The Main Failure Modes of Hot-Work Die Steel and the Development Status of Traditional Strengthening Methods and Nano-Strengthening Technology
Abstract
1. Introduction
2. Study on Main Service Failure Forms and Mechanisms of Die Steel
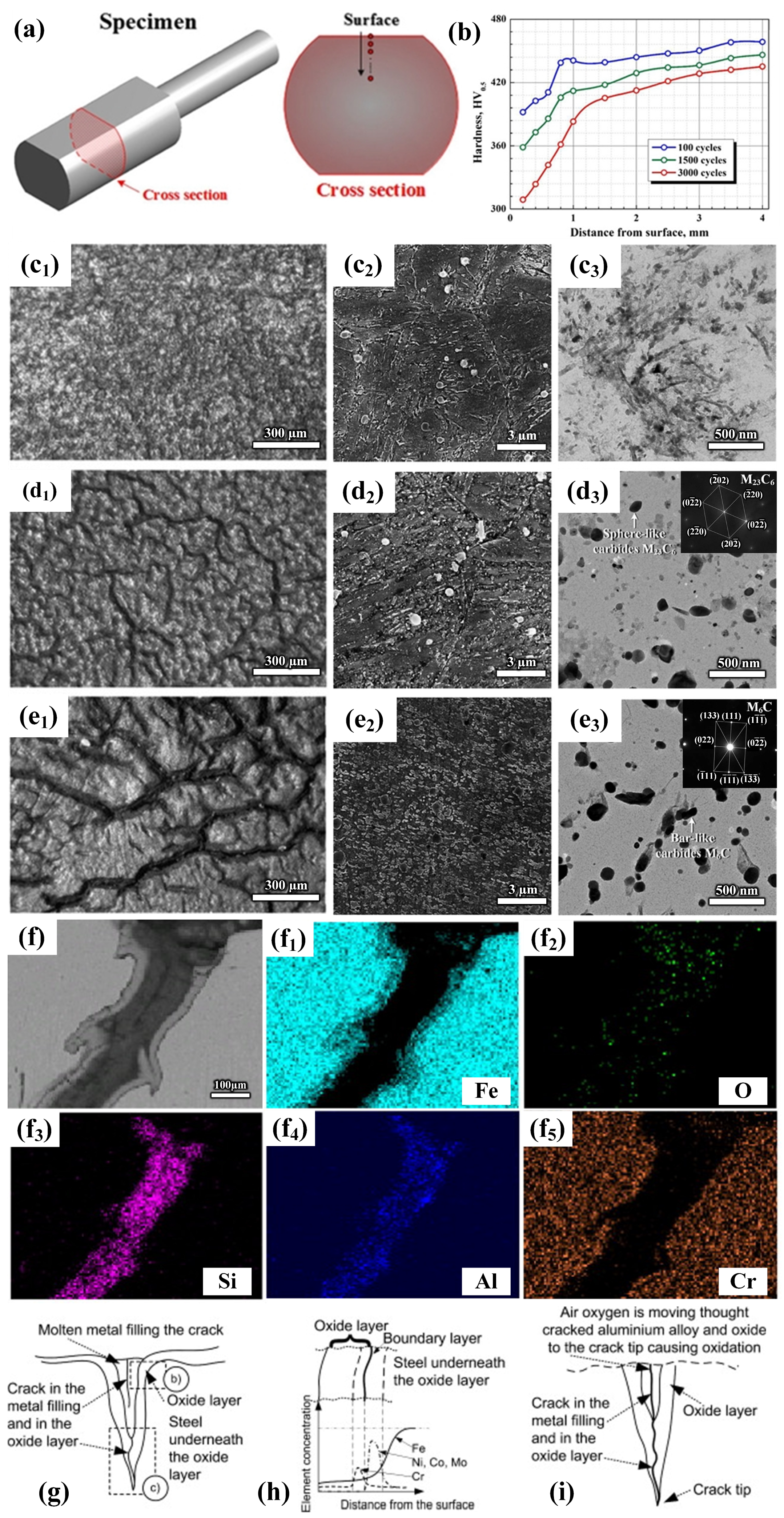
2.1. Study on Fatigue Behavior of Die Steel
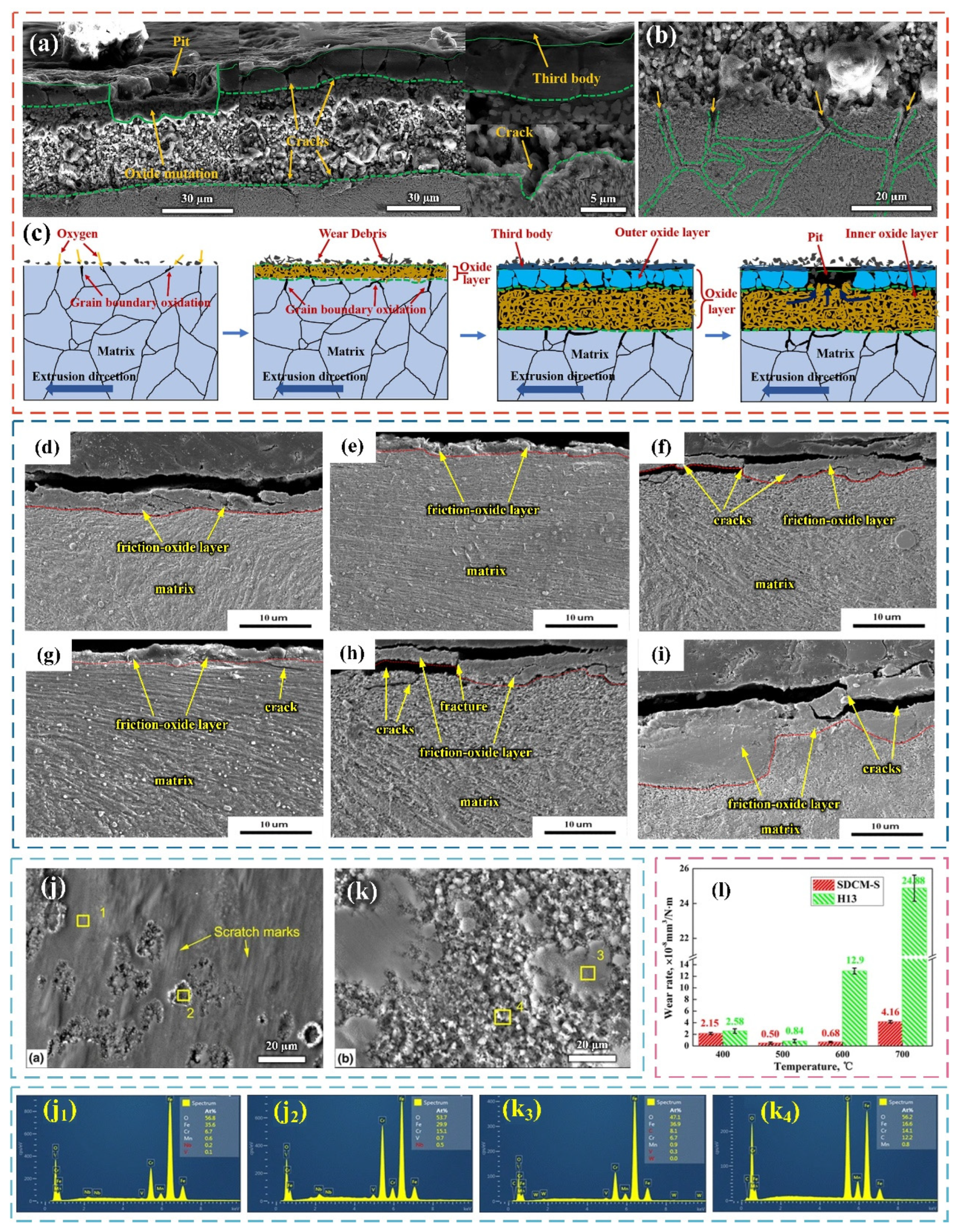
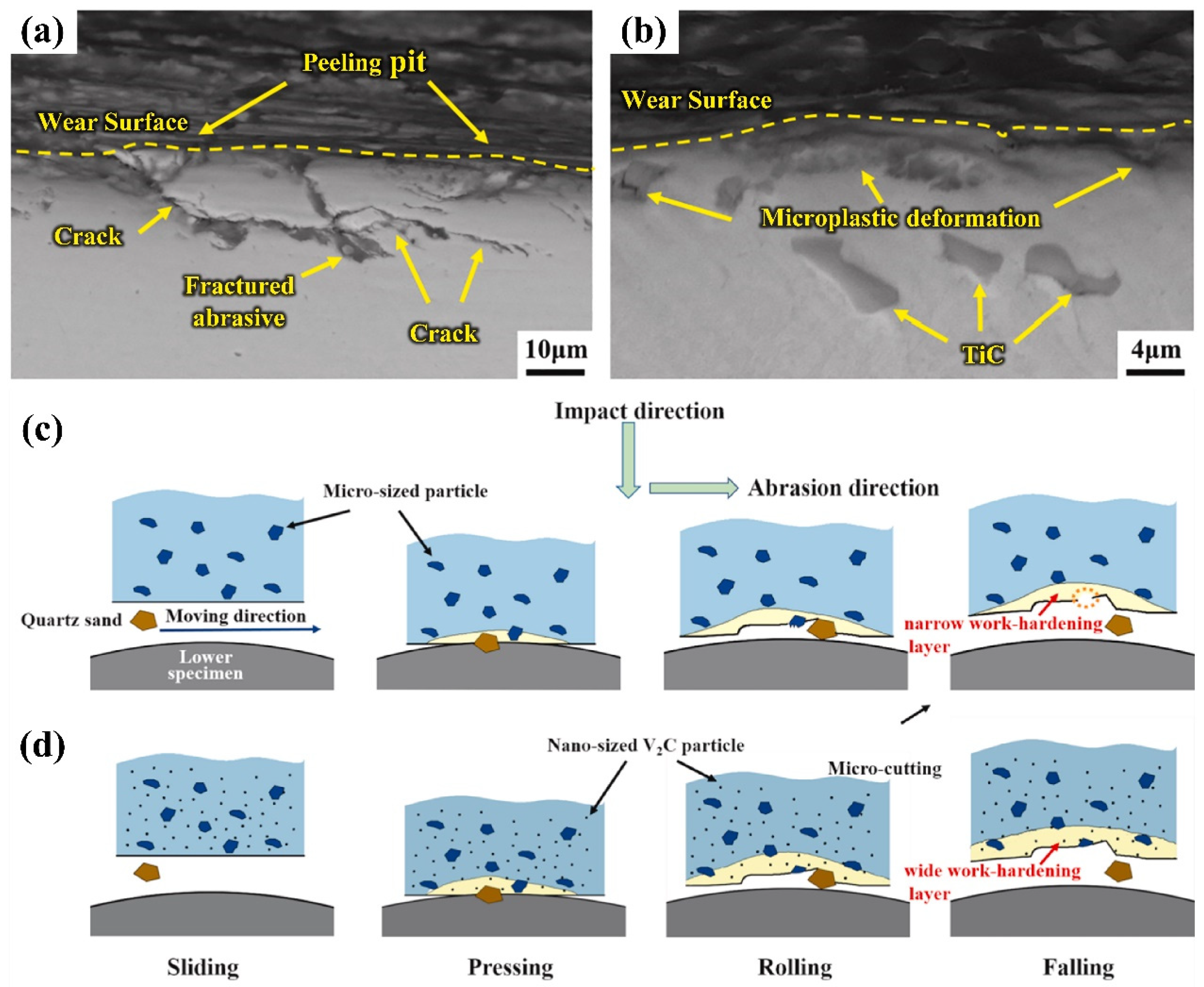
2.2. Study on Wear Behavior of Die Steel
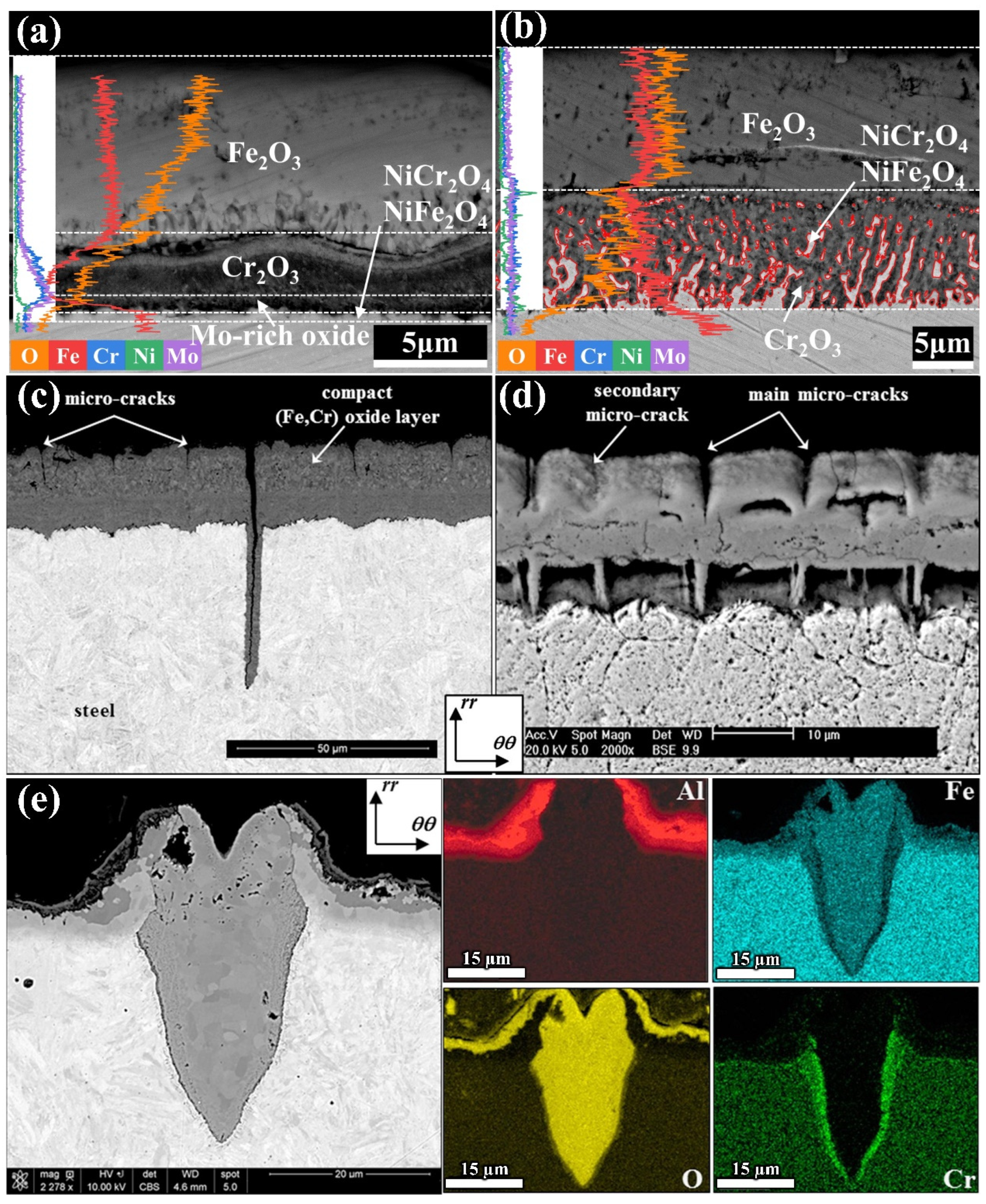
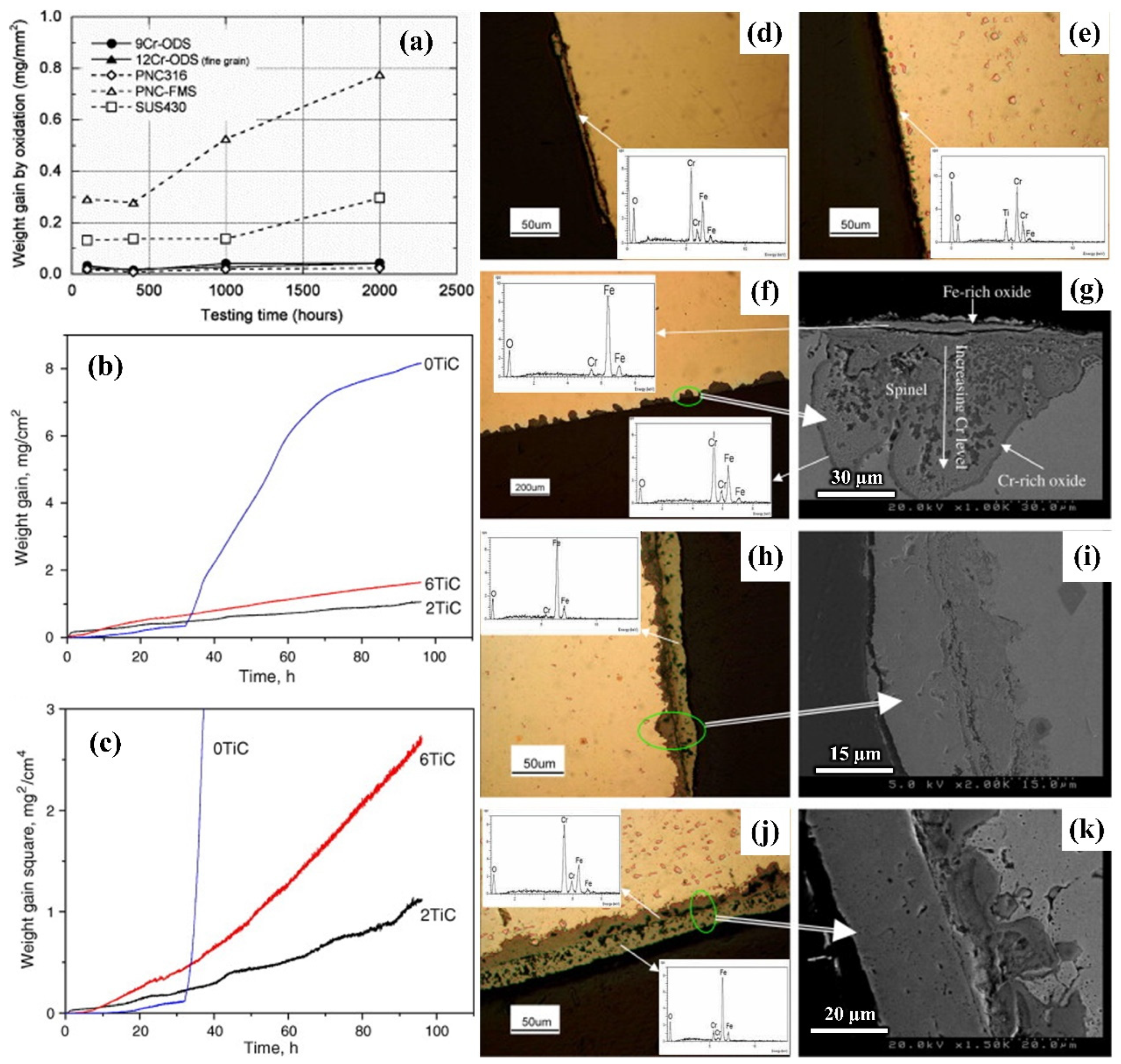
2.3. Study on Oxidation Behavior of Die Steel
3. Main Methods of Toughening Die Steel
3.1. Optimizing Alloying Elements
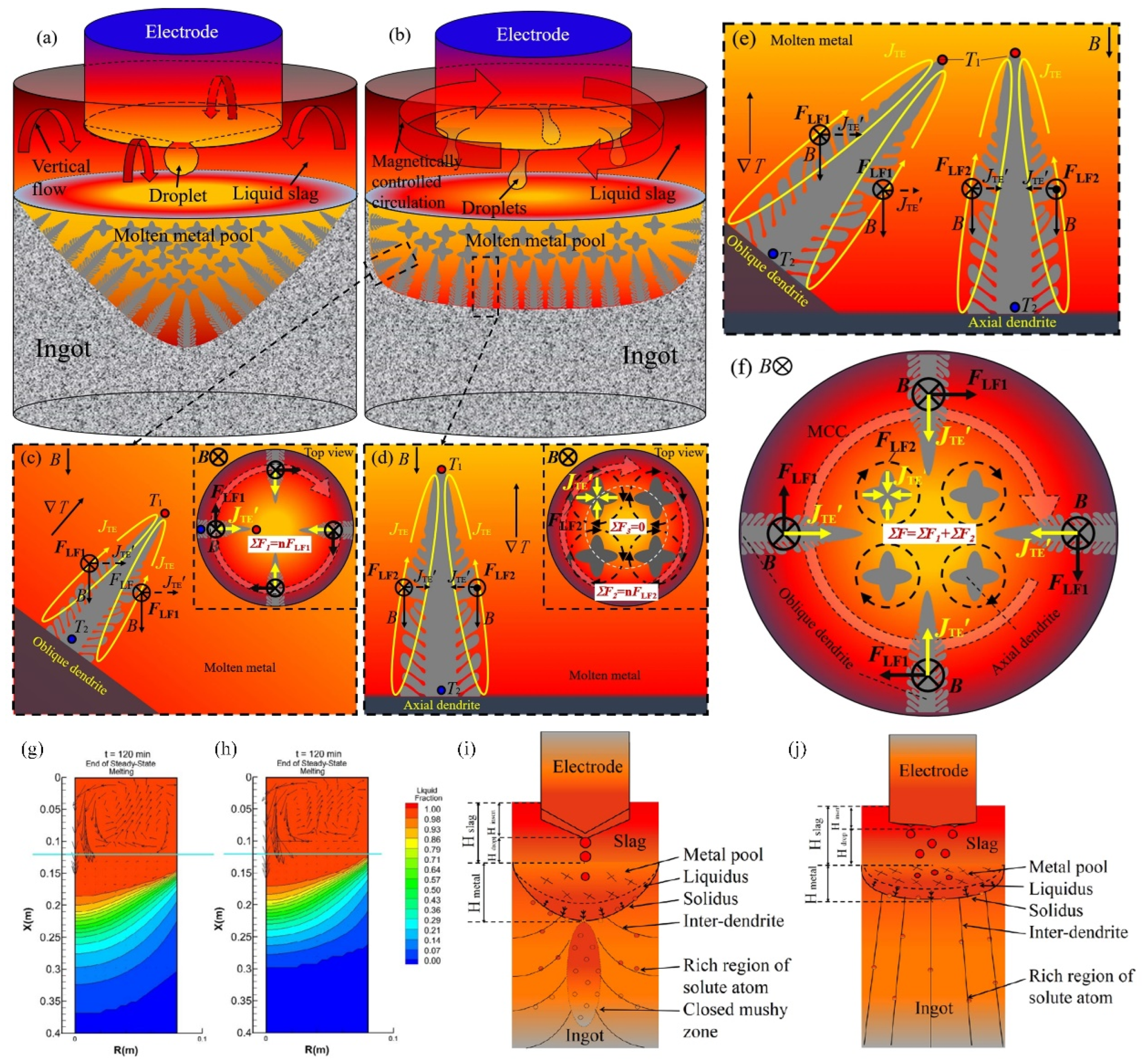
3.2. Electroslag Remelting
3.3. Increasing Forging Ratio
3.4. Heat Treatment Process Enhancement
4. Nanoparticle Strengthening Technology
4.1. Research Status of Nanoparticle Strengthening Technology
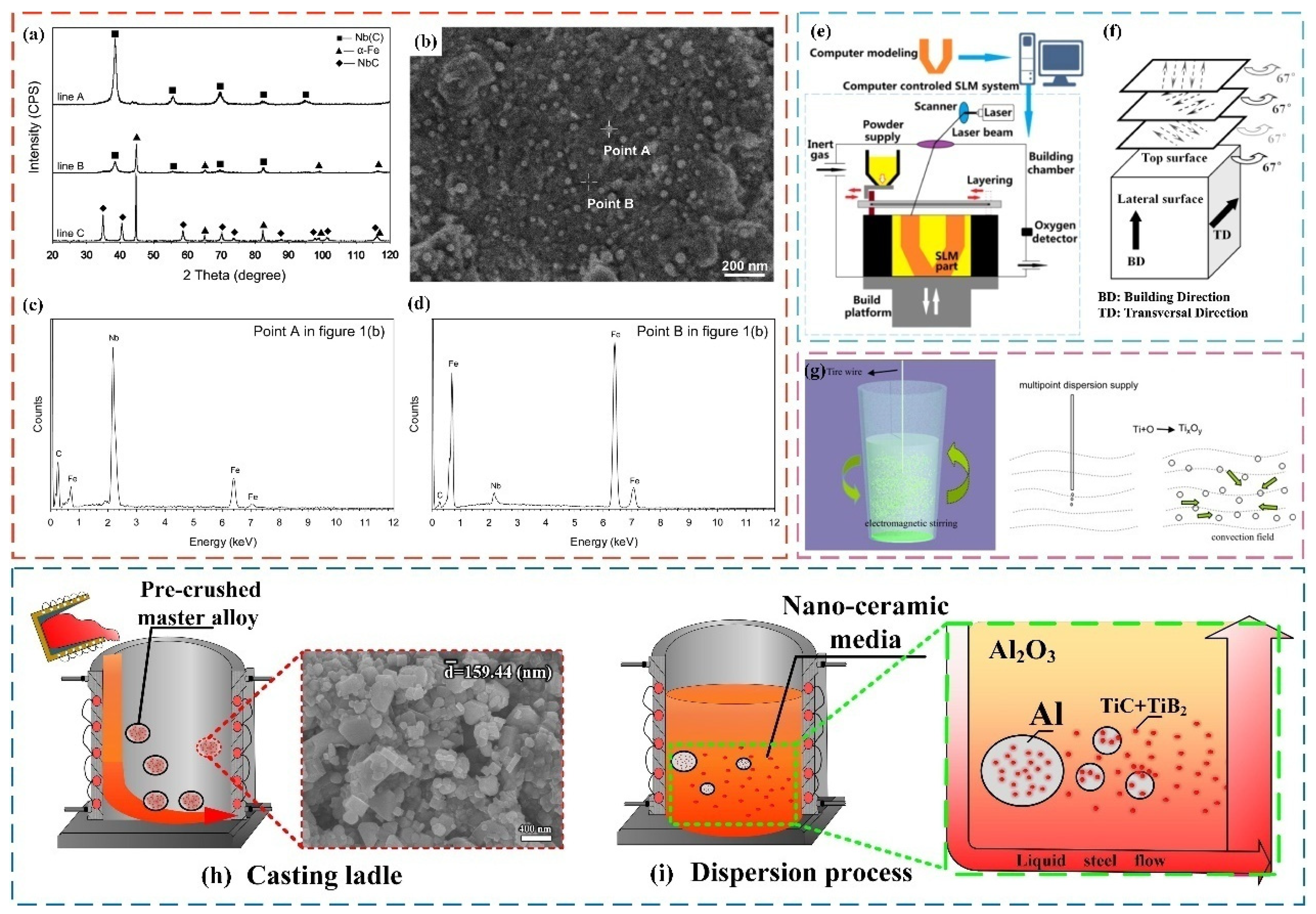
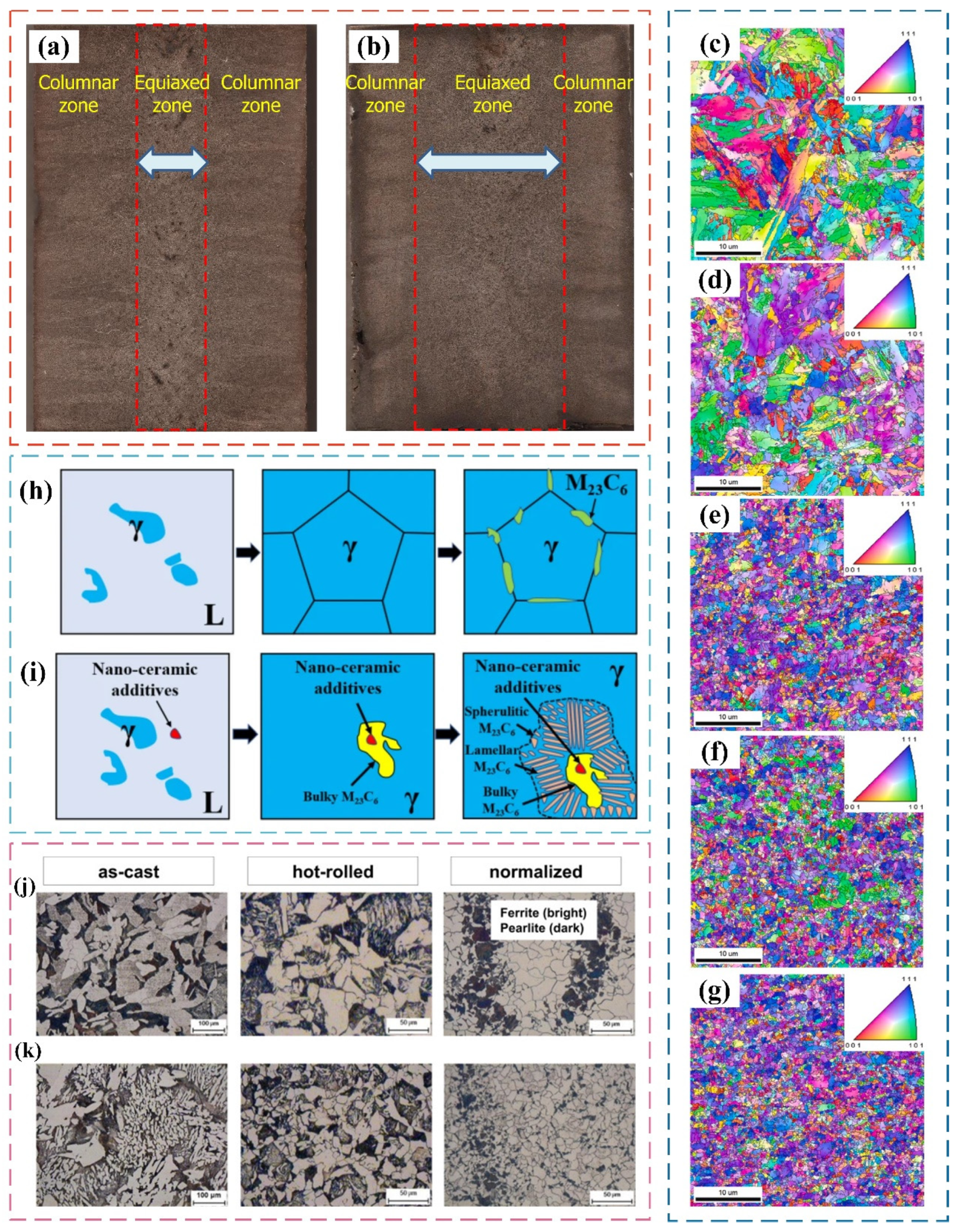
4.2. Effect of Nanoparticles on Microstructure of Steel and Its Mechanism
4.3. Nanoparticle Reinforcement of Service Performance of Steel and Its Reinforcement Mechanism
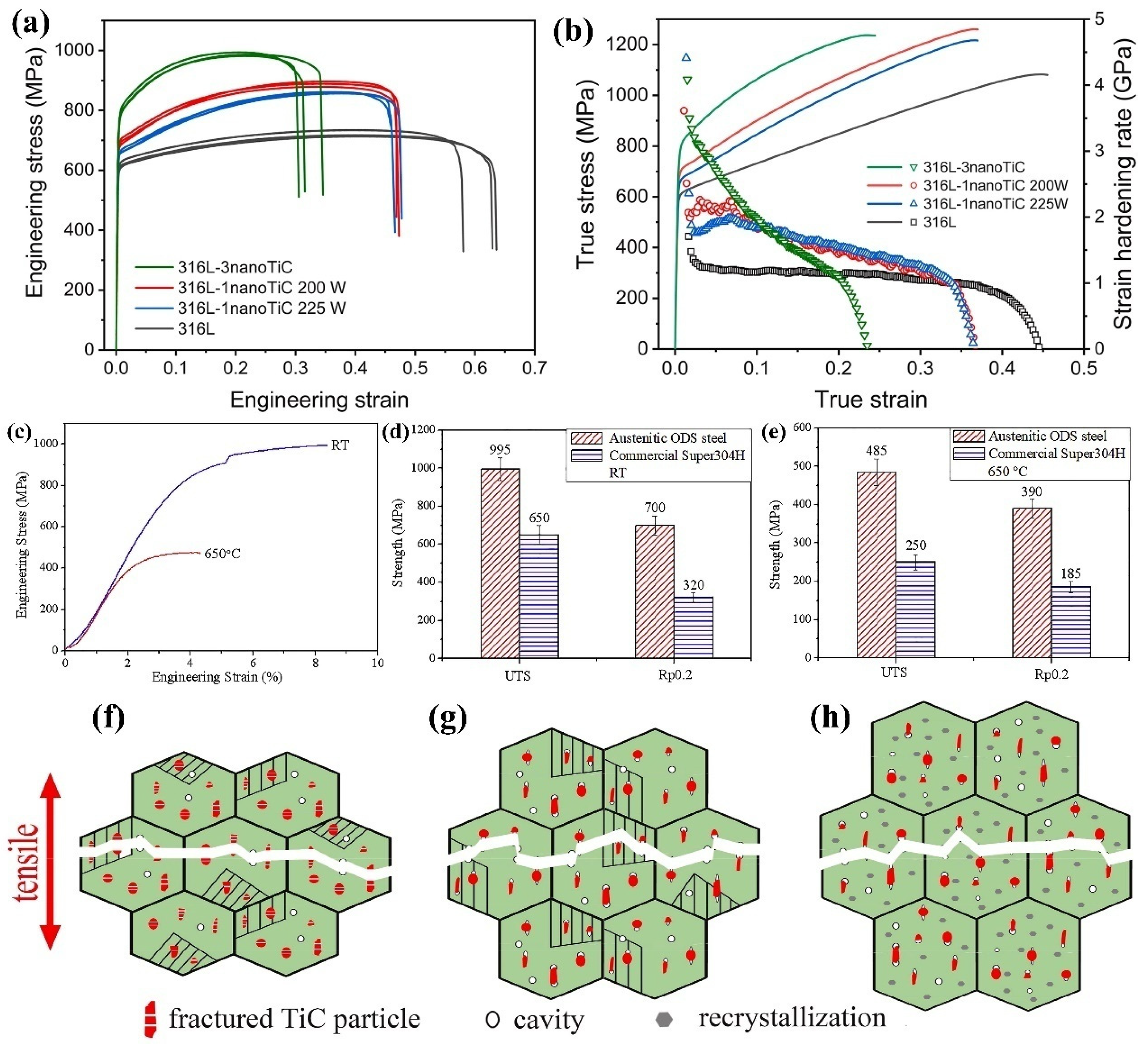
4.3.1. Tensile Properties and Reinforcing Mechanism of Nanoparticle-Reinforced Steel
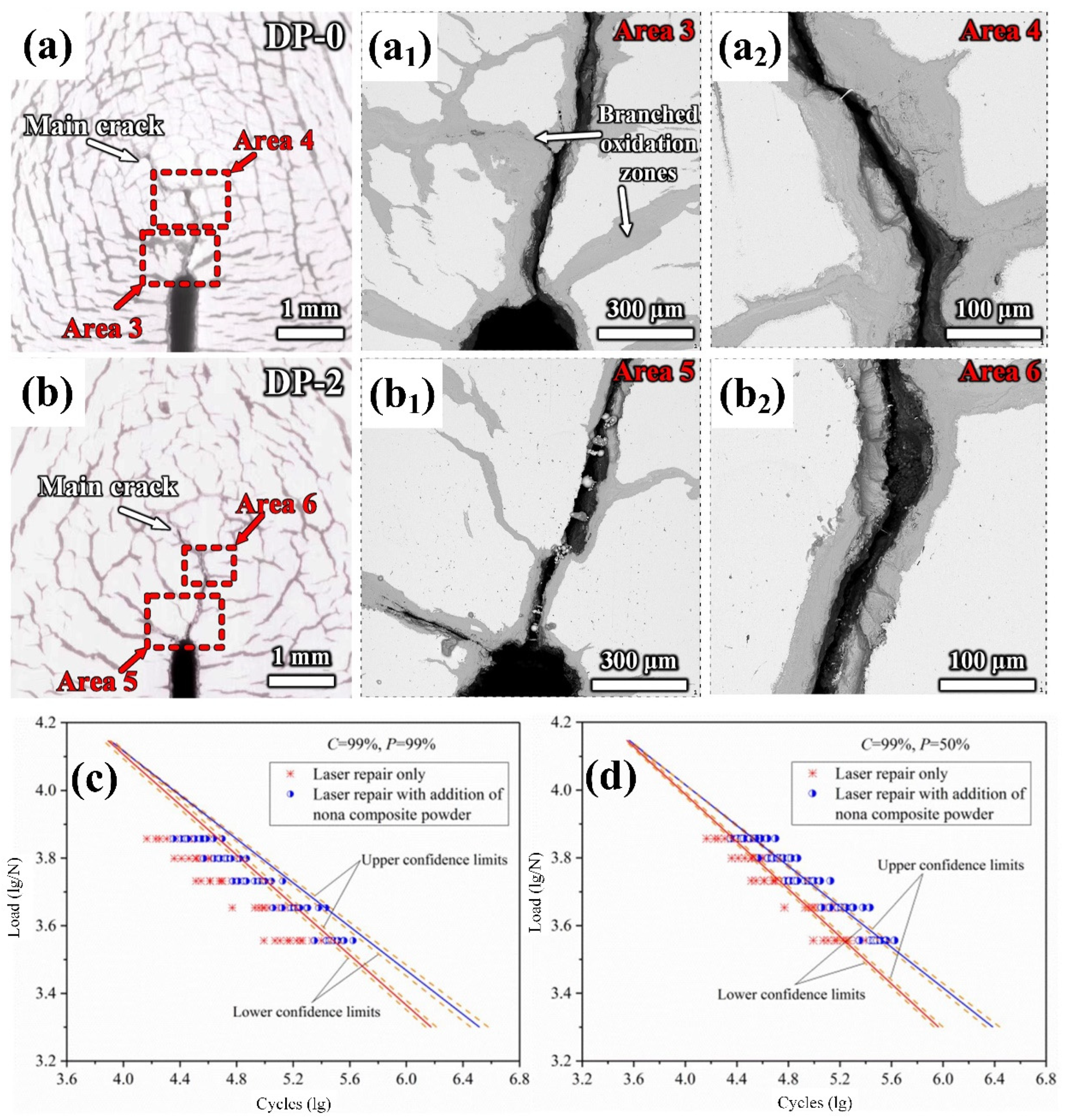
4.3.2. Fatigue Performance and Strengthening Mechanism of Nanoparticle-Reinforced Steel
4.3.3. Wear Properties and Strengthening Mechanism of Nanoparticle-Reinforced Steel
4.3.4. Antioxidant Properties and Strengthening Mechanism of Steel Reinforced by Nanoparticles
5. Summary and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Feng, H.; Li, H.; Zhang, S.; Zhu, H.; Jiao, W.; Jiang, Z. Thermal Stability and Softening Mechanism of a New Type of High Nitrogen Hot-Work Die Steel 3Cr5Mo2SiVN. Mater. Charact. 2024, 210, 113789. [Google Scholar] [CrossRef]
- Li, C.-D.; Wang, W.-X.; Qiu, F.; Zhang, H.; Shu, S.-L.; Li, T.-Y.; Jiang, Q.-C. Application of Ceramic Nanoparticles in Microstructure Manipulation and Simultaneously Strengthening Toughness and Wear Resistance of Tool and Die Steels. Ceram. Int. 2023, 49, 16661–16672. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, H.; Gao, Y.-L.; Shu, S.-L.; Qiu, F.; Jiang, Q.-C. Microstructure Evolution and Mechanical Property Enhancement of High-Cr Hot Work Die Steel Manipulated by Trace Amounts of Nano-Sized TiC. Mater. Sci. Eng. A 2021, 824, 141788. [Google Scholar] [CrossRef]
- Chang, F.; Li, C.-D.; Yang, H.-Y.; Qiu, F.; Shu, S.-L.; Chen, L.-Y.; Jiang, Q.-C. Hot-Work Die Steel with Superior Mechanical Properties at Room and High Temperatures Prepared via a Combined Approach of Composition Design and Nanoparticle Modification. J. Mater. Res. Technol. 2023, 25, 1748–1760. [Google Scholar] [CrossRef]
- Girisha, V.A.; Joshi, M.M.; Kirthan, L.J.; Bharatish, A.; Hegde, R. Thermal Fatigue Analysis of H13 Steel Die Adopted in Pressure-Die-Casting Process. Sādhanā 2019, 44, 148. [Google Scholar] [CrossRef]
- Ghalehbandi, S.M.; Biglari, F. Predicting Damage and Failure under Thermomechanical Fatigue in Hot Forging Tools. Eng. Fail. Anal. 2020, 113, 104545. [Google Scholar] [CrossRef]
- Markežič, R.; Naglič, I.; Mole, N.; Šturm, R. Experimental and Numerical Analysis of Failures on a Die Insert for High Pressure Die Casting. Eng. Fail. Anal. 2019, 95, 171–180. [Google Scholar] [CrossRef]
- Lu, Y.; Ripplinger, K.; Huang, X.; Mao, Y.; Detwiler, D.; Luo, A.A. A New Fatigue Life Model for Thermally-Induced Cracking in H13 Steel Dies for Die Casting. J. Mater. Process. Technol. 2019, 271, 444–454. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Wu, X. Effect of Initial Hardness on the Thermal Fatigue Behavior of AISI H13 Steel by Experimental Sand Numerical Investigations. Fatigue Fract. Eng. Mater. Struct. 2018, 41, 1260–1274. [Google Scholar] [CrossRef]
