Biochar from Co-Pyrolyzed Municipal Sewage Sludge (MSS): Part 1: Evaluating Types of Co-Substrates and Co-Pyrolysis Conditions
Abstract
:1. Introduction
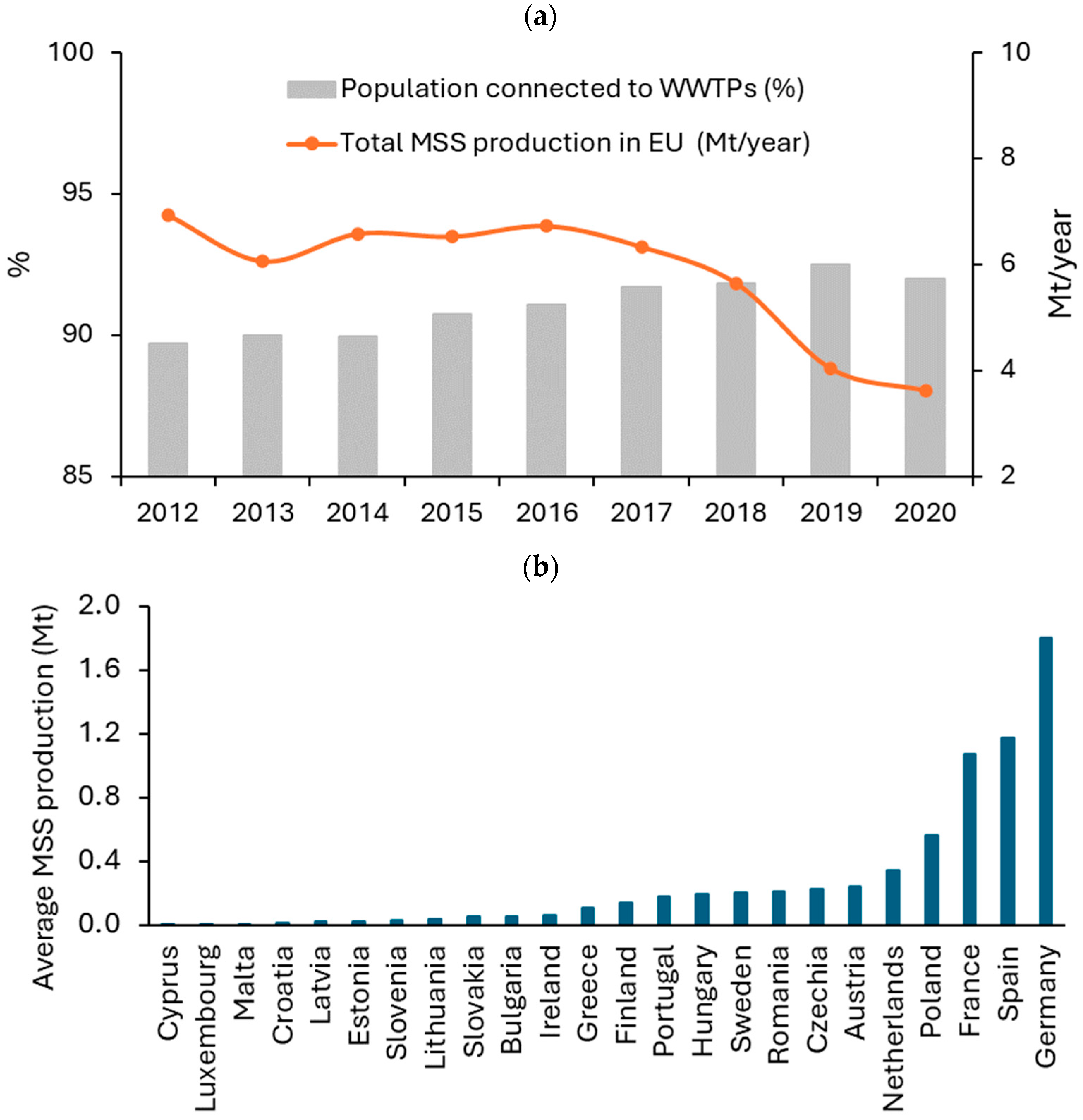
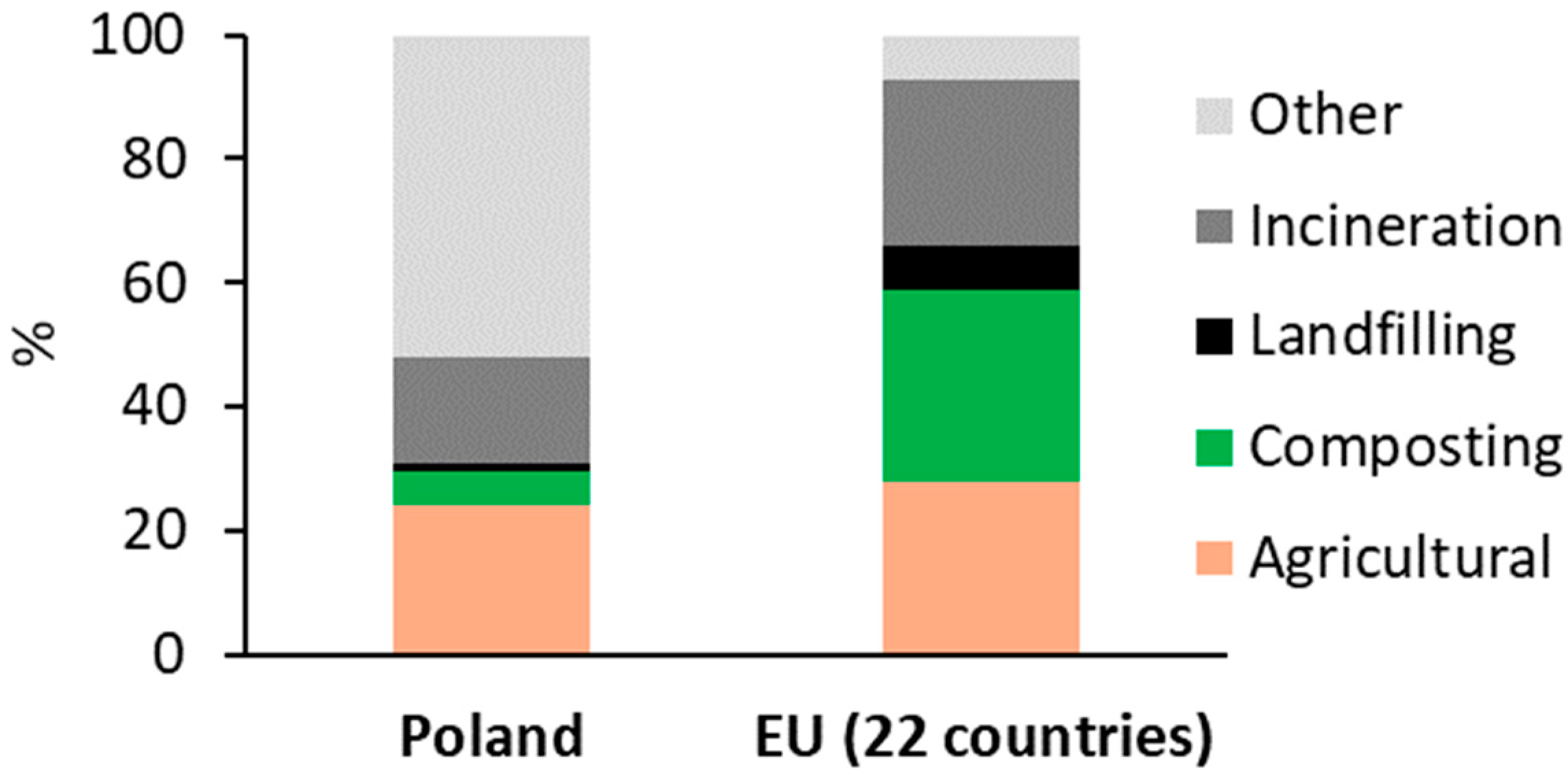
2. MSS as Feedstock for Biochar Production

3. MSS Pyrolysis and Co-Pyrolysis
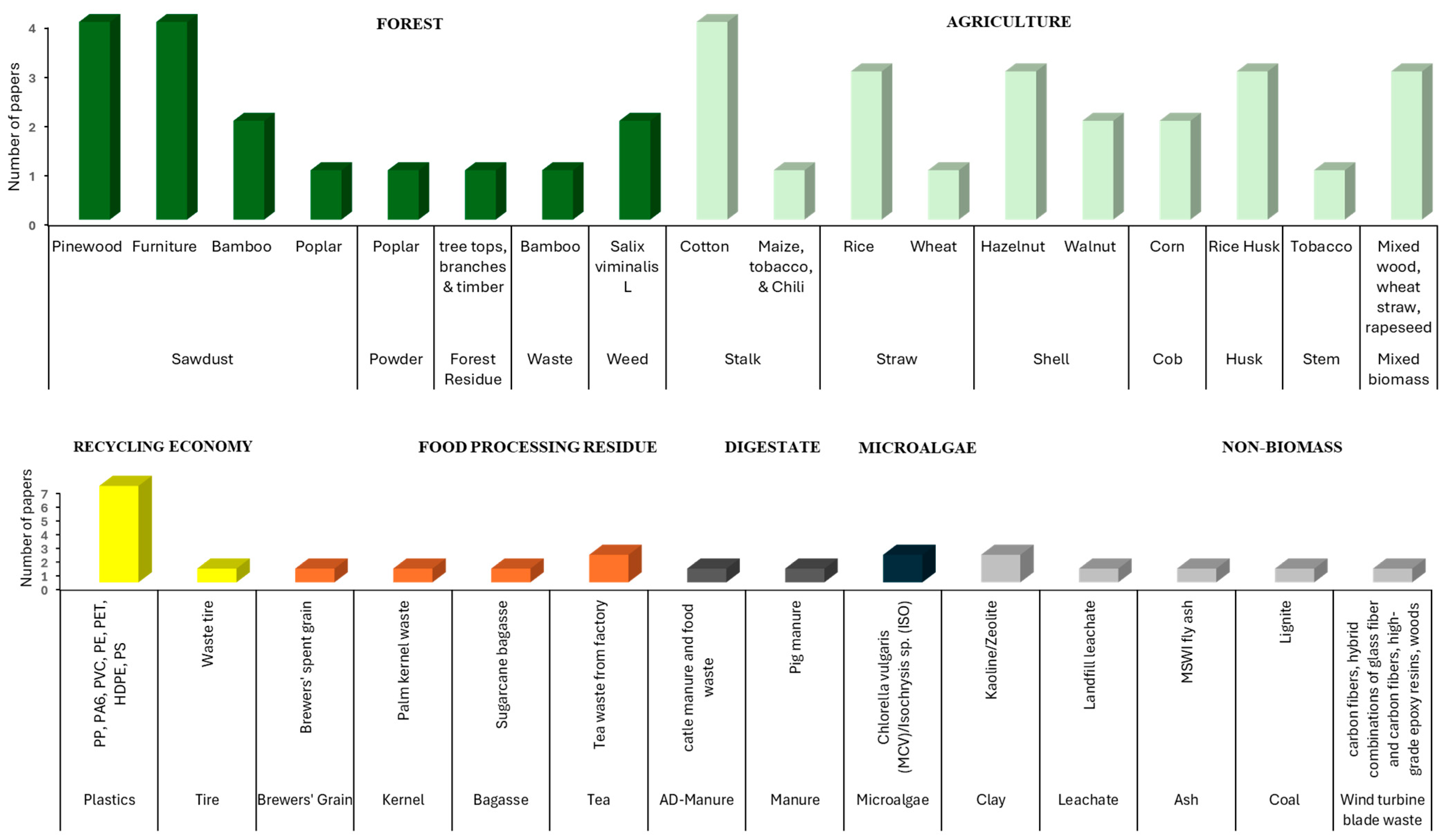
3.1. Types of Co-Substrates Used for MSS Co-Pyrolysis
3.2. Composition of Substrates Used in Co-Pyrolysis With MSS
3.2.1. Proximate Analysis
3.2.2. Ultimate Analysis
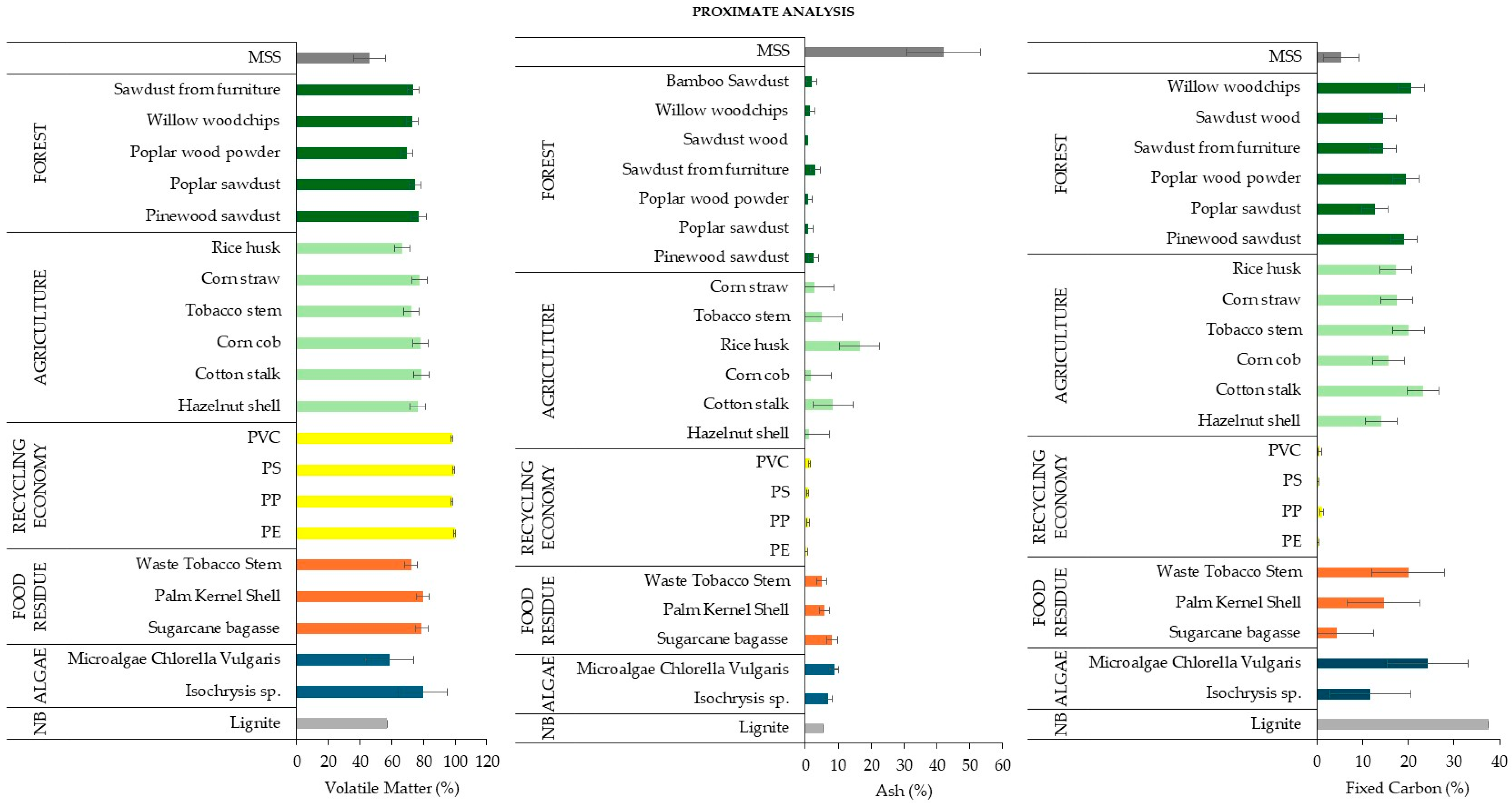
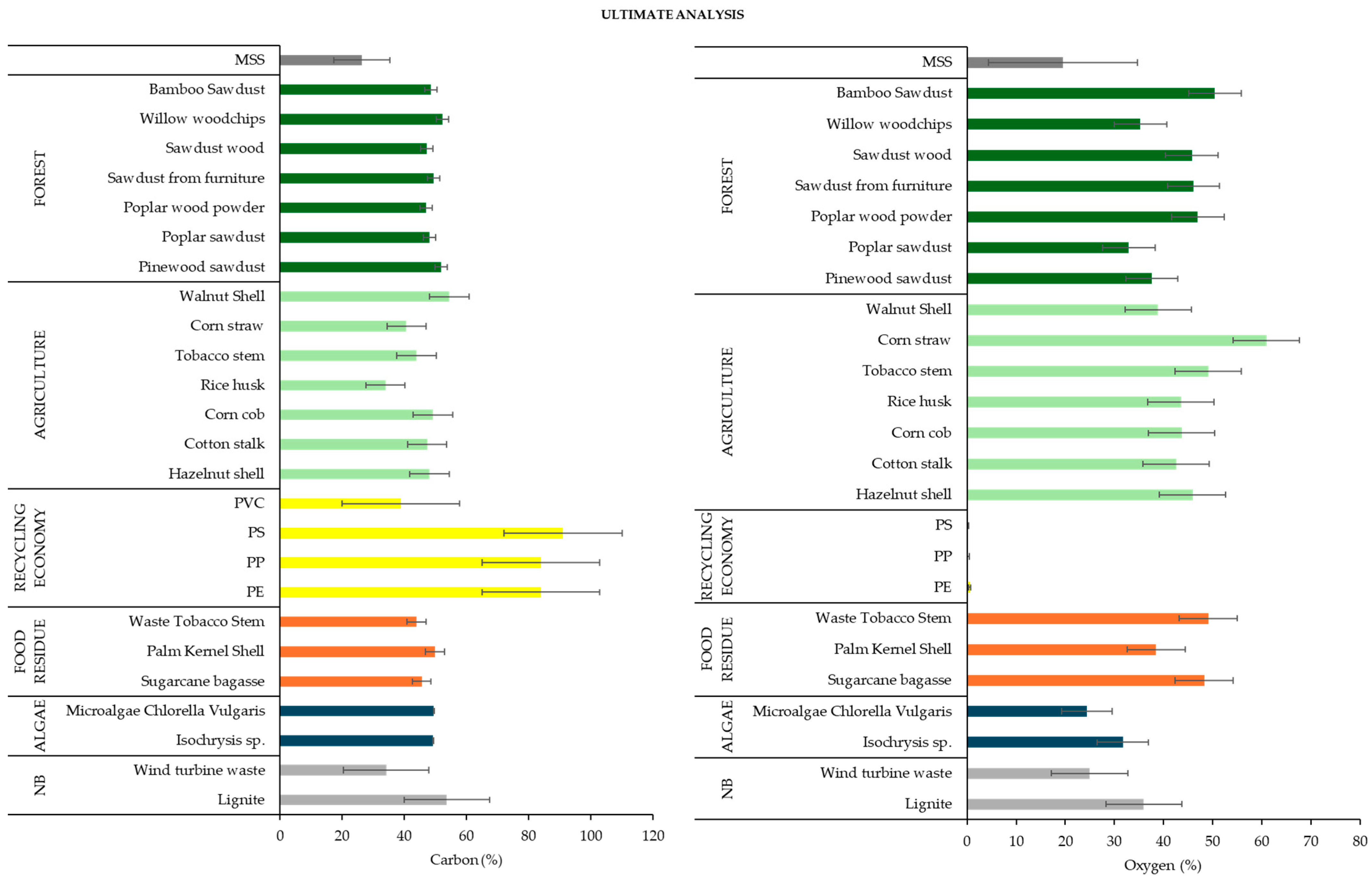
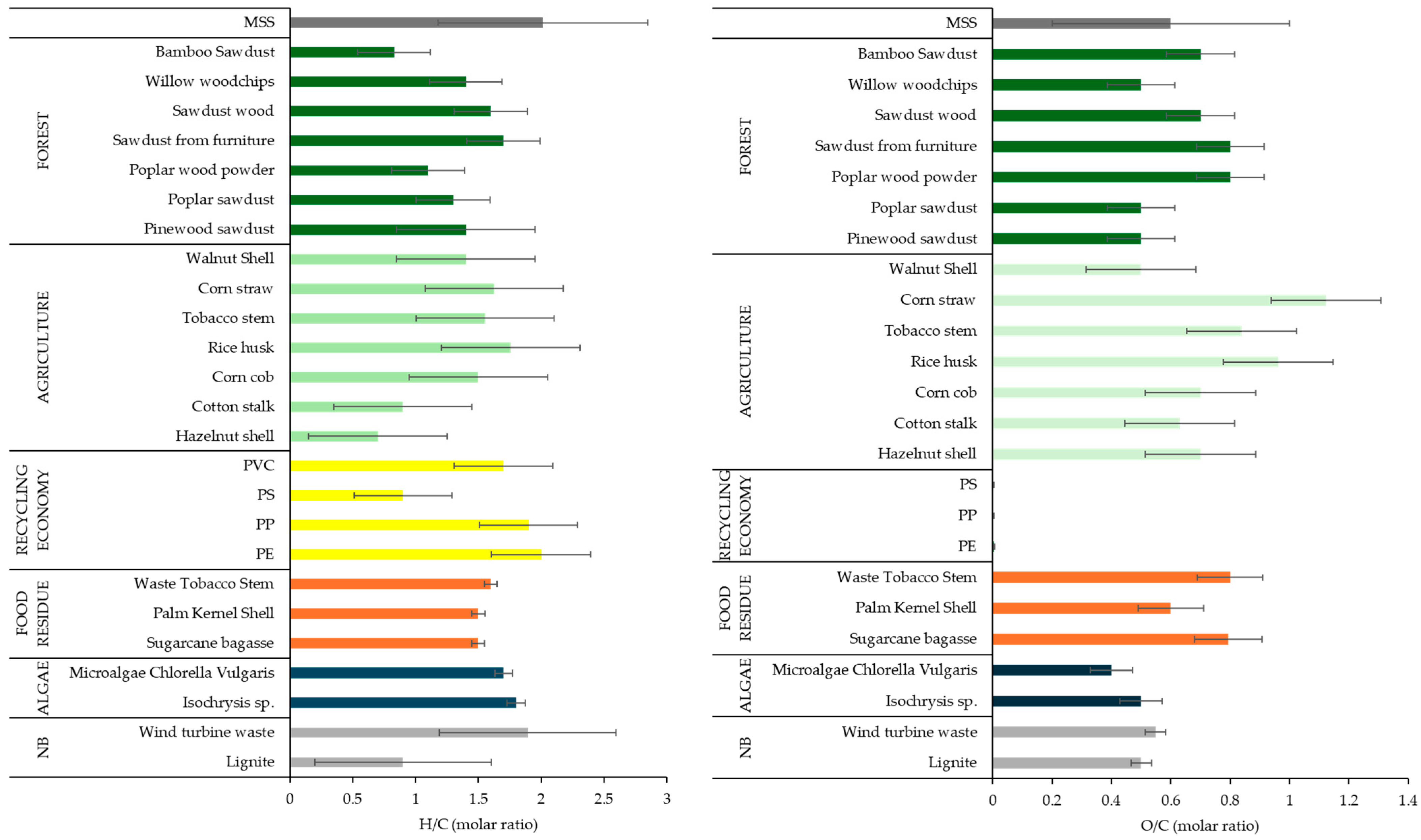
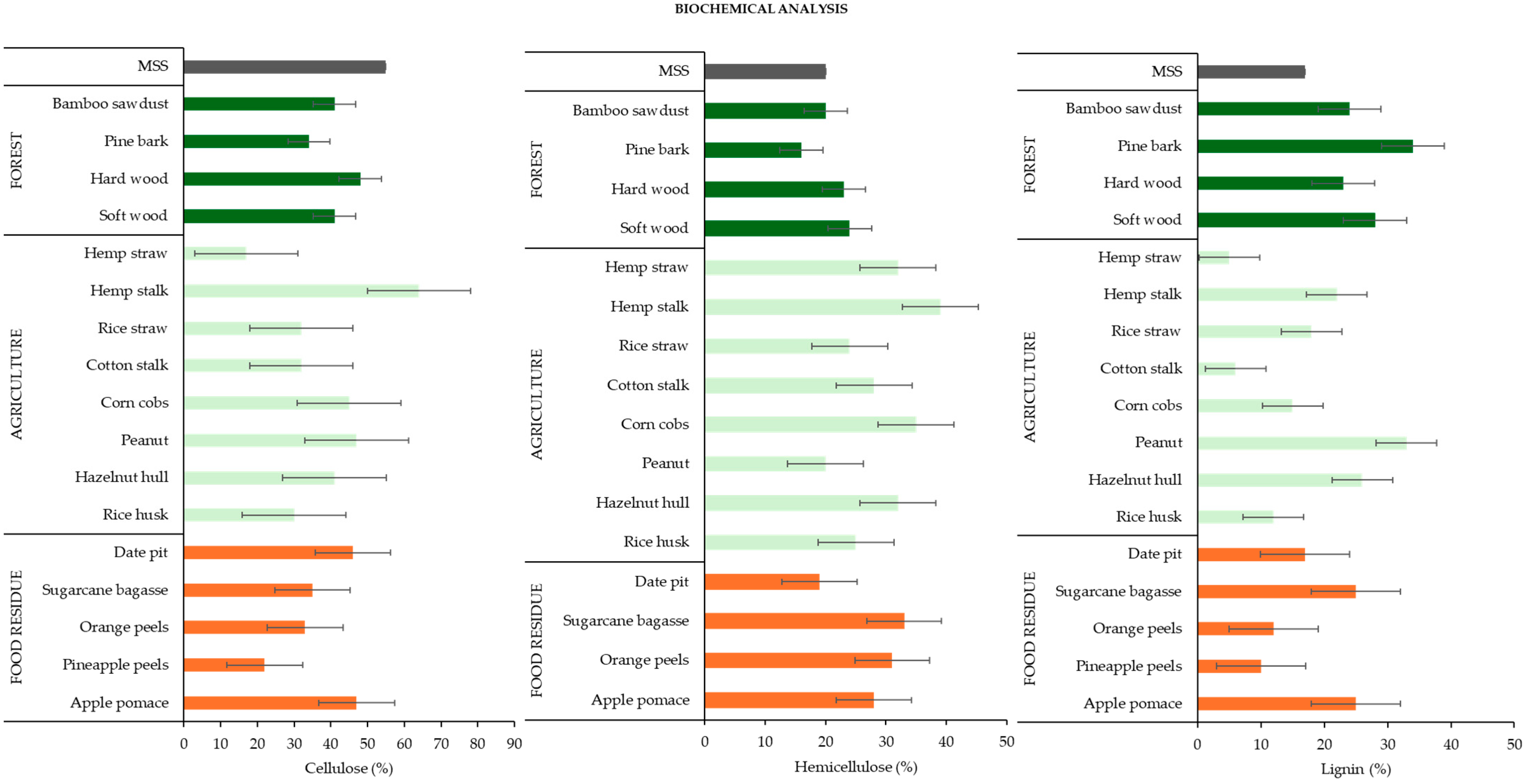
3.2.3. Biochemical Analysis
3.2.4. Particle Size and Density
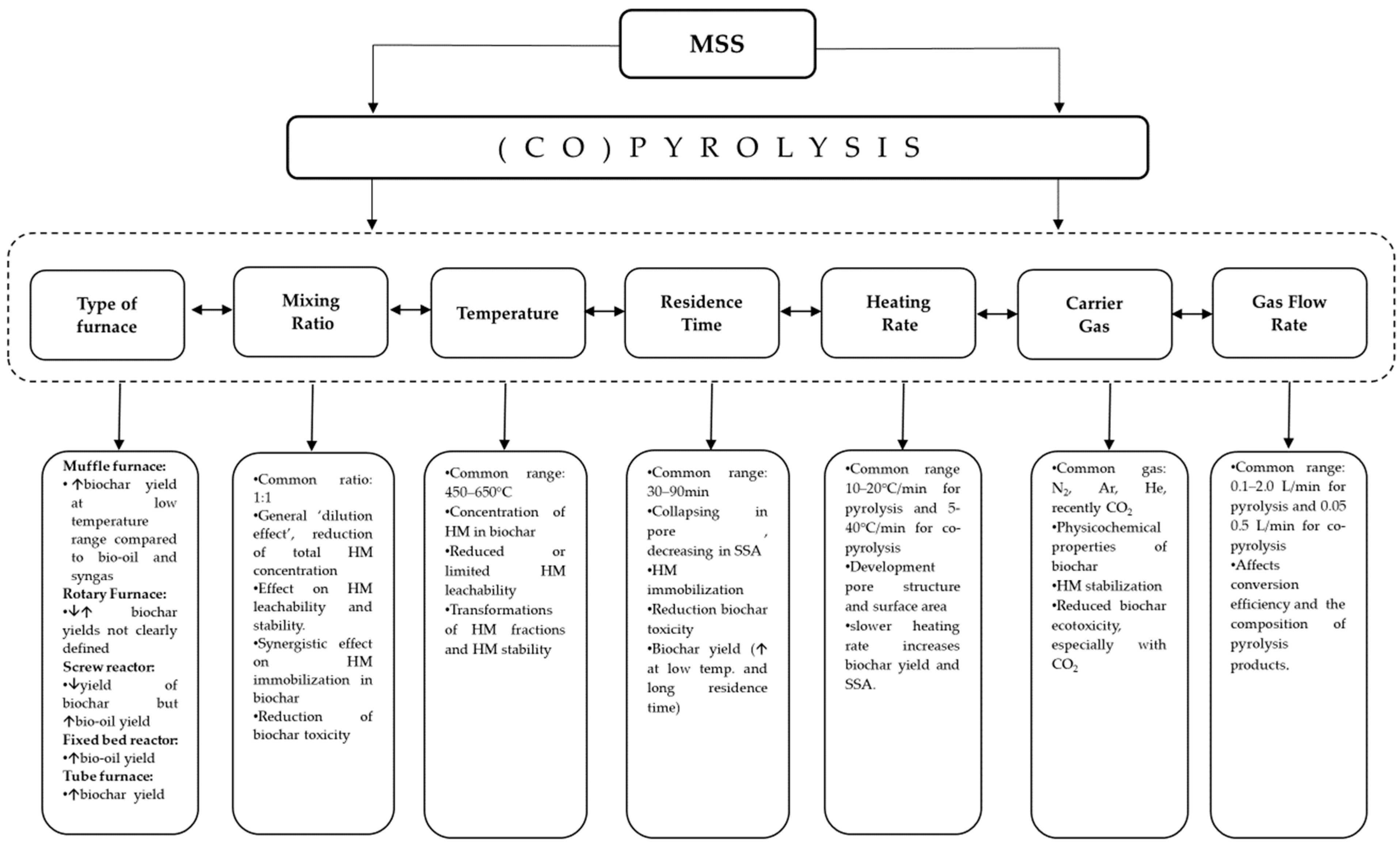
3.3. Conditions of MSS Pyrolysis and Co-Pyrolysis
3.3.1. Type of Furnace
| Feedstock/Mixing Ratio | Temp. (°C) | HR (°C/min) | RT (min) | CG | FR (L/min) | BY (%) | Research Aim | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Muffle Furnace | |||||||||||
| Pyrolysis | |||||||||||
| MSS | 350 | 10 | 120 | nr | nr | 88.3 | The impact of pyrolysis temperature on biochar characteristics | [193] | |||
| 400 | ca. 84 | ||||||||||
| 450 | ca. 82 | ||||||||||
| 500 | 64.7 | ||||||||||
| 200 | 10 | 120 | nr | nr | 92.2 | The impact of biochar produced at different temperatures on urban soil fertility and turf grass growth | [194] | ||||
| 300 | 81.7 | ||||||||||
| 500 | 67.8 | ||||||||||
| 700 | 65.1 | ||||||||||
| 300, 400, 500 | 11 | 30 | nr | nr | nr | The effects of temperature on the agro-chemical and physical properties of biochar | [195] | ||||
| 300 | 17 | 30 | N2 | 0.2 | 62.5 | Investigating the feasibility of biochar production from MSS pyrolysis | [196] | ||||
| 400 | 30 | 28.5 | |||||||||
| 500 | 30 | 27.3 | |||||||||
| 300 | 60 | 58.1 | |||||||||
| 400 | 60 | 25.5 | |||||||||
| 500 | 60 | 27.0 | |||||||||
| 300 | 90 | 64.2 | |||||||||
| 400 | 90 | 27.5 | |||||||||
| 500 | 90 | 31.0 | |||||||||
| Co-pyrolysis | |||||||||||
| MSS + Reed (RD) (P. australis) | 10 | 120 | N2 | 0.05 | Analysis of P and HM transformations in biochar | [154] | |||||
| MSS:RD 75:25 wt.% | 300 | ca. 61 | |||||||||
| 500 | ca. 58 | ||||||||||
| 700 | ca. 56 | ||||||||||
| MSS:RD 50:50 | 300 | ca. 54 | |||||||||
| 500 | ca. 49 | ||||||||||
| 700 | ca. 47 | ||||||||||
| MSS:RD 25:75 | 300 | ca. 49 | |||||||||
| 500 | ca. 45 | ||||||||||
| 700 | ca.42 | ||||||||||
| MSS + Rice Straw (RS) | 400 | nr | 120 | nr | nr | Cd immobilization in paddy soil under biochar amendment | [108] | ||||
| MSS:RS 1:3 wt.% | 51.5 | ||||||||||
| MSS:RS 1:2 | 54.3 | ||||||||||
| MSS:RS 1:1 | 59.3 | ||||||||||
| MSS:RS 2:1 | 64.0 | ||||||||||
| MSS:RS 3:1 | 66.1 | ||||||||||
| MSS + Tea Waste MSS:TW 1:1 | 300 | nr | 120 | nr | nr | 53.2 | Cd removal from aqueous solution | [114] | |||
| 53.2 | Methylene blue removal from aqueous solution | [117] | |||||||||
| MSS + Brewers’ Spent Grain (BSG) | 5 | 120 | N2 | 0.05 | Ammonia-nitrogen removal from aqueous solution | [138] | |||||
| MSS:BSG 8:2 wt.% | 400 | 46.8 | |||||||||
| 500 | 37.4 | ||||||||||
| 600 | 35.9 | ||||||||||
| 700 | 33.1 | ||||||||||
| MSS:BSG 6:4 | 400 | 50.1 | |||||||||
| 500 | 42.2 | ||||||||||
| 600 | 39.4 | ||||||||||
| 700 | 37.3 | ||||||||||
| MSS:BSG 4:6 | 400 | 54 | |||||||||
| 500 | 49.4 | ||||||||||
| 600 | 46.3 | ||||||||||
| 700 | 42.5 | ||||||||||
| MSS:BSG 2:8 | 400 | 59.6 | |||||||||
