Mathematical Modeling of NaCl Scaling Development in Long-Distance Membrane Distillation for Improved Scaling Control
Abstract
:1. Introduction
2. Experimental Section
2.1. Membrane Parameters
2.2. Long-Distance Membrane Distillation Module
2.3. Long-Distance Membrane Distillation Tests
2.4. Characterization of NaCl Scaling
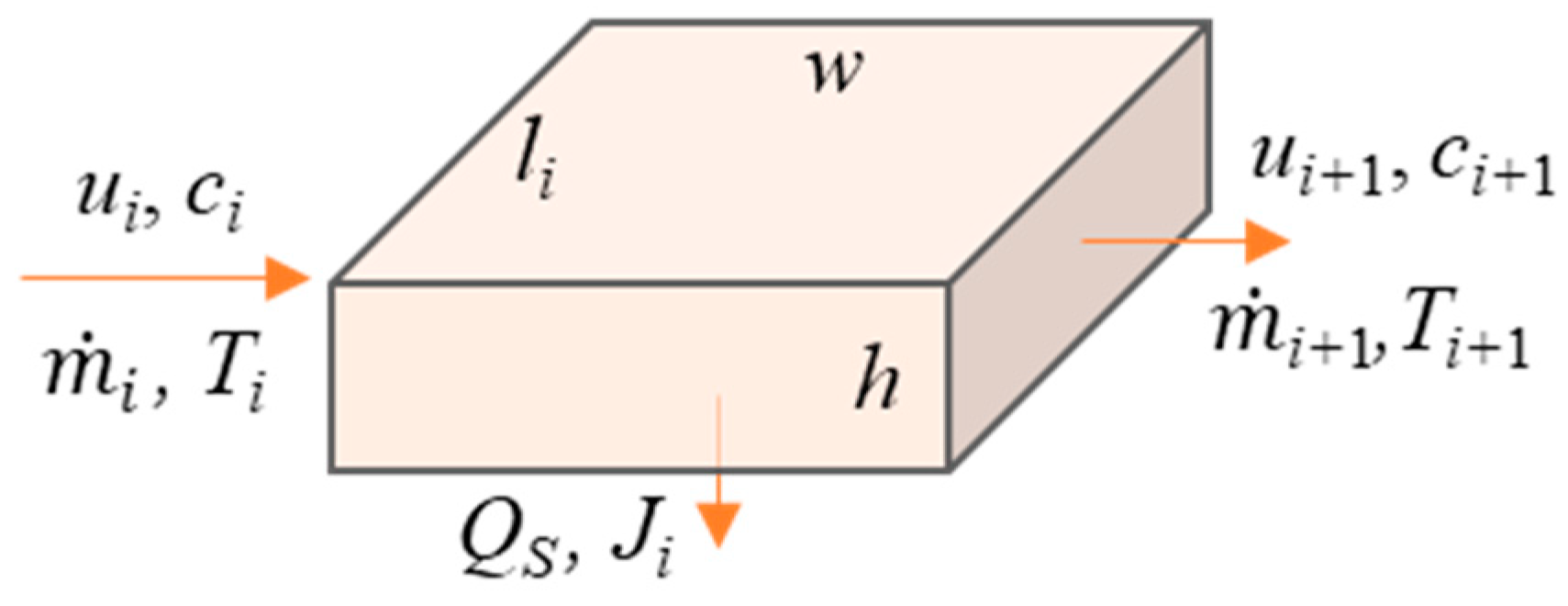
3. Thermodynamic System
- (i)
- The concentration and temperature are considered to be uniform on the flow cross-section of the feed and the coolant.
- (ii)
- When the feed reaches the supersaturated condition for heterogeneous nucleation, the scaling process is initiated on the membrane surface. The induction time of nucleation and the crystal growth process are not considered.
- (iii)
- The heat transfer between the thermodynamic open system and the environment can be neglected. The MD process can be assumed as an adiabatic process when there is no heat input in the feed channel. The MD process can be assumed as an isothermal process after flow development in the entrance region due to the constant heat input along the feed channel.