- Krell, J.; Röttger, A.; Geenen, K.; Theisen, W. General Investigations on Processing Tool Steel X40CrMoV5-1 with Selective Laser Melting. J. Mater. Process. Technol. 2018, 255, 679–688. [Google Scholar] [CrossRef]
- Klobcar, D.; Kosec, L.; Kosec, B.; Tusek, J. Thermo Fatigue Cracking of Die Casting Dies. Eng. Fail. Anal. 2012, 20, 43–53. [Google Scholar] [CrossRef]
- Bai, Z.; Su, N.; Yang, H.; Wu, X. Wear Characteristics of Austenitic Steel and Martensitic Steel at High Temperature. Mater. Res. Express 2022, 9, 086504. [Google Scholar] [CrossRef]
- Youn, K.T.; Rhyim, Y.M.; Lee, J.H.; Lee, C.G.; Jung, Y.C. An Evaluation of Thermal Fatigue Cracking and Chemical Reaction in Die Casting Mould. Key Eng. Mater. 2007, 345–346, 701–704. [Google Scholar] [CrossRef]
- Jiang, B.; Li, X.; Zuo, P.; Wu, X. Effect of Stress-Relief Annealing on Isothermal Fatigue Life of a New Hot Stamping Die Steel 4Cr2Mo2V. Mater. Res. Express 2022, 9, 026516. [Google Scholar] [CrossRef]
- Wang, Y.; Song, K.; Zhang, Y.; Wang, G. Microstructure Evolution and Fracture Mechanism of H13 Steel during High Temperature Tensile Deformation. Mater. Sci. Eng. A 2019, 746, 127–133. [Google Scholar] [CrossRef]
- Fang, J.R.; Jiang, Q.C.; Guan, Q.F.; Wang, S.Q. The Characteristics of Fatigue under Isothermal and Thermo-Mechanical Load in Cr–Ni–Mo Cast Hot Work Die Steel. Fatigue Fract. Eng. Mater. Struct. 2002, 25, 481–488. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Feng, Q. Isothermal Oxidation Behavior of Nb-Bearing Austenitic Cast Steels at 950 °C. Int. J. Miner. Metall. Mater. 2022, 29, 814–824. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Hosseini, S.B.; Åsberg, M.; Dahl-Jendelin, A.; Krakhmalev, P.; Oikonomou, C.; Hatami, S. Evaluation of Post-Treatments of Novel Hot-Work Tool Steel Manufactured by Laser Powder Bed Fusion for Aluminum Die Casting Applications. Mater. Sci. Eng. A 2021, 800, 140305. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, Z.; Ji, J.; Wei, B.; Heng, Y.; Liu, D. Determining the Wear Behavior of H13 Steel Die during the Extrusion Process of Pure Nickel. Eng. Fail. Anal. 2022, 134, 106053. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, L.; Jiang, W.; Cui, S.; Cui, X. Investigation on Elevated-Temperature Wear Performance and Wear Failure Mechanism of a Tungsten-System Hot-Working Die Steel. Surf. Topogr.-Metrol. Prop. 2022, 10, 035007. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, Z.; Kosasih, B.; Wu, H.; Luo, S.; Jiang, L. Influence of Cr-Rich Oxide Scale on Sliding Wear Mechanism of Ferritic Stainless Steel at High Temperature. Tribol. Lett. 2016, 63, 28. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Li, X.; Li, J.; He, X. Wear Characteristics of Mo-W-Type Hot-Work Steel at High Temperature. Tribol. Lett. 2016, 64, 32. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Li, F.; Wang, Z.; Wang, X.; Wang, C.; Zhang, C.; Huang, J.; Mao, F.; Chen, C.; et al. High-Temperature Oxidation Behavior of Cr-Ni-Mo Hot-Work Die Steels. Materials 2022, 15, 5145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qu, K.L.; Luo, S.X.; Liu, S.S. Effect of Cr on the Microstructure and Properties of TiC-TiB2 Particles Reinforced Fe-Based Composite Coatings. Surf. Coat. Technol. 2017, 316, 131–137. [Google Scholar] [CrossRef]
- Oksiuta, Z. High-Temperature Oxidation Resistance of Ultrafine-Grained 14 %Cr ODS Ferritic Steel. J. Mater. Sci. 2013, 48, 4801–4805. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Shen, H.; Chou, W.; Iwata, N.Y.; Kimura, A. The Effects of Cr and Al Concentrations on the Oxidation Behavior of Oxide Dispersion Strengthened Ferritic Alloys. Corros. Sci. 2013, 76, 310–316. [Google Scholar] [CrossRef]
- Qin, J.; Chen, X.; Wang, Y.; Zhu, Y.; Pan, S.; Zhou, W.; Chen, M.; Wang, Z. Fabrication Techniques and the Formation Mechanism of Nanoparticles and Nanoclusters in Metal Materials. Metals 2022, 12, 1420. [Google Scholar] [CrossRef]
