Using Mössbauer Spectroscopy to Evaluate the Influence of Heat Treatment on the Surface Characteristics of Additive Manufactured 316L Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Temperature Treatment
2.2. Mössbauer Spectroscopy
2.3. XRD Analysis, Surface Morphologya and Elemental Composition
3. Results and Discussion
3.1. Surface Characterisationa and Oxide Morphology
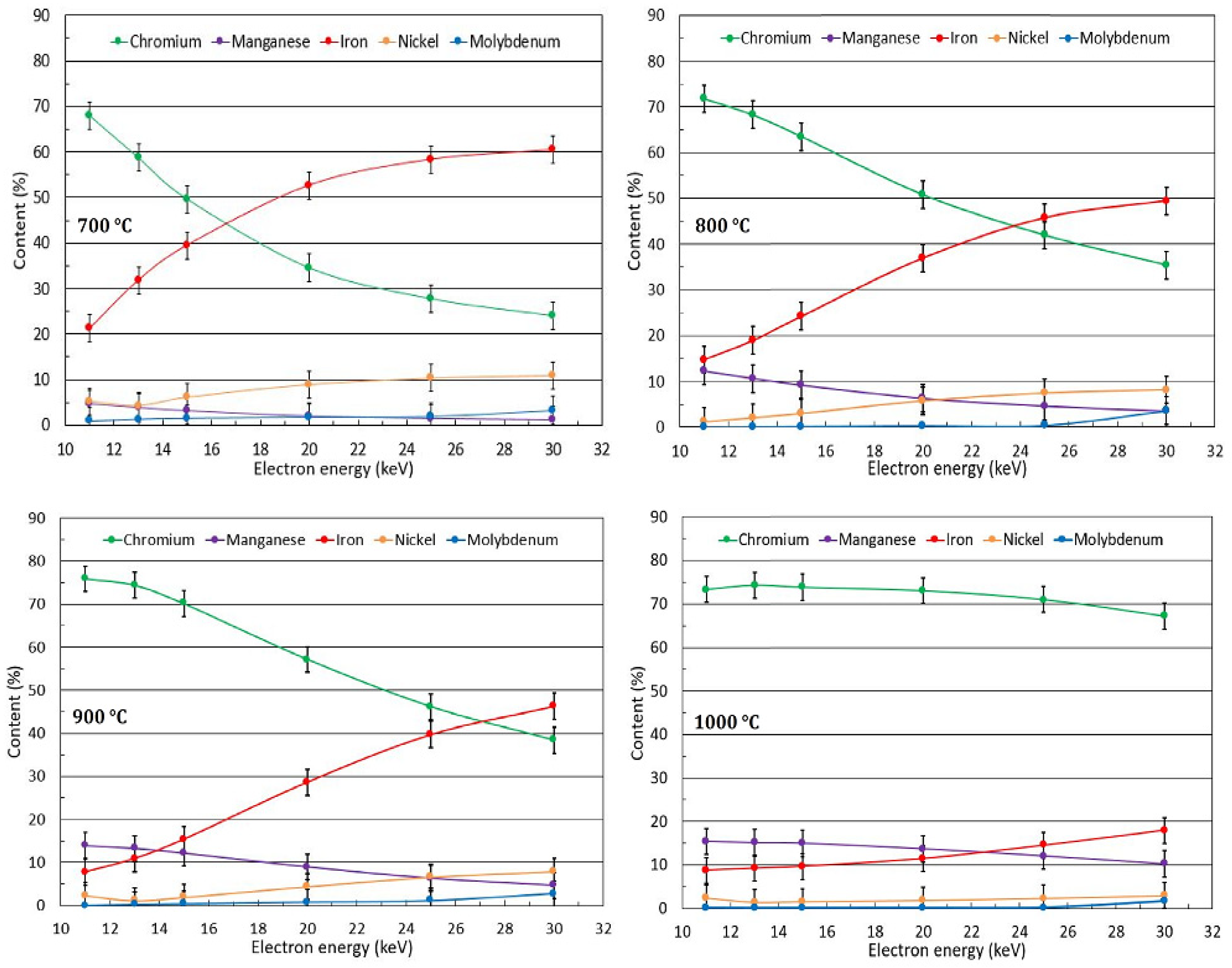
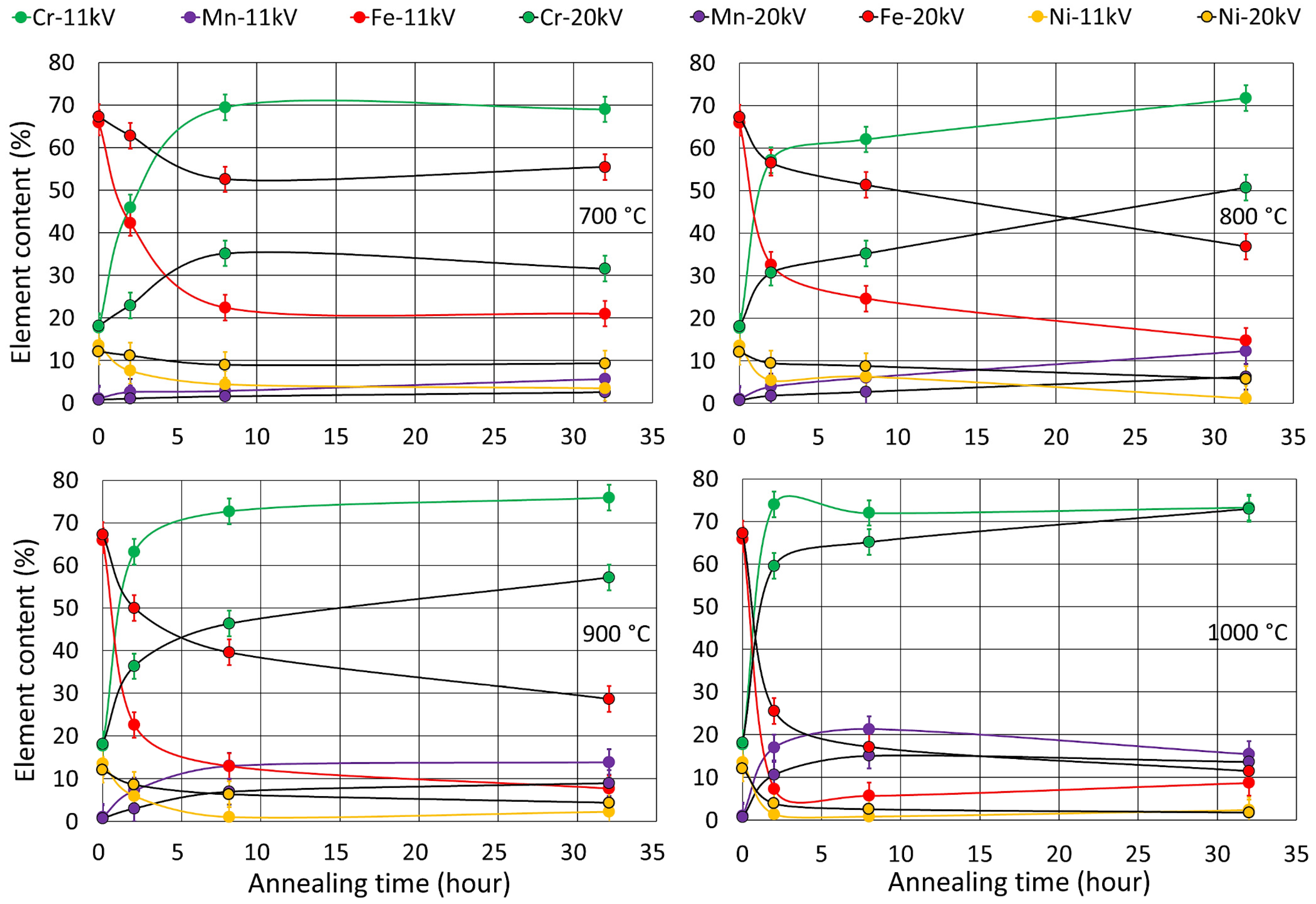
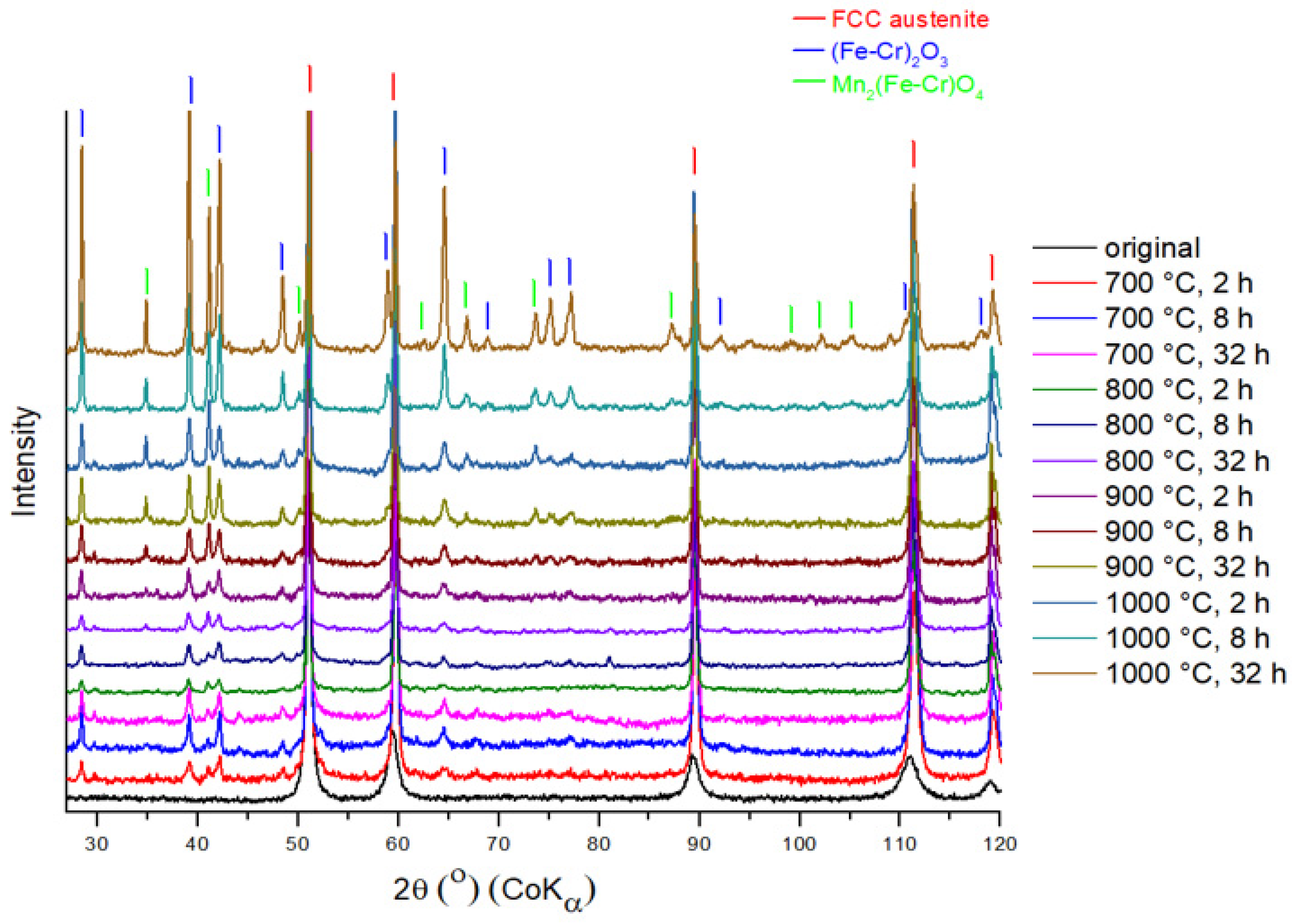
3.2. Oxide Phase Composition
3.3. Mössbauer Spectroscopy
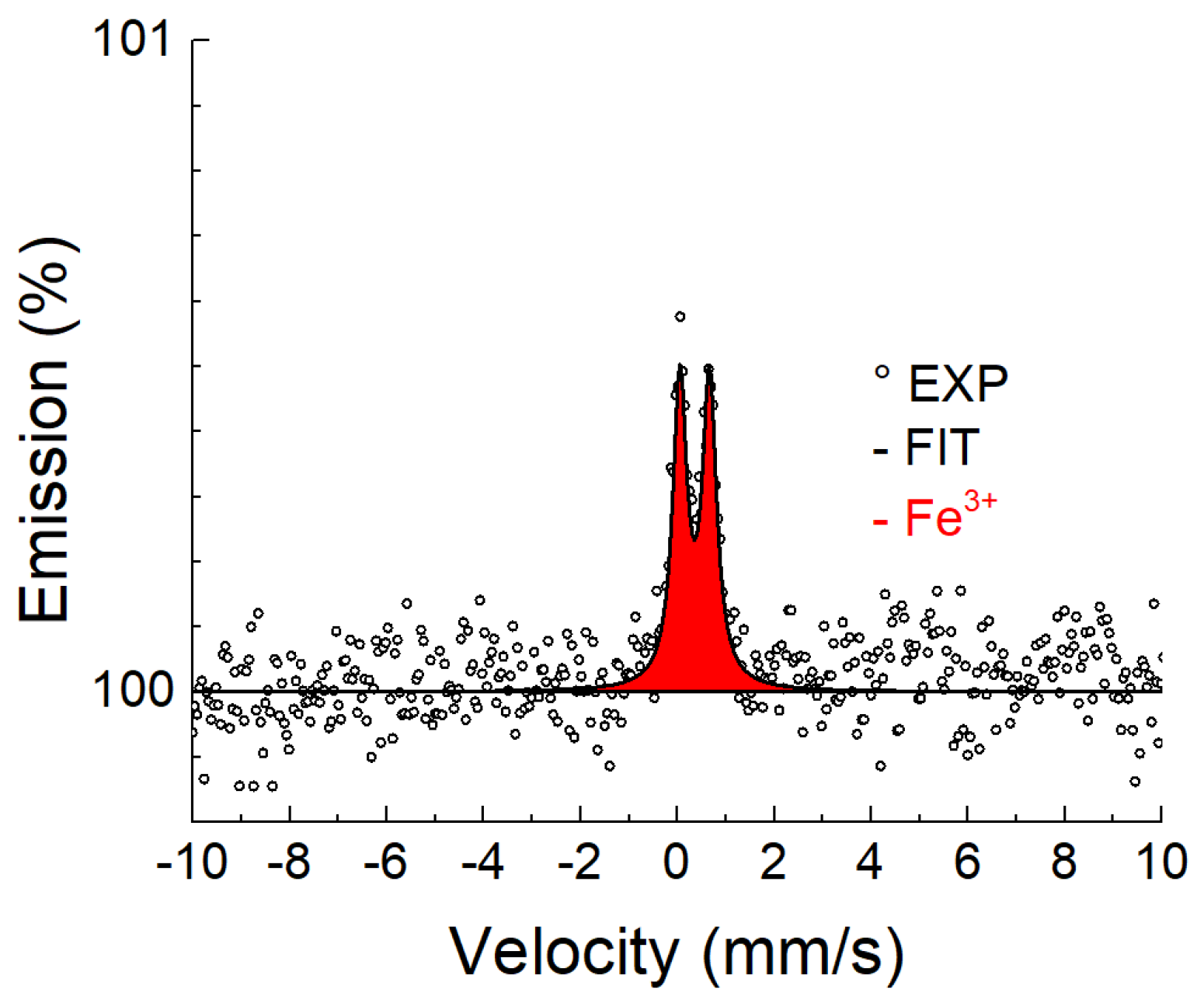
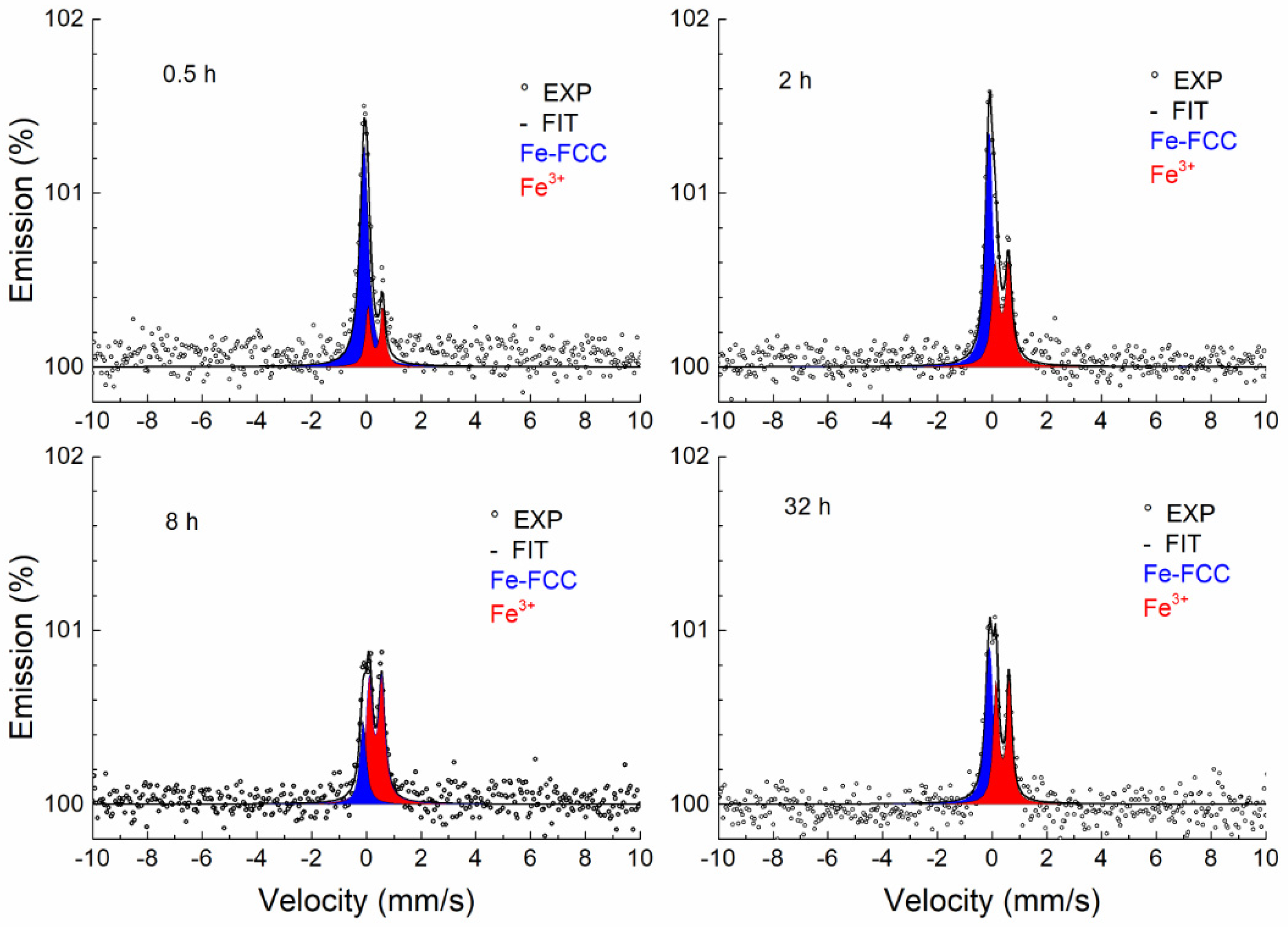
3.3.1. Conversion X-ray Mössbauer Spectroscopy
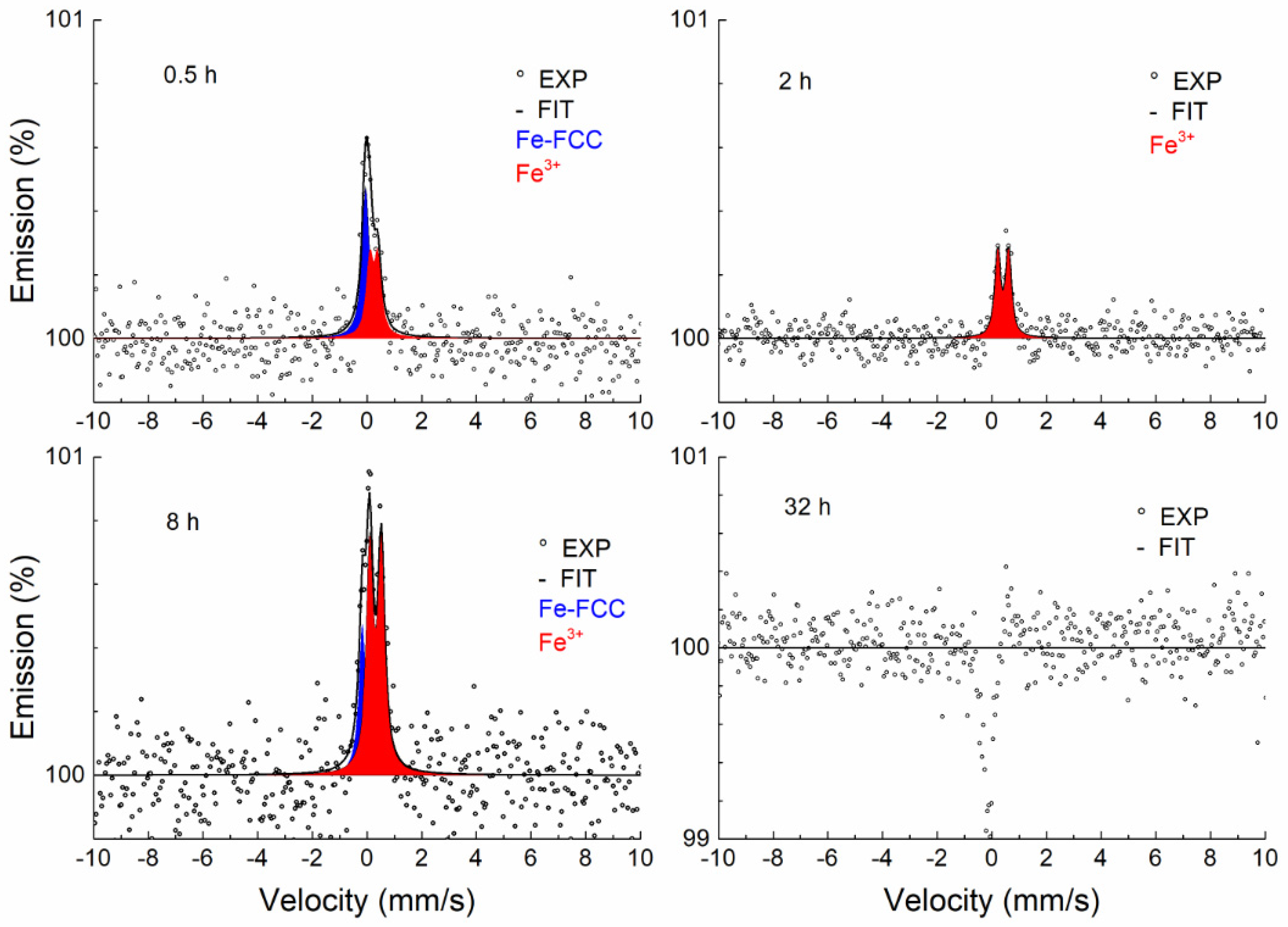
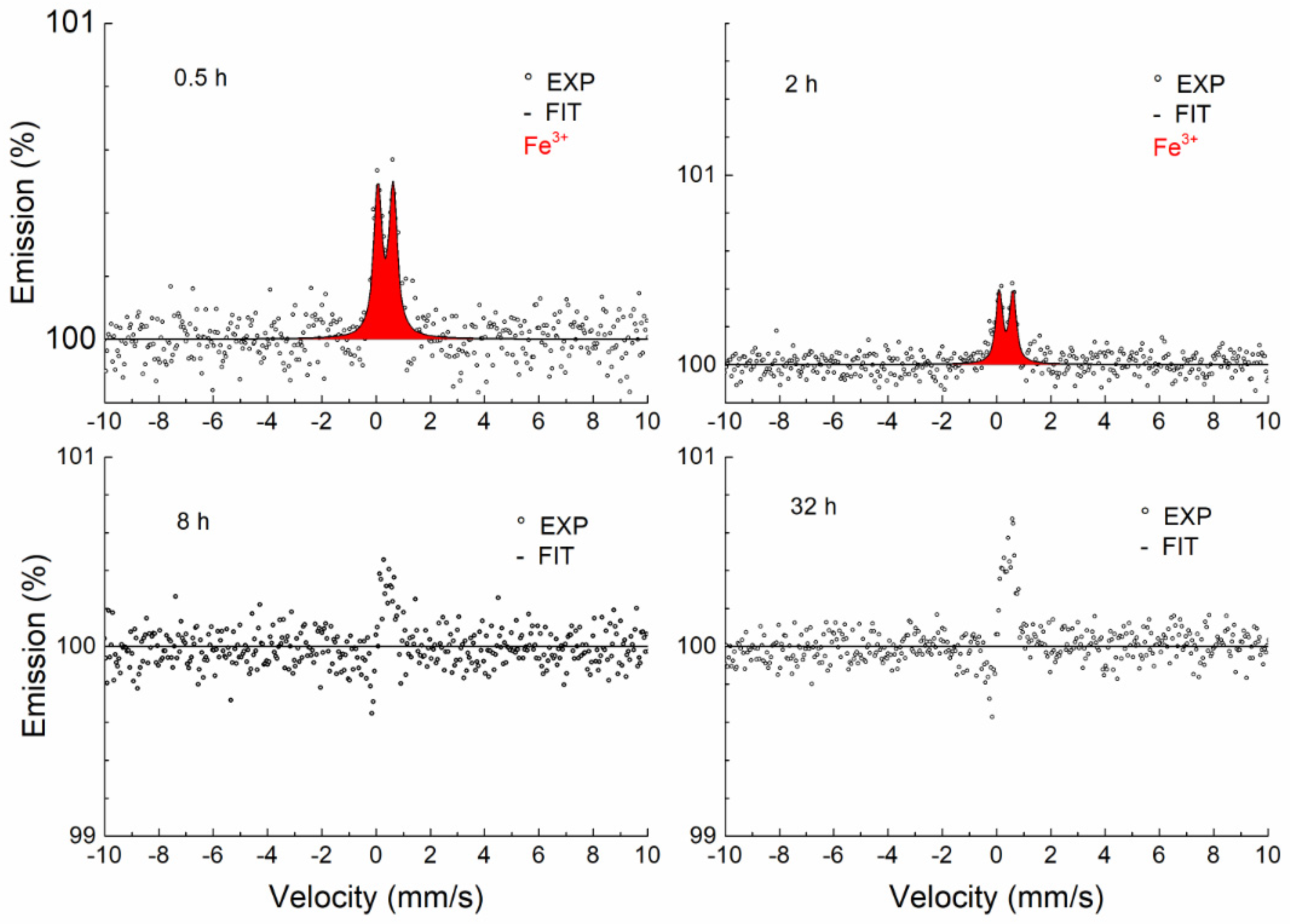
3.3.2. Conversion Electron Mössbauer Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
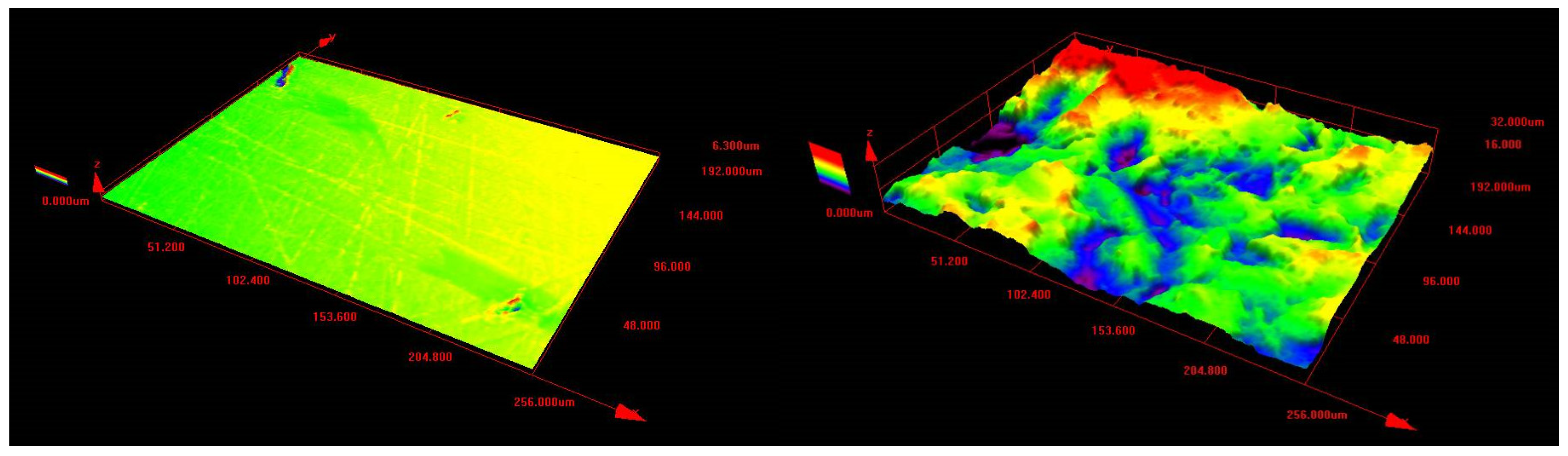
| Sample | Ra | Sa |
|---|---|---|
| polished | 0.07 ± 0.02 | 0.08 |
| unpolished | 2.64 ± 0.37 | 3.44 |
References
- Lewandowski, J.J.; Seifi, M. Metal additive manufacturing: A review of mechanical properties. Annu. Rev. Mater. Res. 2016, 46, 151–168. [Google Scholar] [CrossRef]
- Afkhami, S.; Dabiri, M.; Alavi, S.H.; Björk, T.; Salminen, A. Fatigue characteristics of steels manufactured by selective laser melting. Int. J. Fatigue 2019, 122, 72–83. [Google Scholar] [CrossRef]
- Kurzynowski, T.; Chlebus, E.; Kuznicka, B.; Reiner, J. Parameters in selective laser melting for processing metallic powders. In High Power Laser Materials Processing: Lasers, Beam Delivery, Diagnostics, and Applications; SPIE: Cergy-Pointoise, France, 2012; Volume 8239, p. 823914. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Smurov, I. Surface Morphology in selective laser melting of metal powders. Phys. Procedia 2011, 12, 264–270. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal fabrication by additive manufacturing using laser and electron beam melting technology. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Gunasekaran, J.; Sevvel, P.; Solomon, I.J. Metallic material fabrication by selective laser melting. A review. Mater. Today Proc. 2021, 37, 252–256. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, J.; Bae Lee, H.; Jang, C.; Jang, K. Effect of heat treatment on corrosion behavior of additively manufactured 316L stainless steel in higher-temperature water. Corros. Sci. 2023, 210, 110830. [Google Scholar] [CrossRef]
- Murkute, P.; Pasebani, S.; Isgor, O.B. Production of corrosion-resistant 316L stainless steel clads on carbon steel using powder bed fusion-selective laser melting. J. Mat. Proc. Technol. 2019, 273, 116243. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, C.; Zhang, Z.; Liu, W. Tensile properties of selective laser melted 316L stainless steel. Acta Metall. Sin. 2020, 56, 683–692. [Google Scholar] [CrossRef]
- Chandra-ambhorn, S.; Saranyachot, P.; Thublaor, T. Higher temperature oxidation behavior of Fe-15.7 wt% Cr-8.5 wt% Mn in oxygen without and with water vapor at 700 °C. Corros. Sci. 2019, 148, 39–47. [Google Scholar] [CrossRef]
- Ivanova, T.; Mashlan, M.; Ingr, T.; Doláková, H.; Sarychev, D.; Sedláčková, A. Mössbauer spectroscopy for Additive Manufacturing by Selective Laser Melting. Metals 2022, 12, 551. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, K.; Fang, X.; Xiong, Z.; Wei, L.; Zhu, P.; Li, X. Oxidation behavior of 316L austenitic stainless steel in higher temperature air with long-term exposure. Mater. Res. Express 2020, 7, 066517. [Google Scholar] [CrossRef]
