The Effect of the Initiator/Activator/Accelerator Ratio on the Degree of Conversion, Film Thickness, Flow, and Cytotoxicity of Dual-Cured Self-Adhesive Resin Cements
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition of Experimental Self-Adhesive Resin Cements
2.2. Degree of Conversion
- Mode 1: Each self-adhesive resin cement in the group (n = 6) was self-cured in the dark at room temperature.
- Mode 2: Each self-adhesive resin cement in the group (n = 6) was light cured through 1 mm thickness zirconia ceramic (LAVATM Plus, 3M Deutschland GmbH, Neuss, Germany) for 30 s at room temperature. A light-curing unit (Be-Lite, B&L Biotech, Fairfax, VA, USA) with an irradiance of 1000 mW/cm2 was used.
2.3. Film Thickness
2.4. Flow Ability
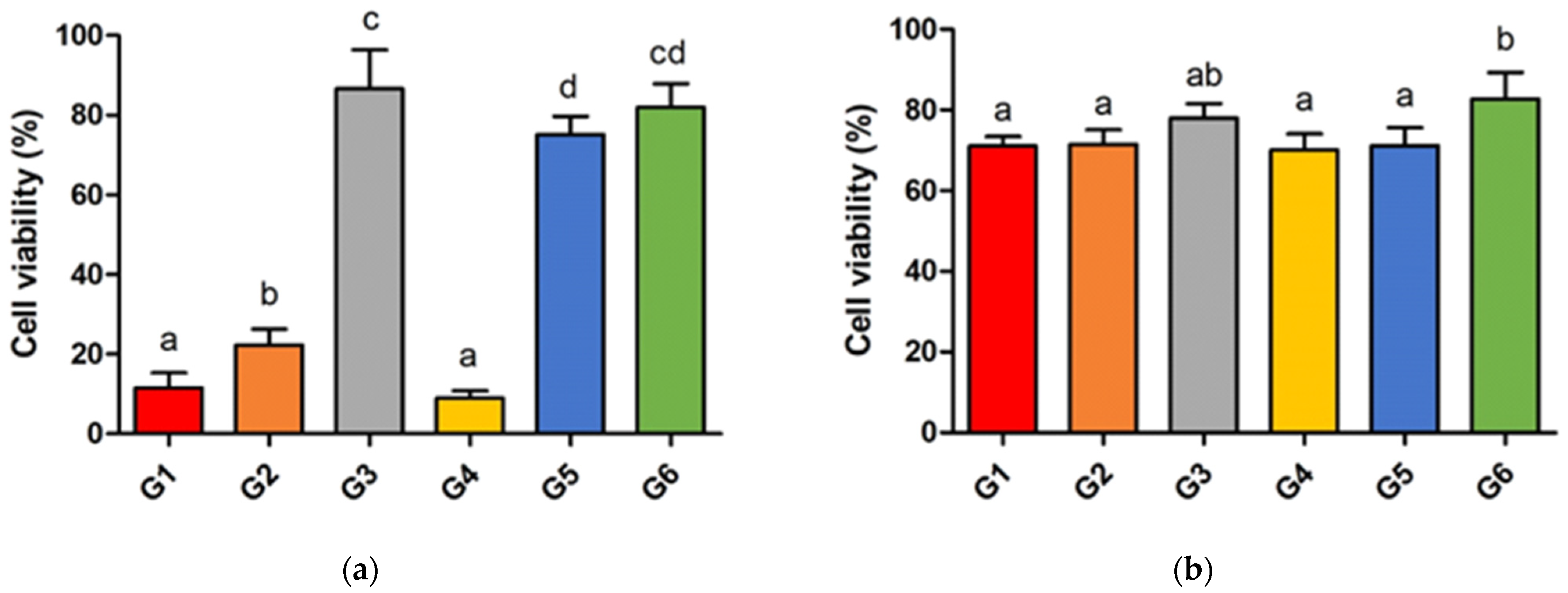
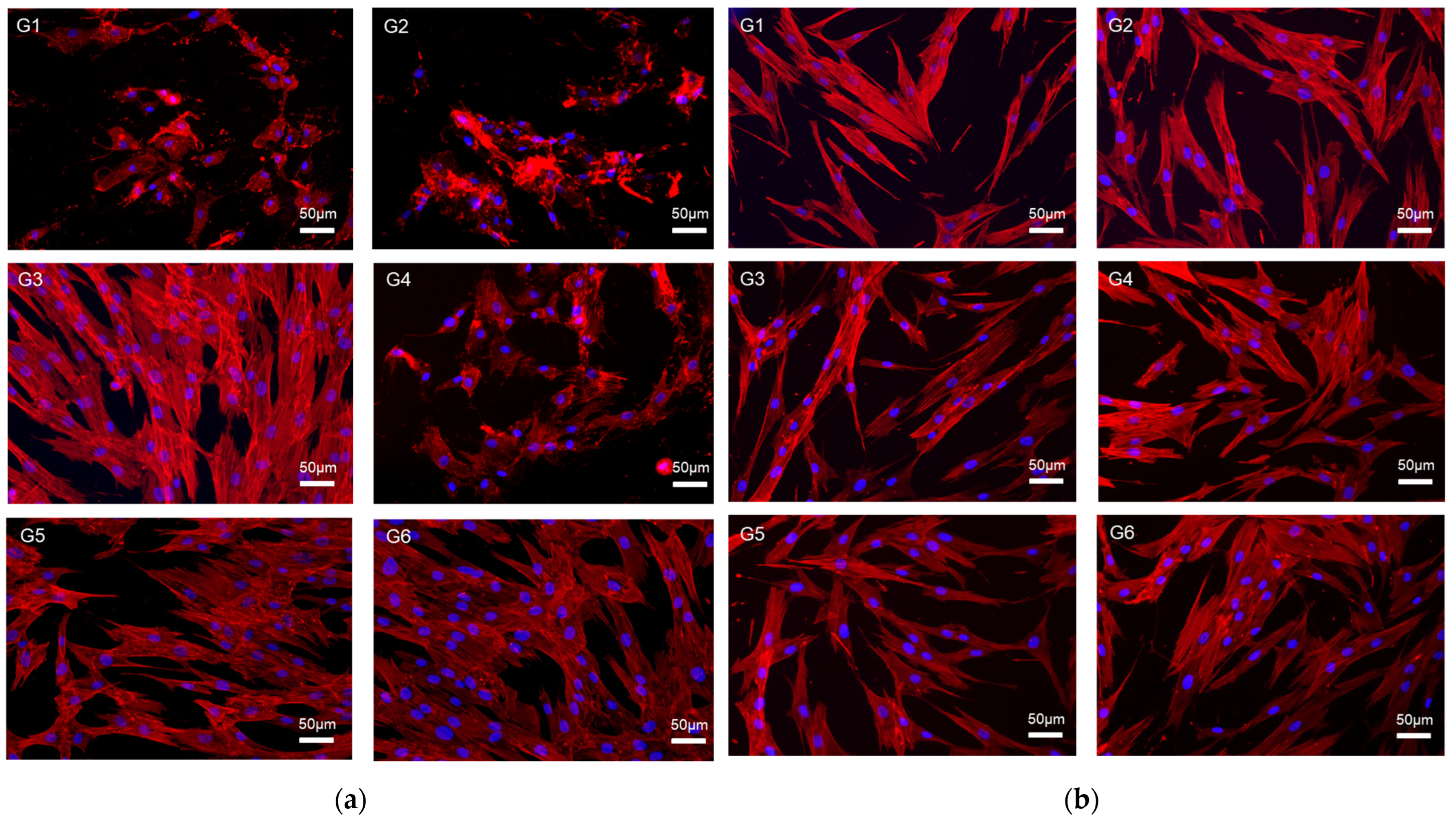
2.5. Cell Viability and Fluorescence Image
2.6. Statistical Analysis
3. Results and Analysis
3.1. Degree of Conversion
3.2. Film Thickness and Flow Distance
3.3. Cell Viability and Fluorescence Image
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferracane, J.L.; Stansbury, J.; Burke, F.J.T. Self-adhesive resin cements–chemistry, properties and clinical considerations. J. Oral Rehabil. 2011, 38, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, T.A.; da Silva, N.R.; Carvalho, R.M. Cements for use in esthetic dentistry. Dent. Clin. N. Am. 2007, 51, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Arrais, C.A.; Rueggeberg, F.A.; Waller, J.L.; de Goes, M.F.; Giannini, M. Effect of curing mode on the polymerization characteristics of dual-cured resin cement systems. J. Dent. 2008, 36, 418–426. [Google Scholar] [CrossRef]
