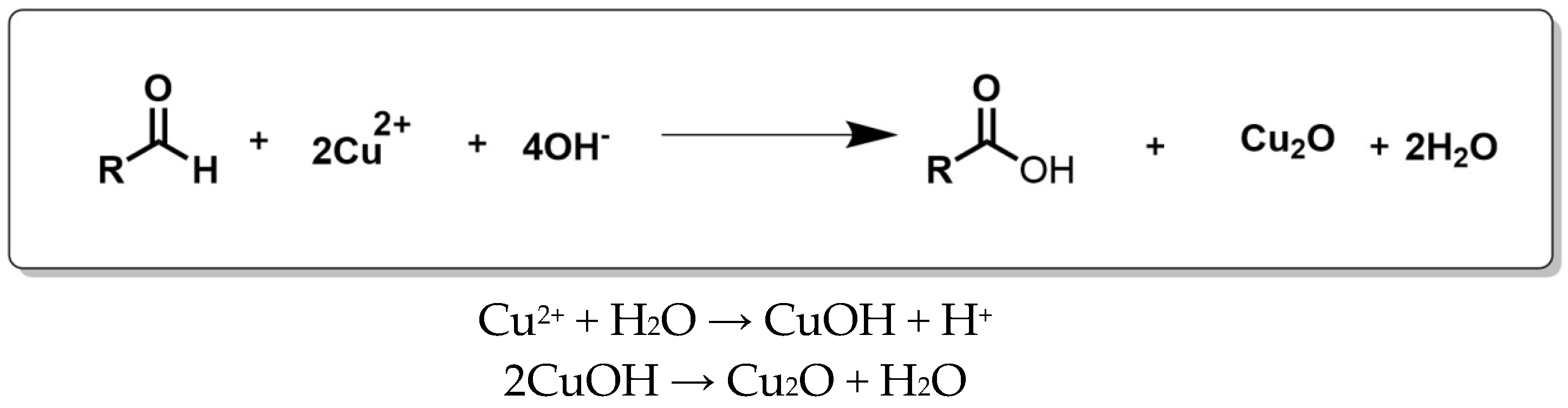
2.3. Synthesis of NPs
2.3.1. Zinc Oxide Nanoparticle Synthesis
2.3.2. Copper Oxide Nanoparticles Synthesis
2.4. Isolation of Bacteria
2.5. DNA Isolation
2.6. DNA Quantification
2.7. Agarose Gel Electrophoresis
2.8. PCR Amplification for 16S rRNA
