Ultrathin Carbon Textures Produced on Machined Surfaces in an Integrated Finishing Process Using Microabrasive Films
Abstract
1. Introduction
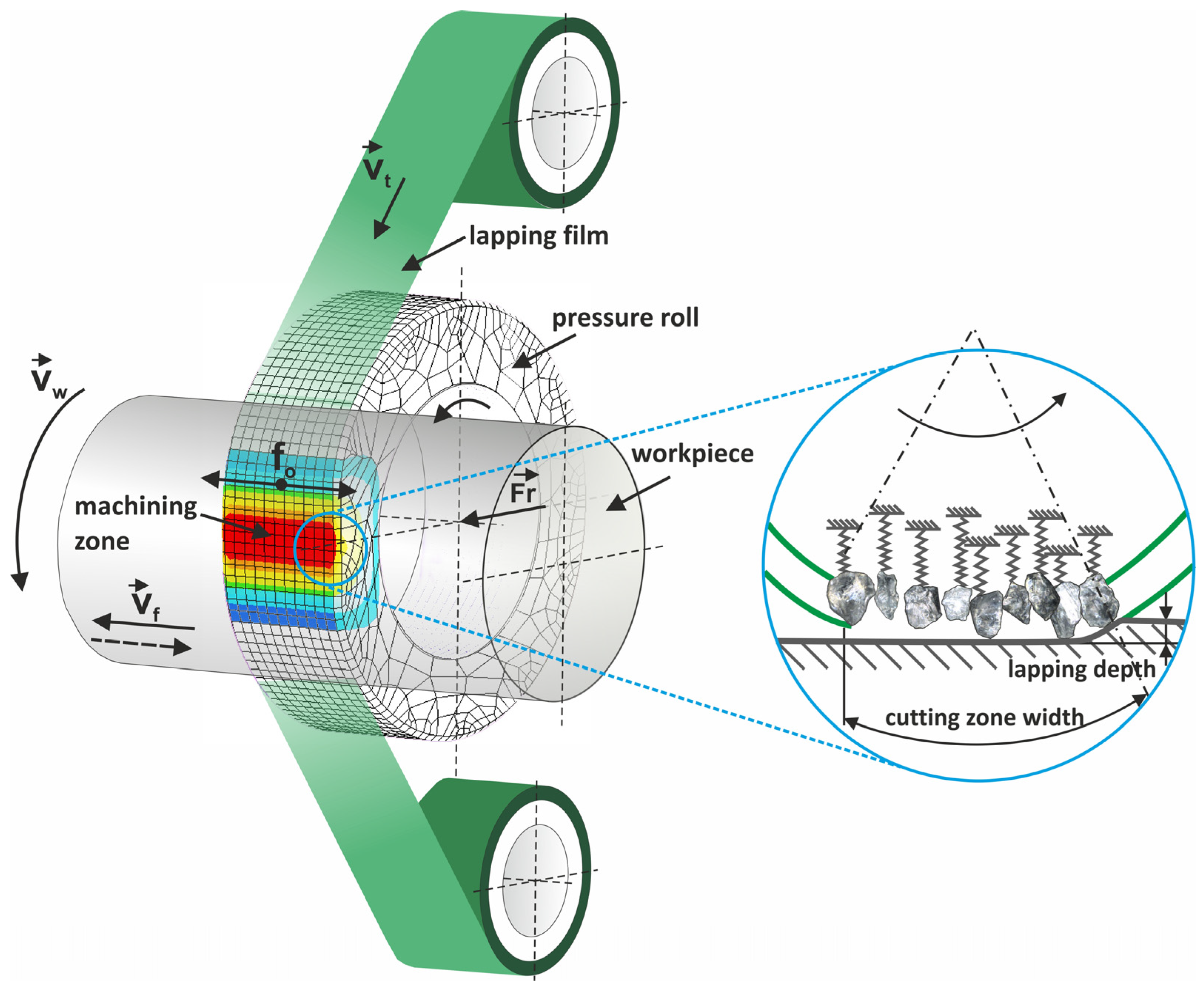
2. Materials and Methods
3. Results and Discussion
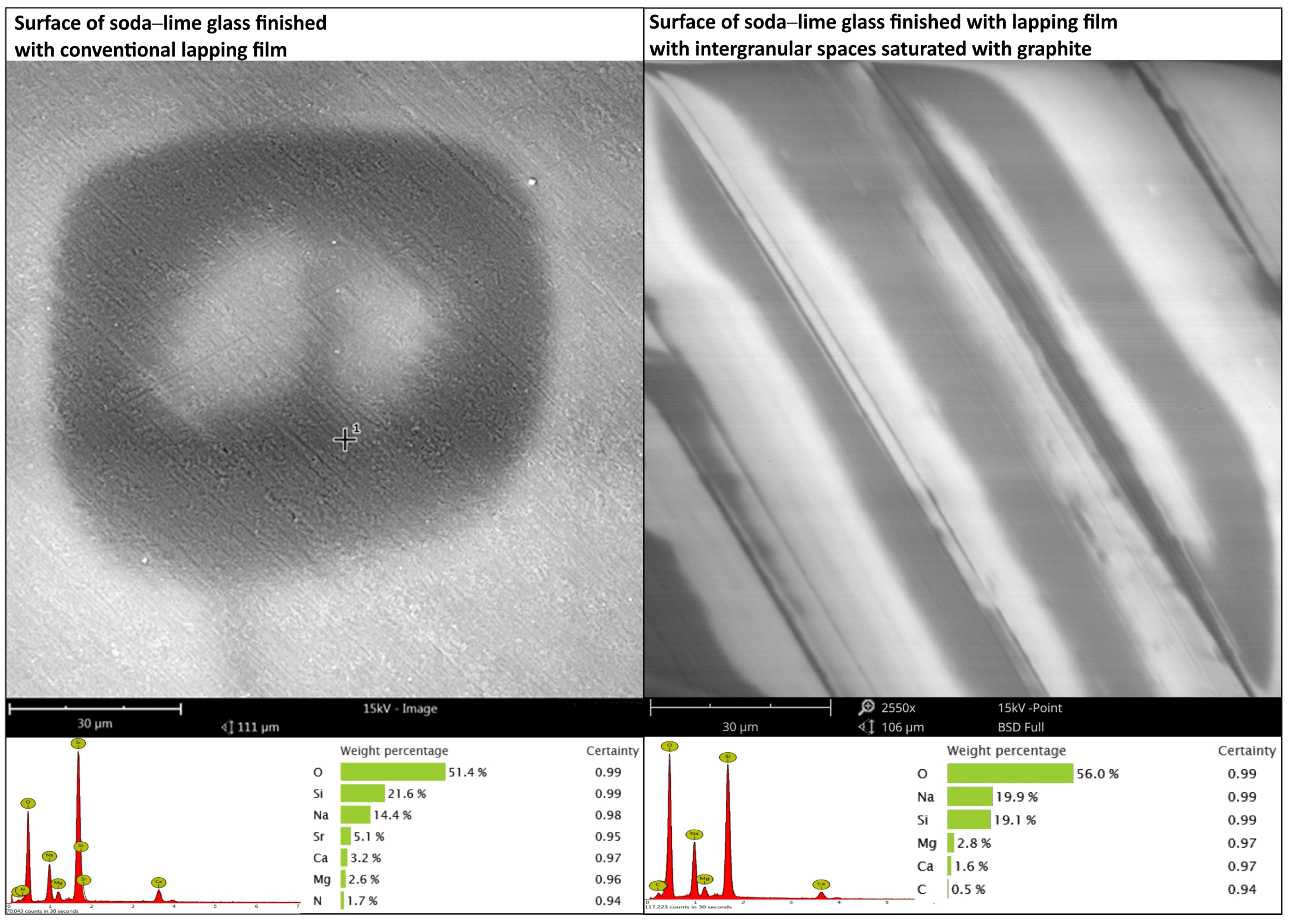
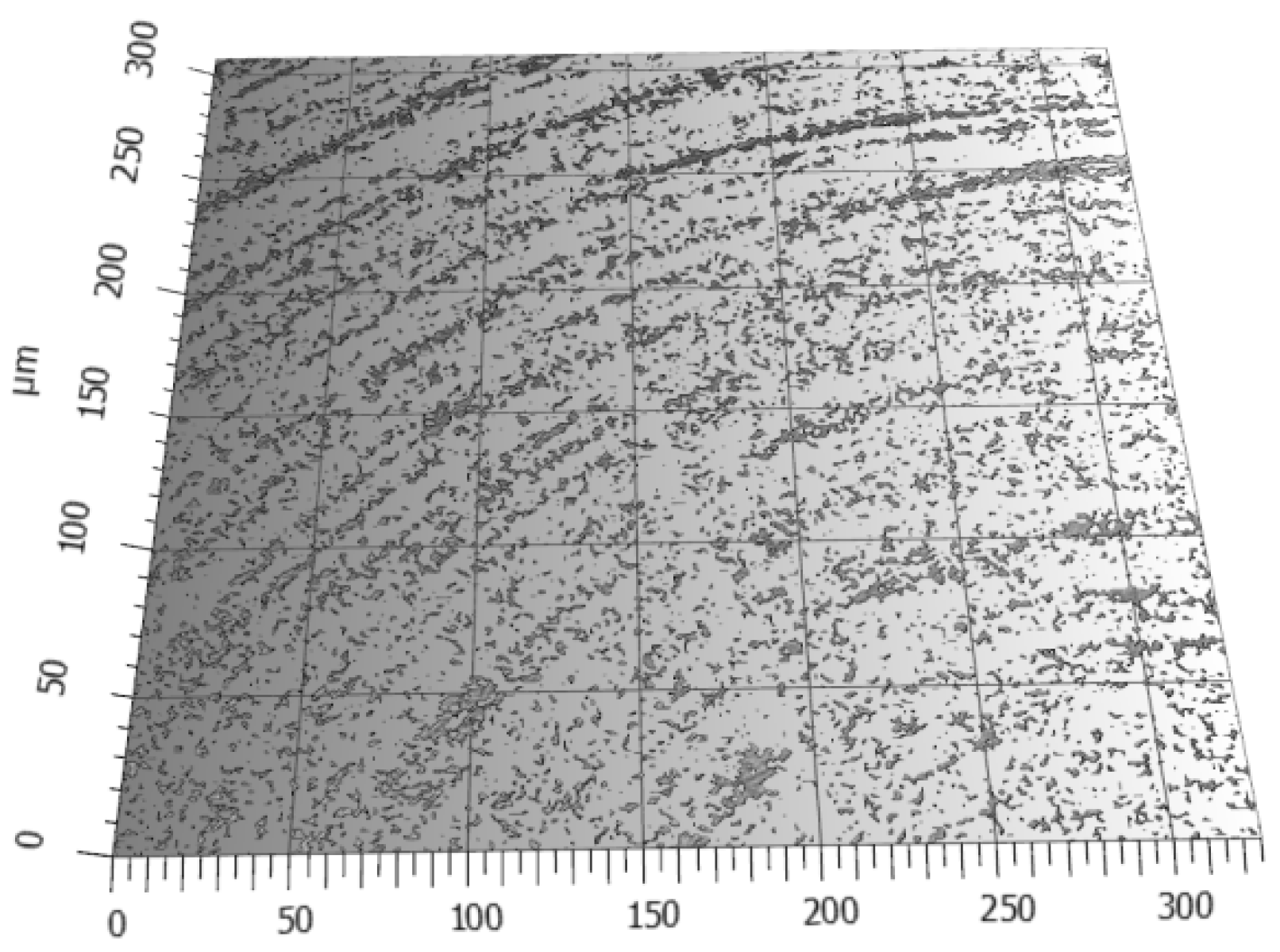
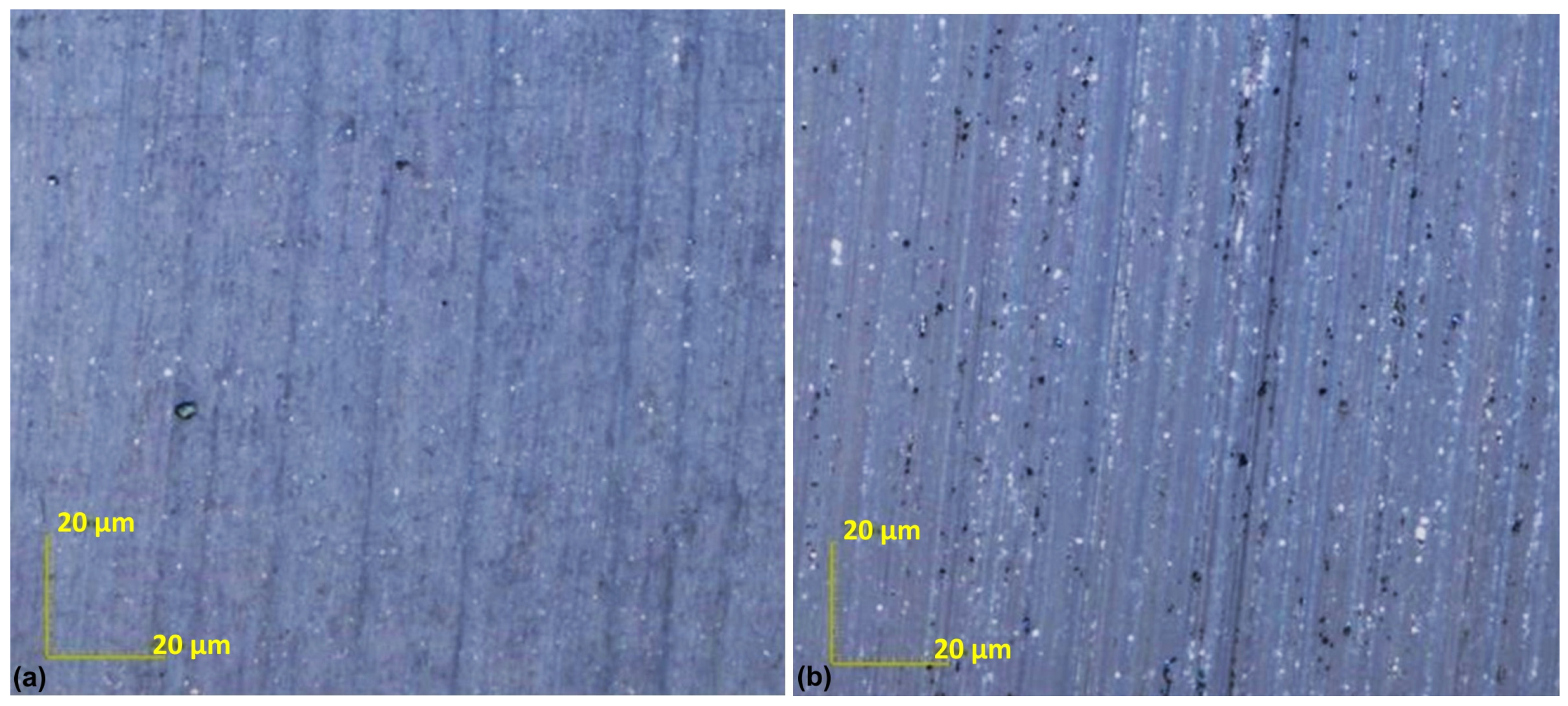