- Giráldez, I.; Ceballos, L.; Garrido, M.A.; Rodríguez, J. Early Hardness of Self-Adhesive Resin Cements Cured under Indirect Resin Composite Restorations. J. Esthet. Restor. Dent. 2011, 23, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Simon, A. Effect of curing mode on the micro-mechanical properties of dual-cured self-adhesive resin cements. Clin. Oral Investig. 2012, 16, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin. Oral Investig. 2008, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Weiser, F.; Behr, M. Self-adhesive resin cements: A clinical review. J. Prosthodont. 2015, 24, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.O.; Ghuman, T.; Cakir, D.; Swift, J.; Edward, J. Self-adhesive resin cements. J. Esthet. Restor. Dent. 2010, 22, 412–419. [Google Scholar] [CrossRef]
- Silva, R.A.T.d.; Coutinho, M.; Cardozo, P.I.; Silva, L.A.d.; Zorzatto, J.R. Conventional dual-cure versus self-adhesive resin cements in dentin bond integrity. J. Appl. Oral Sci. 2011, 19, 355–362. [Google Scholar] [CrossRef][Green Version]
- Ling, L.; Ma, Y.; Chen, Y.; Malyala, R. Physical, mechanical, and adhesive properties of novel self-adhesive resin cement. Int. J. Dent. 2022, 2022, 4475394. [Google Scholar] [CrossRef]
- Vrochari, A.D.; Eliades, G.; Hellwig, E.; Wrbas, K.-T. Curing efficiency of four self-etching, self-adhesive resin cements. Dent. Mater. 2009, 25, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Goracci, C.; Cury, A.H.; Cantoro, A.; Papacchini, F.; Tay, F.R.; Ferrari, M. Microtensile bond strength and interfacial properties of self-etching and self-adhesive resin cements used to lute composite onlays under different seating forces. J. Adhes. Dent. 2006, 8, 327–335. [Google Scholar] [PubMed]