- Salem, M.; Le Roux, S.; Dour, G.; Lamesle, P.; Choquet, K.; Rezai-Aria, F. Effect of Aluminizing and Oxidation on the Thermal Fatigue Damage of Hot Work Tool Steels for High Pressure Die Casting Applications. Int. J. Fatigue 2019, 119, 126–138. [Google Scholar] [CrossRef]
- Zeng, C.; Neils, A.; Lesko, J.; Post, N. Machine Learning Accelerated Discovery of Corrosion-Resistant High-Entropy Alloys. Comput. Mater. Sci. 2024, 237, 112925. [Google Scholar] [CrossRef]
- Gwoździk, M.; Bramowicz, M.; Kulesza, S. Surface Morphology Analysis of Oxide Layers Formed on 10CrMo9-10 Steel Used in the Power Industry. Mater. Res. Express 2020, 7, 026544. [Google Scholar] [CrossRef]
- Şengül, Ü.; Şengül, A.B. Hot Forging Die Material Selection Using Fuzzy Multi-Criteria Decision-Making Methods. Mater. Today Commun. 2024, 38, 108352. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Gu, J.; Li, W.; Xu, F.; Li, J. Effect of Nitrogen-Substituted Carbon on Thermal Stability of Cr-Mo-V Hot-Working Die Steel. Steel Res. Int. 2020, 91, 2000206. [Google Scholar] [CrossRef]
- Wei, M.X.; Wang, F.; Wang, S.Q.; Cui, X.H. Comparative Research on the Elevated-Temperature Wear Resistance of a Cast Hot-Working Die Steel. Mater. Des. 2009, 30, 3608–3614. [Google Scholar] [CrossRef]
- Deng, X.; Wang, Q.; Huang, L.; Cao, Y.; Wang, Z. Effect of Solidification and Hot Rolling Processes on Wear Performance of TiC-Reinforced Wear-Resistant Steel. Materials 2022, 15, 729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, B.; Sun, D.; Li, C.; Han, L.; Gu, J. Effect of Pre-Existing VC Carbides on Nitriding and Wear Behavior of Hot-Work Die Steel. Appl. Surf. Sci. 2019, 486, 179–186. [Google Scholar] [CrossRef]
- Huang, S.; Wu, R.; Li, W.; Min, N.; Li, X. Fluctuations of Properties of Cr-Mo-V Hot Work Die Steels by Artificial Increment of Vanadium. Mater. Today Commun. 2022, 33, 105024. [Google Scholar] [CrossRef]
- Yu, C.; Fu, L.; Xiao, H.; Lv, Q.; Gao, B. Effect of Carbon Content on the Microstructure and Bonding Properties of Hot-Rolling Pure Titanium Clad Carbon Steel Plates. Mater. Sci. Eng. A 2021, 820, 141572. [Google Scholar] [CrossRef]
- Jiang, Y.; Gu, R.C.; Peterlechner, M.; Liu, Y.W.; Wang, J.T.; Wilde, G. Impurity Effect on Recrystallization and Grain Growth in Severe Plastically Deformed Copper. Mater. Sci. Eng. A 2021, 824, 141786. [Google Scholar] [CrossRef]
- Du, N.; Liu, H.; Fu, P.; Liu, H.; Sun, C.; Cao, Y.; Li, D. Microstructural Stability and Softening Resistance of a Novel Hot-Work Die Steel. Crystals 2020, 10, 238. [Google Scholar] [CrossRef]
- Oh, J.; Park, S.; Bae, H.J.; Son, S.; Kim, H.S.; Seol, J.B.; Sung, H.; Kim, J.G. Mechanical Properties and Microstructural Evolution of High-Pressure Torsion-Processed Al7075 Alloy at Elevated Temperatures. Mater. Sci. Eng. A 2022, 835, 142692. [Google Scholar] [CrossRef]
- Zou, J.; Lu, L.; Kolisnichenko, O.V.; Chen, W.; Yu, J.-M. Gas Nitriding of a Plasma Detonation Modified Die Steel. Mater. Lett. 2021, 287, 129297. [Google Scholar] [CrossRef]
- Xiang, S.S.; Wu, R.M.; Hu, T.; Huang, S. Effect of Tempering Temperature on Microstructure and Mechanical Properties of Tungsten-Containing DIEVAR Steel. Die Ind. 2021, 47, 68–72. [Google Scholar]
- Li, Y.; Dong, Y.; Jiang, Z.; Tang, Q.; Du, S.; Hou, Z. Influence of Rare Earth Ce on Hot Deformation Behavior of As-Cast Mn18Cr18N High Nitrogen Austenitic Stainless Steel. Int. J. Miner. Metall. Mater. 2023, 30, 324–334. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; He, J.; Zhu, F.; Li, C. Effects of Rare Earth Elements on Microstructure and Mechanical Properties of H13 Die Steel. Metals 2020, 10, 918. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Xie, J. Improving Strength and Ductility of H13 Die Steel by Pre-Tempering Treatment and Its Mechanism. Mater. Sci. Eng. A 2019, 752, 101–114. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, G.T.; Zhang, Z.H.; Xie, J.X. The Martensitic Crystallography and Strengthening Mechanisms of Ultra-High Strength Rare Earth H13 Steel. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process 2020, 797, 140139. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Z.; Wang, H.; Qi, L.; Zhu, F. Effects of Yttrium on the Microstructures, Internal Fraction and Martensitic Transformation in H13 Die Steel. J. Mater. Sci. 2021, 56, 7753–7764. [Google Scholar] [CrossRef]
