Analyzing the Effects of Cr and Mo on the Pearlite Formation in Hypereutectoid Steel Using Experiments and Phase Field Numerical Simulations
Abstract
:1. Introduction
2. Experimental and Phase Field Simulation Methodologies
2.1. Sample Preparation
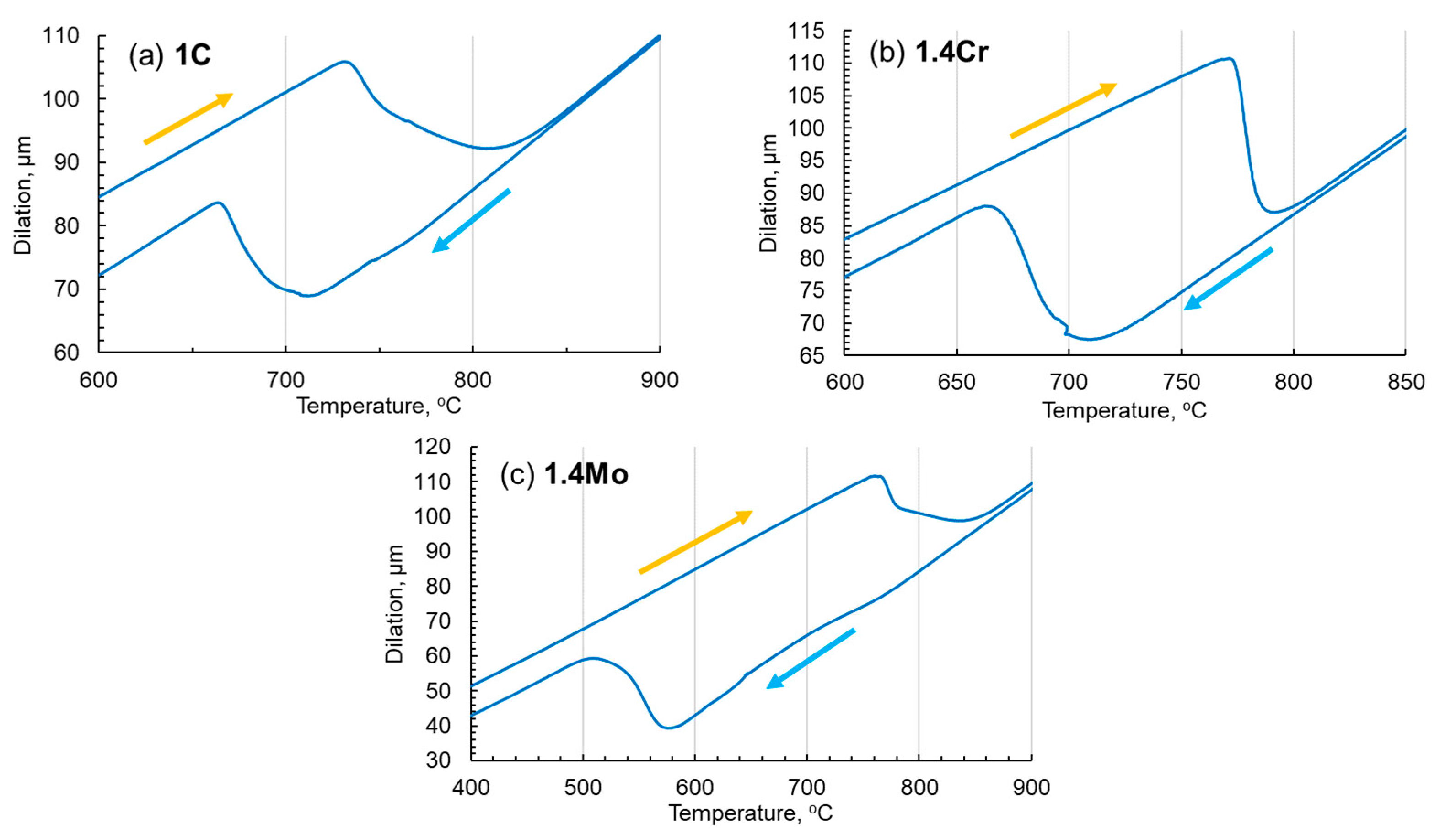
2.2. Analysis of Phase Transformation Behavior
2.3. Modeling and Assumptions
2.4. Identification and Calibration of PFM Simulation Parameters
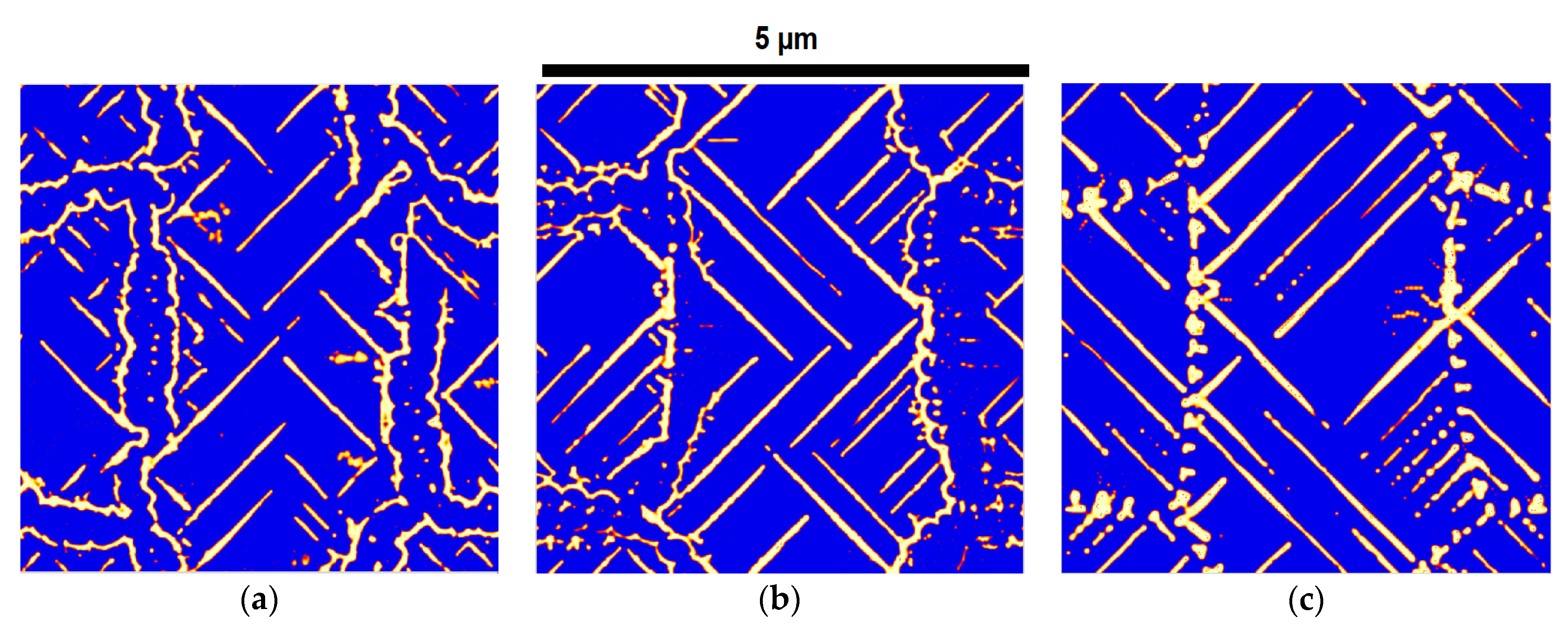
2.5. Microstructural Analysis and Feature Characterization
3. Results
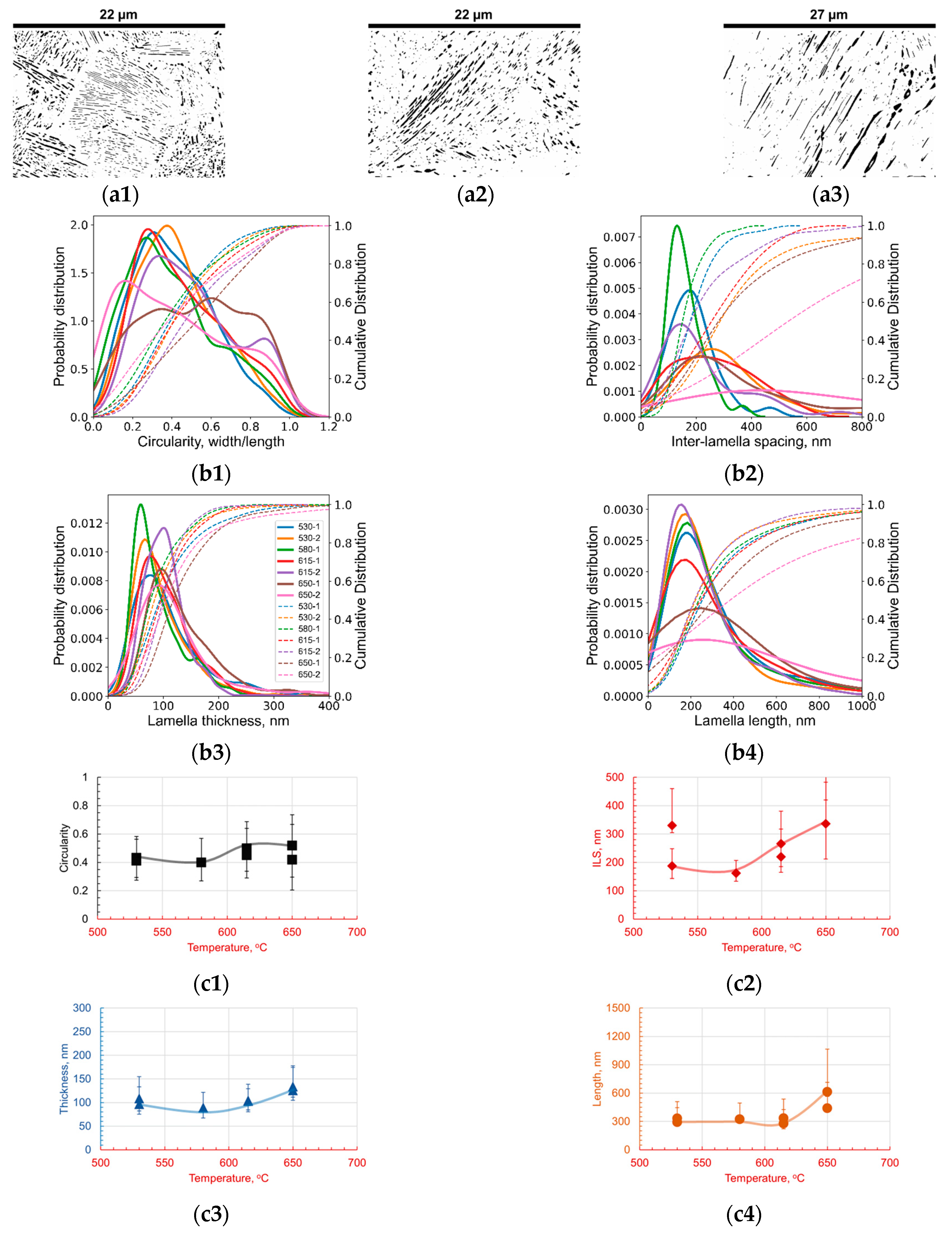
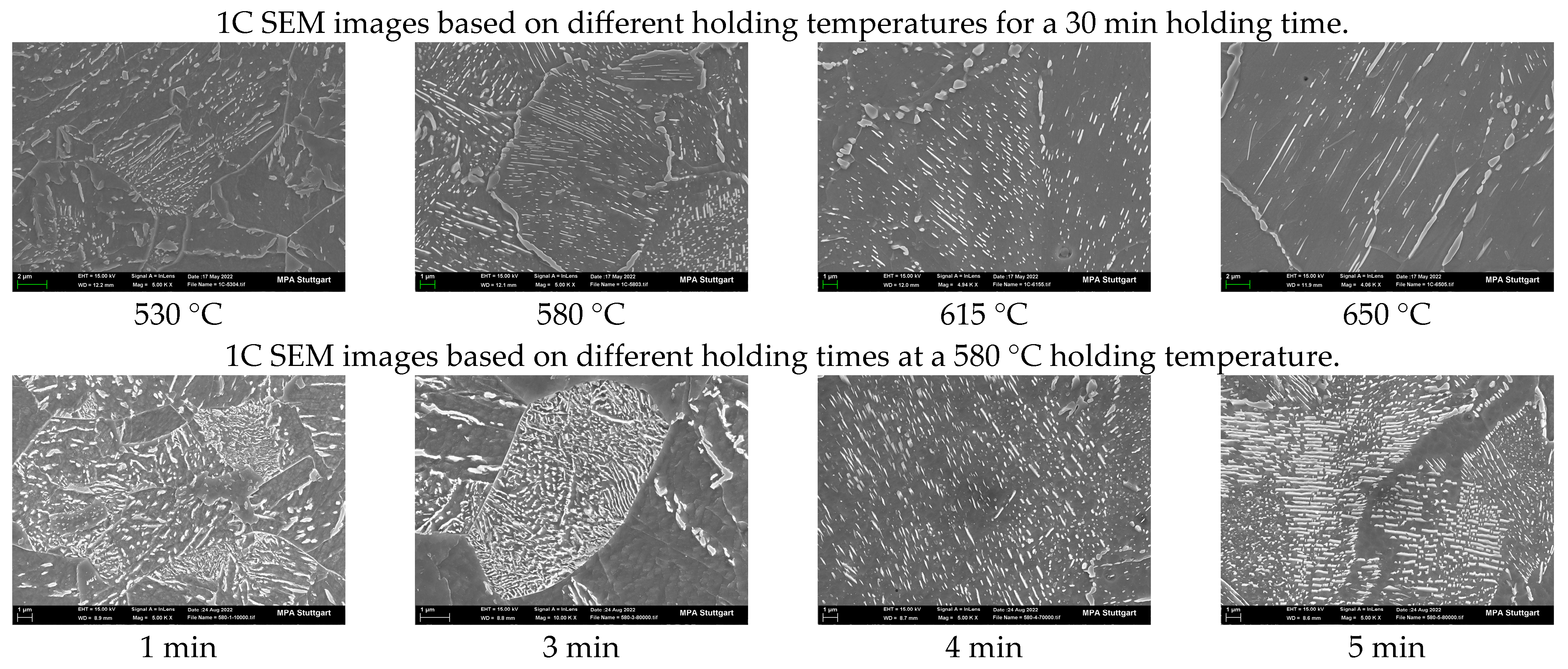
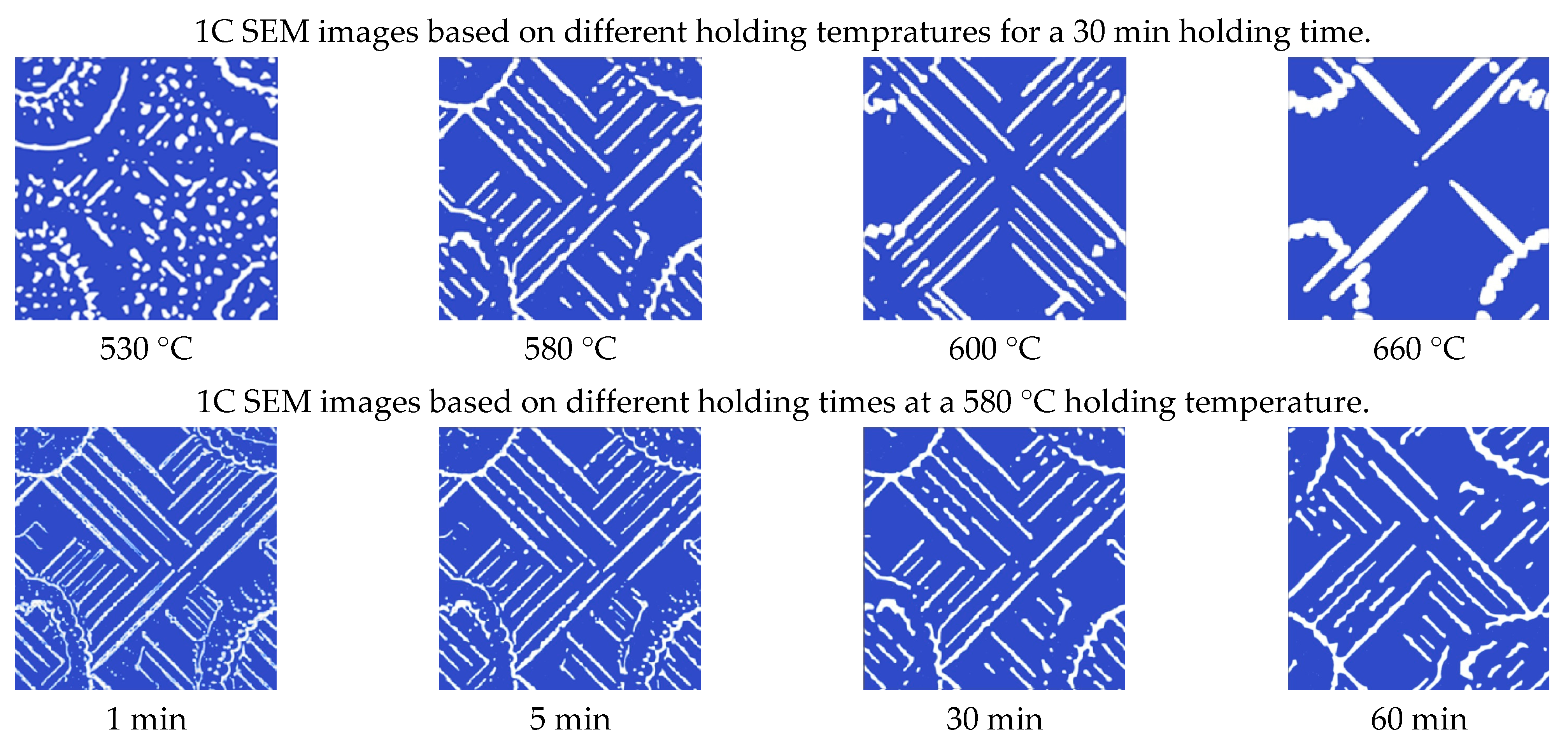
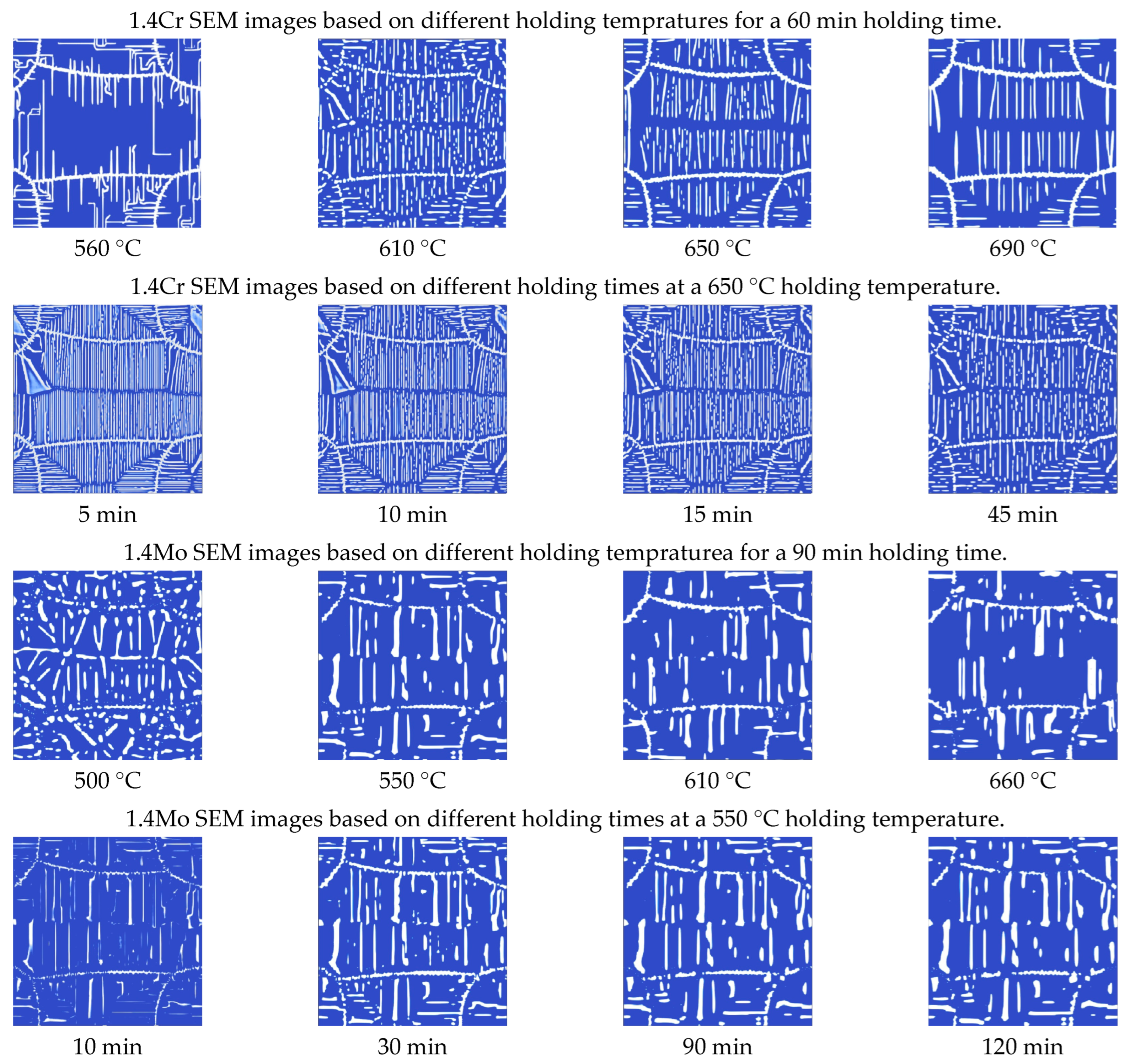
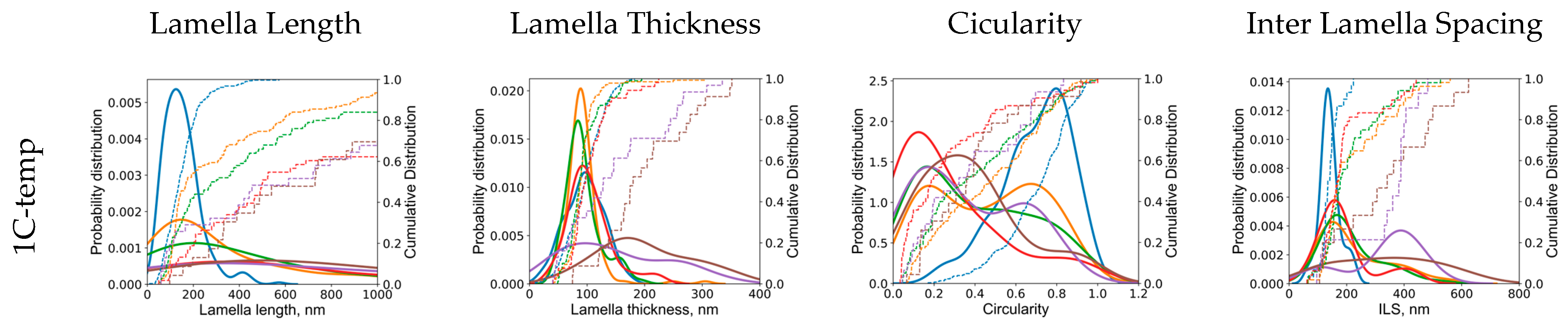
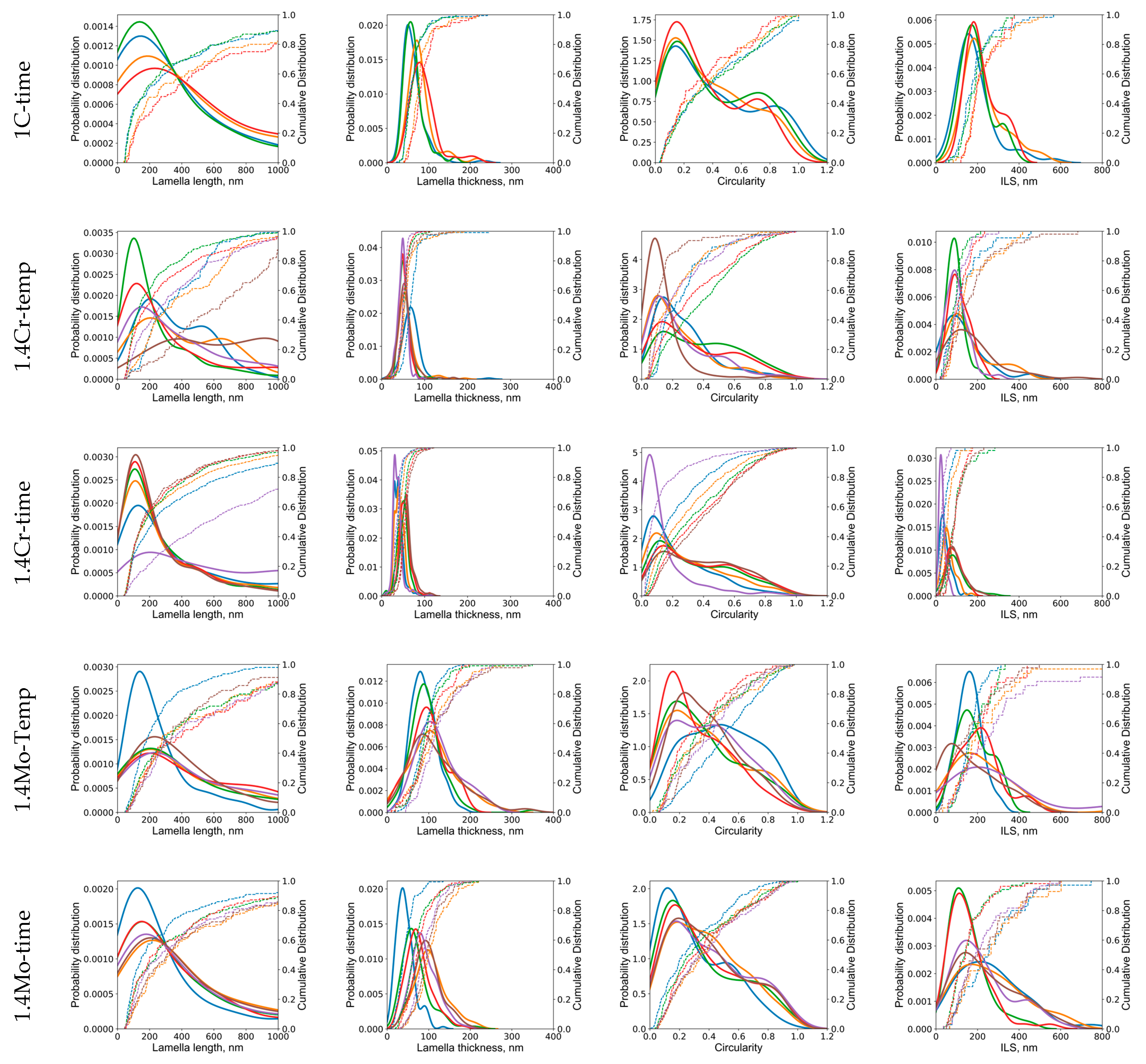
