How Often Should Microbial Contamination Be Detected in Aircraft Fuel Systems? An Experimental Test of Aluminum Alloy Corrosion Induced by Sulfate-Reducing Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Microbes
2.2. Orthogonal Experiments of Various Environmental Factors
2.3. Corrosion Behavior of Aluminum Alloy Influenced by D. bizertensis under Harshest Conditions
2.4. Biofilm and Corrosion Product Film Characterizations
2.5. Weight Loss and Pit Morphology
2.6. Electrochemical Measurements
3. Results
3.1. Statistical Orthogonal Experiments under Various Environmental Conditions
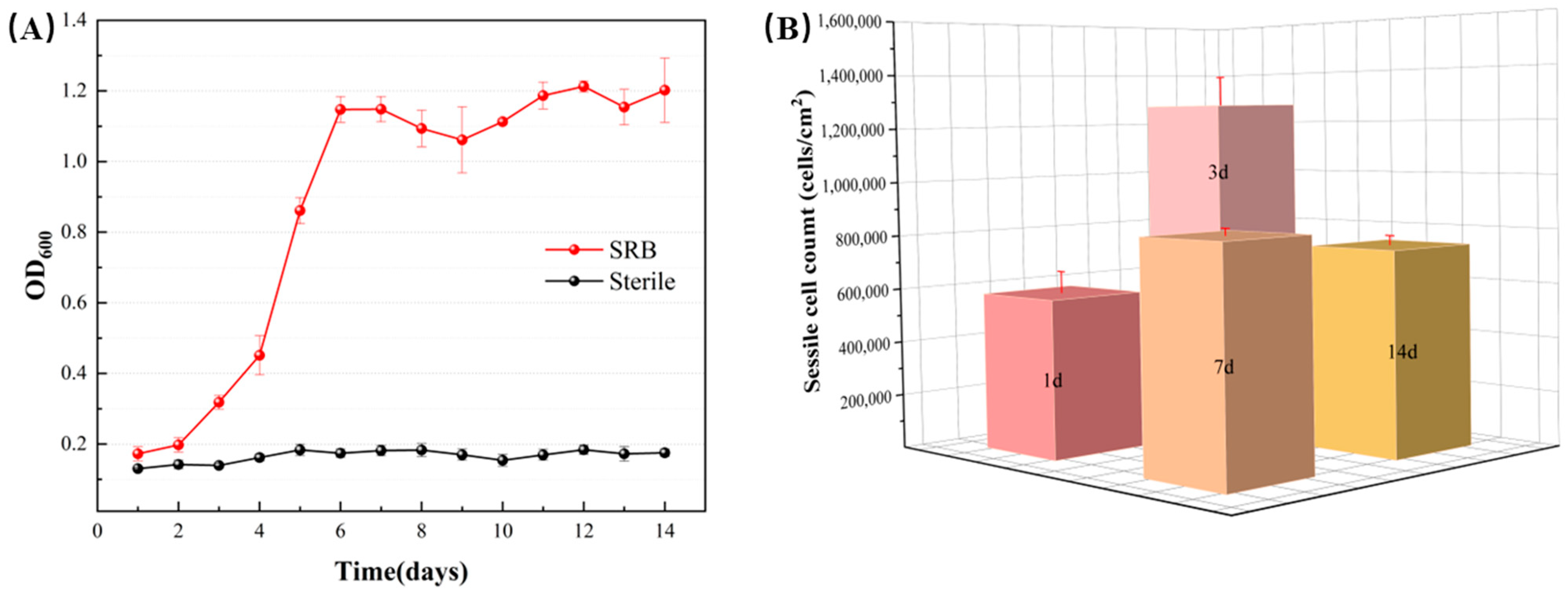
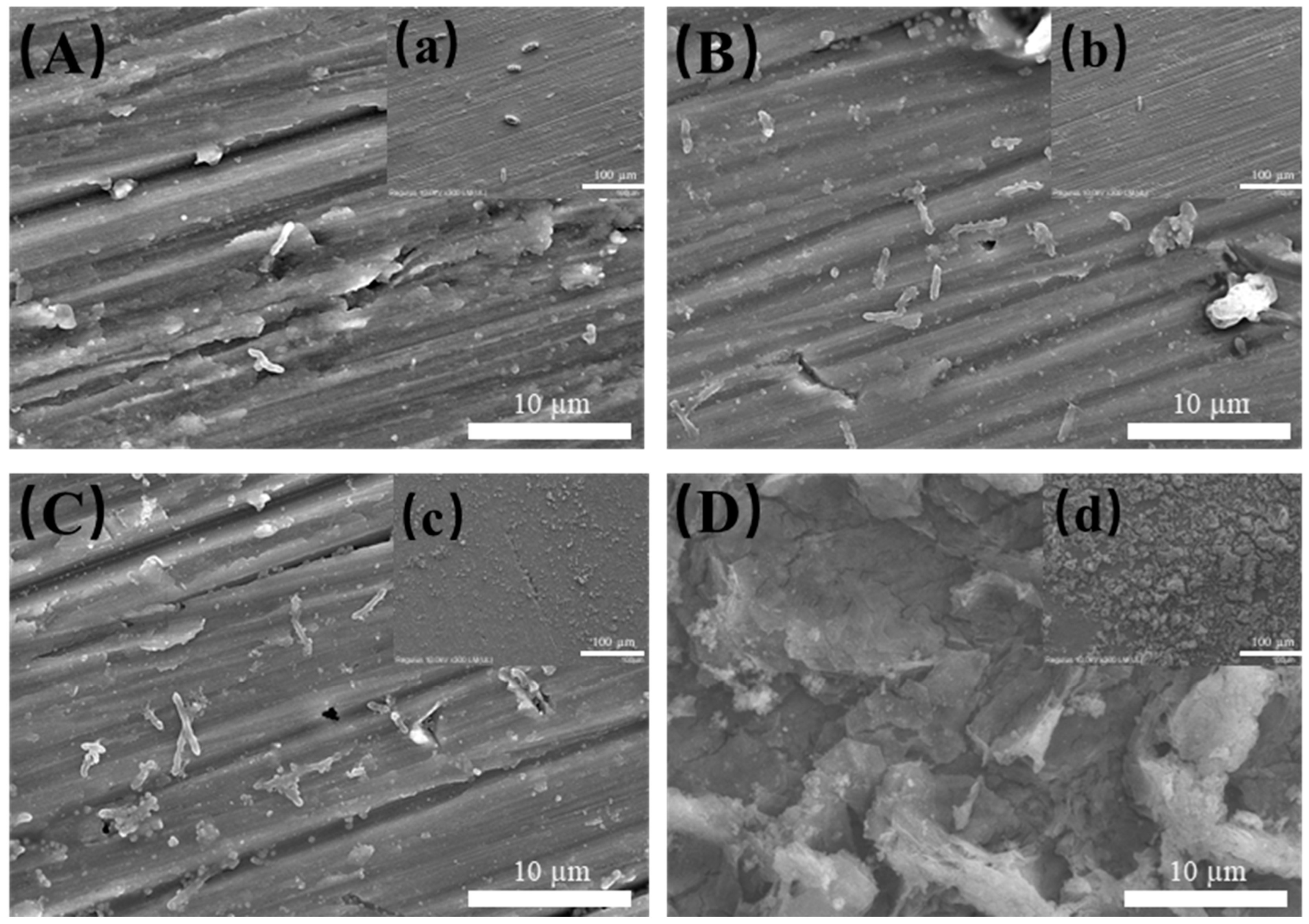
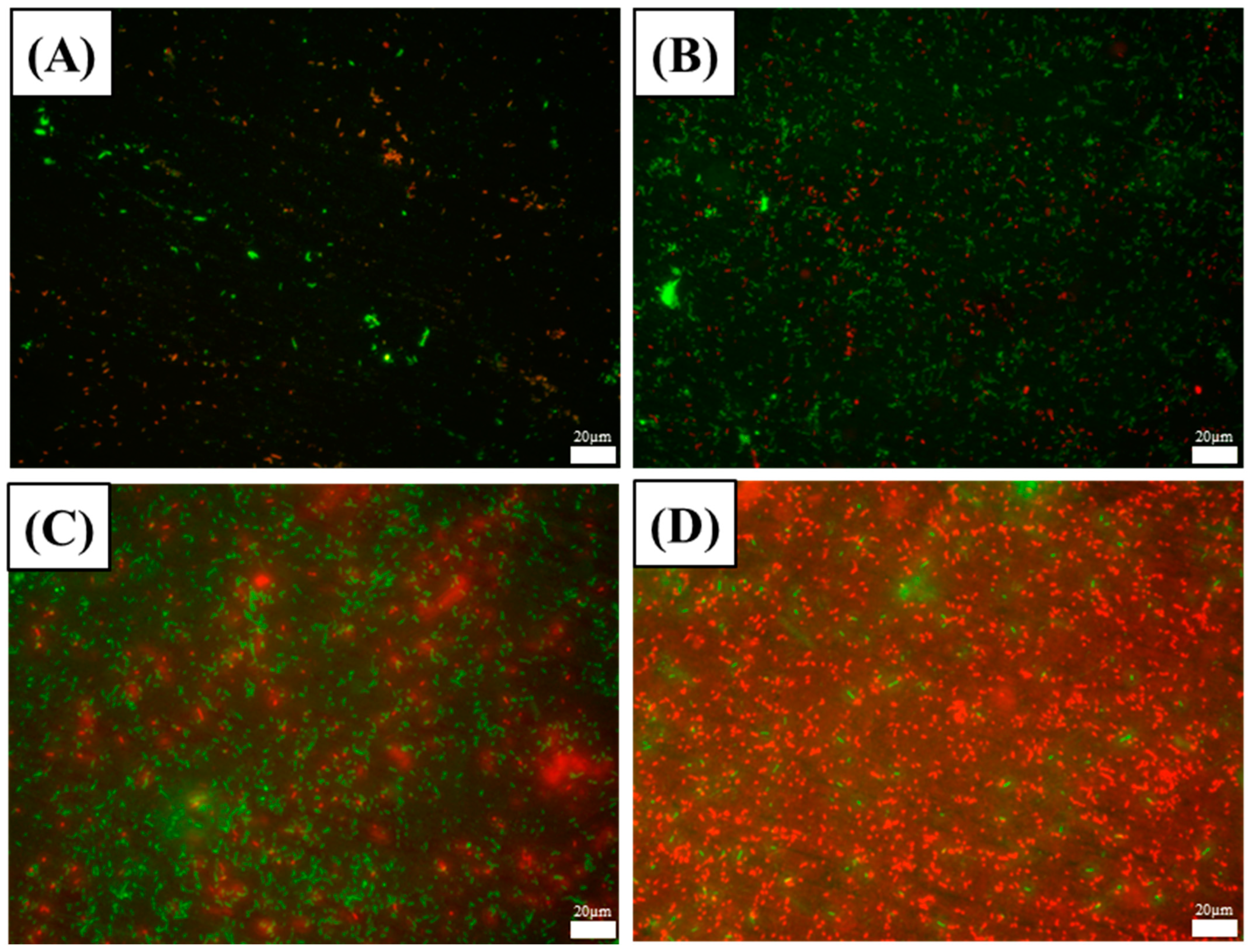
3.2. Bacterial Growth and Biofilm Characterization
3.3. Composition of Corrosion Products
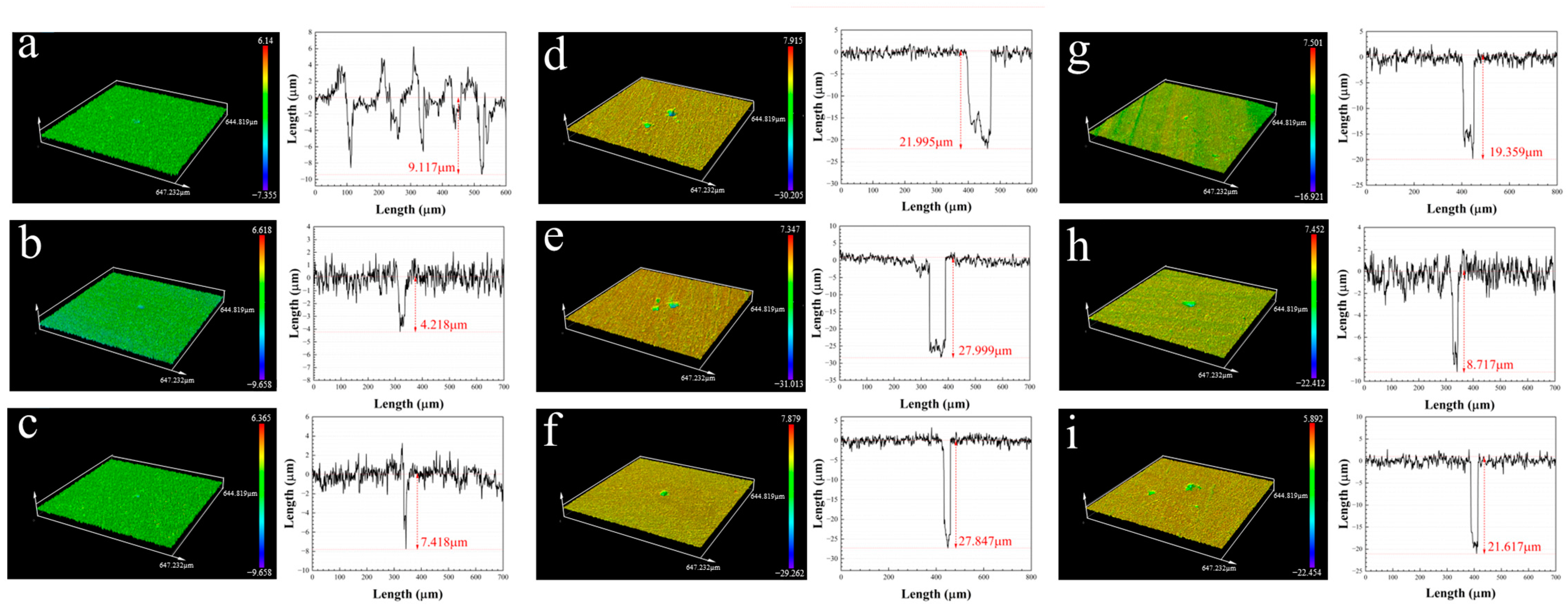
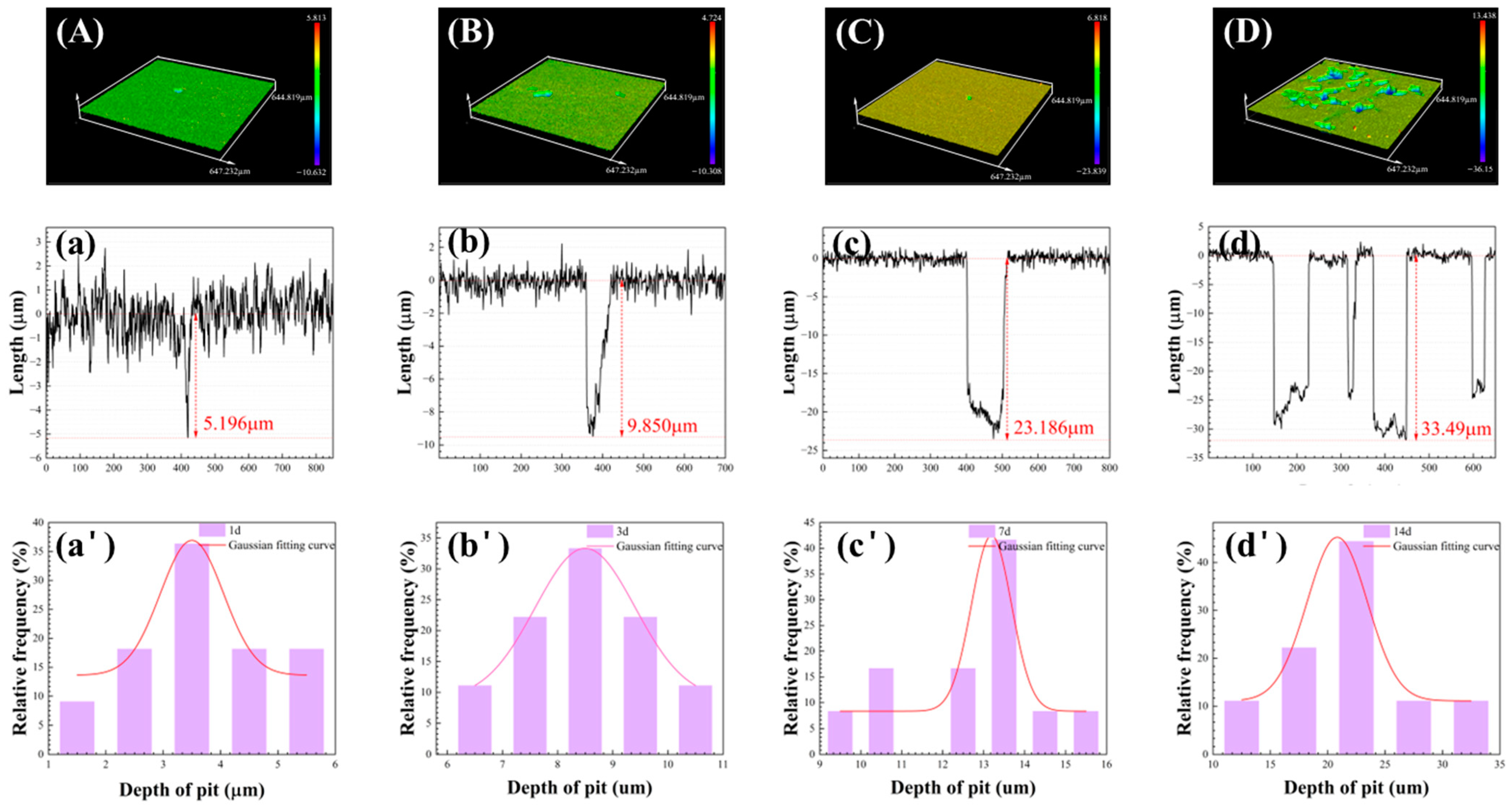
3.4. The Pitting Corrosion
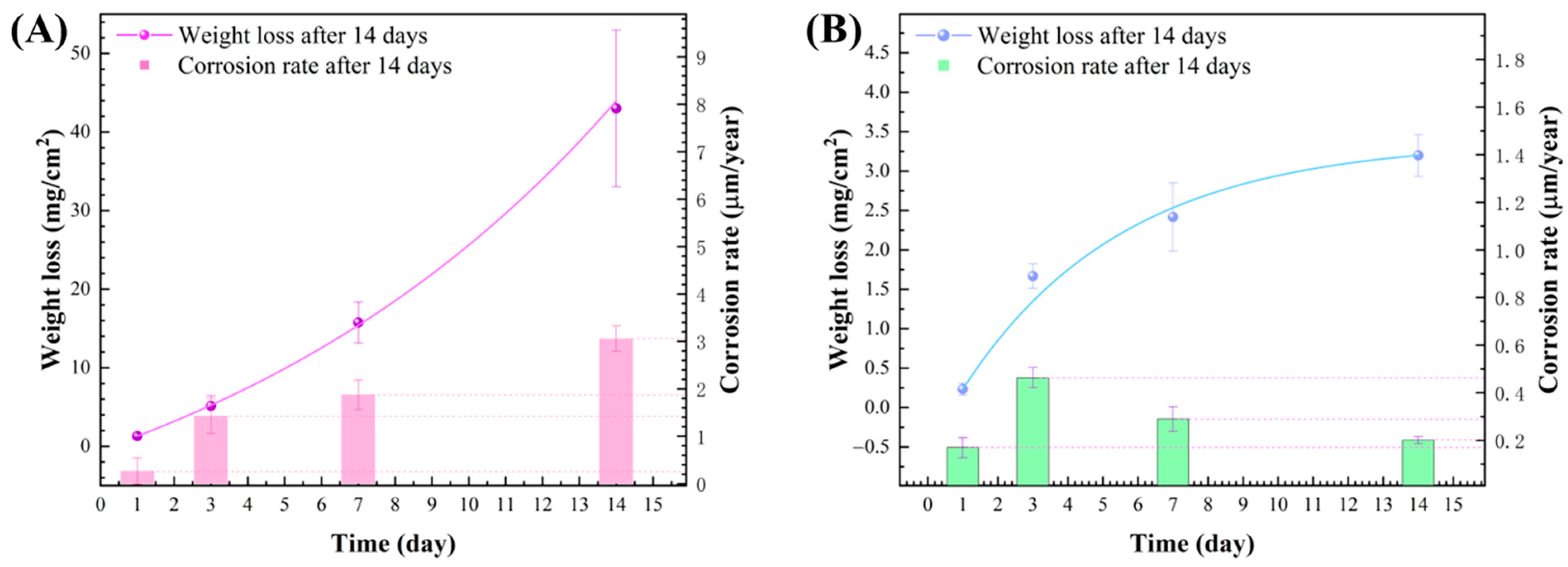
3.5. Weight Loss
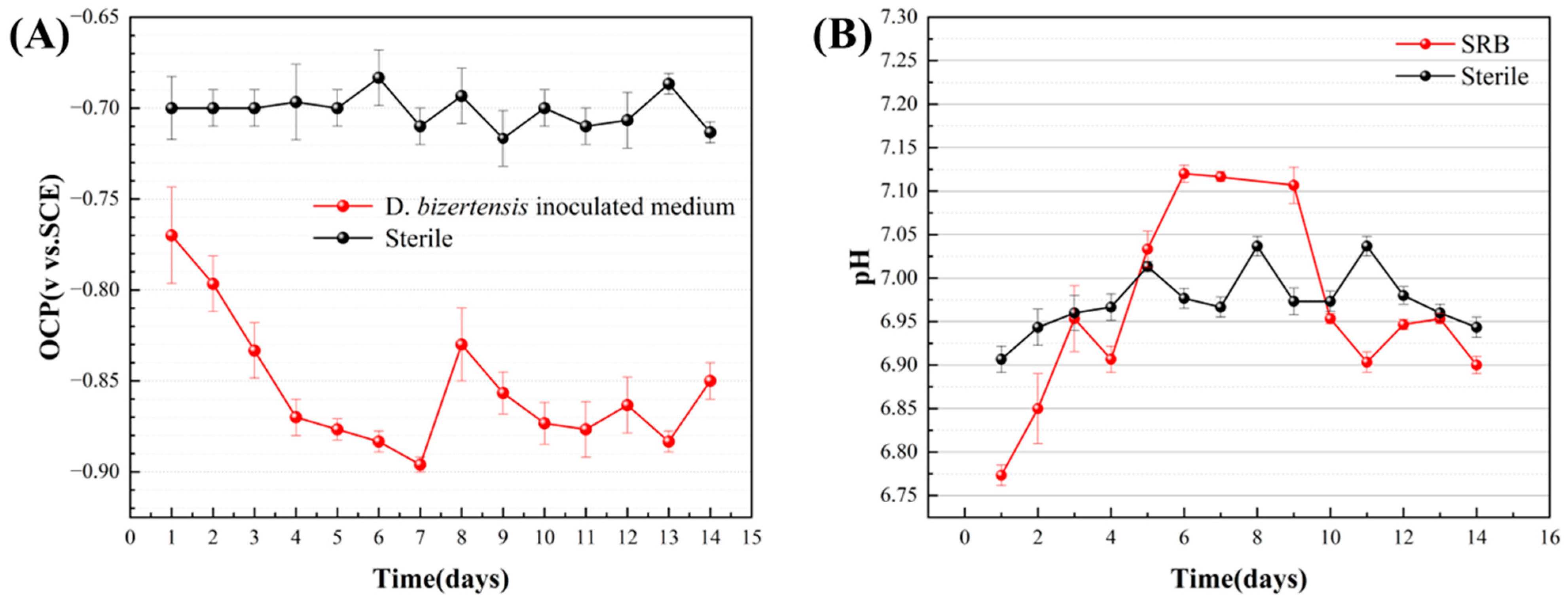
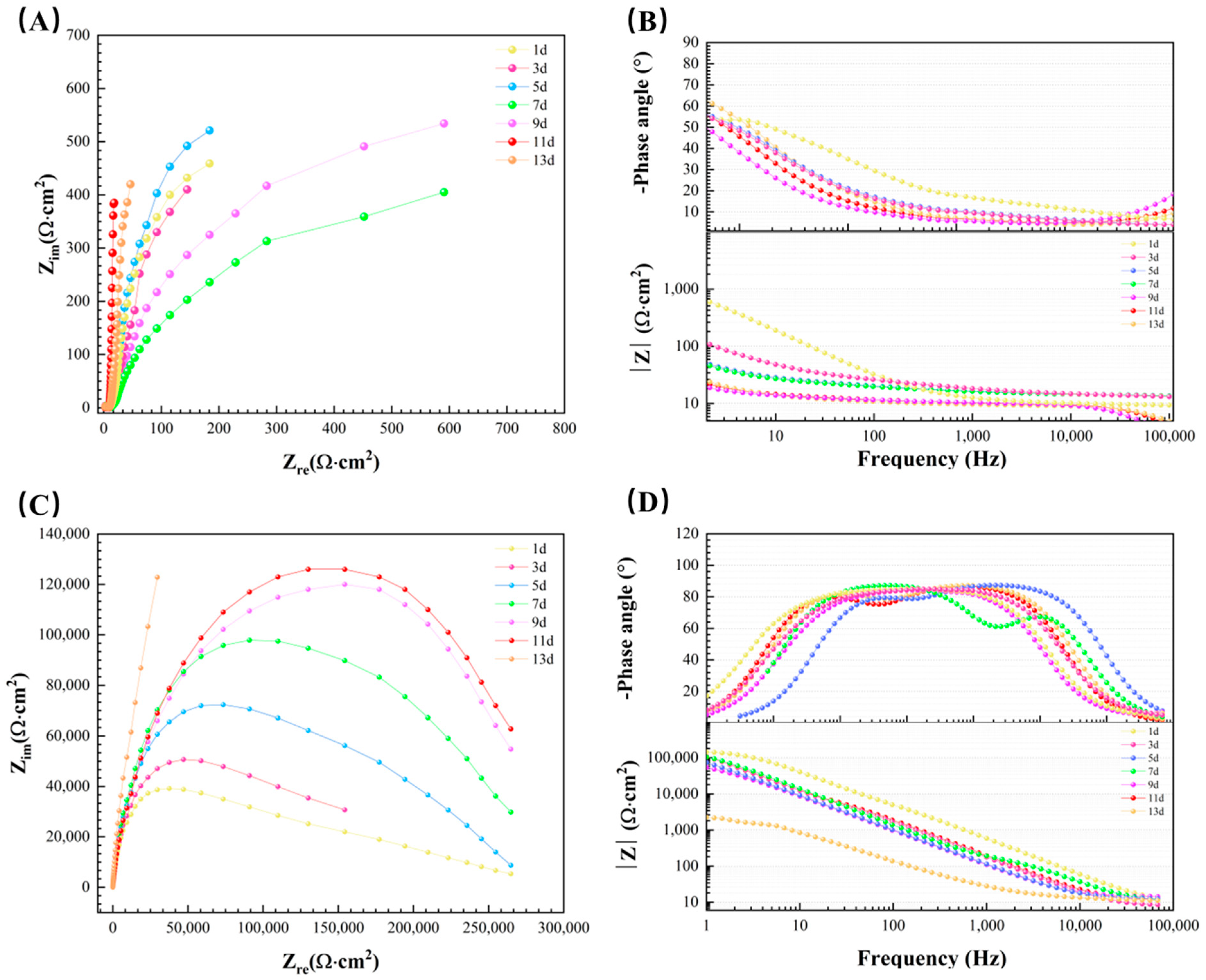
3.6. Electrochemical Measurements
4. Discussion
4.1. Determine the Environmental Factors That Accelerated MIC Most in Aircraft Fuel Systems
4.2. Determination of the Time Node That SRB-Induced Corrosion Is Accelerated Obviously
4.3. Corrosion Mechanism Analysis of Aluminum Alloy in Fuel–Water Mixed Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The Cost of Corrosion in China. Npj Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Li, L.; Chakik, M.; Prakash, R. A Review of Corrosion in Aircraft Structures and Graphene-Based Sensors for Advanced Corrosion Monitoring. Sensors 2021, 21, 2908. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, T.; Zhang, G.; Cheng, Y.; Wang, H.; Liu, H. The Effect of Magneticfield on Biomineralization and Corrosion Behavior of Carbon Steel Induced by Iron-Oxidizing Bacteria. Corros. Sci. 2016, 102, 93–102. [Google Scholar] [CrossRef]