- Liu, F.; Gao, J.; Cao, H.; Li, H.; Jiang, Z.; Geng, X.; Kang, C.; Chen, K.; An, R. Effect of Slag Composition on Elements Oxidation Behavior of GH984G Superalloy for Electroslag Remelting Withdrawal Process. J. Iron Steel Res. Int. 2022, 29, 761–771. [Google Scholar] [CrossRef]
- Li, Q.; Xia, Z.; Guo, Y.; Shen, Z.; Zheng, T.; Ding, B.; Zhong, Y. Carbides Modification and Mechanical Properties Enhancement of Cr12MoV Die Steel by Magnetically Controlled Electroslag Remelting. Metall. Mater. Trans. B 2021, 52, 1495–1507. [Google Scholar] [CrossRef]
- Qi, Y.; Li, J.; Shi, C.; Geng, R.; Zhang, J. Effect of Directional Solidification in Electroslag Remelting on the Microstructure and Cleanliness of an Austenitic Hot-Work Die Steel. ISIJ Int. 2018, 58, 1275–1284. [Google Scholar] [CrossRef]
- Qi, Y.; Li, J.; Shi, C.; Zhang, Y.; Zhu, Q.; Wang, H. Effect of Directional Solidification of Electroslag Remelting on the Microstructure and Primary Carbides in an Austenitic Hot-Work Die Steel. J. Mater. Process. Technol. 2017, 249, 32–38. [Google Scholar] [CrossRef]
- Lu, H.; Li, D.; Li, S.; Chen, Y. Hot Deformation Behavior of Fe-27.34Mn-8.63Al-1.03C Lightweight Steel. Int. J. Miner. Metall. Mater. 2023, 30, 734–743. [Google Scholar] [CrossRef]
- Miyake, M.; Yazaki, T.; Sodani, Y. Development of Load Reduction Technology by Swing-Type Forging and Lubrication for Large-Deformation Forging by High-Speed Large-Reduction Forging—Production Technology for Fine Grained Steel by Large Deformation Forging II. Mater. Trans. 2013, 54, 1951–1956. [Google Scholar] [CrossRef]
- Dyja, H.; Banaszek, G.; Berski, S.; Mróz, S. Effect of Symmetrical and Asymmetrical Forging Processes on the Quality of Forged Products. J. Mater. Process. Technol. 2004, 157–158, 496–501. [Google Scholar] [CrossRef]
- Dindorf, R.; Takosoglu, J.; Wos, P. Prediction of the Parameters and the Hot Open Die Elongation Forging Process on an 80 MN Hydraulic Press. Open Eng. 2021, 11, 528–534. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kwon, S.-C.; Kim, S.-T.; Lee, S.; Jeong, H.-T.; Choi, S.-H. Effect of Forging Type on the Deformation Heterogeneities in Multi-Axial Diagonal Forged AA1100. Met. Mater. Int. 2019, 25, 779–793. [Google Scholar] [CrossRef]
- Chen, F.; Ren, F.; Chen, J.; Cui, Z.; Ou, H. Microstructural Modeling and Numerical Simulation of Multi-Physical Fields for Martensitic Stainless Steel during Hot Forging Process of Turbine Blade. Int. J. Adv. Manuf. Technol. 2016, 82, 85–98. [Google Scholar] [CrossRef]
- Jang, G.; Kim, J.N.; Lee, H.; Lee, T.; Enikeev, N.; Abramova, M.; Valiev, R.Z.; Kim, H.S.; Lee, C.S. Microstructural Evolution and Mechanical Properties of Nanocrystalline Fe–Mn–Al–C Steel Processed by High-Pressure Torsion. Mater. Sci. Eng. A 2021, 827, 142073. [Google Scholar] [CrossRef]
- Alimi, A.; Fajoui, J.; Kchaou, M.; Branchu, S.; Elleuch, R.; Jacquemin, F. Multi-Scale Hot Working Tool Damage (X40CrMoV5-1) Analysis in Relation to the Forging Process. Eng. Fail. Anal. 2016, 62, 142–155. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Z.; Jiang, L.; Wang, X.; Xu, P.; Ma, Y.; Yan, Y.; Yang, C.; Yong, Q. An Investigation into the Role of Non-Metallic Inclusions in Cleavage Fracture of Medium Carbon Pearlitic Steels for High-Speed Railway Wheel. Eng. Fail. Anal. 2022, 131, 105860. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Wang, L.; Li, L. Study on Carbide in Forged and Annealed H13 Hot Work Die Steel. High Temp. Mater. Process. 2015, 34, 593–598. [Google Scholar] [CrossRef]
