Surface Analysis of Stainless Steel Electrodes Cleaned by Atmospheric Pressure Plasma
Abstract
:1. Introduction
2. Experimental
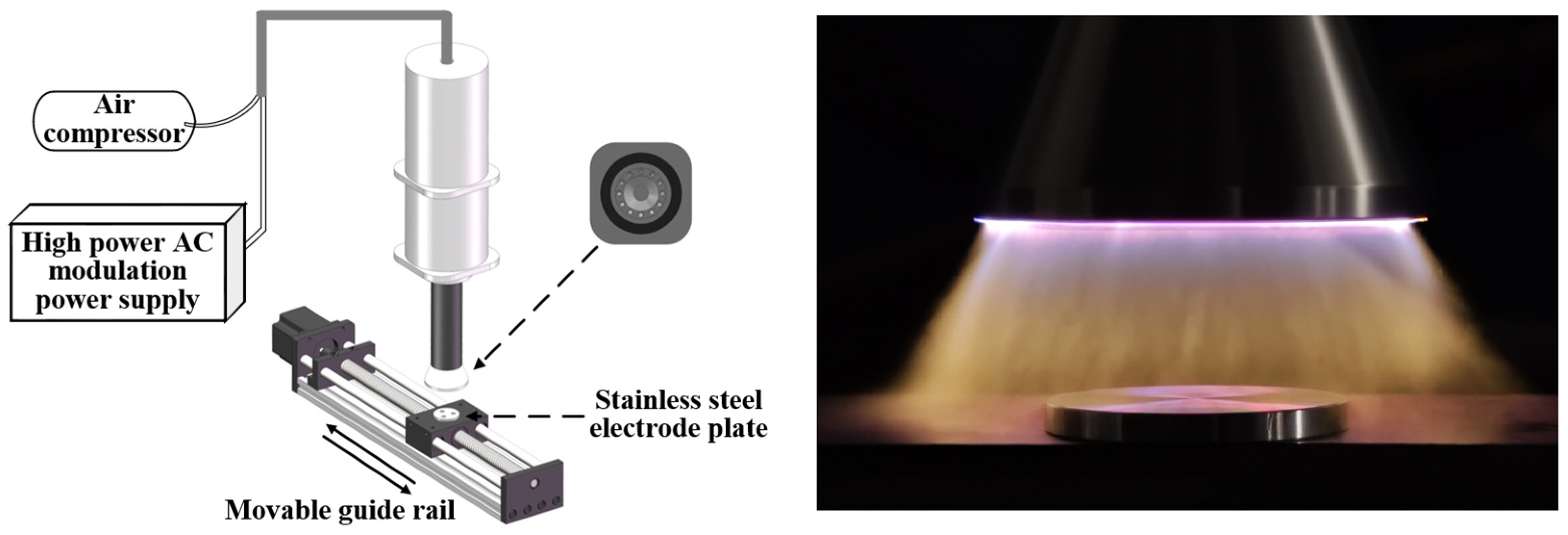
2.1. Plasma Cleaning Platform
2.2. Analysis Methods
2.2.1. X-ray Photoelectron Spectroscopy (XPS)
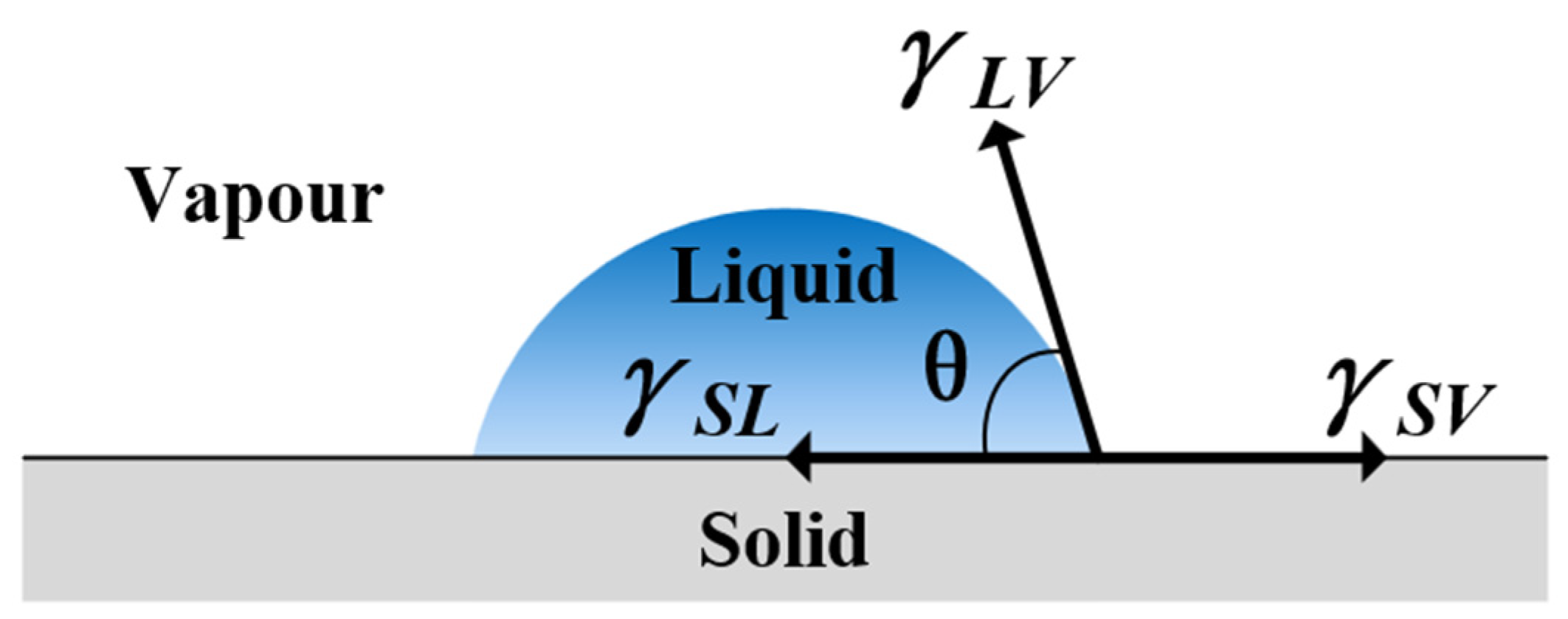
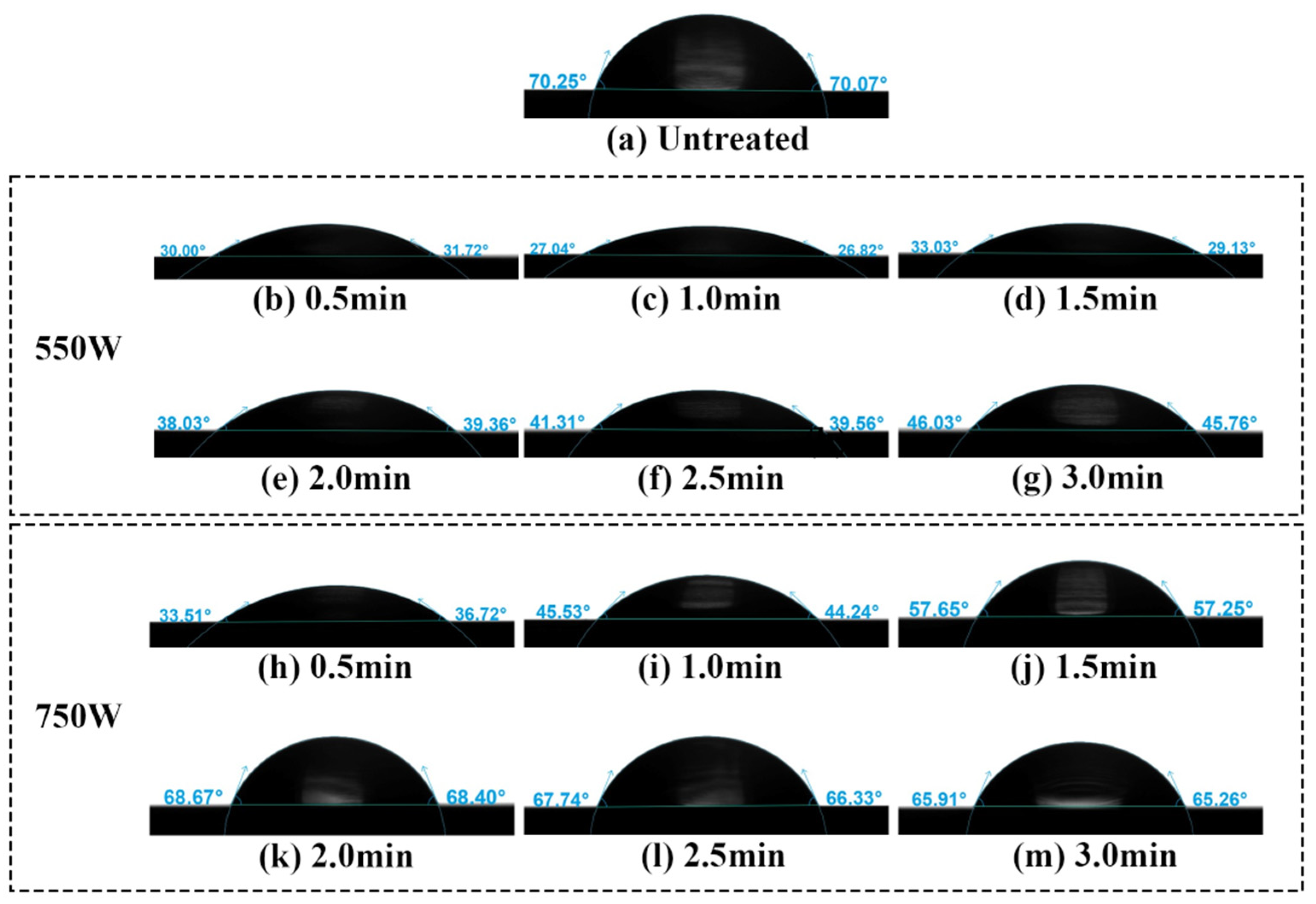
2.2.2. Water Contact Angle (WCA)
3. Results and Discussion
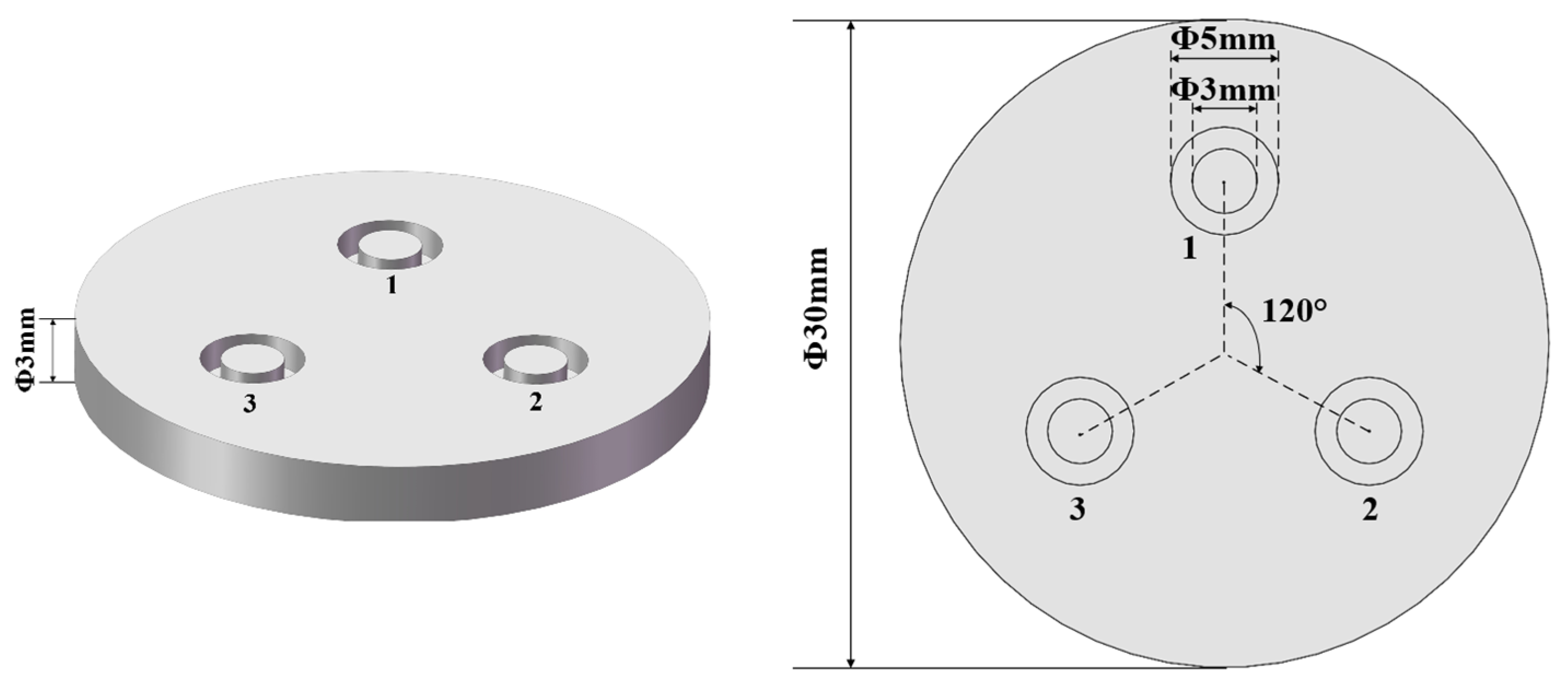
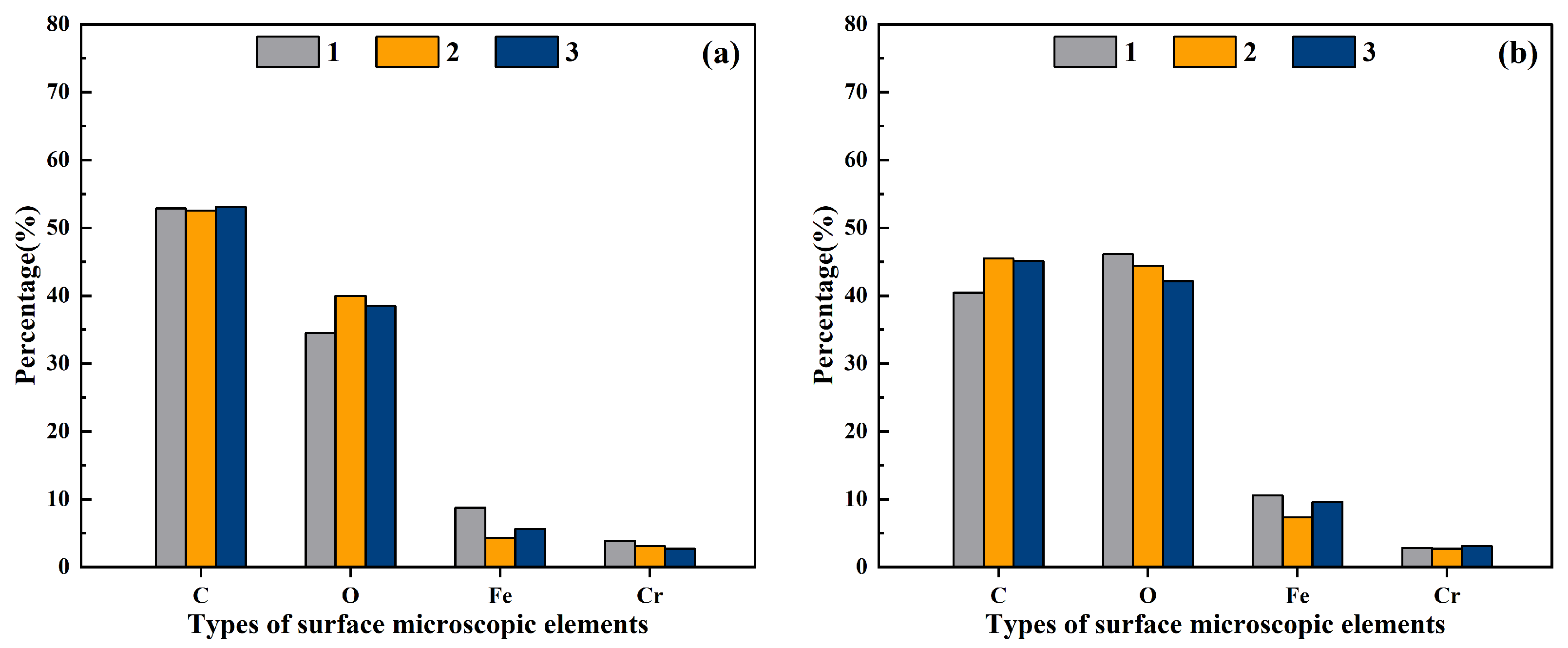
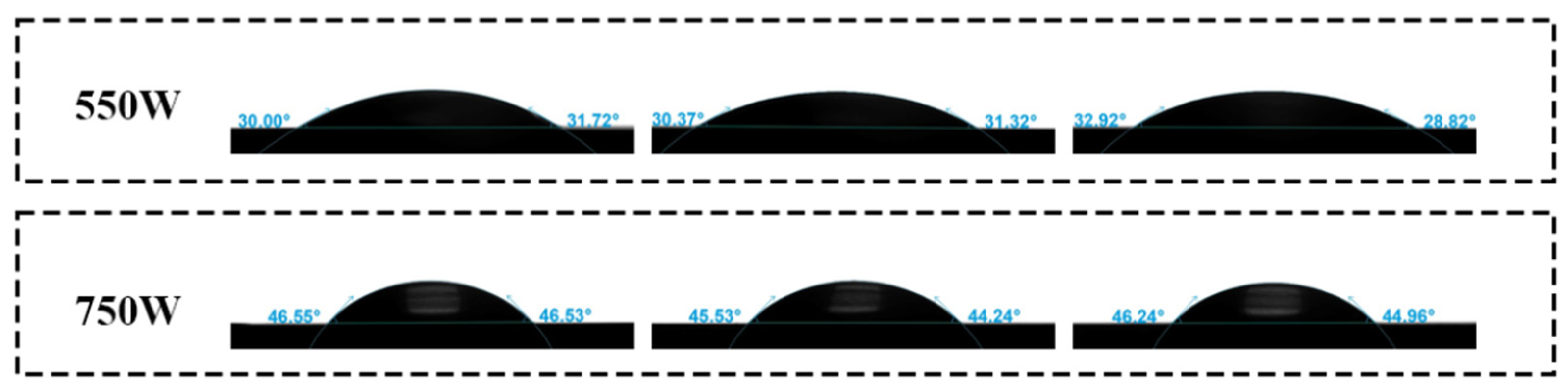
3.1. Uniformity of Plasma Cleaning
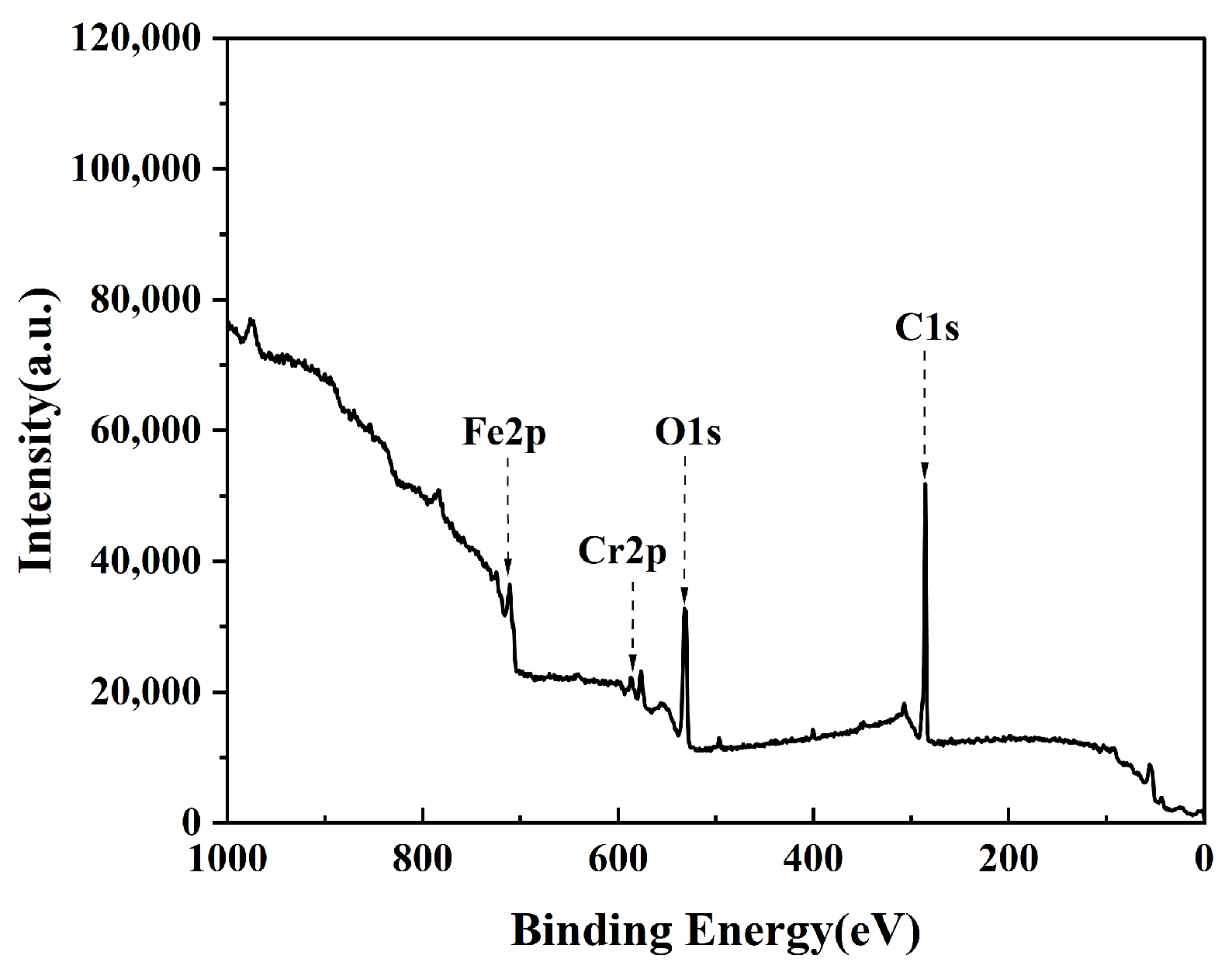
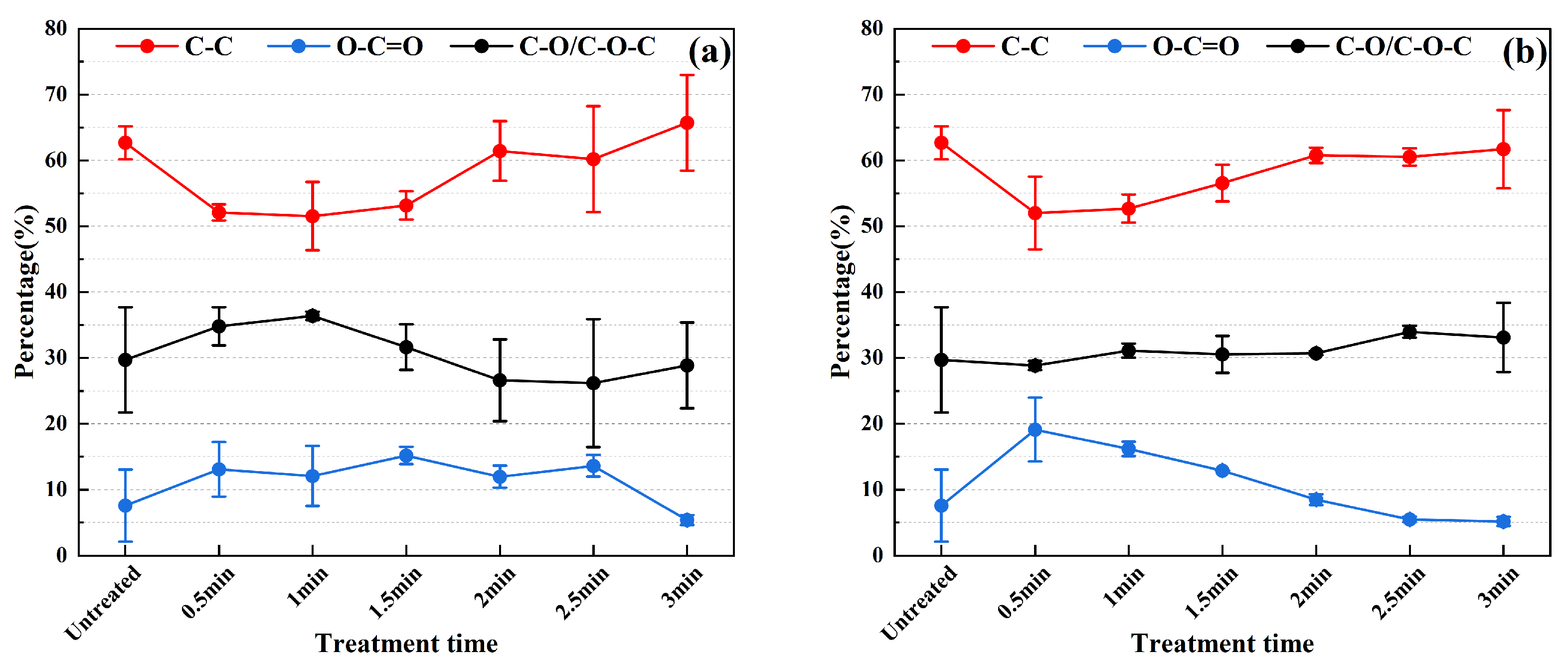
3.2. Chemical Composition Characteristics of the Stainless Steel Surface
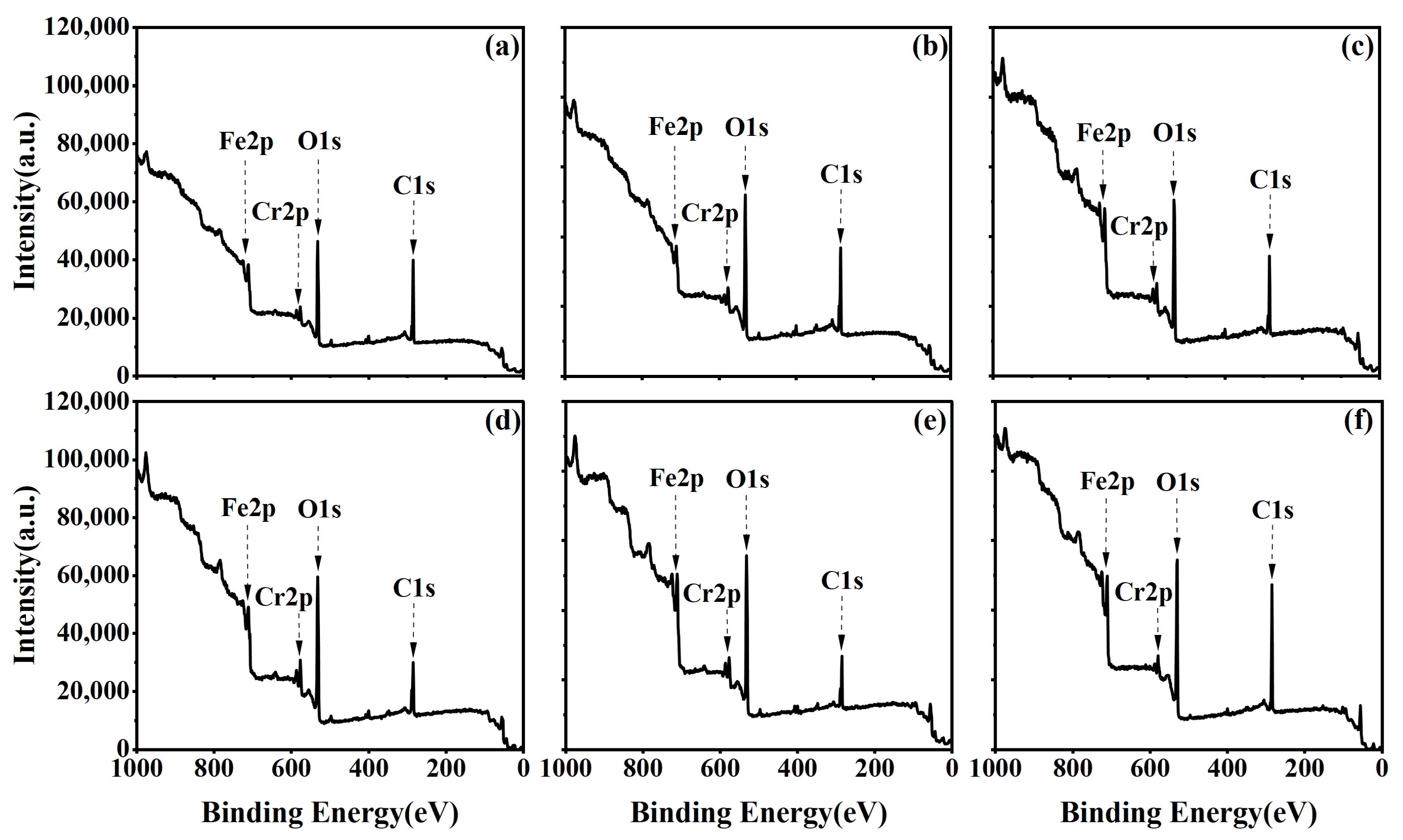
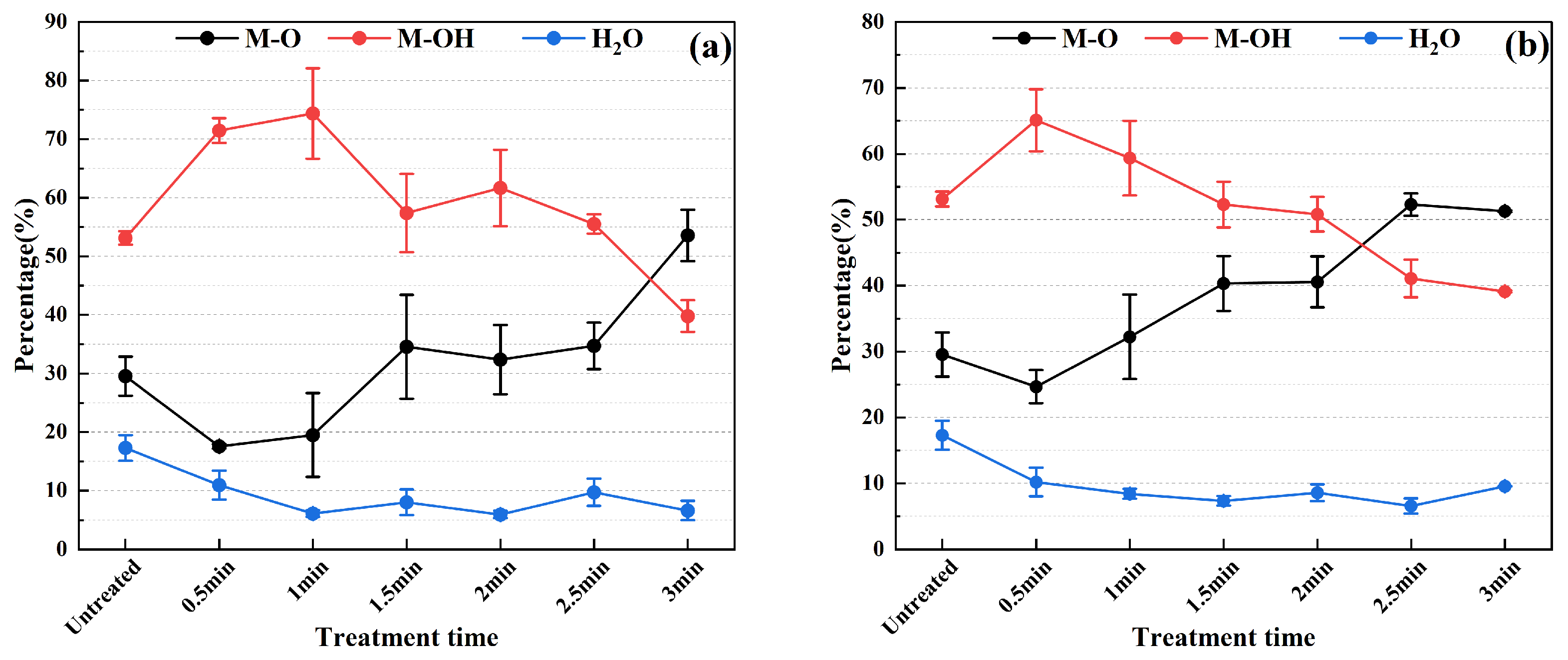
3.3. Evolution of Surface Microscopic Elemental Content and Chemical State
3.4. Evolution of Surface Microscopic Elemental Content and Chemical State
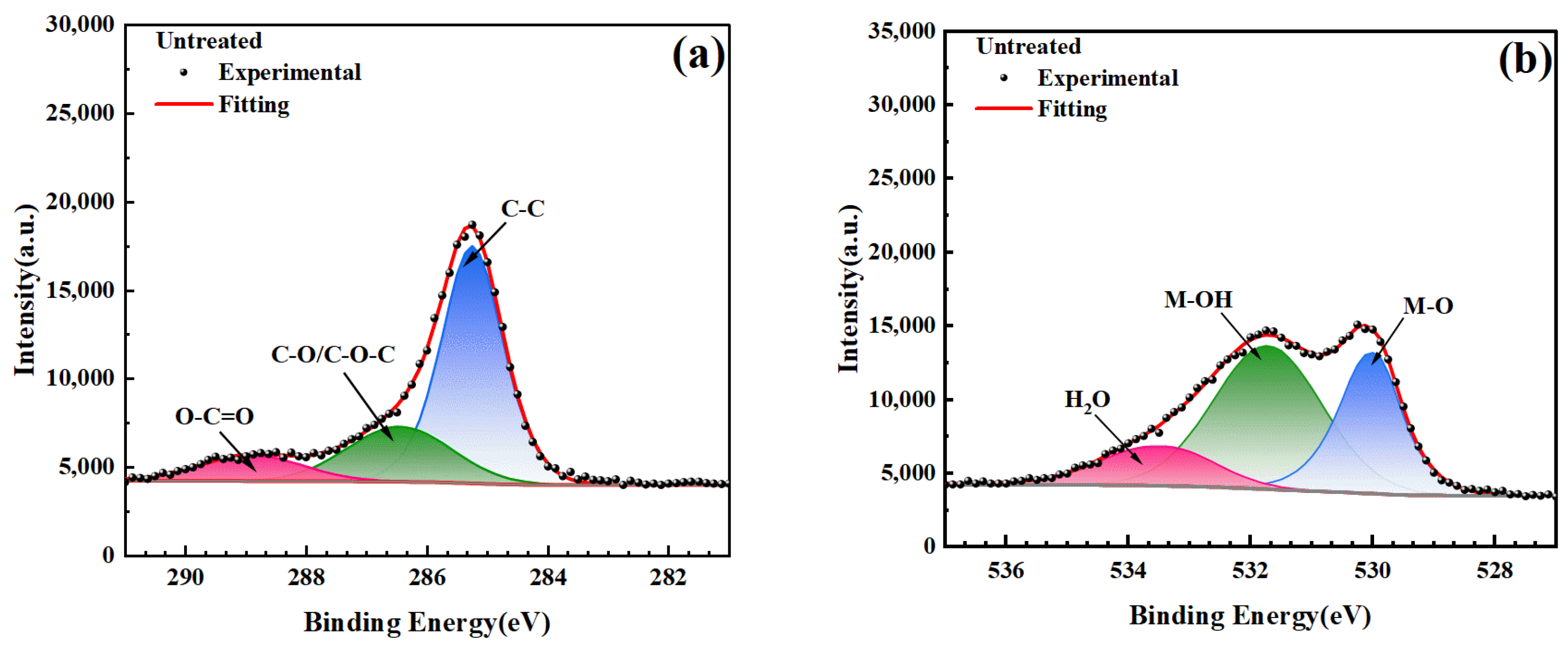
3.5. Mechanism of Surface Wettability Improvement
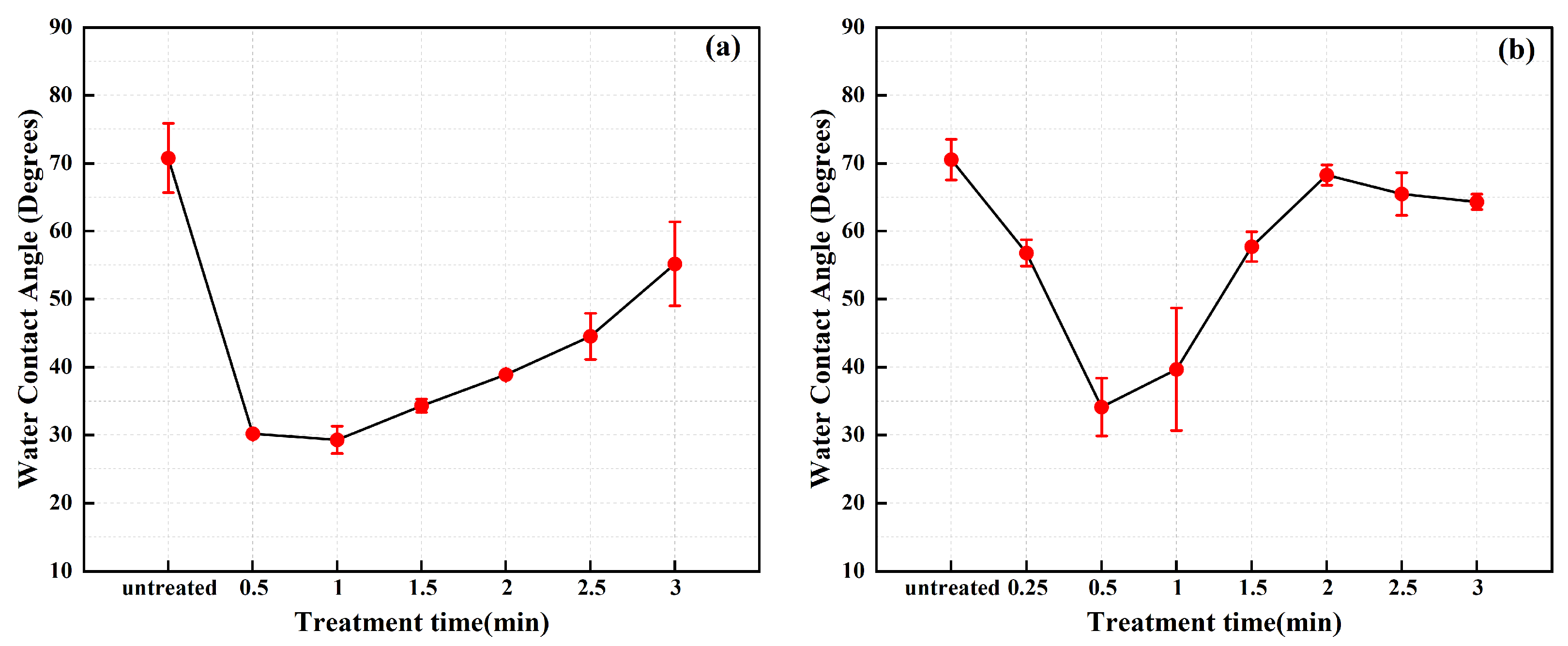
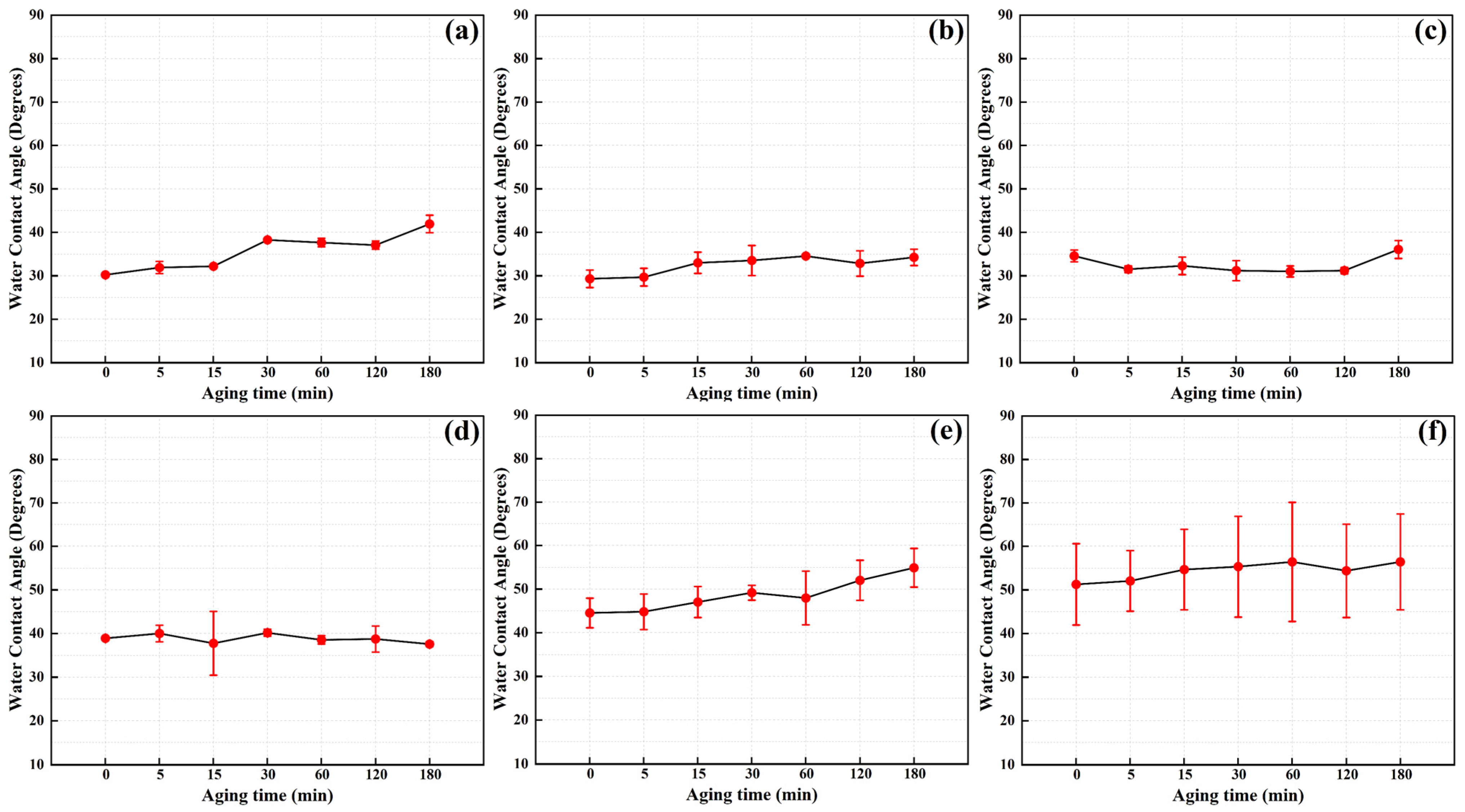
3.6. Time Efficiency Characteristics of the Plasma Cleaning
4. Conclusions
- Plasma cleaning significantly improved the wettability of the stainless steel electrode surface, as evidenced by a decrease in the water contact angle from 70.76° to 29.31°. Additionally, the surface carbon content decreasing from 62.95% to 