Fabrication and Characterization of Biomedical Ti-Mg Composites via Spark Plasma Sintering
(This article belongs to the Section Metals and Alloys)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Evaluation of Powders
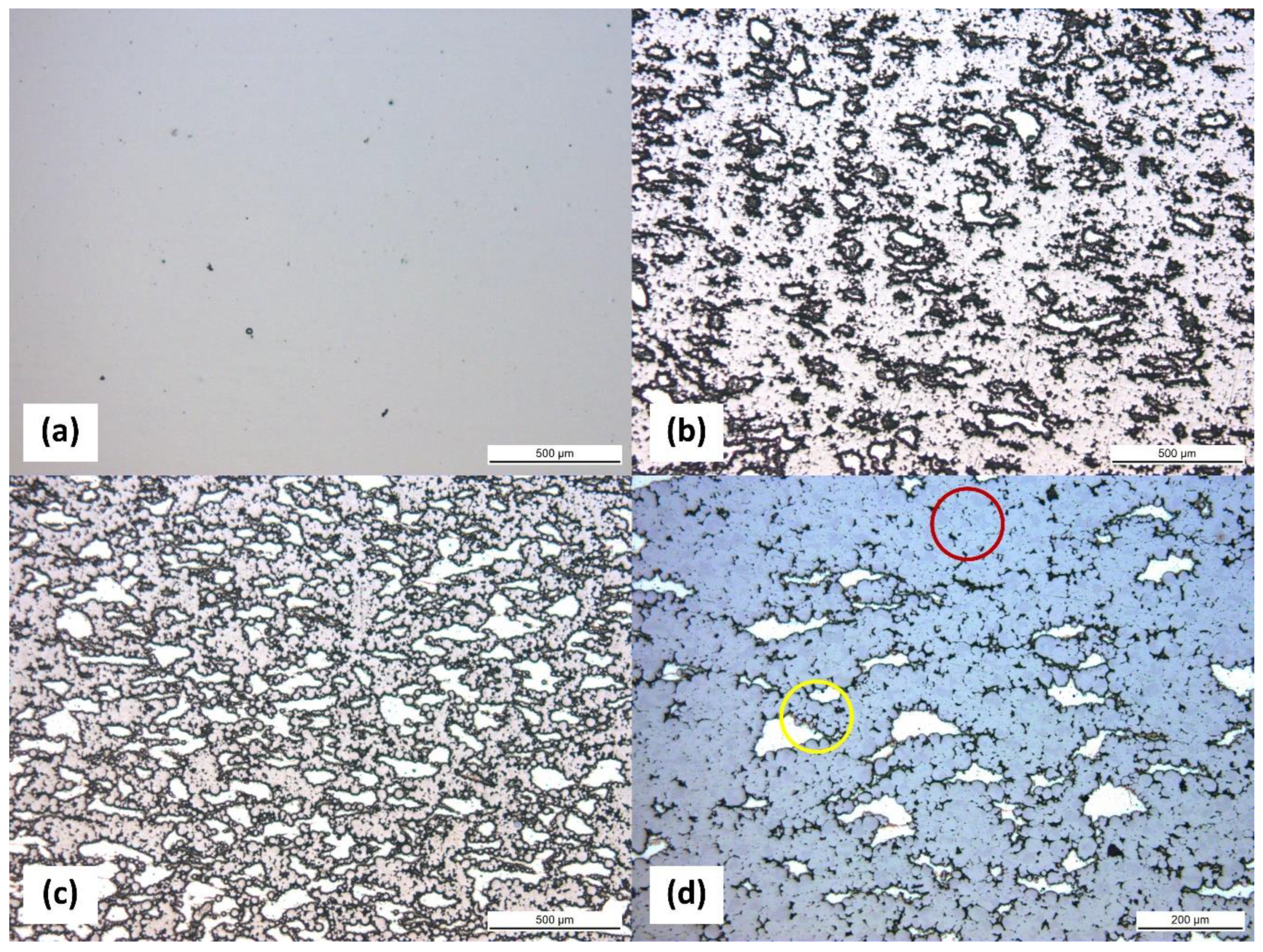
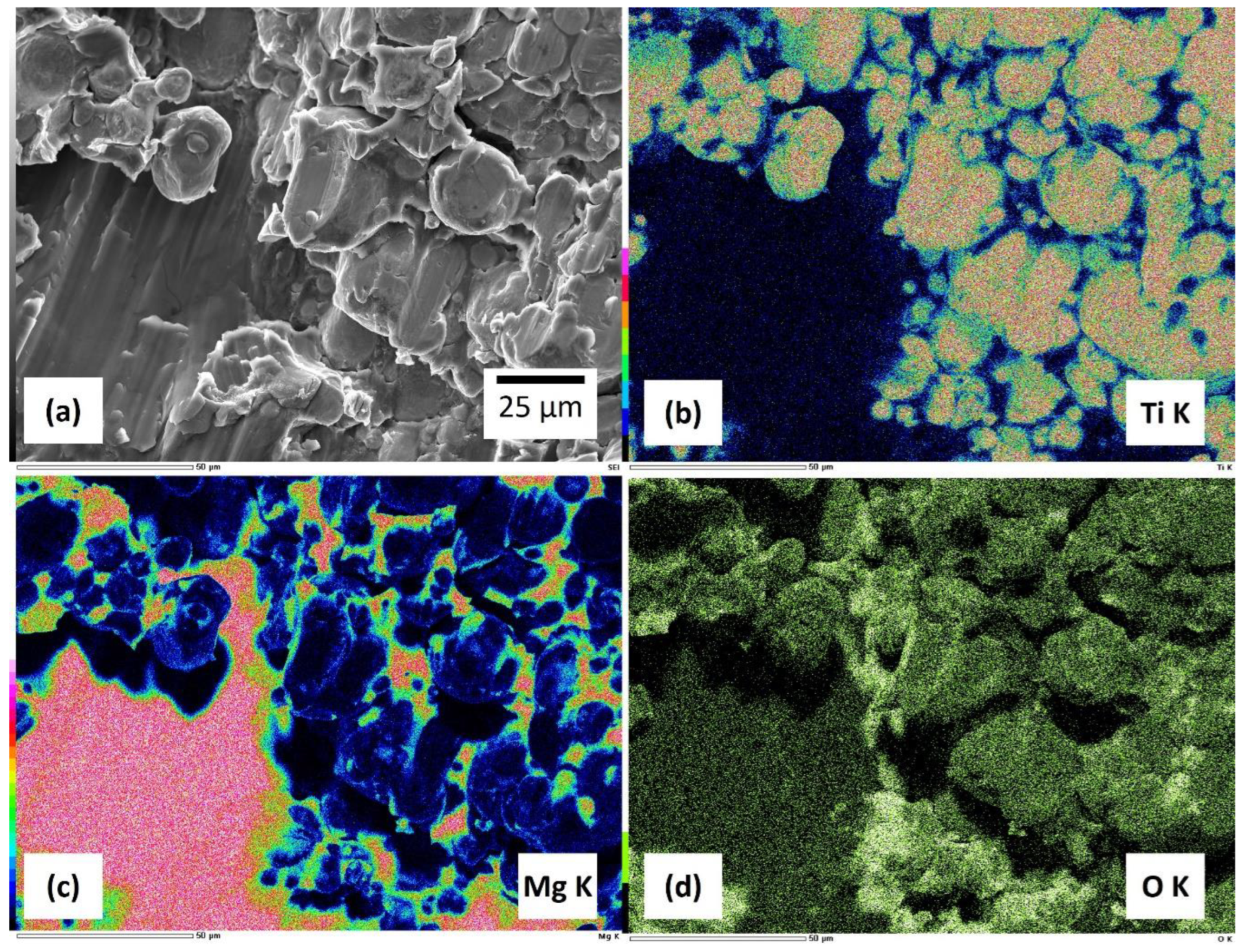
3.2. Microstructure of Sintered Composites
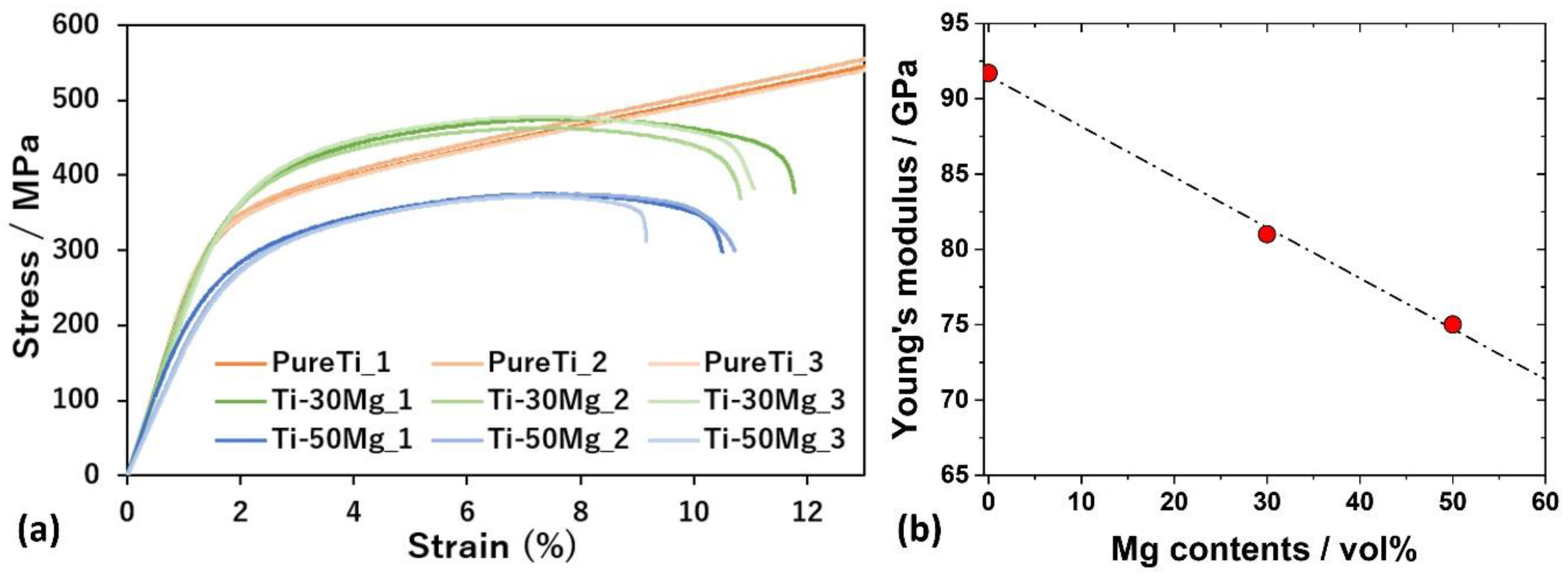
