A Comparative Study on the Wear Performance and High-Temperature Oxidation of Co-Free Cermets and Hardmetals
Abstract
:1. Introduction
- Study the response to high-temperature oxidation and wear of compositions based on Ti(C,N) and WC with a Co-free metallic phase (FeNiCr).
- Assess the effect of Cr in these materials by comparing them with others using an FeNi binder phase.
- Compare the behaviour of these materials with a commercial-grade WC-Co.
2. Materials and Methods
2.1. Starting Materials and Processing
2.2. Microstructural Characterisation
2.3. Hardness, Fracture Toughness, and TRS
2.4. Wear Tests
2.5. High-Temperature Oxidation Tests
- Exposing the samples to a constant temperature for a fixed time. The samples were heated at three different temperatures (500 °C, 800 °C, and 1000 °C) during a fixed time of 120 h.
- Exposing the samples to a constant temperature for different periods. The samples were heated at 650 °C and 800 °C for up to 300 h. Measurements were taken at 5 h, 12 h, 24 h, 48 h, and 148 h. For this purpose, the samples were placed inside the furnace, heated until the test temperature was reached, maintained for the test period duration, cooled to room temperature within the furnace chamber, taken out for weight measurements (one specimen was kept out for FE-SEM characterisation), and then placed back inside the furnace and heated to the test temperature again.
3. Results and Discussion
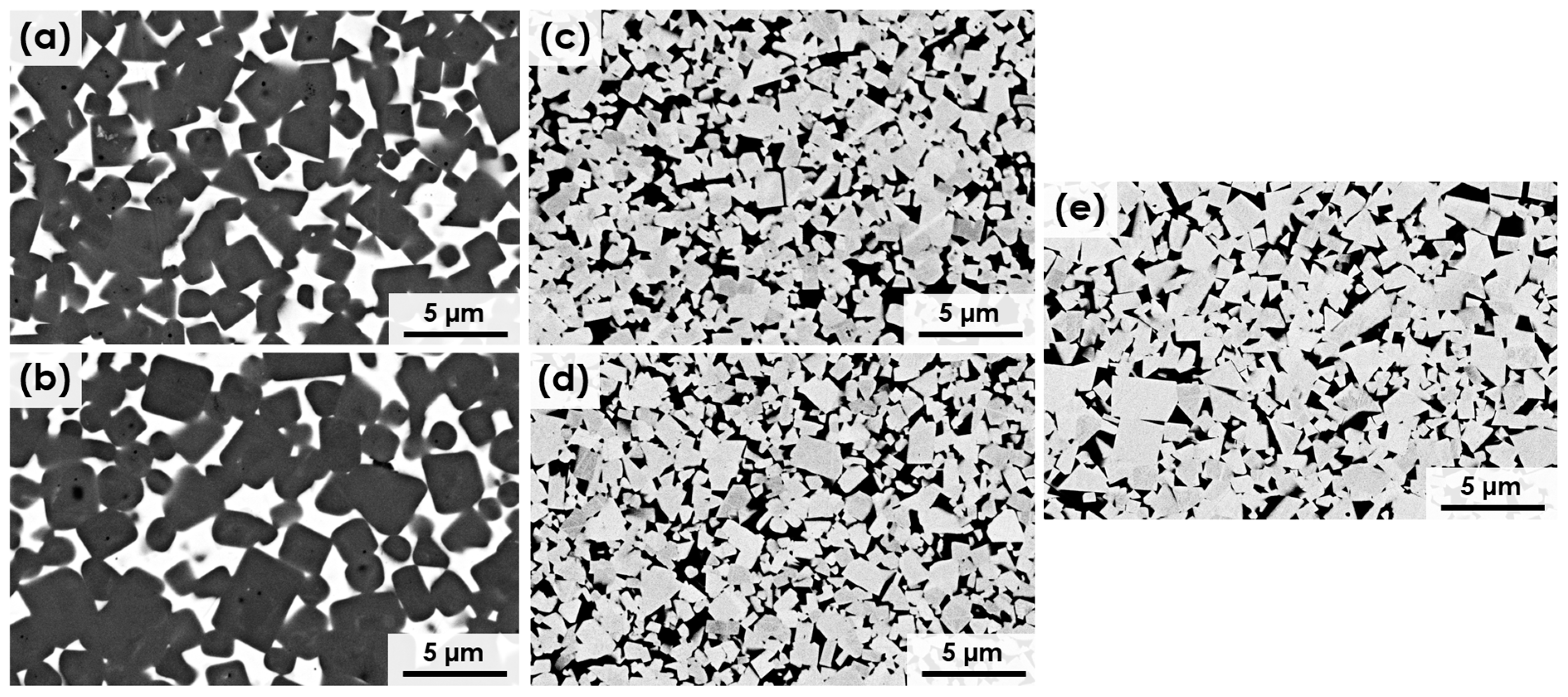
3.1. Microstructure of Bulk Materials
3.2. Mechanical Properties
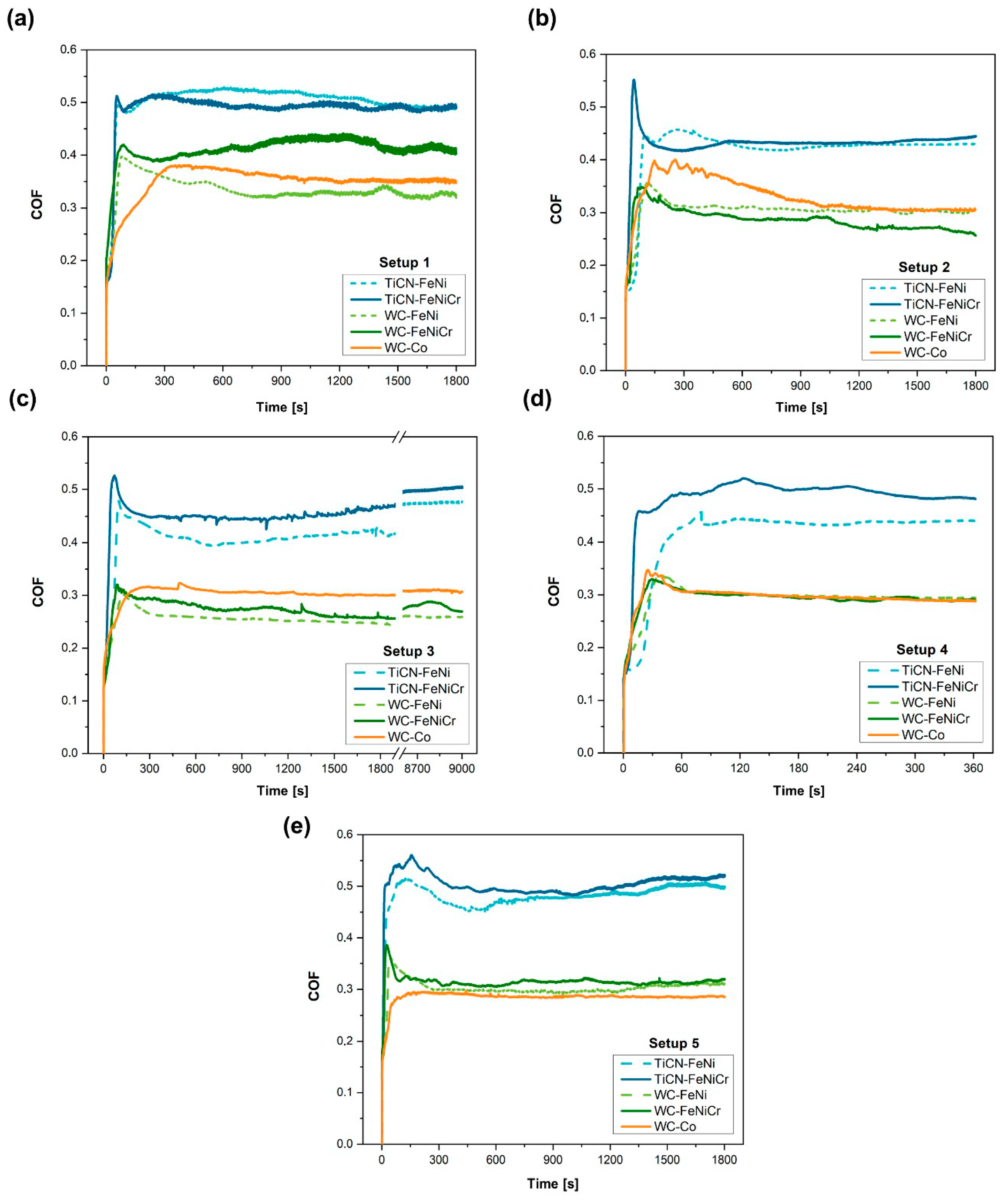
3.3. Wear Tests
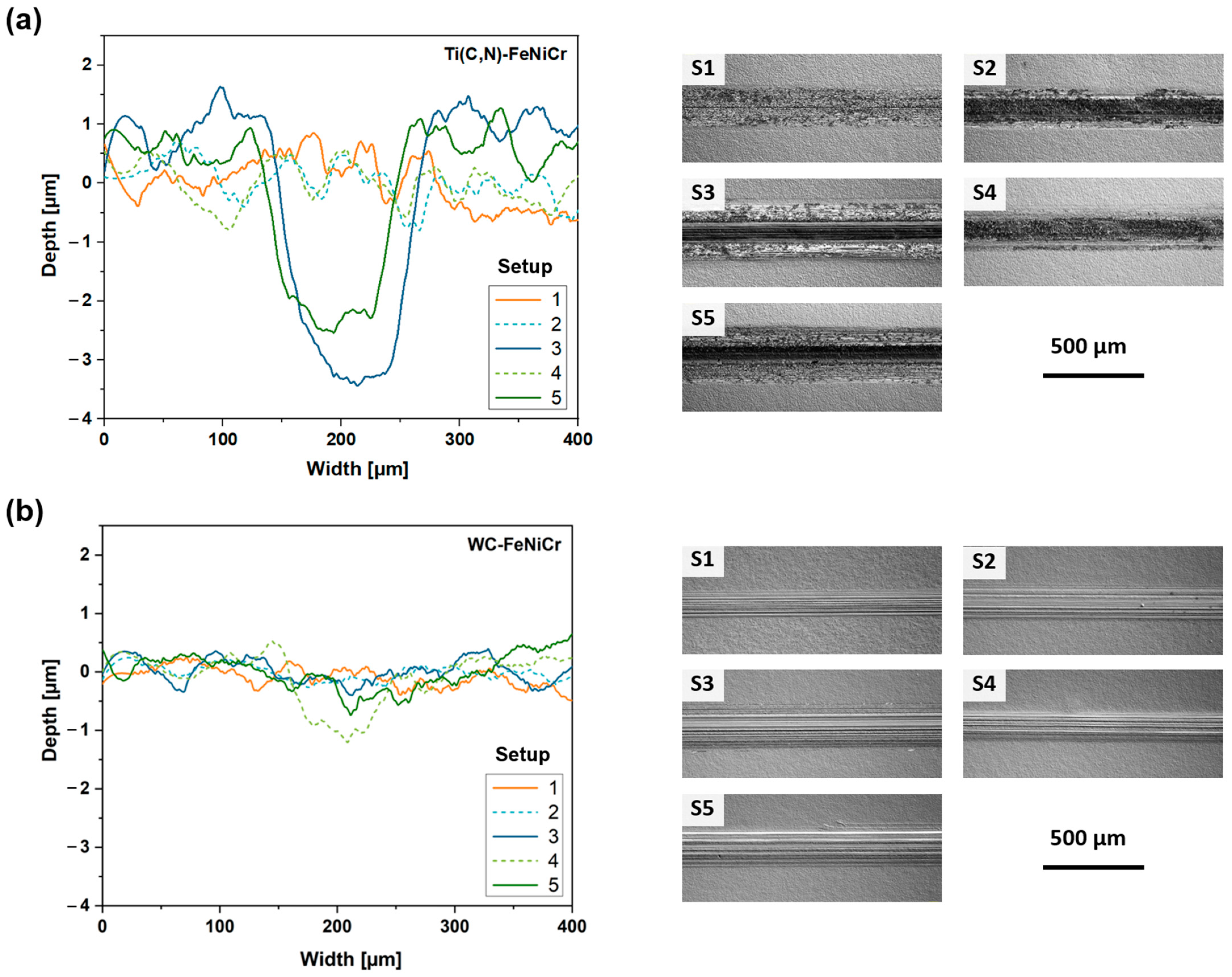
3.3.1. Wear Response
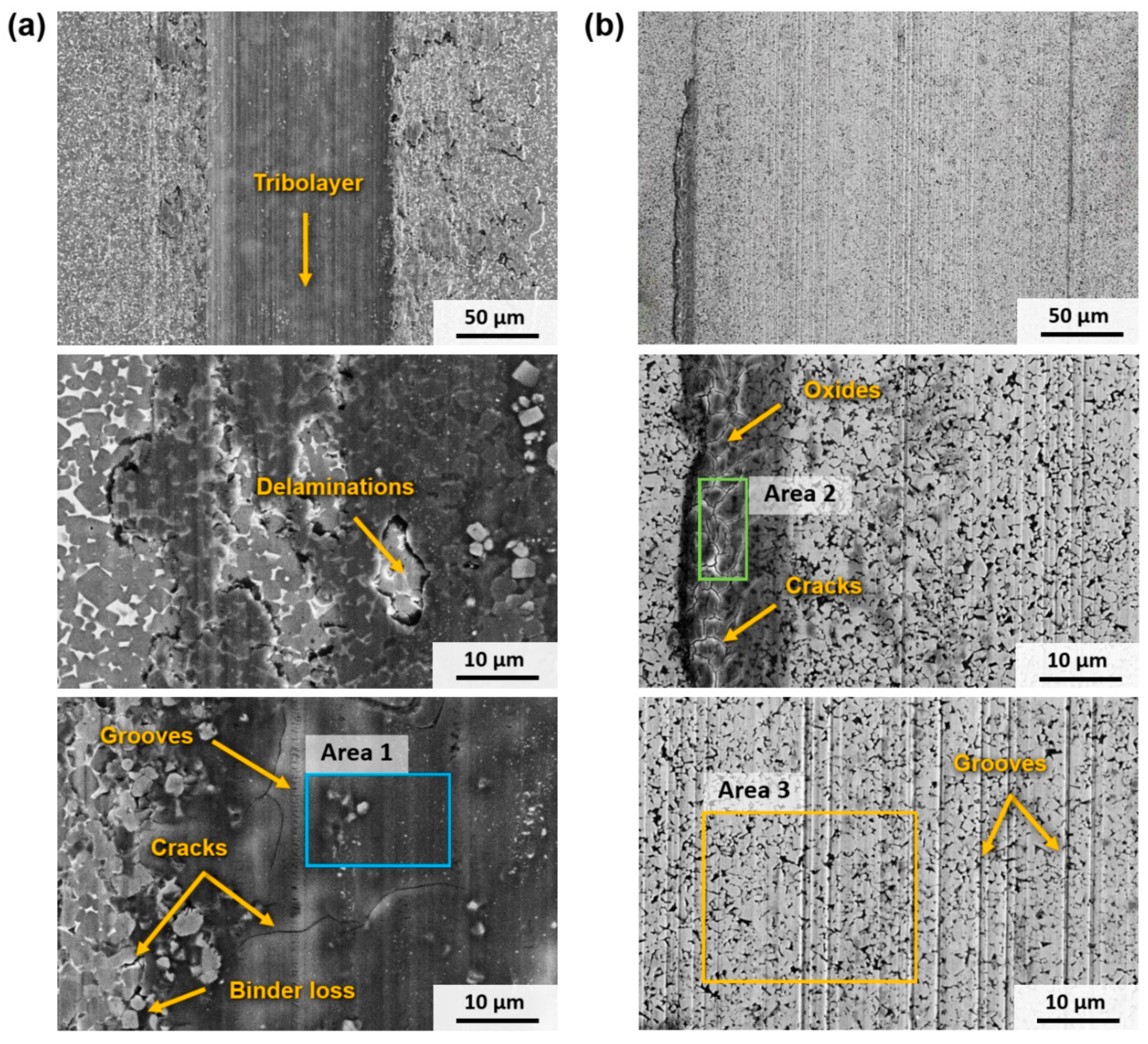
3.3.2. Wear Mechanisms
3.4. High-Temperature Oxidation
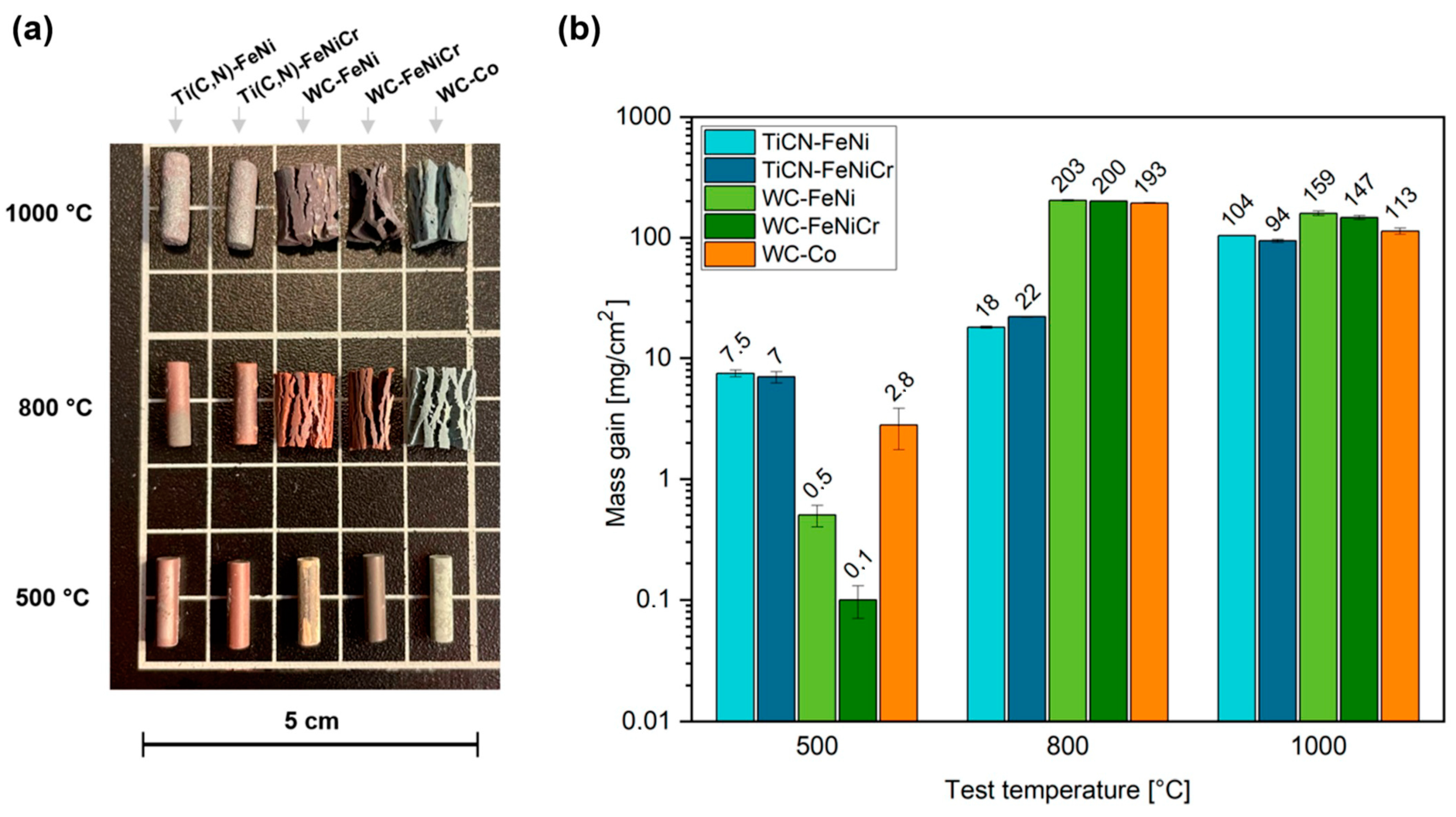
3.4.1. Oxidation Behaviour
