The Impact of Boron Nitride Additive on Thermal and Thermochromic Properties of Organic Thermochromic Phase Change Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Limitations of the Work and Future Scope
5. Conclusions
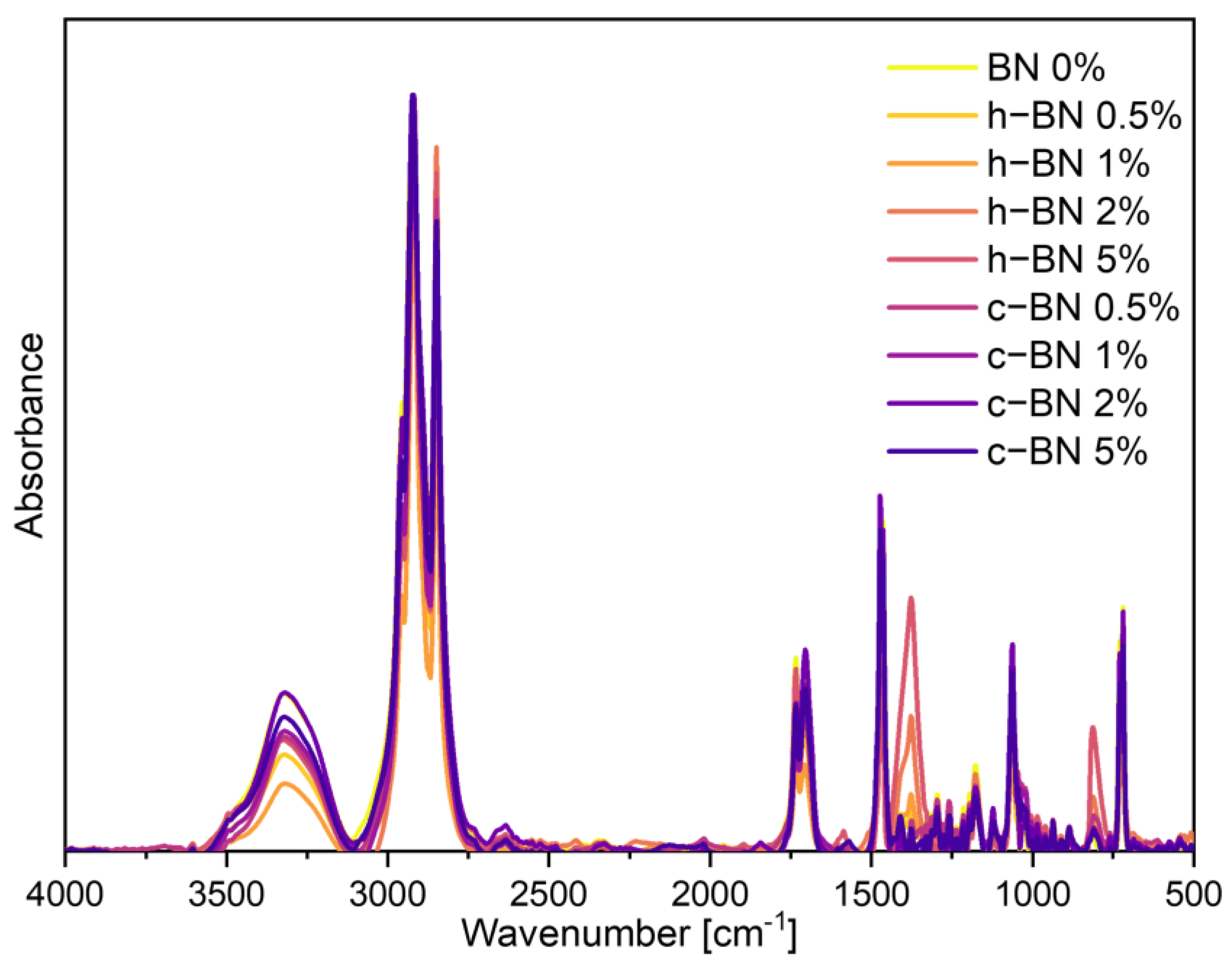
- The addition of boron nitride did not cause significant changes in the positions of the characteristic absorption bands. However, new absorption bands appeared at 1377 cm−1 and 811 cm−1 for samples with hexagonal boron nitride.