3.3. Mechanical Properties Depending on Mg Contents
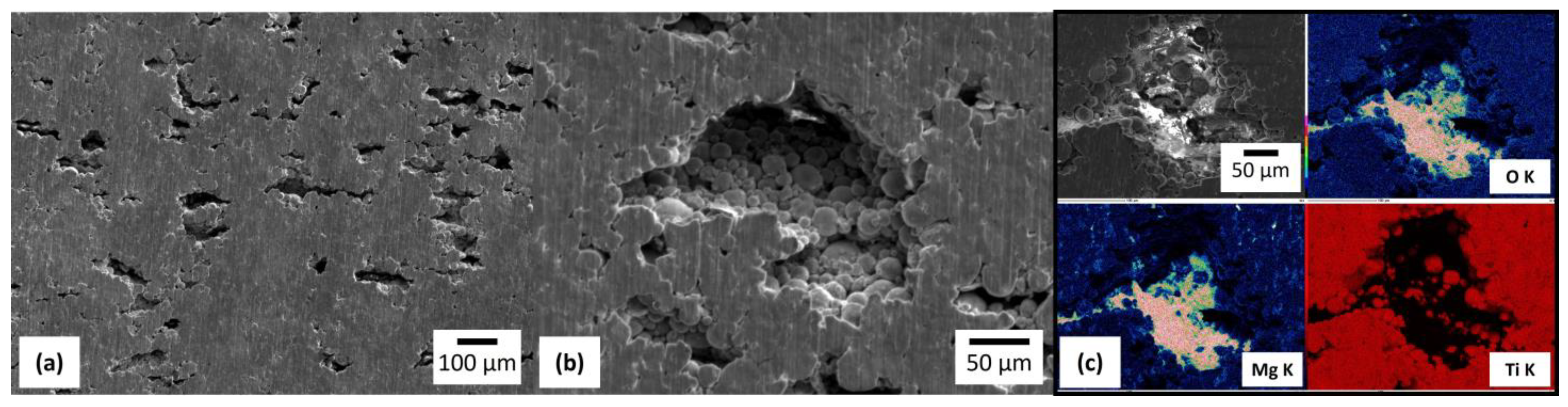
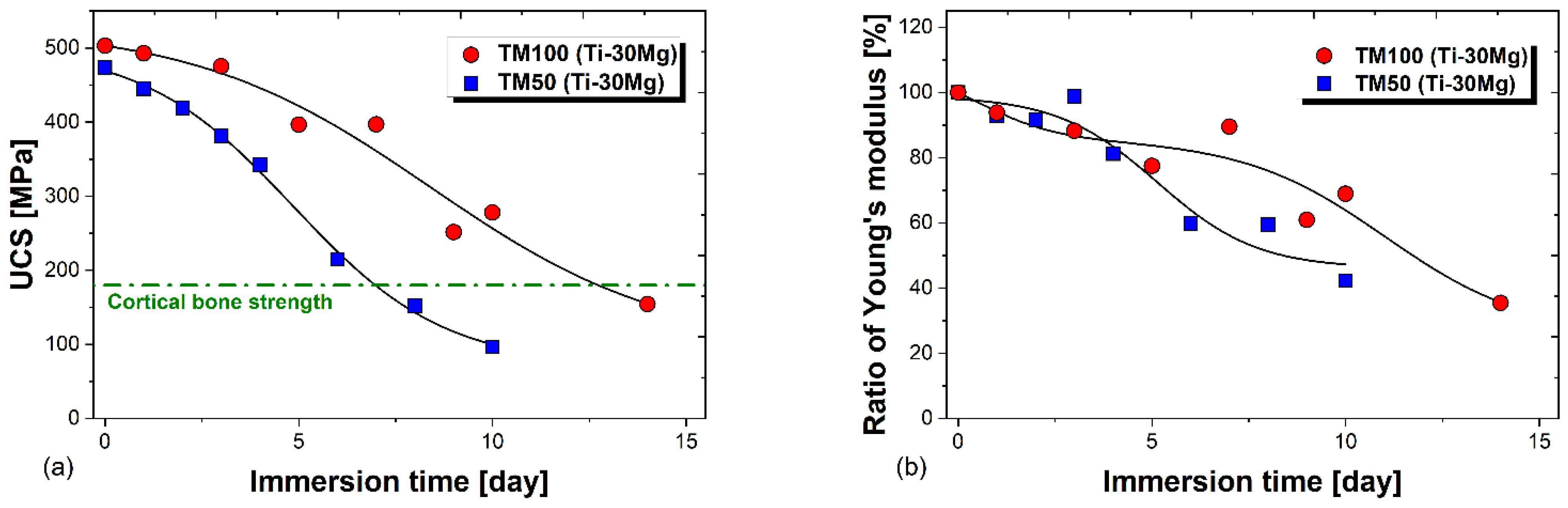
3.4. Immersion Test in Physiological Saline
4. Conclusions
- The uniform dispersion of Mg in Ti-30Mg and Ti-50Mg and the confirmation of the effectiveness of powder mixing with ethanol-added ball milling in producing uniform Ti-Mg composite materials further solidify the reliability of our findings.
- Young’s modulus of pure Ti, Ti-30Mg, and Ti-50Mg is 92 GPa, 81 GPa, and 75 GPa, respectively. This indicates a linear decrease in Young’s modulus of Ti-Mg composite materials with Mg addition.
- From density measurement, compression tests, and Young’s modulus measurement results, only TM25 (Ti-30Mg) showed significantly smaller values, while TM50 (Ti-30Mg), TM75 (Ti-30Mg), and TM100 (Ti-30Mg) showed no significant differences. Therefore, the sinterability of Ti-30vol%Mg saturates around a sintering pressure of approximately 50 MPa.
- TM50 (Ti-30Mg) showed a decrease in Young’s modulus from 81 GPa to 49 GPa after 6 days of immersion, while TM100 (Ti-30Mg) showed a decline from 91 GPa to 63 GPa after 10 days of immersion, indicating a reduction in stress shielding phenomenon.