- Al-Ezzi, A.; Shehab, A.A. Effect of Heat Treatment on the Microstructure and Property of Aerospace Punch Dies. Metallogr. Microstruct. Anal. 2019, 8, 314–328. [Google Scholar] [CrossRef]
- Gu, J.; Li, J.; Yanagimoto, J.; Li, W.; Li, L. Microstructural Evolution and Mechanical Property Changes of a New Nitrogen-Alloyed Cr–Mo–V Hot-Working Die Steel during Tempering. Mater. Sci. Eng. A 2021, 804, 140721. [Google Scholar] [CrossRef]
- Li, J.; Zhao, P.; Yanagimoto, J.; Sugiyama, S.; Chen, Y. Effects of Heat Treatment on the Microstructures and Mechanical Properties of a New Type of Nitrogen-Containing Die Steel. Int. J. Miner. Metall. Mater. 2012, 19, 511–517. [Google Scholar] [CrossRef]
- Yu, X.-S.; Wu, C.; Shi, R.-X.; Yuan, Y.-S. Microstructural Evolution and Mechanical Properties of 55NiCrMoV7 Hot-Work Die Steel during Quenching and Tempering Treatments. Adv. Manuf. 2021, 9, 520–537. [Google Scholar] [CrossRef]
- Yang, D.; Xiong, Z.; Zhang, C.; Feng, G.; Cheng, Z.; Cheng, X. Evolution of Microstructures and Mechanical Properties with Tempering Temperature of a Pearlitic Quenched and Tempered Steel. J. Iron Steel Res. Int. 2022, 29, 1393–1403. [Google Scholar] [CrossRef]
- Gu, J.; Li, J.; Chang, R. Enhanced Refinement of Cr23C6 by Heterogeneous Nucleation in Annealed Nitrogen-Alloyed 4Cr5Mo2V Die Steel. Metall. Mater. Trans. A-Phys. Metall. Mater. Sci. 2019, 50A, 518–522. [Google Scholar] [CrossRef]
- Ning, A.; Yue, S.; Gao, R.; Li, L.; Guo, H. Influence of Tempering Time on the Behavior of Large Carbides’ Coarsening in AISI H13 Steel. Metals 2019, 9, 1283. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, X. Effect of Tempering Temperature on Stress-Assisted Hydrogen Diffusion and Hydrogen-Induced Embrittlement in a High Strength Low Alloy Steel. Mater. Sci. Eng. A 2023, 873, 144948. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.; Wang, F.; Lu, Y.; Chen, Z. Experimental Study on Impact Toughness of Structural Steel and Its Butt-Welded Joint at Low Temperature and Corrosion. J. Constr. Steel Res. 2024, 212, 108298. [Google Scholar] [CrossRef]
- Shinde, T. Influence of Carbide Particle Size on the Wear Performance of Cryogenically Treated H13 Die Steel. Surf. Eng. 2021, 37, 1206–1214. [Google Scholar] [CrossRef]
- Pereira, V.S.M.; Davis, T.P.; Mayoral, M.H.; Kumar, A.; Schut, H.; Sietsma, J. Investigation of Coarsening of Oxide Nanoparticles at 1400 K and Its Effect on the Microstructure Formation of an ODS Eurofer Steel. Mater. Charact. 2022, 185, 111723. [Google Scholar] [CrossRef]
- Yan, G.; Huang, X.; Wang, Y.; Qin, X.; Yang, M.; Chu, Z.; Jin, K. Effects of Heat Treatment on Mechanical Properties of H13 Steel. Met. Sci. Heat Treat. 2010, 52, 393–395. [Google Scholar]
- Yang, Y.-F.; Wang, H.-Y.; Zhao, R.-Y.; Liang, Y.-H.; Jiang, Q.-C. In Situ TiC/TiB2 Particulate Locally Reinforced Steel Matrix Composites Fabricated Via the SHS Reaction of Ni–Ti–B4C System: International Journal of Applied Ceramic Technology. Int. J. Appl. Ceram. Technol. 2009, 6, 437–446. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Y.; Yang, C.; Xue, F.; Song, F. Microstructure and Mechanical Properties of Common Straight Carbon Steels Strengthened by TiC Dispersion. Mater. Trans. 2006, 47, 2393–2398. [Google Scholar] [CrossRef][Green Version]
- Wei, B.; Liu, Z.; Cao, B.; Nong, B.; Zhang, Y.; Ren, Y.; Zhou, H.; Wei, S. Cracking Inhibition of Nano-TiC Reinforced René 104 Superalloy Fabricated by Selective Laser Melting. J. Alloys Compd. 2021, 881, 160413. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Z.; Bai, P.; Du, W.; Liao, H.; Li, Y.; Liang, M.; Huo, P.; Zhang, L.; Tie, D. Effects of Alloying Elements X (Cr, Mn, Mo, Ni, Si) on the Interface Stability of TiC (001)/γ-Fe (001) in TiC/316L Stainless Steel Composite Formed by Selective Laser Melting: First Principles and Experiments. Adv. Compos. Hybrid Mater. 2021, 4, 195–204. [Google Scholar] [CrossRef]
