Investigating the Dewatering Efficiency of Sewage Sludge with Optimized Ratios of Electrolytic Manganese Residue Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Characterization of Sludge Dewatering Performance
- (1)
- CST test
- (2)
- SRF test
- (3)
- wt% test
- (4)
- Zeta Potential Test
2.3. Extraction and Determination of EPS
2.4. Analytical Methods
3. Results and Discussion
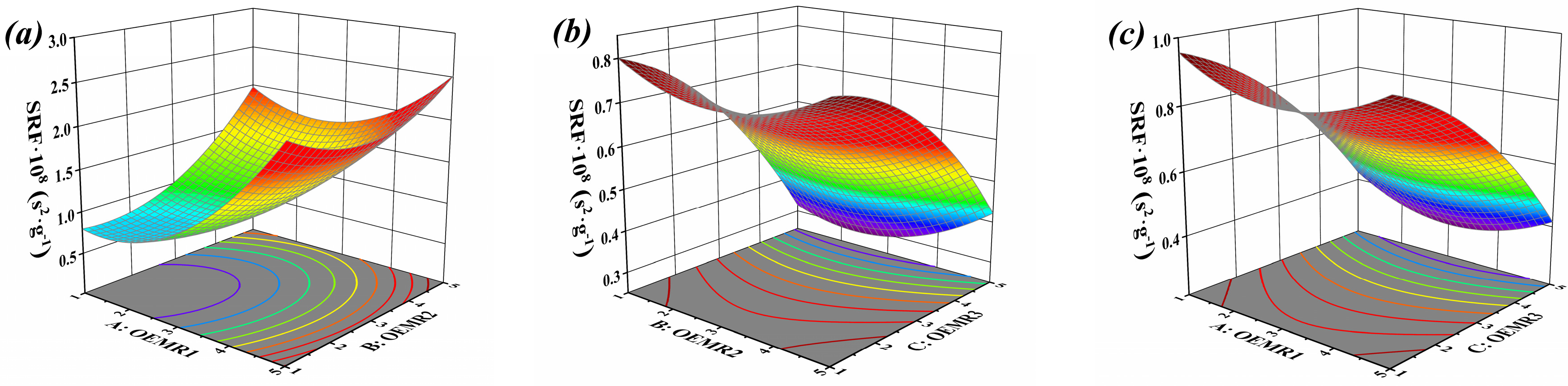
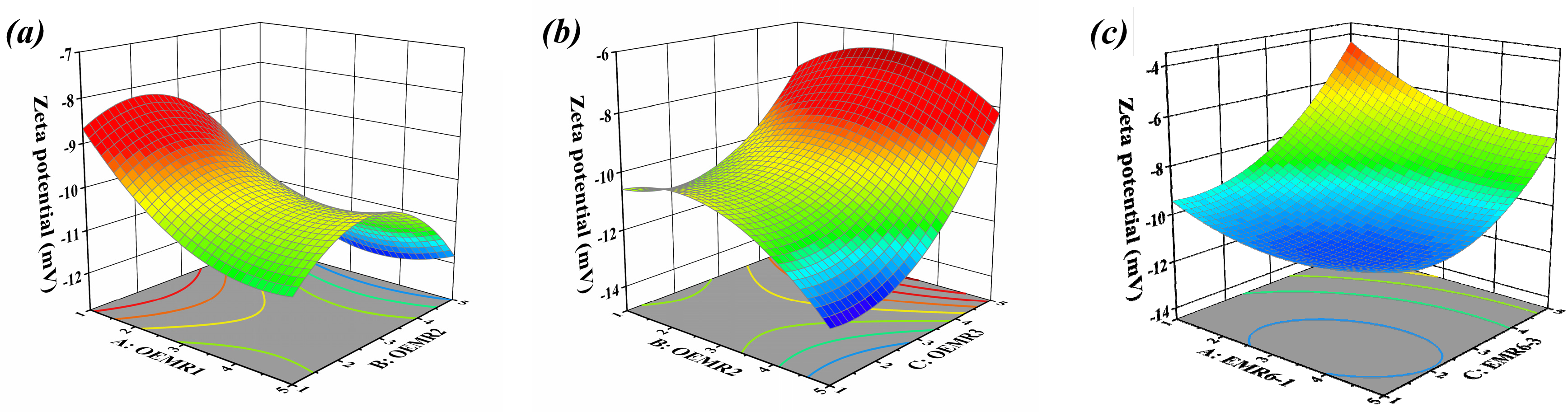
3.1. Result and Analysis of Box-Behnken Test
3.2. XRD Analysis with Internal Standard Method by Blending EMR Components
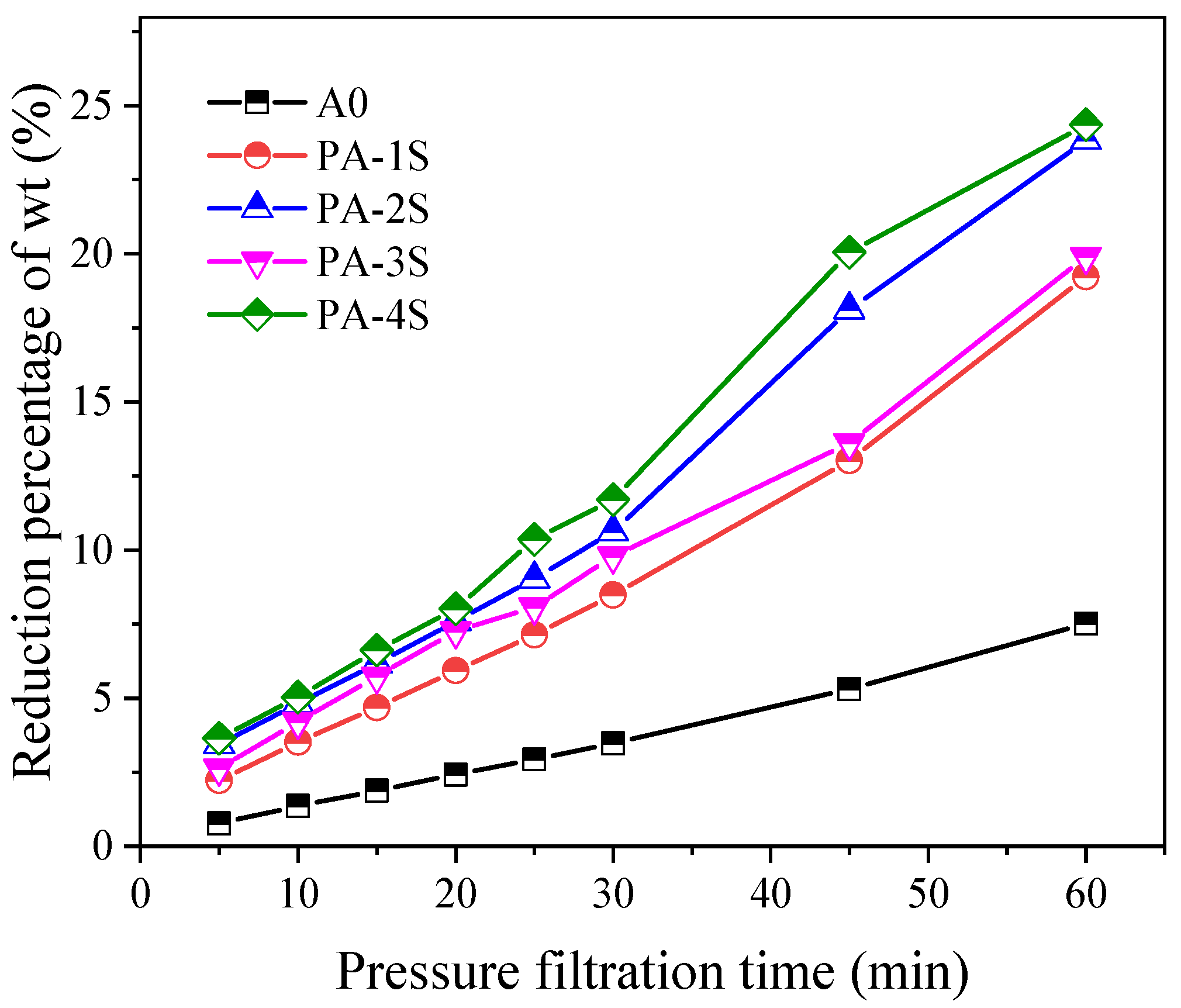
3.3. Dehydration Test of Response Surface Optimization Group with a Filter Press
3.4. Distributions and Compositions of EPS
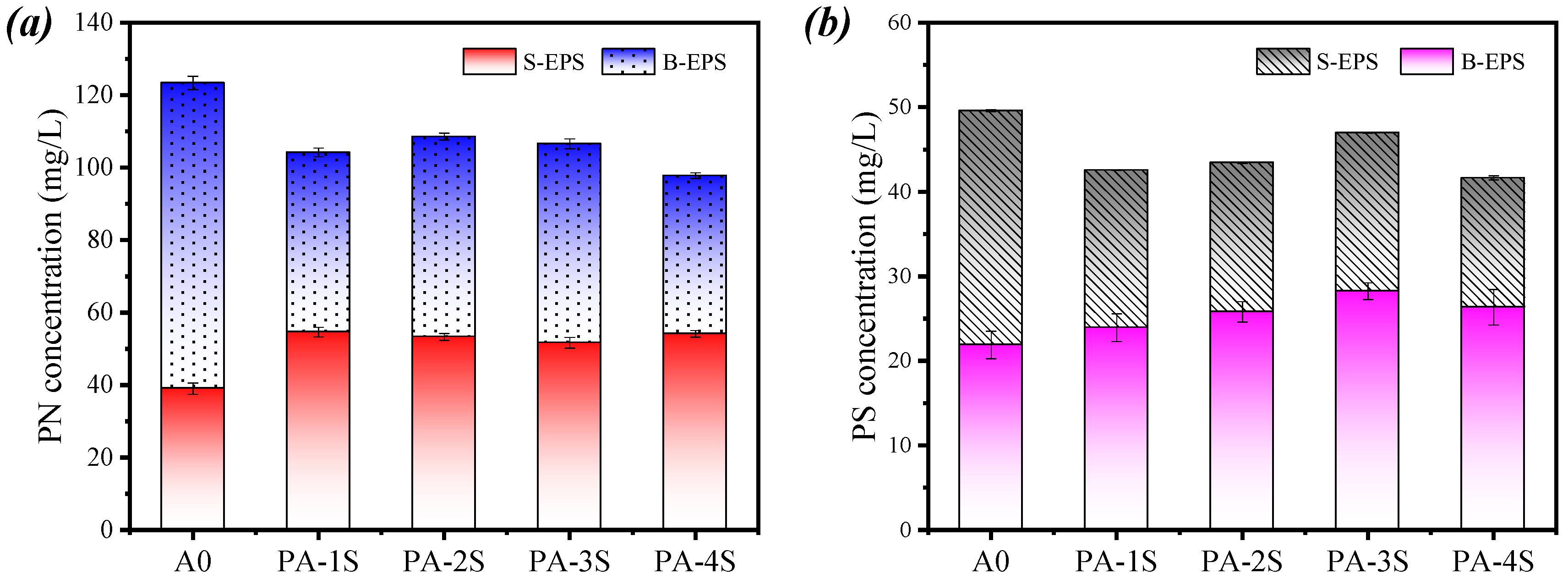
3.4.1. Concentration Distribution of Different Components in EPS
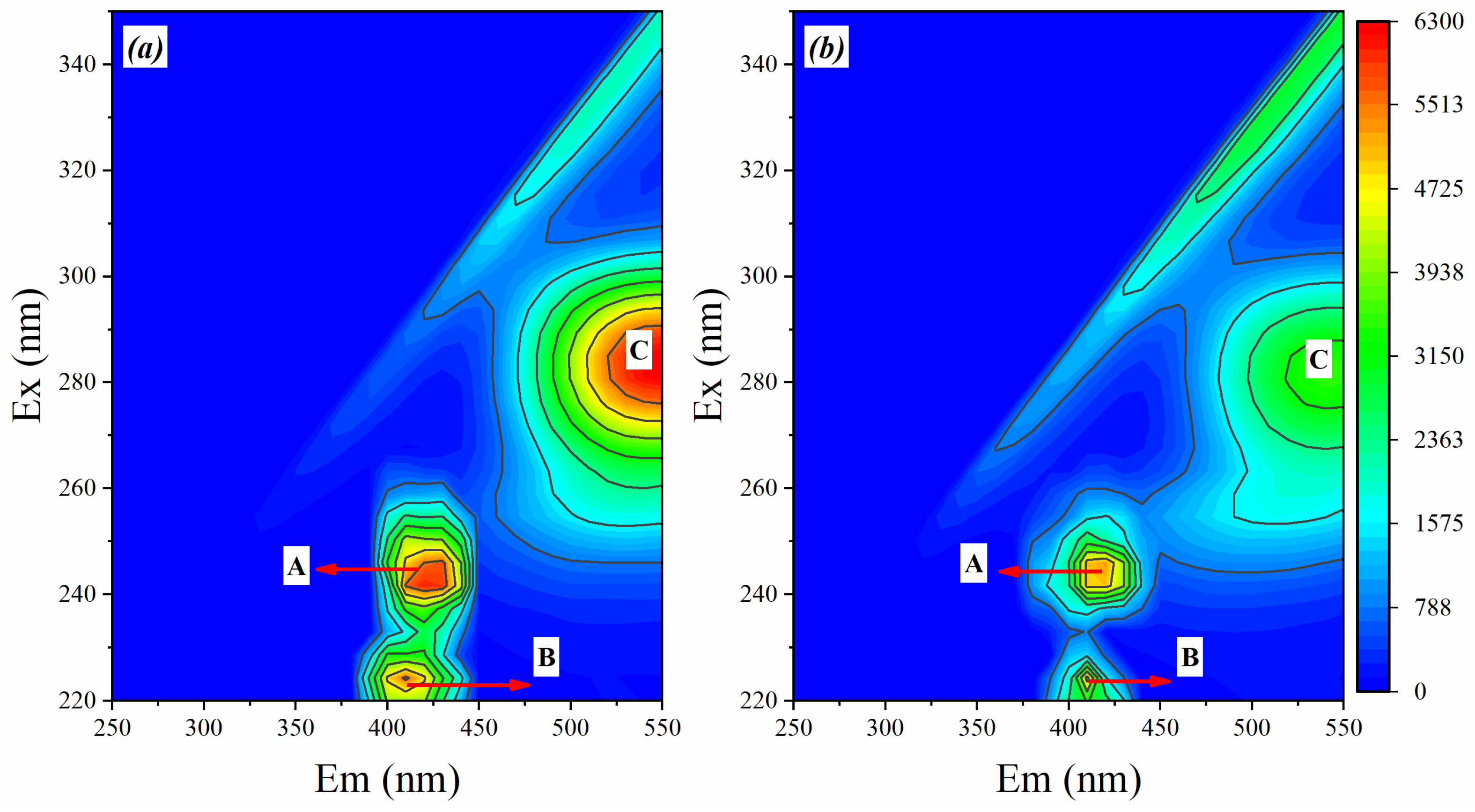
3.4.2. 3D-EEM Fluorescence Spectra of EPS at Different Conditioner
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, R.; Yuan, X.; Wu, Z.; Jiang, L.; Li, Y.; Zeng, G. Principle and application of hydrogen peroxide based advanced oxidation processes in activated sludge treatment: A review. Chem. Eng. J. 2018, 339, 519–530. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Zou, X.; Zhong, Y.; Pan, X.; Pang, H.; Zhang, J.; Cui, X.; Wu, X.; Li, B.; et al. Impact of divalent cations on lysozyme-induced solubilisation of waste-activated sludge: Perspectives of extracellular polymeric substances and surface electronegativity. Chemosphere 2022, 302, 134841. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, J.; Zhang, J.; Dai, T.; Yi, H.; Chen, F.; Zhou, M.; Hou, H. Multiple environmental risk assessments of heavy metals and optimization of sludge dewatering: Red mud–reed straw biochar combined with Fe2+ activated H2O2. J. Environ. Manag. 