- During immersion in physiological saline, TM50 maintained compressive strength above cortical bone for 6 days and TM100 for 10 days. This confirms that the mechanical integrity of Ti-30vol%Mg improves with increasing sintering pressure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otsuka, Y.; Mizutani, M. Introduction of Biomaterials for Beginners: II.Metallic Biomaterials. Zair. J. Soc. Mater. Sci. Jpn. 2014, 63, 480–486. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Hall, D.J.; Pourzal, R.; Garrigues, G.E. The Biomaterials of Total Shoulder Arthroplasty: Their Features, Function, and Effect on Outcomes. JBJS Rev. 2020, 8, e19. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Chen, W.C.; Huang, C.H.; Cheng, C.K.; Chan, K.K.; Chang, T.K. The Effect of Graft Strength on Knee Laxity and Graft In-Situ Forces after Posterior Cruciate Ligament Reconstruction. PLoS ONE 2015, 10, e0127293. [Google Scholar] [CrossRef] [PubMed]
- Noyama, Y.; Miura, T.; Ishimoto, T.; Itaya, T.; Niinomi, M.; Nakano, T. Bone Loss and Reduced Bone Quality of the Human Femur after Total Hip Arthroplasty under Stress-Shielding Effects by Titanium-Based Implant. Mater. Trans. 2012, 53, 565–570. [Google Scholar] [CrossRef]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.H. A Review on Alloy Design, Biological Response, and Strengthening of β-Titanium Alloys as Biomaterials. Mater. Sci. Eng. C 2021, 121, 111661. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Meng, Q.; Zhao, X.; Wei, Q.; Xu, H. Design and Fabrication of a Metastable β-Type Titanium Alloy with Ultralow Elastic Modulus and High Strength. Sci. Rep. 2015, 5, 14688. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Li, S.; Yin, D.; Xie, J.; Rommens, P.M.; Xiang, Z.; Liu, M.; Ritz, U. Recent Progress in Mg-Based Alloys as a Novel Bioabsorbable Biomaterials for Orthopedic Applications. J. Magnes. Alloys 2022, 10, 1428–1456. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, J.; An, X.; Guo, Y.; Xu, X.; Ma, Z.; Yao, W.; Kong, Q. Mechanical and Corrosion Properties of Ti–29Nb–13Ta-4.6Zr Alloy Prepared by Cryomilling and Spark Plasma Sintering. Vacuum 2023, 215, 112316. [Google Scholar] [CrossRef]
- Putra, N.E.; Mirzaali, M.J.; Apachitei, I.; Zhou, J.; Zadpoor, A.A. Multi-Material Additive Manufacturing Technologies for Ti-, Mg-, and Fe-Based Biomaterials for Bone Substitution. Acta Biomater. 2020, 109, 1–20. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of New Metallic Alloys for Biomedical Applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Krishna, B.V.; Bose, S.; Bandyopadhyay, A. Low Stiffness Porous Ti Structures for Load-Bearing Implants. Acta Biomater. 2007, 3, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Huang, Q.; Wu, H.; Ouyang, Z.; Liu, T.; He, H.; Xiao, J.; Lei, G.; Zhou, K. Stimulation of in Vitro and in Vivo Osteogenesis by Ti-Mg Composite Materials with the Sustained-Release Function of Magnesium Ions. Colloids Surf. B Biointerfaces 2021, 197, 111360. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Huang, Q.; Liu, Y.; Ouyang, Z.; Liang, L. Powder Metallurgical Ti-Mg Metal-Metal Composites Facilitate Osteoconduction and Osseointegration for Orthopedic Application. Bioact. Mater. 2019, 4, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Luo, F.; Zhang, Y.; Fang, Y.; Jiang, X. Strengthening Mechanisms of Ti–Mg Composite for Biomaterials: A Review. Adv. Eng. Mater. 2023, 25, 2300620. [Google Scholar] [CrossRef]
- Pham, D.N.; Hiromoto, S.; Yamazaki, T.; O, M.; Kobayashi, E. Enhanced Corrosion Resistance and in Vitro Biocompatibility of Mg-Zn Alloys by Carbonate Apatite Coating. ACS Appl. Bio Mater. 2021, 4, 6881–6892. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.N.; Hiromoto, S.; O, M.; Kobayashi, E. Influence of Substrate Microstructure on Hydroxyapatite Coating and Corrosion Behavior of Coated Mg[Sbnd]Zn Alloys. Surf. Coat. Technol. 2021, 421, 127414. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Pan, S.Q.; Zhang, F.; Wen, C.; Zeng, R.C. Advances in Mg–Al-Layered Double Hydroxide Steam Coatings on Mg Alloys: A Review. J. Magnes. Alloys 2023, 11, 1505–1518. [Google Scholar] [CrossRef]