- Piekoszewski, J.; Sartowska, B.; Barlak, M.; Konarski, P.; Dabrowski, L.; Starosta, W.; Walis, L.; Werner, Z.; Pochrybniak, C.; Bochenska, K.; et al. Improvement of higher temperature oxidation resistance of AISI 316L stainless steel by incorporation of Ce-La elements using intense pulsed plasma beams. Surf. Coat. Technol. 2011, 206, 854–858. [Google Scholar] [CrossRef]
- Hong, M.; Morales, A.L.; Chan, H.L.; Macdonald, D.; Balooch, M.; Xie, Y.; Romanovskaia, E.; Scully, J.R.; Kaoumi, D.; Hosemann, P. Effect of thermal oxidation on helium implanted 316L stainless steel. J. App. Phys. 2022, 132, 185104. [Google Scholar] [CrossRef]
- Elger, R.; Pettersson, R. Effect of addition of 4% Al on the high temperature oxidation and nitridation of a 20Cr-25Ni austenitic stainless steel. Oxid. Met. 2014, 82, 469–490. [Google Scholar] [CrossRef]
- De Las Heras, E.; Egidi, D.A.; Corengia, P.; Gonzalez-Santamaria, D.; Garccia-Luis, A.; Brizuela, M.; Lopez, G.A.; Flores Martinez, M. Duplex surface treatment of an AISI 316L stainless steel; microstructure and tribological behaviour. Surf. Coat. Technol. 2008, 202, 2945–2954. [Google Scholar] [CrossRef]
- Benafia, S.; Retraint, D.; Yapi Brou, S.; Panicaud, B.; Grosseau Poussard, J.L. Influence of Surface Mechanical Attrition Treatment on the oxidation behaviour of 316L stainless steel. Corros. Sci. 2018, 136, 188–200. [Google Scholar] [CrossRef]
- Lei, Y.B.; Wang, Z.B.; Zhang, B.; Luo, Z.P.; Lu, J.; Lu, K. Enhanced mechanical properties and corrosion resistance of 316L stainless steel by preforming a gradient nanostructured surface layer and annealing. Acta Mater. 2021, 208, 116773. [Google Scholar] [CrossRef]
- Terachi, T.; Yamada, T.; Miyamoto, T.; Arioka, K.; Fukuya, K. Corrosion Behavior of Stainless Steel in Simulated PWR Primary Water—Effect of Chromium Content in Alloys and Dissolved Hydrogen. J. Nucl. Sci. Technol. 2008, 45, 975–984. [Google Scholar] [CrossRef]