37.68%, indicating effective removal of carbon contaminants. At discharge powers of 550 W and 750 W, the cleaning times required to achieve the lowest carbon contamination were 2.5 min and 1.5 min, respectively. Regarding wettability and ageing characteristics of the samples, the optimal cleaning condition was observed at a discharge power of 550 W with a cleaning time of 1.0 min.
- The XPS analysis results of the electrode surface, combined with the water contact angle data, demonstrate a highly consistent trend between the content of -OH and O-C=O functional groups and the wettability of the electrode surface. This indicates that -OH and O-C=O are the primary functional groups influencing the wettability of the electrode surface.
- The results of time efficiency characteristics tests indicate that within 3 h of exposure to ambient air following plasma cleaning, there were no significant changes observed in the water contact angle and surface elemental content. This suggests a certain level of stability in the cleaning effectiveness of plasma cleaning on the stainless steel surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutsel, T.; Corcoran, A.; Cuneo, E.; Gomez, R.; Hess, H.; Hinshelwood, D.; Jennings, A.; Laity, R.; Lamppa, C.; McBride, D.; et al. Transmission-line-circuit model of an 85-TW, 25-MA pulsed-power accelerator. Phys. Rev. Accel. Beams 2018, 21, 030401. [Google Scholar] [CrossRef]