| 500 | 54.1 | ||||||||||
| 600 | 52.4 | ||||||||||
| 700 | 49 | ||||||||||
| Fixed-Bed Reactor | |||||||||||
| Pyrolysis | |||||||||||
| MSS | 500 | 20 | 20 | N2 | 0.02 | 59 | Investigating the conversions of MSS-nitrogen into primary product (NH3-N, HCN-N, HCN/NH3) | [197] | |||
| 600 | 52.4 | ||||||||||
| 700 800 | 47.2 45.6 | ||||||||||
| MSS | 700 | 10 | 60 | N2 | 0.2 | 38.7 | Investigating the impacts of organic and inorganic constituents on pyrolysis products | [198] | |||
| Co-pyrolysis | |||||||||||
| MSS + Poplar Wood (PW) | 800 | 10, 30 | 20 | N2 | 0.06 | Evaluating the synergistic effects of poplar wood co-substrate yield | [85] | ||||
| MSS:PW 8:2 wt.% | ca. 59 | ||||||||||
| MSS:PW 6:4 | ca. 53 | ||||||||||
| MSS:PW 4:6 | ca. 44 | ||||||||||
| MSS:PW 2:8 | ca. 35 | ||||||||||
| MSS + Pinewood Sawdust | 450 | 30 | 30 | N2 | 0.4 | ca. 54 | Investigating the synergistic effects of the product yield and distribution | [84] | |||
| 500 | ca. 53 | ||||||||||
| 550 | ca. 49 | ||||||||||
| 600 | ca. 48 | ||||||||||
| MSS + Pine Sawdust | 800 | 40 | 15 | H2 | nr | nr | Methylene blue removal from aqueous solution | [90] | |||
| MSS + Rice Husk | 900 | 10 | 120 | N2 | nr | 20 | Investigating the synergetic effects of co-substrate on gas and biochar production | [118] | |||
| MSS + Microalgae Isochrysis sp. (ISO) | 500 | 5, 10, 15, 20, 25 | nr | N2 | 0.4 | Evaluation of biocrude assessment and biochar yield | [135] | ||||
| MSS:ISO 1:1 wt.% | 35.3 | ||||||||||
| MSS:ISO 1:2 | 41.4 | ||||||||||
| MSS:ISO 2:1 | 46.2 | ||||||||||
| MSS + Lignite | 900 | 10 | 120 | N2 | nr | 24 | Analysis of product yields and composition of co-pyrolysis | [136] | |||
| Tube Furnance | |||||||||||
| Pyrolysis | |||||||||||
| MSS | 220 | nr | 30 | N2 | 1.7 | 91.3 | Analysis of the total and available contents of Ca, K, Mg, P, and S in biochar | [179] | |||
| 320 | 70.5 | ||||||||||
| 420 | 60.0 | ||||||||||
| 520 | 53.2 | ||||||||||
| 620 | 50.3 | ||||||||||
| 500, 700 | 10 | 60 | N2 | 0.1 | nr | Ferrous sulfate modification and treatment on biochar | [199] | ||||
| 300 | 12–13 | 120 | N2 | 2 | 84.4 | P removal from aqueous solution | [186] | ||||
| 400 | 66.4 | ||||||||||
| 500 | 60.5 | ||||||||||
| 600 | 58.4 | ||||||||||
| Co-pyrolysis | |||||||||||
| MSS + Bamboo Waste (4:1 mass ratio) | 700 | 10 | 30 | N2 | nr | nr | Ciprofloxacin removal from aqueous solutions | [103] | |||
| MSS + Hazelnut Shell (4:1 wt.%) | 900 | 10 | 90 | N2 | 0.3 | nr | Analyzing thermal decomposition reaction and interaction of biochar | [100] | |||
| MSS + Walnut Shell (3:1, 1:1, 1:3 mass ratio) | 600 | 10 | 180 | N2 | 0.3 | nr | Ammonium and phosphate removal from water | [101] | |||
| MSS + Wheat Straw (WS) | nr | 30 | N2 | 0.5 | Investigating the combustion reactivity of biochar | [105] | |||||
| MSS:WS 8:2 wt.% | 900 | ca. 25 | |||||||||
| MSS:WS 6:4 | ca. 20 | ||||||||||
| MSS:WS 4:6 | ca. 17 | ||||||||||
| MSS:WS 2:8 | ca. 14 | ||||||||||
| MSS + Waste Tire (0:10, 1:9, 3:7 wt.%) | 300, 500, 700 | 10 | 120 | N2 | 0.3 | nr | Cd and tetracycline removal from water | [123] | |||
| MSS + PP, PA6, PVC (8:2 wt.%) | 800 | 15 | 60 | N2 | 0.1 | nr | Analyzing the release of N, S, and Cl by different plastics share in biochar | [124] | |||
| MSS + Wind Turbine Blade Waste (8:2, 7:3, 4:6, 5:5 wt.%) | 600 | 10 | 60 | N2 | 0.1 | nr | Evaluation of wind blade co-substrate for carbon capture | [134] | |||