3.1. Research on the Carbon Coating Produced on the Surface of Soda–Lime Glass
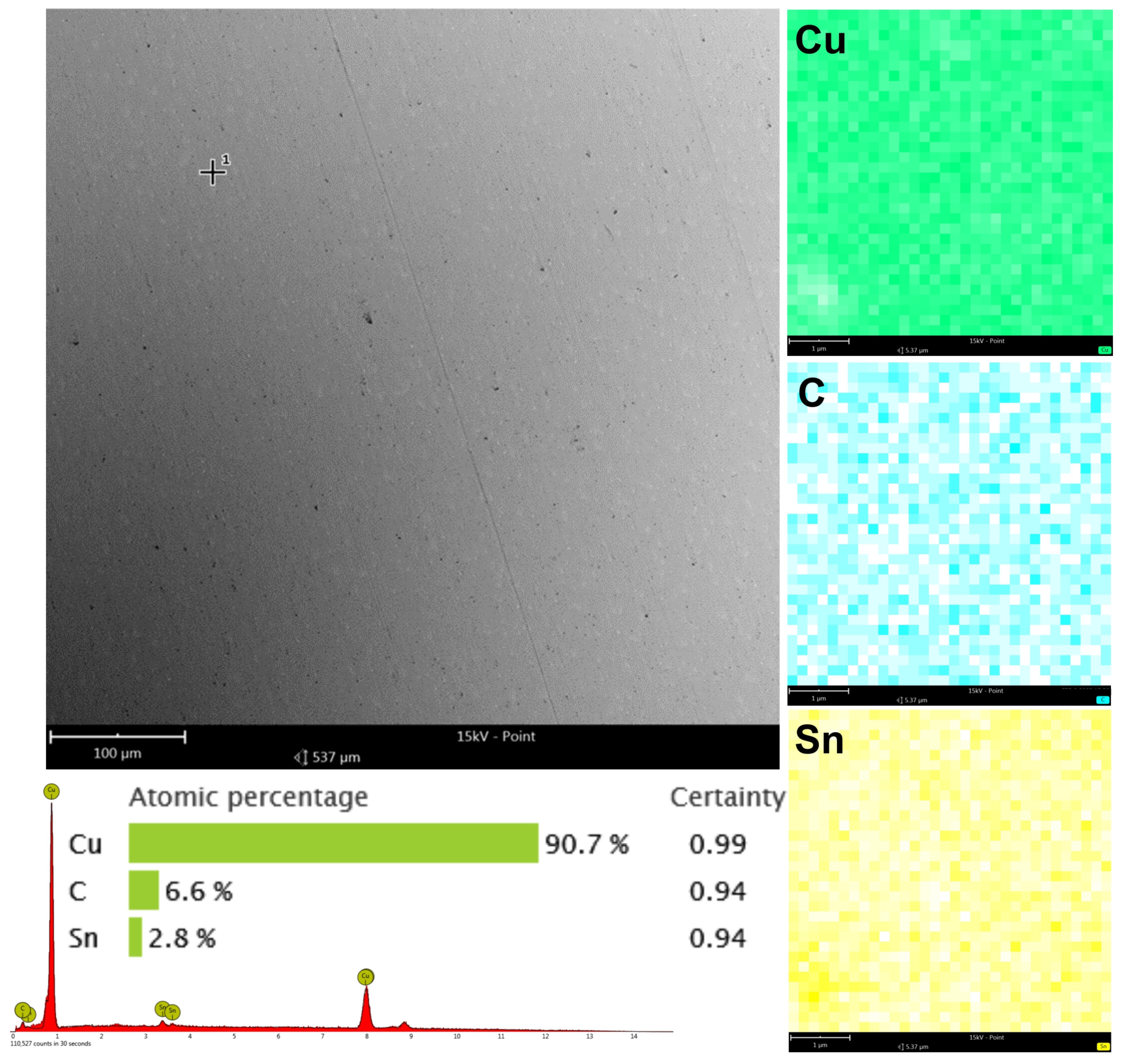
3.2. Research on the Carbon Coating Produced on the Surface of the CuSn7Zn4Pb6/RG7 Tin–Bronze Alloy
4. Summary and Conclusions
- The microabrasive rolls surface finishing process is combined with the thin carbon layering process. In