- Thompson, M.C.; Simpson, S.C.; Beers, C.J.; Dadras, J.; Meier, E.T.; Stoltz, P.H. Electrode durability and sheared-flow-stabilized Z-pinch fusion energy. Phys. Plasmas 2023, 30, 100601. [Google Scholar] [CrossRef]
- Gong, Z.Z.; Wei, H.; Fan, S.Y.; Hong, Y.P.; Wu, H.Y.; Qiu, A.C. Analysis of electron flow current in vacuum magnetically-insulated-transmission-line sheath for 15-MA Z-pinch driver. Acta Phys. Sin. 2023, 72, 035204. [Google Scholar] [CrossRef]
- Waisman, E.M.; Desjarlais, M.P.; Cuneo, M.E. Ion current losses in the convolute and inner magnetically insulated transmission line of the Z machine. Phys. Rev. Accel. Beams 2019, 22, 030402. [Google Scholar] [CrossRef]
- Spielman, R.B.; Reisman, D.B. On the design of magnetically insulated transmission lines for z-pinch loads. Matter Radiat. Extrem. 2019, 4, 027402. [Google Scholar] [CrossRef]
- Chen, L.; Zou, W.; Zhou, L.; Wang, M.; Liu, Y.; Liu, L.; Deng, M.; Liu, D.; Zhu, J.; Lian, K.; et al. Development of a fusion-oriented pulsed power module. Phys. Rev. Accel. Beams 2019, 22, 030401. [Google Scholar] [CrossRef]
- Tao, Z.; Ruonan, W.; Zhang, X.; Han, Y.; Niu, W.; Xue, Z.; Wang, L. Controlling fine particles in flue gas from lead-zinc smelting by plasma technology. Plasma Sci. Technol. 2020, 22, 044004. [Google Scholar]
- Yang, L.; Wang, S.; Wu, A.; Chen, B.; Chen, J.; Wang, H.; Chen, S.; Wei, J.; Zhang, K.; Ye, Z. Cleaning of nitrogen-containing carbon contamination by atmospheric pressure plasma jet. Plasma Sci. Technol. 2022, 24, 105505. [Google Scholar]