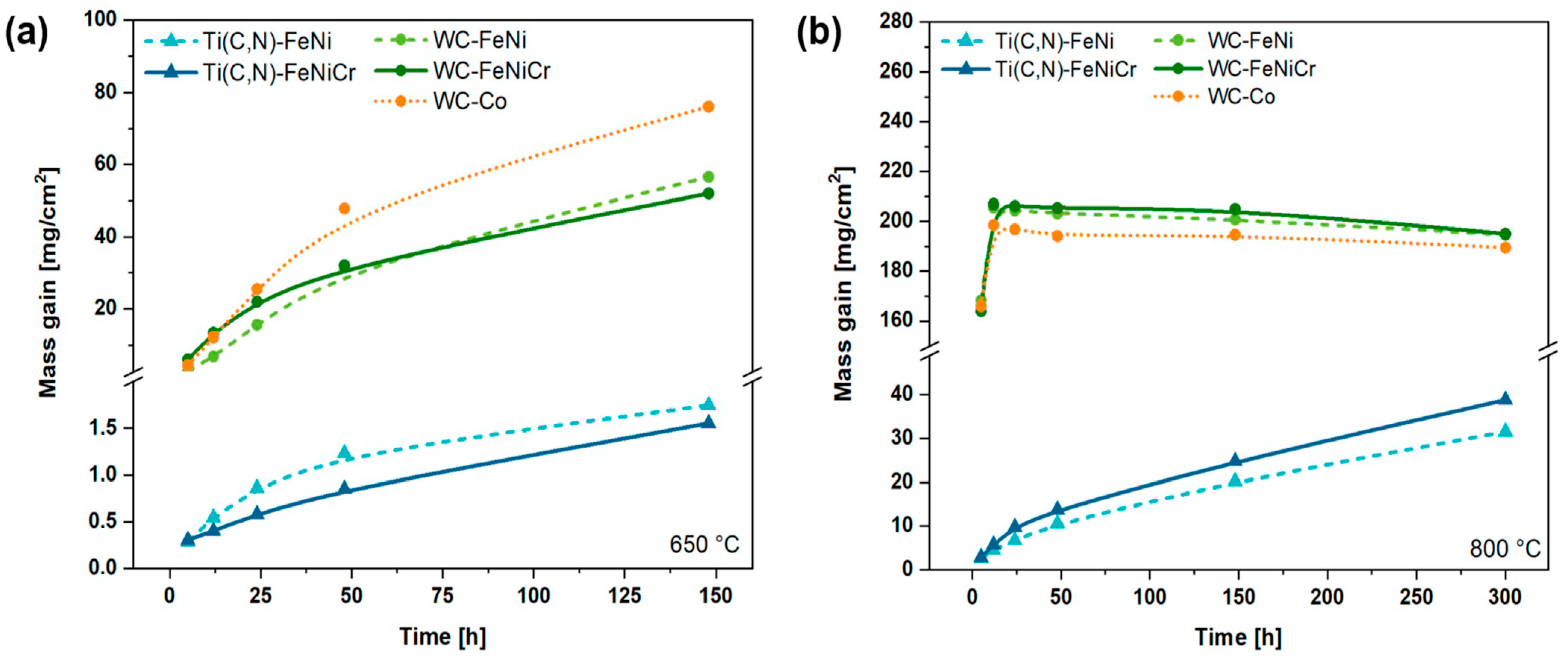
3.4.2. Oxidation Kinetics
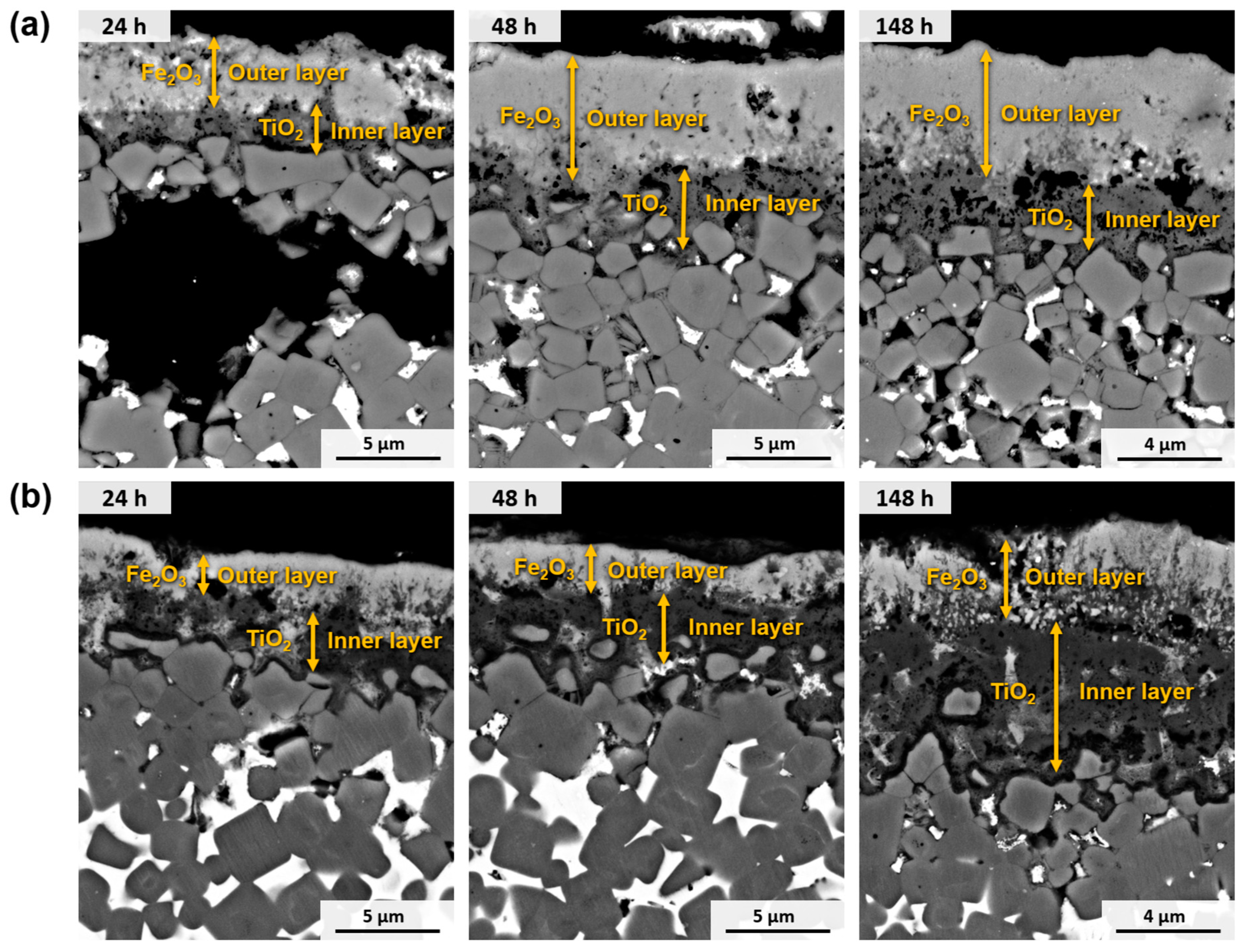
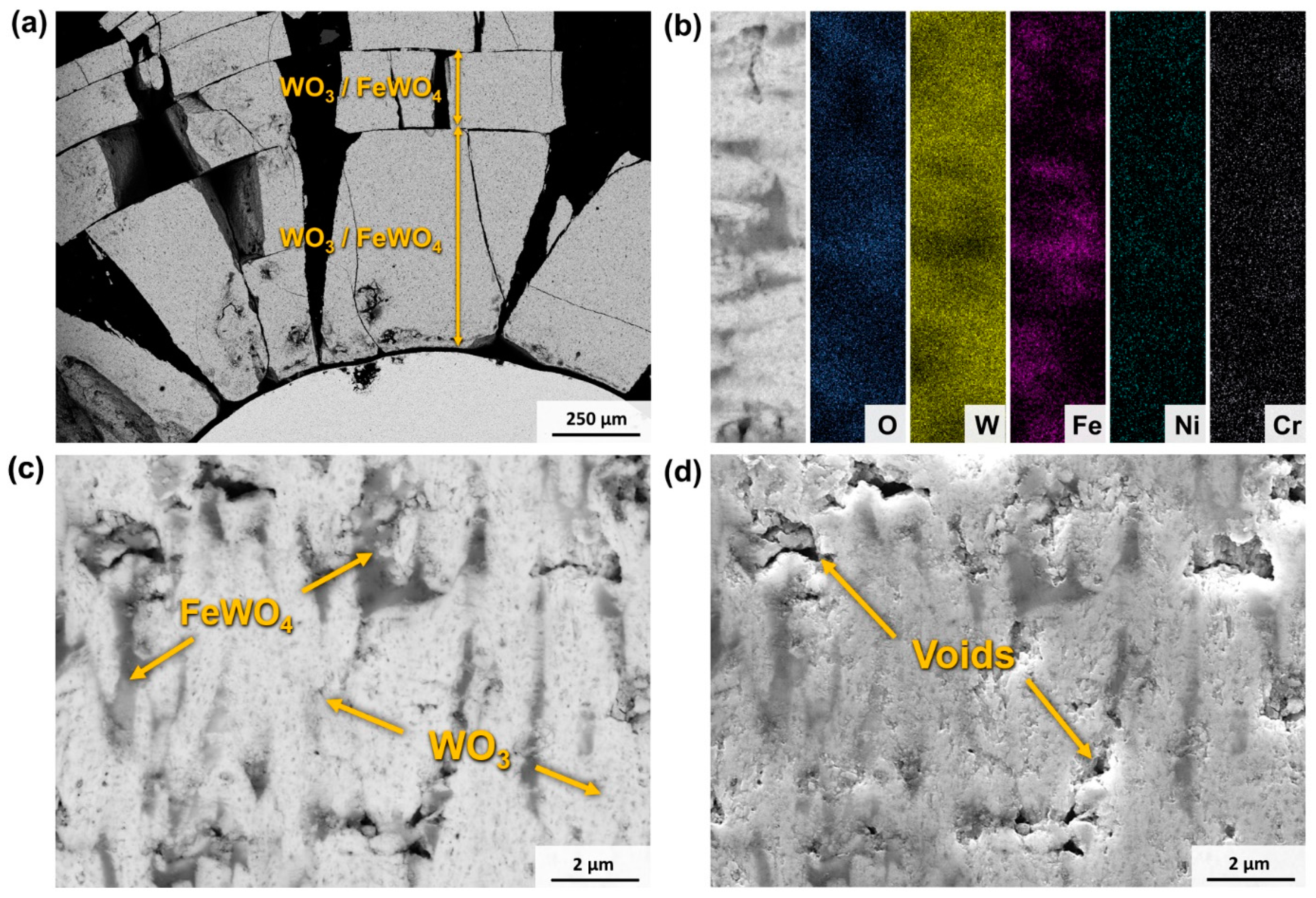
3.4.3. Scale and Transitions of Oxides: Oxidised Surface and Cross-Section Morphologies
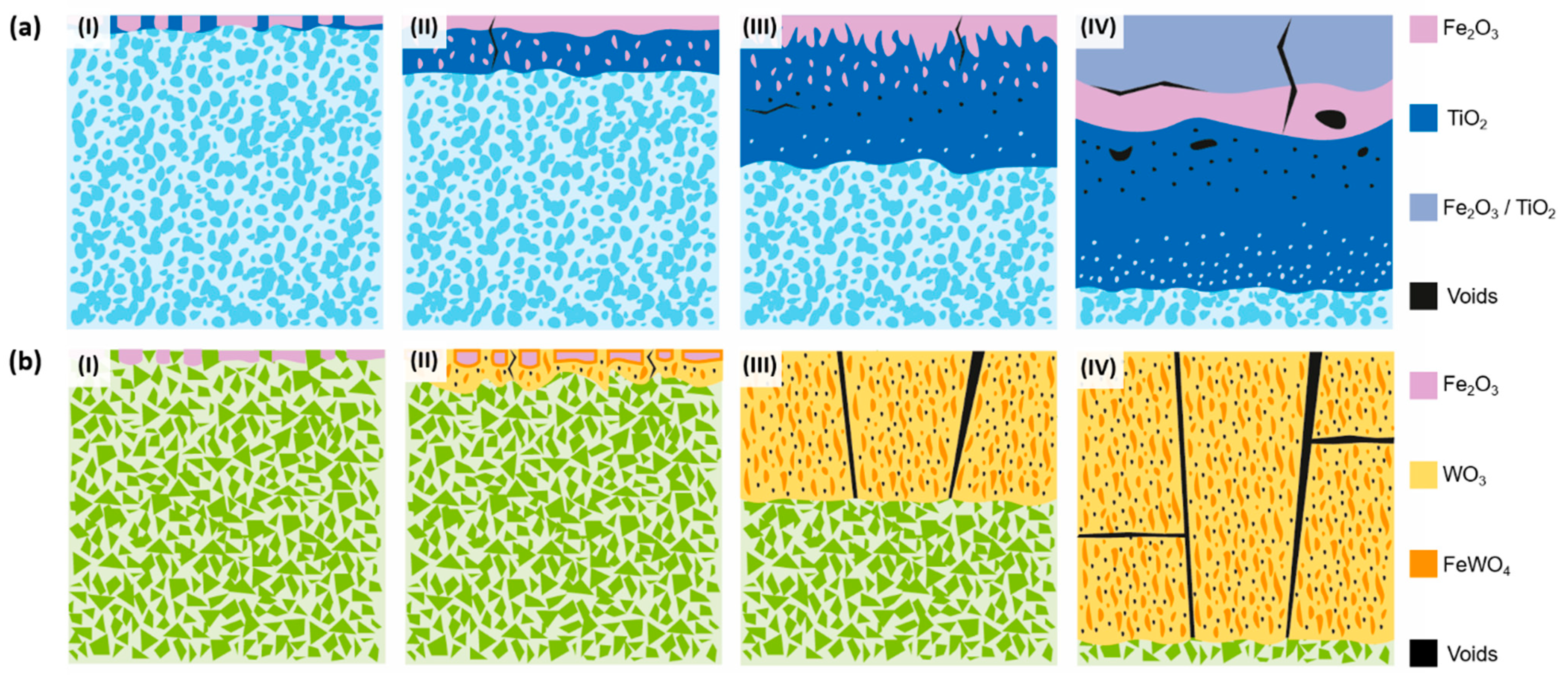
3.4.4. Oxidation Mechanisms
- The oxidation process of Ti(C,N)-based cermets starts with the simultaneous oxidation of both their metallic and ceramic phases through direct contact with atmospheric O2. Consequently, the metallic and ceramic phases undergo oxidation through the formation of Fe2O3 and TiO2, respectively.