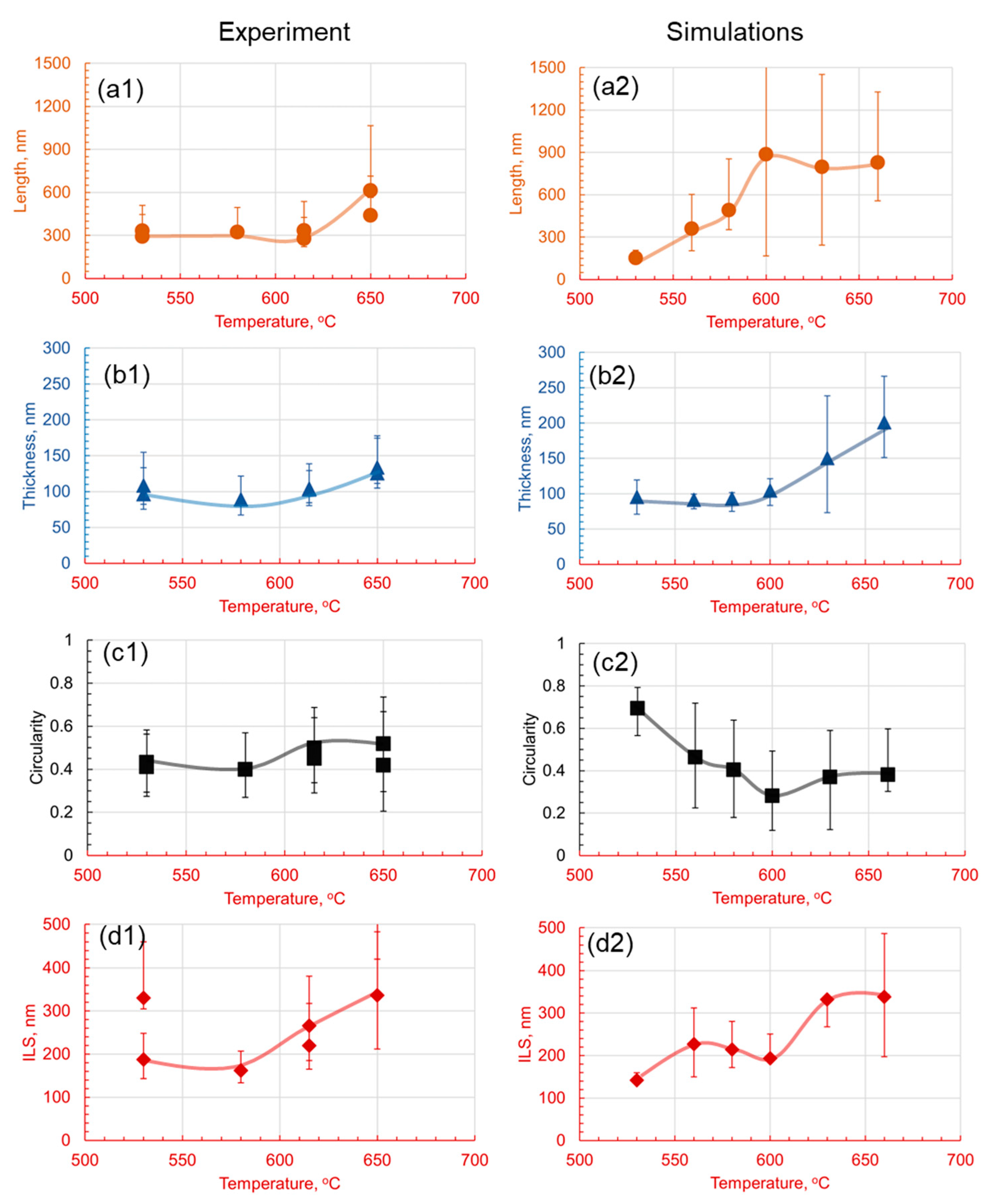
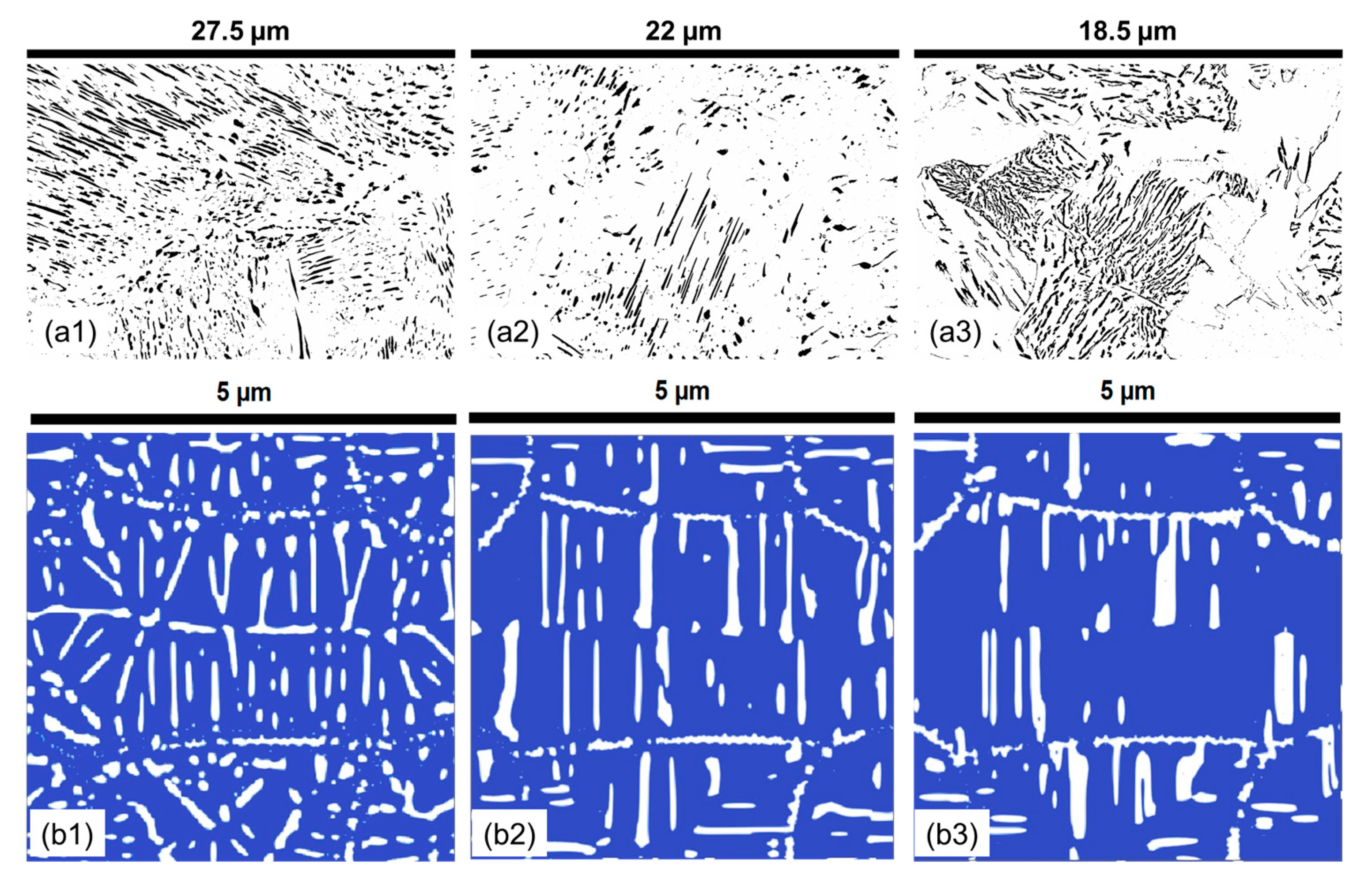
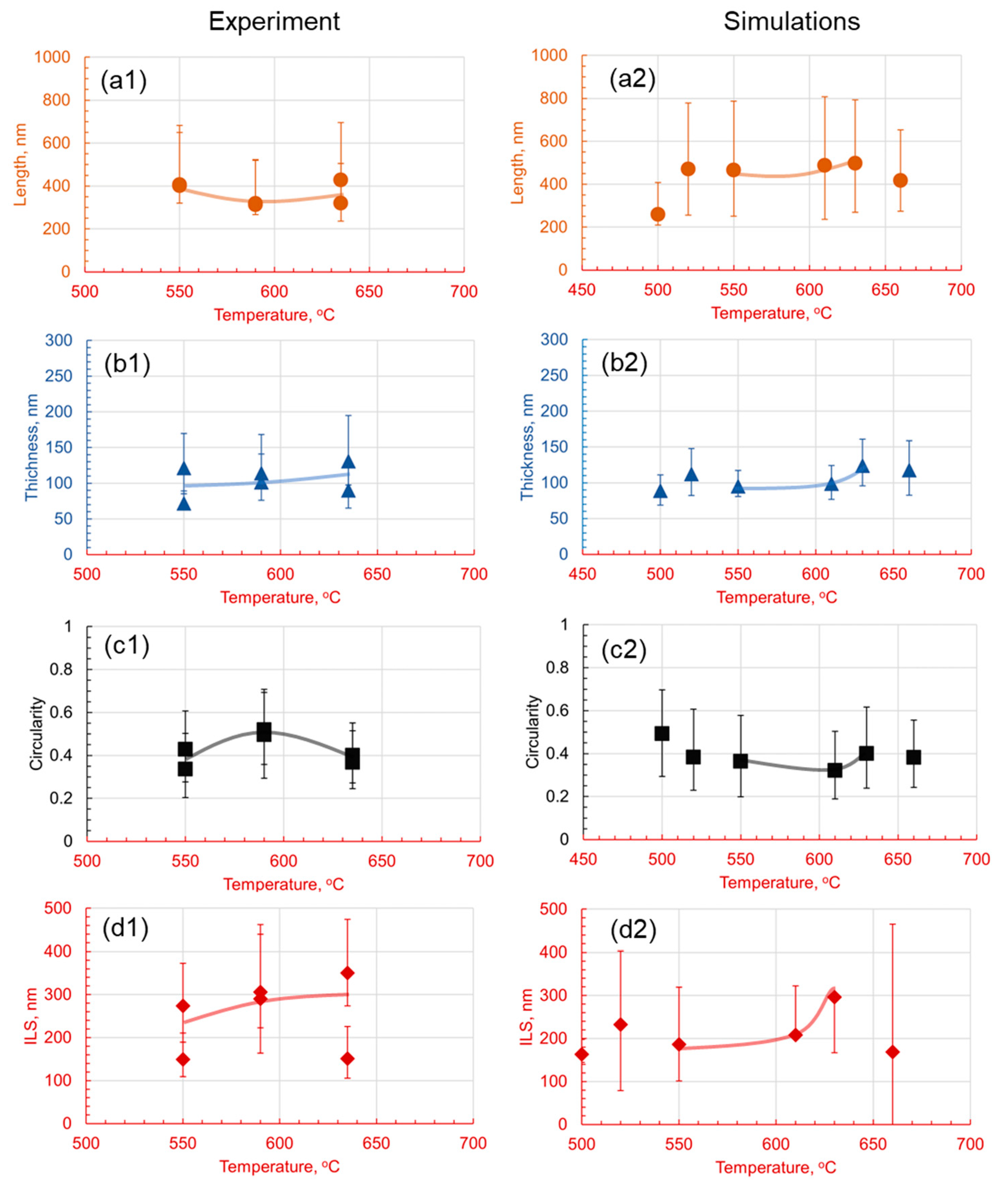
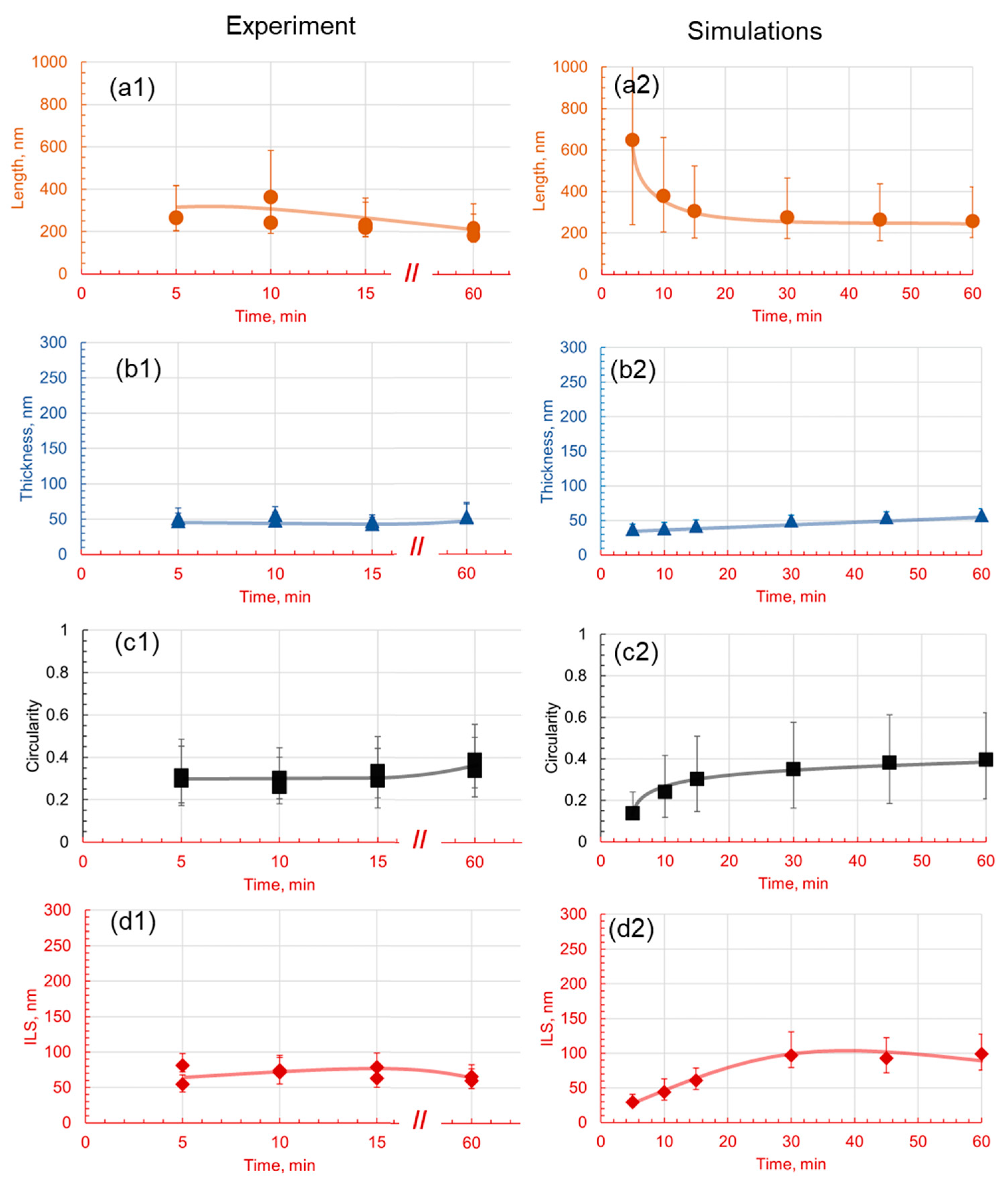
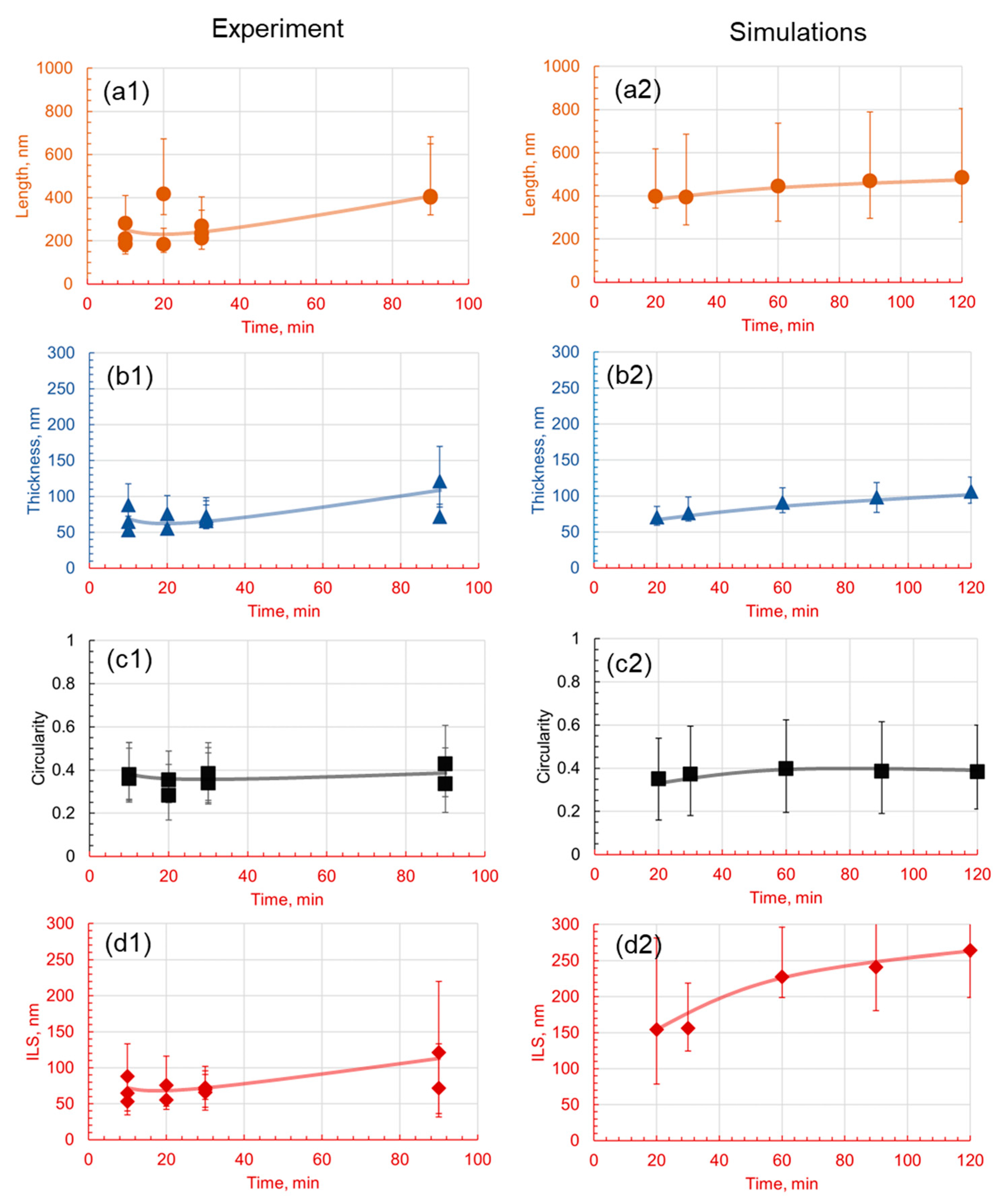
3.1. Effect of Holding Temperature on Pearlite Morphology
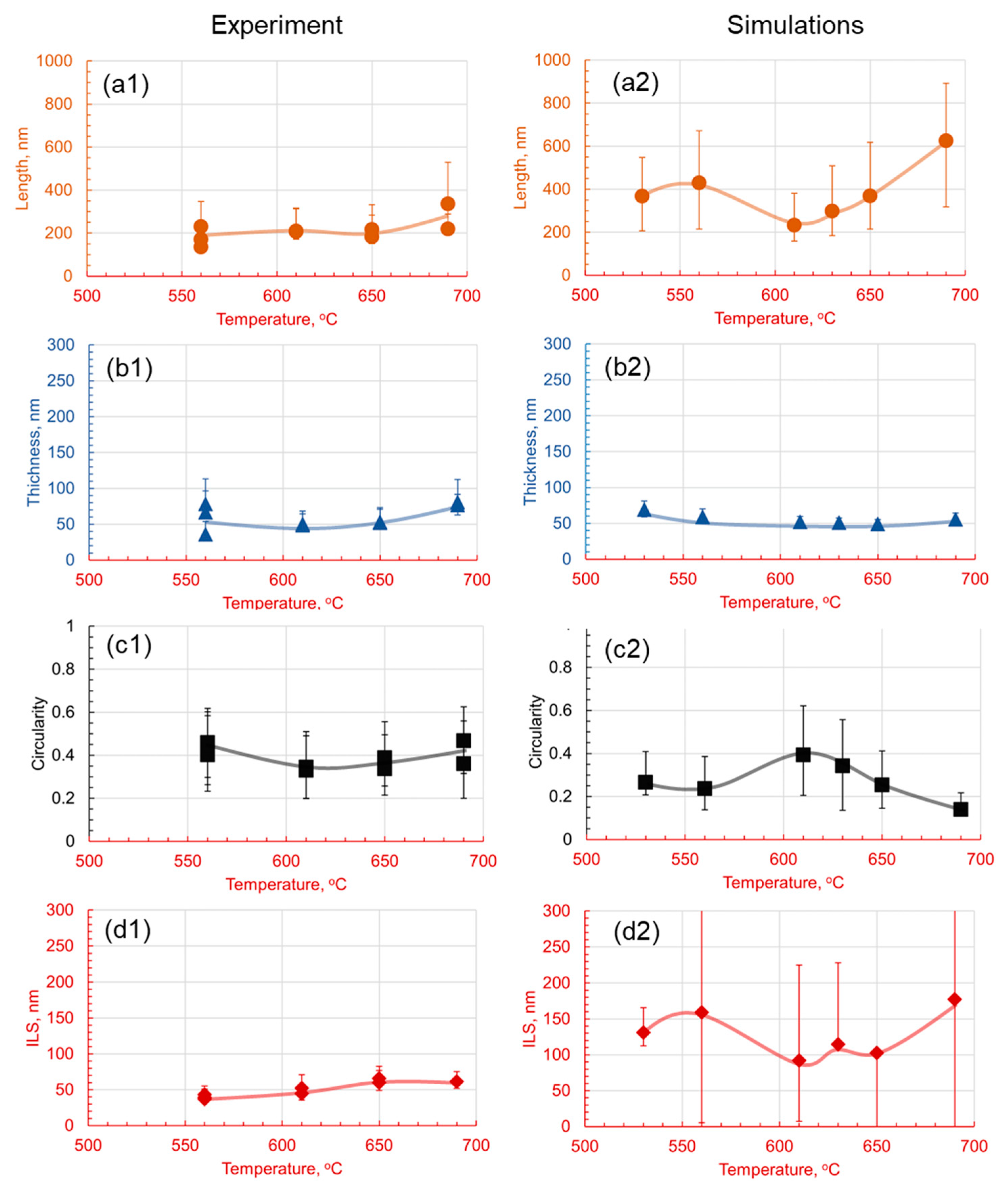
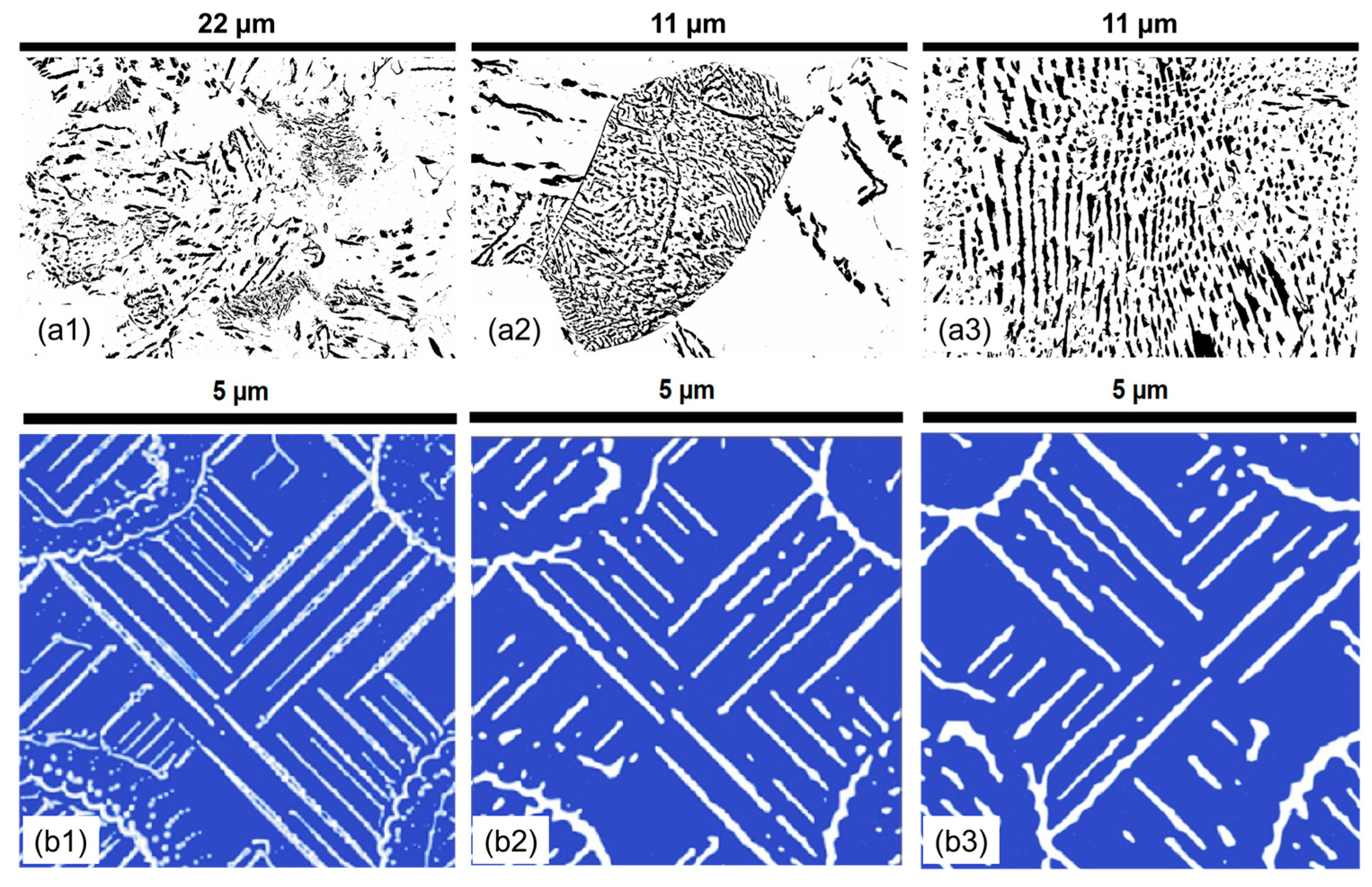
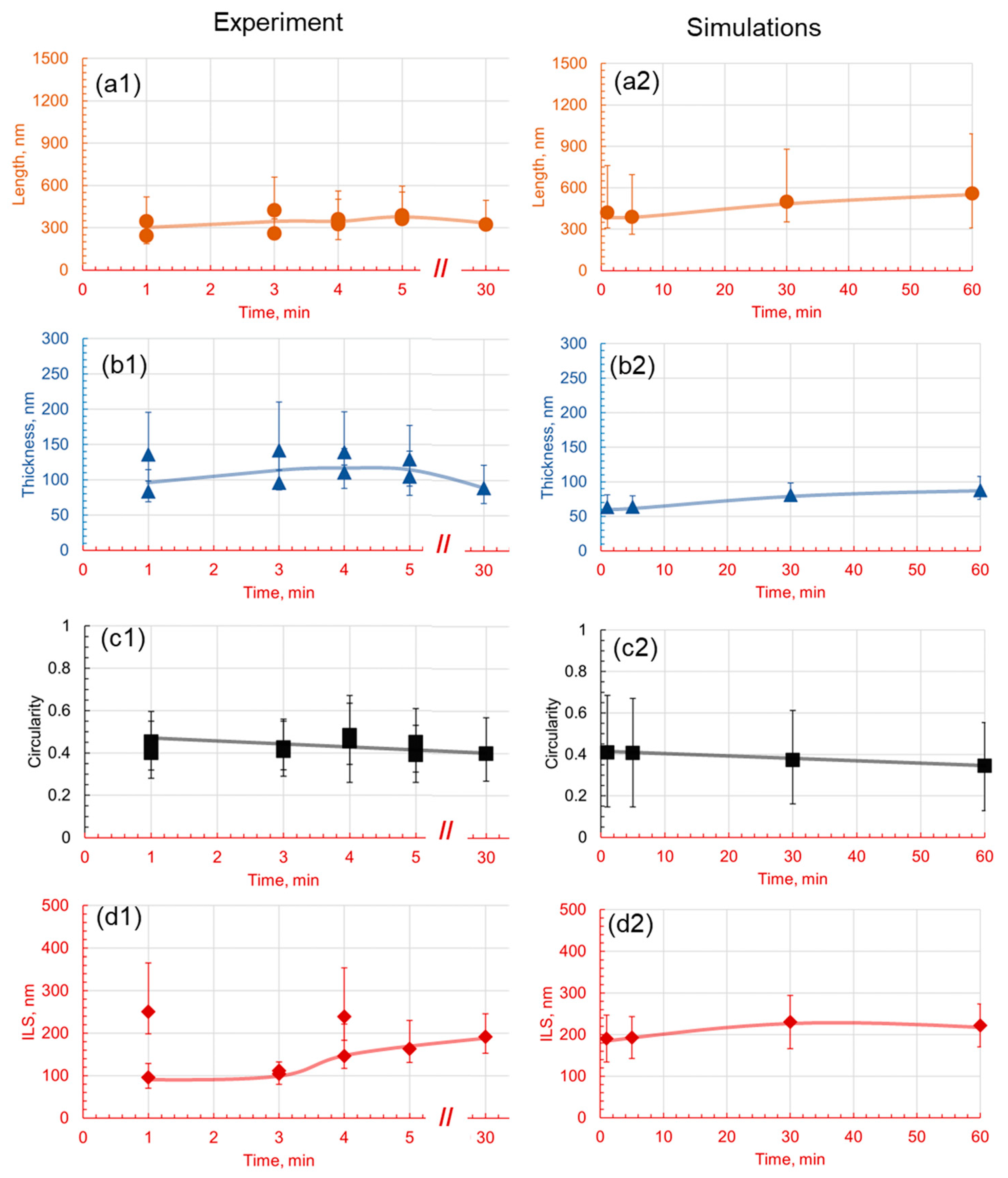
3.2. Effect of Holding Time on Pearlite Morphology
4. Discussion
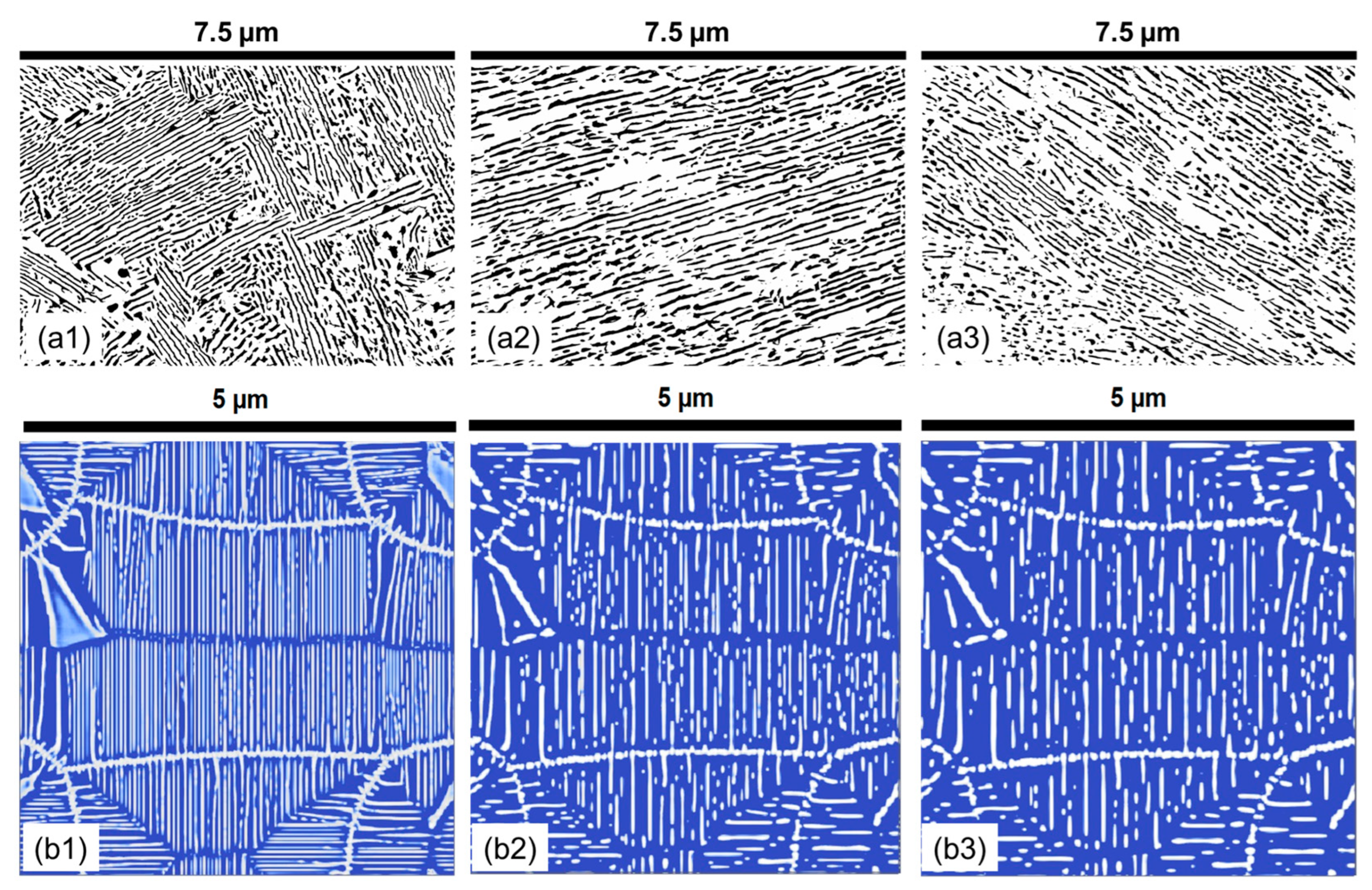
- The morphology of cementite lamellae is not uniform throughout a given steel sample. It exhibits variations in size, shape, and distribution. It can vary significantly depending on the location within the material. To address this, multiple pictures are typically taken and characterized. This approach