- Jafari, R.; Asadollahi, S.; Farzaneh, M. Applications of plasma technology in development of superhydrophobic surfaces. Plasma Chem. Plasma Process. 2013, 33, 177–200. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Bisht, B.; Kumar, V.; Joshi, N.C.; Tomar, M.S. Cold plasma technology: Advanced and sustainable approach for wastewater treatment. Environ. Sci. Pollut. Res. Int. 2021, 28, 65062–65082. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A. Recent advances of cold plasma technology for water and soil remediation: A critical review. Chem. Eng. J. 2022, 428, 131657. [Google Scholar] [CrossRef]
- Lan, Y.; Yang, G.; Zhao, Y.; Liu, Y.; Demir, A. Facet passivation process of high-power laser diodes by plasma cleaning and ZnO film. Appl. Surf. Sci. 2022, 596, 153506. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Xue, S.; Xie, P.; He, J.; Koval, O.; Chen, Z.; Wang, R. Silicon oxide anticorrosion coating deposition on alloy steel surface by low temperature plasma. Surf. Coat. Technol. 2024, 477, 130338. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, Z.; Wang, Z.; Gou, F.; Shen, B.; Wu, A.; He, Y.; He, P.; Wang, H.; Chen, B.; et al. The mechanism study of mixed Ar/O2 plasma-cleaning treatment on niobium surface for work function improvement. Appl. Surf. Sci. 2019, 475, 143–150. [Google Scholar] [CrossRef]