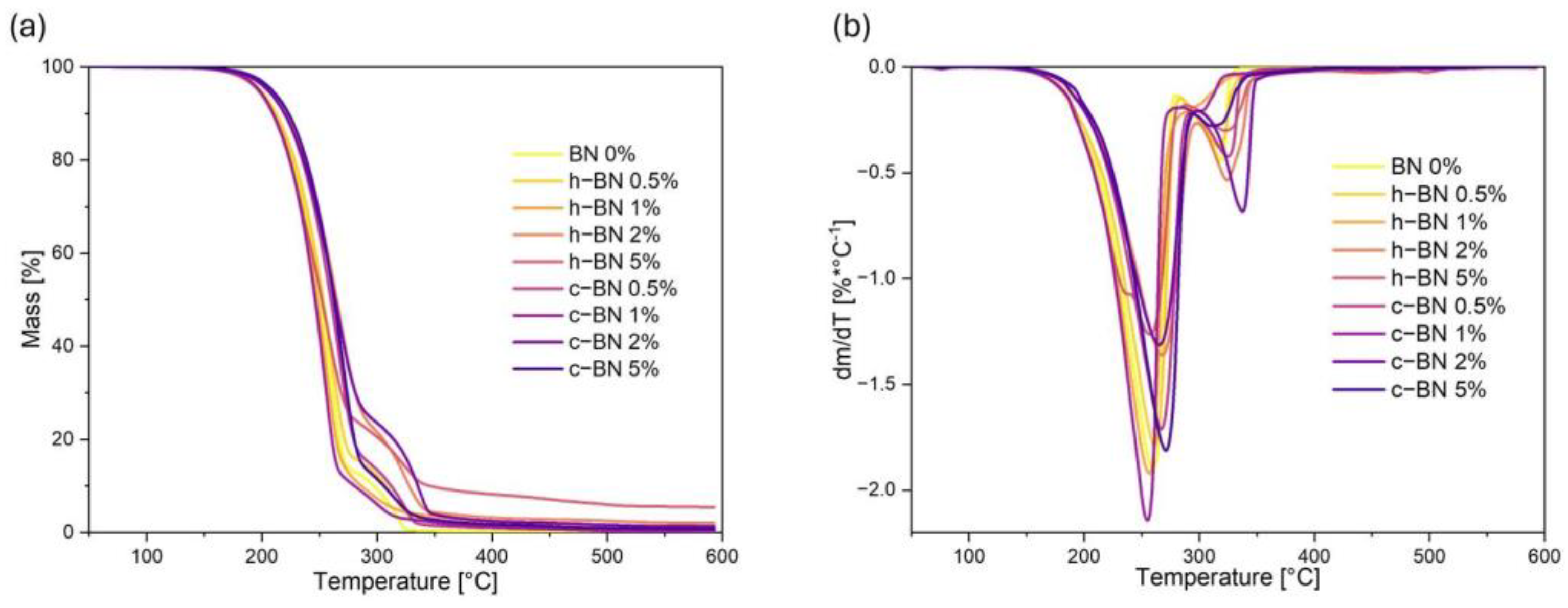
- The thermogravimetric analysis confirmed that the obtained materials are thermally stable in the temperature range corresponding to the potential applications, i.e., at the phase transition temperatures. TPCM mixtures show two-stage thermal degradation. The addition of boron nitride did not significantly influence the thermal stability of the samples.
