Investigation of Using Calcined Coal Gangue as the Co-Blended Precursor in the Alkali-Activated Metakaolin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Testing Methods
2.3.1. Performance Evaluation
2.3.2. Reaction and Reaction Products
2.3.3. Microstructure Analysis
3. Results and Discussion
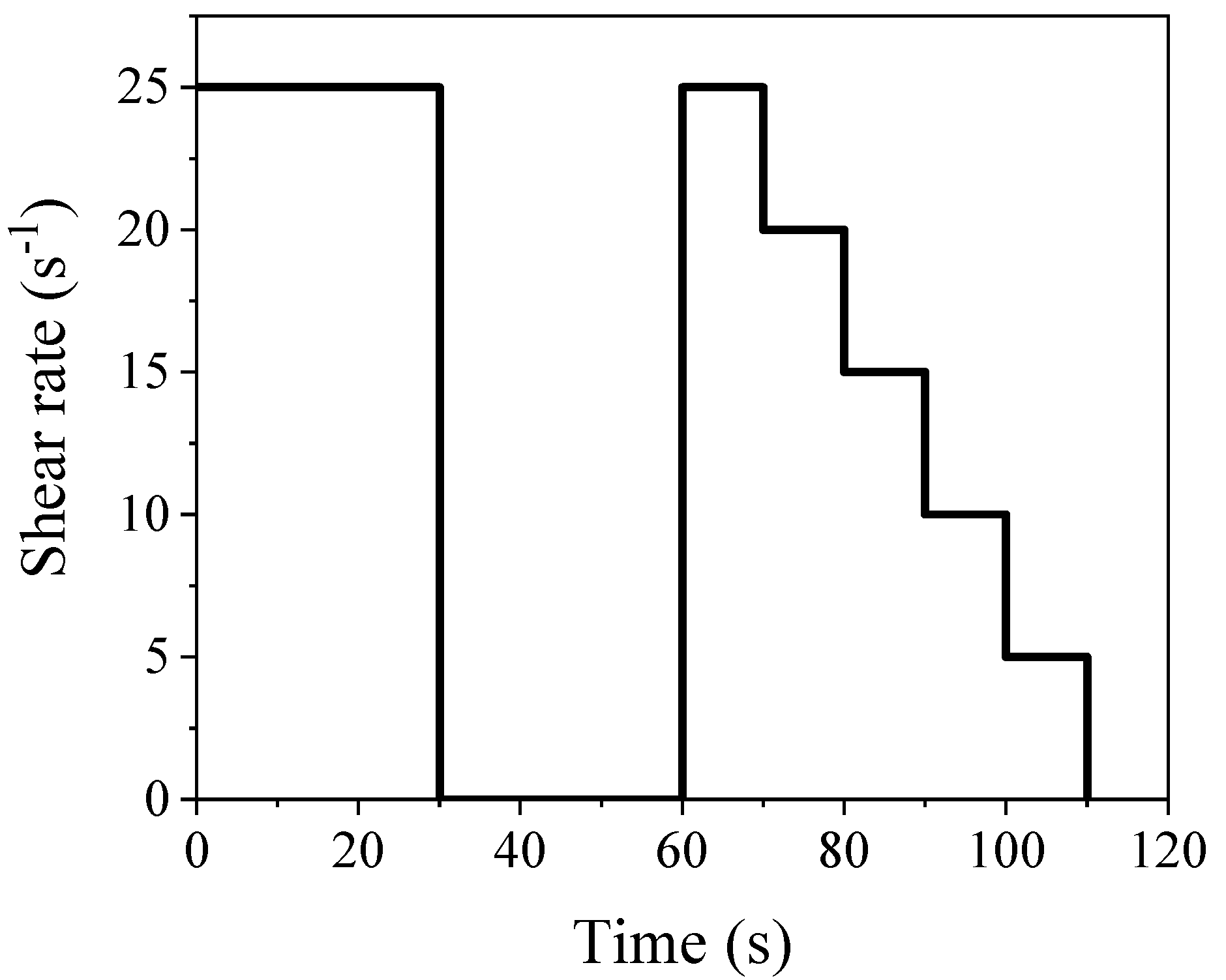
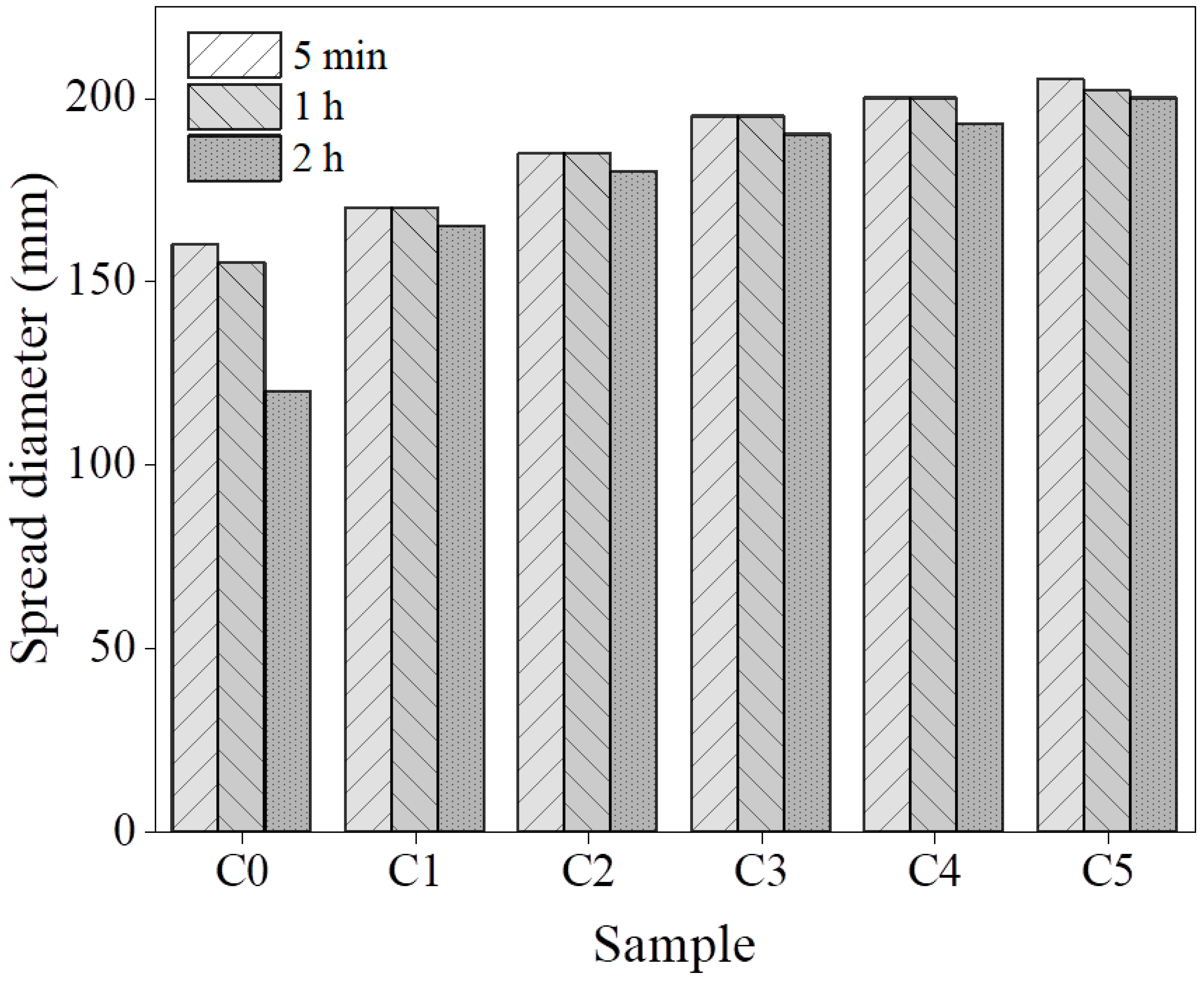
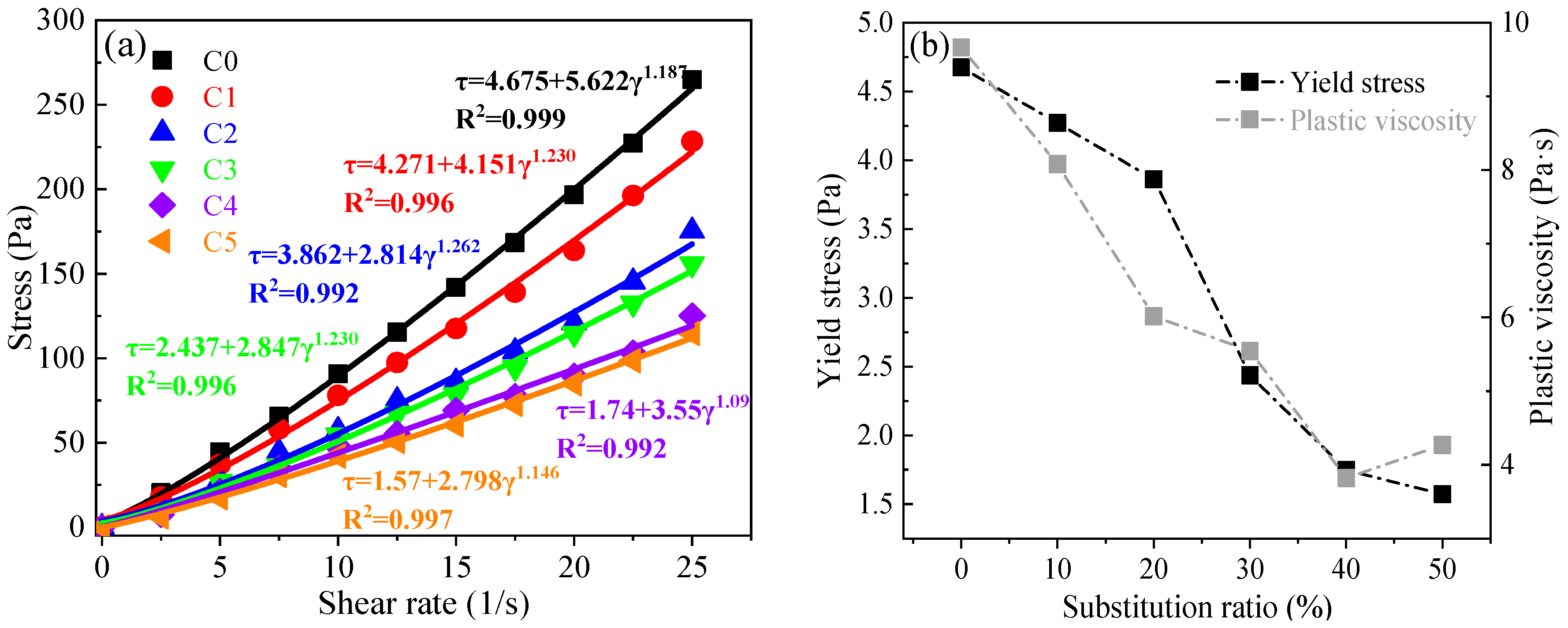
3.1. Flowability and Rheological Properties
3.2. Setting Time and Compressive Strength
3.3. Calorimetry
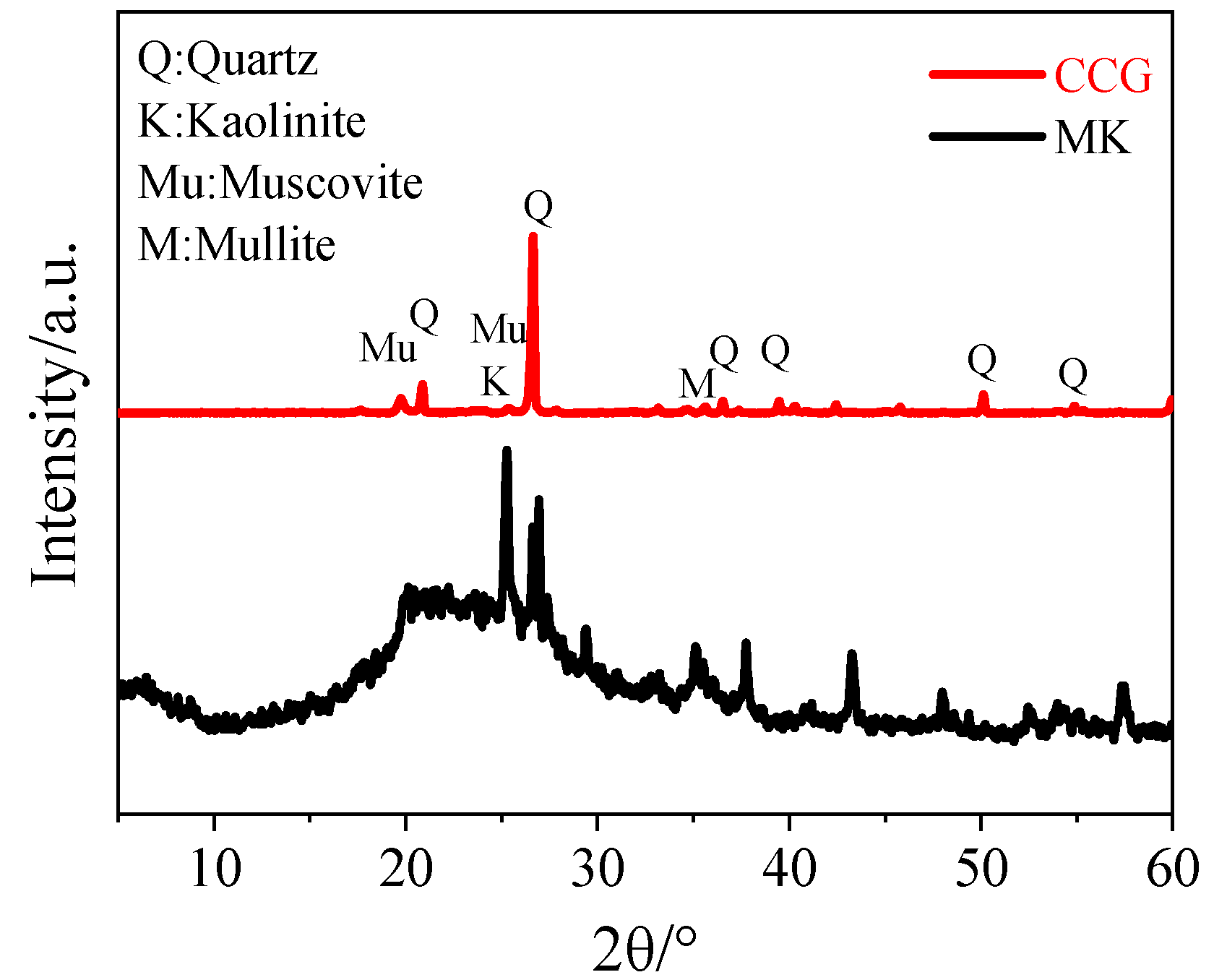
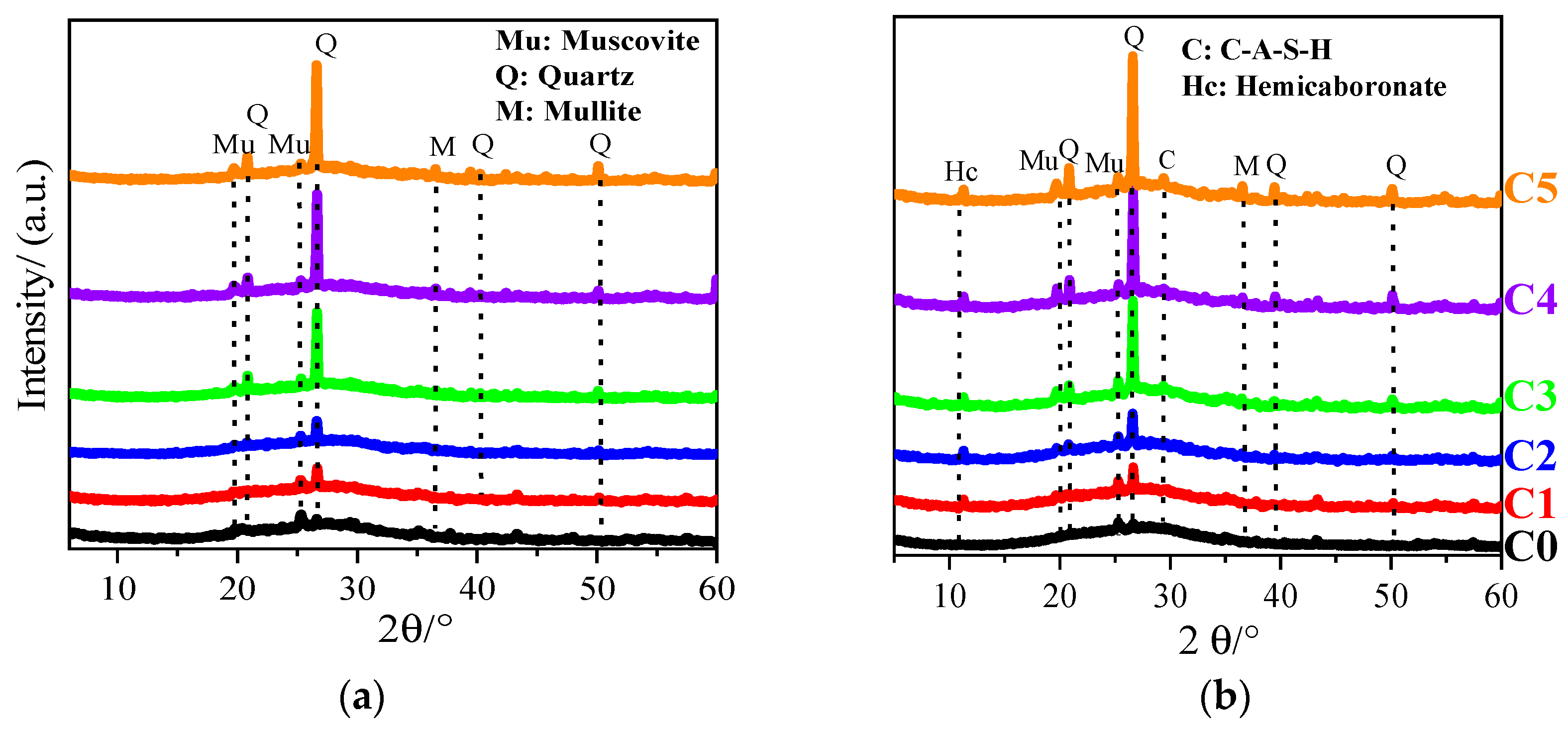
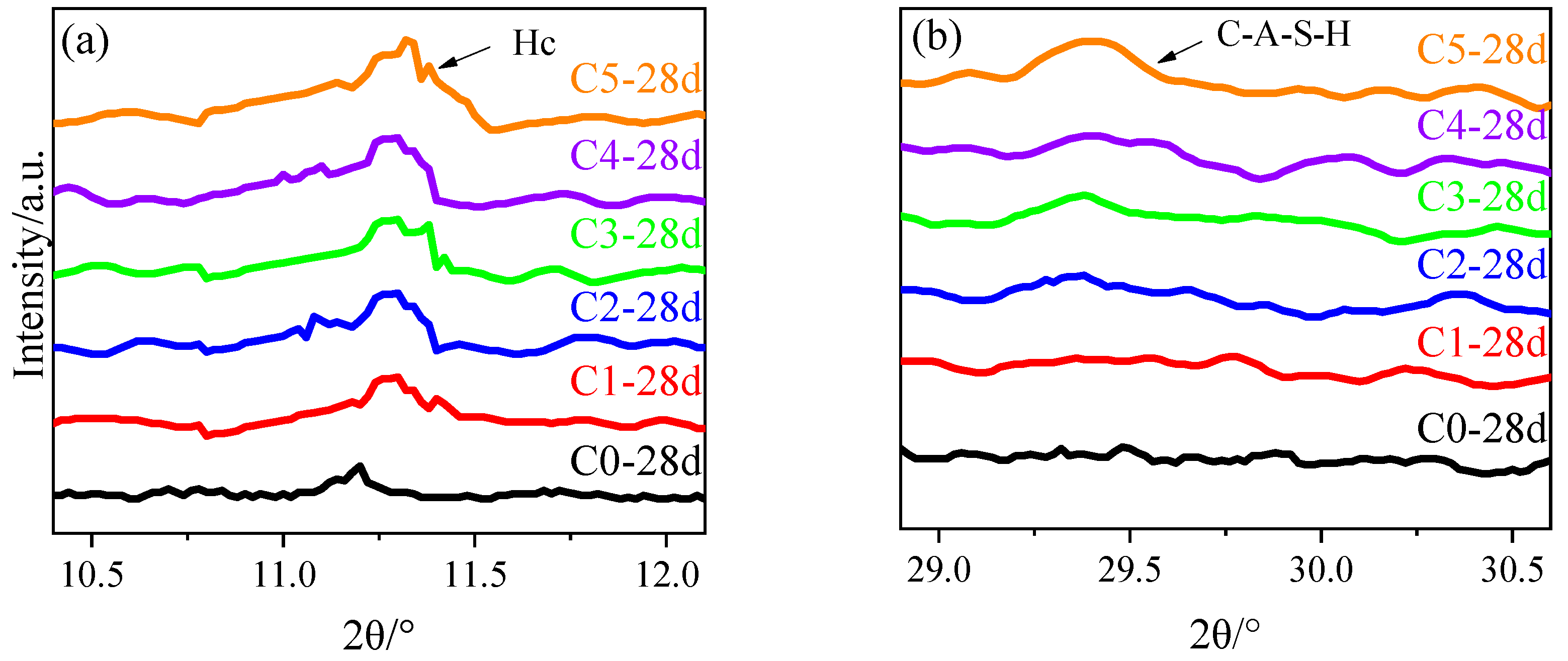
3.4. XRD
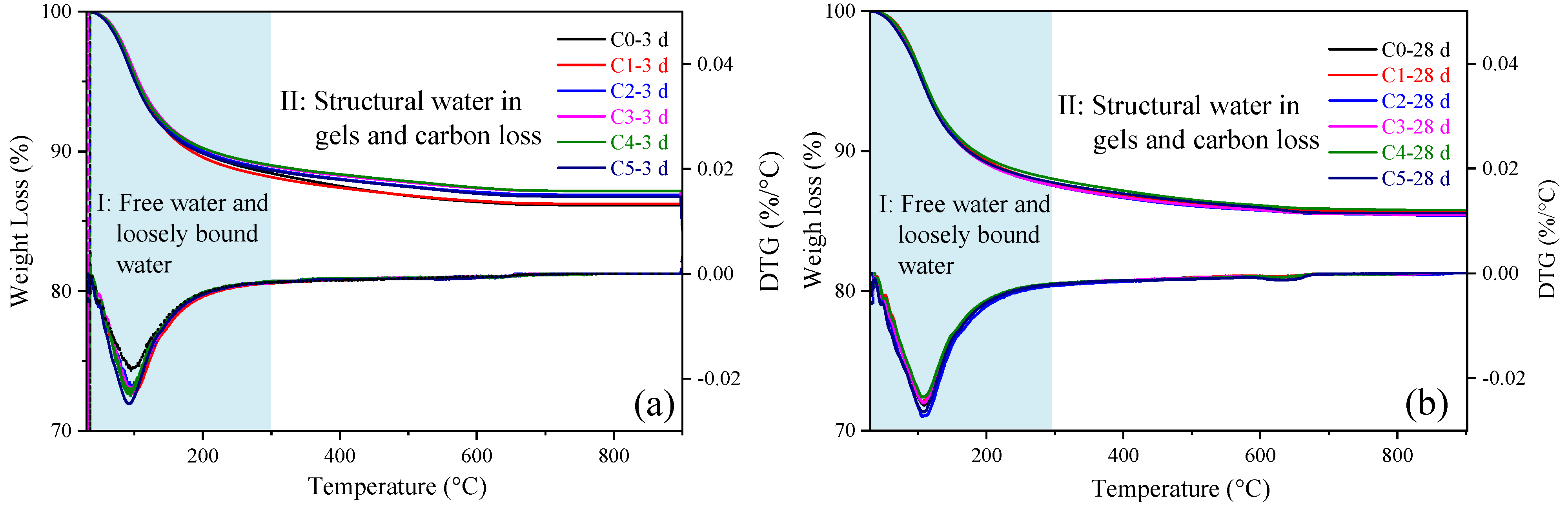
3.5. TGA-DTG
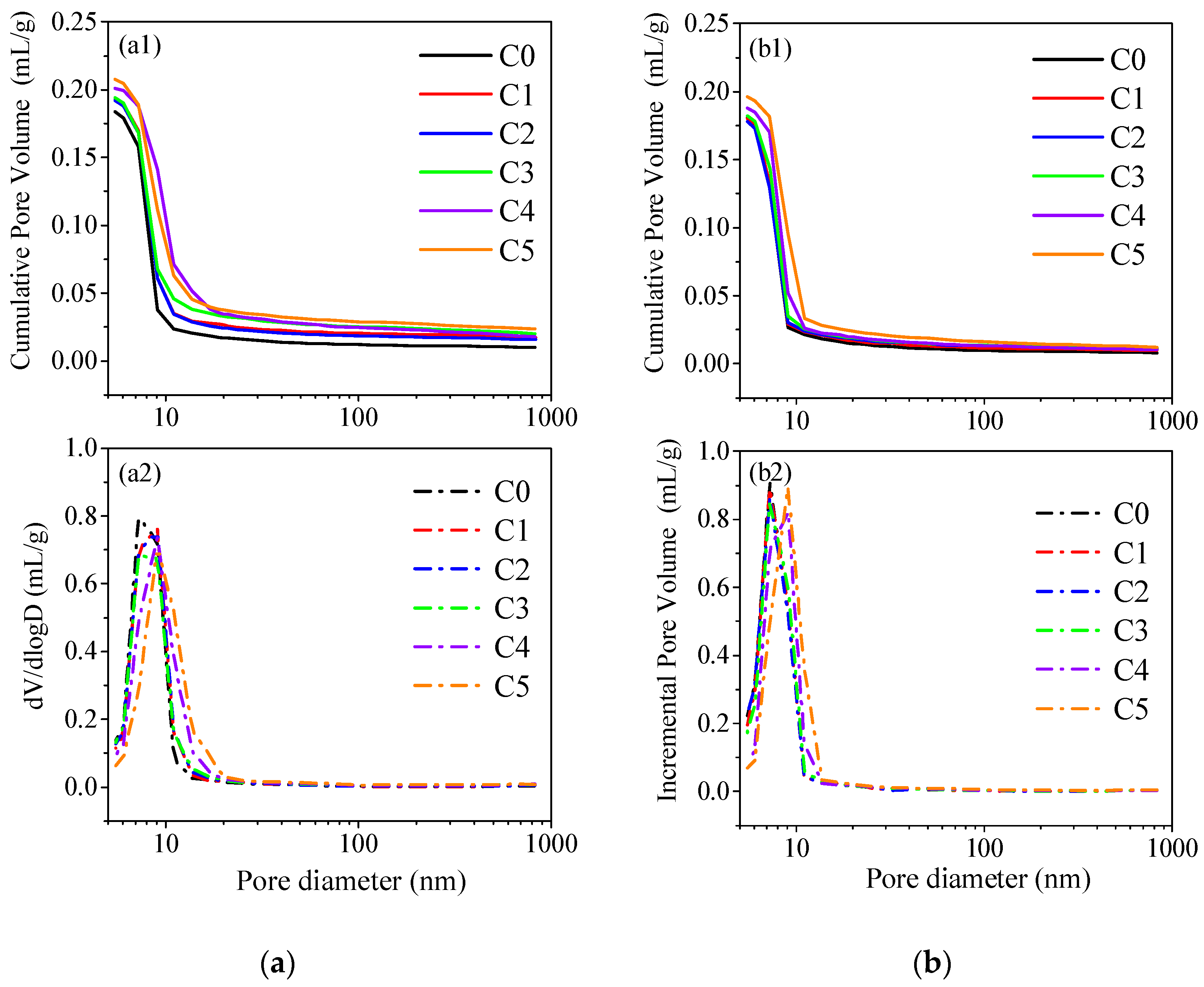
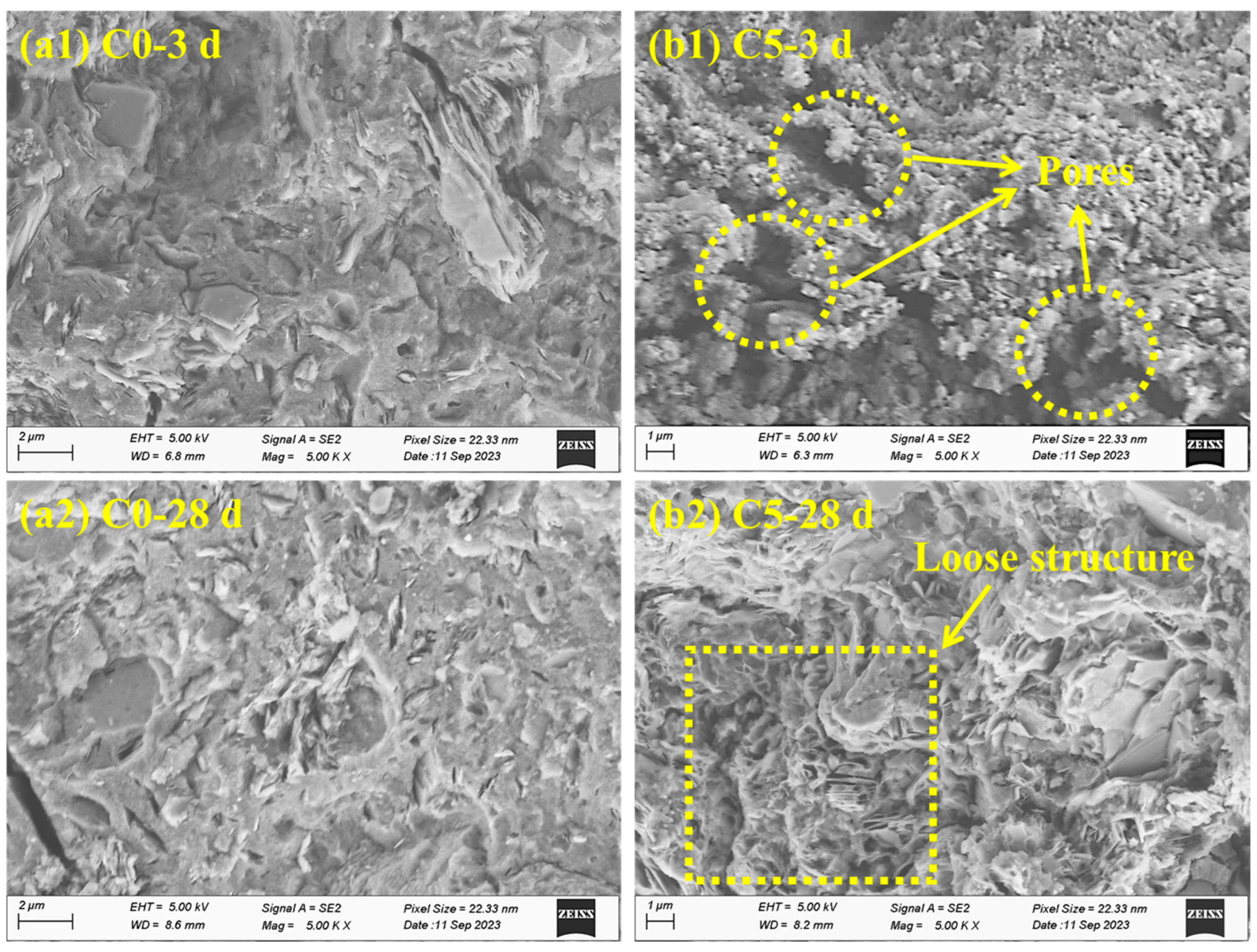
3.6. Microstructure Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Liu, C.; Zhang, L.; Wang, C.; Xu, S.; Yang, J. Exploring calcined coal gangue fines as the total substitute of fly ash in the production of alkali-activated slag/fly ash materials. Case Stud. Constr. Mater. 2022, 17, e01332. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Y.; Ren, H. Intelligent and ecological coal mining as well as clean utilization technology in China: Review and prospects. Int. J. Min. Sci. Technol. 2019, 29, 161–169. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, L.; Wang, Y.; Fang, Y.; Zhang, F.; Xu, Y. Environmentally friendly utilization of coal gangue as aggregates for shotcrete used in the construction of coal mine tunnel. Case Stud. Constr. Mater. 2021, 15, e00751. [Google Scholar] [CrossRef]