- Wang, J.; Dou, J.; Wang, Z.; Hu, C.; Yu, H.; Chen, C. Research Progress of Biodegradable Magnesium-Based Biomedical Materials: A Review. J. Alloys Compd. 2022, 923, 166377. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of Magnesium-Based Biomaterials and Their Applications. J. Magnes. Alloys 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Verma, R.P. Titanium Based Biomaterial for Bone Implants: A Mini Review. Mater. Today: Proc. 2020, 26, 3148–3151. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Wang, N.; Maskomani, S.; Lu, S.; Anantharajan, S.K.; Dheen, S.T.; Nai, S.M.L.; Fuh, J.Y.H.; Wei, J. Fabrication of Ti + Mg Composites by Three-Dimensional Printing of Porous Ti and Subsequent Pressureless Infiltration of Biodegradable Mg. Mater. Sci. Eng. C 2020, 108, 110478. [Google Scholar] [CrossRef]
- Miao, L.; Gao, M.; Chen, S.; Wang, J.; Zhang, H.; Tan, L.; Pan, Y. The Mechanical Properties and Bioactivity of Ti–Mg Composites as Orthopedic Implants: A Pilot Study. J. Mater. Res. Technol. 2024, 28, 1074–1083. [Google Scholar] [CrossRef]
- Yang, H.; Chen, X.; Huang, G.; Song, J.; She, J.; Tan, J.; Zheng, K.; Jin, Y.; Jiang, B.; Pan, F. Microstructures and Mechanical Properties of Titanium-Reinforced Magnesium Matrix Composites: Review and Perspective. J. Magnes. Alloys 2022, 10, 2311–2333. [Google Scholar] [CrossRef]
- Eessaa, A.K.; Elkady, O.A.; El-Shamy, A.M. Powder Metallurgy as a Perfect Technique for Preparation of Cu–TiO2 Composite by Identifying Their Microstructure and Optical Properties. Sci. Rep. 2023, 13, 7034. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhang, Z.H.; Cheng, X.W.; Wang, F.C.; Zhang, Y.F.; Li, S.L. A Review of Multi-Physical Fields Induced Phenomena and Effects in Spark Plasma Sintering: Fundamentals and Applications. Mater. Des. 2020, 191, 108662. [Google Scholar] [CrossRef]
- Ratzker, B.; Sokol, M. Exploring the Capabilities of High-Pressure Spark Plasma Sintering (HPSPS): A Review of Materials Processing and Properties. Mater. Des. 2023, 233, 112238. [Google Scholar] [CrossRef]
- Liu, W.; An, R.; Wang, C.; Zheng, Z.; Tian, Y.; Xu, R.; Wang, Z. Recent Progress in Rapid Sintering of Nanosilver for Electronics Applications. Micromachines 2018, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Anselmi-Tamburini, U.; Garay, J.E.; Munir, Z.A. Fundamental Investigations on the Spark Plasma Sintering/Synthesis Process. Mater. Sci. Eng. A 2005, 407, 24–30. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, G.; Shi, M.; Yang, X.; Li, A. Microstructure and Properties of Porous Titanium Prepared by Spark Plasma Sintering. Metals 2019, 9, 82. [Google Scholar] [CrossRef]
- McDonnell, N.J.; Muchatuta, N.A.; Paech, M.J. Acute Magnesium Toxicity in an Obstetric Patient Undergoing General Anaesthesia for Caesarean Delivery. Int. J. Obs. Anesth. 2010, 19, 226–231. [Google Scholar] [CrossRef]
- Van Le, H.; Hiromoto, S.; O, M.; Kobayashi, E.; Nguyen, N.V.; Cao, N.Q. In Vitro Cellular Biocompatibility and in Vivo Degradation Behavior of Calcium Phosphate-Coated ZK60 Magnesium Alloy. Biomed. Mater. 2023, 18, 035003. [Google Scholar]
- Oh, M.; Iwamoto, H.; Kobayashi, E. Influence of Carbon Nanotubes on the Morphology of Cu6Sn5 in Cu/(Sn–Ag–Cu) Solder Joints. Results Mater. 2024, 21, 100553. [Google Scholar] [CrossRef]
- Wȩglewski, W.; Bochenek, K.; Basista, M.; Schubert, T.; Jehring, U.; Litniewski, J.; Mackiewicz, S. Comparative Assessment of Young’s Modulus Measurements of Metal-Ceramic Composites Using Mechanical and Non-Destructive Tests and Micro-CT Based Computational Modeling. Comput. Mater. Sci. 2013, 77, 19–30. [Google Scholar] [CrossRef]