2.9. Agarose Gel Electrophoresis of PCR Product
3. Results
3.1. Morphological and Physiological Studies
3.2. Studies of rRNA Sequence Analysis
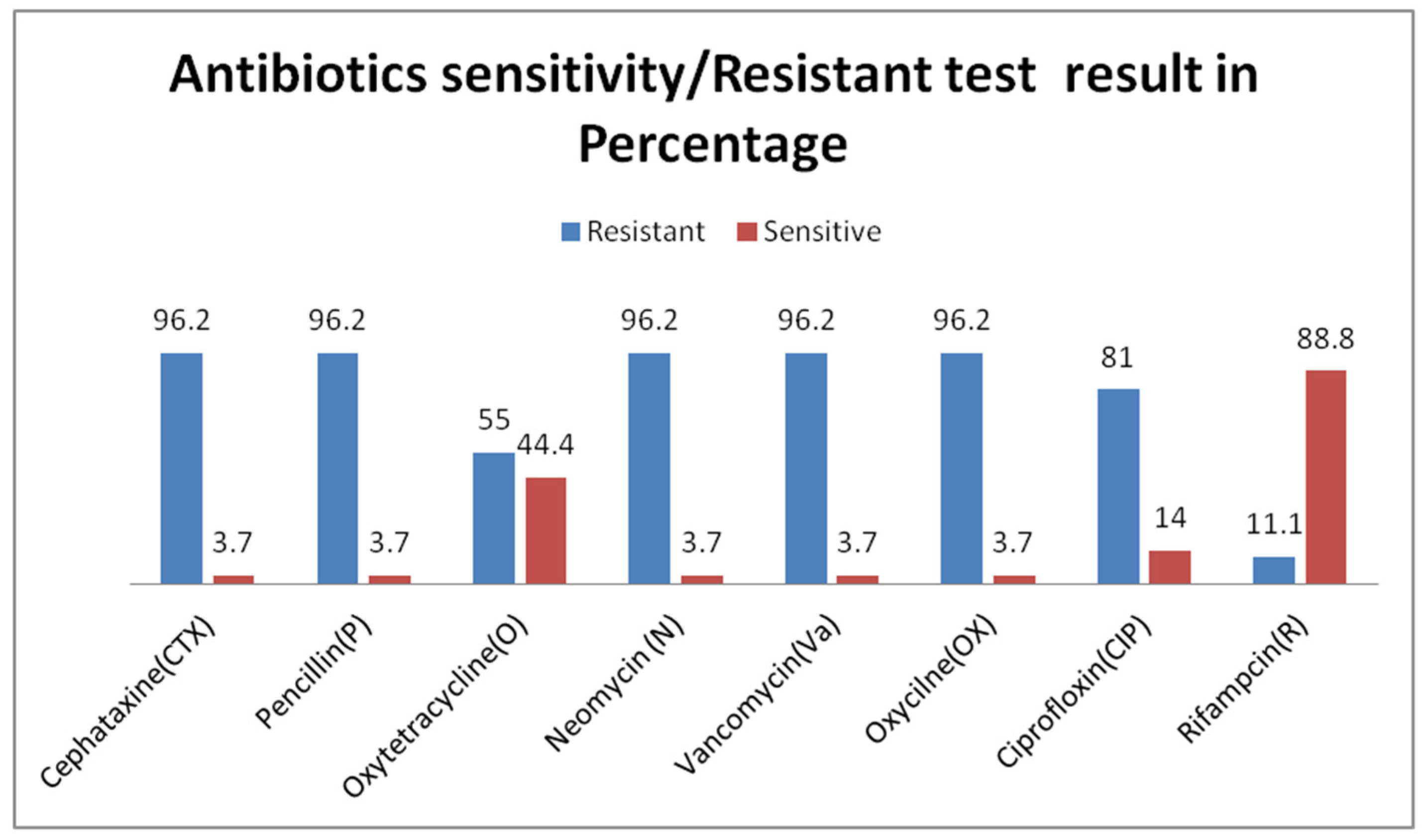
3.3. Antibiotic Resistance Profile
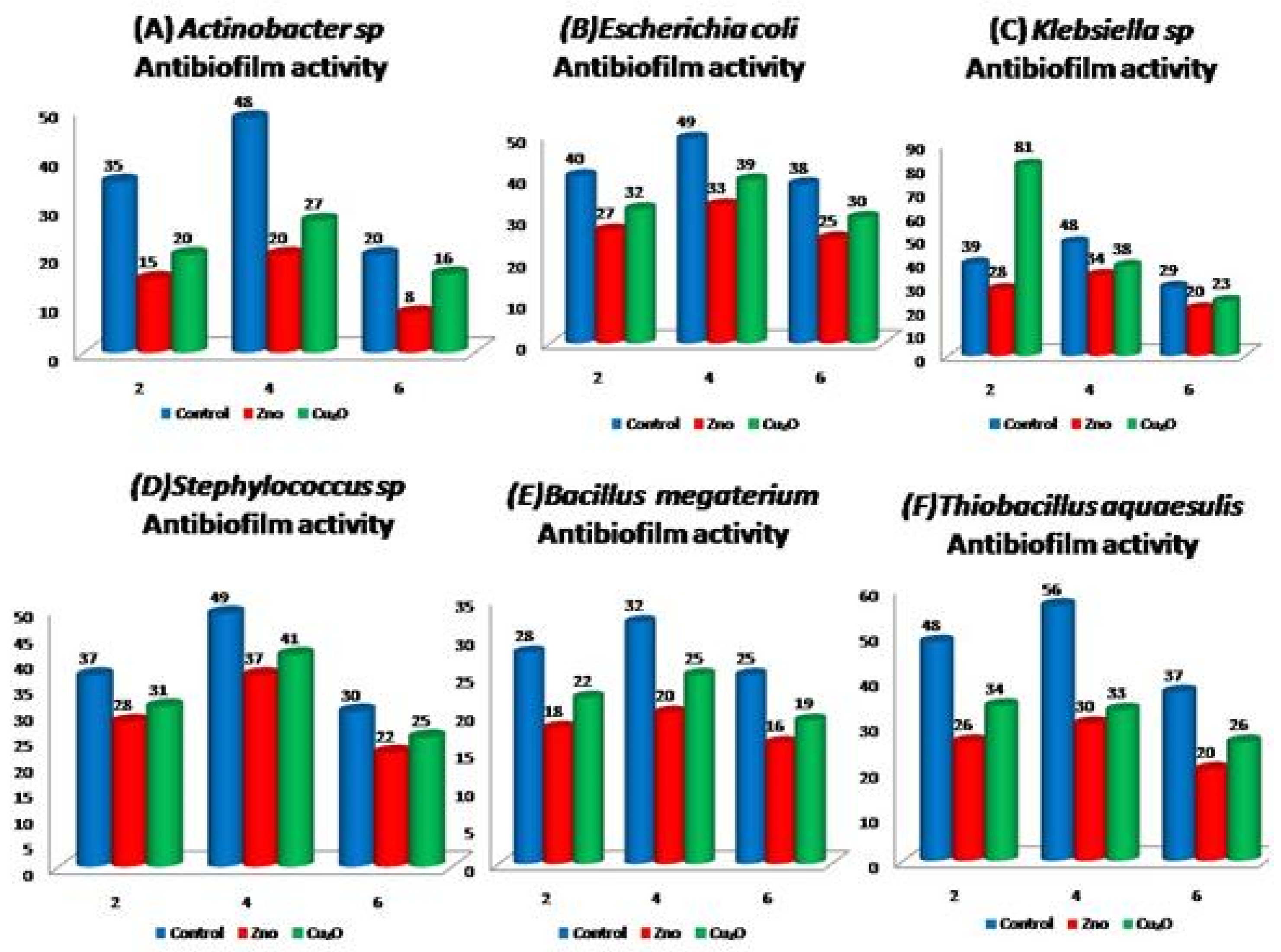
3.4. Biofilm Formation Assay
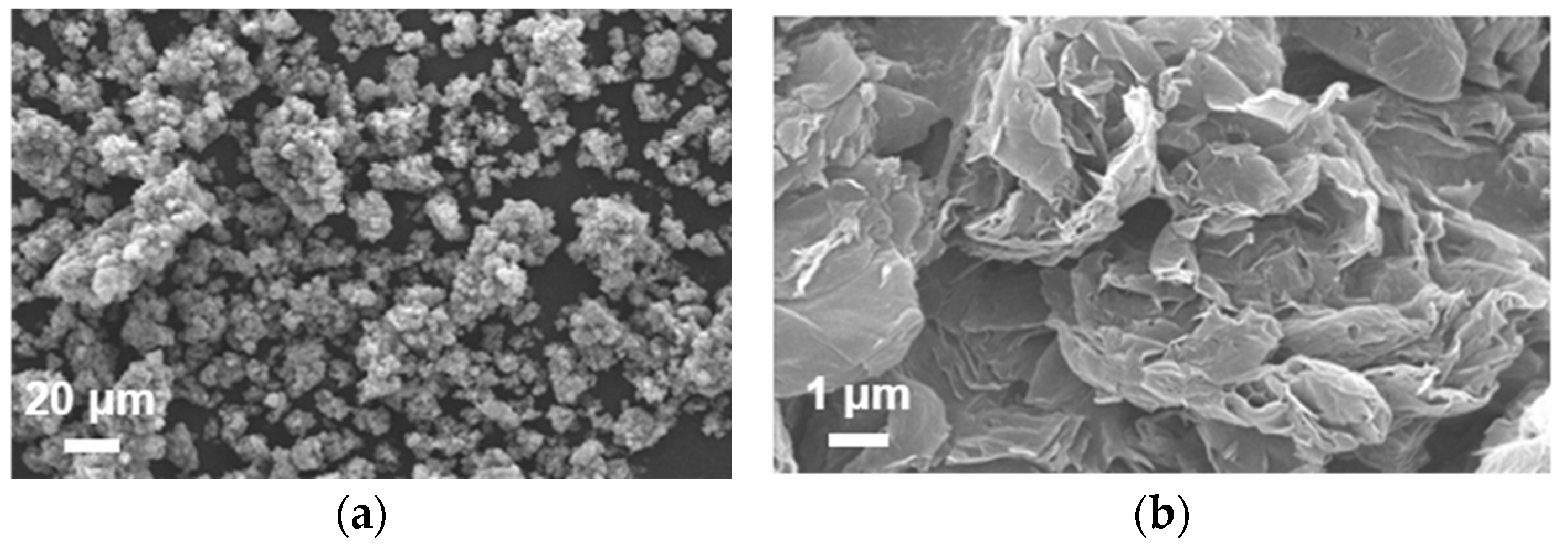
3.5. Characterization of Metal Oxide NPs
- D is the average crystallite size,
- K is the Scherrer constant (typically taken as 0.9),
- λ is the wavelength of the X-ray radiation used (in Å),
- β is the full width at half maximum of the peak (in radians),
- θ is the Bragg angle (in radians).

