Microstructural Evolution of Quaternary AlCoCrNi High-Entropy Alloys during Heat Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
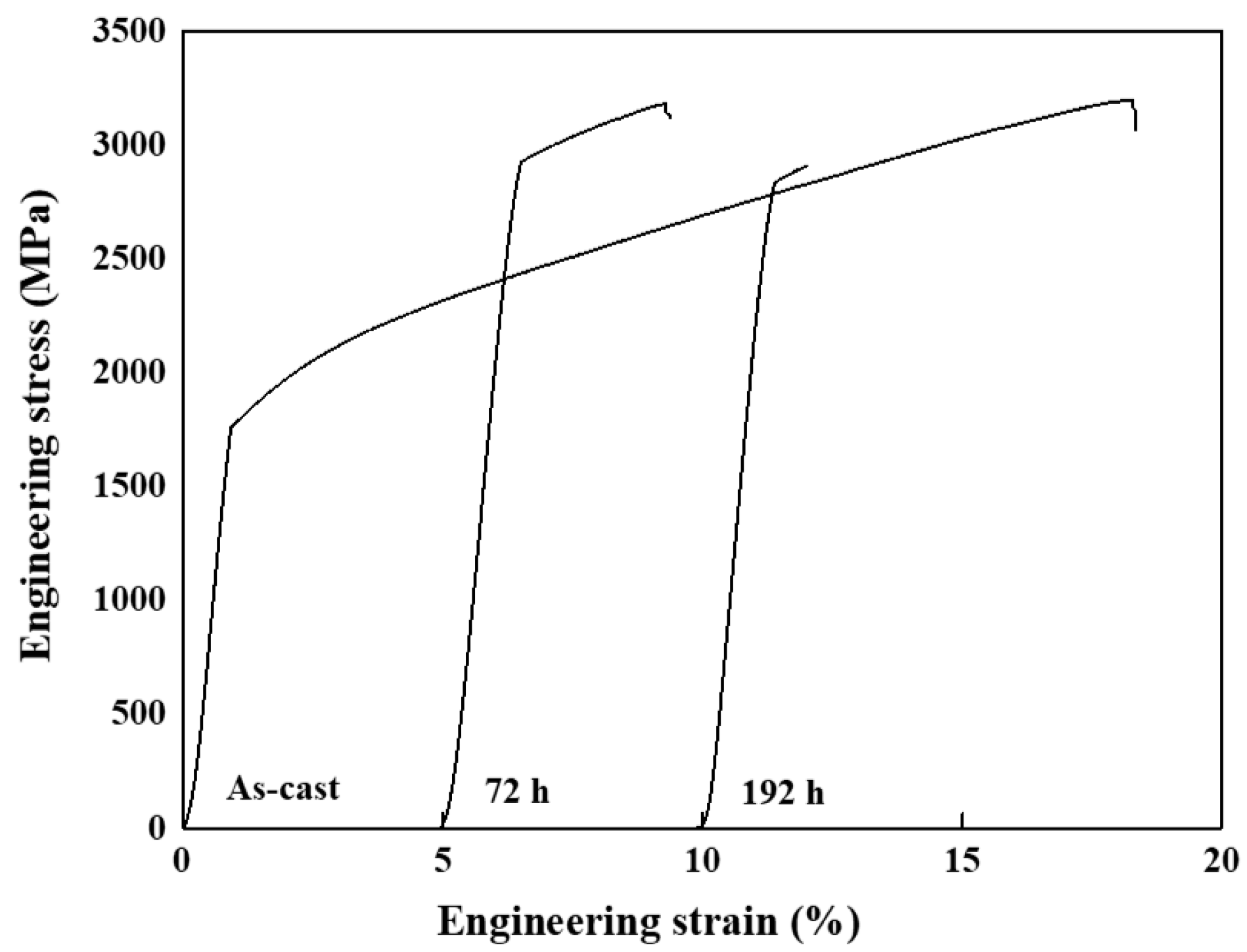
- The highest yield strength for the HEAs was determined to be approximately 2925 MPa in the sample heat-treated at 873 K for 72 h, and remarkable variations in the nano-scale morphology compared to the as-cast alloy were observed.