- Resulting from the inward diffusion of Fe3+, a Fe2O3 layer is generated on the outer surface of the material. However, it remains permeable to O2−, allowing the oxidation of TiO2 to continue within the material.
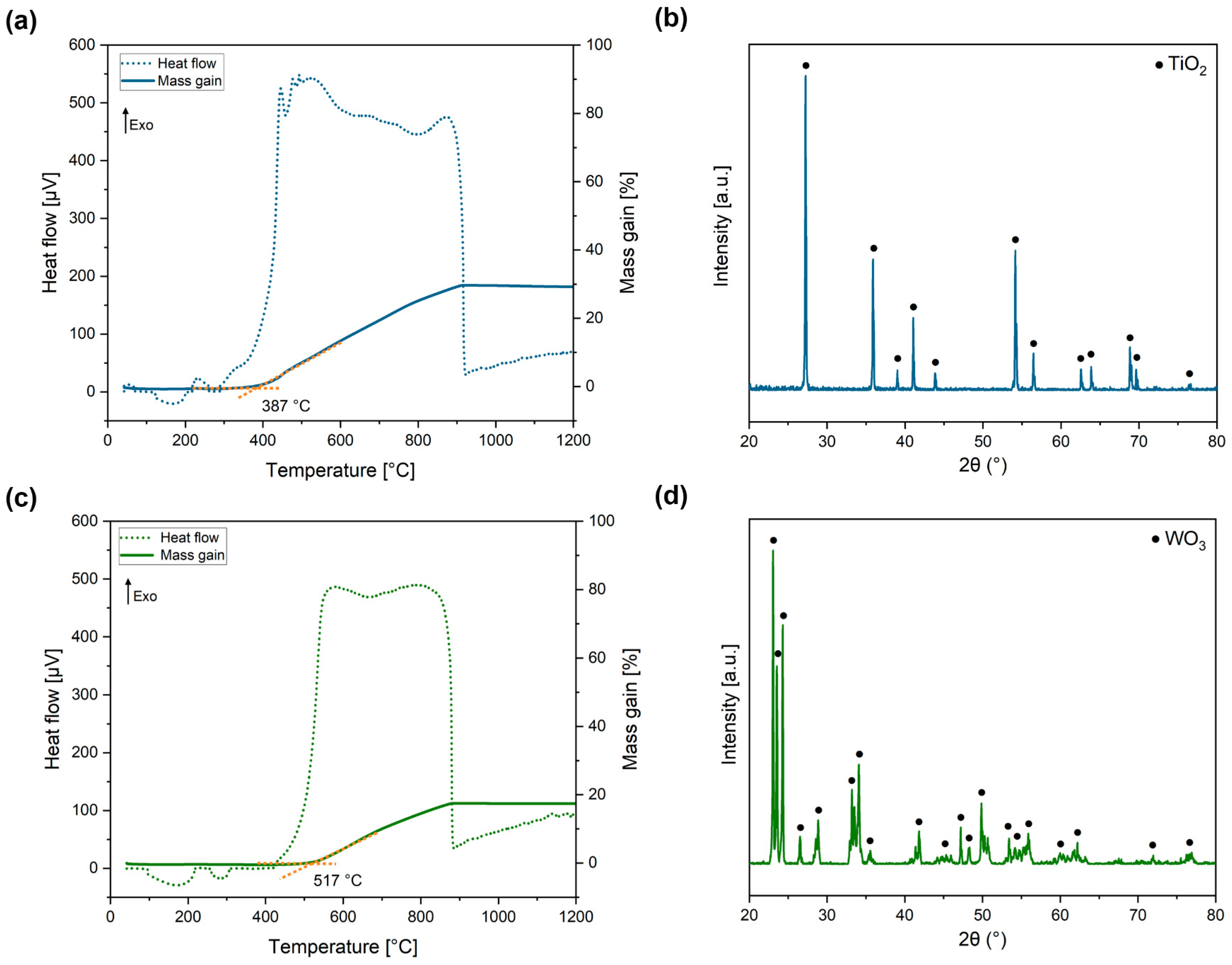
- As the internal TiO2 layer becomes denser, the inward diffusion of O2− is controlled, and the primary mode of oxidation shifts to the outward diffusion of the metal ions. This phenomenon aligns with Wagner’s theory, which suggests that the oxidation rate is governed by the diffusion of ions. This correlation is further supported by the observation that the oxidation kinetics conform to a parabolic function, as previously demonstrated [52]. This reaction, involving Ti(C,N), produces the oxidation product and generates CO, CO2, and N2, which contribute to the defects and voids formed in the oxide layer.
- Over time, the external layer displays a combination of Ti and Fe oxides. Within an intermediate layer, a Fe2O3 region emerges, indicating the diffusion limit of Ni2+ and Cr3+, which are dispersed in the innermost regions of the material, contributing to other Ti and Fe oxides. The innermost region, consisting primarily of TiO2, continues to expand with time. As we near the metallic substrate, Ni-rich metallic regions are present within this oxide layer, given its lower reactivity compared to Fe and Cr. Fe3+ diffuses more rapidly towards the surface, while Cr3+ integrates into adjacent oxides, potentially forming a stoichiometric composition of CrxFe2−xTiO5.
- Initially, there is a selective oxidation of the metallic phase, resulting in the formation of Fe2O3 on the surface.
- Subsequently, the WC component begins to oxidise, leading to void formation and the growth of WO3 in the radial direction. As a consequence, a reaction occurs between WO3 and the Fe2O3 formed during the oxidation of the metallic phase, resulting in the formation of FeWO4 domains within the WO3 oxide.
- During the oxidation of WC, the production of COx leads to a pressure difference within the oxide. This pressure difference induces the formation of cracks, which in turn promote the growth of the oxide. These cracks serve as channels through which O2 can directly react with the substrate material. Furthermore, the porous nature of the oxide facilitates the inward diffusion of O2−, thereby enhancing the overall oxidation rate.
- The oxide continues to grow, leading to catastrophic oxidation and the collapse of the material.
4. Conclusions
- Regarding the mechanical properties of the hard materials, WC-based materials exhibit superior hardness, toughness, and TRS compared to cermets. Specifically, WC-FeNi demonstrates higher levels of hardness and TRS when compared to the commercial-grade material (an increase of 7% and 9%, respectively). The addition of Cr results in a reduction in TRS (13% for the cermet and 17% for the hardmetal), likely attributed to the precipitation of M7C3-type carbides surrounding the Ti(C,N) and WC particles. Nevertheless, the TRS values of WC-FeNiCr are in the range of those found for WC-Co. The impact of Cr on hardness and fracture toughness is less clear.