| MSS + Red Mud and Steel Slag (100:0, 80:20, 60:40, 33:67, 0:100 wt.%) | 900 | 10 | 120 | N2 | nr | nr | Tetracycline removal from wastewater | [200] | |||
| Microwave Reactor | |||||||||||
| Pyrolysis | |||||||||||
| MSS | nr | nr | 10 | N2 | 0.01 | nr | Cu removal from aqueous solution | [201] | |||
| Co-pyrolysis | |||||||||||
| MSS + Cotton Stalk (CS) | nr | 30 | N2 | nr | The effect of co-substrate on biochar properties | [177] | |||||
| 17:3 (MSS:CS) wt.% | 450 | 49.62 | |||||||||
| 550 | ca. 43 | ||||||||||
| 650 | ca. 41.5 | ||||||||||
| 750 | 35.16 | ||||||||||
| 7:3 (MSS:CS) | 450 | 43.25 | |||||||||
| 550 | ca. 39.5 | ||||||||||
| 650 | ca. 36.5 | ||||||||||
| 750 | 31.41 | ||||||||||
| 700 | |||||||||||
| 800 | |||||||||||
| 900 | |||||||||||
| 1000 | |||||||||||
| Categories of co-substrates: | |||||||||||
| Forest | Agriculture | Recycling economy | Food residues | Algae | Non-biomass | ||||||
3.3.2. Mixing Ratio
3.3.3. Temperature
3.3.4. Residence Time
3.3.5. Heating Rate
3.3.6. Type of Inert Gas and Carrier Gas Flowrate
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Hu, J.; Lee, D.-J.; Chang, Y.; Lee, Y.-J. Sludge Treatment: Current Research Trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Ehalt Macedo, H.; Lehner, B.; Nicell, J.; Grill, G.; Li, J.; Limtong, A.; Shakya, R. Distribution and Characteristics of Wastewater Treatment Plants within the Global River Network. Earth Syst. Sci. Data 2022, 14, 559–577. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and Regional Potential of Wastewater as a Water, Nutrient and Energy Source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Shaddel, S.; Bakhtiary-Davijany, H.; Kabbe, C.; Dadgar, F.; Østerhus, S. Sustainable Sewage Sludge Management: From Current Practices to Emerging Nutrient Recovery Technologies. Sustainability 2019, 11, 3435. [Google Scholar] [CrossRef]
- Quan, L.M.; Kamyab, H.; Yuzir, A.; Ashokkumar, V.; Hosseini, S.E.; Balasubramanian, B.; Kirpichnikova, I. Review of the Application of Gasification and Combustion Technology and Waste-to-Energy Technologies in Sewage Sludge Treatment. Fuel 2022, 316, 123199. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Wang, D.; Yang, G.; Pan, L.; Wang, Q.; Ni, B.-J.; Li, H.; Yuan, X.; Jiang, L.; et al. Fe(II) Catalyzing Sodium Percarbonate Facilitates the Dewaterability of Waste Activated Sludge: Performance, Mechanism, and Implication. Water Res. 2020, 174, 115626. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, J.; Zhang, S.; Yang, X.; Huang, S.; Zheng, L.; Ye, M.; Sun, S. A Highly Efficient Conditioning Process to Improve Sludge Dewaterability by Combining Calcium Hypochlorite Oxidation, Ferric Coagulant Re-Flocculation, and Walnut Shell Skeleton Construction. Chem. Eng. J. 2019, 361, 1462–1478. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Ren, X.; Hu, X.; Ji, B. Recent Research on Municipal Sludge as Soil Fertilizer in China: A Review. Water Air Soil Pollut. 2023, 234, 119. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, Y.; Yoochatchaval, W.; Sumino, H.; Banjongproo, P.; Yamaguchi, T.; Onodera, T.; Okadera, T.; Syutsubo, K. Evaluation of the Process Performance of a Down-Flow Hanging Sponge Reactor for Direct Treatment of Domestic Wastewater in Bangkok, Thailand. J. Environ. Sci. Health Part A 2017, 52, 956–970. [Google Scholar] [CrossRef]
- Statistics|Eurostat. Available online: https://Ec.Europa.Eu/Eurostat/Databrowser/View/Sdg_06_20/Default/Table?Lang=en (accessed on 5 March 2024).