- Forsyth, R.A. Microbial Induced Corrosion in Ports and Harbors Worldwide. In Proceedings of the Ports 2010, Jacksonville, FL, USA, 22 April 2010; American Society of Civil Engineers: Reston, VA, USA, 2010; pp. 932–939. [Google Scholar] [CrossRef]
- Rhee, I. Microbiogical Contamination in JP-8 Fuel; SAE Technical Paper 2005-01-1802; SAE Publications: Warrendale, PA, USA, 2005. [Google Scholar] [CrossRef]
- Hill, E.C.; Hill, G.C. Microbial Contamination and Associated Corrosion in Fuels, during Storage, Distribution and Use. Adv. Mater. Res. 2008, 38, 257–268. [Google Scholar] [CrossRef]
- Sun, Y. Analysis of microbial contamination in aviation fuel. Shandong Chem. Ind. 2015, 9, 121–123. [Google Scholar] [CrossRef]
- Wei, X. Diagnosis and treatment of typical faults of aircraft wing integral fuel tank. New Technol. New Process 2019, 06, 9–12. [Google Scholar] [CrossRef]
- Kong, L. Metagenomic Analysis of Petroleum Biodegradation Coupled to Specific N-Cycling Process in Oil-Contaminated Soil. Appl. Soil Ecol. 2024, 193, 105144. [Google Scholar] [CrossRef]
- Kang, M.-J. Potential Natural Attenuation of Petroleum Hydrocarbons in Fuel Contaminated Soils: Focusing on Anaerobic Fuel Biodegradation Involving Microbial Fe(III) Reduction. Chemosphere 2023, 341, 140134. [Google Scholar] [CrossRef]
- Tang, F. New Insights of Crude Oil Biodegradation Construction by Microbial Consortium B10: Responded Substrates, Genomics, Biodegradation Mechanism and Pathways. Chem. Eng. J. 2023, 478, 147143. [Google Scholar] [CrossRef]