4. Results and Discussion
4.1. Theoretical Estimation of NaCl Scaling Process
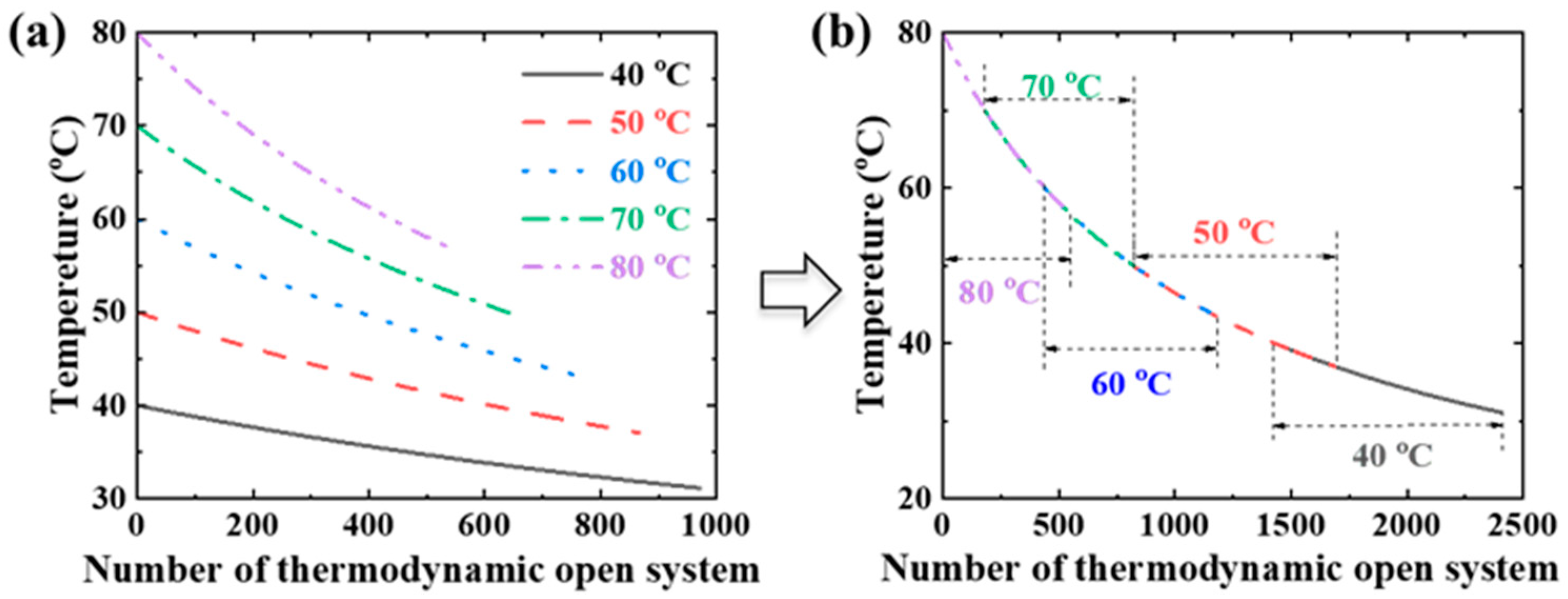
4.2. Simplified Estimation of Initiating Position of NaCl Scaling
- (i)
- The isothermal process
- (ii)
- The adiabatic process
- (i)
- Estimate the supersaturated concentration corresponding to the local temperature (c*) from Equation (9).
- (ii)
- Estimate the total permeate flux (ΔJt) from Equation (8).
- (iii)
- Estimate the outlet feed temperature of the LDMD module (Tout) from Equation (7).
- (iv)
- Estimate the initiating position of the scaling (L) from Equation (6).