- Ghosh, S.; Kiran Kumar, M.; Kain, V. High temperature oxidation behavior of AISI 304L stainless steel—Effect of surface working operation. Appl. Surf. Sci. 2013, 264, 312–319. [Google Scholar] [CrossRef]
- Yasa, E.; Kruth, J.-P.; Deckers, J. Manufacturing by combining Selective Laser Melting and Selective Laser Erosion/laser re-melting. CIRP Ann. 2011, 60, 263–266. [Google Scholar] [CrossRef]
- Marimuthu, S.; Triantaphyllou, A.; Antar, M.; Wimpenny, D.; Morton, H.; Beard, M. Laser polishing of selective laser melted components. Int. J. Mach. Tools Manuf. 2015, 95, 97–104. [Google Scholar] [CrossRef]
- Cuesta, E.; Giganto, S.; Alvarez, B.J.; Barreiro, J.; Martinez-Pellitero, S.; Meana, V. Laser line scanner aptitude for the measurement of Selective Laser Melting parts. Opt. Lasers Eng. 2021, 138, 106406. [Google Scholar] [CrossRef]
- Saeidi, K.; Gao, X.; Lofaj, F.; Kvetkova, L.; Shen, Z.J. Transformation of austenite to duplex austenite-ferrite assembly in annealed stainless steel 316L consolidate by laser melting. J. Alloys Compd. 2015, 633, 463–469. [Google Scholar] [CrossRef]
- Sedláčková, A.; Ivanova, T.; Mashlan, M.; Doláková, H. Phase changes in the Surface Layer of Stainless Steel Annealed at a Temperature of 550 °C. Materials 2022, 15, 8871. [Google Scholar] [CrossRef] [PubMed]
- Biehler, J.; Hoche, H.; Oechsner, M. Nitriding behavior and corrosion properties of AISI 304L and 316L austenitic stainless steel with deformation-induced martensite. Surf. Coat. Technol. 2017, 324, 121–128. [Google Scholar] [CrossRef]
- Principi, G. The Mössbauer Effect: A Romantic Scientific Page. Metals 2020, 10, 992. [Google Scholar] [CrossRef]