- Raikos, V.; Vamvakas, S.S.; Sevastos, D.; Kapolos, J.; Karaiskakis, G.; Koliadima, A. Water Content, Temperature and Biocide Effects on the Growth Kinetics of Bacteria Isolated from JP-8 Aviation Fuel Storage Tanks. Fuel 2012, 93, 559–566. [Google Scholar] [CrossRef]
- Gaylarde, C.C. Microbial contamination of stored hydrocarbon fuels and its control. Rev. Microbiol. 1999, 30, 01–10. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Yuan, W.; Du, Y. Biocorrosion Characteristics of the Copper Alloys BFe30-1-1 and HSn70-1AB by SRB Using Atomic Force Microscopy and Scanning Electron Microscopy. Int. Biodeterior. Biodegrad. 2010, 64, 363–370. [Google Scholar] [CrossRef]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically Influenced Corrosion and Current Mitigation Strategies: A State of the Art Review. Int. Biodeterior. Biodegrad. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Yin, X. Research status of microbial corrosion in fuel tank of civil aviation aircraft. Corros. Prot. 2019, 40, 366–369. [Google Scholar] [CrossRef]
- Xu, D. Stainless steel corrosion via direct iron-to-microbe electron transfer by Geobacter species. In Proceedings of the 1st Corrosion and Materials Degradation Web Conference, Virtual, 17–19 May 2021; MDPI: Basel, Switzerland, 2021. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Holmes, D.E.; Ueki, T.; Palacios, P.A.; Lovley, D.R. Iron Corrosion via Direct Metal-Microbe Electron Transfer. ASM J. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y. Microbial Corrosion Law of 7075 Aluminum Alloy Used for Engine and Oil Tank in Accumulated Water Environment. Mater. Prot. 2014, 47, 29–32. [Google Scholar] [CrossRef]