- Arrais, C.A.; Giannini, M.; Rueggeberg, F.A. Effect of sodium sulfinate salts on the polymerization characteristics of dual-cured resin cement systems exposed to attenuated light-activation. J. Dent. 2009, 37, 219–227. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, P.P.A.C.; Rodrigues, E.C.; Schneider, L.F.; Moraes, R.R.; Cesar, P.F.; Rodrigues Filho, L.E. Effect of an acidic sodium salt on the polymerization behavior of self-adhesive resin cements formulated with different adhesive monomers. Dent. Mater. 2018, 34, 1359–1366. [Google Scholar] [CrossRef]
- Moraes, R.; Boscato, N.; Jardim, P.; Schneider, L. Dual and self-curing potential of self-adhesive resin cements as thin films. Oper. Dent. 2011, 36, 635–642. [Google Scholar] [CrossRef]
- Faria-e-Silva, A.L.; Pfeifer, C.S. Development of dual-cured resin cements with long working time, high conversion in absence of light and reduced polymerization stress. Dent. Mater. 2020, 36, e293–e301. [Google Scholar] [CrossRef]
- Kious, A.R.; Roberts, H.W.; Brackett, W.W. Film thicknesses of recently introduced luting cements. J. Prosthet. Dent. 2009, 101, 189–192. [Google Scholar] [CrossRef]
- Teyagirwa, P.F.; Aquin, C.; Kharouf, N.; Roman, T.; Senger, B.; Reitzer, F.; Etienne, O. Operator versus material influence on film thickness using adhesive resin cement or pre-heated resin composite. J. Esthet. Restor. Dent. 2023, 35, 517–524. [Google Scholar] [CrossRef]
- Bagheri, R. Film thickness and flow properties of resin-based cements at different temperatures. J. Dent. 2013, 14, 57. [Google Scholar]
- Oliveira, M.; Cesar, P.F.; Giannini, M.; Rueggeberg, F.; Rodrigues, J.; Arrais, C. Effect of temperature on the degree of conversion and working time of dual-cured resin cements exposed to different curing conditions. Oper. Dent. 2012, 37, 370–379. [Google Scholar] [CrossRef]
- Stansbury, J.; Dickens, S.H. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent. Mater. 2001, 17, 71–79. [Google Scholar] [CrossRef] [PubMed]
- ISO 4049; Dentistry—Polymer-Based Restorative Materials. ISO: Geneva, Switzerland, 2019.