- Peng, W.; Liu, Y.; Lin, M.; Liu, Y.; Zhu, C.; Sun, L.; Gui, H. Toxicity of coal fly ash and coal gangue leachate to Daphnia magna: Focusing on typical heavy metals. J. Clean. Prod. 2022, 330, 129946. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, T.; Ren, G.; Zhu, Z.; Gao, Y.; Shi, M.; Ding, S.; Fan, H. Reusing waste coal gangue to improve the dispersivity and mechanical properties of dispersive soil. J. Clean. Prod. 2023, 404, 136993. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Shi, X.; Li, Y.; Zhang, X. Co-spontaneous combustion of coal and gangue: Thermal behavior, kinetic characteristics and interaction mechanism. Fuel 2022, 315, 123275. [Google Scholar] [CrossRef]
- Mishra, D.P. Effects of intrinsic properties, particle size and specific surface area on WOP and spontaneous combustion susceptibility of coal. Adv. Powder Technol. 2022, 33, 103454. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Z. Analysis of Coal Gangue Used to Produce Green Materials. Sci. Technol. Eng. 2007, 7, 1443–1445. [Google Scholar]
- Zhao, Y.; Yang, C.; Li, K.; Qu, F.; Yan, C.; Wu, Z. Toward understanding the activation and hydration mechanisms of composite activated coal gangue geopolymer. Constr. Build. Mater. 2022, 318, 125999. [Google Scholar] [CrossRef]
- Cheng, Y.; Hongqiang, M.; Hongyu, C.; Jiaxin, W.; Jing, S.; Zonghui, L.; Mingkai, Y. Preparation and characterization of coal gangue geopolymers. Constr. Build. Mater. 2018, 187, 318–326. [Google Scholar] [CrossRef]
- Yi, C.; Ma, H.; Zhu, H.; Li, W.; Xin, M.; Liu, Y.; Guo, Y. Study on chloride binding capability of coal gangue based cementitious materials. Constr. Build. Mater. 2018, 167, 649–656. [Google Scholar] [CrossRef]