- Jia, L. Microbial Corrosion Behavior of 7075-T6 Magnesium-Aluminum Alloy. Total Corros. Control 2013, 27, 56–60. [Google Scholar]
- Passman, F.J. Microbial contamination and its control in fuels and fuel systems since 1980 a review. Int. Biodeterior. Biodegrad. 2013, 81, 88–104. [Google Scholar] [CrossRef]
- Chung, Y. Temperature and water effects on the biodeterioration for marine fuel oil. Fuel 2000, 79, 1525–1532. [Google Scholar] [CrossRef]
- Abd El Rehim, S.S.; Hassan, H.H.; Amin, M.A. Corrosion Inhibition Study of Pure Al and Some of Its Alloys in 1.0 M HCl Solution by Impedance Technique. Corros. Sci. 2004, 46, 5–25. [Google Scholar] [CrossRef]
- Liu, B. Research status and prospect of aluminum alloys for large aircraft. Chin. J. Nonferrous Met. 2010, 20, 1705–1715. [Google Scholar] [CrossRef]
- Zhou, Z.; Ge, X.; Fan, W.; Shan, B.; Yang, J. Research on accelerating the corrosion of 7B04 aluminum alloy in the Lacticaseibacillus paracasei environment. J. Appl. Electrochem. 2024, 54, 381–392. [Google Scholar] [CrossRef]
- Cordas, C.M.; Guerra, L.T.; Xavier, C.; Moura, J.J.G. Electroactive Biofilms of Sulphate Reducing Bacteria. Electrochim. Acta 2008, 54, 29–34. [Google Scholar] [CrossRef]
- Tuck, B.; Watkin, E.; Somers, A.; Machuca, L.L. A Critical Review of Marine Biofilms on Metallic Materials. Npj Mater. Degrad. 2022, 6, 25. [Google Scholar] [CrossRef]
- Marks, C.R.; Duncan, K.E.; Nanny, M.A.; Harriman, B.H.; Avci, R.; Oldham, A.L.; Suflita, J.M. An Integrated Metagenomic and Metabolite Profiling Study of Hydrocarbon Biodegradation and Corrosion in Navy Ships. Npj Mater. Degrad. 2021, 5, 60. [Google Scholar] [CrossRef]