3.6. Antibacterial and Antibiofilm Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagatin, R.; Klemeš, J.J.; Reverberi, A.P.; Huisingh, D. Conservation and improvements in water resource management: A global challenge. J. Clean. Prod. 2014, 77, 1–9. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Z.; Wu, G.; Wu, Q.; Zhang, F.; Niu, Z.; Hu, H.Y. Characteristics of water quality of municipal wastewater treatment plants in China: Implications for resources utilization and management. J. Clean. Prod. 2016, 131, 1–9. [Google Scholar] [CrossRef]
- Morris, L.; Colombo, V.; Hassell, K.; Kellar, C.; Leahy, P.; Long, S.M.; Myers, J.H.; Pettigrove, V. Municipal wastewater effluent licensing: A global perspective and recommendations for best practice. Sci. Total Environ. 2017, 580, 1327–1339. [Google Scholar] [CrossRef]
- McArdell, C.S.; Molnar, E.; Suter, M.J.F.; Giger, W. Occurrence and fate of macrolide antibiotics in wastewater treatment plants and in the Glatt Valley watershed, Switzerland. Environ. Sci. Technol. 2003, 37, 5479–5486. [Google Scholar] [CrossRef]
- Iwane, T.; Urase, T.; Yamamoto, K. Possible impact of treated wastewater discharge on incidence of antibiotic resistant bacteria in river water. Water Sci. Technol. 2001, 43, 91–99. [Google Scholar] [CrossRef]
- Guardabassi, L.; Dalsgaard, A. Occurrence and Fate of Antibiotic Resistant Bacteria in Sewage; Danish EPA Environmental Project Report 722; Miljøstyrelsen: Odense, Denmark, 2002. [Google Scholar]
- Avci, D.; Altürk, S.; Sönmez, F.; Tamer, Ö.; Başoğlu, A.; Atalay, Y.; Dege, N. A new dinuclear copper (II) complex of 2, 5–Furandicarboxyclic acid with 4 (5)-Methylimidazole as a high potential α-glucosidase inhibitor: Synthesis, Crystal structure, Cytotoxicity study, and TD/DFT calculations. Appl. Organomet. Chem. 2019, 33, e4725. [Google Scholar] [CrossRef]
- Schwartz, T.; Kohnen, W.; Jansen, B.; Obst, U. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol. Ecol. 2003, 43, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, Y.-X.; Liang, P.; Zhou, J.; Yang, Y.; Huang, X. Linkages between Microbial Functional Potential and Wastewater Constituents in Large-Scale Membrane Bioreactors for Municipal Wastewater Treatment. Water Res. 2014, 56, 162–171. [Google Scholar] [CrossRef]
- Dege, N.; Içbudak, H.; Adıyaman, E. Bis (acesulfamato-κ2O4, N) bis (3-methylpyridine) copper (II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2006, 62, m401–m403. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How bacteria stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Alherek, M.; Basu, O.D. Impact of low levels of silver, zinc and copper nanoparticles on bacterial removal and potential synergy in water treatment applications. J. Chem. Technol. Biotechnol. 2023, 98, 1137–1146. [Google Scholar] [CrossRef]
- Alves, D.A.S.; Botelho Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S.; Baltazar, M.D.P.G. Copper and zinc adsorption from bacterial biomass-possibility of low-cost industrial wastewater treatment. Environ. Technol. 2023, 44, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Ilmi, R.; Kansız, S.; Al-Rasbi, N.K.; Dege, N.; Raithby, P.R.; Khan, M.S. Towards white light emission from a hybrid thin film of a self-assembled ternary samarium (III) complex. New J. Chem. 2020, 44, 5673–5683. [Google Scholar] [CrossRef]