2022, 316, 115210. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Huang, C.; Feng, X.; Li, Y.; Yang, H.; Wang, S.; Li, J. Upgradation of sludge deep dewatering conditioners through persulfate activated by ferrous: Compatibility with sludge incineration, dewatering mechanism, ecological risks elimination and carbon emission performance. Environ. Res. 2022, 211, 113024. [Google Scholar] [CrossRef] [PubMed]
- Gahlot, P.; Balasundaram, G.; Tyagi, V.K.; Atabani, A.E.; Suthar, S.; Kazmi, A.A.; Štěpanec, L.; Juchelková, D.; Kumar, A. Principles and potential of thermal hydrolysis of sewage sludge to enhance anaerobic digestion. Environ. Res. 2022, 214, 113856. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liang, J.; Yuan, X.; Li, H.; Li, C.; Xiao, Z.; Huang, H.; Wang, H.; Zeng, G. Co-pelletization of sewage sludge and biomass: The density and hardness of pellet. Bioresour. Technol. 2014, 166, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Ma, L.; Ren, N.; Ning, P.; Guo, Z.; Xie, L.; Gao, H. Investigation on extracellular polymeric substances, sludge flocs morphology, bound water release and dewatering performance of sewage sludge under pretreatment with modified phosphogypsum. Water Res. 2018, 142, 337–346. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Wang, D.; Ma, T.; Bai, R. Enhancement of activated sludge dewatering performance by combined composite enzymatic lysis and chemical re-flocculation with inorganic coagulants: Kinetics of enzymatic reaction and re-flocculation morphology. Water Res. 2015, 83, 367–376. [Google Scholar] [CrossRef]
- Qi, Y.; Thapa, K.B.; Hoadley, A.F.A. Application of filtration aids for improving sludge dewatering properties—A review. Chem. Eng. J. 2011, 171, 373–384. [Google Scholar] [CrossRef]