- O’Boyle, M.; Mohamed, B.A.; Li, L.Y. Co-Pyrolysis of Sewage Sludge and Biomass Waste into Biofuels and Biochar: A Comprehensive Feasibility Study Using a Circular Economy Approach. Chemosphere 2024, 350, 141074. [Google Scholar] [CrossRef]
- GUS Environment. Available online: https://Stat.Gov.Pl/En/Topics/Environment-Energy/Environment/Environment-2023,1,15.Html (accessed on 5 March 2024).
- Kelessidis, A.; Stasinakis, A.S. Comparative Study of the Methods Used for Treatment and Final Disposal of Sewage Sludge in European Countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; He, Z.L.; Stoffella, P.J. Land Application of Biosolids in the USA: A Review. Appl. Environ. Soil Sci. 2012, 2012, 201462. [Google Scholar] [CrossRef]
- Shahbeig, H.; Nosrati, M. Pyrolysis of Municipal Sewage Sludge for Bioenergy Production: Thermo-Kinetic Studies, Evolved Gas Analysis, and Techno-Socio-Economic Assessment. Renew. Sustain. Energy Rev. 2020, 119, 109567. [Google Scholar] [CrossRef]
- Lv, M.; Yu, H.; Shang, X. Sludge-Derived Biochar: A Review on the Influence of Synthesis Conditions on Environmental Risk Reduction and Removal Mechanism of Wastewater Pollutants. Arch. Environ. Prot. 2023, 144, 111068. [Google Scholar]
- Mohamed, B.A.; Ruan, R.; Bilal, M.; Khan, N.A.; Awasthi, M.K.; Amer, M.A.; Leng, L.; Hamouda, M.A.; Vo, D.N.; Li, J. Co-Pyrolysis of Sewage Sludge and Biomass for Stabilizing Heavy Metals and Reducing Biochar Toxicity: A Review. Environ. Chem. Lett. 2023, 21, 1231–1250. [Google Scholar] [CrossRef]
- Fan, Z.; Zhou, X.; Peng, Z.; Wan, S.; Gao, Z.F.; Deng, S.; Tong, L.; Han, W.; Chen, X. Co-Pyrolysis Technology for Enhancing the Functionality of Sewage Sludge Biochar and Immobilizing Heavy Metals. Chemosphere 2023, 317, 137929. [Google Scholar] [CrossRef] [PubMed]
- Volpi, M.P.C.; Silva, J.C.G.; Hornung, A.; Ouadi, M. Review of the Current State of Pyrolysis and Biochar Utilization in Europe: A Scientific Perspective. Clean. Technol. 2024, 6, 152–175. [Google Scholar] [CrossRef]
- Kacprzak, M. Sewage Sludge as a Source of Organic to Be Used as Soil Improvement; Elsevier: Amsterdam, The Netherlands, 2023; pp. 303–316. [Google Scholar]
- Boumalek, W.; Kettab, A.; Bensacia, N.; Bruzzoniti, M.C.; Ben Othman, D.; Mandi, L.; Chabaca, M.N.; Benziada, S. Specification of Sewage Sludge Arising from a Domestic Wastewater Treatment Plant for Agricultural Uses. Desalination Water Treat. 2019, 143, 178–183. [Google Scholar] [CrossRef]
- Demirbas, A.; Edris, G.; Alalayah, W.M. Sludge Production from Municipal Wastewater Treatment in Sewage Treatment Plant. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 999–1006. [Google Scholar] [CrossRef]
- Di Giacomo, G.; Romano, P. Evolution and Prospects in Managing Sewage Sludge Resulting from Municipal Wastewater Purification. Energies 2022, 15, 5633. [Google Scholar] [CrossRef]
- Liu, T.; Liu, B.; Zhang, W. Nutrients and Heavy Metals in Biochar Produced by Sewage Sludge Pyrolysis: Its Application in Soil Amendment. Pol. J. Environ. Stud. 2014, 23, 271–275. [Google Scholar]
- Zhou, D.; Liu, D.; Gao, F.; Li, M.; Luo, X. Effects of Biochar-Derived Sewage Sludge on Heavy Metal Adsorption and Immobilization in Soils. Int. J. Environ. Res. Public Health 2017, 14, 681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, Z.F.; Pan, X.W.; Tan, J.Y.; Yang, S.S.; Wu, J.T.; Chen, C.; Yuan, Y.; Ren, N.Q. Sewage Sludge Derived Biochar for Environmental Improvement: Advances, Challenges, and Solutions. Water Res. X 2023, 18, 100167. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Rene, E.R.; Wang, J.; Ma, W. Biodegradation of Polyaromatic Hydrocarbons and the Influence of Environmental Factors during the Co-Composting of Sewage Sludge and Green Forest Waste. Bioresour. Technol. 2020, 297, 122434. [Google Scholar] [CrossRef] [PubMed]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The Presence of Contaminations in Sewage Sludge—The Current Situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-Z.; Venkatesan, A.K.; Ni, Y.-L.; Steele, J.C.; Wu, L.-L.; Bignert, A.; Bergman, Å.; Halden, R.U. Organic Contaminants in Chinese Sewage Sludge: A Meta-Analysis of the Literature of the Past 30 Years. Environ. Sci. Technol. 2016, 50, 5454–5466. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.O.; Smith, S.R. Review of ‘Emerging’ Organic Contaminants in Biosolids and Assessment of International Research Priorities for the Agricultural Use of Biosolids. Environ. Int. 2011, 37, 226–247. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, X.; Zeng, F.; Li, H.; Chen, X. The Hierarchical Porous Structure Bio-Char Assessments Produced by Co-Pyrolysis of Municipal Sewage Sludge and Hazelnut Shell and Cu(II) Adsorption Kinetics. Environ. Sci. Pollut. Res. 2018, 25, 19423–19435. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Huang, H.; Zhao, D.; Kobayashi, N.; Chen, Y. Influence of Pyrolysis Temperature on Physical and Chemical Properties of Biochar Made from Sewage Sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Pöykiö, R.; Watkins, G.; Dahl, O. Characterisation of Municipal Sewage Sludge as a Soil Improver and a Fertilizer Product. Ecol. Chem. Eng. S 2019, 26, 547–557. [Google Scholar] [CrossRef]
- Fonts, I.; Azuara, M.; Gea, G.; Murillo, M.B. Study of the Pyrolysis Liquids Obtained from Different Sewage Sludge. J. Anal. Appl. Pyrolysis 2009, 85, 184–191. [Google Scholar] [CrossRef]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of Microplastics in a Major Secondary Wastewater Treatment Plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in Sewage Sludge from the Wastewater Treatment Plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, Identification and Removal of Microplastic Particles and Fibers in Conventional Activated Sludge Process and Advanced MBR Technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, Y. Treatment Characteristics of Microplastics at Biological Sewage Treatment Facilities in Korea. Mar. Pollut. Bull. 2018, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and Fate of Microplastic Particles in Wastewater Treatment Plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2014. [Google Scholar]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hurley, R.; Vogelsang, C.; Nizzetto, L.; Olsen, M. Mapping Microplastics in Sludge; Norsk Institutt for Vannforskning: Økernveien, Norway, 2017. [Google Scholar]
- Brandsma, S.H.; Nijssen, P.; van Velzen, M.J.M.; Lesile, H.A. Microplastics in River Suspended Particulate Matter and Sewage Treatment Plants; Institute for Environmental Studies: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Košnář, Z.; Mercl, F.; Pierdonà, L.; Chane, A.D.; Míchal, P.; Tlustoš, P. Concentration of the Main Persistent Organic Pollutants in Sewage Sludge in Relation to Wastewater Treatment Plant Parameters and Sludge Stabilisation. Environ. Pollut. 2023, 333, 122060. [Google Scholar] [CrossRef]
- Wluka, A.-K.; Huang, Y.; Coenen, L.; Dsikowitzky, L.; Schwarzbauer, J. Structural Diversity of Organic Contaminants in Sewage Sludge: A Comparison of Sewage Fingerprints from Germany and China. Discov. Water 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Pakou, C.; Lyberatos, G. Occurrence, Toxicity, and Biodegradation of Selected Emerging Priority Pollutants in Municipal Sewage Sludge; Elsevier: Amsterdam, The Netherlands, 2011; pp. 473–484. [Google Scholar]
- Beduk, F.; Aydin, S.; Ulvi, A.; Aydin, M.E. Persistent Organic Pollutants in Sewage Sludge: Occurrence, Temporal Concentration Variation and Risk Assessment for Sewage Sludge Amended Soils. KSCE J. Civ. Eng. 2023, 27, 3694–3704. [Google Scholar] [CrossRef]
- Riva, F.; Zuccato, E.; Pacciani, C.; Colombo, A.; Castiglioni, S. A Multi-Residue Analytical Method for Extraction and Analysis of Pharmaceuticals and Other Selected Emerging Contaminants in Sewage Sludge. Anal. Methods 2021, 13, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, O.S.; Ventouri, E.I.; Stasinakis, A.S.; Thomaidis, N.S. Occurrence of Different Classes of Perfluorinated Compounds in Greek Wastewater Treatment Plants and Determination of Their Solid–Water Distribution Coefficients. J. Hazard. Mater. 2012, 239–240, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Gassmann, M.; Falk, S.; Brunn, H. Concentrations and Distribution Patterns of Perfluoroalkyl Acids in Sewage Sludge and in Biowaste in Hesse, Germany. J. Agric. Food Chem. 2018, 66, 10147–10153. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarasimman, N.; Gewurtz, S.B.; Parker, W.J.; Smyth, S.A. Removal and Formation of Perfluoroalkyl Substances in Canadian Sludge Treatment Systems—A Mass Balance Approach. Sci. Total Environ. 2021, 754, 142431. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.K.; Halden, R.U. National Inventory of Perfluoroalkyl Substances in Archived U.S. Biosolids from the 2001 EPA National Sewage Sludge Survey. J. Hazard. Mater. 2013, 252–253, 413–418. [Google Scholar] [CrossRef]
- Dąbrowska, L.; Rosińska, A. Change of PCBs and Forms of Heavy Metals in Sewage Sludge during Thermophilic Anaerobic Digestion. Chemosphere 2012, 88, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.J.M.; Gomez, M.J.; Herrera, S.; Hernando, M.D.; Agüera, A.; Fernández-Alba, A.R. Occurrence and Persistence of Organic Emerging Contaminants and Priority Pollutants in Five Sewage Treatment Plants of Spain: Two Years Pilot Survey Monitoring. Environ. Pollut. 2012, 164, 267–273. [Google Scholar] [CrossRef]
- Cristaldi, A.; Fiore, M.; Zuccarello, P.; Oliveri Conti, G.; Grasso, A.; Nicolosi, I.; Copat, C.; Ferrante, M. Efficiency of Wastewater Treatment Plants (WWTPs) for Microplastic Removal: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8014. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A Review of the Removal of Microplastics in Global Wastewater Treatment Plants: Characteristics and Mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Bai, X.; Ye, Z. Removal and Generation of Microplastics in Wastewater Treatment Plants: A Review. J. Clean. Prod. 2021, 291, 125982. [Google Scholar] [CrossRef]
- Wojciula, A.; Boruszko, D.; Pajewska, G. Analysis of Heavy Metal Fraction Content in Sewage Sludge from Selected Wastewater Treatment Plants. J. Ecol. Eng. 2021, 22, 98–105. [Google Scholar] [CrossRef]
- Schmidt, H.; Bucheli, T.; Kammann, C.; Glaser, B. European Biochar Certificate-Guidelines for a Sustainable Production of Biochar; European Biochar Certificate: Arbaz, Switzerland, 2016. [Google Scholar]
- EBC and WBC Guidelines & Documents. Available online: https://www.european-biochar.org/en/ct/2-EBC-and-WBC-guidelines-documents (accessed on 24 January 2024).