helps to ensure that the collected data represent a range of morphological attributes present in different regions of the material.
- Pearlite formation is highly dependent on grain size, grain shape, and orientation. The size and orientation of pearlite colonies can vary on the grain structure of the steel. In many cases, only colonies perpendicular to the viewing direction are recorded and measured. This selective approach is used to maintain consistency in the data, but it can contribute to scatter, especially when colonies in different grain size areas are not considered.
- Cementite lamellae within pearlite colonies can have a wide distribution of characteristics. Some lamellae may be round, while others may be elongated. To maintain the representativeness of the data, it is common practice to measure all visible lamellae, regardless of their shape. This inclusivity can introduce scatter into the data sets due to the diversity of lamella shapes.
4.1. Influence of Alloying Elements on Cementite Morphology
4.2. Interplay between Thermodynamics and Kinetics
4.3. Comparative Analysis of Simulated and Experimental Data
5. Conclusions
- The introduction of 1.4 wt.% Cr and 1.4 wt.% Mo to the base Fe-1C alloy caused significant alterations in pearlite morphology within the hypereutectoid steel. Both Cr and Mo had a profound effect on the morphology of cementite lamellae, resulting in shorter, thinner, and rounder lamellae, with increased density.
- Cr, with its inherent ability to stabilize the pearlite formation process, promoted a more homogeneous microstructure. This