- Samaei, A.; Chaudhuri, S. Multiphysics modeling of metal surface cleaning using atmospheric pressure plasma. J. Appl. Phys. 2020, 128, 054903. [Google Scholar] [CrossRef]
- Noakes, T.C.Q.; Valizadeh, R.; Hannah, A.N.; Jones, L.B.; Militsyn, B.L.; Mistry, S.; Cropper, M.D.; Rossall, A.; Van den Berg, J.A. Oxygen plasma cleaning of copper for photocathode applications: A MEIS and XPS study. Vacuum 2022, 205, 111424. [Google Scholar] [CrossRef]
- Sönmez, T.; Jadidi, M.F.; Kazmanli, K.; Birer, Ö.; Ürgen, M. Role of different plasma gases on the surface chemistry and wettability of RF plasma treated stainless steel. Vacuum 2016, 129, 63–73. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Q.; Guan, Y.; Liu, H.; Zhang, P.; Batelibieke, B.; Shen, R.; Lu, L.; Yuan, X.; Miao, X. The mechanism study of low-pressure air plasma cleaning on large-aperture optical surface unraveled by experiment and reactive molecular dynamics simulation. Plasma Sci. Technol. 2022, 24, 064012. [Google Scholar] [CrossRef]
- Alhomsi, S.; Bauville, G.; Pasquiers, S.; Minea, T. Radio-Frequency linear plasma process for heating of metallic surfaces. Vacuum 2023, 207, 111571. [Google Scholar] [CrossRef]
- Muñoz, J.; Bravo, J.A.; Calzada, M.D. Aluminum metal surface cleaning and activation by atmospheric-pressure remote plasma. Appl. Surf. Sci. 2017, 407, 72–81. [Google Scholar] [CrossRef]