- Linderhof, F.; Mashlan, m.; Doláková, H.; Ingr, T.; Ivanova, T. Surface Micromorphology and Structure of Stainless and Maraging Steel Obtained via Selective Laser Melting: A Mössbauer Spectroscopy Study. Metals 2021, 11, 1028. [Google Scholar] [CrossRef]
- Pechousek, J.; Mashlan, M. Mössbauer spectrometer as a virtual instrument in the PXI/Compact PCI modular system. Czechoslov. J. Phys. 2005, 55, 853. [Google Scholar] [CrossRef]
- Kholmetskii, A.L.; Misevich, O.V.; Mashlan, M.; Chudakov, V.A.; Anashkevich, A.F.; Gurachevskii, V.L. Air scintillation detector for conversion electrons Mössbauer spectroscopy (CEMS). Nucl. Instrum. Methods Phys. Res. B 1997, 129, 110. [Google Scholar] [CrossRef]
- Klencsár, Z.; Kuzmann, E.; Vértes, A. User-Friendly Program for Multifold Evaluation of Mössbauer Spectra. Hyperfine Interact. 1998, 112, 269. [Google Scholar] [CrossRef]
- Klencsár, Z.; Kuzmann, E.; Vértes, A. User-friendly software for Mössbauer spectrum analysis. J. Radioanal. Nucl. Chem. 1996, 210, 105. [Google Scholar] [CrossRef]
- Nezakat, M.; Akhiani, H.; Penttila, S.; Sabet, S.M.; Szpunar, J. Effect of thermo-mechanical processing on oxidation of austenitic stainless steel 316L in supercritical water. Corros. Sci. 2015, 94, 197–206. [Google Scholar] [CrossRef]
- Kain, V.; Chandra, K.; Adhe, K.N.; De, P.K. Effect of cold work on low-temperature sensitization behavior of austenitic stainless steels. J. Nucl. Mater. 2004, 334, 115–132. [Google Scholar] [CrossRef]
- Ostwald, C.; Grabke, H.J. Initial oxidation and chromium diffusion Effects of surface working on 9–20% Cr steels. Corros. Sci. 2004, 46, 1113–1127. [Google Scholar] [CrossRef]
- Tapping, R.L.; Davidson, R.D.; McAlpine, E.; Lister, D.H. The composition and morphology of oxide films formed on type 304 stainless steel in lithiated high temperature water. Corros. Sci. 1986, 26, 563–576. [Google Scholar] [CrossRef]