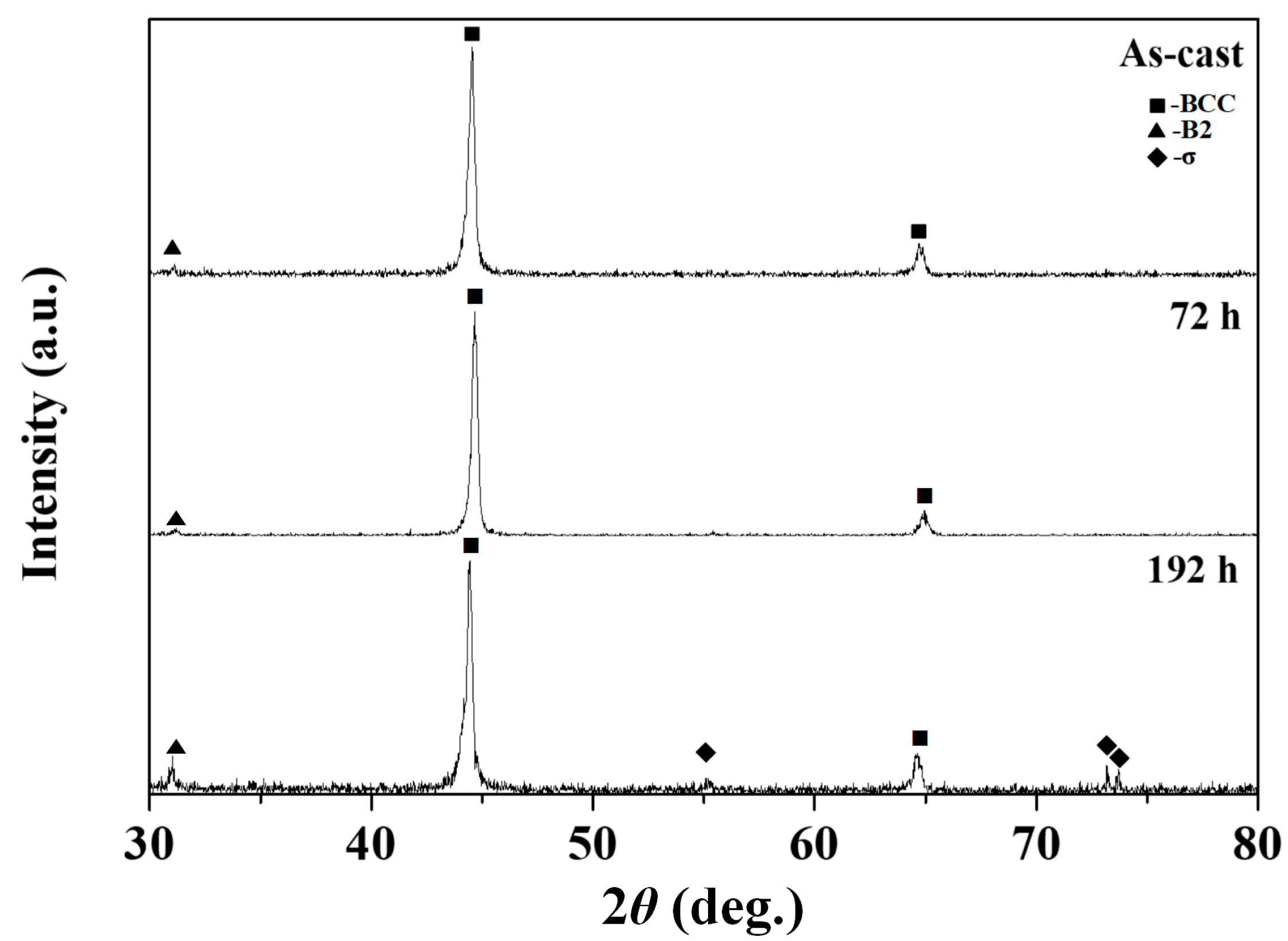
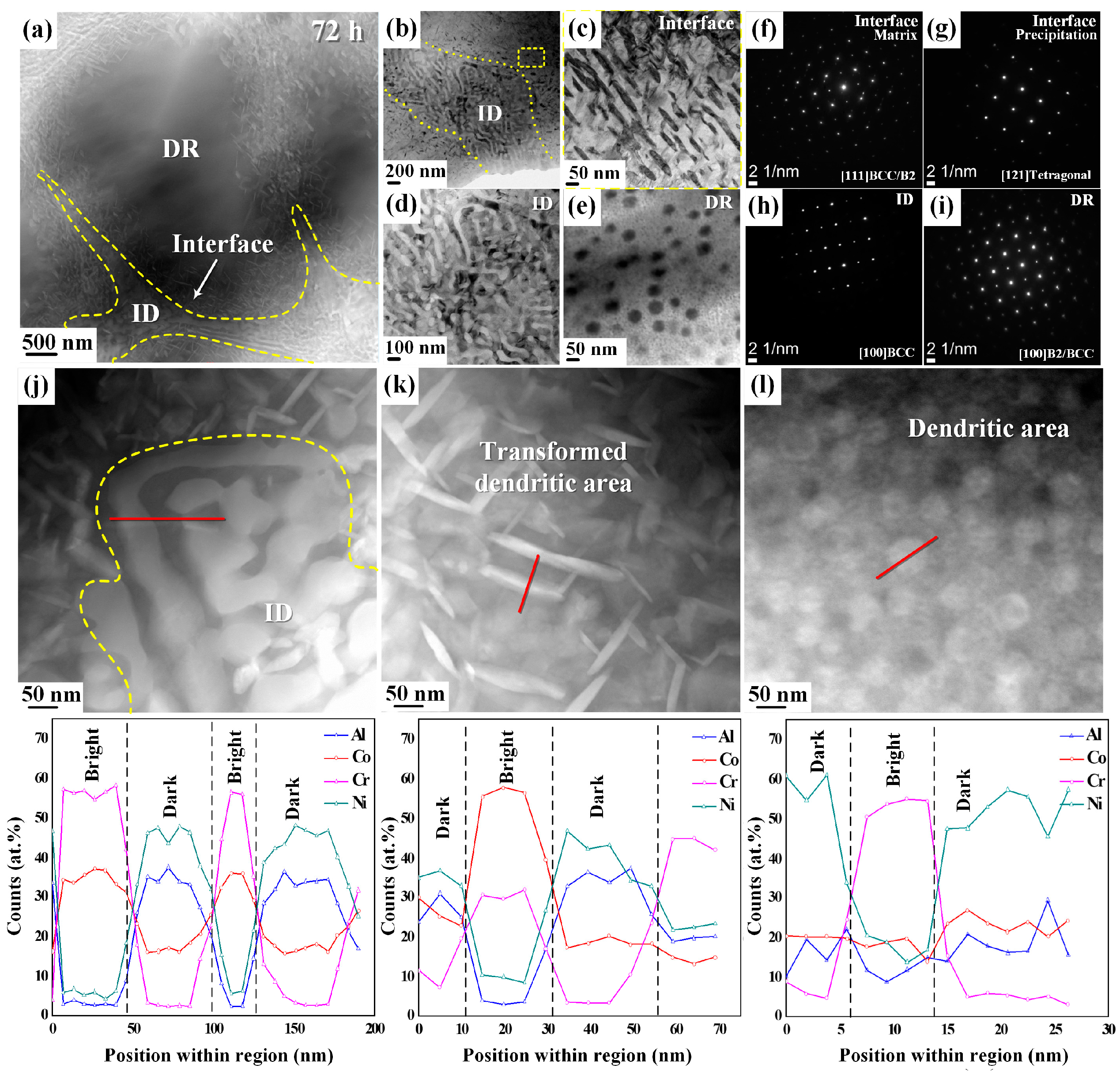
- When the alloy was heat-treated at 873 K for 72 h, an interface with the sigma phase developed between the dendritic and interdendritic regions.