- Shin, D.H.; Bang, C.U.; Kim, J.H.; Hong, Y.C.; Uhm, H.S.; Park, D.K.; Kim, K.H. Treatment of metal surface by atmospheric microwave plasma jet. IEEE Trans. Plasma Sci. 2006, 34, 1241–1246. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, J.; Kong, F.; Hou, X.; Fang, Z.; Yin, Y.; Shao, T. Plasma surface treatment of Cu by nanosecond-pulse diffuse discharges in atmospheric air. Plasma Sci. Technol. 2018, 20, 014011. [Google Scholar] [CrossRef]
- Regula, C.; Ihde, J.; Lommatzsch, U.; Wilken, R. Corrosion protection of copper surfaces by an atmospheric pressure plasma jet treatment. Surf. Coat. Technol. 2011, 205, S355–S358. [Google Scholar] [CrossRef]
- Prysiazhnyi, V. Atmospheric Pressure Plasma Treatment and Following Aging Effect of Chromium Surfaces. J. Surf. Eng. Mater. Adv. Technol. 2013, 2, 138–145. [Google Scholar] [CrossRef]
- Kim, M.C.; Song, D.K.; Shin, H.S.; Baeg, S.H.; Kim, G.S.; Boo, J.H.; Han, J.G.; Yang, S.H. Enhancement of adhesion strength between two AISI 316 L stainless steel plates through atmospheric pressure plasma treatment. Surf. Coat. Technol. 2005, 200, 5220–5228. [Google Scholar]
- Kim, M.C.; Song, D.K.; Shin, H.S.; Choi, H.S. Surface modification for hydrophilic property of stainless steel treated by atmospheric-pressure plasma jet. Surf. Coat. Technol. 2003, 171, 312–316. [Google Scholar] [CrossRef]