- The results of the wear tests indicate that WC-based hardmetals exhibit a lower COF against a cemented carbide ball, as well as a lower wear rate under all test conditions. For instance, under the most aggressive conditions—setup 5—the cermets exhibit a COF of approximately 0.5, while hardmetals have a COF of about 0.3. Additionally, the wear rate of cermets is six times greater than that of hardmetals. Increasing the contact load resulted in a higher wear volume and a marginal reduction in the COF. The primary disparity in wear behaviour can be attributed to the distinct characteristics of the materials’ ceramic phase, as the metallic binder does not appear to have a significant impact on the wear process. In the case of Ti(C,N)-based cermets, the wear mechanism is attributed to the formation of a tribolayer consisting of Ti and Fe oxides, as well as wear debris. Conversely, the wear mechanism in WC-based hardmetals primarily involves abrasion through ploughing.
- Ti(C,N)-based materials exhibit favourable high-temperature oxidation behaviour by forming a protective layer composed of TiO2. This characteristic is due to the fact that the oxidation kinetics of cermets can be adjusted to an exponential law that closely resembles parabolic behaviour but is influenced by various factors. This behaviour provides a significant advantage over the volatile oxides of W. Thus, cermets present a much lower mass gain compared to WC-based hardmetals, which experience catastrophic oxidation at high temperatures (e.g., at 800 °C for 120 h, cermets experience a 10-times lower mass gain than their respective alternative hardmetals). In addition, the addition of 10 wt.% of Cr to the FeNi binder does not seem to be enough for the formation of a protective layer in any scenario. Nevertheless, it does influence the solubilities and distribution of oxides.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzo, A.; Goel, S.; Grilli, M.L.; Iglesias, R.; Jaworska, L.; Lapkovskis, V.; Novak, P.; Postolnyi, B.O.; Valerini, D. The Critical Raw Materials in Cutting Tools for Machining Applications: A Review. Materials 2020, 13, 1377. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Internal Market, Industry, Entrepreneurship and SME, M. Grohol, C.V. Study on the Critical Raw Materials for the EU 2023: Final Report. Publ. Off. Eur. Union 2023, 33–34. [CrossRef]
- Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Petranikova, M. Sustainability Evaluation of Essential Critical Raw Materials: Cobalt, Niobium, Tungsten and Rare Earth Elements. J. Phys. D Appl. Phys. 2018, 51, 203001. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt Toxicity in Humans—A Review of the Potential Sources and Systemic Health Effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Lison, D.; Lauwerys, R. The Interaction of Cobalt Metal with Different Carbides and Other Mineral Particles on Mouse Peritoneal Macrophages. Toxicol. In Vitr. 1995, 9, 341–347. [Google Scholar] [CrossRef]
- Lengauer, W.; Scagnetto, F. Ti(C,N)-Based Cermets: Critical Review of Achievements and Recent Developments. Solid State Phenom. 2018, 274, 53–100. [Google Scholar] [CrossRef]
- de Nicolás, M.; Besharatloo, H.; Alvaredo, P.; Roa, J.J.; Llanes, L.; Gordo, E. Design of Alternative Binders for Hard Materials. Int. J. Refract. Met. Hard Mater. 2020, 87, 105089. [Google Scholar] [CrossRef]
- Aramian, A.; Sadeghian, Z.; Narimani, M.; Razavi, N.; Berto, F. A Review on the Microstructure and Properties of TiC and Ti(C,N) Based Cermets. Int. J. Refract. Met. Hard Mater. 2023, 115, 106320. [Google Scholar] [CrossRef]
- Clark, E.B.; Roebuck, B. Extending the Application Areas for Titanium Carbonitride Cermets. Int. J. Refract. Met. Hard Mater. 1992, 11, 23–33. [Google Scholar] [CrossRef]
- Ortner, H.M.; Ettmayer, P.; Kolaska, H. The History of the Technological Progress of Hardmetals. Int. J. Refract. Met. Hard Mater. 2014, 44, 148–159. [Google Scholar] [CrossRef]
- Kübarsepp, J.; Klaasen, H.; Pirso, J. Behaviour of TiC-Base Cermets in Different Wear Conditions. Wear 2001, 249, 229–234. [Google Scholar] [CrossRef]
- Pirso, J.; Viljus, M.; Letunovitš, S. Friction and Dry Sliding Wear Behaviour of Cermets. Wear 2006, 260, 815–824. [Google Scholar] [CrossRef]
- Kübarsepp, J.; Juhani, K.; Tarraste, M. Abrasion and Erosion Resistance of Cermets: A Review. Materials 2022, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Klaasen, H.; Kübarsepp, J. Abrasive Wear Performance of Carbide Composites. Wear 2006, 261, 520–526. [Google Scholar] [CrossRef]
- Pereira, P.; Vilhena, L.M.; Sacramento, J.; Senos, A.M.R.; Malheiros, L.F.; Ramalho, A. Tribological Behaviour of Different Formulations of WC Composites. Wear 2022, 506–507, 204415. [Google Scholar] [CrossRef]
- Manoj Kumar, B.V.; Basu, B.; Kalin, M.; Vizintin, J. Load-Dependent Transition in Sliding Wear Properties of TiCN-WC-Ni Cermets. J. Am. Ceram. Soc. 2007, 90, 1534–1540. [Google Scholar] [CrossRef]
- Manoj Kumar, B.V.; Basu, B.; Vizintin, J.; Kalin, M. Tribochemistry in Sliding Wear of TiCN-Ni-Based Cermets. J. Mater. Res. 2008, 23, 1214–1227. [Google Scholar] [CrossRef]
- Onuoha, C.C.; Jin, C.; Farhat, Z.N.; Kipouros, G.J.; Plucknett, K.P. The Effects of TiC Grain Size and Steel Binder Content on the Reciprocating Wear Behaviour of TiC-316L Stainless Steel Cermets. Wear 2016, 350–351, 116–129. [Google Scholar] [CrossRef]
- Boukantar, A.R.; Djerdjare, B.; Guiberteau, F.; Ortiz, A.L. Spark Plasma Sinterability and Dry Sliding-Wear Resistance of WC Densified with Co, Co + Ni, and Co + Ni + Cr. Int. J. Refract. Met. Hard Mater. 2020, 92, 105280. [Google Scholar] [CrossRef]
- Bonny, K.; de Patrick, B.; Vleugels, J.; Huang, S.; Lauwers, B. Tribological Characteristics of WC-Ni and WC-CO Cemented Carbide in Dry Reciprocating Sliding Contact. Tribol. Trans. 2009, 52, 481–491. [Google Scholar] [CrossRef]
- Gómez, B.; Jiménez-Suarez, A.; Gordo, E. Oxidation and Tribological Behaviour of an Fe-Based MMC Reinforced with TiCN Particles. Int. J. Refract. Met. Hard Mater. 2009, 27, 360–366. [Google Scholar] [CrossRef]