- Hu, M.; Guo, D.; Ma, C.; Luo, S.; Chen, X.; Cheng, Q.; Laghari, M.; Xiao, B. A Novel Pilot-Scale Production of Fuel Gas by Allothermal Biomass Gasification Using Biomass Micron Fuel (BMF) as External Heat Source. Clean. Technol. Environ. Policy 2016, 18, 743–751. [Google Scholar] [CrossRef]
- Inguanzo, M.; Domínguez, A.; Menéndez, J.A.; Blanco, C.G.; Pis, J.J. On the Pyrolysis of Sewage Sludge: The Influence of Pyrolysis Conditions on Solid, Liquid and Gas Fractions. J. Anal. Appl. Pyrolysis 2002, 63, 209–222. [Google Scholar] [CrossRef]
- Gao, N.; Quan, C.; Liu, B.; Li, Z.; Wu, C.; Li, A. Continuous Pyrolysis of Sewage Sludge in a Screw-Feeding Reactor: Products Characterization and Ecological Risk Assessment of Heavy Metals. Energy Fuels 2017, 31, 5063–5072. [Google Scholar] [CrossRef]
- Jaramillo-Arango, A.; Fonts, I.; Chejne, F.; Arauzo, J. Product Compositions from Sewage Sludge Pyrolysis in a Fluidized Bed and Correlations with Temperature. J. Anal. Appl. Pyrolysis 2016, 121, 287–296. [Google Scholar] [CrossRef]
- Chen, X.; Jeyaseelan, S. Study of Sewage Sludge Pyrolysis Mechanism and Mathematical Modeling. J. Environ. Eng. 2001, 127, 585–593. [Google Scholar] [CrossRef]
- Shao, J.; Yan, R.; Chen, H.; Wang, B.; Lee, D.H.; Liang, D.T. Pyrolysis Characteristics and Kinetics of Sewage Sludge by Thermogravimetry Fourier Transform Infrared Analysis. Energy Fuels 2008, 22, 38–45. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; dos Santos Lins, P.V.; Oliveira, L.M.T.d.M.; Sepulveda, P.; Ighalo, J.O.; Rajapaksha, A.U.; Meili, L. Sewage Sludge-Derived Biochar for the Adsorptive Removal of Wastewater Pollutants: A Critical Review. Environ. Pollut. 2022, 293, 118581. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P. The Conversion of Sewage Sludge into Biochar Reduces Polycyclic Aromatic Hydrocarbon Content and Ecotoxicity but Increases Trace Metal Content. Biomass Bioenergy 2015, 75, 235–244. [Google Scholar] [CrossRef]
- Wang, X.; Chang, V.W.C.; Li, Z.; Chen, Z.; Wang, Y. Co-Pyrolysis of Sewage Sludge and Organic Fractions of Municipal Solid Waste: Synergistic Effects on Biochar Properties and the Environmental Risk of Heavy Metals. J. Hazard. Mater. 2021, 412, 125200. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, T.; Lai, F.; Wu, G. Co-Pyrolysis of Sewage Sludge and Sawdust/Rice Straw for the Production of Biochar. J. Anal. Appl. Pyrolysis 2017, 125, 61–68. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Towards Sewage Sludge Based Biofuels via Thermochemical Conversion—A Review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Jin, J.; Wang, M.; Cao, Y.; Wu, S.; Liang, P.; Li, Y.; Zhang, J.; Zhang, J.; Wong, M.H.; Shan, S.; et al. Cumulative Effects of Bamboo Sawdust Addition on Pyrolysis of Sewage Sludge: Biochar Properties and Environmental Risk from Metals. Bioresour. Technol. 2017, 228, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, K.; Xie, L.; Zhu, H.; Ji, S.; Shu, X. Effects of Residence Time on Characteristics of Biochars Prepared via Co-Pyrolysis of Sewage Sludge and Cotton Stalks. J. Anal. Appl. Pyrolysis 2019, 142, 104659. [Google Scholar] [CrossRef]
- Kończak, M.; Oleszczuk, P.; Różyło, K. Application of Different Carrying Gases and Ratio between Sewage Sludge and Willow for Engineered (Smart) Biochar Production. J. CO2 Util. 2019, 29, 20–28. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, L.; Liu, K.; Wang, J.; Zhu, H.; Song, Q.; Shu, X. Co-Pyrolysis of Sewage Sludge and Cotton Stalks. Waste Manag. 2019, 89, 430–438. [Google Scholar] [CrossRef]
- Li, K.; Zhang, D.; Niu, X.; Guo, H.; Yu, Y.; Tang, Z.; Lin, Z.; Fu, M. Insights into CO2 Adsorption on KOH-Activated Biochars Derived from the Mixed Sewage Sludge and Pine Sawdust. Sci. Total Environ. 2022, 826, 154133. [Google Scholar] [CrossRef]
- Ndoung, O.C.N.; de Souza, L.R.; Fachini, J.; Leão, T.P.; Sandri, D.; Figueiredo, C.C. de Dynamics of Potassium Released from Sewage Sludge Biochar Fertilizers in Soil. J. Environ. Manag. 2023, 346, 119057. [Google Scholar] [CrossRef]
- Han, H.; Buss, W.; Zheng, Y.; Song, P.; Khalid Rafiq, M.; Liu, P.; Mašek, O.; Li, X. Contaminants in Biochar and Suggested Mitigation Measures—A Review. Chem. Eng. J. 2022, 429, 132287. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Bai, L.; Han, C.; Sun, X. Application of Biochar-Based Materials for Remediation of Arsenic Contaminated Soil and Water: Preparation, Modification, and Mechanisms. J. Environ. Chem. Eng. 2022, 10, 108292. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, L.; Luo, J.; Gong, H.; Zhu, N. A Sustainable Reuse Strategy of Converting Waste Activated Sludge into Biochar for Contaminants Removal from Water: Modifications, Applications and Perspectives. J. Hazard. Mater. 2022, 438, 129437. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Q.; Cui, M.-H.; Guo, J.-C.; Du, J.-J.; Zheng, Z.-Y.; Liu, H. Effects of Co-Pyrolysis of Rice Husk and Sewage Sludge on the Bioavailability and Environmental Risks of Pb and Cd. Environ. Technol. 2021, 42, 2304–2312. [Google Scholar] [CrossRef]
- Wang, X.; Wei-Chung Chang, V.; Li, Z.; Song, Y.; Li, C.; Wang, Y. Co-Pyrolysis of Sewage Sludge and Food Waste Digestate to Synergistically Improve Biochar Characteristics and Heavy Metals Immobilization. Waste Manag. 2022, 141, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Fu, X.; Lv, Q.; Chen, F.; Yang, Y.; Wang, J.; Gan, W.; Deng, F.; Zhu, C. Co-Pyrolysis of Sewage Sludge and Pinewood Sawdust: The Synergistic Effect and Bio-Oil Characteristics. Biomass Convers. Biorefinery 2023, 13, 9205–9212. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, R.; Du, Y.; Chen, L.; Chen, Z.; Xiao, N.; Wu, Z. Study on the Co-Pyrolysis Characteristics of Sewage Sludge and Wood Powder and Kinetic Analysis. Biomass Convers. Biorefinery 2024, 14, 1593–1605. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Cui, M.-H.; Ren, Y.-G.; Guo, J.-C.; Zheng, Z.-Y.; Liu, H. Towards Understanding the Mechanism of Heavy Metals Immobilization in Biochar Derived from Co-Pyrolysis of Sawdust and Sewage Sludge. Bull. Environ. Contam. Toxicol. 2020, 104, 489–496. [Google Scholar] [CrossRef]
- Urych, B.; Smoliński, A. Sewage Sludge and Phytomass Co-Pyrolysis and the Gasification of Its Chars: A Kinetics and Reaction Mechanism Study. Fuel 2021, 285, 119186. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, Y.; Yang, L.; Zhu, Y. High Quality Syngas Produced from the Co-Pyrolysis of Wet Sewage Sludge with Sawdust. Int. J. Hydrogen Energy 2018, 43, 5463–5472. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wang, M.; Naidu, R.; Liu, Y.; Man, Y.B.; Liang, X.; Wong, M.; Christie, P.; Zhang, Y.; et al. Co-Pyrolysis of Sewage Sludge and Rice Husk/Bamboo Sawdust for Biochar with High Aromaticity and Low Metal Mobility. Environ. Res. 2020, 191, 110034. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Sun, L.; Jiao, L.; Peng, L.; Lei, Z.; Wang, Y.; Lin, J. Adsorption of Methylene Blue by Residue Biochar from Copyrolysis of Dewatered Sewage Sludge and Pine Sawdust. Desalin. Water Treat. 2013, 51, 7081–7087. [Google Scholar] [CrossRef]
- Zuo, W.; Jin, B.; Huang, Y.; Sun, Y. Characterization of Top Phase Oil Obtained from Co-Pyrolysis of Sewage Sludge and Poplar Sawdust. Environ. Sci. Pollut. Res. 2014, 21, 9717–9726. [Google Scholar] [CrossRef] [PubMed]
- Kwapinska, M.; Horvat, A.; Agar, D.A.; Leahy, J.J. Energy Recovery through Co-Pyrolysis of Wastewater Sludge and Forest Residues—The Transition from Laboratory to Pilot Scale. J. Anal. Appl. Pyrolysis 2021, 158, 105283. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Zhu, G.; Yang, L.; Zhu, Y. The Effect of High Temperature on Syngas Production by Immediate Pyrolysis of Wet Sewage Sludge with Sawdust. J. Therm. Anal. Calorim. 2018, 132, 1783–1794. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, L.; Chen, H.; Zhu, Y.; Yang, L. Pilot Test of Co-Pyrolysis Characteristics of Wet Sewage Sludge and Sawdust in an External Heating Moving Bed. Energy Procedia 2017, 105, 570–575. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Z.; Xiao, B.; Hu, Z.; Hu, M.; Liu, C.; Zhang, Q. Co-Pyrolysis Behaviors and Kinetics of Sewage Sludge and Pine Sawdust Blends under Non-Isothermal Conditions. J. Therm. Anal. Calorim. 2015, 119, 2269–2279. [Google Scholar] [CrossRef]
- Alvarez, J.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Fast Co-Pyrolysis of Sewage Sludge and Lignocellulosic Biomass in a Conical Spouted Bed Reactor. Fuel 2015, 159, 810–818. [Google Scholar] [CrossRef]
- Wang, S.; Mandfloen, P.; Jönsson, P.; Yang, W. Synergistic Effects in the Copyrolysis of Municipal Sewage Sludge Digestate and Salix: Reaction Mechanism, Product Characterization and Char Stability. Appl. Energy 2021, 289, 116687. [Google Scholar] [CrossRef]
- Duan, X.-Y.; Cao, Y.; Liu, T.-Z.; Li, L.; Wang, B.; Wang, X.-D. Nutrient Stability and Sorption of Sewage Sludge Biochar Prepared from Co-Pyrolysis of Sewage Sludge and Stalks Mineral Materials. Environ. Pollut. Bioavail 2020, 32, 12–18. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Yue, X.-G.; Yin, Z.-Y.; Pan, T.-T.; Dong, M.-J.; Sun, T.-Y. Study of the Co-Pyrolysis Behavior of Sewage-Sludge/Rice-Straw and the Kinetics. Procedia Earth Planet. Sci. 2009, 1, 661–666. [Google Scholar]
- Xu, X.; Zhao, B.; Sun, M.; Chen, X.; Zhang, M.; Li, H.; Xu, S. Co-Pyrolysis Characteristics of Municipal Sewage Sludge and Hazelnut Shell by TG-DTG-MS and Residue Analysis. Waste Manag. 2017, 62, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Liu, M.; Ren, H. Biochar Produced from the Co-Pyrolysis of Sewage Sludge and Walnut Shell for Ammonium and Phosphate Adsorption from Water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Xie, L.; Zhu, H.; Shu, X. Influence of the Addition of Cotton Stalk during Co-Pyrolysis with Sewage Sludge on the Properties, Surface Characteristics, and Ecological Risks of Biochars. J. Therm. Sci. 2019, 28, 755–762. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Pan, L.; Li, C.; You, F.; Wang, Y. Ciprofloxacin Adsorption by Biochar Derived from Co-Pyrolysis of Sewage Sludge and Bamboo Waste. Environ. Sci. Pollut. Res. 2020, 27, 22806–22817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shu, X.; Zhu, H.; Xie, L.; Cheng, S.; Zhang, Y. Characteristics of Biochars Prepared by Co-Pyrolysis of Sewage Sludge and Cotton Stalk Intended for Use as Soil Amendments. Environ. Technol. 2020, 41, 1347–1357. [Google Scholar] [CrossRef]
- Deng, S.; Tan, H.; Wang, X.; Yang, F.; Cao, R.; Wang, Z.; Ruan, R. Investigation on the Fast Co-Pyrolysis of Sewage Sludge with Biomass and the Combustion Reactivity of Residual Char. Bioresour. Technol. 2017, 239, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, F.; Hu, J.; Wang, H.; Yang, S.; Liu, H. Co-Pyrolysis of Sewage Sludge and Waste Tobacco Stem: Gas Products Analysis, Pyrolysis Kinetics, Artificial Neural Network Modeling, and Synergistic Effects. Bioresour. Technol. 2023, 389, 129816. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wu, Y.; Li, T.; Lan, G.; Shen, J.; Yu, Y.; Xue, P.; Chen, D.; Wang, M.; Fu, C. A Study of Co-Pyrolysis of Sewage Sludge and Rice Husk for Syngas Production Based on a Cyclic Catalytic Integrated Process System. Renew. Energy 2023, 215, 118946. [Google Scholar] [CrossRef]
- Sun, J.; Wang, P.; Guo, Y.; Hu, B.; Wang, X. Effect of Biochar Derived from Co-Pyrolysis of Sewage Sludge and Rice Straw on Cadmium Immobilization in Paddy Soil. Environ. Sci. Pollut. Res. 2023, 30, 74808–74819. [Google Scholar] [CrossRef]