- Stadler, R.; Fuerbeth, W.; Harneit, K.; Grooters, M.; Woellbrink, M.; Sand, W. First Evaluation of the Applicability of Microbial Extracellular Polymeric Substances for Corrosion Protection of Metal Substrates. Electrochim. Acta 2008, 54, 91–99. [Google Scholar] [CrossRef]
- Little, B.J.; Perez-Ibarra, B.M.; Aktas, D.F.; Lee, J.S.; Duncan, K.E. Effects of oxygen on biodegradation of fuels in a corroding environment. Int. Biodeterior. Biodegrad. 2013, 81, 114–126. [Google Scholar] [CrossRef]
- Duan, J. Corrosion of carbon steel influenced by anaerobic biofilm in natural seawater. Electrochim. Acta 2008, 54, 22–28. [Google Scholar] [CrossRef]
- Dong, X.; Zhai, X.; Zhang, Y.; Yang, J.; Guan, F.; Duan, J.; Sun, J.; Zhang, R.; Hou, B. Steel rust layers immersed in the South China Sea with a highly corrosive Desulfovibrio strain. Npj Mater. Degrad. 2022, 6, 91. [Google Scholar] [CrossRef]
- Pu, Y. Biogenic H2S and extracellular electron transfer resulted in two-coexisting mechanisms in 90/10 Cu-Ni alloy corrosion by a sulfate-reducing bacteria. Corros. Sci. 2023, 211, 110911. [Google Scholar] [CrossRef]
- GB/T 16545-2015; Corrosion of Metals and Alloys-Removal of Corrosion Products from Corrosion Test Specimens. Chinese National: Beijing, China, 2015.
- Babič, M.N. Microorganisms Populating the Water-Related Indoor Biome. Appl. Microbiol. Biotechnol. 2020, 104, 6443–6462. [Google Scholar] [CrossRef] [PubMed]
- Igo, M.J. Quantifying the Influence of Relative Humidity, Temperature, and Diluent on the Survival and Growth of Enterobacter Aerogenes. J. Food Prot. 2019, 82, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A. Crude Oil Biodegradation Potential of Lipase Produced by Bacillus subtilis and Pseudomonas aeruginosa Isolated from Hydrocarbon Contaminated Soil. Environ. Chem. Ecotoxicol. 2024, 6, 26–32. [Google Scholar] [CrossRef]