- In comparison, in the alloy heat-treated at 873 K for 192 h, the morphology of the dendritic area completely transformed from spherical to plate-like, and a tetragonal crystal structure developed, which is the sigma phase. At the same time, the engineering strain of the HEAs also reduced with an increase in the sigma phase.
- The formation of the sigma phase due to varying heat treatment durations can affect the mechanical properties, and prolonged heat treatment may result in a diminished strengthening effect on the alloy.
- Prolonged annealing or extended heat treatment plays a crucial role in the microstructural evolution and phase stability of HEAs. This process can lead to significant phase transformations, resulting in the formation of ordered structures and secondary phases. Further studies are necessary to induce similar phase transformations, balancing improved strength and plasticity with the potential brittleness caused by the sigma phase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murty, B.S.; Yeh, J.-W.; Ranganathan, S.; Bhattacharjee, P.P. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 0128160683. [Google Scholar]
- Yeh, J.; Chen, S.; Lin, S.; Gan, J.; Chin, T.; Shun, T.; Tsau, C.; Chang, S. Nanostructured High-entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Hsu, C.; Yeh, J.; Chen, S.; Shun, T. Wear Resistance and High-Temperature Compression Strength of Fcc CuCoNiCrAl0.5Fe Alloy with Boron Addition. Metall. Mater. Trans. A 2004, 35, 1465–1469. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical Properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 Refractory High Entropy Alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Miracle, D.B.; Woodward, C. Mechanical Properties of Low-Density, Refractory Multi-Principal Element Alloys of the Cr-Nb-Ti-V-Zr System. Mater. Sci. Eng. A 2013, 565, 51–62. [Google Scholar] [CrossRef]
- Gali, A.; George, E.P. Tensile Properties of High- and Medium-Entropy Alloys. Intermetallics 2013, 39, 74–78. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The Influences of Temperature and Microstructure on the Tensile Properties of a CoCrFeMnNi High-Entropy Alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Shaysultanov, D.G.; Stepanov, N.D.; Salishchev, G.A.; Senkov, O.N. Tensile Properties of an AlCrCuNiFeCo High-Entropy Alloy in as-Cast and Wrought Conditions. Mater. Sci. Eng. A 2012, 533, 107–118. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Shaisultanov, D.G.; Stepanov, N.; Salishchev, G.A.; Senkov, O.N. Superplasticity of AlCoCrCuFeNi High Entropy Alloy. Mater. Sci. Forum 2013, 735, 146–151. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, B.S.; Fu, H.Z. Solid Solution or Intermetallics in a High-Entropy Alloy. Adv. Eng. Mater. 