- Li, L.; Shao, X.; Ling, T.-C. Life cycle assessment of coal gangue composite cements: From sole OPC towards low-carbon quaternary binder. J. Clean. Prod. 2023, 414, 137674. [Google Scholar] [CrossRef]
- Li, L.; Xie, J.; Zhang, B.; Feng, Y.; Yang, J. A state-of-the-art review on the setting behaviours of ground granulated blast furnace slag- and metakaolin-based alkali-activated materials. Constr. Build. Mater. 2023, 368, 130389. [Google Scholar] [CrossRef]
- Li, Z.; Delsaute, B.; Lu, T.; Kostiuchenko, A.; Staquet, S.; Ye, G. A comparative study on the mechanical properties, autogenous shrinkage and cracking proneness of alkali-activated concrete and ordinary Portland cement concrete. Constr. Build. Mater. 2021, 292, 123418. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, H.; Wu, C.; Fan, J.; Yang, S.; Hang, Z. Effect of shrinkage reducing admixture on drying shrinkage and durability of alkali-activated coal gangue-slag material. Constr. Build. Mater. 2021, 270, 121372. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, H.; Chen, H.; Ni, Y.; Xu, X.; Huo, Q. Shrinkage-reducing measures and mechanisms analysis for alkali-activated coal gangue-slag mortar at room temperature. Constr. Build. Mater. 2020, 252, 119001. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, B.; Li, Y. Study on mechanical properties, freeze–thaw and chlorides penetration resistance of alkali activated granulated blast furnace slag-coal gangue concrete and its mechanism. Constr. Build. Mater. 