- Abe, F.; Murata, M.; Miyazaki, H. Effect of TiC and NbC Carbides on Creep Life of Stainless Steels. Mater. High Temp. 2019, 36, 35–47. [Google Scholar] [CrossRef]
- Chen, T.; Ji, C.; Zhu, M. Effect of Cooling Rate on the Nucleation and Growth of Large TiC Particles in Ti-Mo Steel. J. Alloys Compd. 2020, 823, 153650. [Google Scholar] [CrossRef]
- Li, G.B.; Wu, J.J.; Jiang, Y.F.; Li, G.Y. The Nucleation and Propagation of a Thermal Fatigue Crack in 4Cr2NiMoV Steel. J. Mater. Process. Technol. 2000, 100, 63–66. [Google Scholar]
- Zhou, Y.; Li, L.; Hu, T.; Wang, Q.; Shao, W.; Rao, L.; Xing, X.; Yang, Q. Role of TiC Nanocrystalline and Interface of TiC and Amorphous Carbon on Corrosion Mechanism of Titanium Doped Diamond-like Carbon Films: Exploration by Experimental and First Principle Calculation. Appl. Surf. Sci. 2021, 542, 148740. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Liang, Y.; Zhao, R.; Jiang, Q. Fabrication of Steel Matrix Composites Locally Reinforced with Different Ratios of TiC/TiB2 Particulates Using SHS Reactions of Ni–Ti–B4C and Ni–Ti–B4C–C Systems during Casting. Mater. Sci. Eng. A 2007, 445–446, 398–404. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, Q.; Zhang, Z.; Li, X.; Ren, L. Effect of B4C Particle Size on the Reaction Behavior of Self-Propagation High-Temperature Synthesis of TiC-TiB2 Ceramic/Cu Composites from a Cu-Ti-B4C System. Int. J. Refract. Met. Hard Mater. 2014, 46, 71–79. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, J.; Liu, X.; Li, X.; Li, L.; Shan, B.; Chen, R. A Combined Multiscale Modeling and Experimental Study on Surface Modification of High-Volume Micro-Nanoparticles with Atomic Accuracy. Int. J. Extrem. Manuf. 2022, 4, 025101. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, Y.; Chen, F.; Shen, L.; Tang, X.; Sun, L.; Fan, M.; Huang, P. Effects of Ion Irradiation on Microstructure of 316L Stainless Steel Strengthened by Disperse Nano TiC through Selective Laser Melting. Mater. Charact. 2021, 180, 111420. [Google Scholar] [CrossRef]
- Yuan, J.; Yao, Y.; Zhuang, M.; Du, Y.; Wang, L.; Yu, Z. Effects of Cu and WS2 Addition on Microstructural Evolution and Tribological Properties of Self-Lubricating Anti-Wear Coatings Prepared by Laser Cladding. Tribol. Int. 2021, 157, 106872. [Google Scholar] [CrossRef]
- Qin, S.; Liao, B.; Mao, L.; Xiao, F. A Novel Method for Preparing Nano-NbC/Fe Powder and Nano-NbC Particle Reinforced Cast Low-Carbon Steel. Mater. Lett. 2014, 121, 162–165. [Google Scholar] [CrossRef]
- Wei, C.; Gu, H.; Gu, Y.; Liu, L.; Huang, Y.; Cheng, D.; Li, Z.; Li, L. Abnormal Interfacial Bonding Mechanisms of Multi-Material Additive-Manufactured Tungsten–Stainless Steel Sandwich Structure. Int. J. Extrem. Manuf. 2022, 4, 025002. [Google Scholar] [CrossRef]
- Li, B.; Qian, B.; Xu, Y.; Liu, Z.; Zhang, J.; Xuan, F. Additive Manufacturing of Ultrafine-Grained Austenitic Stainless Steel Matrix Composite via Vanadium Carbide Reinforcement Addition and Selective Laser Melting: Formation Mechanism and Strengthening Effect. Mater. Sci. Eng. A 2019, 745, 495–508. [Google Scholar] [CrossRef]
- Bhowmik, A.; Zhai, W.; Zhou, W.; Nai, S.M.L. Characterization of Carbide Particle-Reinforced 316L Stainless Steel Fabricated by Selective Laser Melting. Mater. Charact. 2021, 179, 111360. [Google Scholar] [CrossRef]
- Saeedi, R.; Shoja Razavi, R.; Bakhshi, S.R.; Erfanmanesh, M.; Ahmadi Bani, A. Optimization and Characterization of Laser Cladding of NiCr and NiCr–TiC Composite Coatings on AISI 420 Stainless Steel. Ceram. Int. 2021, 47, 4097–4110. [Google Scholar] [CrossRef]
- Tang, H.; Chen, X.; Chen, M.; Zuo, L.; Hou, B.; Wang, Z. Microstructure and Mechanical Property of In-Situ Nano-Particle Strengthened Ferritic Steel by Novel Internal Oxidation. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2014, 609, 293–299. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Wang, F.; An, X.; Chang, Y. Microstructure and Tensile Properties of Nano-Sized ZrC Particle Strengthened RAFM Steels. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2022, 859, 144241. [Google Scholar] [CrossRef]