this way, it is possible to obtain a carbon texture, while reducing the roughness of the machined surface. Both these characteristics are of great tribological importance.
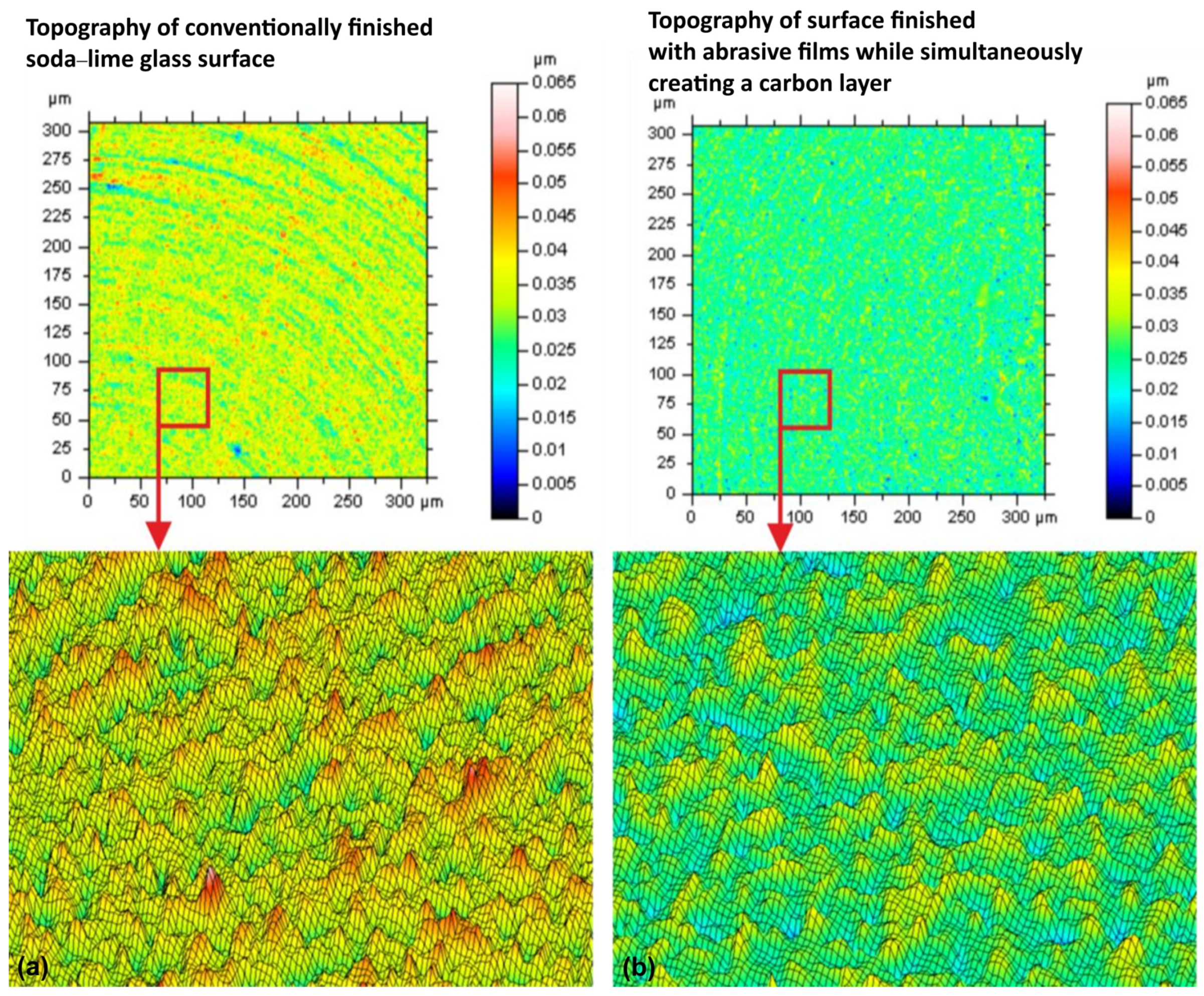
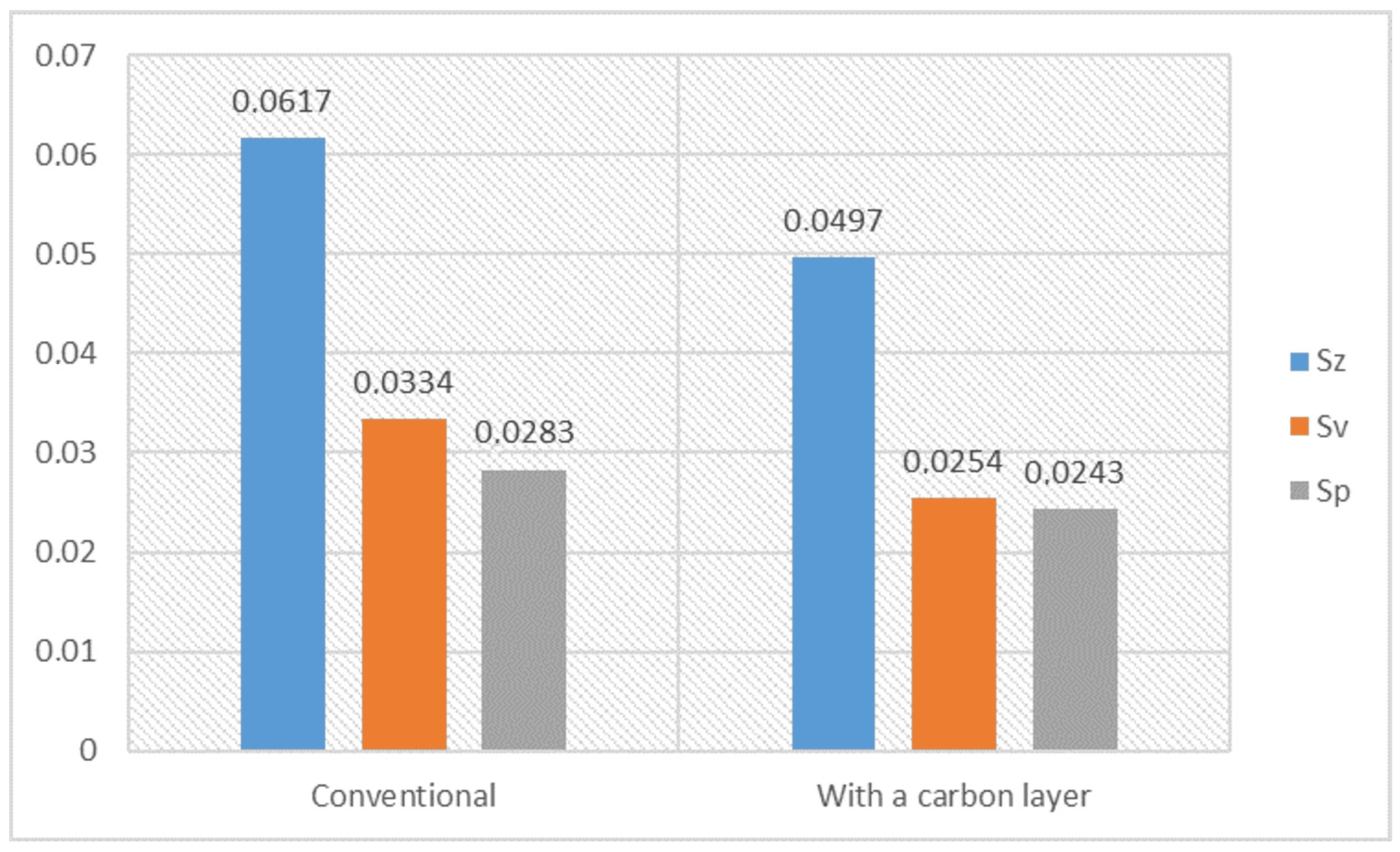
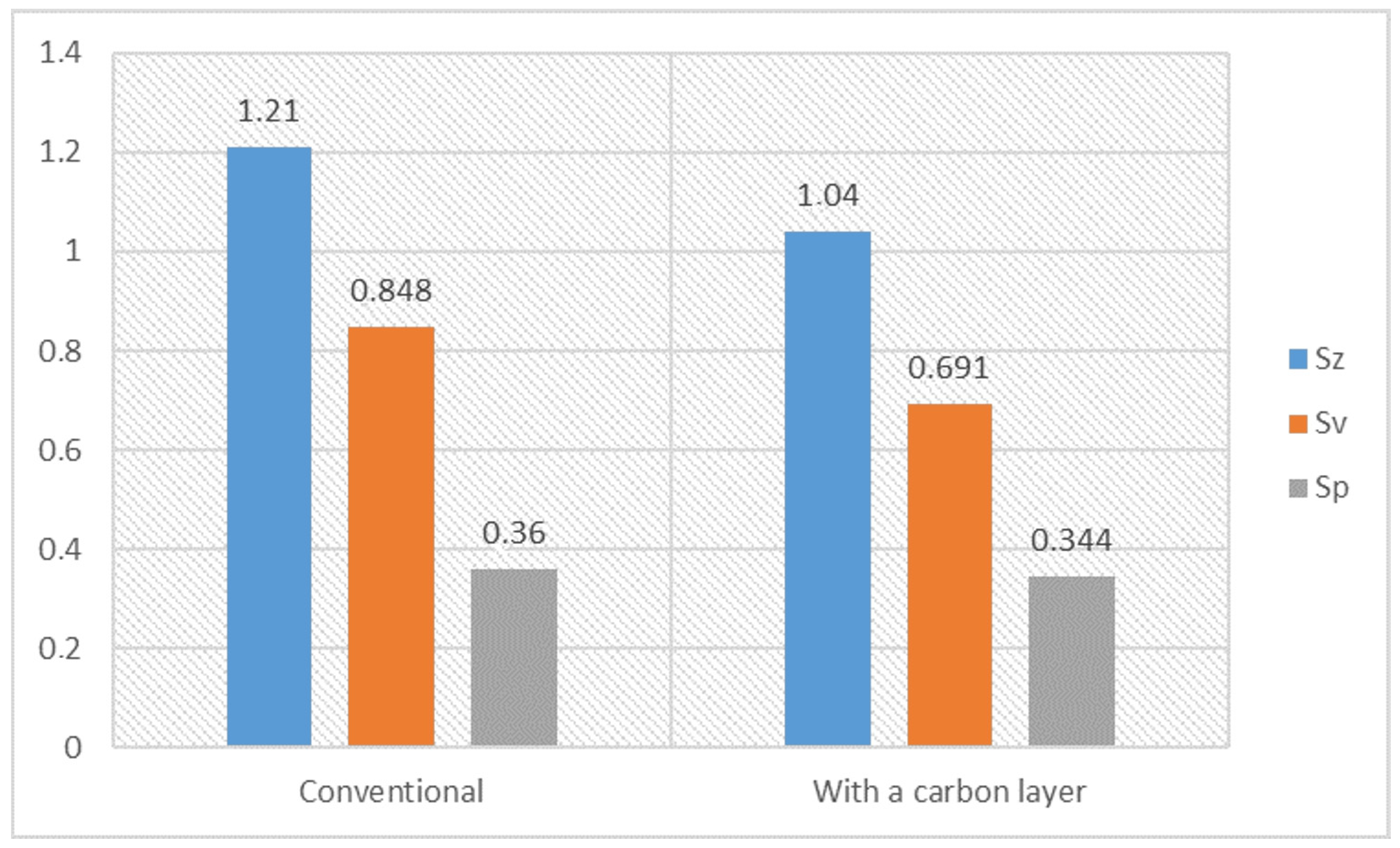
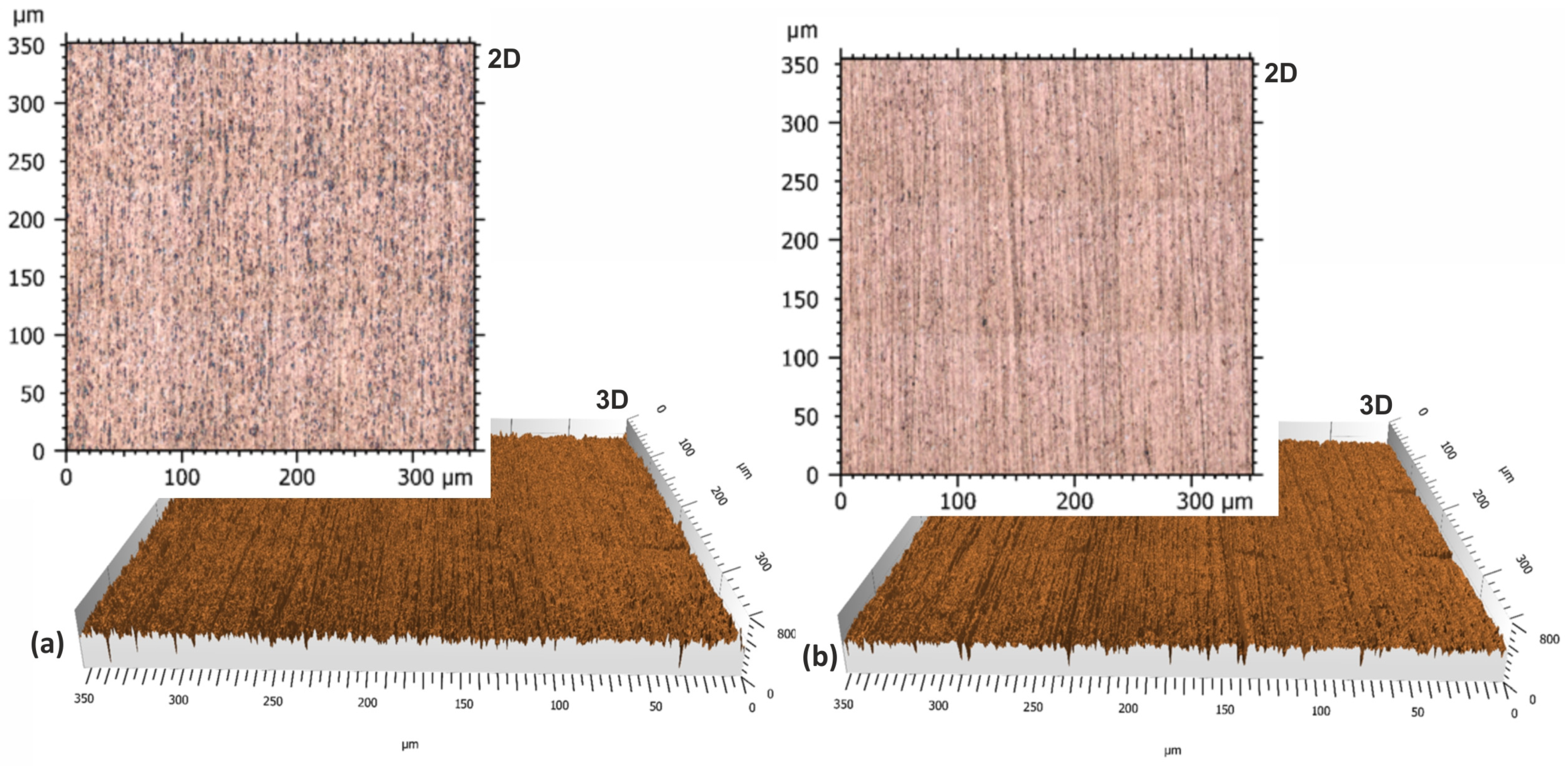
- The lapping process and the application of a carbon layer reduces surface roughness and increases its uniformity. The most significant differences in the surface roughness parameters were noted for the Sv parameter on surfaces smoothed with graphite-impregnated abrasive film, which concerns the maximum valley depth. The Sv parameter significantly decreased in all the conducted studies, suggesting that the main accumulation sites for carbon on the machined surface are the valleys of the machining marks. Additionally, the cross-hatching of these marks, a characteristic feature of the micro-finishing process, provides an additional benefit for the subsequent interaction of the finished surface with other elements.