- Chen, K.; Sun, Y.; Fan, J.; Gu, Y. The dewatering performance and cracking-flocculation-skeleton mechanism of bioleaching-coal fly ash combined process for sewage sludge. Chemosphere 2022, 307, 135994. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, P.; Wang, H.; Ye, J. Conditioning of sewage sludge via combined ultrasonication-flocculation-skeleton building to improve sludge dewaterability. Ultrason. Sonochem. 2018, 40, 353–360. [Google Scholar] [CrossRef]
- Deng, Y.; Shu, J.; Lei, T.; Zeng, X.; Li, B.; Chen, M. A green method for Mn2+ and NH4+-N removal in electrolytic manganese residue leachate by electric field and phosphorus ore flotation tailings. Sep. Purif. Technol. 2021, 270, 118820. [Google Scholar] [CrossRef]
- Lu, T.; Wei, Z.A.; Li, S.L.; Wang, Y.; Wang, W.S.; Yang, Y.H.; Zheng, B.B. Effect of soluble salts in electrolytic manganese residue on its geotechnical characteristics. J. Environ. Manag. 2023, 340, 117999. [Google Scholar] [CrossRef]
- Ayala, J.; Fernández, B. Recovery of manganese from silicomanganese slag by means of a hydrometallurgical process. Hydrometallurgy 2015, 158, 68–73. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, R.; Shu, J.; Chen, M.; Xu, Z.; Xue, T.; Zeng, X.; He, D.; Tan, D.; Deng, Z.; et al. Enhanced manganese leaching from electrolytic manganese residue by electrochemical process and Na2SO3. Miner. Eng. 2022, 189, 107862. [Google Scholar] [CrossRef]
- Lan, J.; Zhang, S.; Mei, T.; Dong, Y.; Hou, H. Mechanochemical modification of electrolytic manganese residue: Ammonium nitrogen recycling, heavy metal solidification, and baking-free brick preparation. J. Clean. Prod. 2021, 329, 129727. [Google Scholar] [CrossRef]
- Chen, H.; Long, Q.; Zhou, F.; Shen, M. Elec-accumulating behaviors of manganese in the electrokinetics-processed electrolytic manganese residue with carbon dioxide and oxalic acid. J. Electroanal. Chem. 2020, 865, 114162. [Google Scholar] [CrossRef]