2023, 366, 130218. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Nepal, J.; Das, B.B. Properties, compatibility, environmental benefits and future directions of limestone calcined clay cement (LC3) concrete: A review. J. Build. Eng. 2023, 79, 107794. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, Understanding and Unlocking Supplementary Cementitious Materials. RILEM Tech. Lett. 2016, 1, 50–55. [Google Scholar] [CrossRef]
- Sarkar, M.; Dana, K.; Das, S. Microstructural and phase evolution in metakaolin geopolymers with different activators and added aluminosilicate fillers. J. Mol. Struct. 2015, 1098, 110–118. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Y.; Zhang, Z.; Liu, K.; Li, Y.; Shi, L.; Sun, D. The Durability of Alkali-Activated Materials in Comparison with Ordinary Portland Cements and Concretes: A Review. Engineering 2020, 6, 695–706. [Google Scholar] [CrossRef]
- Vu, T.H.; Dang, L.C.; Kang, G.; Sirivivatnanon, V. Chloride induced corrosion of steel reinforcement in alkali activated slag concretes: A critical review. Case Stud. Constr. Mater. 2022, 16, e01112. [Google Scholar]
- Pradhan, P.; Dwibedy, S.; Pradhan, M.; Panda, S.; Panigrahi, S.K. Durability characteristics of geopolymer concrete—Progress and perspectives. J. Build. Eng. 2022, 59, 105100. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, D.; Nath, S.; Alex, T.; Kumar, R. From grey waste to green geopolymer. Sci. Cult. 2012, 78, 511–516. [Google Scholar]