- Li, C.-D.; Li, Y.-L.; Zou, Y.-Z.; Lin, Y.-H.; Yang, H.-Y.; Meng, J.; Chen, L.-Y.; Qiu, F.; Jiang, Q.-C. Thermal Fatigue Performance Enhancement of New High-Cr Martensitic Die Steels Based on Overall Microstructure Manipulation by Trace TiC–TiB2 Nanoparticles. Mater. Sci. Eng. A 2024, 901, 146468. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Qiu, F.; Cui, W.; Hu, Z.; Barber, G.C.; Hu, M. Microstructure Refinement and Strengthening-Toughening Mechanisms of Gray Cast Irons Reinforced by In Situ Nanosized TiB2-TiC/Al Master Alloy. Adv. Eng. Mater. 2022, 24, 2100731. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Qiu, F.; Cai, G.; Cui, W.; Hu, Z.; Zhang, H.; Tyrer, N.; Barber, G.C. Role of Trace Nanoparticles in Manipulating the Widmanstatten Structure of Low Carbon Steel. Mater. Lett. 2022, 306, 130853. [Google Scholar] [CrossRef]
- Song, C.; Wang, H.; Sun, Z.; Yu, H. Analysis of Precipitation Characteristics of TiC at Different Quenching and Partitioning Temperatures and Its Effect on the Mechanical Properties. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2021, 824, 141868. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Zhou, H.; Zhou, T. Effect of Nano TiC on Microstructure and Microhardness of Composite Additive Manufacturing 316L Stainless Steel. Mater. Res. Express 2021, 8, 126521. [Google Scholar] [CrossRef]
- Dogan, Ö.; Hawk, J.A.; Schrems, K.K. TiC-Reinforced Cast Cr Steels. J. Mater. Eng. Perform. 2006, 15, 320–327. [Google Scholar] [CrossRef]
- Park, J.-J.; Hong, S.-M.; Park, E.-K.; Kim, K.-Y.; Lee, M.-K.; Rhee, C.-K. Microstructure and Properties of SA 106B Carbon Steel after Treatment of the Melt with Nano-Sized TiC Particles. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process 2014, 613, 217–223. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, M.; Mi, Z.; Zhang, Q.; Yang, X.; Yang, Y.; Wu, Y. Effects of Nano-Ceramic Additives on High-Temperature Mechanical Properties and Corrosion Behavior of 310S Austenitic Stainless Steel. J. Iron Steel Res. Int. 2023, 30, 591–600. [Google Scholar] [CrossRef]
- Hong, S.-M.; Park, E.-K.; Park, J.-J.; Lee, M.-K.; Lee, J.G. Effect of Nano-Sized TiC Particle Addition on Microstructure and Mechanical Properties of SA-106B Carbon Steel. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2015, 643, 37–46. [Google Scholar] [CrossRef]
- Huo, X.; Lv, Z.; Ao, C.; Li, L.; Xia, J.; Chen, S. Effect of Strain-Induced Precipitation on Microstructure and Properties of Titanium Micro-Alloyed Steels. J. Iron Steel Res. Int. 2022, 29, 983–993. [Google Scholar] [CrossRef]
- Zhai, W.; Zhou, W.; Nai, S.M.L. Grain Refinement and Strengthening of 316L Stainless Steel through Addition of TiC Nanoparticles and Selective Laser Melting. Mater. Sci. Eng. A 2022, 832, 142460. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, L.; Liu, Y.; Ma, Z.; Li, H.; Liu, C.; Wu, J. Microstructures and Tensile Properties of an Austenitic ODS Heat Resistance Steel. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process 2019, 767, 138419. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Deng, X.; Wang, Z. Revealing the Role of Micron-Sized in Situ TiC Particles on Tensile Properties and Fracture Mechanism of Martensitic Wear-Resistant Steel at Elevated Temperature. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process 2022, 832, 142503. [Google Scholar]
- Wang, J.; Jiang, W. Fatigue Failure Analysis on Precracked 304 Stainless Steel Components Repaired by Laser with Addition of Nanocomposites. Soldag. Insp. 2019, 24, e2408. [Google Scholar]
- Zhang, H.; Cao, S.; Li, C.; Li, B.; Qian, G. Laser Powder Bed Fused 304L Steel Shot-Peened with Various Ceramic Shot Sizes: Surface/Subsurface Characteristics, Tensile Behavior, and Fatigue Behavior. Int. J. Plast. 2023, 171, 103784. [Google Scholar] [CrossRef]