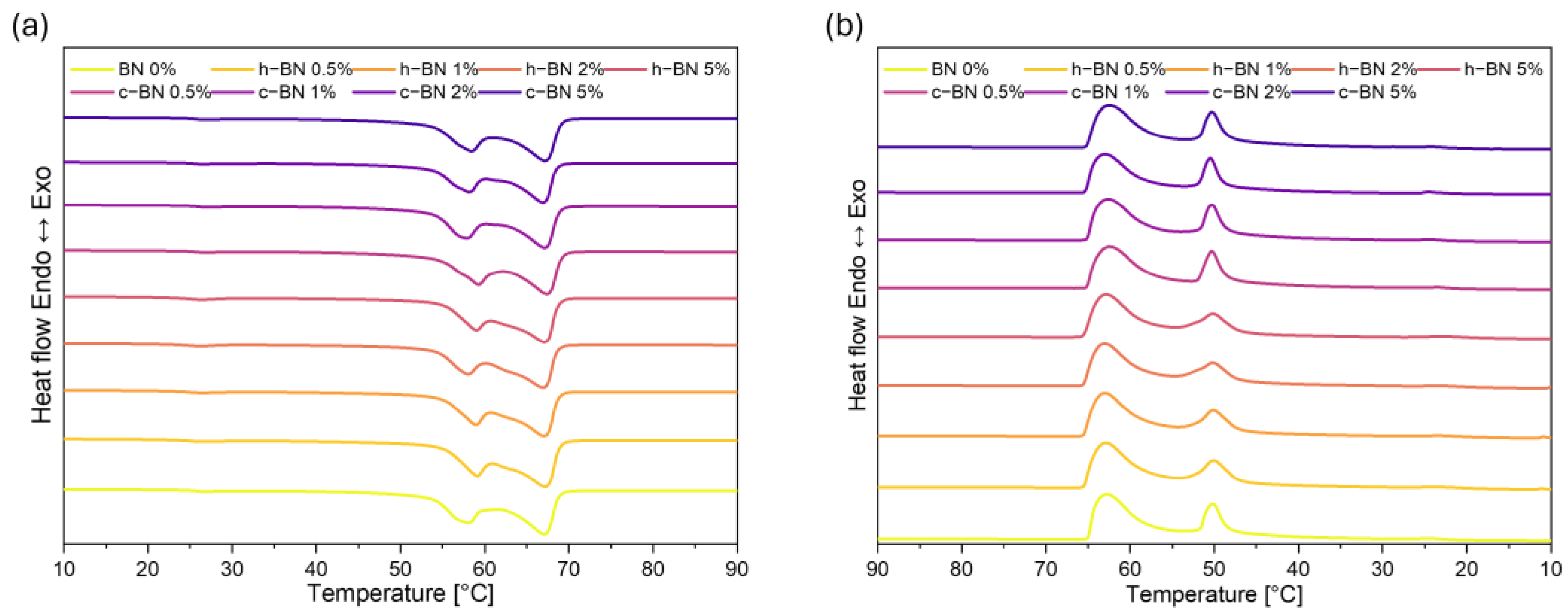
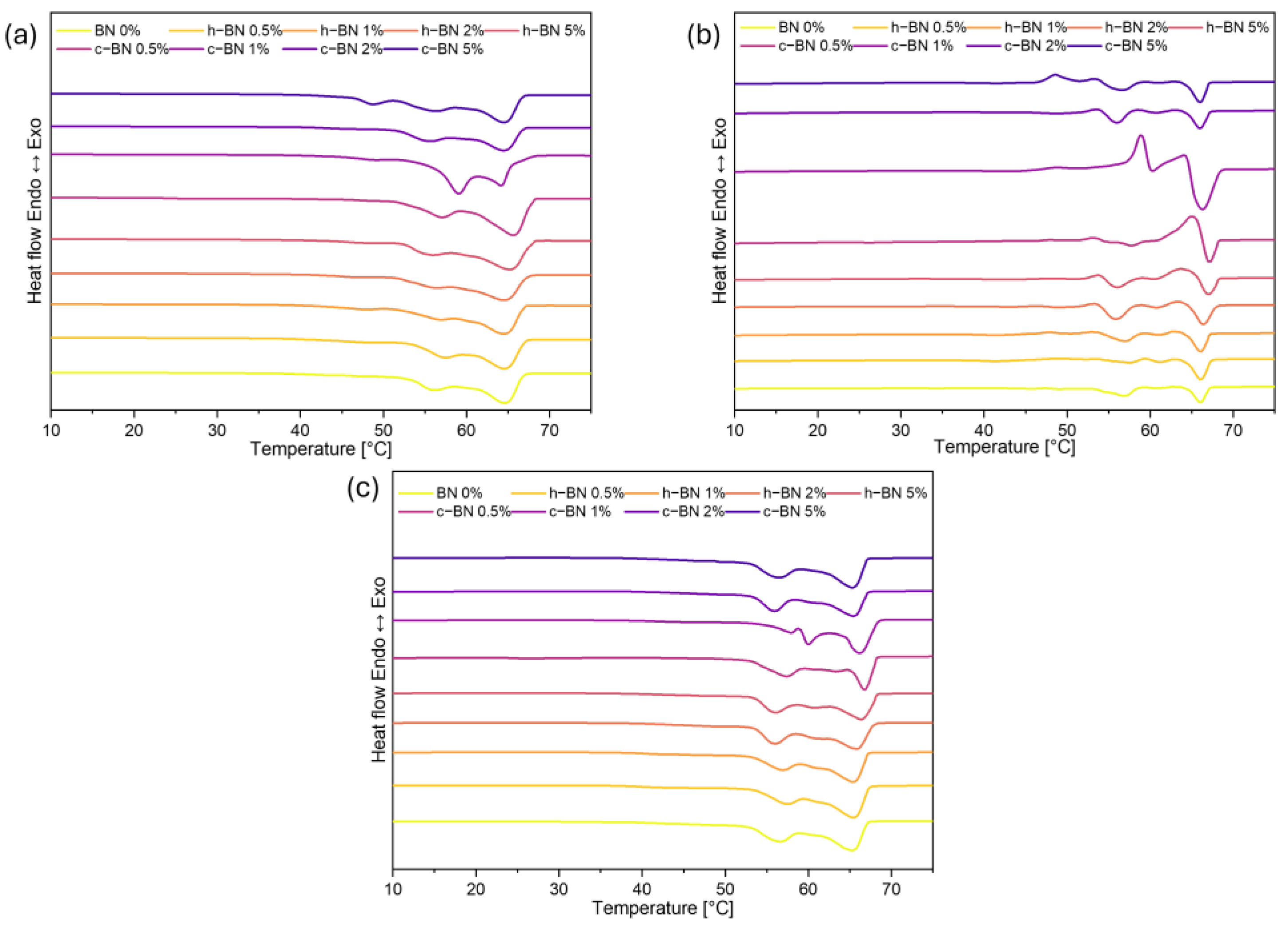
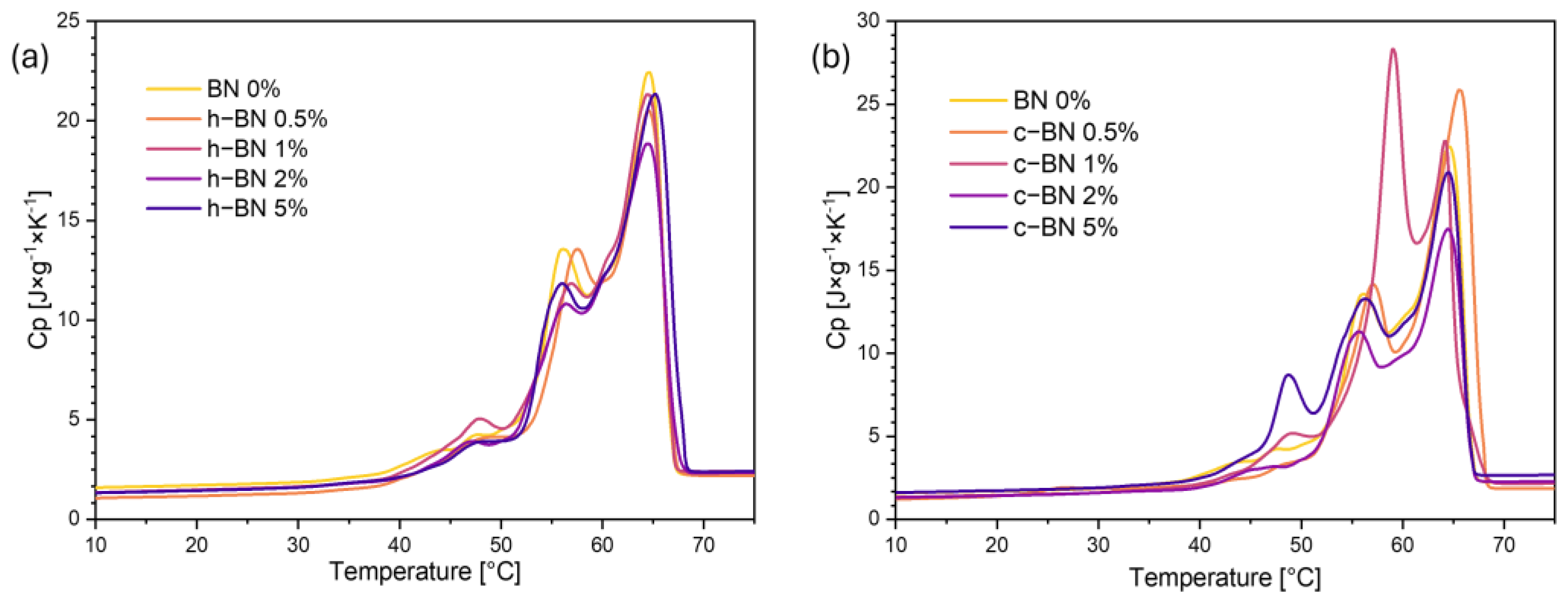
- Samples are characterized by high enthalpies of phase transitions, which is crucial in thermal energy storage. The addition of boron nitride did not cause changes in the temperatures of phase transitions; however, in most samples, the heat of phase transitions dropped with the increased amount of the modificator. The step-mode DSC confirmed that during phase change material can store a high amount of thermal energy (over 300 J/g) but in most samples, the amount of stored heat dropped with the addition of the boron nitride. Therefore, it is crucial to select the right amount of modifier that will improve some other properties without causing too much heat loss.
- The determination of the colour parameters confirmed a clear change in the colour of the TPCM mixtures after the reversible processes of melting and solidification. The incorporation of boron nitride into samples caused the change in their colours (positive in the case of the hexagonal BN as the difference in these colours is greater and negative for the cubic one, because the colour difference is smaller). However, the thermochromic properties of the obtained materials are still satisfactory, since the colour change during the phase transition is visible.
- The LFA results confirmed that the addition of the boron nitride improved the thermal conductivity by almost 30% for samples with 5 mass% BN.