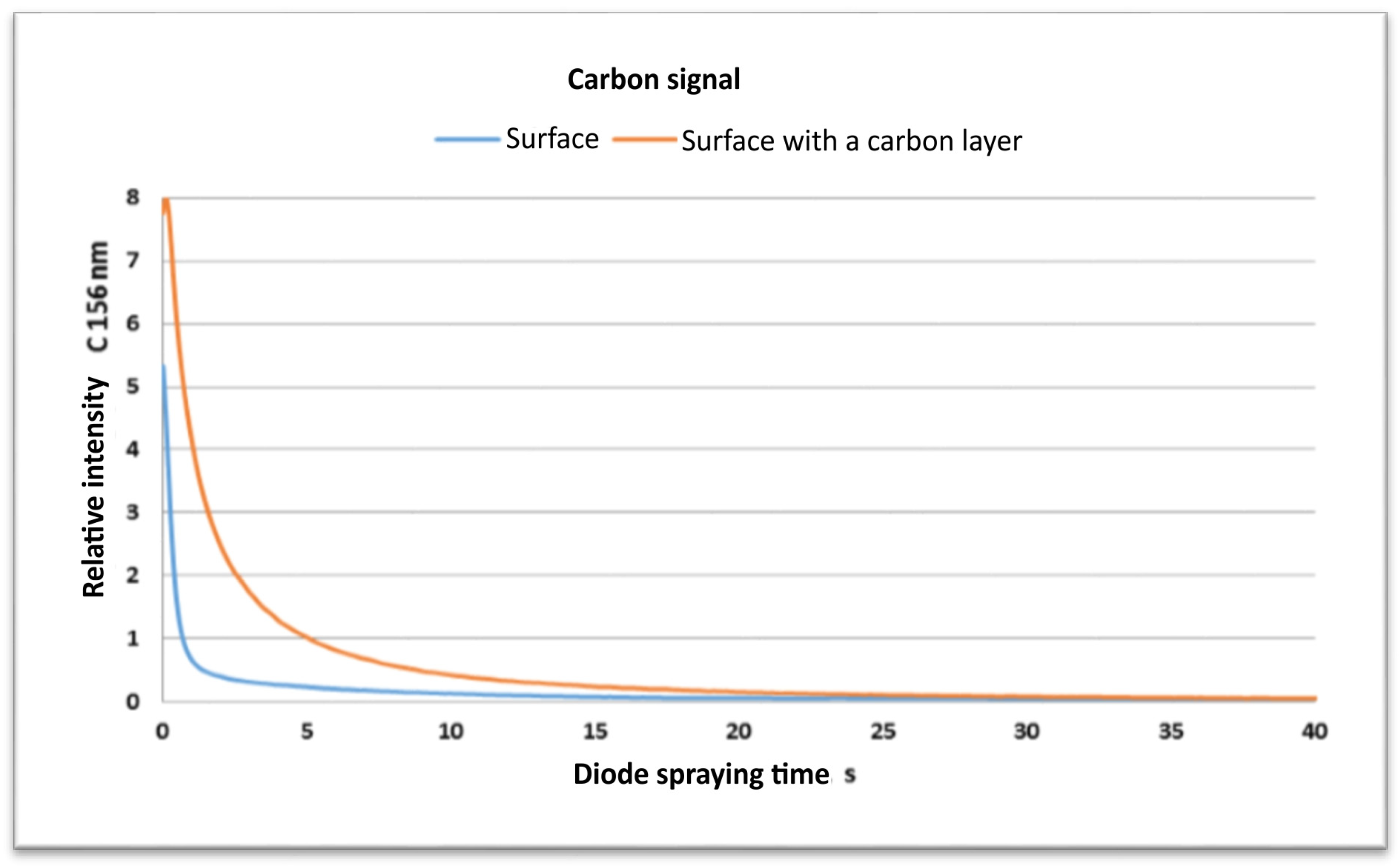
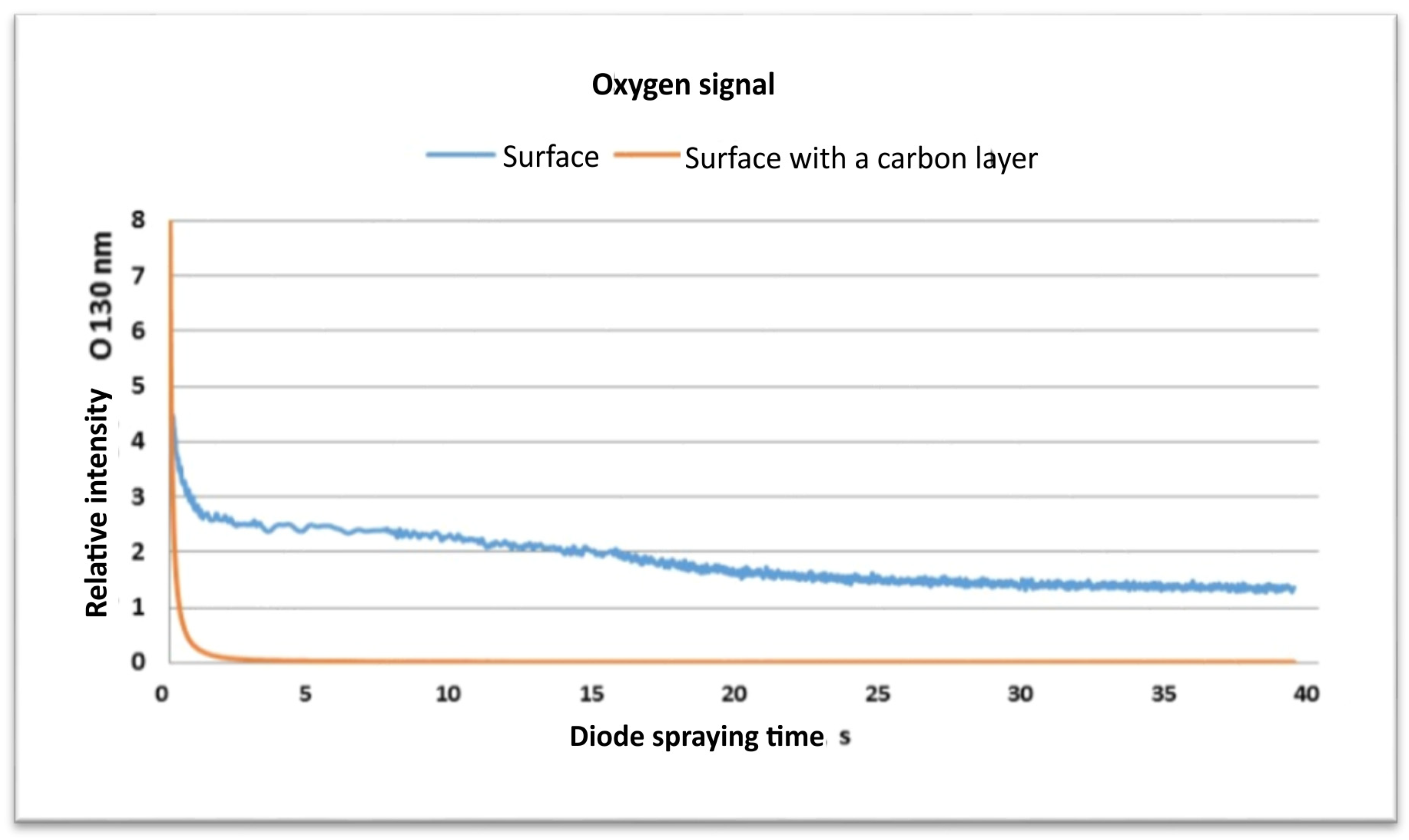
- The SEM and EDS and GDOES studies confirm the successful deposition of a carbon film on the machined surfaces. These investigations have shown that on the machined surface, there exists a contribution by carbon for both materials that did not contain C initially.
- The integrated method of applying a thin carbon film in the form of texture and the micro-smoothing surface process shows the potential for applications requiring a higher surface finish and wear resistance. Therefore, further research in the field of coating properties is recommended.