- Zheng, J.; Li, K.; Zhang, Y.; Zhan, K.; Yang, Z.; Zhao, B.; Wang, Z.; Ji, V. Surface Characteristic and Corrosion Resistance of Different Plasma-Sprayed Coatings (Zn, Al6061and Zn/23Al) on S960 High Strength Steel with Subsequent Micro-Shot Peening. Surf. Coat. Technol. 2022, 451, 129046. [Google Scholar] [CrossRef]
- Huang, L.; Deng, X.; Li, C.; Jia, Y.; Wang, Q.; Wang, Z. Effect of TiC Particles on Three-Body Abrasive Wear Behaviour of Low Alloy Abrasion-Resistant Steel. Wear 2019, 434–435, 202971. [Google Scholar] [CrossRef]
- Deng, X.; Huang, L.; Wang, Q.; Fu, T.; Wang, Z. Three-Body Abrasion Wear Resistance of TiC-Reinforced Low-Alloy Abrasion-Resistant Martensitic Steel under Dry and Wet Sand Conditions. Wear 2020, 452, 203310. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.; Sun, Z.; Zhao, Y.; Ma, Z.; Chen, L.; Yu, T.; Zhao, J. Grinding Performance Oriented Experimental Evaluation on TiC-Steel Cermet with Vitrified Bond cBN Wheel. Ceram. Int. 2021, 47, 34949–34958. [Google Scholar] [CrossRef]
- Zhang, T.; Shan, Q.; Li, Z.; Wu, H.; Jiang, Y. Effects of TiC and Residual Austenite Synergistic Strengthening Mechanism on Impact-Abrasive Wear Behavior of Bainite Steel. Wear 2021, 486, 204088. [Google Scholar] [CrossRef]
- Shan, Q.; Ge, R.; Li, Z.; Zhou, Z.; Jiang, Y.; Lee, Y.-S.; Wu, H. Wear Properties of High-Manganese Steel Strengthened with Nano-Sized V2C Precipitates. Wear 2021, 482, 203922. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Liu, J.; Kondo, S.; Okubo, N.; Kasada, R. Characterization and Corrosion Behavior of Al-Added High Mn ODS Austenitic Steels in Oxygen-Saturated Lead–Bismuth Eutectic. Corros. Sci. 2022, 209, 110818. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.M.; Byun, T.S.; Lee, D.W.; Park, C.H. High-Temperature Oxidation Behavior of Nano-Structured Ferritic Oxide Dispersion-Strengthened Alloys. Thermochim. Acta 2014, 579, 1–8. [Google Scholar]
- Huang, L.; Deng, X.; Wang, Q.; Wang, Z. Microstructure, Mechanical Properties and Wear Resistance of Low Alloy Abrasion Resistant Martensitic Steel Reinforced with TiC Particles. ISIJ Int. 2020, 60, 2586–2595. [Google Scholar] [CrossRef]
- Kaito, T.; Narita, T.; Ukai, S.; Matsuda, Y. High Temperature Oxidation Behavior of ODS Steels. J. Nucl. Mater. 2004, 329–333, 1388–1392. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, J.; Sun, Y. Oxidation Behavior of TiC Particle-Reinforced 304 Stainless Steel. Corros. Sci. 2010, 52, 1003–1010. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.-Y.; Bao, Z.-J.; Gong, Q.; Bao, S.-Z.; Zou, Y.-Z.; Li, A.-M.; Yang, H.-Y.; Wang, C.-G.; Li, Z.-G.; Chang, F.; et al. The Main Failure Modes of Hot-Work Die Steel and the Development Status of Traditional Strengthening Methods and Nano-Strengthening Technology. Materials 2024, 17, 3455. https://doi.org/10.3390/ma17143455
Cui H-Y, Bao Z-J, Gong Q, Bao S-Z, Zou Y-Z, Li A-M, Yang H-Y, Wang C-G, Li Z-G, Chang F, et al. The Main Failure Modes of Hot-Work Die Steel and the Development Status of Traditional Strengthening Methods and Nano-Strengthening Technology. Materials. 2024; 17(14):3455. https://doi.org/10.3390/ma17143455
Chicago/Turabian StyleCui, Hong-Yu, Ze-Ju Bao, Qin Gong, Shi-Zhe Bao, Yun-Zhi Zou, Ai-Min Li, Hong-Yu Yang, Cheng-Gang Wang, Zhi-Gang Li, Fang Chang, and et al. 2024. "The Main Failure Modes of Hot-Work Die Steel and the Development Status of Traditional Strengthening Methods and Nano-Strengthening Technology" Materials 17, no. 14: 3455. https://doi.org/10.3390/ma17143455
APA StyleCui, H.-Y., Bao, Z.-J., Gong, Q., Bao, S.-Z., Zou, Y.-Z., Li, A.-M., Yang, H.-Y., Wang, C.-G., Li, Z.-G., Chang, F., Shu, S.-L., Kang, J., Zhu, M., Qiu, F., & Jiang, Q.-C. (2024). The Main Failure Modes of Hot-Work Die Steel and the Development Status of Traditional Strengthening Methods and Nano-Strengthening Technology. Materials, 17(14), 3455. https://doi.org/10.3390/ma17143455