- Alisir, S.H.; Ozdemir, N.; Burgaz, E.; Dege, N.; Canavar, Y.E. Fabrication and antimicrobial activity of poly (lactic acid) nanofibers containing firstly synthesized silver diclofenac complex with (2-methylimidazole) for wound dressing applications. Fibers Polym. 2021, 22, 2738–2749. [Google Scholar] [CrossRef]
- Hamamci Alisir, S.; Dege, N.; Tapramaz, R. Synthesis, crystal structures and characterizations of three new copper (II) complexes including anti-inflammatory diclofenac. Acta Crystallogr. Sect. C Struct. Chem. 2019, 75, 388–397. [Google Scholar] [CrossRef]
- Hoiby, N.; Bjarnsholt, T.; Givskov, M.; Mo lin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Theivasanthi, T.; Alagar, M. Studies of Copper Nanoparticles Effects on Micro-organisms. Ann. Biol. Res. 2011, 2, 368–379. [Google Scholar]
- Sogukomerogullari, H.G.; Aytar, E.; Ulusoy, M.; Demir, S.; Dege, N.; Richeson, D.S.; Sönmez, M. Synthesis of complexes Fe, Co and Cu supported by “SNS” pincer ligands and their ability to catalytically form cyclic carbonates. Inorganica Chim. Acta 2018, 471, 290–296. [Google Scholar] [CrossRef]
- Nagaraj, K.; Kamalesu, S.; Lokhandwala, S.; Parekh, N.M.; Sakthinathan, S.; Chiu, T.-W. Green synthesis, characterization and efficient photocatalytic study of hydrothermal-assisted Ag@ TiO2 nanocomposites. Inorg. Chem. Commun. 2023, 148, 110362. [Google Scholar] [CrossRef]
- Nagaraj, K.; Naman, J.; Dixitkumar, M.; Priyanshi, J.; Thangamuniyandi, P.; Kamalesu, S.; Lokhandwala, S.; Parekh, N.M.; Panda, S.R.; Sakthinathan, S.; et al. Chelladurai Karuppiah Ammasai Karthikeyan, Iruthaya Kalai Selvam. Inorg. Chem. Commun. 2023, 151, 110635. [Google Scholar] [CrossRef]
- Nagaraj, K.; Thangamuniyandi, P.; Kamalesu, S.; Lokhandwala, S.; Parekh, N.M.; Panda, S.R.; Sakthinathan, S.; Chiu, T.-W.; Chelladurai, K.; Karthikeyan, A.; et al. Metallo-Surfactant assisted silver nanoparticles: A new approach for the colorimetric detection of amino acids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 296, 122693. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, K.; Dixitkumar, M.; Naman, J.; Priyanshi, J.; Thangamuniyandi, P.; Kamalesu, S.; Lokhandwala, S.; Parekh, N.M.; Sakthinathan, S.; Chiu, T.-W.; et al. Silver nanoparticles using Cassia Alata and its catalytic reduction activities of rhodamine6G, methyl orange and methylene blue dyes. Inorg. Chem. Commun. 2023, 155, 110985. [Google Scholar] [CrossRef]
- Radha, S.; Christygnanatheeba, P.; Nagaraj, K.; Prasad, S.; AlSalhi, M.S.; Kumar, J.V.; Arunachalam, P.; Karuppiah, C. Titanium(III) Oxide Doped with meta-Aminophenol Formaldehyde Magnetic Microspheres: Enhancing Dye Adsorption toward Methyl Violet. Processes 2023, 11, 1250. [Google Scholar] [CrossRef]
- Koiki, B.A.; Arotiba, O.A. Cu2O as an emerging semiconductor in photocatalytic and photoelectrocatalytic treatment of water contaminated with organic substances: A review. RSC Adv. 2020, 10, 36514–36525. [Google Scholar] [CrossRef]
- Moezzi, A. Michael Cortie and Andrew McDonagh Aqueous pathways for the formation of zinc oxide nanoparticles. Dalton Trans. 2011, 36, 40–4871. [Google Scholar]