- ISO 6876; Dentistry—Root Canal Sealing Materials. ISO: Geneva, Switzerland, 2012.
- ISO 10993; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. ISO: Geneva, Switzerland, 2018.
- Kumbuloglu, O.; Lassila, L.V.; User, A.; Vallittu, P.K. A study of the physical and chemical properties of four resin composite luting cements. Int. J. Prosthodont. 2004, 17, 357. [Google Scholar] [PubMed]
- Tezvergil-Mutluay, A.; Lassila, L.V.; Vallittu, P.K. Degree of conversion of dual-cure luting resins light-polymerized through various materials. Acta Odontol. Scand. 2007, 65, 201–205. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, D.; Zhang, J.; Li, J.; Chen, X. Optimization of initiator contents in room temperature polymerization of methyl methacrylate. Polym. Polym. Compos. 2022, 30, 09673911221143201. [Google Scholar] [CrossRef]
- Shim, J.S.; Kang, J.K.; Jha, N.; Ryu, J.J. Polymerization mode of self-adhesive, dual-cured dental resin cements light cured through various restorative materials. J. Esthet. Restor. Dent. 2017, 29, 209–214. [Google Scholar] [CrossRef]
- Shim, J.S.; Lee, S.Y.; Song, S.-Y.; Jha, N.; Ryu, J.J. Polymerization efficiency of dental dual-cured resin cement light-cured at various times after the initiation of chemical activation. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 622–628. [Google Scholar] [CrossRef]
- Schmid-Schwap, M.; Franz, A.; König, F.; Bristela, M.; Lucas, T.; Piehslinger, E.; Watts, D.C.; Schedle, A. Cytotoxicity of four categories of dental cements. Dent. Mater. 2009, 25, 360–368. [Google Scholar] [CrossRef]
- Price, R.B.; Shortall, A.C.; Palin, W.M. Contemporary issues in light curing. Oper. Dent. 2014, 39, 4–14. [Google Scholar] [CrossRef]
- Shim, J.S.; Han, S.H.; Jha, N.; Hwang, S.T.; Ahn, W.; Lee, J.Y.; Ryu, J.J. Effect of irradiance and exposure duration on temperature and degree of conversion of dual-cure resin cement for ceramic restorations. Oper. Dent. 2018, 43, E280–E287. [Google Scholar] [CrossRef]
- Putzeys, E.; Duca, R.C.; Coppens, L.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. In-vitro transdentinal diffusion of monomers from adhesives. J. Dent. 2018, 75, 91–97. [Google Scholar] [CrossRef]
- Kurt, A.; Altintas, S.H.; Kiziltas, M.V.; Tekkeli, S.E.; Guler, E.M.; Kocyigit, A.; Usumez, A. Evaluation of residual monomer release and toxicity of self-adhesive resin cements. Dent. Mater. J. 2018, 37, 40–48. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Poubel, D.L.; da Silva, R.C.; Ribeiro, A.P.D.; Garcia, F.C.P. Effect of preheating on the viscosity of composite resins. J. Conserv. Dent. Endod. 2024, 27, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-B.; Son, H.-H.; Um, C.