- Zhu, X.; Li, W.; Du, Z.; Zhou, S.; Zhang, Y.; Li, F. Recycling and utilization assessment of steel slag in metakaolin based geopolymer from steel slag by-product to green geopolymer. Constr. Build. Mater. 2021, 305, 124654. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Zou, J.; Reid, A.; Wang, H. Toward an indexing approach to evaluate fly ashes for geopolymer manufacture. Cem. Concr. Res. 2016, 85, 163–173. [Google Scholar] [CrossRef]
- GB/T 1346; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. Standardization Administration of the People’s Republic of China: Beijing, China, 2021.
- GB/T 2419; Test Method for Fluidity of Cement Mortar. Standardization Administration of the People’s Republic of China: Beijing, China, 2005.
- Feys, D.; Verhoeven, R.; De Schutter, G. Evaluation of time independent rheological models applicable to fresh self-compacting concrete. Appl. Rheol. 2007, 17, 56244-1–56244-10. [Google Scholar] [CrossRef]
- Wang, W.; Fan, C.; Wang, B.; Zhang, X.; Liu, Z. Workability, rheology, and geopolymerization of fly ash geopolymer: Role of alkali content, modulus, and water–binder ratio. Constr. Build. Mater. 2023, 367, 130357. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Wang, D.-M.; Xie, F.-Z. Microrheology of fresh geopolymer pastes with different NaOH amounts at room temperature. Constr. Build. Mater. 2019, 207, 284–290. [Google Scholar] [CrossRef]
- Zhong, Q.; Nie, H.; Xie, G.; Peng, H. Experimental study on the characteristics, rheological factors, and flowability of MK-GGBFS geopolymer slurry. J. Build. Eng. 2023, 76, 107300. [Google Scholar] [CrossRef]