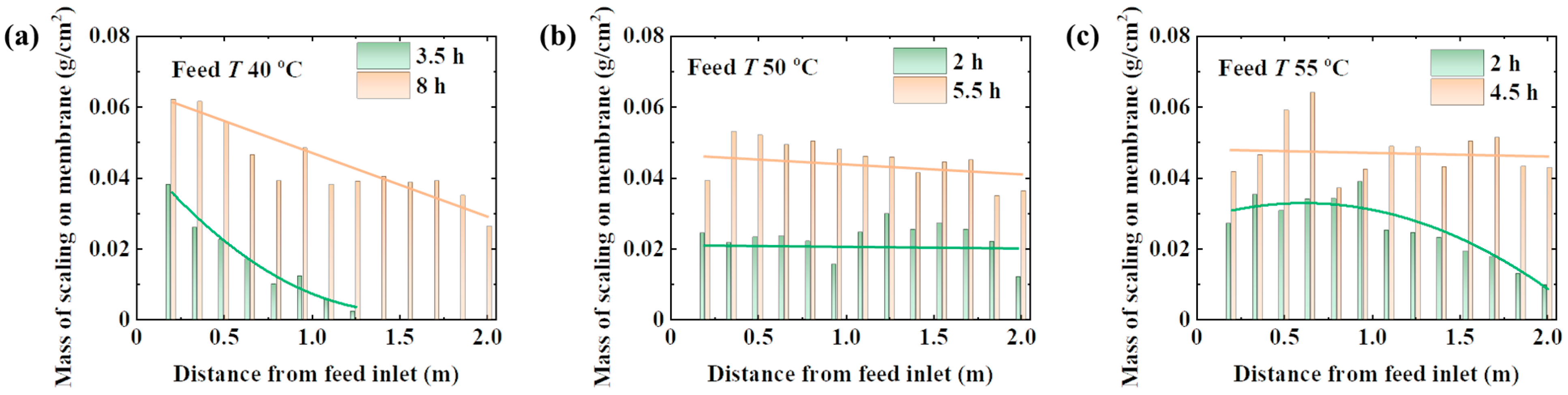
4.3. Evolutionary Process of NaCl Scaling
4.4. Behaviors of NaCl Scaling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adham, S.; Hussain, A.; Matar, J.M.; Dores, R.; Janson, A. Application of Membrane Distillation for Desalting Brines from Thermal Desalination Plants. Desalination 2013, 314, 101–108. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane Distillation: Recent Developments and Perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Lu, D.; Li, P.; Xiao, W.; He, G.; Jiang, X. Simultaneous Recovery and Crystallization Control of Saline Organic Wastewater by Membrane Distillation Crystallization. AIChE J. 2017, 63, 2187–2197. [Google Scholar] [CrossRef]
- Ni, W.; Li, Y.; Zhao, J.; Zhang, G.; Du, X.; Dong, Y. Simulation Study on Direct Contact Membrane Distillation Modules for High-Concentration NaCl Solution. Membranes 2020, 10, 179. [Google Scholar] [CrossRef]
- Venzke, C.D.; Rizzana, D.U.; Giacobbo, A.; Rodrigues, M.A.S.; He, T.; Bernardes, A.M. Membrane Distillation Treating a Real Petrochemical Reverse Osmosis Concentrate: Influence of Membrane Characteristics on the Process Performance. J. Water Process. Eng. 2021, 39, 101722. [Google Scholar] [CrossRef]
- Yan, Z.; Qu, F.; Liang, H.; Yu, H.; Pang, H.; Rong, H.; Fan, G.; Van der Bruggen, B. Effect of Biopolymers and Humic Substances on Gypsum Scaling and Membrane Wetting during Membrane Distillation. J. Memb. Sci. 2021, 617, 118638. [Google Scholar] [CrossRef]
- Gilron, J.; Ladizansky, Y.; Korin, E. Silica Fouling in Direct Contact Membrane Distillation. Ind. Eng. Chem. Res. 2013, 52, 10521–10529. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Skuse, C.; Zaragoza, G.; Gryta, M.; Gorgojo, P. Membrane Cleaning and Pretreatments in Membrane Distillation—A Review. Chem. Eng. J. 2021, 422, 129696. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Chen, Y.; Gao, B.; Wang, Z. Scaling Control of Forward Osmosis-Membrane Distillation (FO-MD) Integrated Process for Pre-Treated Landfill Leachate Treatment. Desalination 2021, 520, 115342. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ooi, B.S.; Ahmad, A.L.; Leo, C.P.; Lyly Leow, H.T.; Abdullah, M.Z.; Aziz, N.A. Correlating Scalants Characteristic and Air Bubbling Rate in Submerged Vacuum Membrane Distillation: A Fouling Control Strategy. J. Memb. Sci. 2021, 621, 118991. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard, V.J.H. Scaling and Fouling in Membrane Distillation for Desalination Applications: A Review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Zhou, M.; Ji, C.; Ji, F.; Chen, M.; Zhong, Z.; Xing, W. Micro-Octahedron Cu2O-Based Photocatalysis-Fenton for Organic Pollutant Degradation: Proposed Coupling Mechanism in a Membrane Reactor. Ind. Eng. Chem. Res. 2022, 61, 7255–7265. [Google Scholar] [CrossRef]
- Addanki Tirumala, R.T.; Khatri, N.; Ramakrishnan, S.B.; Mohammadparast, F.; Khan, M.T.; Tan, S.; Wagle, P.; Puri, S.; McIlroy, D.N.; Kalkan, A.K.; et al. Tuning Catalytic Activity and Selectivity in Photocatalysis on Mie-Resonant Cuprous Oxide Particles: Distinguishing Electromagnetic Field Enhancement Effect from the Heating Effect. ACS Sustain. Chem. Eng. 2023, 11, 15931–15940. [Google Scholar] [CrossRef]
- Yu, Y.; Kipkorir, A.; Choi, M.Y.; Kamat, P.V. Directional Electron Transfer across In2S3/ZnS-Embedded Photocatalytic Membranes. ACS Appl. Energy Mater. 2024, 7, 681–688. [Google Scholar] [CrossRef]
- Chen, G.; Lu, Y.; Krantz, W.B.; Wang, R.; Fane, A.G. Optimization of Operating Conditions for a Continuous Membrane Distillation Crystallization Process with Zero Salty Water Discharge. J. Memb. Sci. 2014, 450, 1–11. [Google Scholar] [CrossRef]
- Lu, K.J.; Cheng, Z.L.; Chang, J.; Luo, L.; Chung, T.S. Design of Zero Liquid Discharge Desalination (ZLDD) Systems Consisting of Freeze Desalination, Membrane Distillation, and Crystallization Powered by Green Energies. Desalination 2019, 458, 66–75. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane Distillation Crystallization Technology for Zero Liquid Discharge and Resource Recovery: Opportunities, Challenges and Futuristic Perspectives. Sci. Total Environ. 2022, 806, 150692. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Tuo, L.; Lu, D.; Hou, B.; Chen, W.; He, G. Progress in Membrane Distillation Crystallization: Process Models, Crystallization Control and Innovative Applications. Front. Chem. Sci. Eng. 2017, 11, 647–662. [Google Scholar] [CrossRef]
- Duong, H.C.; Chivas, A.R.; Nelemans, B.; Duke, M.; Gray, S.; Cath, T.Y.; Nghiem, L.D. Treatment of RO Brine from CSG Produced Water by Spiral-Wound Air Gap Membrane Distillation—A Pilot Study. Desalination 2015, 366, 121–129. [Google Scholar] [CrossRef]
