Underwater Electrochemical Offshore Tests of a Paint Coating Applied in Water on the Legs of an Oil Production Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Research
2.2. Research on the Oil Production Platform
3. Results and Discussion
3.1. Laboratory Research
3.2. Research on the Oil Production Platform
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, A.; Zhang, H.; Qadrdan, M.; Yang, W.; Jin, X.; Wu, J. Optimal Planning of Integrated Energy Systems for Offshore Oil Extraction and Processing Platforms. Energies 2019, 12, 756. [Google Scholar] [CrossRef]
- Priest, T.; Pratt, J. Keys to the Evolution of Offshore Platforms. In Proceedings of the 3rd National Congress on Civil Engineering History and Heritage, Houston, TX, USA, 10–13 October 2001; pp. 263–265. [Google Scholar] [CrossRef]
- Arana HA, H.; Warwick, R.M.; Attrill, M.J.; Rowden, A.A.; Gold-Bouchot, G. Assessing the impact of oil-related activities on benthic macroinfauna assemblages of the Campeche shelf, southern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2005, 289, 89–107. [Google Scholar] [CrossRef]
- Esaklul, K.A.; Ahmed, T.M. Prevention of failures of high strength fasteners in use in offshore and subsea applications. Eng. Fail. Anal. 2009, 16, 1195–1202. [Google Scholar] [CrossRef]
- Melchers, R.E. The effect of corrosion on the structural reliability of steel offshore structures. Corros. Sci. 2005, 47, 2391–2410. [Google Scholar] [CrossRef]
- Skogdalen, J.E.; Utne, I.B.; Vinnem, J.E. Developing safety indicators for preventing offshore oil and gas deepwater drilling blowouts. Saf. Sci. 2011, 49, 1187–1199. [Google Scholar] [CrossRef]
- Garverick, L. Corrosion in the Petrochemical Industry, 2nd ed.; ASM International: Chardon, OH, USA, 2015; ISBN 978-1-62708-094-1. [Google Scholar]
- Viscusi, W.; Zeckhauser, R.J. Deterring and Compensating Oil-Spill Catastrophes: The Need for Strict and Two-Tier Liability. Vanderbilt Law Rev. 2011, 64, 1717. [Google Scholar] [CrossRef]
- Brkic, D.; Stajic, Z. Offshore Oil and Gas Safety: Protection against Explosions. J. Mar. Sci. Eng. 2021, 9, 331. [Google Scholar] [CrossRef]
- Hughes, S.A.; Naile, J.; Pinza, M.; Ray, C.; Hester, B.; Baum, J.; Gardiner, W.; Kallestad, W.; Brzuzy, L. Characterization of Miscellaneous Effluent Discharges from a Mobile Offshore Drilling Unit to the Marine Environment. Environ. Toxicol. Chem. 2019, 38, 2811–2823. [Google Scholar] [CrossRef]
- Husain, A.; Al-Shamali, O.; Abduljaleel, A. Investigation of marine environmental related deterioration of coal tar epoxy paint on tubular steel pilings. Desalination 2004, 166, 295–304. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Bierwagen, G. Flow accelerated degradation of organic clear coat: The effect of fluid shear. Electrochim. Acta 2014, 142, 25–33. [Google Scholar] [CrossRef]
- Fredj, N.; Cohendoz, S.; Feaugas, X.; Touzain, S. Ageing of marine coating in natural and artificial seawater under mechanical stresses. Prog. Org. Coat. 2012, 74, 391–399. [Google Scholar] [CrossRef]
- Fredj, N.; Cohendoz, S.; Feaugas, X.; Touzain, S. Some consequences of saline solution immersion on mechanical behaviour of two marine epoxy-based coatings. Prog. Org. Coat. 2010, 69, 82–91. [Google Scholar] [CrossRef]
- Alessi, L.; Correia, J.; Fantuzzi, N. Initial Design Phase and Tender Designs of a Jacket Structure Converted into a Retrofitted Offshore Wind Turbine. Energies 2019, 12, 659. [Google Scholar] [CrossRef]
- Orlikowski, J.; Szociński, M.; Żakowski, K.; Igliński, P.; Domańska, K.; Darowicki, K. Actual field corrosion rate of offshore structures in the Baltic Sea along depth profile from water surface to sea bed. Ocean Eng. 2022, 265, 112545. [Google Scholar] [CrossRef]
- Żakowski, K. Studying the effectiveness of a modernized cathodic protection system for an offshore platform. Anti-Corros. Methods Mater. 2011, 58, 167–172. [Google Scholar] [CrossRef]