- Wang, A.; Hao, F.; Liu, P.; Mo, L.; Liu, K.; Li, Y.; Cao, J.; Sun, D. Separation of calcined coal gangue and its influence on the performance of cement-based materials. J. Build. Eng. 2022, 51, 104293. [Google Scholar] [CrossRef]
- Liang, G.; Yao, W.; She, A. New insights into the early-age reaction kinetics of metakaolin geopolymer by 1H low-field NMR and isothermal calorimetry. Cem. Concr. Compos. 2023, 137, 104932. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Peng, K.-D.; Alrefaei, Y.; Dai, J.-G. The bond between geopolymer repair mortars and OPC concrete substrate: Strength and microscopic interactions. Cem. Concr. Compos. 2021, 119, 103991. [Google Scholar] [CrossRef]
- Chithiraputhiran, S.; Neithalath, N. Isothermal reaction kinetics and temperature dependence of alkali activation of slag, fly ash and their blends. Constr. Build. Mater. 2013, 45, 233–242. [Google Scholar] [CrossRef]
- Lancellotti, I.; Catauro, M.; Ponzoni, C.; Bollino, F.; Leonelli, C. Inorganic polymers from alkali activation of metakaolin: Effect of setting and curing on structure. J. Solid State Chem. 2013, 200, 341–348. [Google Scholar] [CrossRef]
- Georget, F.; Lothenbach, B.; Wilson, W.; Zunino, F.; Scrivener, K.L. Stability of hemicarbonate under cement paste-like conditions. Cem. Concr. Res. 2022, 153, 106692. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Zhang, H.; Li, M.; Wu, Y.; Guo, L.; Wang, W.; Duan, P.; Zhang, W.; Zhang, Z. Thermal stability and microstructure of metakaolin-based geopolymer blended with rice husk ash. Appl. Clay Sci. 2020, 196, 105769. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, G.; Zhang, Z.; Wu, Q.; Du, J. Partial replacement of metakaolin with thermally treated rice husk ash in metakaolin-based geopolymer. Constr. Build. Mater. 2019, 221, 527–538. [Google Scholar] [CrossRef]