was empirically evident through the observed decrease in the length of cementite lamellae in the 1.4Cr alloy, further reinforcing the role of Cr as a stabilizing agent.
- On the contrary, the addition of Mo, while reducing data variance, led to changes in pearlite morphology without necessarily enhancing it. This was established through a relatively non-homogeneous length of lamellae in the 1.4Mo alloy, revealing the influence of Mo on pearlite formation.
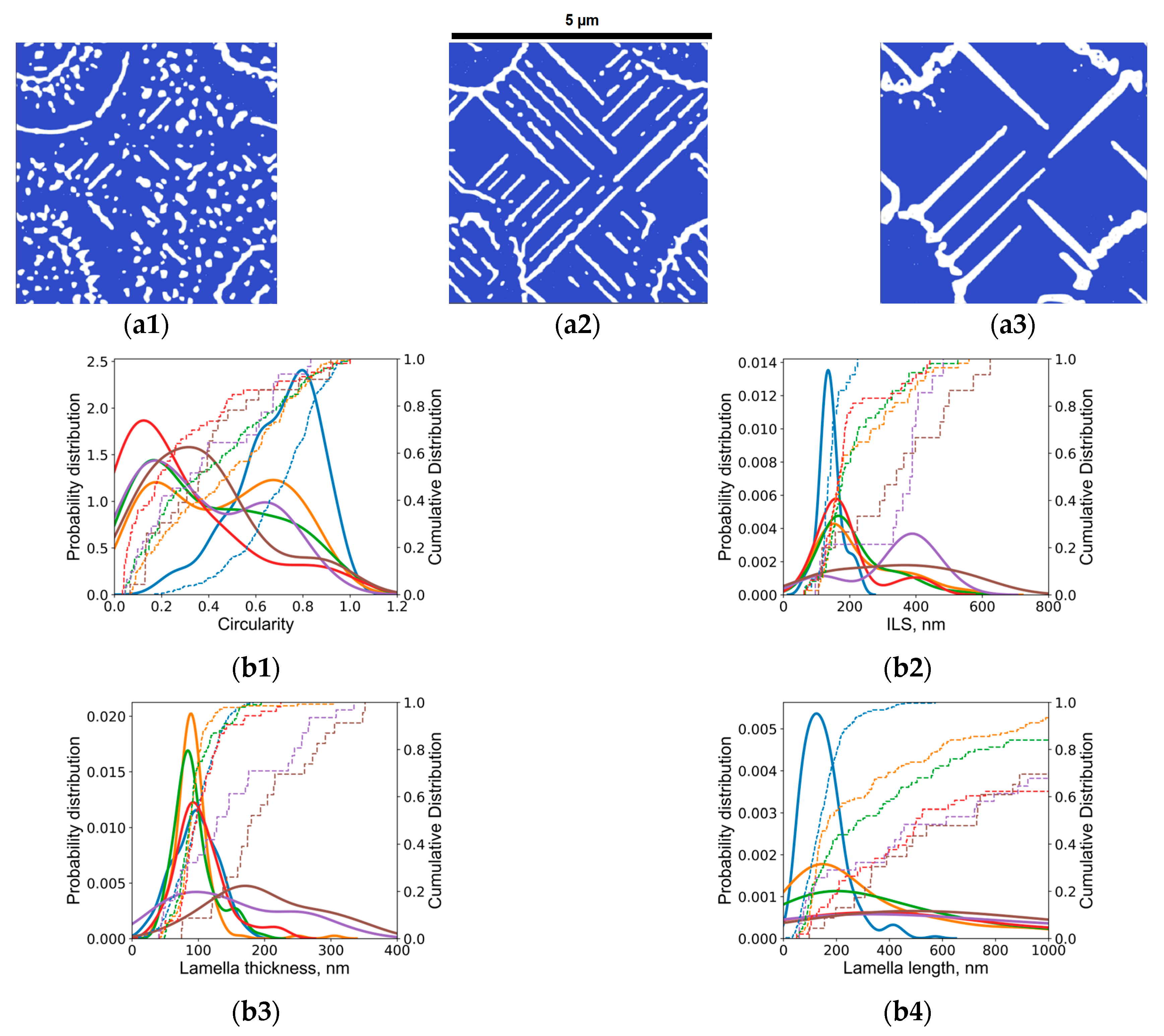
- It is observed that the developed phase field numerical model with identified parameters and given methodology can accurately capture the trends observed experimentally, and can be useful in providing a detailed dynamic understanding of why a certain morphology is observed and evolves.