- Rivard, P.; Saint-Pierre, F. Assessing Alkali-Silica Reaction Damage to Concrete with Non-Destructive Methods: From the Lab to the Field. Constr. Build. Mater. 2009, 23, 902–909. [Google Scholar] [CrossRef]
- Trang, L.T.; Quang Cao, N.; Hiromoto, S.; O, M.; Kobayashi, E. Formation and Corrosion Behavior of Calcium Phosphate Coating Layers on ZK60 Alloy Coated at Various PH Conditions by Chemical Conversion Method. Surf. Coat. Technol. 2022, 444, 128639. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of Pore Size and Morphology in Musculo-Skeletal Tissue Regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous Scaffold Design for Tissue Engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Cai, X.; Ding, S.; Li, Z.; Zhang, X.; Wen, K.; Xu, L.; Zhang, Y.; Peng, Y.; Shen, T. Simultaneous Sintering of Low-Melting-Point Mg with High-Melting-Point Ti via a Novel One-Step High-Pressure Solid-Phase Sintering Strategy. J. Alloys Compd. 2021, 858, 158344. [Google Scholar] [CrossRef]
- Oh, M.; Sakaguchi, H.; Kobayashi, E.; Kajihara, M. Understanding Intermetallic Compound Growth at Ag/Zn Interfaces: Kinetics and Mechanisms. Intermetallics 2024, 172, 108378. [Google Scholar] [CrossRef]
- Tavakol, M.; Mahnama, M.; Naghdabadi, R. Mechanisms Governing Microstructural Evolution during Consolidation of Nanoparticles. Mater. Manuf. Process. 2015, 30, 1397–1402. [Google Scholar] [CrossRef]
- Oh, M.; Kim, H.-S.; Kobayashi, E.; Kajihara, M. Understanding Kirkendall Effect in Ni(W) Diffusion-Induced Recrystallization Region. J. Alloys Compd. 2024, 991, 174556. [Google Scholar] [CrossRef]
- Oh, M.; Tokunaga, N.; Kobayashi, E. Reactive Diffusion at the Interface between Cu and Sn–Ag Alloys. J. Mater. Res. Technol. 2024, 30, 9531–9541. [Google Scholar] [CrossRef]
- Mehrer, H. Diffusion in Solids: Fundamentals, Methods, Materials, Diffusion-Controlled Processes; Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- O, M.; Sato, K.; Kobayashi, E. Investigation of the Rate-Controlling Process of Intermetallic Layer Growth at the Interface between Ferrous Metal and Molten Al–Mg–Si Alloy. Intermetallics 2023, 163, 108069. [Google Scholar] [CrossRef]
- Inomata, S.; O, M.; Kajihara, M. Diffusion-Induced Recrystallization in the Cu(Pd) System at Complete Solid-Solution Temperatures. J. Mater. Sci. 2011, 46, 2410–2421. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Si, C.; Xu, Q.; Liu, D.; Le, Q.; Fu, L.; Jia, Z.; Ma, Z.; Yuan, R. The Microstructure Evolution, Mechanical Property and Deformation Mechanism of Rolled Al3La/Mg–Li–Al–Zn Composites Prepared by in-Situ Synthesis. Mater. Sci. Eng. A 2024, 894, 146182. [Google Scholar] [CrossRef]
- Chen, X.; O, M.; Kobayashi, E. Enhanced Mechanical Properties in an Al-Mg-Cu Alloy Processed by the Combination of Cyclic Deformation and Aging Heat Treatment. J. Alloys Compd. 2022, 911, 165070. [Google Scholar] [CrossRef]
- Rezanezhad, M.; Lajevardi, S.A.; Karimpouli, S. Effects of Pore(s)-Crack Locations and Arrangements on Crack Growth Modeling in Porous Media. Theor. Appl. Fract. Mech. 2020, 107, 102529. [Google Scholar] [CrossRef]
- Zhu, H.X.; Fan, T.X.; Zhang, D. Composite Materials with Enhanced Dimensionless Young’s Modulus and Desired Poisson’s Ratio. Sci Rep 2015, 5, 14103. [Google Scholar] [CrossRef]
- Zeng, F.; Malzbender, J.; Baumann, S.; Nijmeijer, A.; Winnubst, L.; Ziegner, M.; Guillon, O.; Schwaiger, R.; Meulenberg, W.A. Optimization of Sintering Conditions for Improved Microstructural and Mechanical Properties of Dense Ce0.8Gd0.2O2-δ-FeCo2O4 Oxygen Transport Membranes. J. Eur. Ceram. Soc. 2021, 41, 509–516. [Google Scholar] [CrossRef]