- Liang, G.; Li, H.; Zhu, H.; Liu, T.; Chen, Q.; Guo, H. Reuse of waste glass powder in alkali-activated metakaolin/fly ash pastes: Physical properties, reaction kinetics and microstructure. Resour. Conserv. Recycl. 2021, 173, 105721. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Vogel, M.; Müller, H.S.; Zhao, T. Influence of freeze-thaw cycles on capillary absorption and chloride penetration into concrete. Cem. Concr. Res. 2017, 100, 60–67. [Google Scholar] [CrossRef]



| Oxide | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | Na2O | MgO | SO3 | P2O5 | TiO2 | ZrO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MK | 44.78 | 50.51 | 1.51 | 0.41 | 0.18 | 0.08 | 0.18 | 0.14 | 0.09 | 2.01 | 0.04 | 1.02 |
| CCG | 56.22 | 29.11 | 4.50 | 3.62 | 2.45 | 0.54 | 1.16 | 1.08 | 0.13 | 0.92 | 0.02 | 1.46 |
| Materials | d50 a (μm) | D [3,2] b (μm) | D [4,3] c (μm) |
|---|---|---|---|
| MK | 6.38 | 3.79 | 8.57 |
| CCG | 13.16 | 4.12 | 17.00 |
| Samples | Mk Content (wt.%) | CCG Content (wt.%) | Water/Binder | Na2O/Binder |
|---|---|---|---|---|
| C0 | 100 | 0 | 0.6 | 0.15 |
| C1 | 90 | 10 | 0.6 | 0.15 |
| C2 | 80 | 20 | 0.6 | 0.15 |
| C3 | 70 | 30 | 0.6 | 0.15 |
| C4 | 60 | 40 | 0.6 | 0.15 |
| C5 | 50 | 50 | 0.6 | 0.15 |
| Samples | Mass Loss (wt. %) | |||||
|---|---|---|---|---|---|---|
| 3 d | 28 d | |||||
| I | II | Sum | I | II | Sum | |
| C0 | 11.6 | 2.3 | 13.9 | 12.2 | 2.2 | 14.4 |
| C1 | 11.6 | 1.7 | 13.7 | 12.3 | 2.0 | 14.4 |
| C2 | 11.2 | 1.8 | 13.0 | 12.4 | 2.1 | 14.6 |