- Shenton, M.J.; Stevens, G.C. Surface modification of polymer surfaces: Atmospheric plasma versus vacuum plasma treatments. J. Phys. D Appl. Phys. 2001, 34, 2761. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, S.; Liu, Y.; Hu, X.; Ostrikov, K.K.; Shao, T. Energy-efficient pathways for pulsed-plasma-activated sustainable ammonia synthesis. ACS Sustain. Chem. Eng. 2023, 11, 1110–1120. [Google Scholar] [CrossRef]
- Castle, J.E. The composition of metal surfaces after atmospheric exposure: An historical perspective. J. Adhes. 2008, 84, 368–388. [Google Scholar] [CrossRef]
- Williams, D.F.; Kellar, E.J.C.; Jesson, D.A.; Watts, J.F. Surface analysis of 316 stainless steel treated with cold atmospheric plasma. Appl. Surf. Sci. 2017, 403, 240–247. [Google Scholar] [CrossRef]
- Al-Hamarneh, I.; Pedrow, P.; Eskhan, A.; Abu-Lail, N. Hydrophilic property of 316 L stainless steel after treatment by atmospheric pressure corona streamer plasma using surface-sensitive analyses. Appl. Surf. Sci. 2012, 259, 424–432. [Google Scholar] [CrossRef]
- Bónová, L.; Zhu, W.; Patel, D.K.; Krogstad, D.V.; Ruzic, D.N. Atmospheric pressure microwave plasma for aluminum surface cleaning. J. Vac. Sci. Technol. A 2020, 38, 023002. [Google Scholar] [CrossRef]
- Yao, H.; Ke, H.; Zhang, X.; Pan, S.J.; Li, M.S.; Yang, L.P.; Schreckenbach, G.; Jiang, W. Molecular Recognition of Hydrophilic Molecules in Water by Combining the Hydrophobic Effect with Hydrogen Bonding. J. Am. Chem. Soc. 2018, 140, 13466–13477. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Jain, S.K.; Jin, Q.; Peng, X. Adsorption and structure of benzene confined in disordered porous carbons: Effect of pore heterogeneity and surface chemistry. Mol. Simul. 2018, 44, 1291–1303. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.H.; Kim, B.J.; Lee, H.M. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater. Des. 2023, 230, 111952. [Google Scholar]


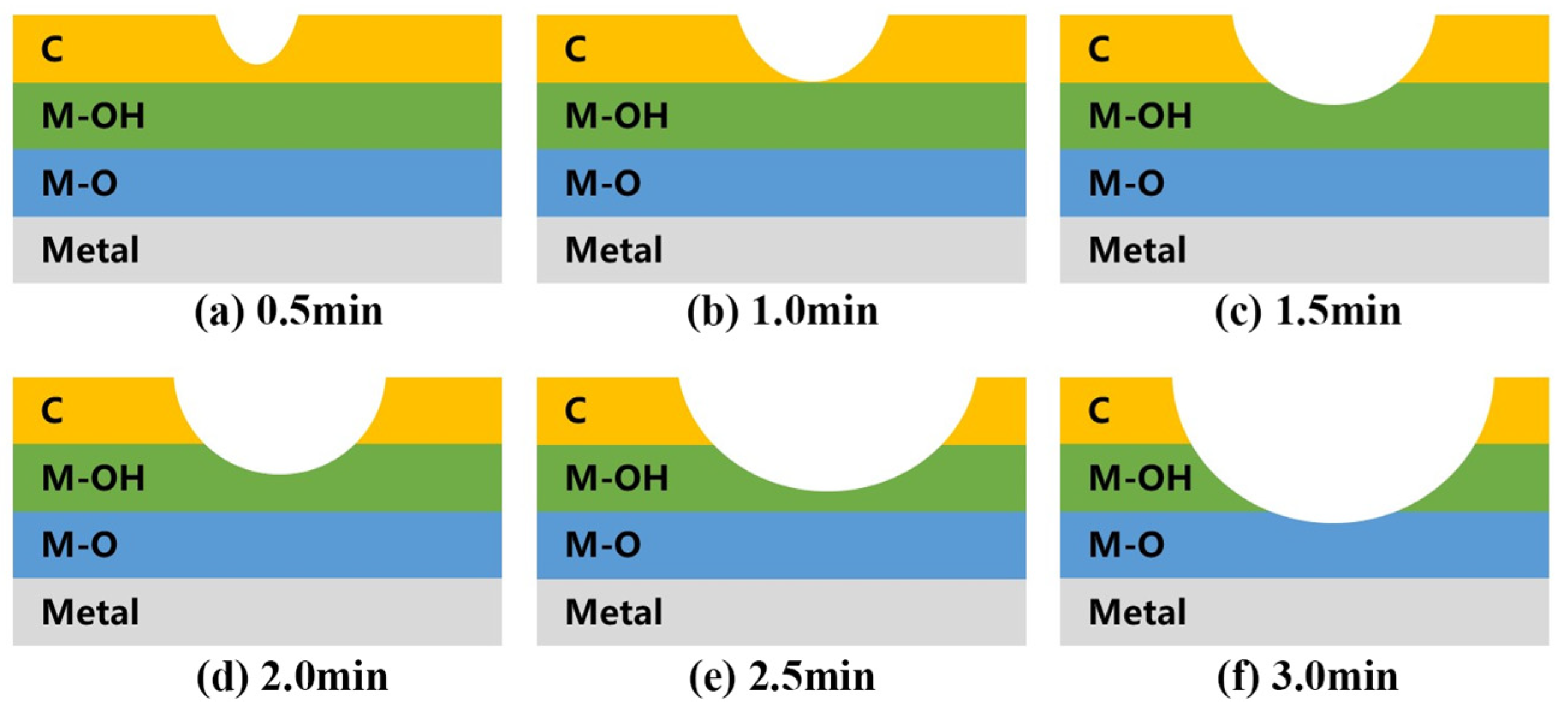
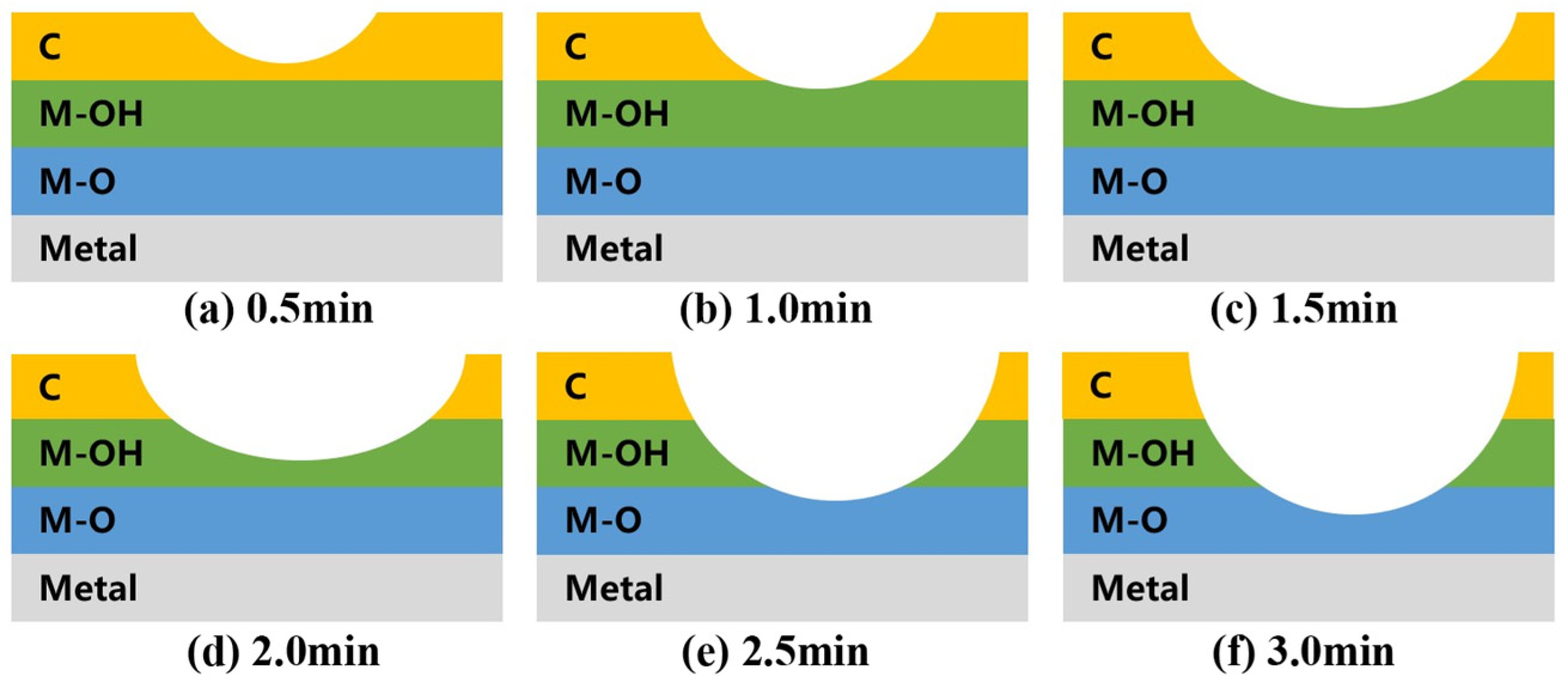


| Power | Cleaning Time | Cleaning Scope | Power | Cleaning Time | Cleaning Scope |
|---|---|---|---|---|---|
| 550 W | 0.5 min | C | 750 W | 0.5 min | C |
| 1.0 min | C | 1.0 min | C, M-OH | ||
| 1.5 min | C, M-OH | 1.5 min | C, M-OH | ||
| 2.0 min | C, M-OH | 2.0 min | M-OH | ||
| 2.5 min | C, M-OH | 2.5 min | M-OH, M-O | ||
| 3.0 min | M-OH, M-O | 3.0 min | M-OH, M-O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Dang, M.; Luo, C.; Ba, Y.; Li, Q. Surface Analysis of Stainless Steel Electrodes Cleaned by Atmospheric Pressure Plasma. Materials 2024, 17, 3621. https://doi.org/10.3390/ma17143621
Zhang J, Dang M, Luo C, Ba Y, Li Q. Surface Analysis of Stainless Steel Electrodes Cleaned by Atmospheric Pressure Plasma. Materials. 2024; 17(14):3621. https://doi.org/10.3390/ma17143621
Chicago/Turabian StyleZhang, Jia, Mengjia Dang, Cheng Luo, Yongshan Ba, and Qingkai Li. 2024. "Surface Analysis of Stainless Steel Electrodes Cleaned by Atmospheric Pressure Plasma" Materials 17, no. 14: 3621. https://doi.org/10.3390/ma17143621