-M. Rheologic properties of flowable, conventional hybrid, and condensable composite resins. Dent. Mater. 2003, 19, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Beketova, A.; Tzanakakis, E.-G.C.; Vouvoudi, E.; Anastasiadis, K.; Rigos, A.E.; Pandoleon, P.; Bikiaris, D.; Tzoutzas, I.G.; Kontonasaki, E. Zirconia nanoparticles as reinforcing agents for contemporary dental luting cements: Physicochemical properties and shear bond strength to monolithic zirconia. Int. J. Mol. Sci. 2023, 24, 2067. [Google Scholar] [CrossRef] [PubMed]
- White, S.N.; Yu, Z. Film thickness of new adhesive luting agents. J. Prosthet. Dent. 1992, 67, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Okamoto, A.; Fukushima, M.; Okiji, T. Evaluation of physical properties and surface degradation of self-adhesive resin cements. Dent. Mater. J. 2007, 26, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Lozada, J.; Urquía-Morales, M.d.C. In vitro evaluation of the film thickness of self-etching resin cements. Acta Odontol. Latinoam. 2014, 27, 145–150. [Google Scholar] [PubMed]
- Alofi, R.S. Comparative Evaluation of Film Thickness and Temperature of Different Luting Cements: An In Vitro Study. World 2019, 10, 429. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Inokoshi, S.; Davidson, C.; De Gee, A.; Lambrechts, P.; Braem, M.; Vanherle, G. Dual cure luting composites–part II: Clinically related properties. J. Oral Rehabil. 1994, 21, 57–66. [Google Scholar] [CrossRef]
- Arrais, C.; Chagas, C.; Munhoz, A.; Oliveira, M.; Reis, A.; Rodrigues, J. Effect of simulated tooth temperature on the degree of conversion of self-adhesive resin cements exposed to different curing conditions. Oper. Dent. 2014, 39, 204–212. [Google Scholar] [CrossRef]
- França, F.Á.; Oliveira, M.D.; Rodrigues, J.A.; Arrais, C.A.G. Pre-heated dual-cured resin cements: Analysis of the degree of conversion and ultimate tensile strength. Braz. Oral Res. 2011, 25, 174–179. [Google Scholar] [CrossRef] [PubMed]




| Components | |
|---|---|
| Paste A | TEGDMA, UDMA, 10-MDP, BPO, TP, barium glass filler |
| Paste B | TEGDMA, UDMA, TP, CQ, EDMAB, DMPT, SPTS, barium glass filler |
| G1 | G2 | G3 | G4 | G5 | G6 | |
|---|---|---|---|---|---|---|
| BPO (self-curing initiator) | 1 | 2 | 1 | 1 | 2 | 1 |
| DMTP (self-curing activator) | 1 | 1 | 2 | 1 | 1 | 2 |
| SPTS (accelerator) | 1 | 1 | 1 |
| Group | G1 | G2 | G3 | G4 | G5 | G6 | |
|---|---|---|---|---|---|---|---|
| Time | |||||||
| 1 min | 0.91 (0.68) A,a | 0.84 (0.50) A,a | 0.93 (1.08) A,a | 0.95 (0.49) A,a | 0.69 (0.61) A,a | 0.93 (0.77) A,a | |
| 2 min | 2.65 (1.53) A,a | 2.85 (1.47) A,a | 1.91 (1.10) A,a | 1.16 (0.58) A,a | 1.61 (0.73) A,a | 2.77 (2.24) AB,a | |
| 3 min | 3.18 (1.43) A,a | 4.14 (1.29) A,a | 3.96 (2.37) A,a | 1.61 (0.88) A,a | 4.46 (2.71) A,a | 12.61 (8.32) B,a | |