- Sun, H.; Bi, H.; Jiang, C.; Ni, Z.; Tian, J.; Zhou, W.; Qiu, Z.; Lin, Q. Experimental Study of the Co-Pyrolysis of Sewage Sludge and Wet Waste via TG-FTIR-GC and Artificial Neural Network Model: Synergistic Effect, Pyrolysis Kinetics and Gas Products. Renew. Energy 2022, 184, 1–14. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, R.; Ji, S.; Xie, L.; Zhang, H. Effects of Biochar Derived from Sewage Sludge and Sewage Sludge/Cotton Stalks on the Immobilization and Phytoavailability of Pb, Cu, and Zn in Sandy Loam Soil. J. Hazard. Mater. 2021, 419, 126468. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Jiang, W.; Shao, L.; Zhang, L.; Feng, L. Effects of Pyrolysis Temperature and Addition Proportions of Corncob on the Distribution of Products and Potential Energy Recovery during the Preparation of Sludge Activated Carbon. Chemosphere 2019, 221, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, R.; Cheng, Z.; Yan, B.; Ma, W. Nitric Oxide Formation during Corn Straw/Sewage Sludge Co-Pyrolysis/Gasification. J. Clean. Prod. 2018, 197, 97–105. [Google Scholar] [CrossRef]
- Hameed, Z.; Aman, Z.; Naqvi, S.R.; Tariq, R.; Ali, I.; Makki, A.A. Kinetic and Thermodynamic Analyses of Sugar Cane Bagasse and Sewage Sludge Co-Pyrolysis Process. Energy Fuels 2018, 32, 9551–9558. [Google Scholar] [CrossRef]
- Fan, S.; Li, H.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Cadmium Removal from Aqueous Solution by Biochar Obtained by Co-Pyrolysis of Sewage Sludge with Tea Waste. Res. Chem. Intermed. 2018, 44, 135–154. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Chen, M.; Ma, L.; Yang, B.; Chen, J.; Lv, Z.; Liang, Q.; Yang, P. Evaluation of Migration of Heavy Metals and Performance of Product during Co-Pyrolysis Process of Municipal Sewage Sludge and Walnut Shell. Environ. Sci. Pollut. Res. 2017, 24, 22082–22090. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xu, X.; Xu, S.; Chen, X.; Li, H.; Zeng, F. Surface Characteristics and Potential Ecological Risk Evaluation of Heavy Metals in the Bio-Char Produced by Co-Pyrolysis from Municipal Sewage Sludge and Hazelnut Shell with Zinc Chloride. Bioresour. Technol. 2017, 243, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar Prepared from Co-Pyrolysis of Municipal Sewage Sludge and Tea Waste for the Adsorption of Methylene Blue from Aqueous Solutions: Kinetics, Isotherm, Thermodynamic and Mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, C.; Xu, J.; Yang, X. Beneficial Synergetic Effect on Gas Production during Co-Pyrolysis of Sewage Sludge and Biomass in a Vacuum Reactor. Bioresour. Technol. 2015, 183, 255–258. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Shih, C.-H.; Chiueh, P.-T.; Lo, S.-L. Microwave Co-Pyrolysis of Sewage Sludge and Rice Straw. Energy 2015, 87, 638–644. [Google Scholar] [CrossRef]
- Samanya, J.; Hornung, A.; Apfelbacher, A.; Vale, P. Characteristics of the Upper Phase of Bio-Oil Obtained from Co-Pyrolysis of Sewage Sludge with Wood, Rapeseed and Straw. J. Anal. Appl. Pyrolysis 2012, 94, 120–125. [Google Scholar] [CrossRef]
- Chen, X.; Wu, R.; Sun, Y.; Jian, X. Synergistic Effects on the Co-Pyrolysis of Agricultural Wastes and Sewage Sludge at Various Ratios. ACS Omega 2022, 7, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gómez, N.; Quispe, V.; Ábrego, J.; Atienza-Martínez, M.; Murillo, M.B.; Gea, G. Co-Pyrolysis of Sewage Sludge and Manure. Waste Manag. 2017, 59, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, J.; Xie, Y.; Xu, D.; Liu, Y.; Liu, J.; Hou, J. Biochar Produced from the Co-Pyrolysis of Sewage Sludge and Waste Tires for Cadmium and Tetracycline Adsorption from Water. Water Sci. Technol. 2021, 83, 1429–1445. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wu, Y.; Lan, G.; Xia, Y.; Yan, B.; Li, Y.; Zhang, Y.; Yu, Y.; Fu, C.; Xu, A.; et al. Effect of Co-Pyrolysis of Sewage Sludge with Different Plastics on the Nitrogen, Sulfur, and Chlorine Releasing Characteristics and the Heavy Metals Ecological Risk of Biochar. J. Environ. Chem. Eng. 2023, 11, 110406. [Google Scholar] [CrossRef]
- Li, W.; Meng, J.; Zhang, Y.; Haider, G.; Ge, T.; Zhang, H.; Li, Z.; Yu, Y.; Shan, S. Co-Pyrolysis of Sewage Sludge and Metal-Free/Metal-Loaded Polyvinyl Chloride (PVC) Microplastics Improved Biochar Properties and Reduced Environmental Risk of Heavy Metals. Environ. Pollut. 2022, 302, 119092. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Liu, G.; Arif, M.; Yousaf, B.; Akhtar, P.; Rashid, A.; Gulzaman, H.; Safeer, R.; Rashid, M.S.; Haider, M.I.S. The Development of Plastic Waste and Sewage Sludge Co-Pyrolyzed Biochar Composites with Improved Interfacial Characteristics for the Effective Removal of Ciprofloxacin. Process Saf. Environ. Prot. 2024, 184, 766–781. [Google Scholar] [CrossRef]
- Ling, C.C.Y.; Li, S.F.Y. Synergistic Interactions between Sewage Sludge, Polypropylene, and High-Density Polyethylene during Co-Pyrolysis: An Investigation Based on Iso-Conversional Model-Free Methods and Master Plot Analysis. J. Hazard. Mater. 2023, 455, 131600. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.; Li, Z.; Ding, X.; Qi, S.; Zhang, Y.; Liu, J.; Gao, J. Characteristics of Three-Phase Products from Co-Pyrolysis of Sewage Sludge and PVC. Chem. Ind. Eng. Prog. 2023, 42, 2122–2129. [Google Scholar]
- Li, Z.; Huang, Y.; Zhu, Z.; Cheng, H.; Zhao, J.; Yu, M.; Xu, W.; Yuan, Q.; He, T.; Wang, S. Co-Pyrolysis of Sewage Sludge with Polyvinyl Chloride (PVC)/CaO: Effects on Heavy Metals Behavior and Ecological Risk. Fuel 2023, 333, 126281. [Google Scholar] [CrossRef]
- Wang, G.; YU, G.; Xie, S.; Jiang, R.; Wang, Y. Effect of Co-Pyrolysis of Different Plastics with Sewage Sludge on Heavy Metals in the Biochar. J. Fuel Chem. Technol. 2019, 47, 611–620. [Google Scholar]
- Li, Q.; Zhong, Z.; Du, H.; Zheng, X.; Zhang, B.; Jin, B. Co-Pyrolysis of Sludge and Kaolin/Zeolite in a Rotary Kiln: Analysis of Stabilizing Heavy Metals. Front. Environ. Sci. Eng. 2022, 16, 85. [Google Scholar] [CrossRef]
- Saleh Khodaparasti, M.; Reza Shirazvatan, M.; Tavakoli, O.; Ali Khodadadi, A. Co-Pyrolysis of Municipal Sewage Sludge and Microalgae Chlorella Vulgaris: Products’ Optimization; Thermo-Kinetic Study, and ANN Modeling. Energy Convers. Manag. 2022, 254, 115258. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, F.; Chen, F.; Feng, Y.; Chen, D.; Dai, X. Pyrolysis of the Mixture of MSWI Fly Ash and Sewage Sludge for Co-Disposal: Effect of Ferrous/Ferric Sulfate Additives. Waste Manag. 2018, 75, 340–351. [Google Scholar] [CrossRef]
- Hu, J.; Danish, M.; Lou, Z.; Zhou, P.; Zhu, N.; Yuan, H.; Qian, P. Effectiveness of Wind Turbine Blades Waste Combined with the Sewage Sludge for Enriched Carbon Preparation through the Co-Pyrolysis Processes. J. Clean. Prod. 2018, 174, 780–787. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.; Yang, X. Co-Pyrolysis of Microalgae and Sewage Sludge: Biocrude Assessment and Char Yield Prediction. Energy Convers. Manag. 2016, 117, 326–334. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, C.; Xu, J.; Zhang, W. Potential Method for Gas Production: High Temperature Co-Pyrolysis of Lignite and Sewage Sludge with Vacuum Reactor and Long Contact Time. Bioresour. Technol. 2015, 179, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Ischia, M.; Maschio, R.D.; Grigiante, M.; Baratieri, M. Clay–Sewage Sludge Co-Pyrolysis. A TG–MS and Py–GC Study on Potential Advantages Afforded by the Presence of Clay in the Pyrolysis of Wastewater Sewage Sludge. Waste Manag. 2011, 31, 71–77. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q. Sustainable Mechanisms of Biochar Derived from Brewers’ Spent Grain and Sewage Sludge for Ammonia–Nitrogen Capture. J. Clean. Prod. 2016, 112, 3927–3934. [Google Scholar] [CrossRef]
- Meng, J.; Tao, M.; Wang, L.; Liu, X.; Xu, J. Changes in Heavy Metal Bioavailability and Speciation from a Pb-Zn Mining Soil Amended with Biochars from Co-Pyrolysis of Rice Straw and Swine Manure. Sci. Total Environ. 2018, 633, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, S.; Wang, Y.; Pan, X.; Yu, G.; Zhang, Y. Simultaneous Heavy Metal Immobilization and Antibiotics Removal during Synergetic Treatment of Sewage Sludge and Pig Manure. Environ. Sci. Pollut. Res. 2020, 27, 30323–30332. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Lv, H.; Fan, L.; Chen, L.; Hu, Y.; Wang, X.; Guo, Q.; Cui, X.; Zhou, N.; Jiao, L. Co-Pyrolysis of Sewage Sludge and Poplar Sawdust under Controlled Low-Oxygen Conditions: Biochar Properties and Heavy Metals Behavior. J. Anal. Appl. Pyrolysis 2023, 169, 105868. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, S.; Wu, B.; Pi, M.; Xiong, Y.; Zhang, H. Co-Pyrolysis of Sewage Sludge and Rice Straw: Thermal Behavior and Char Characteristic Evaluations. Energy Fuels 2020, 34, 607–615. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A Review of Pyrolysis Technologies and Feedstock: A Blending Approach for Plastic and Biomass towards Optimum Biochar Yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Mujtaba Munir, M.A.; Yousaf, B.; Ali, M.U.; Dan, C.; Abbas, Q.; Arif, M.; Yang, X. In Situ Synthesis of Micro-Plastics Embedded Sewage-Sludge Co-Pyrolyzed Biochar: Implications for the Remediation of Cr and Pb Availability and Enzymatic Activities from the Contaminated Soil. J. Clean. Prod. 2021, 302, 127005. [Google Scholar] [CrossRef]
- Mohamed, B.A.; O’Boyle, M.; Li, L.Y. Co-Pyrolysis of Sewage Sludge with Lignocellulosic and Algal Biomass for Sustainable Liquid and Gaseous Fuel Production: A Life Cycle Assessment and Techno-Economic Analysis. Appl. Energy 2023, 346, 121318. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Li, L.Y. Biofuel Production by Co-Pyrolysis of Sewage Sludge and Other Materials: A Review. Environ. Chem. Lett. 2023, 21, 153–182. [Google Scholar] [CrossRef]
- Li, D.C.; Jiang, H. The Thermochemical Conversion of Non-Lignocellulosic Biomass to Form Biochar: A Review on Characterizations and Mechanism Elucidation. Bioresour. Technol. 2017, 246, 57–68. [Google Scholar] [CrossRef]
- Yin, X.; Xi, M.; Li, Y.; Kong, F.; Jiang, Z. Improvements in Physicochemical and Nutrient Properties of Sewage Sludge Biochar by the Co-Pyrolysis with Organic Additives. Sci. Total Environ. 2021, 779, 146565. [Google Scholar] [CrossRef]
- Chen, W.H.; Naveen, C.; Ghodke, P.K.; Sharma, A.K.; Bobde, P. Co-Pyrolysis of Lignocellulosic Biomass with Other Carbonaceous Materials: A Review on Advance Technologies, Synergistic Effect, and Future Prospectus. Fuel 2023, 345, 128177. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Iwuozor, K.O.; Emenike, E.C.; Ajala, O.J.; Ogunniyi, S.; Muritala, K.B. Thermochemical Co-Conversion of Biomass-Plastic Waste to Biochar: A Review. Green. Chem. Eng. 2024, 5, 31–49. [Google Scholar] [CrossRef]
- Chen, G.B.; Wu, F.H.; Fang, T.L.; Lin, H.T.; Chao, Y.C. A Study of Co-Gasification of Sewage Sludge and Palm Kernel Shells. Energy 2021, 218, 119532. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Quicker, P.; Weber, K. (Eds.) Biokohle; Springer Fachmedien: Wiesbaden, Germany, 2016; ISBN 978-3-658-03688-1. [Google Scholar]
- Gbouri, I.; Yu, F.; Wang, X.; Wang, J.; Cui, X.; Hu, Y.; Yan, B.; Chen, G. Co-Pyrolysis of Sewage Sludge and Wetland Biomass Waste for Biochar Production: Behaviors of Phosphorus and Heavy Metals. Int. J. Environ. Res. Public Health 2022, 19, 2818. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Xiang, W.; Liu, Q.; Wang, M.; Zhang, X.; Zhang, X. Co-Pyrolysis Biochar Derived from Sewage Sludge and Lignin: Synergetic Effect and Adsorption Properties. J. Environ. Chem. Eng. 2022, 10, 107898. [Google Scholar] [CrossRef]