- Alvaredo, P.; Abajo, C.; Tsipas, S.A.; Gordo, E. Influence of Heat Treatment on the High Temperature Oxidation Mechanisms of an Fe-TiCN Cermet. J. Alloys Compd. 2014, 591, 72–79. [Google Scholar] [CrossRef]
- Chicardi, E.; Córdoba, J.M.; Gotor, F.J. Kinetics of High-Temperature Oxidation of (Ti,Ta)(C,N)-Based Cermets. Corros. Sci. 2016, 102, 168–177. [Google Scholar] [CrossRef]
- He, L.; Liu, Q.; Gao, Y.; Li, Y.; Liu, Z.; Zhai, W.; Zhou, C.; Yuan, W.; Chen, W.; Yan, W. Abnormal Oxidation Behavior of Fe in Ti(C,N)-304ss Cermet during Early Oxidation Stage. J. Alloys Compd. 2019, 790, 20–26. [Google Scholar] [CrossRef]
- Yang, Q.; Xiong, W.; Li, S.; Dai, H.; Li, J. Characterization of Oxide Scales to Evaluate High Temperature Oxidation Behavior of Ti(C,N)-Based Cermets in Static Air. J. Alloys Compd. 2010, 506, 461–467. [Google Scholar] [CrossRef]
- Basu, S.N.; Sarin, V.K. Oxidation Behavior of WC-Co. Mater. Sci. Eng. A 1996, 209, 206–212. [Google Scholar] [CrossRef]
- del Campo, L.; Pérez-Sáez, R.B.; González-Fernández, L.; Tello, M.J. Kinetics Inversion in Isothermal Oxidation of Uncoated WC-Based Carbides between 450 and 800 °C. Corros. Sci. 2009, 51, 707–712. [Google Scholar] [CrossRef]
- Karimi, H.; Hadi, M.; Ebrahimzadeh, I.; Farhang, M.R.; Sadeghi, M. High-Temperature Oxidation Behaviour of WC-FeAl Composite Fabricated by Spark Plasma Sintering. Ceram. Int. 2018, 44, 17147–17153. [Google Scholar] [CrossRef]
- Long, J.; Yang, J.; Xu, T.; Zeng, R.; Zhang, R.; Cao, Y.; Yuan, M.; Tan, L.; Cui, Y.; Wei, X. Influences of Cr Contents on Oxidation Behavior of WC-Co-Ni-Cr Cemented Carbides at 900 °C. Int. J. Refract. Met. Hard Mater. 2024, 118, 106466. [Google Scholar] [CrossRef]
- de Nicolás, M.; Besharatloo, H.; Pereira, L.; Müller-Grunz, A.; Bertalan, C.; Useldinger, R.; Llanes, L.; Gordo, E. Ti(C,N)-Fe15Ni10Cr Cermets as Alternative Hard Materials: Influence of the Processing Route and Composition on Their Microstructure and Properties. Ceram. Int. 2021, 47, 23318–23331. [Google Scholar] [CrossRef]
- de Nicolás-Morillas, M.; Besharatloo, H.; Cabezas, L.; de la Mata, M.; Sales, D.L.; Pereira, L.; Müller-Grunz, A.; Bertalan, C.; Useldinger, R.; Llanes, L.; et al. Processing of WC with Fe-Based Alternative Binders: Adjustment of C Content and Effect of Cr Addition. Int. J. Refract. Met. Hard Mater. 2023, 118, 106444. [Google Scholar] [CrossRef]
- de Nicolás-Morillas, M.; Llanes, L.; Gordo, E. High-Temperature Wettability in Hard Materials: Comparison of Systems with Different Binder/Carbide Phases and Evaluation of C Addition. Int. J. Refract. Met. Hard Mater. 2023, 111, 106081. [Google Scholar] [CrossRef]
- Bozzini, B.; Gianoncelli, A.; Kourousias, G.; Boniardi, M.; Casaroli, A.; Dal Zilio, S.; Hussain, R.; Abyaneh, M.K.; Kiskinova, M.; Mele, C.; et al. The Role of Chromium in the Corrosion Performance of Cobalt- and Cobalt-Nickel Based Hardmetal Binders: A Study Centred on X-Ray Absorption Microspectroscopy. Int. J. Refract. Met. Hard Mater. 2020, 92, 105320. [Google Scholar] [CrossRef]
- ISO 3327:2009; Hardmetals—Determination of Transverse Rupture Strength. ISO: Geneva, Switzerland, 2009.
- Roebuck, B.; Bennett, E.G. Phase Size Distribution in WC/Co Hardmetal. Metallography 1986, 19, 27–47. [Google Scholar] [CrossRef]
- Shetty, D.K.; Wright, I.G.; Mincer, P.N.; Clauer, A.H. Indentation Fracture of WC-Co Cermets. J. Mater. Sci. 1985, 20, 1873–1882. [Google Scholar] [CrossRef]
- ASTM G133-05; Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear. ASTM: West Conshohocken, PA, USA, 2016.
- Doni, Z.; Alves, A.C.; Toptan, F.; Gomes, J.R.; Ramalho, A.; Buciumeanu, M.; Palaghian, L.; Silva, F.S. Dry Sliding and Tribocorrosion Behaviour of Hot Pressed CoCrMo Biomedical Alloy as Compared with the Cast CoCrMo and Ti6Al4V Alloys. Mater. Des. 2013, 52, 47–57. [Google Scholar] [CrossRef]
- Roulon, Z.; Missiaen, J.M.; Lay, S. Carbide Grain Growth in Cemented Carbides Sintered with Alternative Binders. Int. J. Refract. Met. Hard Mater. 2020, 86, 105088. [Google Scholar] [CrossRef]
- Genga, R.M.; Rokebrand, P.; Cornish, L.A.; Nelwalani, N.; Brandt, G.; Kelling, N.; Woydt, M.; van Vuuren, A.J.; Polese, C. High-Temperature Sliding Wear, Elastic Modulus and Transverse Rupture Strength of Ni Bonded NbC and WC Cermets. Int. J. Refract. Met. Hard Mater. 2020, 87, 105143. [Google Scholar] [CrossRef]
- Fang, Z.Z. Correlation of Transverse Rupture Strength of WC–Co with Hardness. Int. J. Refract. Met. Hard Mater. 2005, 23, 119–127. [Google Scholar] [CrossRef]
- Kolnes, M.; Mere, A.; Kübarsepp, J.; Viljus, M.; Maaten, B.; Tarraste, M. Microstructure Evolution of TiC Cermets with Ferritic AISI 430L Steel Binder. Powder Metall. 2018, 61, 197–209. [Google Scholar] [CrossRef]
- Lin, T.; Guo, Y.; Wang, Z.; Shao, H.; Lu, H.; Li, F.; He, X. Effects of Chromium and Carbon Content on Microstructure and Properties of TiC-Steel Composites. Int. J. Refract. Met. Hard Mater. 2018, 72, 228–235. [Google Scholar] [CrossRef]
- Gao, Y.; Luo, B.H.; He, K.J.; Zhang, W.W.; Bai, Z.H. Effect of Fe/Ni Ratio on the Microstructure and Properties of WC-Fe-Ni-Co Cemented Carbides. Ceram. Int. 2018, 44, 2030–2041. [Google Scholar] [CrossRef]
- Chand Yadav, P.; Kumar Sharma, S.; Venkata Manoj Kumar, B.; Kang, S. Effect of Linear Velocity on Sliding Wear Behavior of TiCN Based Cermets. Mater. Today Proc. 2018, 5, 17342–17349. [Google Scholar] [CrossRef]
- Pereira, P.; Vilhena, L.M.; Sacramento, J.; Senos, A.M.R.; Malheiros, L.F.; Ramalho, A. Abrasive Wear Resistance of WC-Based Composites, Produced with Co or Ni-Rich Binders. Wear 2021, 482–483, 203924. [Google Scholar] [CrossRef]
- Larsen-Basse, J. Binder Extrusion in Sliding Wear of WC-Co Alloys. Wear 1985, 105, 247–256. [Google Scholar] [CrossRef]
- Kurlov, A.S.; Gusev, A.I. Oxidation of Tungsten Carbide Powders in Air. RMHM 2013, 41, 300–307. [Google Scholar] [CrossRef]