- Tang, B.; Gao, S.; Wang, Y.; Liu, X.; Zhang, N. Pore structure analysis of electrolytic manganese residue based permeable brick by using industrial CT. Constr. Build. Mater. 2019, 208, 697–709. [Google Scholar] [CrossRef]
- He, W.L.; Li, R.; Yang, Y.P.; Zhang, Y.; Nie, D.P. Kinetic and thermodynamic analysis on preparation of belite-calcium sulphoaluminate cement using electrolytic manganese residue and barium slag by TGA. Environ. Sci. Pollut. Res. 2023, 30, 95901–95916. [Google Scholar] [CrossRef]
- Song, N.N.; Chen, Z.W.; Yu, Y.; Yang, B.R.; Guo, Y.; Chu, Y.P.; Chen, T.T.; Bi, Y.H.; Zhang, Q.W.; Feng, C.; et al. Electrolytic manganese residue to metal phosphides via ball milling: Enhancing flame retardancy, mechanical properties, and electrical conductivity of epoxy resin. Appl. Surf. Sci. 2023, 638, 158154. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Zhang, Z. A novel conversion process for waste residue: Synthesis of zeolite from electrolytic manganese residue and its application to the removal of heavy metals. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 258–267. [Google Scholar] [CrossRef]
- Lv, Y.; Li, J.; Ye, H.; Du, D.; Li, J.; Sun, P.; Ma, M.; Wen, J. Bioleaching behaviors of silicon and metals in electrolytic manganese residue using silicate bacteria. J. Clean. Prod. 2019, 228, 901–909. [Google Scholar] [CrossRef]
- Wang, F.; Long, G.C.; Zhou, J.L. Enhanced green remediation and refinement disposal of electrolytic manganese residue using air-jet milling and horizontal-shaking leaching. J. Hazard. Mater. 2024, 465, 133419. [Google Scholar] [CrossRef] [PubMed]