- Puntus, I.F. Contribution of Soil Bacteria Isolated from Different Regions into Crude Oil and Oil Product Degradation. J. Soils Sediments 2019, 19, 3166–3177. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Y.Y.; Zhu, Y.; Fei, C.; Shen, Z.; Ying, Y. Impact of Biodegradation on Polar Compounds in Crude Oil: Comparative Simulation of Biodegradation from Two Aerobic Bacteria Using Ultrahigh-Resolution Mass Spectrometry. Energy Fuels 2020, 34, 5553–5565. [Google Scholar] [CrossRef]
- Guan, F. Interaction between Sulfate-Reducing Bacteria and Aluminum Alloys Corrosion Mechanisms of 5052 and Al-Zn-In-Cd Aluminum Alloys. J. Mater. Sci. 2020, 36, 55–64. [Google Scholar] [CrossRef]
- Chen, Z. Influence of Nutrition on Cu Corrosion by Desulfovibrio vulgaris in Anaerobic Environment. Bioelectrochemistry 2022, 144, 108040. [Google Scholar] [CrossRef]
- Dou, W. Corrosion of Cu by a Sulfate Reducing Bacterium in Anaerobic Vials with Different Headspace Volumes. Bioelectrochemistry 2020, 133, 107478. [Google Scholar] [CrossRef]
- Pu, Y. Enhancement of Exogenous Riboflavin on Microbiologically Influenced Corrosion of Nickel by Electroactive Desulfovibrio vulgaris Biofilm. Npj Mater. Degrad. 2023, 7, 7. [Google Scholar] [CrossRef]
- Xu, D. Laboratory Investigation of Microbiologically Influenced Corrosion of C1018 Carbon Steel by Nitrate Reducing Bacterium Bacillus licheniformis. Corros. Sci. 2013, 77, 385–390. [Google Scholar] [CrossRef]
- Örnek, D. Pitting corrosion control using regenerative biofilms on aluminum 2024 in artificial seawater. Corros. Sci. 2001, 43, 2121–2133. [Google Scholar] [CrossRef]