- Choren, J.A.; Heinrich, S.M.; Silver-Thorn, M.B. Young’s Modulus and Volume Porosity Relationships for Additive Manufacturing Applications. J. Mater. Sci. 2013, 48, 5103–5112. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and Properties of Hydroxyapatite-Based Biomaterials for Use as Hard Tissue Replacement Implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Luo, T.; Song, M.; Wu, H.; Xiao, J.; Tan, Y.; Cheng, M.; Chen, B.; Niu, X.; et al. Powder Metallurgical Low-Modulus Ti-Mg Alloys for Biomedical Applications. Mater. Sci. Eng. C 2015, 56, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ding, S.; Li, Z.; Zhang, X.; Wen, K.; Xu, L.; Zhang, Y.; Shen, T. Mg-Ti Composites Fabricated by a Novel One-Step High-Pressure Sintering: The Correlation between Microstructures and Mechanical Properties. Compos. B Eng. 2021, 215, 108743. [Google Scholar] [CrossRef]
- Zeng, R.C.; Li, X.T.; Li, S.Q.; Zhang, F.; Han, E.H. In Vitro Degradation of Pure Mg in Response to Glucose. Sci. Rep. 2015, 5, 13026. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Zhao, X.; Zhang, X.; Miao, Y.; Yang, N.; Yang, B.; Zhang, L.; Kuang, W.; Li, J.; et al. Turning a Native or Corroded Mg Alloy Surface into an Anti-Corrosion Coating in Excited CO2. Nat. Commun. 2018, 9, 4058. [Google Scholar] [CrossRef]




| Feature | Spark Plasma Sintering (SPS) | Liquid Mg Infiltration |
|---|---|---|
| Temperature Control | Precise, localized heating reduces thermal gradients. | High temperatures required for Mg melting (>650 °C). |
| Reaction Time | Short sintering times due to efficient heating and pressure. | Longer processing times are needed for complete infiltration. |
| Microstructure Control | Fine control over microstructure through adjustable parameters. | Dependent on porous Ti structure and Mg flow dynamics. |
| Porosity Control | Ability to produce dense and controlled porosity composites. | Porosity depends on the initial Ti structure. |
| Material Homogeneity | Uniform distribution of Mg within Ti matrix. | Potential for uneven Mg distribution. |
| Mechanical Properties | Tunable properties through precise control of sintering parameters. | Limited by the inherent properties of porous Ti and Mg. |
| Scalability | Suitable for small-to-medium-scale production. | It can be challenging for large-scale uniform production. |
| Equipment and Cost | Requires SPS equipment, potentially high initial cost. | Lower equipment cost but higher operational complexity. |
| Post-processing Needs | Minimal, often no additional machining is required. | It may require additional machining to achieve the final shape. |
| Applications | Ideal for biomedical implants with tailored properties. | Suitable for large implants where high porosity is needed. |
| Specimen | Mg Content (Vol%) | Sintering Pressure (MPa) |
|---|---|---|
| Pure Ti | 0 | 50 |
| Ti-30Mg | 30 | 50 |
| Ti-50Mg | 50 | 50 |
| Ti-30Mg (TM25) | 30 | 25 |
| Ti-30Mg (TM75) | 30 | 75 |
| Ti-30Mg (TM100) | 30 | 100 |
| Specimen | Porosity of Sample (%) |
|---|---|
| Pure Ti | 9.0 |
| Ti-30Mg | 0 |
| Ti-50Mg | 0 |
| Ti-30Mg (TM25) | 6.4 |
| Ti-30Mg (TM75) | 0 |
| Ti-30Mg (TM100) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuda, T.; Oh, M.; Kobayashi, E. Fabrication and Characterization of Biomedical Ti-Mg Composites via Spark Plasma Sintering. Materials 2024, 17, 3470. https://doi.org/10.3390/ma17143470
Masuda T, Oh M, Kobayashi E. Fabrication and Characterization of Biomedical Ti-Mg Composites via Spark Plasma Sintering. Materials. 2024; 17(14):3470. https://doi.org/10.3390/ma17143470
Chicago/Turabian StyleMasuda, Taisei, Minho Oh, and Equo Kobayashi. 2024. "Fabrication and Characterization of Biomedical Ti-Mg Composites via Spark Plasma Sintering" Materials 17, no. 14: 3470. https://doi.org/10.3390/ma17143470
APA StyleMasuda, T., Oh, M., & Kobayashi, E. (2024). Fabrication and Characterization of Biomedical Ti-Mg Composites via Spark Plasma Sintering. Materials, 17(14), 3470. https://doi.org/10.3390/ma17143470