- From a processing point of view, it can be concluded that adding 1.4 wt.% chromium to Fe-1C can significantly improve the stability and morphology of pearlite while increasing the length of the pearlite nose in the TTT diagram, and hence can make this alloy suitable for production through a variety of processes, like casting, 3D printing, and forming in wider temperature windows while maintaining a consistent fully pearlitic morphology for desired properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
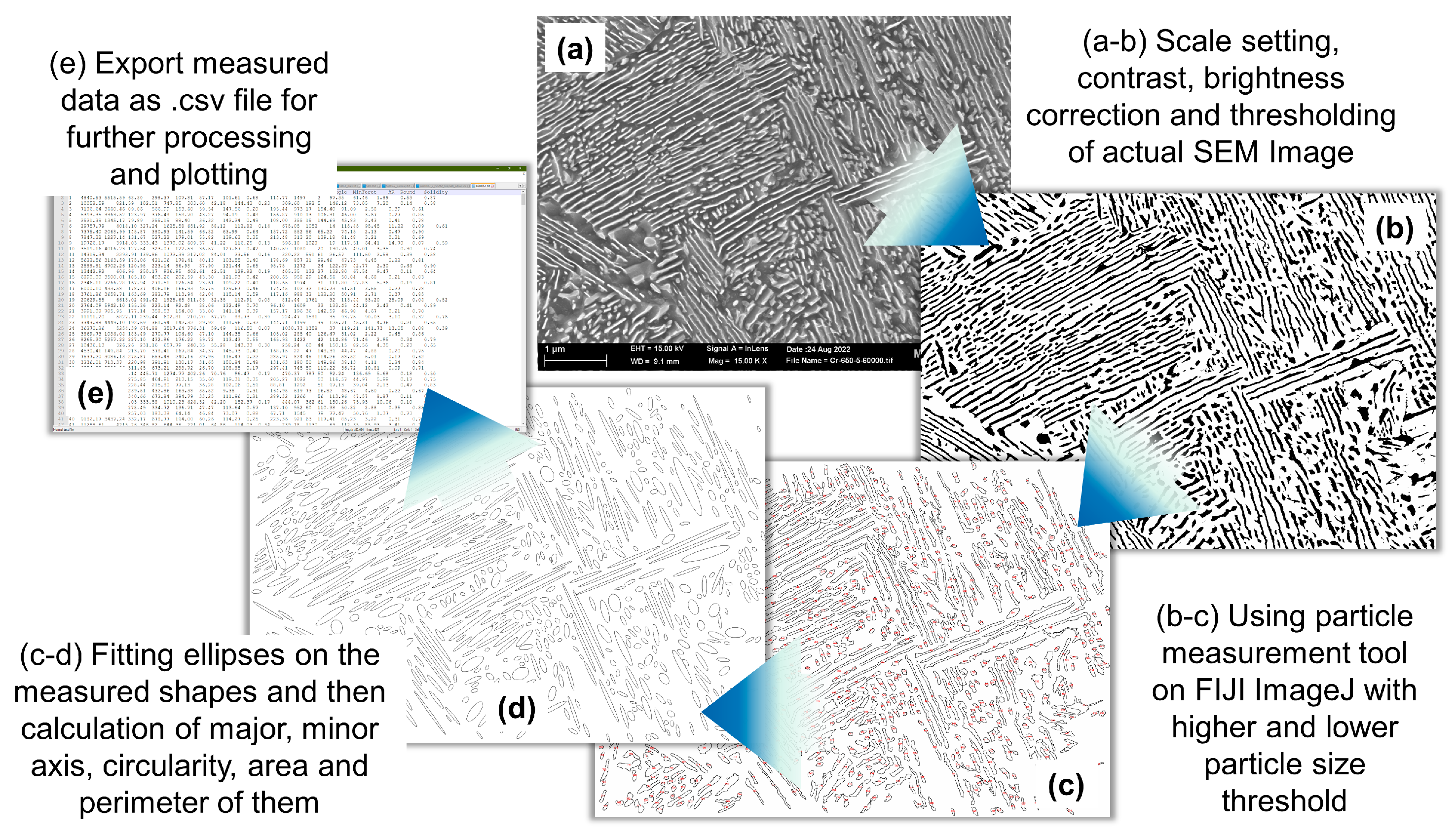
- First, an SE image is imported, and the scale is set based on the provided scale in the SEM image.
- The SEM image is cropped to remove the bottom ribbon and transformed into a grayscale image.
- The contrast of the cropped image is adjusted by using threshold values of the gray-scale image. The grain boundaries or large clusters of particles are removed by manual touch-up.
- The particle analysis script is used to identify cementite particles within certain size and aspect ratio ranges to avoid any noise. The identified particles are then arranged with ellipses of equivalent length, width, and area.
- The major axes of the fitted ellipses represent the length of the particles, and the minor axes of the ellipses represent the thickness of the particles. Apart from these two parameters, other derived parameters, such as total area and circularity, are also calculated for each feature.
- The interlamellar spacing between two adjacent parallel lamellae is challenging to obtain automatically, and therefore is manually measured for at least 50 points per image.
- These data sets for each particle along with the centroid position coordinates are exported in a text file for further processing, analysis, and plotting using adequate Python scripts.
- Adequate python scripts are used to optimally read desired data sets from all the saved text files; the PDFs and CDFs are plotted to understand the attribute distributions with changing external quantities.
- The means of the first and third quartiles of the PDFs are calculated and recorded in separate files for plotting and comparison.

Appendix B
Appendix B.1. Experimental Data

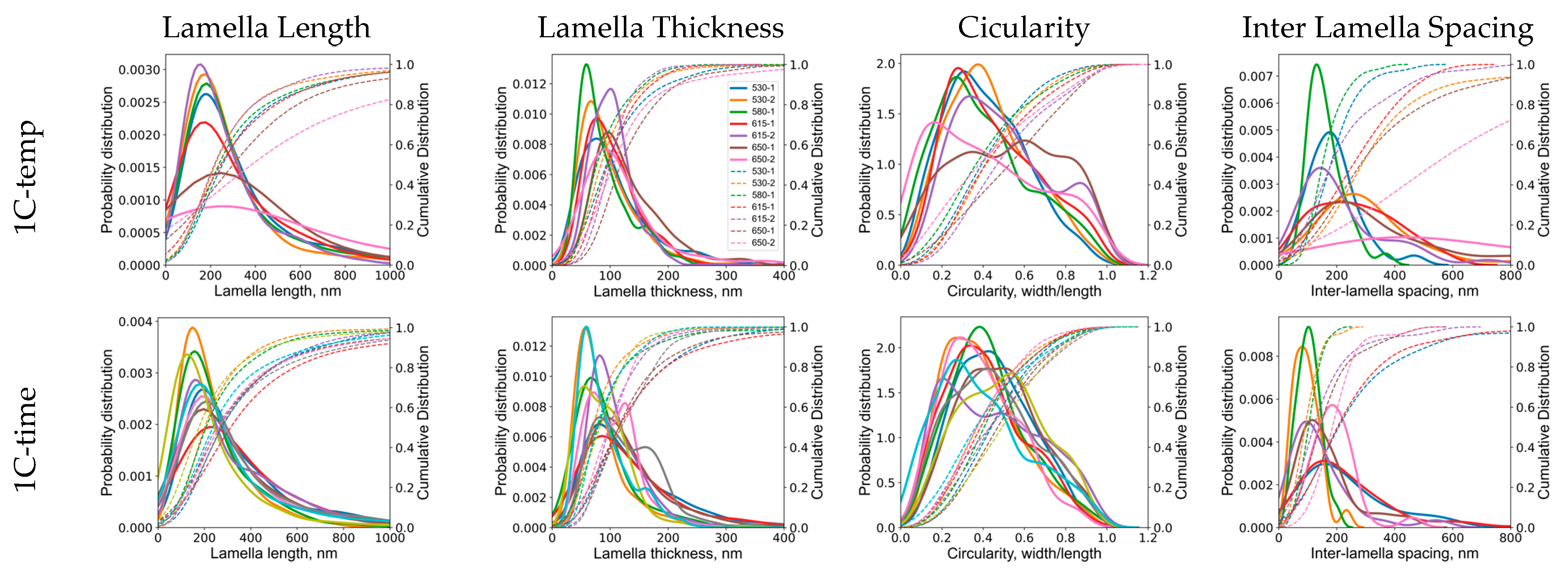
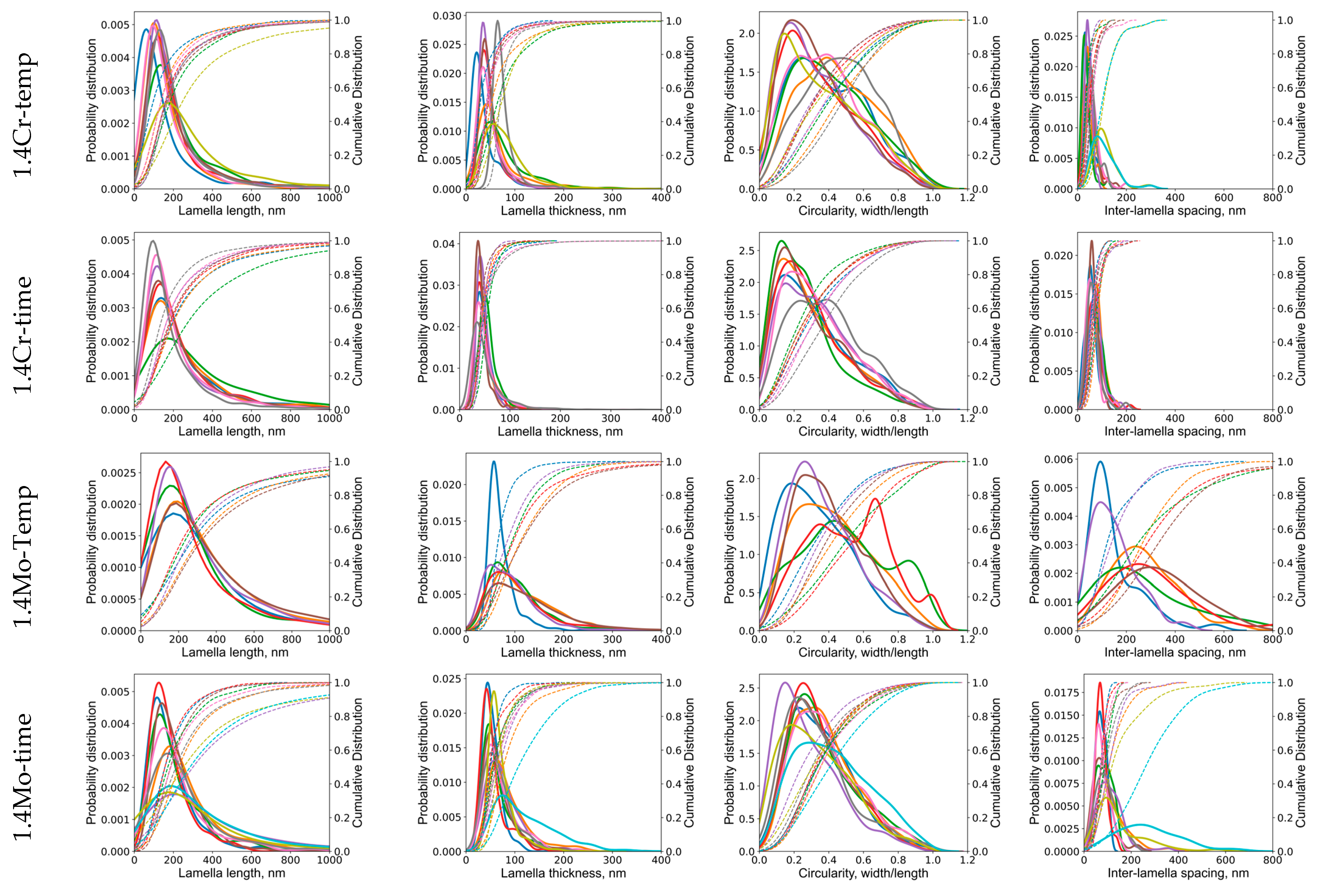
Appendix B.2. Phase Field Simulation Results
References
- Samuels, L.E. Light Microscopy of Carbon Steels; ASM International: Materials Park, OH, USA, 1999. [Google Scholar]
- Hyzak, J.; Bernstein, I. The role of microstructure on the strength and toughness of fully pearlitic steels. Metall. Trans. A 1976, 7, 1217–1224. [Google Scholar] [CrossRef]
- Alza, V.A. Spheroidizing in Steels: Processes, Mechanisms, Kinetic and Microstructure—A Review. IOSR J. Mech. Civ. Eng. 2021, 18, 63–81. [Google Scholar]
- O’Brien, J.; Hosford, W.F. Spheroidization of medium-carbon steels. J. Mater. Eng. Perform. 1997, 6, 69–72. [Google Scholar] [CrossRef]
- Taleff, E.M.; Lewandowski, J.J.; Pourladian, B. Microstructure-property relationships in pearlitic eutectoid and hypereutectoid carbon steels. JOM 2002, 54, 25–30. [Google Scholar] [CrossRef]
- Zheng, H.; Fu, L.; Ji, X.; Ding, Y.; Wang, W.; Wen, M.; Shan, A. Microstructural evolution and mechanical property of ultrafine-grained pearlitic steel by cold rolling: The influence of cementite morphology. Mater. Sci. Eng. A 2021, 824, 141860. [Google Scholar] [CrossRef]
- Qayyum, F.; Guk, S.; Kawalla, R.; Prahl, U. Experimental investigations and multiscale modeling to study the effect of sulfur content on formability of 16MnCr5 alloy steel. Steel Res. Int. 2019, 90, 1800369. [Google Scholar] [CrossRef]
- El-Shabasy, A.B.; Lewandowski, J.J. Effects of load ratio, R, and test temperature on fatigue crack growth of fully pearlitic eutectoid steel (fatigue crack growth of pearlitic steel). Int. J. Fatigue 2004, 26, 305–309. [Google Scholar] [CrossRef]
- Pina, J.; Kouznetsova, V.; Geers, M. Elevated temperature creep of pearlitic steels: An experimental–numerical approach. Mech. Time-Depend. Mater. 2014, 18, 611–631. [Google Scholar] [CrossRef]
- Qayyum, F.; Chiu, C.; Tseng, S.; Rustamov, U.; Berndorf, S.; Shen, F.; Guk, S.; Chao, C.; Prahl, U. Local strain heterogeneity and damage mechanisms in zirconia particle-reinforced TRIP steel MMCs: In situ tensile testing with digital image processing. J. Mater. Sci. 2024, 1–19. [Google Scholar] [CrossRef]
- Ullah, M.; Pasha, R.A.; Chohan, G.Y.; Qayyum, F. Numerical simulation and experimental verification of CMOD in CT specimens of TIG welded AA2219-T87. Arab. J. Sci. Eng. 2015, 40, 935–944. [Google Scholar] [CrossRef]
- Zhang, G.H.; Suh, D.W.; Wu, K.M. Effects of Mn, Si and Cr Addition on the Spheroidization of Cementite in Hypereutectoid Fe-1mass%C Steel. Mater. Sci. Forum 2014, 783–786, 1053–1057. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Chae, J.-Y.; Kim, K.-H.; Suh, D.W. Effects of Mn, Si and Cr addition on the dissolution and coarsening of pearlitic cementite during intercritical austenitization in Fe-1mass%C alloy. Mater. Charact. 2013, 81, 56–67. [Google Scholar] [CrossRef]
- Bae, C.; Lee, C.; Nam, W. Effect of carbon content on mechanical properties of fully pearlitic steels. Mater. Sci. Technol. 2002, 18, 1317–1321. [Google Scholar] [CrossRef]
- Herian, J.; Aniołek, K. The structure and properties of steel with different pearlite morphology and its resistance to abrasive wear. Arch. Mater. Sci. Eng. 2008, 31, 83–86. [Google Scholar]
- Harisha, S.; Sharma, S.; Kini, U.A.; Shankar, M.G. Study on Spheroidization and Related Heat Treatments of Medium Carbon Alloy Steels; MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; p. 02008. [Google Scholar]
- Sinha, A.K. Ferrous Physical Metallurgy; Butterworth-Heinemann: Oxford, UK, 1989. [Google Scholar]
- Ayer, R.; Machmeier, P. Microstructural basis for the effect of chromium on the strength and toughness of AF1410-based high performance steels. Metall. Mater. Trans. A 1996, 27, 2510–2517. [Google Scholar] [CrossRef]
- Ding, H.; Yuan, Z.; Liu, T.; Chen, L.; Zhou, Y.; Cao, Y.; Cao, F.; Luo, R.; Cheng, X. Microstructure and high-temperature tensile behavior of modified H13 steel with the addition of tungsten, molybdenum, and lowering of chromium. Mater. Sci. Eng. A 2023, 866, 144655. [Google Scholar] [CrossRef]
- Nora, J.V.P.D.; Medeiros, J.L.B.; Biehl, L.V.; Lemos, G.V.B.; Martins, C.O.D.; de Souza, J.; de Jesus Pacheco, D.A. The Influence of Thermal Cycles on the Performance and Spheroidization Process of SAE 8640 Steels. J. Mater. Eng. Perform. 2023, 32, 4932–4940. [Google Scholar] [CrossRef]
- Mohrbacher, H.; Kern, A. Nickel alloying in carbon steel: Fundamentals and applications. Alloys 2023, 2, 1–28. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Honeycombe, R.W.K. Steels: Structure, Properties, and Design; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Siitonen, S.; Tuomaala, M.; Ahtila, P. Variables affecting energy efficiency and CO2 emissions in the steel industry. Energy Policy 2010, 38, 2477–2485. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Arens, M.; Cardenas, J.C.R.; Price, L.; Triolo, R. Comparison of carbon dioxide emissions intensity of steel production in China, Germany, Mexico, and the United States. Resour. Conserv. Recycl. 2016, 113, 127–139. [Google Scholar] [CrossRef]
- Sachs, J.D.; Schmidt-Traub, G.; Mazzucato, M.; Messner, D.; Nakicenovic, N.; Rockström, J. Six transformations to achieve the sustainable development goals. Nat. Sustain. 2019, 2, 805–814. [Google Scholar] [CrossRef]
- United Nations General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/inequality (accessed on 20 June 2022).
- Ullah, M.; Wu, C.S.; Qayyum, F. Prediction of crack tip plasticity induced due to variation in solidification rate of weld pool and its effect on fatigue crack propagation rate (FCPR). J. Mech. Sci. Technol. 2018, 32, 3625–3635. [Google Scholar] [CrossRef]
- Hussein, T.; Umar, M.; Qayyum, F.; Guk, S.; Prahl, U. Micromechanical Effect of Martensite Attributes on Forming Limits of Dual-Phase Steels Investigated by Crystal Plasticity-Based Numerical Simulations. Crystals 2022, 12, 155. [Google Scholar] [CrossRef]
- Umar, M.; Qayyum, F.; Farooq, M.U.; Guk, S.; Prahl, U. Qualitative Investigation of Damage Initiation at Meso-Scale in Spheroidized C45EC Steels by Using Crystal Plasticity-Based Numerical Simulations. J. Compos. Sci. 2021, 5, 222. [Google Scholar] [CrossRef]
- Umar, M.; Qayyum, F.; Farooq, M.U.; Guk, S.; Kirschner, M.; Korpala, G.; Prahl, U. Exploring the Structure–Property Relationship in Spheroidized C45EC Steel Using Full Phase Crystal Plasticity Numerical Simulations. Steel Res. Int. 2022, 93, 2100452. [Google Scholar] [CrossRef]
- Chiu, C.; Tseng, S.; Qayyum, F.; Guk, S.; Chao, C.; Prahl, U. Local deformation and interfacial damage behavior of partially stabilized zirconia-reinforced metastable austenitic steel composites: Numerical simulation and validation. Mater. Des. 2023, 225, 111515. [Google Scholar] [CrossRef]
- Podor, R.; Bouala, G.N.; Ravaux, J.; Lautru, J.; Clavier, N. Working with the ESEM at high temperature. Mater. Charact. 2019, 151, 15–26. [Google Scholar] [CrossRef]
- Lypchanskyi, O.; Chiu, C.-C.; Qayyum, F.; Korpała, G.; Guk, S.; Chao, C.-K.; Prahl, U. Temperature dependent deformation behavior and texture evolution in AA6082 aluminum alloy: An integrated experimental and crystal plasticity simulation approach. Int. J. Plast. 2024, 176, 103942. [Google Scholar] [CrossRef]