- He, L.; Gao, Y.; Li, Y.; Liu, Z.; Yuan, W.; Chen, W.; Zhao, S.; Liu, H.; Yan, W. Effect of TiB2/WC Addition on the Oxidation Behavior of Ti(C,N)-304ss Cermets during the Early Oxidation Stage. Corros. Sci. 2019, 159, 108118. [Google Scholar] [CrossRef]
- Lassner, E.; Schubert, W.D. Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; ISBN 0306450534. [Google Scholar]
- Chen, L.; Yi, D.; Wang, B.; Liu, H.; Wu, C. Mechanism of the Early Stages of Oxidation of WC-Co Cemented Carbides. Corros. Sci. 2016, 103, 75–87. [Google Scholar] [CrossRef]
- Gesmundo, F.; Viani, F. Application of Wagner’s Theory to the Parabolic Growth of Oxides Containing Different Kinds of Defects: II. Doped Oxides. J. Electrochem. Soc. 1981, 128, 470–479. [Google Scholar] [CrossRef]








| Raw Powders | Provider | Characteristics |
|---|---|---|
| Ti(C0.5,N0.5) | Treibacher Industrie AG (Althofen, Austria) | FSSS = 1.66 μm |
| WC | – | – |
| Fe | – | FSSS = 2.32 μm |
| Ni | Vale International SA (Saint-Prex, Switzerland) | FSSS = 2.25 μm |
| Cr | SkySpring Nanomaterials (Houston, TX, USA) | D50 = 5 μm |
| C | Thermax®, Cancarb (Medicine Hat, AB, Canada) | FSSS = 0.40 μm |
| Material | Ceramic Phase 80 vol.% | Metallic Phase 20 vol.% | ||
|---|---|---|---|---|
| Type | [wt.%] | Type | [wt.%] | |
| Ti(C,N)-FeNi | Ti(C,N) | 71.77 | Fe15Ni | 28.23 |
| Ti(C,N)-FeNiCr | Ti(C,N) | 71.97 | Fe15Ni10Cr | 28.03 |
| WC-FeNi | WC | 88.62 | Fe15Ni | 11.38 |
| WC-FeNiCr | WC | 88.71 | Fe15Ni10Cr | 11.29 |
| WC-Co | WC | 87.54 | Co | 12.46 |
| Setup | Counter Material | Load [N] | Frequency [Hz] | Time [s] | Stroke Length [mm] | Sliding Distance [m] |
|---|---|---|---|---|---|---|
| 1 | WC-Co 6 mm ⌀ Ball | 10 | 1 | 1800 | 5 | 18 |
| 2 | 30 | 1 | 1800 | 5 | 18 | |
| 3 | 30 | 1 | 9000 | 5 | 90 | |
| 4 | 30 | 5 | 360 | 5 | 18 | |
| 5 | 30 | 5 | 1800 | 5 | 90 |
| Material | Ceramic Diameter [µm] | Metallic Phase [%] | Free Mean Path [µm] | Contiguity |
|---|---|---|---|---|
| Ti(C,N)-FeNi | 1.60 ± 0.66 | 20.5 ± 0.3 | 0.82 ± 0.13 | 0.38 |
| Ti(C,N)-FeNiCr | 1.92 ± 0.79 | 20.4 ± 0.6 | 0.92 ± 0.26 | 0.39 |
| WC-FeNi | 0.94 ± 0.45 | 20.2 ± 0.5 | 0.39 ± 0.05 | 0.42 |
| WC-FeNiCr | 0.87 ± 0.44 | 19.7 ± 0.4 | 0.36 ± 0.01 | 0.42 |
| WC-Co | 1.00 ± 0.50 | 19.8 ± 0.2 | 0.41 ± 0.05 | 0.42 |
| Material | HV30 [kg/mm2] | KIC [MPa·m1/2] | TRS [N/mm2] |
|---|---|---|---|
| Ti(C,N)-FeNi | 1275 ± 36 | 12.2 ± 1.5 | 2210 ± 132 |
| Ti(C,N)-FeNiCr | 1246 ± 56 | 11.4 ± 1.5 | 1915 ± 47 |
| WC-FeNi | 1400 ± 22 | 13.5 ± 0.2 | 3533 ± 101 |
| WC-FeNiCr | 1458 ± 25 | 13.7 ± 0.5 | 2924 ± 145 |
| WC-Co | 1298 ± 14 | 15.3 ± 0.7 | 3198 ± 122 |
| Material | Increase in Roughness, ΔRa [%] | |||||
|---|---|---|---|---|---|---|
| Initial Ra [µm] | Setup 1 | Setup 2 | Setup 3 | Setup 4 | Setup 5 | |
| Ti(C,N)-FeNi | 0.50 ± 0.06 | 128 | 136 | 170 | 144 | 174 |
| Ti(C,N)-FeNiCr | 0.49 ± 0.03 | 143 | 145 | 127 | 167 | 124 |
| WC-FeNi | 0.35 ± 0.04 | 106 | 114 | 134 | 134 | 137 |
| WC-FeNiCr | 0.36 ± 0.03 | 125 | 139 | 150 | 139 | 144 |
| WC-Co | 0.33 ± 0.04 | 121 | 142 | 151 | 145 | 158 |
| Material | COF | ||||
|---|---|---|---|---|---|
| Setup 1 | Setup 2 | Setup 3 | Setup 4 | Setup 5 | |
| Ti(C,N)-FeNi | 0.50 | 0.42 | 0.44 | 0.41 | 0.48 |
| Ti(C,N)-FeNiCr | 0.49 | 0.43 | 0.48 | 0.48 | 0.50 |
| WC-FeNi | 0.33 | 0.30 | 0.25 | 0.29 | 0.31 |
| WC-FeNiCr | 0.41 | 0.29 | 0.26 | 0.29 | 0.32 |
| WC-Co | 0.35 | 0.33 | 0.30 | 0.30 | 0.28 |
| Material | Width of Wear Track [µm] | ||||
|---|---|---|---|---|---|
| Setup 1 | Setup 2 | Setup 3 | Setup 4 | Setup 5 | |
| Ti(C,N)-FeNi | 206 ± 2 | 214 ± 2 | 348 ± 7 | 243 ± 25 | 323 ± 20 |
| Ti(C,N)-FeNiCr | 197 ± 8 | 206 ± 9 | 344 ± 26 | 231 ± 17 | 309 ± 14 |
| WC-FeNi | 132 ± 6 | 182 ± 5 | 227 ± 9 | 214 ± 24 | 271 ± 35 |
| WC-FeNiCr | 144 ± 14 | 195 ± 9 | 239 ± 5 | 193 ± 6 | 249 ± 3 |
| WC-Co | 99 ± 5 | 192 ± 26 | 225 ± 26 | 200 ± 23 | 223 ± 19 |
| Material | Wear Rate [10−8 mm3/mm] | ||||
|---|---|---|---|---|---|
| Setup 1 | Setup 2 | Setup 3 | Setup 4 | Setup 5 | |
| Ti(C,N)-FeNi | 2.10 ± 0.29 | 6.42 ± 1.15 | 3.79 ± 0.18 | 6.73 ± 2.58 | 3.20 ± 0.84 |
| Ti(C,N)-FeNiCr | 1.98 ± 0.09 | 3.48 ± 0.23 | 3.45 ± 0.90 | 3.05 ± 0.26 | 2.96 ± 0.63 |
| WC-FeNi | 1.19 ± 0.04 | 1.85 ± 0.25 | 0.56 ± 0.03 | 2.38 ± 0.08 | 0.56 ± 0.03 |
| WC-FeNiCr | 1.34 ± 0.07 | 1.83 ± 0.05 | 0.62 ± 0.05 | 2.24 ± 0.39 | 0.50 ± 0.01 |
| WC-Co | 1.74 ± 0.09 | 2.32 ± 0.09 | 0.50 ± 0.15 | 2.70 ± 0.05 | 0.49 ± 0.01 |
| Area | Element [wt.%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ti | W | C | N | Fe | Ni | Cr | O | Co | |
| 1 | 37.4 | 2.4 | 5.6 | 3.7 | 9.8 | 2.3 | 1.3 | 37.5 | - |
| 2 | - | 67.4 | 2.0 | - | 7.7 | 2.2 | 1.6 | 17.4 | 1.7 |
| 3 | - | 77.4 | 9.2 | - | 9.2 | 1.9 | 1.5 | 0.1 | 0.7 |
| Material | Exponential Coefficient, n (R2) | Oxidation Rate Constant, kn [mgn/cm2n·s] | ||
|---|---|---|---|---|
| 650 °C | 800 °C | 650 °C | 800 °C | |
| Ti(C,N)-FeNi | 0.4327 (0.9964) | 0.6013 (0.9998) | 0.1444 | 1.9114 |
| Ti(C,N)-FeNiCr | 0.6111 (0.9992) | 0.5530 (0.9977) | 0.1279 | 2.3308 |
| WC-FeNi | 0.3463 (0.9915) | - | 5.6042 | - |
| WC-FeNiCr | 0.2782 (0.9995) | - | 4.5694 | - |
| WC-Co | 0.2976 (0.9886) | - | 7.3862 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biedma, Á.; Sánchez, G.; de Nicolás, M.; Bertalan, C.; Useldinger, R.; Llanes, L.; Gordo, E. A Comparative Study on the Wear Performance and High-Temperature Oxidation of Co-Free Cermets and Hardmetals. Materials 2024, 17, 3615. https://doi.org/10.3390/ma17143615
Biedma Á, Sánchez G, de Nicolás M, Bertalan C, Useldinger R, Llanes L, Gordo E. A Comparative Study on the Wear Performance and High-Temperature Oxidation of Co-Free Cermets and Hardmetals. Materials. 2024; 17(14):3615. https://doi.org/10.3390/ma17143615
Chicago/Turabian StyleBiedma, Ángel, Gabriel Sánchez, María de Nicolás, Claudio Bertalan, Ralph Useldinger, Luis Llanes, and Elena Gordo. 2024. "A Comparative Study on the Wear Performance and High-Temperature Oxidation of Co-Free Cermets and Hardmetals" Materials 17, no. 14: 3615. https://doi.org/10.3390/ma17143615