2009, 11, 641–644. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in Multi-Component AlCoCrCuFeNi High-Entropy Alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative Effects of Enthalpy and Entropy on the Phase Stability of Equiatomic High-Entropy Alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, Y.S.; Mun, S.C.; Hong, S.H.; Wang, W.-M.; Kim, K.B. Designing of Fe-Containing (Ti33Zr33Hf33)-(Ni50Cu50) High Entropy Alloys Developed by Equiatomic Substitution: Phase Evolution and Mechanical Properties. J. Mater. Res. Technol. 2020, 9, 7732–7739. [Google Scholar] [CrossRef]
- Park, H.J.; Na, Y.S.; Hong, S.H.; Kim, J.T.; Kim, Y.S.; Lim, K.R.; Park, J.M.; Kim, K.B. Phase Evolution, Microstructure and Mechanical Properties of Equi-Atomic Substituted TiZrHfNiCu and TiZrHfNiCuM (M = Co, Nb) High-Entropy Alloys. Met. Mater. Int. 2016, 22, 551–556. [Google Scholar] [CrossRef]
- Jumaev, E.; Abbas, M.A.; Mun, S.C.; Song, G.; Hong, S.-J.; Kim, K.B. Nano-Scale Structural Evolution of Quaternary AlCrFeNi Based High Entropy Alloys by the Addition of Specific Minor Elements and Its Effect on Mechanical Characteristics. J. Alloys Compd. 2021, 868, 159217. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Zhu, M.; Li, J.; Dang, Y.; Wang, J. The Phase Stability of Ni2CrFeMox Multi-Principal-Component Alloys with Medium Configurational Entropy. Mater. Des. 2015, 85, 1–6. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Microstructure and Compressive Properties of Multicomponent Alx(TiVCrMnFeCoNiCu)100-x High-Entropy Alloys. Mater. Sci. Eng. A 2007, 454–455, 260–265. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Sheu, T.-S.; Yeh, J.-W.; Chen, S.-K. Effect of Iron Content on Wear Behavior of AlCoCrFexMo0. 5Ni High-Entropy Alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Li, C.; Li, J.C.; Zhao, M.; Jiang, Q. Effect of Alloying Elements on Microstructure and Properties of Multiprincipal Elements High-Entropy Alloys. J. Alloys Compd. 2009, 475, 752–757. [Google Scholar] [CrossRef]
- Wu, Y.D.; Cai, Y.H.; Chen, X.H.; Wang, T.; Si, J.J.; Wang, L.; Wang, Y.D.; Hui, X.D. Phase Composition and Solid Solution Strengthening Effect in TiZrNbMoV High-Entropy Alloys. Mater. Des. 2015, 83, 651–660. [Google Scholar] [CrossRef]
- Sistla, H.R.; Newkirk, J.W.; Frank Liou, F. Effect of Al/Ni Ratio, Heat Treatment on Phase Transformations and Microstructure of AlxFeCoCrNi2-x (X = 0.3, 1) High Entropy Alloys. Mater. Des. 2015, 81, 113–121. [Google Scholar] [CrossRef]