- Leão, P.B.P.; de Barros Neto, J.R.; Rodrigues, S.F.; Rodrigues, M.V.G.; Xavier, J.V.B.; Lima, T.N.; Reis, G.S.; Ramirez, A.J.; de Abreu, H.F.G. In-situ and interrupted cyclic three-point bending tests in SEM of two pearlitic steel microstructures: Lamellar and partially spheroidized. J. Mater. Res. Technol. 2024, 28, 2990–3012. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, J.; Xu, Y.; Qiao, P.; Lu, Y.; Gong, J. Evolution of mechanical properties of ferrite and pearlite phases during spheroidization process and their relationship to the overall properties of low alloy steel. J. Mater. Res. Technol. 2024, 29, 5437–5446. [Google Scholar] [CrossRef]
- Benke, S. Modeling of solid state transformations using a phase field model with transformation plasticity. In III European Conference on Computational Mechanics: Solids, Structures and Coupled Problems in Engineering: Book of Abstracts; Springer: Berlin/Heidelberg, Germany, 2006; p. 57. [Google Scholar]
- Böttger, B.; Apel, M.; Eiken, J.; Schaffnit, P.; Steinbach, I. Phase-Field simulation of solidification and solid-state transformations in multicomponent steels. Steel Res. Int. 2008, 79, 608–616. [Google Scholar] [CrossRef]
- Suwanpinij, P.; Rudnizki, J.; Prahl, U.; Bleck, W. Investigation of the Effect of Deformation on γ-α Phase Transformation Kinetics in Hot-Rolled Dual Phase Steel by Phase Field Approach. Steel Res. Int. 2009, 80, 616–622. [Google Scholar]
- Rudnizki, J.; Böttger, B.; Prahl, U.; Bleck, W. Phase-field modeling of austenite formation from a ferrite plus pearlite microstructure during annealing of cold-rolled dual-phase steel. Metall. Mater. Trans. A 2011, 42, 2516–2525. [Google Scholar] [CrossRef]
- Rudnizki, J.; Prahl, U.; Bleck, W. Phase-field modelling of microstructure evolution during processing of cold-rolled dual phase steels. Integr. Mater. Manuf. Innov. 2012, 1, 19–31. [Google Scholar] [CrossRef]
- Steinbach, I.; Böttger, B.; Eiken, J.; Warnken, N.; Fries, S. CALPHAD and phase-field modeling: A successful liaison. J. Phase Equilibria Diffus. 2007, 28, 101–106. [Google Scholar] [CrossRef]
- Novick-Cohen, A. The cahn-hilliard equation. In Handbook of Differential Equations: Evolutionary Equations; Elsevier: Amsterdam, The Netherlands, 2008; Volume 4, pp. 201–228. [Google Scholar]
- Allen, S.M.; Cahn, J.W. Ground state structures in ordered binary alloys with second neighbor interactions. Acta Metall. 1972, 20, 423–433. [Google Scholar] [CrossRef]
- Moelans, N.; Blanpain, B.; Wollants, P. An introduction to phase-field modeling of microstructure evolution. Calphad 2008, 32, 268–294. [Google Scholar] [CrossRef]
- Lin, M.; Prahl, U. A parallelized model for coupled phase field and crystal plasticity simulation. Comput. Methods Mater. Sci. 2016, 16, 156–162. [Google Scholar] [CrossRef]
- Kamachali, R.D.; Schwarze, C.; Lin, M.; Diehl, M.; Shanthraj, P.; Prahl, U.; Steinbach, I.; Raabe, D. Numerical benchmark of phase-field simulations with elastic strains: Precipitation in the presence of chemo-mechanical coupling. Comput. Mater. Sci. 2018, 155, 541–553. [Google Scholar] [CrossRef]
- Kundin, J.; Ramazani, A.; Prahl, U.; Haase, C. Microstructure evolution of binary and multicomponent manganese steels during selective laser melting: Phase-field modeling and experimental validation. Metall. Mater. Trans. A 2019, 50, 2022–2040. [Google Scholar] [CrossRef]
- Ramazani, A.; Kundin, J.; Haase, C.; Prahl, U. Phase-Field Modeling of Microstructure Evolution of Binary and Multicomponent Alloys During Selective Laser Melting (SLM) Process. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Berlin/Heidelberg, Germany, 2019; pp. 301–309. [Google Scholar]
- Santillana, B.; Paruchuri, V.; Prahl, U. Phase Field Simulation of Segregation and Precipitates Formation during Steel Solidification; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2023; p. 012060. [Google Scholar]
- Amos, P.K.; Bhattacharya, A.; Nestler, B.; Ankit, K. Mechanisms of pearlite spheroidization: Insights from 3D phase-field simulations. Acta Mater. 2018, 161, 400–411. [Google Scholar] [CrossRef]
- Mecozzi, M.; Eiken, J.; Santofimia, M.; Sietsma, J. Phase field modelling of microstructural evolution during the quenching and partitioning treatment in low-alloy steels. Comput. Mater. Sci. 2016, 112, 245–256. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, G.; Li, C.; Gu, J. Analysis of interface migration and isothermal martensite formation for quenching and partitioning process in a low-carbon steel by phase field modeling. Model. Simul. Mater. Sci. Eng. 2019, 27, 075011. [Google Scholar] [CrossRef]
- Kalina, K.A.; Metsch, P.; Kästner, M. Microscale modeling and simulation of magnetorheological elastomers at finite strains: A study on the influence of mechanical preloads. Int. J. Solids Struct. 2016, 102–103, 286–296. [Google Scholar] [CrossRef]
- Yamanaka, A. Phase-field modeling and simulation of solid-state phase transformations in steels. ISIJ Int. 2023, 63, 395–406. [Google Scholar] [CrossRef]
- Tseng, S.; Qayyum, F.; Guk, S.; Chao, C.; Prahl, U. Transformation of 2D RVE local stress and strain distributions to 3D observations in full phase crystal plasticity simulations of dual-phase steels. Crystals 2022, 12, 955. [Google Scholar] [CrossRef]
- Qayyum, F.; Umar, M.; Dölling, J.; Guk, S.; Prahl, U. 3.03—Mechanics of New-Generation Metals and Alloys. In Comprehensive Mechanics of Materials, 1st ed.; Silberschmidt, V., Ed.; Elsevier: Oxford, UK, 2024; pp. 31–57. [Google Scholar]
- Tseng, S.; Ali, M.; Qayyum, F.; Guk, S.; Overhagen, C.; Chao, C.; Prahl, U. Improved method for generation of hot working process maps of metals. J. Chin. Inst. Eng. 2023, 46, 580–590. [Google Scholar] [CrossRef]
- Saunders, N.; Guo, U.; Li, X.; Miodownik, A.; Schillé, J.-P. Using JMatPro to model materials properties and behavior. JOM 2003, 55, 60–65. [Google Scholar] [CrossRef]
- Steinbach, I.; Pezzolla, F. A generalized field method for multiphase transformations using interface fields. Phys. D Nonlinear Phenom. 1999, 134, 385–393. [Google Scholar] [CrossRef]
- Pariser, G.; Schaffnit, P.; Steinbach, I.; Bleck, W. Simulation of the γ–α-transformation using the phase-field method. Steel Res. 2001, 72, 354–360. [Google Scholar] [CrossRef]
- Zhu, B. Phase-Field Modeling of Microstructure Evolution in Low-Carbon Steels during Intercritical Annealing. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2015. [Google Scholar]
- Bos, C.; Mecozzi, M.; Sietsma, J. A microstructure model for recrystallisation and phase transformation during the dual-phase steel annealing cycle. Comput. Mater. Sci. 2010, 48, 692–699. [Google Scholar] [CrossRef]
- Bellavoine, M.; Dumont, M.; Dehmas, M.; Stark, A.; Schell, N.; Drillet, J.; Hébert, V.; Maugis, P. Ferrite recrystallization and austenite formation during annealing of cold-rolled advanced high-strength steels: In situ synchrotron X-ray diffraction and modeling. Mater. Charact. 2019, 154, 20–30. [Google Scholar] [CrossRef]
- Bouzouni, M.; Gavalas, E.; Papaefthymiou, S. Modeling the microstructure evolution during quenching & partitioning of a conventional CrMo alloy steel. Comput. Mater. Sci. 2022, 206, 111265. [Google Scholar]
- Mushongera, L.; Amos, P.K.; Nestler, B.; Ankit, K. Phase-field simulations of pearlitic divergence in Fe-C-Mn steels. Acta Mater. 2018, 150, 78–87. [Google Scholar] [CrossRef]
- Azizi-Alizamini, H.; Militzer, M. Phase field modelling of austenite formation from ultrafine ferrite–carbide aggregates in Fe–C. Int. J. Mater. Res. 2010, 101, 534–541. [Google Scholar] [CrossRef]
- Eiken, J.; Böttger, B.; Steinbach, I. Multiphase-field approach for multicomponent alloys with extrapolation scheme for numerical application. Phys. Rev. E 2006, 73, 066122. [Google Scholar] [CrossRef]
- Steinbach, I.; Apel, M. The influence of lattice strain on pearlite formation in Fe–C. Acta Mater. 2007, 55, 4817–4822. [Google Scholar] [CrossRef]
- Zurob, H.; Hutchinson, C.; Brechet, Y.; Purdy, G. Solid-Solid Phase Transformations in Inorganic Materials 2005; TMS: Warrendale, PA, USA, 2005. [Google Scholar]
- Jiang, Z.; Wang, P.; Li, D.; Li, Y. The evolutions of microstructure and mechanical properties of 2.25Cr-1Mo-0.25V steel with different initial microstructures during tempering. Mater. Sci. Eng. A 2017, 699, 165–175. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.; Liu, Y.; Wang, F.; Jia, N. Tailoring Strength and Ductility of a Cr-Containing High Carbon Steel by Cold-Working and Annealing. Materials 2019, 12, 4136. [Google Scholar] [CrossRef]
- Harisha, S.R.; Sharma, S.; Kini, U.A.; Sharma, A.; Joshi, N. Mechanical properties-morphology corroboration of spheroidized AISI1040 steel. Mater. Res. Express 2019, 6, 0865g8. [Google Scholar] [CrossRef]
- Wanalerkngam, A.; Boonmee, S.; Krataitong, R.; Morakotjinda, M.; Pongsak, W.; Tosangthum, N.; Tongsri, R. Effect of Carbon Content on Microstructure and Mechanical Properties of Sintered Fe-Mo-Mn-C Alloys; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; p. 012029. [Google Scholar]
- Qayyum, F. Fast Particle Analysis with FIJI ImageJ: Measure Lamella Thickness, Length, Circularity, and Spacing. Youtube, 2023. Available online: https://youtu.be/xSs0C-GcAOE?si=RuO3MH4yHBNdMlxS (accessed on 10 July 2024).