- Castaing, R. Application des Sondes Electronique a une Methode D’analyse Ponctuelle Chimique et Cristallographique. Ph.D. Thesis, University of Paris, Paris, France, 1952. [Google Scholar]
- Riffard, F.; Buscail, H.; Rabaste, F.; Caudron, E.; Cueff, R.; Issartel, C.; Karimi, N.; Perrier, S. Manganese effect on isothermal high temperature oxidation behavior of AISI 304 stainless steel. Mater. Sci. Forum 2008, 595, 1127–1134. [Google Scholar] [CrossRef]
- Lothongkum, G.; Tiyawatwitthaya, C.; Prawetpai, N.; Lothongkum, A.W. XRD investigation of the Cu and Mn effects on the oxide scale of hot rolled AISI 304L stainless steel after annealing and shot-blasting. Mater. Test. 2020, 62, 568–572. [Google Scholar] [CrossRef]
- Lobnig, R.E.; Schmidt, H.P.; Hennesen, K.; Grabke, H.J. Diffusion of Cations in Chromia Layers Grown on Iron-Base Alloys. Oxid. Met. 1992, 37, 81–93. [Google Scholar] [CrossRef]
- Wei, F.I.; Stott, F.H. The oxidation performance of Cr2O3-forming commercial iron-nickel alloys at high temperature. High Temp. Technol. 2016, 7, 59–71. [Google Scholar] [CrossRef]
- Apell, J.; Wonneberger, R.; Seyring, M.; Stöcker, H.; Rettenmayr, M.; Undisz, A. Early oxidation stages of a Co-Cr-Fe-Mn-Ni-Si complex concentrated alloy with Cr, Mn, and Si contents matching those of 316L stainless steel. Corros. Sci. 2021, 190, 109642. [Google Scholar] [CrossRef]
- Cook, D.C. Strain induced martensite formation in stainless steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1987, 18, 201–210. [Google Scholar] [CrossRef]
- Kanaya, K.; Okayama, S. Penetration and energy-loss theory of electrons in solid targets. J. Phys. D Appl. Phys. 1972, 5, 43–58. [Google Scholar] [CrossRef]