- Zhang, J.W.; Fang, H.; Wang, J.W.; Hao, L.Y.; Xu, X.; Chen, C.S. Preparation and Characterization of Silicon Nitride Hollow Fiber Membranes for Seawater Desalination. J. Memb. Sci. 2014, 450, 197–206. [Google Scholar] [CrossRef]
- Winter, D.; Koschikowski, J.; Gross, F.; Maucher, D.; Düver, D.; Jositz, M.; Mann, T.; Hagedorn, A. Comparative Analysis of Full-Scale Membrane Distillation Contactors—Methods and Modules. J. Memb. Sci. 2017, 524, 758–771. [Google Scholar] [CrossRef]
- Amaya-Vías, D.; López-Ramírez, J.A. Techno-Economic Assessment of Air and Water Gap Membrane Distillation for Seawater Desalination under Different Heat Source Scenarios. Water 2019, 11, 2117. [Google Scholar] [CrossRef]
- Guillén-Burrieza, E.; Zaragoza, G.; Miralles-Cuevas, S.; Blanco, J. Experimental Evaluation of Two Pilot-Scale Membrane Distillation Modules Used for Solar Desalination. J. Memb. Sci. 2012, 409–410, 264–275. [Google Scholar] [CrossRef]
- Winter, D.; Koschikowski, J.; Wieghaus, M. Desalination Using Membrane Distillation: Experimental Studies on Full Scale Spiral Wound Modules. J. Memb. Sci. 2011, 375, 104–112. [Google Scholar] [CrossRef]
- Schwantes, R.; Bauer, L.; Chavan, K.; Dücker, D.; Felsmann, C.; Pfafferott, J. Air Gap Membrane Distillation for Hypersaline Brine Concentration: Operational Analysis of a Full-Scale Module–New Strategies for Wetting Mitigation. Desalination 2018, 444, 13–25. [Google Scholar] [CrossRef]
- Cai, J.; Luo, Y.; Chen, J.; Guo, F. Investigation of Interfacial Crystallization Fouling Behaviors and Membrane Re-Functionalization Based on a Long-Distance Membrane Distillation Module. Desalination 2022, 534, 115800. [Google Scholar] [CrossRef]
- Ruiz Salmón, I.; Luis, P. Membrane Crystallization via Membrane Distillation. Chem. Eng. Process. Process. Intensif. 2018, 123, 258–271. [Google Scholar] [CrossRef]
- Meng, S.; Ye, Y.; Mansouri, J.; Chen, V. Crystallization Behavior of Salts during Membrane Distillation with Hydrophobic and Superhydrophobic Capillary Membranes. J. Memb. Sci. 2015, 473, 165–176. [Google Scholar] [CrossRef]
- Zou, T.; Kang, G.; Zhou, M.; Li, M.; Cao, Y. Submerged Vacuum Membrane Distillation Crystallization (S-VMDC) with Turbulent Intensification for the Concentration of NaCl Solution. Sep. Purif. Technol. 2019, 211, 151–161. [Google Scholar] [CrossRef]