| 4 min | 4.34 (0.81) A,a | 4.95 (1.31) A,a | 10.90 (5.52) A,a | 1.84 (1.05) A,a | 10.41 (7.70) A,a | 28.44 (11.26) C,b | |
| 5 min | 4.11 (1.30) A,a | 5.04 (1.15) A,a | 21.53 (10.55) B,b | 3.92 (2.01) A,a | 23.33 (11.39) B,b | 40.81 (10.64) D,c | |
| 7 min | 5.15 (0.59) A,a | 6.58 (1.36) A,a | 37.73 (10.33) C,b | 6.48 (3.15) AB,a | 39.34 (13.71) C,b | 52.72 (7.93) E,c | |
| 10 min | 6.75 (1.49) A,a | 9.91 (2.50) A,a | 53.01 (4.90) D,b | 14.55 (4.83) B,a | 51.79 (8.02) D,b | 61.02 (4.41) EF,b | |
| 15 min | 7.64 (1.68) A,a | 21.36 (9.68) B,b | 61.23 (2.49) DE,d | 33.38 (5.79) C,c | 60.89 (5.43) DE,d | 65.98 (2.56) FG,d | |
| 20 min | 10.71 (2.55) AB,a | 36.53 (10.17) C,b | 64.64 (2.10) EF,d | 46.97 (10.56) D,bc | 65.74 (3.14) EF,d | 68.62 (1.65) FG,d | |
| 25 min | 18.59 (8.23) B,a | 51.06 (6.38) D,b | 67.12 (2.19) EF,d | 55.93 (7.48) DE,bc | 68.39 (2.83) EF,d | 70.67 (1.32) FGH,d | |
| 30 min | 32.25 (15.35) C,a | 57.47 (4.09) DE,b | 68.75 (1.54) EF,c | 63.01 (2.79) EF,bc | 70.22 (2.01) EF,c | 71.51 (1.29) GH,c | |
| 40 min | 50.13 (11.54) D,a | 63.55 (2.07) EF,b | 70.37 (1.76) EFG,b | 67.08 (1.86) F,b | 72.04 (1.59) F,b | 73.13 (0.83) GH,b | |
| 50 min | 59.66 (4.28) DE,a | 66.86 (2.02) EF,a | 72.34 (1.30) FG,b | 69.96 (1.25) F,a | 73.76 (1.17) FG,b | 73.70 (0.99) GHI,b | |
| 60 min | 64.33 (3.37) E,a | 69.19 (1.78) F,a | 73.40 (1.43) FG,a | 71.11 (2.00) FG,a | 75.22 (1.75) FGH,a | 74.52 (0.58) GHI,a | |
| 1 day | 80.35 (1.38) F,a | 82.73 (1.45) G,a | 80.63 (0.69) GH,a | 80.71 (0.75) GH,a | 82.96 (0.79) GHI,a | 80.76 (0.92) HIJ,a | |
| 1 week | 83.45 (0.54) F,a | 85.52 (1.91) G,a | 83.76 (0.64) H,a | 83.74 (0.33) H,a | 85.38 (0.66) HI,a | 83.91 (0.63) IJ,a | |
| 2 weeks | 84.08 (0.73) F,a | 86.53 (0.30) G,a | 84.77 (0.46) H,a | 84.77 (0.53) H,a | 86.10 (0.38) I,a | 84.83 (0.44) J,a | |
| Group | Group1 | Group2 | Group3 | Group4 | Group5 | Group6 | |
|---|---|---|---|---|---|---|---|
| Time | |||||||
| 1 min | 62.30 (1.79) A,a | 63.59 (1.72) A,ab | 65.80 (1.59) A,b | 64.57 (2.31) A,ab | 64.12 (3.06) A,ab | 66.31 (1.08) A,b | |
| 2 min | 65.56 (0.80) AB,a | 67.31 (1.32) AB,ab | 68.91 (0.79) AB,ab | 68.07 (2.15) AB,ab | 68.21 (2.51) B,ab | 69.36 (1.33) AB,b | |
| 3 min | 66.79 (0.86) B,a | 69.28 (1.19) BC,ab | 71.13 (1.25) BC,b | 68.94 (1.69) B,ab | 68.83 (2.47) BC,ab | 70.46 (1.56) B,b | |
| 4 min | 66.75 (1.13) B,a | 70.58 (1.55) BCD,b | 71.21 (1.30) BC,b | 70.97 (1.96) BC,b | 70.18 (3.41) BCD,b | 71.46 (2.02) BC,b | |
| 5 min | 68.26 (1.01) BC,a | 70.65 (2.00) BCD,ab | 72.52 (1.45) BCD,b | 71.52 (2.01) BCD,ab | 70.04 (3.20) BCD,ab | 72.04 (2.13) BCD,b | |
| 7 min | 69.07 (1.52) BC,a | 71.20 (2.11) CDE,ab | 72.88 (0.53) CD,b | 72.13 (1.68) CD,ab | 71.26 (2.79) BCDE,ab | 72.63 (1.80) BCD,b | |