| C3 | 11.0 | 1.8 | 12.8 | 12.5 | 2.1 | 14.5 |
| C4 | 10.5 | 1.8 | 12.3 | 12.0 | 2.2 | 14.2 |
| C5 | 10.6 | 1.7 | 12.3 | 12.3 | 2.2 | 14.4 |
| Parameter | Curing Ages | C0 | C1 | C2 | C3 | C4 | C5 |
|---|---|---|---|---|---|---|---|
| MPD/volume (nm) 1 | 3 d | 8.1 | 8.3 | 8.3 | 8.4 | 9.3 | 10.0 |
| 28 d | 7.8 | 7.9 | 7.9 | 8.0 | 8.3 | 9.0 | |
| APD/(4 V/A) (nm) 2 | 3 d | 8.8 | 9.5 | 9.5 | 9.9 | 10.7 | 11.4 |
| 28 d | 8.4 | 8.5 | 8.6 | 8.8 | 9.2 | 9.9 | |
| Porosity (%) | 3 d | 28.2 | 28.9 | 28.9 | 29.3 | 30.7 | 31.4 |
| 28 d | 27.4 | 27.2 | 27.2 | 28.0 | 28.5 | 29.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Lu, Z.; Zhang, L.; Zhang, H.; Zhang, Q.; Sun, Z. Investigation of Using Calcined Coal Gangue as the Co-Blended Precursor in the Alkali-Activated Metakaolin. Materials 2024, 17, 3610. https://doi.org/10.3390/ma17143610
Pan Y, Lu Z, Zhang L, Zhang H, Zhang Q, Sun Z. Investigation of Using Calcined Coal Gangue as the Co-Blended Precursor in the Alkali-Activated Metakaolin. Materials. 2024; 17(14):3610. https://doi.org/10.3390/ma17143610
Chicago/Turabian StylePan, Ye, Zichen Lu, Liheng Zhang, Hui Zhang, Qin Zhang, and Zhenping Sun. 2024. "Investigation of Using Calcined Coal Gangue as the Co-Blended Precursor in the Alkali-Activated Metakaolin" Materials 17, no. 14: 3610. https://doi.org/10.3390/ma17143610
APA StylePan, Y., Lu, Z., Zhang, L., Zhang, H., Zhang, Q., & Sun, Z. (2024). Investigation of Using Calcined Coal Gangue as the Co-Blended Precursor in the Alkali-Activated Metakaolin. Materials, 17(14), 3610. https://doi.org/10.3390/ma17143610