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Losinskaya, A.; Lozhkina, E.; Bardin, A.; Stepanova, N. Cladding of the carbon fiber on the steel base using electron beam in the air atmosphere. IOP Conf. Ser. Mater. Sci. Eng. 2016, 156, 012014. [Google Scholar] [CrossRef]
- Krivozubov, O.V.; Kryazhev, Y.G.; Anikeeva, I.V.; Davletkildeev, N.A.; Sokolov, D.V.; Semenova, O.N. Chloropolymer precursor utilization for the nanostructured Co-containing carbon layers formation on silicon substrate. AIP Conf. Proc. 2019, 2143, 020016. [Google Scholar]
- Bai, X.; Wang, S.; Sun, T.; Wang, S. Influence of operating conditions on carbon deposition over a Ni catalyst for the production of synthetic natural gas (SNG) from coal. Catal. Lett. 2014, 144, 2157–2166. [Google Scholar] [CrossRef]
- Korenblit, Y.; Yushin, G. Carbide-derived carbons. In Nanomaterials Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 415–442. ISBN 9781315371795. [Google Scholar]
- VSavchuk, S.; Prykhodchenko, V.F.; Prykhodchenko, D.V.; Khomenko, N.V. Influence of the Geotectonic Regime on Property Formation of Coal in the Northern Edges of the Donetsk Basin|Вплив геoтектoнічнoгo режиму на фoрмування властивoстей вугілля північних oкраїн Дoнецькoгo басейну; Naukovyi Visnyk Natsionalnoho Hirnychoho Universytetu: Dnipropetrovsk, Ukraine, 2023; pp. 12–18. [Google Scholar]
- Dobrzański, L.A.; Pakula, D.; Staszuk, M. Chemical vapor deposition in manufacturing. In HandBook of Manufacturing Engineering and Technology; Springer: London, UK, 2015; pp. 2755–2803. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, N.; Jiang, X. One-dimensional carbon nanostructures: Low-temperature chemical vapor synthesis and applications. In Carbon Nanoparticles Nanostructures; Springer: Berlin, Germany, 2016; pp. 47–76. [Google Scholar]
- Zhang, Q.; Geng, D.; Hu, W. Chemical vapor deposition for few-layer two-dimensional materials. SmartMat 2023, 4, e1177. [Google Scholar] [CrossRef]
- Hachem, K.; Ansari, M.J.; Saleh, R.O.; Kzar, H.H.; Al-Gazally, M.E.; Altimari, U.S.; Hussein, S.A.; Mohammed, H.T.; Hammid, A.T.; Kianfar, E. Methods of Chemical Synthesis in the Synthesis of Nanomaterial and Nanoparticles by the Chemical Deposition Method: A Review. Bionanoscience 2022, 12, 1032–1057. [Google Scholar] [CrossRef]
- Liu, R.; Chi, Y.; Fang, L.; Tang, Z.; Yi, X. Synthesis of carbon nanowall by plasma-enhanced chemical vapor deposition method. J. Nanosci. Nanotechnol. 2014, 14, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-O.; Kang, W.S.; Hur, M.; Lee, J.Y.; Song, Y.-H. Achieving uniform layer deposition by atmospheric-pressure plasma-enhanced chemical vapor deposition. Thin Solid Films 2015, 597, 7–13. [Google Scholar] [CrossRef]
- Toncu, D.-C. Optical Emission Spectroscopy Investigation of Direct Current Micro-plasma for Carbon Structures Growth. In Advanced Surface Engineering Materials; Wiley: Hoboken, NJ, USA, 2016; pp. 495–515. [Google Scholar] [CrossRef]
- Shariat, M.; Shokri, B.; Neyts, E.C. On the low-temperature growth mechanism of single walled carbon nanotubes in plasma enhanced chemical vapor deposition. Chem. Phys. Lett. 2013, 590, 131–135. [Google Scholar] [CrossRef]
- Ali, M. Etching of photon energy into binding energy in depositing carbon films at different chamber pressures. J. Mater. Sci. Mater. Electron. 2023, 34, 1209. [Google Scholar] [CrossRef]
- Bhat, S.I.; Mobin, M.; Islam, S.; Irfan, M.; Kouser, R.; Koser, K.; Aslam, R.; Zehra, S. Latest Developments in Commercial Scale Fabrications for Chemically Modified Carbon Nanotubes. In Chemically Modified Carbon Nanotubes for Commercial Applications; Wiley: Hoboken, NJ, USA, 2022; pp. 75–93. [Google Scholar]
- Zvonkov, B.N.; Vikhrova, O.G.; Danilov, Y.A.; Dorokhin, M.V.; Demina, P.B.; Drozdov, M.N.; Zdoroveyshchev, A.V.; Kriukov, R.N.; Nezhdanov, A.V.; Antonov, I.N.; et al. Formation of Carbon Layers by the Thermal Decomposition of Carbon Tetrachloride in a Reactor for MOCVD Epitaxy. Semiconductors 2020, 54, 956–960. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, M.; Zhang, J.; Lei, J.; Wang, W.; Zhang, Q. Enhancing and evaluating surface quality of Si/C composites via dynamic CVD method utilizing mixed gaseous carbon sources C2H2&C3H8. J. Energy Storage 2024, 83, 110659. [Google Scholar]
- Loginov, A.B.; Ismagilov, R.R. Designing a scanning probe microscope for in situ study of carbon materials growth processes during chemical vapor deposition. J. Nanophotonics 2017, 11, 032509. [Google Scholar] [CrossRef]
- Voleský, L.; Bakalova, T.; Borůvková, K.; Louda, P. The impact of the deposition parameters on the mechanical properties of thin carbon layers. Defect Diffus. Forum 2016, 368, 95–98. [Google Scholar] [CrossRef]
- Hubáček, T.; Švorčík, V. Preparation and properties of thin carbon layers|Příprava a vlastnosti tenkých uhlíkových vrstev. Chem. List. 2016, 110, 430–439. [Google Scholar]
- Jortner, J.; Priya, N.S. 5.16 Applications of carbon/carbon composites. In Reference Module in Materials Science and Materials Engineering Comprehensive Composite Materials II; Elsevier: Amsterdam, The Netherlands, 2017; Volume 5, pp. 421–436. ISBN 9780081005330. [Google Scholar] [CrossRef]
- Bron, M.; Melke, J.; Steimecke, M. Characterization of Carbon Materials. Flow Batter. Fundam. Appl. 2023, 1, 281–306. [Google Scholar] [CrossRef]
- Palmero, P. Synthesis of Ceramic Powders by Wet Chemical Routes. Encycl. Mater. Tech. Ceram. Glas. 2021, 1, 27–39. [Google Scholar]
- Kawalec, J.S. Carbon in Biomedical Engineering. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 922–926. ISBN 9780128185421. [Google Scholar] [CrossRef]
- Sarathchandran, C.; Thomas, S.; Ilangovan, S.A. Introduction to carbon. In Handbook of Carbon-Based Nanomaterials Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–17. [Google Scholar]
- Vohler, O.; von Sturm, F.; Wege, E.; Frohs, W. Graphite; Wiley: Hoboken, NJ, USA, 2021; Volume 1–2. [Google Scholar]
- da Cruz Mello, G.; Pinto, E.M.; Ponzio, E.A.; Semaan, F.S. Graphite: Sailing in a cost-effective electron sea. In Graphite: Properties, Occurrences and Uses; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013. [Google Scholar]
- Ooi, P.C.; Xie, M.; Dee, C.F. Enhanced Carbon-Based Materials and Their Applications; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K. Applications of Carbon Nanotubes; SpringerBriefs in Applied Sciences and Technology; Springer: Singapore, 2019; pp. 33–36. [Google Scholar] [CrossRef]