- Ziyao, S.; Xiaorong, Z.; Zaiqian, W.; Yihan, H.; Yimin, L.; Xuquan, H. Comprehensive effects of grain-size modification of electrolytic manganese residue on deep dehydration performance and microstructure of sludge. J. Environ. Manag. 2023, 326, 116793. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, J.K.; Shi, Y.F.; Li, Y.; He, S.; Yang, C.Z.; Yao, H. Conditioning of sewage sludge by Fenton’s reagent combined with skeleton builders. Chemosphere 2012, 88, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, L.; Rodríguez, M.; Prats, D. Effect of different extraction methods on bound EPS from MBR sludges. Part I: Influence of extraction methods over three-dimensional EEM fluorescence spectroscopy fingerprint. Desalination 2010, 261, 19–26. [Google Scholar] [CrossRef]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 2002, 28, 350–356. [Google Scholar] [CrossRef]
- Jiang, N.; Zhao, Y.; Qiu, C.; Shang, K.; Lu, N.; Li, J.; Wu, Y.; Zhang, Y. Enhanced catalytic performance of CoOx-CeO2 for synergetic degradation of toluene in multistage sliding plasma system through response surface methodology (RSM). Appl. Catal. B Environ. 2019, 259, 118061. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Moradi, S.; Shahlaei, M.; Farhadian, N. Application of carbon dots as efficient catalyst for the green oxidation of phenol: Kinetic study of the degradation and optimization using response surface methodology. J. Hazard. Mater. 2018, 353, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Morero, B.; Groppelli, E.S.; Campanella, E.A. Evaluation of biogas upgrading technologies using a response surface methodology for process simulation. J. Clean. Prod. 2017, 141, 978–988. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, K.; Hu, X.; He, Q.; Yan, J.; Xue, Y. Cadmium removal by MgCl2 modified biochar derived from crayfish shell waste: Batch adsorption, response surface analysis and fixed bed filtration. J. Hazard. Mater. 2021, 408, 124860. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, J.; Zhou, Y.; Luo, Y.; Zhou, B.; Fang, D.; Li, J.; Zhou, L. Enhancing sludge dewatering efficiency through bioleaching facilitated by increasing reactive oxygen species. Water Res. 2023, 231, 119622. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chadetrik, R.; Kumar, R.; Bishnoi, K.; Bhatia, D.; Kumar, A.; Bishnoi, N.R.; Singh, N. Biosorption optimization of lead(II), cadmium(II) and copper(II) using response surface methodology and applicability in isotherms and thermodynamics modeling. J. Hazard. Mater. 2009, 174, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Wilén, B.-M.; Keiding, K.; Nielsen, P.H. Anaerobic deflocculation and aerobic reflocculation of activated sludge. Water Res. 2000, 34, 3933–3942. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Q.; Zhang, M.; Guo, J.; Zheng, J.; Chen, Z.; Jia, Y.; Zhang, J.; Li, Z.; Zhang, H. Mg2+ distribution in activated sludge and its effects on the nitrifying activity and the characteristics of extracellular polymeric substances and sludge flocs. Process Biochem. 2020, 88, 120–128. [Google Scholar] [CrossRef]


| Name | Al2O3 | SiO2 | Fe2O3 | P2O5 | CaO | SO3 | K2O | TiO2 | Cl | MnO | ZnO | V2O5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| value | 46.73 | 24.36 | 10.33 | 7.57 | 5.57 | 2.59 | 0.95 | 0.92 | 0.26 | 0.19 | 0.16 | 0.11 |
| Name | Al2O3 | SiO2 | Fe2O3 | P2O5 | CaO | SO3 | K2O | TiO2 | MgO | MnO | ZnO | SrO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| value | 8.812 | 29.04 | 4.022 | 1.087 | 5.929 | 19.494 | 2.171 | 0.398 | 1.82 | 2.976 | 0.011 | 0.022 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 4729.48 | 9 | 525.50 | 75.08 | <0.0001 |
| A | 603.61 | 1 | 603.61 | 86.24 | <0.0001 |
| B | 3868.04 | 1 | 3868.04 | 552.64 | <0.0001 |