- Jiang, X.; Ruan, X.; Xiao, W.; Lu, D.; He, G. A Novel Membrane Distillation Response Technology for Nucleation Detection, Metastable Zone Width Measurement and Analysis. Chem. Eng. Sci. 2015, 134, 671–680. [Google Scholar] [CrossRef]
- Qtaishat, M.R.; Matsuura, T. Modelling of Pore Wetting in Membrane Distillation Compared with Pervaporation; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Chan, M.T.; Fane, A.G.; Matheickal, J.T.; Sheikholeslami, R. Membrane Distillation Crystallization of Concentrated Salts—Flux and Crystal Formation. J. Memb. Sci. 2005, 257, 144–155. [Google Scholar] [CrossRef]
- Gryta, M. Mitigation of Membrane Wetting by Applying a Low Temperature Membrane Distillation. Membranes 2020, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zheng, R.; Liu, Y.; He, H.; Yuan, X.; Ji, Y.; Li, D.; Yin, H.; Zhang, Y.; Li, X.-M.; et al. Slippery for Scaling Resistance in Membrane Distillation: A Novel Porous Micropillared Superhydrophobic Surface. Water Res. 2019, 155, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, X.; Lu, Y.; Wang, R.; Fane, A.G. Heat Transfer Intensification and Scaling Mitigation in Bubbling-Enhanced Membrane Distillation for Brine Concentration. J. Memb. Sci. 2014, 470, 60–69. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S.; Rahman, M.; Anwar, N.; Jassby, D.; Rahaman, S. Fouling and Wetting in the Membrane Distillation Driven Wastewater Reclamation Process—A Review. Adv. Colloid Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef] [PubMed]
- Tijing, L.D.; Woo, Y.C.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Fouling and Its Control in Membrane Distillation-A Review. J. Memb. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Lienhard, V.J.H.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting Phenomena in Membrane Distillation: Mechanisms, Reversal, and Prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A Review of Membrane Wettability for the Treatment of Saline Water Deploying Membrane Distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Panagopoulos, A. Study and Evaluation of the Characteristics of Saline Wastewater (Brine) Produced by Desalination and Industrial Plants. Environ. Sci. Pollut. Res. Int. 2021, 29, 23736–23749. [Google Scholar] [CrossRef]
- Kiefer, F.; Präbst, A.; Rodewald, K.S.; Sattelmayer, T. Membrane Scaling in Vacuum Membrane Distillation—Part 1: In-Situ Observation of Crystal Growth and Membrane Wetting. J. Memb. Sci. 2019, 590, 117294. [Google Scholar] [CrossRef]