- Consequently, obtained thermochromic phase change materials exhibit great potential for thermal energy storage applications with additional indicator possibilities. They are characterized by high enthalpy of phase change enthalpy, good thermochromic reversible properties, and satisfactory thermal conductivity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.-W.; Wu, N.; Fan, Y.-Y.; Sun, X.-C.; Song, T.-T.; Zhong, Q. Shape-stabilized thermochromic phase-change materials. J. Thermophys. Heat Transf. 2018, 32, 269–272. [Google Scholar] [CrossRef]
- Wu, Z.; Zhai, X.; Rui, Z.; Xu, X.; Wang, C.; Peng, H. Thermochromic microencapsulated phase change materials for cold energy storage application in vaccine refrigerator. J. Energy Storage 2023, 73, 109027. [Google Scholar] [CrossRef]
- Li, Y.; Yang, A.; Li, Y.; Jiang, Z.; He, F.; Chen, Z.; Li, X.; Said, Z.; Shehzad, N.; Waqas, A.; et al. TC@MF phase change microcapsules with reversibly thermochromic property for temperature response and thermoregulation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677 Pt A, 132333. [Google Scholar] [CrossRef]
- Lin, Y.; Jia, Y.; Alva, G.; Fang, G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew. Sustain. Energy Rev. 2018, 82, 2730–2742. [Google Scholar] [CrossRef]
- Liu, L.; Su, D.; Tang, Y.; Fang, G. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2016, 62, 305–317. [Google Scholar] [CrossRef]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2020, 25, 251–295. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ali, H.M.; Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: A review. Int. J. Heat Mass Transf. 2018, 127 Pt C, 838–856. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Xin, Z. Thermal properties of paraffin based composites containing multi-walled carbon nanotubes. Thermochim. Acta 2009, 488, 39–42. [Google Scholar] [CrossRef]
- Bing, N.; Yang, J.; Gao, H.; Xie, H.; Yu, W. Unsaturated polyester resin supported form-stable phase change materials with enhanced thermal conductivity for solar energy storage and conversion. Renew. Energy 2021, 173, 926–933. [Google Scholar] [CrossRef]
- Fang, X.; Fan, L.-W.; Ding, Q.; Yao, X.-L.; Wu, Y.-Y.; Hou, J.-F.; Wang, X.; Yu, Z.-T.; Cheng, G.-H.; Hu, Y.-C. Thermal energy storage performance of paraffin-based composite phase change materials filled with hexagonal boron nitride nanosheets. Energy Convers. Manag. 2014, 80, 103–109. [Google Scholar] [CrossRef]
- Yung, K.C.; Liem, H. Enhanced thermal conductivity of boron nitride epoxy-matrix composite through multi-modal particle size mixing. J. Appl. Polym. Sci. 2007, 106, 3587–3591. [Google Scholar] [CrossRef]
- Wei, X.; Xue, F.; Qi, X.-D.; Yang, J.-H.; Zhou, Z.-W.; Yuan, Y.-P.; Wang, Y. Photo- and electro-responsive phase change materials based on highly anisotropic microcrystalline cellulose/graphene nanoplatelet structure. Appl. Energy 2019, 236, 70–80. [Google Scholar] [CrossRef]
- Wang, B.; Li, G.; Xu, L.; Liao, J.; Zhang, X. Nanoporous Boron Nitride Aerogel Film and Its Smart Composite with Phase Change Materials. ACS Nano 2020, 14, 16590–16599. [Google Scholar] [CrossRef] [PubMed]
- Günther, E.; Hiebler, S.; Mehling, H.; Redlich, R. Enthalpy of phase change materials as a function of temperature: Required accuracy and suitable measurement methods. Int. J. Thermophys. 2009, 30, 1257–1269. [Google Scholar] [CrossRef]