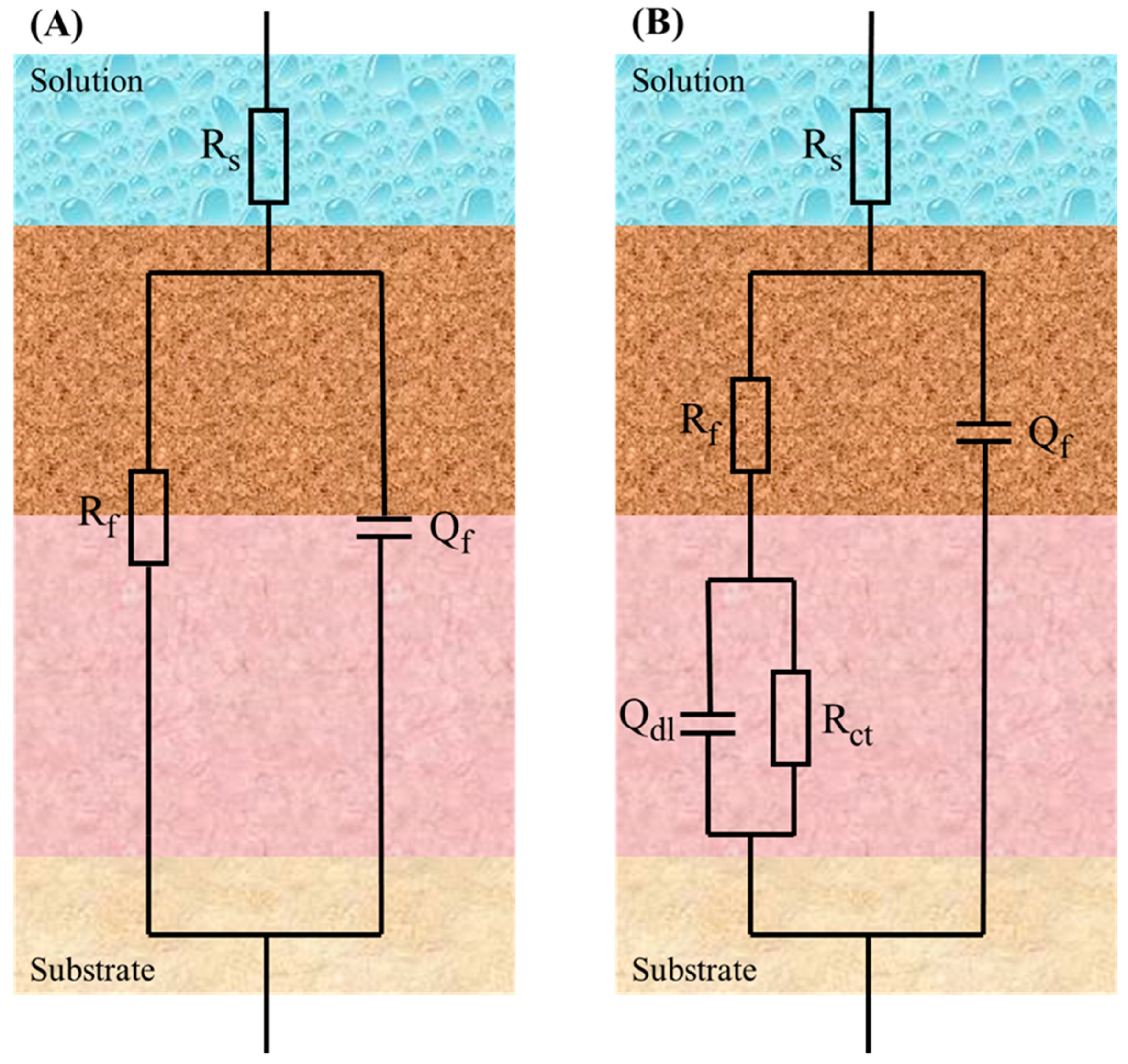
| Group | Water Content | Oxygen Content | Temperature (°C) |
|---|---|---|---|
| 1 | 20% | 10% | 15 |
| 2 | 20% | 20% | 35 |
| 3 | 20% | 0% | 25 |
| 4 | 50% | 20% | 15 |
| 5 | 50% | 10% | 25 |
| 6 | 50% | 0% | 35 |
| 7 | 90% | 10% | 35 |
| 8 | 90% | 0% | 15 |
| 9 | 90% | 20% | 25 |
| Time | RS (Ω cm2) | Qf × 10−5 (Ω−1 cm2 Sn) | Rf (Ω cm2) | Qdl × 10−5 (Ω−1 cm2 Sn) | Rct (KΩ cm2) |
|---|---|---|---|---|---|
| 1 | 12.51 ± 0.89 | 3.89 ± 0.69 | 73.21 ± 16.08 | 5.84 ± 1.20 | 14.98 ± 2.38 |
| 3 | 10.77 ± 0.61 | 1.49 ± 2.52 | 33.62 ± 5.58 | 6.06 ± 1.42 | 19.66 ± 3.52 |
| 5 | 12.72 ± 0.82 | 1.86 ± 1.60 | 50.66 ± 5.10 | 3.72 ± 2.18 | 26.82 ± 1.51 |
| 7 | 11.32 ± 1.49 | 2.99 ± 0.95 | 59.85 ± 2.91 | 4.27 ± 0.38 | 16.92 ± 2.82 |
| 9 | 16.92 ± 0.41 | 5.58 ± 3.86 | 57.27 ± 4.10 | 4.92 ± 1.52 | 15.87 ± 4.14 |
| 11 | 18.44 ± 2.02 | 2.67 ± 1.40 | 22.69 ± 6.10 | 4.74 ± 1.14 | 23.12 ± 1.58 |
| 13 | 11.08 ± 0.18 | 4.09 ± 0.21 | 26.12 ± 2.92 | 9.20 ± 1.04 | 16.69 ± 1.73 |
| Time | RS (Ω cm2) | Qf × 10−5 (Ω−1 cm2 Sn) | Rf (Ω cm2) | Qdl × 10−5 (Ω−1 cm2 Sn) | Rct (KΩ cm2) |
|---|---|---|---|---|---|
| 1 | 8.61 ± 0.25 | 22.39 ± 3.23 | 21.38 ± 2.78 | 3.03 ± 0.41 | 22.37 ± 1.48 |
| 3 | 8.75 ± 0.54 | 41.65 ± 3.11 | 7.62 ± 6.67 | 18.91 ± 1.23 | 10.93 ± 0.88 |
| 5 | 10.11 ± 1.33 | 54.13 ± 3.43 | 4.05 ± 6.81 | 14.94 ± 1.74 | 8.35 ± 1.19 |
| 7 | 11.94 ± 1.93 | 19.48 ± 2.94 | 25.02 ± 4.24 | 11.62 ± 1.43 | 3.74 ± 0.31 |
| 9 | 9.63 ± 0.97 | 53.90 ± 2.45 | 31.24 ± 3.4 | 10.34 ± 11.88 | 3.58 ± 0.43 |
| 11 | 14.61 ± 0.19 | 69.61 ± 0.68 | 44.93 ± 8.71 | 32.47 ± 1.73 | 5.93 ± 0.47 |
| 13 | 19.10 ± 3.97 | 63.70 ± 6.96 | 30.89 ± 3.56 | 28.42 ± 4.56 | 8.16 ± 1.04 |