| C | 92.07 | 1 | 92.07 | 13.15 | 0.0084 |
| AB | 24.70 | 1 | 24.70 | 3.53 | 0.1024 |
| AC | 2.36 | 1 | 2.36 | 0.34 | 0.5800 |
| BC | 19.67 | 1 | 19.67 | 2.81 | 0.1376 |
| A2 | 46.14 | 1 | 46.14 | 6.59 | 0.0371 |
| B2 | 22.48 | 1 | 22.48 | 3.21 | 0.1162 |
| /C2 | 55.70 | 1 | 55.70 | 7.96 | 0.0257 |
| Residual | 48.99 | 7 | 7.00 | ||
| Lack of Fit | 35.95 | 3 | 11.98 | 3.68 | 0.1204 |
| Pure Error | 13.04 | 4 | 3.26 | ||
| Cor Total | 4778.47 | 16 | |||
| Std. Dev. | 2.65 | R-Squared | 0.9897 | ||
| Mean | 83.81 | Adj R-Squared | 0.9766 | ||
| C. V. % | 3.16 | Pred R-Squared | 0.8754 | ||
| PRESS | 595.63 | Adeq Precisior | 33.368 | ||
| Source | Sum of Squares | df | Mean Square | F Value | p-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 0.50 | 9 | 0.055 | 58.26 | <0.0001 |
| A | 0.016 | 1 | 0.016 | 16.71 | 0.0046 |
| B | 5.500 × 10−³ | 1 | 5.500 × 10−³ | 5.78 | 0.0472 |
| C | 0.37 | 1 | 0.37 | 389.17 | <0.0001 |
| AB | 9.947 × 10−³ | 1 | 9.947 × 10−³ | 10.45 | 0.0144 |
| AC | 4.391 × 10−³ | 1 | 4.391 × 10−³ | 4.61 | 0.0689 |
| BC | 2.682 × 10−³ | 1 | 2.682 × 10−³ | 2.82 | 0.1372 |
| A2 | 0.042 | 1 | 0.042 | 44.31 | 0.0003 |
| B2 | 0.023 | 1 | 0.023 | 24.36 | 0.0017 |
| C2 | 0.027 | 1 | 0.027 | 28.86 | 0.0010 |
| Residual | 6.666 × 10−³ | 7 | 9.522 × 10−4 | ||
| Lack of Fit | 2.206 × 10−³ | 3 | 7.355 × 10−4 | 0.66 | 0.6185 |
| Pure Error | 4.459 × 10−³ | 4 | 1.115 × 10−³ | ||
| Cor Total | 0.51 | 16 | |||
| Std. Dev. | 0.031 | R-Squared | 0.9868 | ||
| Mean | 0.79 | Adj R-Squared | 0.9699 | ||
| C. V. % | 3.93 | Pred R-Squared | 0.9165 | ||
| PRESS | 0.042 | Adeq Precisior | 21.968 | ||
| Source | Sum of Squares | df | Mean Square | F Value | p-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 63.22 | 9 | 7.02 | 8.47 | 0.0051 |
| A | 5.27 | 1 | 5.27 | 6.35 | 0.0398 |
| B | 4.62 | 1 | 4.62 | 5.57 | 0.0503 |