- Joubert, J.-M. Crystal Chemistry and Calphad Modeling of the σ Phase. Prog. Mater. Sci. 2008, 53, 528–583. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, Y.-L.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Mechanical Performance of the AlxCoCrCuFeNi High-Entropy Alloy System with Multiprincipal Elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Boesch WJ, S.J. Preventing Sigma Phase Embrittlement in Nickel-Base Superalloys. Met Prog 1964, 86, 109–111. [Google Scholar]
- Yunjun, Z.; Yong, Z.; Yanli, W.; Guoliang, C. Others Microstructure Characterization of Alx(TiVCrMnFeCoNiCu)100-x High-Entropy Alloy System with Multi-Principal Elements. Rare Met. Mater. Eng. 2007, 36, 2136. [Google Scholar]
- Chen, M.-R.; Lin, S.-J.; Yeh, J.-W.; Chuang, M.-H.; Chen, S.-K.; Huang, Y.-S. Effect of Vanadium Addition on the Microstructure, Hardness, and Wear Resistance of Al 0.5 CoCrCuFeNi High-Entropy Alloy. Metall. Mater. Trans. A 2006, 37, 1363–1369. [Google Scholar] [CrossRef]
- Tsai, M.H.; Tsai, K.Y.; Tsai, C.W.; Lee, C.; Juan, C.C.; Yeh, J.W. Criterion for Sigma Phase Formation in Cr- and V-Containing High-Entropy Alloys. Mater. Res. Lett. 2013, 1, 207–212. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Otto, F.; Dlouh`y, A.; Pradeep, K.G.; Kuběnová, M.; Raabe, D.; Eggeler, G.; George, E.P. Decomposition of the Single-Phase High-Entropy Alloy CrMnFeCoNi after Prolonged Anneals at Intermediate Temperatures. Acta Mater. 2016, 112, 40–52. [Google Scholar] [CrossRef]
- Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Salishchev, G.A.; Senkov, O.N. Phase Composition and Superplastic Behavior of a Wrought AlCoCrCuFeNi High-Entropy Alloy. JOM 2013, 65, 1815–1828. [Google Scholar] [CrossRef]
- Jumaev, E.; Hong, S.H.; Kim, J.T.; Park, H.J.; Kim, Y.S.; Mun, S.C.; Park, J.-Y.; Song, G.; Lee, J.K.; Min, B.H.; et al. Chemical Evolution-Induced Strengthening on AlCoCrNi Dual-Phase High-Entropy Alloy with High Specific Strength. J. Alloys Compd. 2019, 777, 828–834. [Google Scholar] [CrossRef]
- Christofidou, K.A.; McAuliffe, T.P.; Mignanelli, P.M.; Stone, H.J.; Jones, N.G. On the Prediction and the Formation of the Sigma Phase in CrMnCoFeNix High Entropy Alloys. J. Alloys Compd. 2019, 770, 285–293. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Pickering, E.J.; Muñoz-Moreno, R.; Stone, H.J.; Jones, N.G. Precipitation in the Equiatomic High-Entropy Alloy CrMnFeCoNi. Scr. Mater. 2016, 113, 106–109. [Google Scholar] [CrossRef]

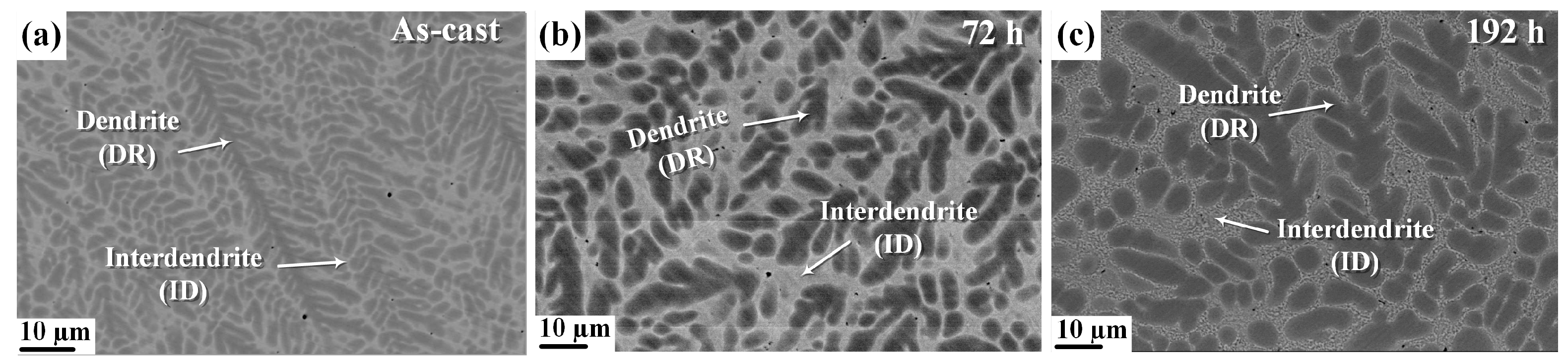

| Alloy | Dendrite Volume Fraction (%) | Interdendrite Volume Fraction (%) |
|---|---|---|
| As-cast [33] | 59.6 | 40.4 |
| Heat-treated for 72 h | 53.1 | 46.9 |
| Heat-treated for 192 h | 50.9 | 49.1 |
| Region | Element Composition (%) | |||
|---|---|---|---|---|
| Al | Co | Cr | Ni | |
| As-cast [33] | ||||
| Dendrite | 28.4 | 25.3 | 16 | 30.3 |
| Interdendrite | 18.4 | 26.7 | 31.7 | 23.2 |