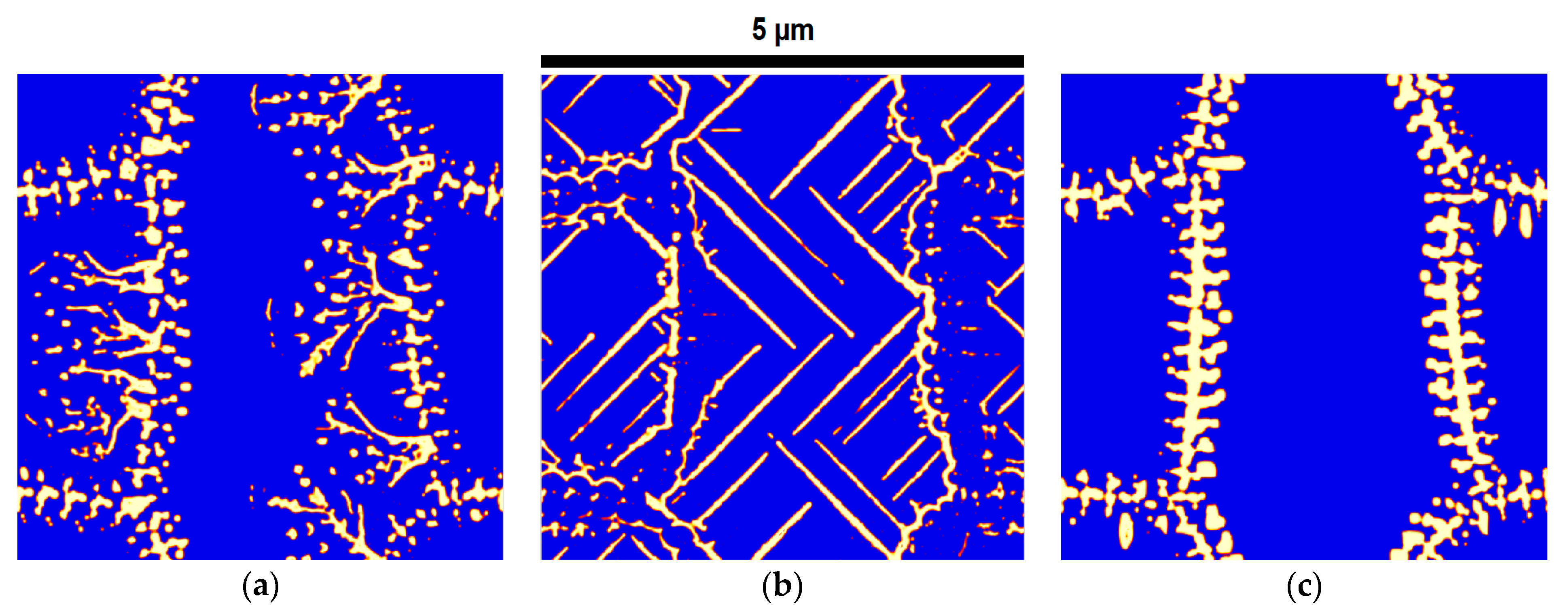

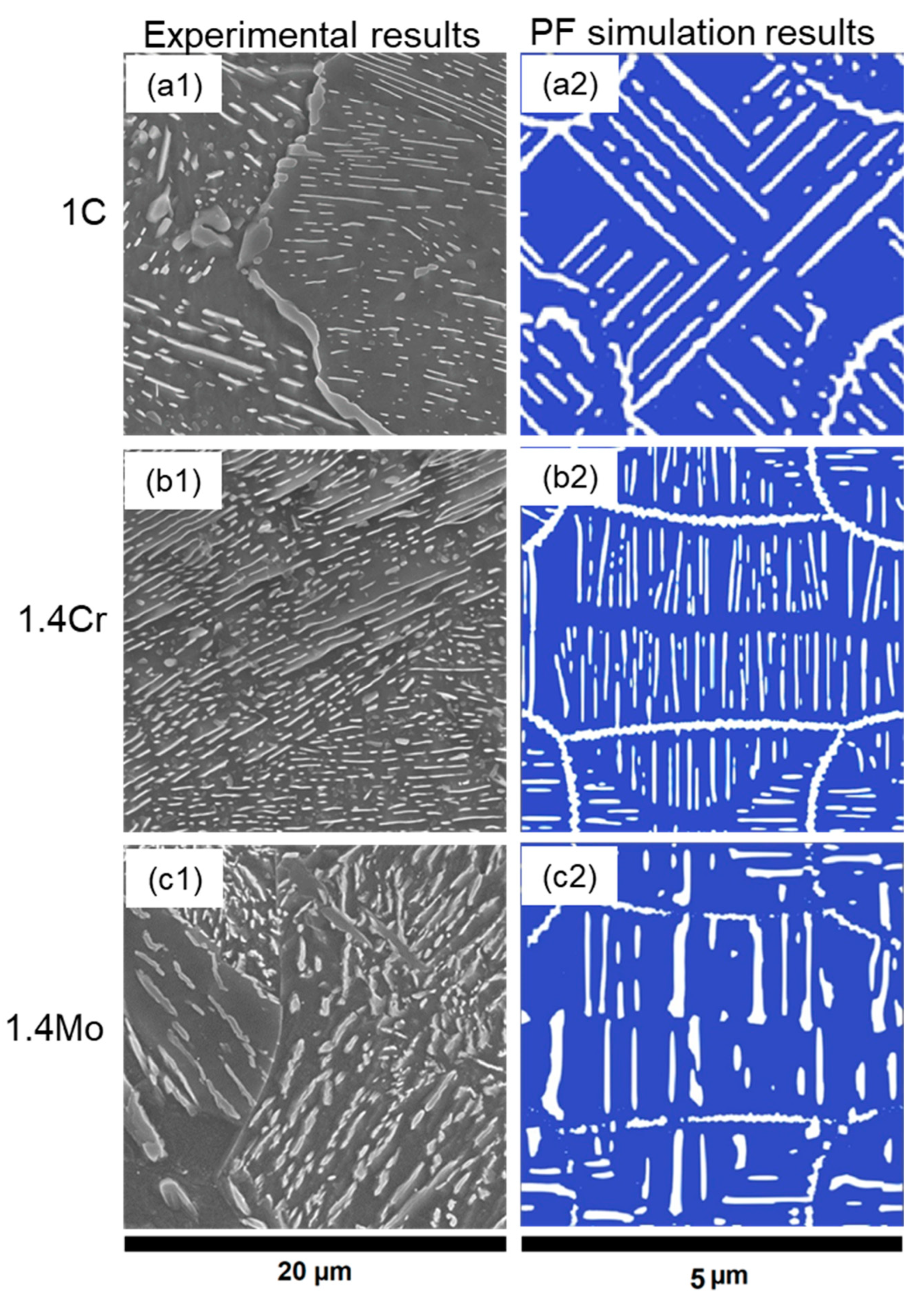



| Alloy Name | C | Cr | Mo | Si | Mn | Fe | Acronym |
|---|---|---|---|---|---|---|---|
| Fe-1C | 1.01 | 0.003 | 0.005 | 0.005 | 0.016 | Bal. | 1C |
| Fe-1C-1.4Cr | 0.97 | 1.42 | 0.005 | 0.005 | 0.02 | Bal. | 1.4Cr |
| Fe-1C-1.4Mo | 0.96 | 0.01 | 1.4 | 0.005 | 0.02 | Bal. | 1.4Mo |
| AC1-B | AC1-F | AC1 | AC3-B | AC3-F | ACM | |
| 1C | 730 | 738 | 734 | 790 | 831 | 810.5 |
| 1.4Cr | 768 | 776 | 772 | 781 | 801 | 791 |
| 1.4Mo | 755 | 769 | 762 | 822 | 860 | 841 |
| AR1-B | AR1-F | AR1 | AR3-B | AR3-F | AR3 | |
| 1C | 667 | 659 | 663 | 725 | 689 | 707 |
| 1.4Cr | 675 | 659 | 667 | 733 | 689 | 711 |
| 1.4Mo | 546 | 492 | 519 | 590 | 563 | 576.5 |
| Type | Phase | 1C | 1.4Cr | 1.4Mo | |
|---|---|---|---|---|---|
| Interface | [59,66,67] | α/α | |||
| α/γ | |||||
| α/θ | |||||
| γ/γ | |||||
| γ/θ | |||||
| Mobility, [66] | α/α | ||||
| α/γ | Variable | Variable | Variable | ||
| α/θ | Variable | Variable | Variable | ||
| γ/γ | |||||
| γ/θ | Variable | Variable | Variable | ||
| Carbon Diffusion | [68,69] | γ | |||
| α | |||||
| θ | |||||
| [68,69] | α | ||||
| γ | |||||
| θ | |||||
| Selected Mobility Parameters | 1C | 1.4Cr | 1.4Mo |
|---|---|---|---|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qayyum, F.; Darabi, A.C.; Guk, S.; Guski, V.; Schmauder, S.; Prahl, U. Analyzing the Effects of Cr and Mo on the Pearlite Formation in Hypereutectoid Steel Using Experiments and Phase Field Numerical Simulations. Materials 2024, 17, 3538. https://doi.org/10.3390/ma17143538
Qayyum F, Darabi AC, Guk S, Guski V, Schmauder S, Prahl U. Analyzing the Effects of Cr and Mo on the Pearlite Formation in Hypereutectoid Steel Using Experiments and Phase Field Numerical Simulations. Materials. 2024; 17(14):3538. https://doi.org/10.3390/ma17143538
Chicago/Turabian StyleQayyum, Faisal, Ali Cheloee Darabi, Sergey Guk, Vinzenz Guski, Siegfried Schmauder, and Ulrich Prahl. 2024. "Analyzing the Effects of Cr and Mo on the Pearlite Formation in Hypereutectoid Steel Using Experiments and Phase Field Numerical Simulations" Materials 17, no. 14: 3538. https://doi.org/10.3390/ma17143538