| 10 min | 70.60 (1.83) CD,a | 72.77 (2.32) CDEF,ab | 74.22 (0.97) CDE,b | 73.48 (2.78) CDE,ab | 72.21 (3.22) CDEF,ab | 74.38 (2.20) CDE,b | |
| 15 min | 71.81 (1.54) CDE,a | 73.73 (2.05) DEFG,ab | 75.27 (0.79) DEF,b | 73.36 (2.60) CDE,ab | 73.17 (3.26) DEFG,ab | 75.34 (1.76) DEF,b | |
| 20 min | 73.01 (1.81) DEF,a | 74.71 (1.89) EFGH,ab | 75.93 (0.49) DEF,ab | 74.14 (2.20) CDE,ab | 74.59 (2.71) EFGH,ab | 76.51 (1.63) EFG,b | |
| 25 min | 73.78 (1.88) DEF,a | 75.29 (1.81) FGH,ab | 76.06 (0.77) DEF,ab | 74.93 (2.50) DE,ab | 75.04 (2.15) FGHI,ab | 77.80 (1.43) EFG,b | |
| 30 min | 74.20 (1.91) DEF,a | 76.22 (0.99) FGH,a | 76.72 (0.72) EF,a | 75.19 (2.65) DE,a | 75.15 (2.46) FGHI,a | 77.56 (1.15) EFG,a | |
| 40 min | 75.27 (1.43) EF,a | 77.15 (1.47) GH,a | 76.96 (0.88) EF,a | 75.93 (1.97) E,a | 76.31 (1.80) GHI,a | 78.66 (1.15) FG,a | |
| 50 min | 75.89 (1.37) F,a | 77.86 (1.20) H,ab | 77.41 (0.56) EF,ab | 76.29 (1.98) E,ab | 77.01 (1.19) HI,ab | 79.31 (1.08) G,b | |
| 60 min | 76.59 (1.75) F,a | 77.59 (1.38) H,a | 78.15 (1.00) F,a | 76.43 (2.36) E,a | 78.54 (0.78) I,a | 79.59 (0.75) G,a | |
| 1 day | 83.82 (0.51) G,a | 84.65 (1.05) I,a | 84.26 (0.55) G,a | 83.15 (1.29) F,a | 84.93 (0.77) J,a | 84.35 (0.65) H,a | |
| 1 week | 85.44 (0.75) G,a | 86.32 (1.08) I,a | 86.67 (0.50) G,a | 85.57 (1.10) F,a | 87.07 (0.49) J,a | 87.04 (0.39) H,a | |
| 2 weeks | 86.70 (0.84) G,a | 87.76 (0.69) I,a | 87.29 (0.68) G,a | 86.57 (0.94) F,a | 87.49 (0.41) J,a | 87.61 (0.78) H,a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, H.K.; Won, J.-E.; Ryu, J.J.; Shim, J.S. The Effect of the Initiator/Activator/Accelerator Ratio on the Degree of Conversion, Film Thickness, Flow, and Cytotoxicity of Dual-Cured Self-Adhesive Resin Cements. Materials 2024, 17, 3572. https://doi.org/10.3390/ma17143572
Moon HK, Won J-E, Ryu JJ, Shim JS. The Effect of the Initiator/Activator/Accelerator Ratio on the Degree of Conversion, Film Thickness, Flow, and Cytotoxicity of Dual-Cured Self-Adhesive Resin Cements. Materials. 2024; 17(14):3572. https://doi.org/10.3390/ma17143572
Chicago/Turabian StyleMoon, Hyun Kyung, Jong-Eun Won, Jae Jun Ryu, and Ji Suk Shim. 2024. "The Effect of the Initiator/Activator/Accelerator Ratio on the Degree of Conversion, Film Thickness, Flow, and Cytotoxicity of Dual-Cured Self-Adhesive Resin Cements" Materials 17, no. 14: 3572. https://doi.org/10.3390/ma17143572
APA StyleMoon, H. K., Won, J.-E., Ryu, J. J., & Shim, J. S. (2024). The Effect of the Initiator/Activator/Accelerator Ratio on the Degree of Conversion, Film Thickness, Flow, and Cytotoxicity of Dual-Cured Self-Adhesive Resin Cements. Materials, 17(14), 3572. https://doi.org/10.3390/ma17143572