| Heat-treated sample (at 873 K for 72 h) | ||||
| Dendrite | 26.3 | 27.6 | 17.7 | 28.4 |
| Interdendrite | 20.8 | 25.5 | 30.2 | 23.5 |
| Heat-treated sample (at 873 K for 192 h) | ||||
| Dendrite | 24.6 | 29.2 | 19.3 | 26.9 |
| Interdendrite | 22.3 | 24.3 | 28.9 | 24.5 |
| Region | Brightness | Element Composition (%) | |||
|---|---|---|---|---|---|
| Al | Co | Cr | Ni | ||
| As-cast [33] | |||||
| Dendrite | Dark | 23.2 | 28.3 | 9.2 | 39.3 |
| Bright | 8.9 | 28.9 | 48.2 | 14 | |
| Interdendrite | Dark | 20.3 | 27.3 | 15.2 | 37.4 |
| Bright | 3.1 | 30.7 | 60.6 | 5.6 | |
| Heat-treated sample (at 873 K for 72 h) | |||||
| Dendrite | Dark | 18.7 | 20.3 | 7.8 | 53.7 |
| Bright | 9.8 | 21.1 | 49.5 | 19.6 | |
| Interface | Dark | 24.6 | 25.3 | 9.6 | 40.5 |
| Bright | 3.8 | 55.7 | 30.2 | 10.3 | |
| Interdendrite | Dark | 33.4 | 16.2 | 2.8 | 47.6 |
| Bright | 2.9 | 34.5 | 56.9 | 5.7 | |
| Heat-treated sample (at 873 K for 192 h) | |||||
| Dendrite | Dark | 28.9 | 18.7 | 16.3 | 36.1 |
| Bright | 9.2 | 36.7 | 40.8 | 13.3 | |
| Interdendrite | Dark | 30.1 | 19.4 | 6.8 | 43.7 |
| Bright | 8.4 | 30.5 | 44.9 | 16.2 | |
| Alloy | Compressive Yield Stress [MPa] | Compressive Strain (%) |
|---|---|---|
| As-cast [33] | 1753 | 16.71 |
| Heat-treated for 72 h | 2925 | 2.78 |
| Heat-treated for 192 h | 2834 | 0.62 |
| Alloys | VEC | Phase | Heat-Treatment Condition |
|---|---|---|---|
| AlCoCrNi | 7.0 | B2 + BCC + σ | 873 K 72, 192 h |
| AlCoCeFeNi [30] | 7.2 | B2 + FCC + σ | 973 K, 20 h |
| Al0.3CrFe1.5MnNi0.5 [23] | 7.19 | B2 + FCC + σ | 973 K, 2 h |
| Al5Cr32Fe35Ni22Ti6 [24] | 7.31 | B2 + FCC + σ | 973 K, 20 h |
| CoCrCuFeMnNiTiV [27] | 8.5 | B2 + FCC + σ | 973 K, 20 h |
| Al0.5CoCrCuFeNiV [28] | 7.77 | B2 + FCC + σ | 973 K, 20 h |
| AlCoCrCuFeNi [32] | 7.83 | B2 + FCC + σ | 973 K, 20 h |
| Cr2CuFe2MnNi [30] | 8.0 | FCC + σ | 973 K, 20 h |
| Al0.5CoCrCuFeNi [27] | 8.27 | FCC + σ | 973 K, 24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumaev, E.; Park, H.-J.; Abbas, M.A.; Yusupov, D.; Hong, S.-H.; Kim, K.-B. Microstructural Evolution of Quaternary AlCoCrNi High-Entropy Alloys during Heat Treatment. Materials 2024, 17, 3617. https://doi.org/10.3390/ma17143617
Jumaev E, Park H-J, Abbas MA, Yusupov D, Hong S-H, Kim K-B. Microstructural Evolution of Quaternary AlCoCrNi High-Entropy Alloys during Heat Treatment. Materials. 2024; 17(14):3617. https://doi.org/10.3390/ma17143617
Chicago/Turabian StyleJumaev, Elyorjon, Hae-Jin Park, Muhammad Aoun Abbas, Dilshodbek Yusupov, Sung-Hwan Hong, and Ki-Buem Kim. 2024. "Microstructural Evolution of Quaternary AlCoCrNi High-Entropy Alloys during Heat Treatment" Materials 17, no. 14: 3617. https://doi.org/10.3390/ma17143617