- Machala, L.; Tuček, J.; Zbořil, R. Polymorphous Transformations of Nanometric Iron(III) Oxide: A Review. Chem. Mater. 2011, 23, 3255–3272. [Google Scholar] [CrossRef]





| Element Concentration, wt % | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe | C | Si | Mn | P | S | Cr | Mo | Ni |
| Balance | ≤0.03 | 0–1.0 | 0–2.0 | ≤0.045 | ≤0.03 | 16.5–18.5 | 2.0–2.5 | 10.0–13.0 |
| Temperature (°C) | Time (h) | Phase | IS (mm/s) ±0.01 | QS (mm/s) ±0.01 | FWHM (mm/s) ±0.01 |
|---|---|---|---|---|---|
| Unannealed | - | Austenite | −0.10 | −0.17 | 0.29 |
| 700 °C | 0.5 | Austenite | −0.10 | −0.16 | 0.28 |
| 2 | Austenite | −0.10 | −0.15 | 0.28 | |
| 8 | Austenite | −0.10 | −0.16 | 0.27 | |
| 32 | Austenite | −0.10 | −0.15 | 0.28 | |
| 800 °C | 0.5 | Austenite | −0.08 | −0.16 | 0.27 |
| 2 | Austenite | −0.10 | −0.15 | 0.26 | |
| 8 | Austenite | −0.10 | −0.15 | 0.29 | |
| 32 | Austenite | −0.10 | −0.14 | 0.29 | |
| 900 °C | 0.5 | Austenite | −0.08 | −0.15 | 0.28 |
| 2 | Austenite | −0.08 | −0.16 | 0.27 | |
| 8 | Austenite | −0.08 | −0.16 | 0.27 | |
| 32 | Austenite | −0.10 | −0.15 | 0.27 | |
| 1000 °C | 0.5 | Austenite | −0.10 | −0.15 | 0.27 |
| 2 | Austenite | −0.08 | −0.15 | 0.28 | |
| 8 | Austenite | −0.10 | −0.16 | 0.29 | |
| 32 | Austenite | −0.10 | −0.14 | 0.29 |
| Temperature, (°C) | Time, (h) | Phase | IS (mm/s) ±0.02 | QS (mm/s) ±0.03 | FWHM (mm/s) ±0.03 |
|---|---|---|---|---|---|
| Unannealed | - | Austenite | −0.12 | 0.15 | 0.25 |
| 700 °C | 0.5 | Austenite | −0.12 * | 0.15 * | 0.25 * |
| 2 | Austenite | −0.12 * | 0.15 * | 0.25 * | |
| Fe3+ | 0.33 | 0.57 | 0.25 | ||
| 8 | Austenite | −0.12 * | 0.15 * | 0.25 * | |
| Fe3+ | 0.33 | 0.48 | 0.24 | ||
| 32 | Austenite | −0.12 * | 0.15 * | 0.25 * | |
| Fe3+ | 0.36 | 0.48 | 0.25 | ||