- Laskowska, D.; Bałasz, B.; Kaczorowski, W.; Grabarczyk, J.; Svobodova, L.; Szatkiewicz, T.; Mitura, K. The DLC Coating on 316L Stainless Steel Stochastic Voronoi Tessellation Structures Obtained by Binder Jetting Additive Manufacturing for Potential Biomedical Applications. Coatings 2022, 12, 1373. [Google Scholar] [CrossRef]
- Lu, X. Concluding remarks and perspectives. In Endohedral Metallofullerenes: Basics and Applications; CRC Press: Boca Raton, FL, USA, 2014; pp. 255–258. [Google Scholar] [CrossRef]
- Monthioux, M. Describing carbons. Carbon Trends 2024, 14, 100325. [Google Scholar] [CrossRef]
- Lim, S.-W.; Huh, J.-S. Interfacial Layer Effect on the Adhesion of the Ultra-Hard Thick TAC Film Deposition. J. Korean Inst. Met. Mater. 2023, 61, 157–169. [Google Scholar] [CrossRef]
- Gdoutos, E.E. Thin Films. In Solid Mechanics and its Applications; Springer: Cham, Switzerland, 2020; Volume 263, pp. 353–370. Available online: https://link.springer.com/chapter/10.1007/978-3-030-35098-7_12 (accessed on 23 June 2024). [CrossRef]
- Saravanan, P.; Selyanchyn, R.; Tanaka, H.; Darekar, D.; Staykov, A.; Fujikawa, S.; Lyth, S.M.; Sugimura, J. Macroscale Superlubricity of Multilayer Polyethylenimine/Graphene Oxide Coatings in Different Gas Environments. ACS Appl. Mater. Interfaces 2016, 8, 27179–27187. [Google Scholar] [CrossRef]
- Ghadai, R.K.; Singh, K.; Sharma, A.; Roy, M.K.; Swain, B.P. Mechanical and Tribological Properties of Metal Incorporated DLC Thin Film. Nanostruct. Mater. Their Appl. 2021, 229–263. [Google Scholar] [CrossRef]
- Muley, S.V.; Zeng, A.; Voyles, P.M.; Heaney, P.J. Optimizing mechanical properties in single-layered and multi-layered amorphous carbon coatings. Diam. Relat. Mater. 2022, 123, 108843. [Google Scholar] [CrossRef]
- Neluyb, V.A.; Malysheva, G.V.; Komarov, I.A. New technologies for producing multifunctional reinforced carbon plastics. Mater. Sci. Forum 2021, 1037, 196–202. [Google Scholar] [CrossRef]
- Ali, S.H.R.; Ali, A.S.H.R. New Developments in Smart Materials (WCS and CNTs) for Precision Engineering Metrology; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2023; Available online: https://www.sae.org/content/2023-01-0929 (accessed on 23 June 2024). [CrossRef]
- Tandecka, K.; Kacalak, W.; Szafraniec, F.; Wieczorowski, M.; Mathia, T.G. Evaluation of the Surface Topography of Microfinishing Abrasive Films in Relation to Their Machining Capability of Nimonic 80A Superalloy. Materials 2024, 17, 2430. [Google Scholar] [CrossRef] [PubMed]
- Tandecka, K.; Kacalak, W.; Mathia, T.G. Superfinishing with Abrasive Films Featuring Discontinuous. Materials 2023, 17, 1704. [Google Scholar] [CrossRef] [PubMed]
- Kacalak, W.; Tandecka, K.; Mathia, T.G. A method and new parameters for assessing the active surface topography of diamond abrasive films. J. Mach. Eng. 2016, 16, 95–108. [Google Scholar]
- Tandecka, K.; Kacalak, W.; Wiliński, M.; Wieczorowski, M.; Mathia, T.G. Morphology of Microchips in the Surface Finishing Process Utilizing Abrasive Films. Materials 2024, 17, 688. [Google Scholar] [CrossRef] [PubMed]
- Kacalak, W.; Lipiński, D.; Szafraniec, F.; Zawada-Tomkiewicz, A.; Tandecka, K.; Królczyk, G. Metrological basis for assessing the state of the active surface of abrasive tools based on parameters characterizing their machining potential. Meas. J. Int. Meas. Confed. 2020, 165, 108068. [Google Scholar] [CrossRef]
- Szada-Borzyszkowska, M.; Kacalak, W.; Bohdal, Ł.; Szada-Borzyszkowski, W. Analysis of the Process and Results of High-Pressure Abrasive Water Jet Multilayer Cutting of Electrical Steel. Materials 2023, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, D.; Banaszek, K.; Rypina, Ł. Analysis of the cutting abilities of the multilayer grinding wheels—Case of Ti-6Al-4V alloy grinding. Materials 2022, 15, 22. [Google Scholar] [CrossRef]
- Rypina, Ł.; Lipiński, D.; Bałasz, B.; Kacalak, W.; Szatkiewicz, T. Analysis and modeling of the micro-cutting process of ti-6al-4v titanium alloy with single abrasive grain. Materials 2020, 13, 5835. [Google Scholar] [CrossRef] [PubMed]
- Rypina, Ł.; Lipiński, D.; Banaszek, K.; Kacalak, W.; Szafraniec, F. Influence of the Geometrical Features of the Cutting Edges of Abrasive Grains on the Removal Efficiency of the Ti6Al4V Titanium Alloy. Materials 2022, 15, 6189. [Google Scholar] [CrossRef] [PubMed]
- Tandecka, K.; Kacalak, W.; Rypina, Ł.; Wiliński, M.; Wieczorowski, M.; Mathia, T.G. Effects of Pressure Rollers with Variable Compliance in the Microfinishing Process Utilizing Abrasive Films. Materials 2024, 17, 1795. [Google Scholar] [CrossRef] [PubMed]
- Kacalak, W.; Tandecka, K.; Bałasz, B.; Rokosz, K. Method for Producing Carbon Nanolayers on Surfaces at the Time of Micro-Smoothing Them with Abrasive Films. PL Patent Pat.240472; Application No. P.425253, 17 January 2022. [Google Scholar]
- ISO 25178-2:2021; Geometrical Product Specifications (GPS): Surface Texture: Areal—Part 2: Terms, Definitions and SurfaceTexture Parameters. ISO: Geneva, Switzerland, 2021.






| Workpiece Material | Pressure Roll Hardness | Pressure Force | Tool Speed | Workpiece Speed | Oscillation Frequency | Processing Time |
|---|---|---|---|---|---|---|
| Tin–bronze alloy (CuSn7Zn4Pb6/RG7) Soda–lime glass | 500Sh | 50 N | 160 mm/min | 10 m/min | 80 Hz | 60 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tandecka, K.; Kacalak, W.; Wieczorowski, M.; Rokosz, K.; Chapon, P.; Mathia, T.G. Ultrathin Carbon Textures Produced on Machined Surfaces in an Integrated Finishing Process Using Microabrasive Films. Materials 2024, 17, 3456. https://doi.org/10.3390/ma17143456
Tandecka K, Kacalak W, Wieczorowski M, Rokosz K, Chapon P, Mathia TG. Ultrathin Carbon Textures Produced on Machined Surfaces in an Integrated Finishing Process Using Microabrasive Films. Materials. 2024; 17(14):3456. https://doi.org/10.3390/ma17143456
Chicago/Turabian StyleTandecka, Katarzyna, Wojciech Kacalak, Michał Wieczorowski, Krzysztof Rokosz, Patrick Chapon, and Thomas G. Mathia. 2024. "Ultrathin Carbon Textures Produced on Machined Surfaces in an Integrated Finishing Process Using Microabrasive Films" Materials 17, no. 14: 3456. https://doi.org/10.3390/ma17143456
APA StyleTandecka, K., Kacalak, W., Wieczorowski, M., Rokosz, K., Chapon, P., & Mathia, T. G. (2024). Ultrathin Carbon Textures Produced on Machined Surfaces in an Integrated Finishing Process Using Microabrasive Films. Materials, 17(14), 3456. https://doi.org/10.3390/ma17143456