- Ibraheem, N.A.; Hasan, M.M.; Khan, R.Z.; Mishra, P.K. Understanding Color Models: A Review. ARPN J. Sci. Technol. 2012, 2, 265–275. [Google Scholar]
- Parker, W.J.; Jenkins, R.J.; Butler, C.P.; Abbott, G.L. Flash method of determining thermal diffusivity, heat capacity, and thermal conductivity. J. Appl. Phys. 1961, 32, 1679–1684. [Google Scholar] [CrossRef]
- Yüksel, N. The Review of Some Commonly Used Methods and Techniques to Measure the Thermal Conductivity of Insulation Materials. In Insulation Materials in Context of Sustainability; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Raditoiu, V.; Eugenia, W.L.; Nicolae, C.-A. Thermochromic Systems based on Complexes of Some Triarylmethane Dyes. Rev. Chim. 2013, 64, 147–151. [Google Scholar]
- Kimura, F.; Umemura, J.; Takenaka, T. FTIR-ATR Studies on Langmuir-Blodgett Films of Stearic Acid with 1–9 Monolayers. Langmuir 1986, 2, 96–101. [Google Scholar] [CrossRef]
- Pudney, P.; Mutch, K.; Zhu, S. Characterising the phase behaviour of stearic acid and its triethanolamine soap and acid–soap by infrared spectroscopy. Phys. Chem. Chem. Phys. 2009, 11, 5010–5018. [Google Scholar] [CrossRef]
- Ao, C.; Yan, S.; Zhao, X.; Zhang, N.; Wu, Y. Stearic acid/boron nitride as a composite phase change material for thermal energy storage. Diam. Relat. Mater. 2023, 133, 109671. [Google Scholar] [CrossRef]
- Su, D.; Jia, Y.; Alva, G.; Tang, F.; Fang, G. Preparation and thermal properties of n–octadecane/stearic acid eutectic mixtures with hexagonal boron nitride as phase change materials for thermal energy storage. Energy Build. 2016, 131, 35–41. [Google Scholar] [CrossRef]
- Zuo, P.; Liu, Z.; Zhang, H.; Dai, D.; Fu, Z.; Corker, J.; Fan, M. Formulation and phase change mechanism of Capric acid/Octadecanol binary composite phase change materials. Energy 2023, 270, 126943. [Google Scholar] [CrossRef]
- Sarı, A.; Sarı, H.; Önal, A. Thermal properties and thermal reliability of eutectic mixtures of some fatty acids as latent heat storage materials. Energy Convers. Manag. 2004, 45, 365–376. [Google Scholar] [CrossRef]
- Gandolfo, F.G.; Bot, A.; Flöter, E. Phase diagram of mixtures of stearic acid and stearyl alcohol. Thermochim. Acta 2003, 404, 9–17. [Google Scholar] [CrossRef]
- Fraga, I.; Montserrat, S.; Hutchinson, J. TOPEM, a new temperature modulated DSC technique Application to the glass transition of polymers. J. Therm. Anal. Calorim. 2007, 87, 119–124. [Google Scholar] [CrossRef]
- Ma, B.; Zhou, X.-Y.; Liu, J.; You, Z.; Wei, K.; Huang, X.-F. Determination of specific heat capacity on composite shape-stabilized phase change materials and asphalt mixtures by heat exchange system. Materials 2016, 9, 389. [Google Scholar] [CrossRef]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12.e1. [Google Scholar] [CrossRef] [PubMed]
- Rumyantsev, S.L.; Levinshtein, M.E.; Shur, M.S. Properties of Advanced Semiconductor Materials: GaN, AIN, InN, BN, SiC, SiG; John Wiley & Sons: Hoboken, NJ, USA, 2001; pp. 67–91. [Google Scholar]