| C | 30.46 | 1 | 30.64 | 36.73 | 0.0005 |
| AB | 0.59 | 1 | 0.59 | 0.71 | 0.4258 |
| AC | 1.38 | 1 | 1.38 | 1.66 | 0.2379 |
| BC | 1.00 | 1 | 1.00 | 1.21 | 0.3085 |
| A2 | 2.78 | 1 | 2.78 | 3.35 | 0.1099 |
| B2 | 7.85 | 1 | 7.85 | 9.46 | 0.0179 |
| C2 | 10.08 | 1 | 10.08 | 12.15 | 0.0102 |
| Residual | 5.81 | 7 | 0.83 | ||
| Lack of Fit | 4.54 | 3 | 1.51 | 4.79 | 0.0821 |
| Pure Error | 1.26 | 4 | 0.32 | ||
| Cor Total | 69.03 | 16 | |||
| Std. Dev. | 0.91 | R-Squared | 0.9159 | ||
| Mean | −9.61 | Adj R-Squared | 0.8078 | ||
| C. V. % | 9.47 | Pred R-Squared | −0.0813 | ||
| PRESS | 74.64 | Adeq Precisior | 12.512 | ||
| OEMR1 | OEMR2 | OEMR3 | |
|---|---|---|---|
| PA-1 | 4.5 | 5.0 | 1.0 |
| PA-2 | 2.2 | 1.2 | 5.0 |
| PA-3 | 1.1 | 1.4 | 4.9 |
| PA-4 | 2.3 | 3.7 | 5.0 |
| Components of Crystals (%) | OEMR | PA-4 |
|---|---|---|
| OEMR1 | 37.7 | 14.3 |
| OEMR2 | 21.5 | 36.2 |
| OEMR3 | 10.8 | 19.5 |
| Rutile | 30.0 | 30.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Wang, J.; Xue, F.; Zhao, X.; Shi, Z.; Liang, Q.; Wang, H.; Zhao, Z. Investigating the Dewatering Efficiency of Sewage Sludge with Optimized Ratios of Electrolytic Manganese Residue Components. Materials 2024, 17, 3605. https://doi.org/10.3390/ma17143605
Huang X, Wang J, Xue F, Zhao X, Shi Z, Liang Q, Wang H, Zhao Z. Investigating the Dewatering Efficiency of Sewage Sludge with Optimized Ratios of Electrolytic Manganese Residue Components. Materials. 2024; 17(14):3605. https://doi.org/10.3390/ma17143605
Chicago/Turabian StyleHuang, Xuquan, Jun Wang, Fei Xue, Xiaorong Zhao, Ziyao Shi, Qingyang Liang, Haojie Wang, and Ziyu Zhao. 2024. "Investigating the Dewatering Efficiency of Sewage Sludge with Optimized Ratios of Electrolytic Manganese Residue Components" Materials 17, no. 14: 3605. https://doi.org/10.3390/ma17143605