- Zakowski, K.; Iglinski, P.; Orlikowski, J.; Darowicki, K.; Domanska, K. Modernized cathodic protection system for legs of the production rig—Evaluation during ten years of service. Ocean Eng. 2020, 218, 108074. [Google Scholar] [CrossRef]
- Zakowski, K.; Orlikowski, J.; Darowicki, K.; Czekajlo, M.; Iglinski, P.; Domanska, K. The Effect of Increasing the Amount of Indium Alloying Material on the Efficiency of Sacrificial Aluminium Anodes. Materials 2021, 14, 1755. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Narożny, M.; Żakowski, K.; Darowicki, K. Time evolution of Electrochemical Impedance spectra of cathodically protected steel in artificial seawater. Constr. Build. Mater. 2017, 154, 88–94. [Google Scholar] [CrossRef]
- Bordziłowski, J.; Darowicki, K.; Krakowiak, S.; Królikowska, A. Impedance measurements of coating properties on bridge structures. Prog. Org. Coat. 2003, 46, 216–219. [Google Scholar] [CrossRef]
- Jorda, R.M. Epoxy-polyamide Mixture Resists Splash Zone Corrosion. Mater. Perform. 1963, 2, 56–61. [Google Scholar]
- Kausar, A. Polymer coating technology for high performance applications: Fundamentals and advances. J. Macromol. Sci. Part A 2018, 55, 440–448. [Google Scholar] [CrossRef]
- Monetta, T.; Bellucci, F.; Nicodemo, L.; Nicolais, L. Protective properties of epoxy-based organic coatings on mild steel. Prog. Org. Coat. 1993, 21, 353–369. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Maruthan, K.; Jayakrishnan, P.; Rengaswamy, N.S. Coatings for underwater and wet surface application. Anti-Corros. Methods Mater. 1997, 44, 393–399. [Google Scholar] [CrossRef]
- Mally, T.S.; Johnston, A.L.; Chann, M.; Walker, R.H.; Keller, M.W. Performance of a carbon-fiber/epoxy composite for the underwater repair of pressure equipment. Compos. Struct. 2013, 100, 542–547. [Google Scholar] [CrossRef]
- Stark, E.B.; Ibrahim, A.M.; Munns, T.E.; Seferis, J.C. Moisture effects during cure of high-performance epoxy matrices. J. Appl. Polym. Sci. 1985, 30, 1717–1731. [Google Scholar] [CrossRef]
- Renato, I.; Grubisa, M.; Miskovic, D. Protection Coatings for the Underwater Part of Ship’s Hull. J. Marit. Transp. Sci. 2019, 55, 59–70. [Google Scholar] [CrossRef]
- Orlikowski, J.; Jażdżewska, A.; Jurak, K. Research on organic coatings designed for underwater applications. Arch. Metall. Mater. 2020, 65, 169–174. [Google Scholar] [CrossRef]
- Królikowska, A.; Komorowski, L.; Langer, E.; Zubielewicz, M. Promising Results of the Comparison of Coatings on Aged Bridges and of Same Coatings in Laboratory. Materials 2022, 15, 3064. [Google Scholar] [CrossRef]
- Eugenio, A.; Barbara, P.; Stefania, D.; Alfonso, M. Thermally Mendable Self-Healing Epoxy Coating for Corrosion Protection in Marine Environments. Materials 2023, 16, 1775. [Google Scholar] [CrossRef]
- Feng, Z.; Song, G.L.; Wang, Z.M.; Xu, Y.; Zheng, D.; Wu, P.; Chen, X. Sallt crystallization-assisted degradation of epoxy resin surface in simulated marine environments. Prog. Org. Coat. 2020, 149, 105932. [Google Scholar] [CrossRef]
- Xu, Y.; Song, G.; Zheng, D. The Combined Effect of Direct Current Polarization, Ultraviolet Radiation, and Saline Immersion on the Degradation of an Epoxy Coating. J. Mater. Eng. Perform. 2023, 33, 3570–3581. [Google Scholar] [CrossRef]
- Pagore, I.F.; Kol, G.R.; Betchewe, J.G. Analysis and numerical investigations on the temperature-dependent creep behavior of polypropylene matrix composite used for coating of offshore pipeline. J. Thermoplast. Compos. Mater. 2023, 36, 2076–2092. [Google Scholar] [CrossRef]
- Fan, H.M.; Zheng, T.; Chen, H.; Huang, J.; Wei, Z.; Kang, W.; Dai, C.; Zeng, H. Viscoelastic Surfactants with High Salt Tolerance, Fast-Dissolving Property, and Ultralow Interfacial Tension for Chemical Flooding in Offshore Oilfields. J. Surfactants Deterg. 2018, 21, 475–488. [Google Scholar] [CrossRef]
- Belzona 5831. Available online: www.belzona.com/viewfile.aspx?id=46367 (accessed on 29 June 2024).