- Krylova, A.Y.; Zaitchenko, V.M. Hydrothermal Carbonization of Biomass: A Review. Solid Fuel Chem. 2018, 52, 91–103. [Google Scholar] [CrossRef]
- Chaowana, P.; Hnoocham, W.; Chaiprapat, S.; Yimlamai, P.; Chitbanyong, K.; Wanitpinyo, K.; Chaisan, T.; Paopun, Y.; Pisutpiched, S.; Khantayanuwong, S.; et al. Utilization of Hemp Stalk as a Potential Resource for Bioenergy. Mater. Sci. Energy Technol. 2024, 7, 19–28. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. Influence of Mineral Matter on Pyrolysis of Palm Oil Wastes. Combust. Flame 2006, 146, 605–611. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Cañellas, J.; Garcia-Raso, J. Determination of Hemicellulose, Cellulose and Lignin Contents of Dietary Fibre and Crude Fibre of Several Seed Hulls. Data Comparison. Z. Lebensm. Unters. Forsch. 1983, 177, 200–202. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, A.; Joshi, H.C. Ethanol as an Alternative Fuel from Agricultural, Industrial and Urban Residues. Resour. Conserv. Recycl. 2007, 50, 1–39. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Fu, J.; Ao, W.; Ali Siyal, A.; Zhou, C.; Liu, C.; Yu, M.; Zhang, Y.; Dai, J.; et al. Co-Pyrolysis of Sewage Sludge and Lignocellulosic Biomass: Synergistic Effects on Products Characteristics and Kinetics. Energy Convers. Manag. 2022, 268, 116061. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-Industrial Lignocellulosic Biomass a Key to Unlock the Future Bio-Energy: A Brief Review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Guerrero, M.R.B.; Marques Da Silva Paula, M.; Zaragoza, M.M.; Gutiérrez, J.S.; Velderrain, V.G.; Ortiz, A.L.; Collins-Martínez, V. Thermogravimetric Study on the Pyrolysis Kinetics of Apple Pomace as Waste Biomass. Int. J. Hydrogen Energy 2014, 39, 16619–16627. [Google Scholar] [CrossRef]
- Sánchez, J.D.; Ramírez, G.E.; Barajas, M.J. Comparative Kinetic Study of the Pyrolysis of Mandarin and Pineapple Peel. J. Anal. Appl. Pyrolysis 2016, 118, 192–201. [Google Scholar] [CrossRef]
- AlNouss, A.; Parthasarathy, P.; Mackey, H.R.; Al-Ansari, T.; McKay, G. Pyrolysis Study of Different Fruit Wastes Using an Aspen Plus Model. Front. Sustain. Food Syst. 2021, 5, 604001. [Google Scholar] [CrossRef]
- Compositional Analysis of Lignocellulosic Materials: Evaluation of an Economically Viable Method Suitable for Woody and Non-Woody Biomass. Available online: https://www.researchgate.net/publication/280641412_Compositional_analysis_of_lignocellulosic_materials_Evaluation_of_an_economically_viable_method_suitable_for_woody_and_non-woody_biomass (accessed on 22 April 2024).
- Kuryntseva, P.; Karamova, K.; Galitskaya, P.; Selivanovskaya, S.; Evtugyn, G. Biochar Functions in Soil Depending on Feedstock and Pyrolyzation Properties with Particular Emphasis on Biological Properties. Agriculture 2023, 13, 2003. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, J.; Yue, F.; Ren, J.; Liu, C. Chemical Composition Analysis and Isolated Lignin Structural Characterization of 10 Wild Bamboo Species in Guangxi. Chung-Kuo Tsao Chih/China Pulp Pap. 2023, 42, 46–54+68. [Google Scholar]
- Šurić, J.; Brandić, I.; Peter, A.; Bilandžija, N.; Leto, J.; Karažija, T.; Kutnjak, H.; Poljak, M.; Voća, N. Wastewater Sewage Sludge Management via Production of the Energy Crop Virginia Mallow. Agronomy 2022, 12, 1578. [Google Scholar] [CrossRef]
- Branca, C.; Albano, A.; Di Blasi, C. Critical Evaluation of Global Mechanisms of Wood Devolatilization. Thermochim. Acta 2005, 429, 133–141. [Google Scholar] [CrossRef]
- Uzun, B.B.; Pütün, A.E.; Pütün, E. Rapid Pyrolysis of Olive Residue. 1. Effect of Heat and Mass Transfer Limitations on Product Yields and Bio-Oil Compositions. Energy Fuels 2007, 21, 1768–1776. [Google Scholar] [CrossRef]
- Dupont, C.; Chen, L.; Cances, J.; Commandre, J.-M.; Cuoci, A.; Pierucci, S.; Ranzi, E. Biomass Pyrolysis: Kinetic Modelling and Experimental Validation under High Temperature and Flash Heating Rate Conditions. J. Anal. Appl. Pyrolysis 2009, 85, 260–267. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, Y.T.; Tsang, Y.F.; Lee, J. Sustainable Ethylene Production: Recovery from Plastic Waste via Thermochemical Processes. Sci. Total Environ. 2023, 903, 166789. [Google Scholar] [CrossRef] [PubMed]
- Sadrameli, S.M. Thermal/Catalytic Cracking of Hydrocarbons for the Production of Olefins: A State-of-the-Art Review I: Thermal Cracking Review. Fuel 2015, 140, 102–115. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Zhang, G.; Li, J.; Li, Z.; Yu, G.; Wang, Y. A Process Combining Hydrothermal Pretreatment, Anaerobic Digestion and Pyrolysis for Sewage Sludge Dewatering and Co-Production of Biogas and Biochar: Pilot-Scale Verification. Bioresour. Technol. 2018, 254, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Mohd Taib, R.; Mohamad Aziz, N.S.; Omar, M.R.; Md Disa, N. Banana Pseudo-Stem Biochar Derived from Slow and Fast Pyrolysis Process. Heliyon 2023, 9, e12940. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wang, D.; Deng, Z.; Yu, H.; Dai, J.; Bi, X. Biochar Prepared by Microwave-Assisted Co-Pyrolysis of Sewage Sludge and Cotton Stalk: A Potential Soil Conditioner. Sustainability 2023, 15, 7265. [Google Scholar] [CrossRef]
- Mohamad Aziz, N.A.; Mohamed, H.; Kania, D.; Ong, H.C.; Zainal, B.S.; Junoh, H.; Ker, P.J.; Silitonga, A.S. Bioenergy Production by Integrated Microwave-Assisted Torrefaction and Pyrolysis. Renew. Sustain. Energy Rev. 2024, 191, 114097. [Google Scholar] [CrossRef]
- Mercl, F.; Košnář, Z.; Pierdonà, L.; Ulloa-Murillo, L.M.; Száková, J.; Tlustoš, P. Changes in Availability of Ca, K, Mg, P and S in Sewage Sludge as Affected by Pyrolysis Temperature. Plant Soil Environ. 2020, 66, 143–148. [Google Scholar] [CrossRef]
- Working Principle of Muffle Furnace 2023. Flair Pharma the Knowledge Kit. Available online: https://flairpharma.com/muffle-furnace/ (accessed on 22 January 2024).
- Henan Sante Furnace Techincal Co., Ltd. Different Types of Muffle Furnace. Available online: https://www.saftherm.com/news/company-news/different-types-of-muffle-furnace/ (accessed on 24 April 2024).
- Vacuum Muffle Furnaces|Up to 1500 °C and 31 Liters in Capacity. Available online: https://labandfurnace.com/product-category/muffle-furnace/vacuum-muffle-furnace/ (accessed on 24 April 2024).
- Vacuum Muffle Furnace for Biomass Decomposition. Available online: https://labandfurnace.com/muffle-furnace/biomass-decompositions-pyrolysis-vaccum-muffle-furnace/ (accessed on 24 April 2024).
- de Oliveira Paiva, I.; de Morais, E.G.; Jindo, K.; Silva, C.A. Biochar N Content, Pools and Aromaticity as Affected by Feedstock and Pyrolysis Temperature. Waste Biomass Valoriz. 2024, 15, 3599–3619. [Google Scholar] [CrossRef]
- Everything about Muffle Furnace. Available online: https://www.mrclab.com/everything-about-muffle-furnace (accessed on 22 January 2024).
- Januševičius, T.; Mažeikienė, A.; Danila, V.; Paliulis, D. The Characteristics of Sewage Sludge Pellet Biochar Prepared Using Two Different Pyrolysis Methods. Biomass Convers. Biorefinery 2024, 14, 891–900. [Google Scholar] [CrossRef]
- Rotary Furnace Working Principle. Available online: https://thermcraftinc.com/working-principle-rotary-furnace/ (accessed on 22 January 2024).
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Murthy Konda, N.V.S.N.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N. The Multi-Scale Challenges of Biomass Fast Pyrolysis and Bio-Oil Upgrading: Review of the State of Art and Future Research Directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Brown, J.N.; Brown, R.C. Process Optimization of an Auger Pyrolyzer with Heat Carrier Using Response Surface Methodology. Bioresour. Technol. 2012, 103, 405–414. [Google Scholar] [CrossRef]
- Garcia-Nunez, J.A.; Pelaez-Samaniego, M.R.; Garcia-Perez, M.E.; Fonts, I.; Abrego, J.; Westerhof, R.J.M.; Garcia-Perez, M. Historical Developments of Pyrolysis Reactors: A Review. Energy Fuels 2017, 31, 5751–5775. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Piddubniak, O. The Non-Stationary Heat Transport inside a Shafted Screw Conveyor Filled with Homogeneous Biomass Heated Electrically. Energies 2022, 15, 6164. [Google Scholar] [CrossRef]
- Slezak, R.; Unyay, H.; Szufa, S.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Reactors Torrefaction vs. Biomass Pyrolizers—Part 2. Energies 2023, 16, 2212. [Google Scholar] [CrossRef]
- Raj, A.; Yadav, A.; Arya, S.; Sirohi, R.; Kumar, S.; Rawat, A.P.; Thakur, R.S.; Patel, D.K.; Bahadur, L.; Pandey, A. Preparation, Characterization and Agri Applications of Biochar Produced by Pyrolysis of Sewage Sludge at Different Temperatures. Sci. Total Environ. 2021, 795, 148722. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cui, L.; Lin, Q.; Li, G.; Zhao, X. The Sewage Sludge Biochar at Low Pyrolysis Temperature Had Better Improvement in Urban Soil and Turf Grass. Agronomy 2019, 9, 156. [Google Scholar] [CrossRef]
- Figueiredo, C.; Lopes, H.; Coser, T.; Vale, A.; Busato, J.; Aguiar, N.; Novotny, E.; Canellas, L. Influence of Pyrolysis Temperature on Chemical and Physical Properties of Biochar from Sewage Sludge. Arch. Agron. Soil Sci. 2018, 64, 881–889. [Google Scholar] [CrossRef]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar Production by Sewage Sludge Pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Wei, L.; Wen, L.; Yang, T.; Zhang, N. Nitrogen Transformation during Sewage Sludge Pyrolysis. Energy Fuels 2015, 29, 5088–5094. [Google Scholar] [CrossRef]
- Chanaka Udayanga, W.D.; Veksha, A.; Giannis, A.; Lisak, G.; Lim, T.-T. Effects of Sewage Sludge Organic and Inorganic Constituents on the Properties of Pyrolysis Products. Energy Convers. Manag. 2019, 196, 1410–1419. [Google Scholar] [CrossRef]
- Aihemaiti, A.; Liang, S.; Cai, Y.; Li, R.; Yan, F.; Zhang, Z. Effects of Ferrous Sulfate Modification on the Fate of Phosphorous in Sewage Sludge Biochar and Its Releasing Mechanisms in Heavy Metal Contaminated Soils. Environ. Sci. Pollut. Res. 2023, 30, 106214–106226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, X.; Han, W.; Han, Y.; Guo, M.; Peng, Z.; Fan, Z.; Shi, Y.; Wan, S. Tetracycline Removal by Hercynite-Biochar from the Co-Pyrolysis of Red Mud-Steel Slag-Sludge. Nanomaterials 2022, 12, 2595. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Xu, X.M.; Liang, X.; Wang, Y.; Liu, M.; Wang, X.; Xia, S.Q.; Zhao, J.F.; Yin, D.Q.; Zhang, Y.L. Adsorption of Copper(II) onto Sewage Sludge-Derived Materials via Microwave Irradiation. J. Hazard. Mater. 2011, 192, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Jahirul, M.; Rasul, M.; Chowdhury, A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Direktor, L.B.; Zaichenko, V.M.; Is’emin, R.L.; Chernyavskii, A.A.; Shevchenko, A.L. Comparison of the Efficiency of the Reactors for Low-Temperature Pyrolysis of Biomass. Therm. Eng. 2020, 67, 296–303. [Google Scholar] [CrossRef]
- Stass, D.V.; Woodward, J.R.; Timmel, C.R.; Hore, P.J.; McLauchlan, K.A. Radiofrequency Magnetic Field Effects on Chemical Reaction Yields. Chem. Phys. Lett. 2000, 329, 15–22. [Google Scholar] [CrossRef]
- Rana, K.K.; Rana, S. Microwave Reactors: A Brief Review on Its Fundamental Aspects and Applications. OAlib 2014, 1, 1–20. [Google Scholar] [CrossRef]
- Cravotto, G.; Carnaroglio, D. (Eds.) Microwave Chemistry; De Gruyter: Berlin, Germany, 2017; ISBN 9783110479935. [Google Scholar]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef]
- Carbolite Gero Launches New Tube Furnace Range. Available online: https://www.carbolite-gero.com/news/new-tube-furnace-range/ (accessed on 17 January 2024).