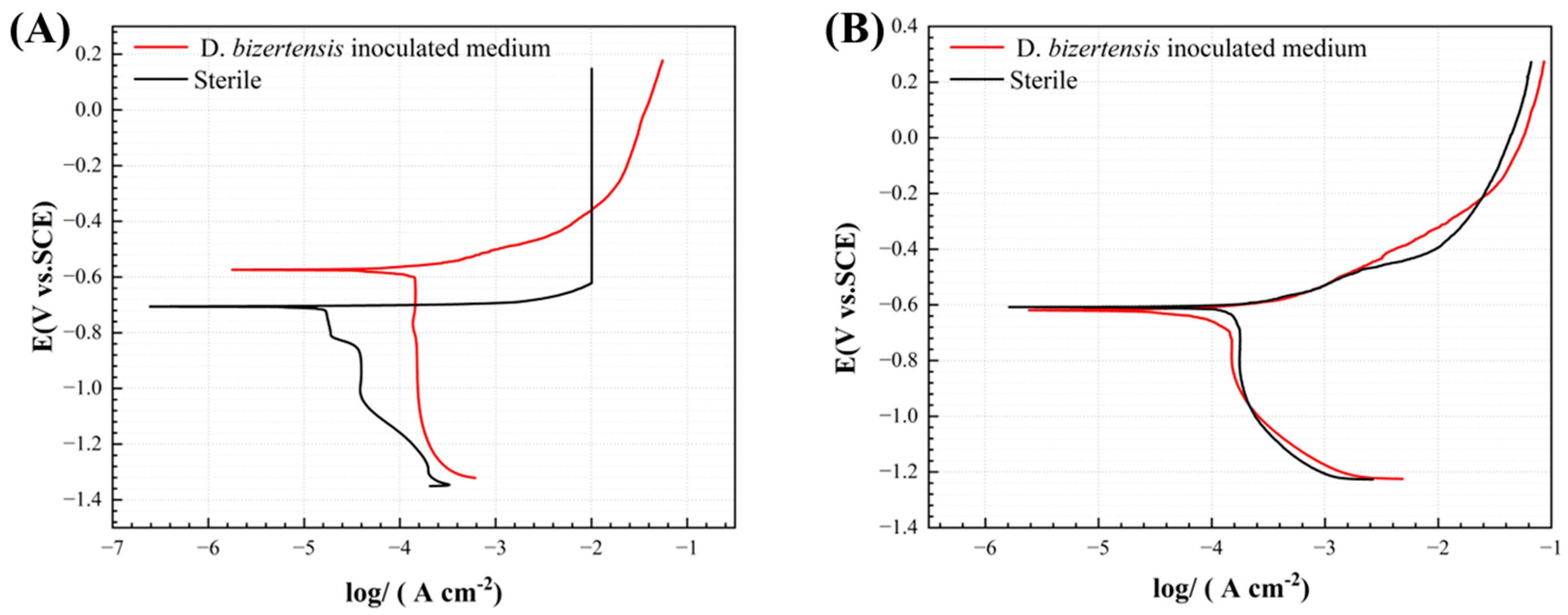
| System | Time | Ecorr/V | Icorr × 10−5/A/cm2 | Epit/V | ∆Ep/V |
|---|---|---|---|---|---|
| Sterile | 3 | −0.6671 ± 0.04 | 1.51 ± 0.43 | −0.5572 ± 0.12 | 0.1099 ± 0.07 |
| 14 | −0.5922 ± 0.01 | 4.21 ± 0.96 | −0.3981 ± 0.01 | 0.2150 ± 0.03 | |
| SRB | 3 | −0.7658 ± 0.06 | 0.91 ± 0.19 | −0.4332 ± 0.08 | 0.4673 ± 0.01 |
| 14 | −0.6254 ± 0.01 | 6.42 ± 3.08 | −0.4724 ± 0.03 | 0.3485 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, B.; Zhang, Y.; Guo, D.; Li, Y.; Zhang, R.; Cui, N.; Duan, J. How Often Should Microbial Contamination Be Detected in Aircraft Fuel Systems? An Experimental Test of Aluminum Alloy Corrosion Induced by Sulfate-Reducing Bacteria. Materials 2024, 17, 3523. https://doi.org/10.3390/ma17143523
Lu B, Zhang Y, Guo D, Li Y, Zhang R, Cui N, Duan J. How Often Should Microbial Contamination Be Detected in Aircraft Fuel Systems? An Experimental Test of Aluminum Alloy Corrosion Induced by Sulfate-Reducing Bacteria. Materials. 2024; 17(14):3523. https://doi.org/10.3390/ma17143523
Chicago/Turabian StyleLu, Bochao, Yimeng Zhang, Ding Guo, Yan Li, Ruiyong Zhang, Ning Cui, and Jizhou Duan. 2024. "How Often Should Microbial Contamination Be Detected in Aircraft Fuel Systems? An Experimental Test of Aluminum Alloy Corrosion Induced by Sulfate-Reducing Bacteria" Materials 17, no. 14: 3523. https://doi.org/10.3390/ma17143523
APA StyleLu, B., Zhang, Y., Guo, D., Li, Y., Zhang, R., Cui, N., & Duan, J. (2024). How Often Should Microbial Contamination Be Detected in Aircraft Fuel Systems? An Experimental Test of Aluminum Alloy Corrosion Induced by Sulfate-Reducing Bacteria. Materials, 17(14), 3523. https://doi.org/10.3390/ma17143523