- Specification S-715. In Supplementary Specification to NORSOK M-501 for Coating and Painting for Offshore, Marine, Coastal and Subsea Environment; International Association of Oil and Gas Producers: London, UK, 2020.
- Policastro, S.A.; Anderson, R.M.; Hangarter, C.M.; Arcari, A.; Iezzi, E.B. Incorporating Physics-Based Models into Equivalent Circuit Analysis of EIS Data from Organic Coatings. Coatings 2023, 13, 1285. [Google Scholar] [CrossRef]
- Schalenbach, M.; Durmus, Y.E.; Tempel, H.; Kungl, H.; Eichel, R.A. Double Layer Capacitances Analysed with Impedance Spectroscopy and Cyclic Voltammetry: Validity and Limits of the Constant Phase Element Parameterization. Phys. Chem. Chem. Phys. 2021, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Šolić, T.; Marić, D.; Peko, I.; Samardžić, I. Influence of Anticorrosive Pigment, Dry-Film Thickness and Conditioning Time on Protective Properties of Two-Component Epoxy Primer. Materials 2022, 15, 3041. [Google Scholar] [CrossRef]
- Clematis, D.; Marroccu, A.; Panizza, M.; Barbucci, A. A Critical Analysis on the Current Design Criteria for Cathodic Protection of Ships and Superyachts. Materials 2022, 15, 2645. [Google Scholar] [CrossRef]
- EN 12473:2014-04; General Principles of Cathodic Protection in Seawater. ISO: Geneva, Swizterland, 2014.








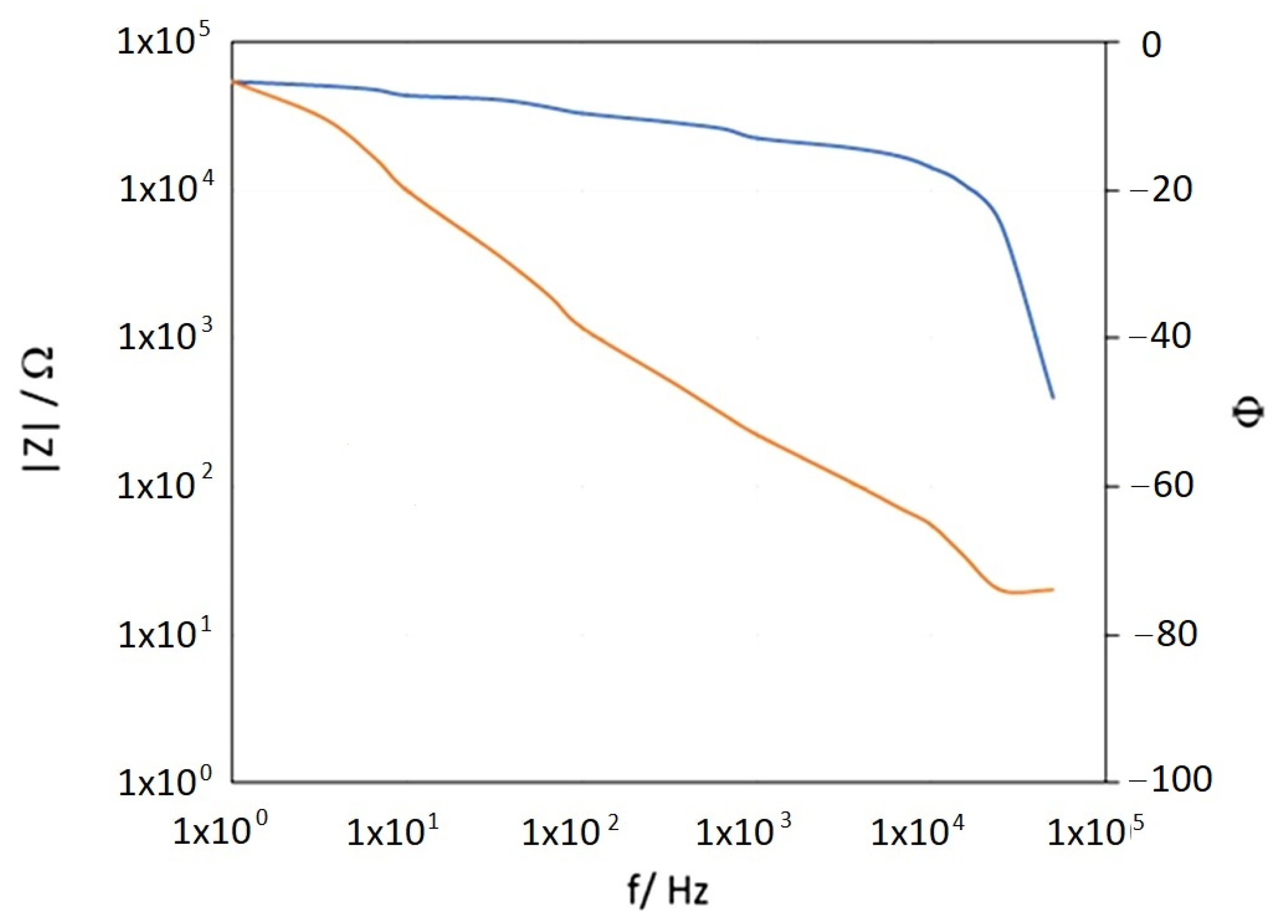
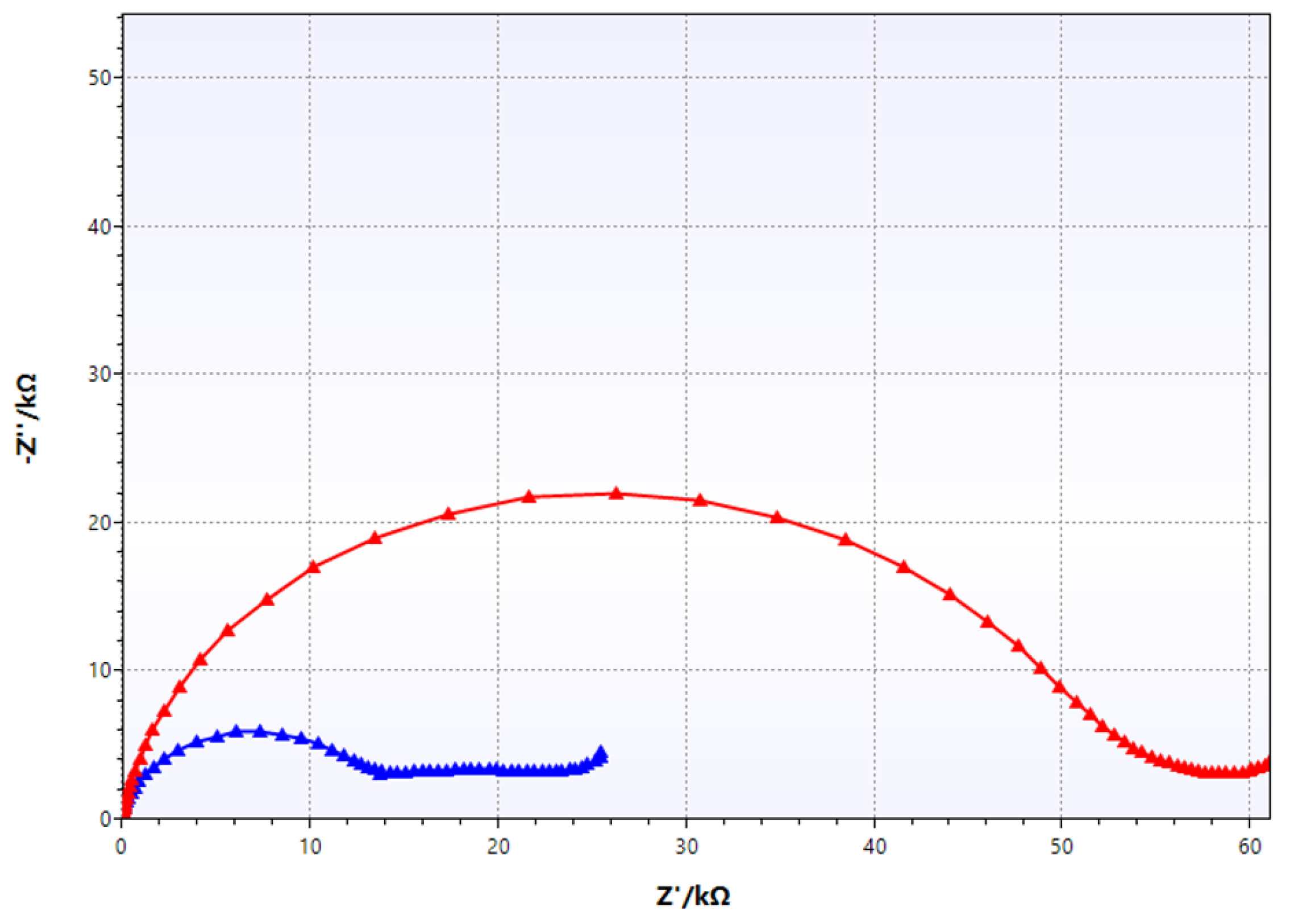
| Coating Application Conditions | Number of Coating Layers Applied | Average Coating Thickness [µm] | Coating Hardness [° Barcol] | Coating Detachment Force [N/mm2] |
|---|---|---|---|---|
| in water | 1 | 139 ± 18 | 48 ± 3 | 7.2 ± 0.5 |
| 2 | 229 ± 7 | 57 ± 3 | 12.2 ± 1.2 | |
| in air | 1 | 116 ± 17 | 36 ± 4 | 19.6 ± 0.9 |
| 2 | 304 ± 32 | 65 ± 2 | 23.4 ± 1.4 |
| Coating Application Conditions | Number of Coating Layers Applied | Coating Capacitance [F/cm2] | Coating Resistance [Ω∙cm2] |
|---|---|---|---|
| in water | 1 | 9.1 · 10−10 | 1.4 · 104 |
| 2 | 8.1 · 10−11 | 5.2 · 106 | |
| in air | 1 | 2.2 · 10−10 | 7.0 · 108 |
| 2 | 4.9 · 10−11 | 2.1 · 1010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlikowski, J.; Żakowski, K.; Szociński, M.; Igliński, P.; Jażdżewska, A.; Gaweł, Ł. Underwater Electrochemical Offshore Tests of a Paint Coating Applied in Water on the Legs of an Oil Production Platform. Materials 2024, 17, 3580. https://doi.org/10.3390/ma17143580
Orlikowski J, Żakowski K, Szociński M, Igliński P, Jażdżewska A, Gaweł Ł. Underwater Electrochemical Offshore Tests of a Paint Coating Applied in Water on the Legs of an Oil Production Platform. Materials. 2024; 17(14):3580. https://doi.org/10.3390/ma17143580
Chicago/Turabian StyleOrlikowski, Juliusz, Krzysztof Żakowski, Michał Szociński, Piotr Igliński, Agata Jażdżewska, and Łukasz Gaweł. 2024. "Underwater Electrochemical Offshore Tests of a Paint Coating Applied in Water on the Legs of an Oil Production Platform" Materials 17, no. 14: 3580. https://doi.org/10.3390/ma17143580