| 800 °C | 0.5 | Austenite | −0.12 * | 0.15 * | 0.25 * |
| Fe3+ | 0.32 | 0.52 | 0.26 | ||
| 2 | Austenite | −0.12 * | 0.15 * | 0.25 * | |
| Fe3+ | 0.35 | 0.49 | 0.31 | ||
| 8 | Austenite | −0.12 * | 0.15 * | 0.25 * | |
| Fe3+ | 0.34 | 0.43 | 0.26 | ||
| 32 | Austenite | −0.12 * | 0.15 * | 0.25 * | |
| Fe3+ | 0.38 | 0.49 | 0.24 | ||
| 900 °C | 0.5 | Austenite | −0.12 * | 0.15 * | 0.25 * |
| Fe3+ | 0.21 | 0.30 | 0.29 | ||
| 2 | Fe3+ | 0.40 | 0.40 | 0.25 | |
| 8 | Austenite | −0.12 * | 0.15 * | 0.25 * | |
| Fe3+ | 0.32 | 0.42 | 0.35 | ||
| 32 | - | - | - | - | |
| 1000 °C | 0.5 | Fe3+ | 0.33 | 0.58 | 0.36 |
| 2 | Fe3+ | 0.36 | 0.52 | 0.28 | |
| 8 | - | - | - | - | |
| 32 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, T.; Kořenek, M.; Mashlan, M. Using Mössbauer Spectroscopy to Evaluate the Influence of Heat Treatment on the Surface Characteristics of Additive Manufactured 316L Stainless Steel. Materials 2024, 17, 3494. https://doi.org/10.3390/ma17143494
Ivanova T, Kořenek M, Mashlan M. Using Mössbauer Spectroscopy to Evaluate the Influence of Heat Treatment on the Surface Characteristics of Additive Manufactured 316L Stainless Steel. Materials. 2024; 17(14):3494. https://doi.org/10.3390/ma17143494
Chicago/Turabian StyleIvanova, Tatiana, Michal Kořenek, and Miroslav Mashlan. 2024. "Using Mössbauer Spectroscopy to Evaluate the Influence of Heat Treatment on the Surface Characteristics of Additive Manufactured 316L Stainless Steel" Materials 17, no. 14: 3494. https://doi.org/10.3390/ma17143494
APA StyleIvanova, T., Kořenek, M., & Mashlan, M. (2024). Using Mössbauer Spectroscopy to Evaluate the Influence of Heat Treatment on the Surface Characteristics of Additive Manufactured 316L Stainless Steel. Materials, 17(14), 3494. https://doi.org/10.3390/ma17143494