- Gonzalez-Aguilar, A.M.; Cabrera-Madera, V.P.; Vera-Rozo, J.R.; Riesco-Ávila, J.M. Effects of Heating Rate and Temperature on the Thermal Pyrolysis of Expanded Polystyrene Post-Industrial Waste. Polymers 2022, 14, 4957. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Zhuo, J.; Zhou, H.; Pang, R.; Yao, Q. Study on the Co-Pyrolysis of High Density Polyethylene and Potato Blends Using Thermogravimetric Analyzer and Tubular Furnace. J. Anal. Appl. Pyrolysis 2015, 112, 66–73. [Google Scholar] [CrossRef]
- Gustafsson, M. Pyrolysis for Heat Production: Biochar—The Primary Byproduct. Master’s Thesis, University of Gävle, Gävle, Sweden, 2013. [Google Scholar]
- Aschjem, C.W.S. Modeling and Optimization of Pyrolysis Reactors. Master’s Thesis, Norwegian University of Life Sciences, Ås, Norway, 2019. [Google Scholar]
- EU Emission Standard ‘Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe’. Available online: http://data.europa.eu/eli/dir/2008/50/oj/ (accessed on 10 March 2024).
- PYREG GmbH. Waste-to-Value: From Sewage Sludge to Natural Fertilizer and Carbon Capture. Available online: https://pyreg.com/waste-to-value-from-sewage-sludge-to-natural-fertilizer-and-carbon-capture/ (accessed on 17 April 2024).
- EBC standard ‘European Biochar Certificate—Guidelines for a Sustainable Production of Biochar’. Carbon Standards International (CSI), Frick, Switzerland. Version 10.3 from 5th April 2022. Available online: http://european-biochar.org (accessed on 10 March 2024).
- Andersson, A.; Isaksson, W.; Norström, T.; Widlund, A. Biochar and District Heating The Path to Negative Emissions. Earth’s Future 2023, 11, e2022EF003246. [Google Scholar]
- Baltar, R. Report on Options for Utilization of Surplus Biomass Coming from the Usal Forest; Redwood Forest Foundation, Inc.: Fort Bragg, CA, USA, 2018. [Google Scholar]
- Gustavsson, D. Perspectives for Pyrolysis of Sewage Sludges. In ESPP SCOPE Newsletter Special Issue on BIOCHARS 2022; European Sustainable Phosphorus Platform: Bruxelles, Belgium, 2022; pp. 11–12. [Google Scholar]
- Kern, S.; Halwachs, M.; Kampichler, G.; Pfeifer, C.; Pröll, T.; Hofbauer, H. Rotary Kiln Pyrolysis of Straw and Fermentation Residues in a 3MW Pilot Plant—Influence of Pyrolysis Temperature on Pyrolysis Product Performance. J. Anal. Appl. Pyrolysis 2012, 97, 1–10. [Google Scholar] [CrossRef]
- Technology—AquaGreen. Available online: https://aquagreen.dk/technology/ (accessed on 12 April 2024).
- Sludge Pyrolysis—AquaGreen. Available online: https://aquagreen.dk/ (accessed on 12 April 2024).
- Aquagreen Aps|Aquatech. Available online: https://company.aquatechtrade.com/Aquagreen-Aps?Language=EN&account=00643207-0&eventid=22841 (accessed on 12 April 2024).
- Ravenni, G.; Thomsen, T.P.; Smith, A.M.; Ambye-Jensen, M.; Rohde-Nielsen, K.T.; Henriksen, U.B. Integration of a Drying and Pyrolysis System in a Green Biorefinery: Biochar Product Quality and Impacts on the Overall Energy Balance and Climate Footprint. Biomass Convers. Biorefinery 2023, 1, 1–17. [Google Scholar] [CrossRef]
- Aboyade, A.O.; Hugo, T.J.; Carrier, M.; Meyer, E.L.; Stahl, R.; Knoetze, J.H.; Görgens, J.F. Non-Isothermal Kinetic Analysis of the Devolatilization of Corn Cobs and Sugar Cane Bagasse in an Inert Atmosphere. Thermochim. Acta 2011, 517, 81–89. [Google Scholar] [CrossRef]
- Han, L.; Li, J.; Qu, C.; Shao, Z.; Yu, T.; Yang, B. Recent Progress in Sludge Co-Pyrolysis Technology. Sustainability 2022, 14, 7574. [Google Scholar] [CrossRef]
- Jellali, S.; Khiari, B.; Usman, M.; Hamdi, H.; Charabi, Y.; Jeguirim, M. Sludge-Derived Biochars: A Review on the Influence of Synthesis Conditions on Pollutants Removal Efficiency from Wastewaters. Renew. Sustain. Energy Rev. 2021, 144, 111068. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, J.; Zhang, S.; Shi, K.; Liu, X.; Zhao, S.; Yang, H.; Jia, J. Carbonization Characteristics of Co-Pyrolysis of Sewage Sludge and Corn Stalks and Its Agricultural Benefits. J. Soils Sediments 2023, 23, 1674–1686. [Google Scholar] [CrossRef]
- Fassinou, W.F.; Van de Steene, L.; Toure, S.; Volle, G.; Girard, P. Pyrolysis of Pinus Pinaster in a Two-Stage Gasifier: Influence of Processing Parameters and Thermal Cracking of Tar. Fuel Process. Technol. 2009, 90, 75–90. [Google Scholar] [CrossRef]
- Al-Mrayat, T.; Al-Hamaiedeh, H.; El-Hasan, T.; Aljbour, S.H.; Al-Ghazawi, Z.; Mohawesh, O. Pyrolysis of Domestic Sewage Sludge: Influence of Operational Conditions on the Product Yields Using Factorial Design. Heliyon 2022, 8, e09418. [Google Scholar] [CrossRef]
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties. Energies 2023, 16, 6936. [Google Scholar] [CrossRef]
- Choi, H.S.; Choi, Y.S.; Park, H.C. Fast Pyrolysis Characteristics of Lignocellulosic Biomass with Varying Reaction Conditions. Renew. Energy 2012, 42, 131–135. [Google Scholar] [CrossRef]
- Cai, Y.; Jiao, X.; Aihemaiti, A.; Su, Y.; Sun, Y.; Chen, J.; Shen, X.; Yan, F.; Li, H.; Chen, H.; et al. Effect of Carbon Dioxide on Pyrolytic Products Characteristics and DOM Binding Heavy Metals Copper in Sewage Sludge Biochar. Energy 2024, 291, 130439. [Google Scholar] [CrossRef]
- Heidari, A.; Stahl, R.; Younesi, H.; Rashidi, A.; Troeger, N.; Ghoreyshi, A.A. Effect of Process Conditions on Product Yield and Composition of Fast Pyrolysis of Eucalyptus Grandis in Fluidized Bed Reactor. J. Ind. Eng. Chem. 2014, 20, 2594–2602. [Google Scholar] [CrossRef]
- Ertaş, M.; Hakkı Alma, M. Pyrolysis of Laurel (Laurus nobilis L.) Extraction Residues in a Fixed-Bed Reactor: Characterization of Bio-Oil and Bio-Char. J. Anal. Appl. Pyrolysis 2010, 88, 22–29. [Google Scholar] [CrossRef]
- Namdari, M.; Soleimani, M.; Mirghaffari, N.; Kharrazi, S.M. Effect of Biological Sewage Sludge and Its Derived Biochar on Accumulation of Potentially Toxic Elements by Corn (Zea mays L.). Sci. Rep. 2024, 14, 5985. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Xie, W.; Zhang, D.; Gao, X.; Qiao, Y.; Xu, M. Pyrolysis of Sewage Sludge under Conditions Relevant to Applied Smouldering Combustion. Proc. Combust. Inst. 2023, 39, 3447–3456. [Google Scholar] [CrossRef]
- Altıkat, A.; Alma, M.H.; Altıkat, A.; Bilgili, M.E.; Altıkat, S. A Comprehensive Study of Biochar Yield and Quality Concerning Pyrolysis Conditions: A Multifaceted Approach. Sustainability 2024, 16, 937. [Google Scholar] [CrossRef]
- Kończak, M.; Pan, B.; Ok, Y.S.; Oleszczuk, P. Carbon Dioxide as a Carrier Gas and Mixed Feedstock Pyrolysis Decreased Toxicity of Sewage Sludge Biochar. Sci. Total Environ. 2020, 723, 137796. [Google Scholar] [CrossRef]
| Treatment | Czechia | Denmark | France | Germany | Greece | Ireland | Italy | Poland | Portugal | Spain | Sweden | U.K. | USA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stabilization | ||||||||||||||
| Aerobic | ||||||||||||||
| Anaerobic | ||||||||||||||
| Lime | ||||||||||||||
| Composting | ||||||||||||||
| Dewatering | ||||||||||||||
| Filter press | ||||||||||||||
| Centrifuges | ||||||||||||||
| Belt filter | ||||||||||||||
| Others | ||||||||||||||
| Thermal drying | ||||||||||||||
| Solar drying | ||||||||||||||
| Long-term storage | ||||||||||||||
| Most commonly used | Commonly used | Not used | ||||||||||||
| Origin of Feedstock | Examples of Feedstock | Biochar’s Class | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | AO | A | U | CM | BM | |||||
| Agriculture | Energy crops, short rotation woody crops, harvest residues | |||||||||
| Old straw and grain dust, vegetables, seeds | ||||||||||
| Forestry and wood processing | Bark, wood chips, and residues from mechanical processing, sawdust | |||||||||
| Landscape management | Biomasses from nature conservation, landscaping residues | |||||||||
| Foliage, root stocks | ||||||||||
| Recycling residues | Untreated waste wood, paper fiber sludge, residues from industrial biomass | |||||||||
| Waste paper, waste wood without PVC, heavy metals, or wood preservative | ||||||||||
| Waste wood with PVC and/or HMs, with/without wood preservatives | ||||||||||
| Food processing residues on vegetable basis | Pomace, kernels, husk, grist, residues from potatoes, corn, etc. | |||||||||
| Different residues from food production | ||||||||||
| Kitchen waste | Kitchen, canteen, and restaurant residue | |||||||||
| Water maintenance biomass | Aquatic plants and algae | |||||||||
| Screening, floating debris, mowed material | ||||||||||
| Textiles | Cellulose, cotton, and plant fibers, fibers of hemp, sisal | |||||||||
| Anaerobic digestion | Plant-based digestate | |||||||||
| Digestate from secondary plant biomass | ||||||||||
| Manure digestate, animal byproduct digestate | ||||||||||
| Animal byproduct | Bones, manures | |||||||||
| Other animal byproducts | ||||||||||
| Sludges from wastewater treatment | Sludge from municipal wastewater treatment | |||||||||
| Sludge from other wastewater treatment | ||||||||||
| Permissible | Allowed with some restrictions | Not recommended | ||||||||
| Feedstock Property | Main Effects on Biochar |
|---|---|
| Moisture | Low content is crucial for higher biochar yield |
| Volatile matter (VM) | High VM content may lead to higher gas and tar yields during pyrolysis, resulting in lower biochar yield |
| Ash | Low ash content minimizes impurities in the biochar, which can affect its properties such as porosity, surface area, and reactivity. High ash content reduces biochar yield |
| Fixed carbon (FC) | High FC content contributes to the carbon stability and heating value of the biochar, resulting in a higher biochar yield and quality |
| Elemental composition (C, H, N, O) | Higher C content and lower O content generally result in higher biochar yield with higher stability, porosity, and surface area |
| Lignin, cellulose, and hemicellulose | Higher lignin content is associated with higher biochar yield and stability, while cellulose and hemicellulose content can influence biochar porosity and surface area |
| Particle size and density | Smaller particle size and higher density generally result in higher biochar yield and better control over biochar properties |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biney, M.; Gusiatin, M.Z. Biochar from Co-Pyrolyzed Municipal Sewage Sludge (MSS): Part 1: Evaluating Types of Co-Substrates and Co-Pyrolysis Conditions. Materials 2024, 17, 3603. https://doi.org/10.3390/ma17143603
Biney M, Gusiatin MZ. Biochar from Co-Pyrolyzed Municipal Sewage Sludge (MSS): Part 1: Evaluating Types of Co-Substrates and Co-Pyrolysis Conditions. Materials. 2024; 17(14):3603. https://doi.org/10.3390/ma17143603
Chicago/Turabian StyleBiney, Michael, and Mariusz Z. Gusiatin. 2024. "Biochar from Co-Pyrolyzed Municipal Sewage Sludge (MSS): Part 1: Evaluating Types of Co-Substrates and Co-Pyrolysis Conditions" Materials 17, no. 14: 3603. https://doi.org/10.3390/ma17143603