- Sparenberg, M.C.; Chergaoui, S.; Sang Sefidi, V.; Luis, P. Crystallization Control via Membrane Distillation-Crystallization: A Review. Desalination 2022, 519, 115315. [Google Scholar] [CrossRef]
- Edwie, F.; Chung, T.S. Development of Simultaneous Membrane Distillation-Crystallization (SMDC) Technology for Treatment of Saturated Brine. Chem. Eng. Sci. 2013, 98, 160–172. [Google Scholar] [CrossRef]
- Cai, J.; Liu, X.; Zhao, Y.; Guo, F. Membrane Desalination Using Surface Fluorination Treated Electrospun Polyacrylonitrile Membranes with Nonwoven Structure and Quasi-Parallel Fibrous Structure. Desalination 2018, 429, 70–75. [Google Scholar] [CrossRef]
- Shaulsky, E.; Nejati, S.; Boo, C.; Perreault, F.; Osuji, C.O.; Elimelech, M. Post-Fabrication Modification of Electrospun Nanofiber Mats with Polymer Coating for Membrane Distillation Applications. J. Memb. Sci. 2017, 530, 158–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, F. Breaking the Saturated Vapor Layer with a Thin Porous Membrane. Membranes 2022, 12, 1231. [Google Scholar] [CrossRef]
- Mersmann, A. Crystallization Technology Handbook; CRC Press: New York, NY, USA, 2001; Volume 13. [Google Scholar] [CrossRef]
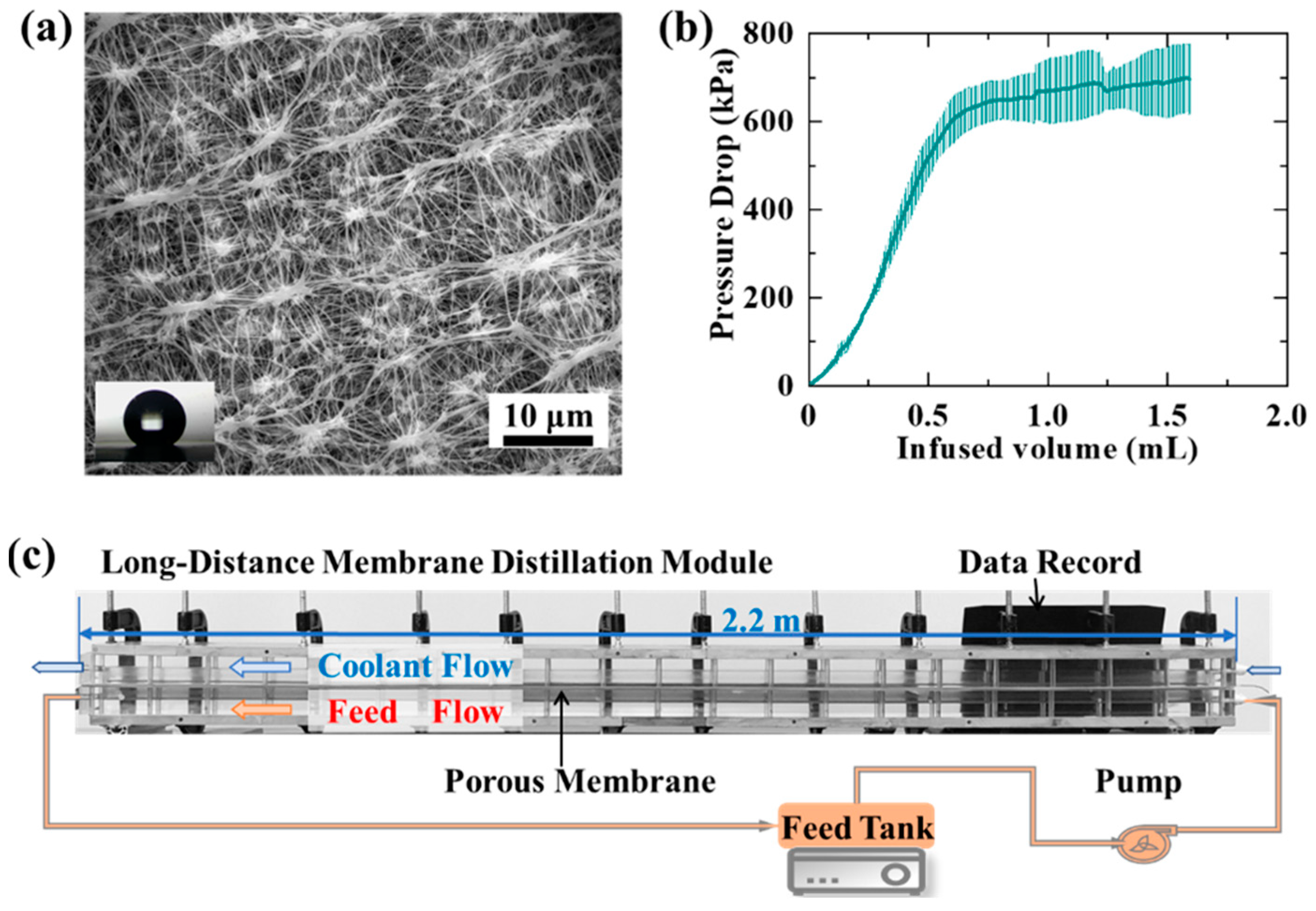


| Membrane Thickness | Contact Angle | LEP | Porosity |
|---|---|---|---|
| 60 ± 5 μm | 153 ± 5° | 600 ± 50 kPa | 75 ± 5% |
| Isothermal Process | Adiabatic Process |
|---|---|
| Equation (5) | Equation (9) Equation (8) Equation (7) Equation (6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Mu, X.; Xue, J.; Chen, J.; Liu, Z.; Guo, F. Mathematical Modeling of NaCl Scaling Development in Long-Distance Membrane Distillation for Improved Scaling Control. Materials 2024, 17, 3629. https://doi.org/10.3390/ma17153629
Cai J, Mu X, Xue J, Chen J, Liu Z, Guo F. Mathematical Modeling of NaCl Scaling Development in Long-Distance Membrane Distillation for Improved Scaling Control. Materials. 2024; 17(15):3629. https://doi.org/10.3390/ma17153629
Chicago/Turabian StyleCai, Jingcheng, Xingsen Mu, Jian Xue, Jiaming Chen, Zeman Liu, and Fei Guo. 2024. "Mathematical Modeling of NaCl Scaling Development in Long-Distance Membrane Distillation for Improved Scaling Control" Materials 17, no. 15: 3629. https://doi.org/10.3390/ma17153629