- Kooti, M.; Matouri, L. Fabrication of Nanosized Cuprous Oxide Using Fehling’s Solution. Nanotechnology 2010, 6, 73–78. [Google Scholar]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Zhang, A.; Zhang, L.; Niu, H. Jinyou Duan Inhibitory effects of chitosan in combination with antibiotics on Listeria monocytogenes biofilm. Food Control 2014, 38, 215–220. [Google Scholar] [CrossRef]
- Hu, M.; Wang, X.; Wen, X.; Xia, Y. Microbial community structures in different wastewater treatment plants as revealed by 454-pyrosequencing analysis. Bioresour. Technol. 2012, 117, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Nolvak, H.; Truu, M.; Tiirik, K.; Oopkaup, K.; Sildvee, T.; Kaasik, A.; Mander, U.; Truu, J. Dynamics of antibiotic resistance genes and their relationships with system treatment efficiency in a horizontal subsurface flow constructed wetland. Sci. Total Environ. 2013, 461–462, 636–644. [Google Scholar] [CrossRef]
- Top, A.; Ulkü, S. Silver, zinc, and copper exchange in a Na-clinoptilolite and resulting effect on antibacterial activity. Appl. Clay Sci. 2004, 27, 13–19. [Google Scholar] [CrossRef]
- Taglietti, A.; Arciola, C.R.; D’Agostino, A.; Dacarro, G.; Montanaro, L.; Campoccia, D.; Cucca, L.; Vercellino, M.; Poggi, A.; Pallavicini, P.; et al. Antibiofilm activity of a monolayer of silver nanoparticles anchored to an amino-silanized glass surface. Biomaterials 2014, 35, 1779–1788. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef]
| Primer | Target for Amplification | Oligonucleotide 5′–3′ |
|---|---|---|
| 8F | 16S rRNA | AGAGTTTGATCCTGGCTCAG |
| 1492R | 16S rRNA | TACGGCTACTTGTTACGACTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uthra, C.; Nagaraj, K.; Wadaan, M.A.; Karuppiah, C.; Maity, P.; Baabbad, A.; Kaliyaperumal, R.; Venkatachalapathy, R.; Shah, F.; Kumar, P. Zinc and Copper Oxide Nanoparticles: Pioneering Antibacterial and Antibiofilm Strategies for Environmental Restoration against Antibiotic-Resistant Bacteria. Materials 2024, 17, 3444. https://doi.org/10.3390/ma17143444
Uthra C, Nagaraj K, Wadaan MA, Karuppiah C, Maity P, Baabbad A, Kaliyaperumal R, Venkatachalapathy R, Shah F, Kumar P. Zinc and Copper Oxide Nanoparticles: Pioneering Antibacterial and Antibiofilm Strategies for Environmental Restoration against Antibiotic-Resistant Bacteria. Materials. 2024; 17(14):3444. https://doi.org/10.3390/ma17143444
Chicago/Turabian StyleUthra, Chandrabose, Karuppiah Nagaraj, Mohammad Ahmad Wadaan, Chelladurai Karuppiah, Prasenjit Maity, Almohannad Baabbad, Raja Kaliyaperumal, Renuka Venkatachalapathy, Flora Shah, and Puneet Kumar. 2024. "Zinc and Copper Oxide Nanoparticles: Pioneering Antibacterial and Antibiofilm Strategies for Environmental Restoration against Antibiotic-Resistant Bacteria" Materials 17, no. 14: 3444. https://doi.org/10.3390/ma17143444
APA StyleUthra, C., Nagaraj, K., Wadaan, M. A., Karuppiah, C., Maity, P., Baabbad, A., Kaliyaperumal, R., Venkatachalapathy, R., Shah, F., & Kumar, P. (2024). Zinc and Copper Oxide Nanoparticles: Pioneering Antibacterial and Antibiofilm Strategies for Environmental Restoration against Antibiotic-Resistant Bacteria. Materials, 17(14), 3444. https://doi.org/10.3390/ma17143444