- Kim, H.; Hong, J.; Choi, H.; Oh, J.; Lee, H. Development of PCM-based shell-and-tube thermal energy storages for efficient EV thermal management. Int. Commun. Heat Mass Transf. 2024, 154, 107401. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A.; Kaygusuz, K. Thermal conductivity improvement of stearic acid using expanded graphite and carbon fiber for energy storage applications. Renew. Energy 2007, 32, 2201–2210. [Google Scholar] [CrossRef]
- Yang, J.; Tang, L.-S.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Largely enhanced thermal conductivity of poly (ethylene glycol)/boron nitride composite phase change materials for solar-thermal-electric energy conversion and storage with very low content of graphene nanoplatelets. Chem. Eng. J. 2017, 315, 481–490. [Google Scholar] [CrossRef]
- Wong, T.L.; Vallés, C.; Nasser, A.; Abeykoon, C. A critical experimental evaluation of hexagonal boron nitride, graphene oxide and graphite as thermally conductive fillers in organic PCMs. J. Energy Storage 2023, 72, 108523. [Google Scholar] [CrossRef]


| Sample Name | Bromocresol Purple [-] | Stearic Acid [-] | Behenyl Alcohol [-] | h-BN [Mass %] | c-BN [Mass %] |
|---|---|---|---|---|---|
| BN 0% | 1 | 10 | 40 | 0 | 0 |
| h−BN 0.5% | 1 | 10 | 40 | 0.5 | 0 |
| h−BN 1% | 1 | 10 | 40 | 1 | 0 |
| h−BN 2% | 1 | 10 | 40 | 2 | 0 |
| h−BN 5% | 1 | 10 | 40 | 5 | 0 |
| c−BN 0.5% | 1 | 10 | 40 | 0 | 0.5 |
| c−BN 1% | 1 | 10 | 40 | 0 | 1 |
| c−BN 2% | 1 | 10 | 40 | 0 | 2 |
| c−BN 5% | 1 | 10 | 40 | 0 | 5 |
| Sample Name | Diameter [mm] | Thickness [mm] |
|---|---|---|
| BN 0% | 12.8 | 2.09 |
| h−BN 0.5% | 12.52 | 1.94 |
| h−BN 1% | 12.51 | 1.81 |
| h−BN 2% | 12.85 | 1.85 |
| h−BN 5% | 12.48 | 1.67 |
| c−BN 0.5% | 12.46 | 1.57 |
| c−BN 1% | 12.50 | 2.09 |
| c−BN 2% | 12.30 | 1.83 |
| c−BN 5% | 12.38 | 1.70 |
| BN 0% | h–BN 0.5% | h–BN 1% | h–BN 2% | h–BN 5% | c–BN 0.5% | c–BN 1% | c–BN 2% | c–BN 5% | |
|---|---|---|---|---|---|---|---|---|---|
| 3322 | 3322 | 3319 | 3323 | 3325 | 3320 | 3320 | 3320 | 3321 | OH stretching (alcohol) |
| 2924 | 2919 | 2918 | 2921 | 2919 | 2919 | 2921 | 2924 | 2921 | CH2 asymmetric stretching |
| 2849 | 2849 | 2849 | 2849 | 2849 | 2849 | 2849 | 2849 | 2849 | CH2 symmetric stretching |
| 1734 | 1734 | 1734 | 1734 | 1734 | 1734 | 1734 | 1734 | 1734 | stretching vibrations of C=O (stearic acid) |
| 1705 | 1705 | 1705 | 1705 | 1705 | 1705 | 1705 | 1705 | 1705 | |
| 1472 | 1473 | 1473 | 1473 | 1473 | 1473 | 1472 | 1472 | 1472 | methylene C-H bend/C=C-C aromatic ring stretch |
| - | - | 1377 | 1377 | 1377 | - | - | - | - | h-BN |
| 1296 | 1296 | 1296 | 1296 | 1296 | 1296 | 1296 | 1296 | 1296 | C(O)-O stretching vibrations (stearic acid) |
| 1177 | 1178 | 1178 | 1177 | 1176 | 1177 | 1178 | 1177 | 1177 | structure of the benzene sulphonate group, suggesting that the ester ring group of BCP is opened |
| 1063 | 1063 | 1063 | 1063 | 1062 | 1063 | 1063 | 1063 | 1063 | |
| - | 809 | 811 | 814 | 813 | - | - | - | - | h-BN |
| 720 | 720 | 720 | 720 | 719 | 720 | 720 | 720 | 720 | OH out-of-plane bend (alcohol) |
| Sample Code | T1% | T2% | T3% | T10% | T50% | TDTGmax1 | TDTGmax2 | Mresid |
|---|---|---|---|---|---|---|---|---|
| [°C] | [°C] | [°C] | [°C] | [°C] | [°C] | [°C] | [%] | |
| BN 0% | 172 | 184 | 191 | 213 | 251 | 259 | 319 | 0.185 |
| h–BN 0.5% | 170 | 182 | 189 | 212 | 253 | 261 | 317 | 0.830 |
| h–BN 1% | 173 | 184 | 190 | 211 | 248 | 257 | 301 | 1.370 |
| h–BN 2% | 177 | 190 | 197 | 221 | 265 | 266 | 324 | 2.017 |
| h–BN 5% | 168 | 180 | 188 | 209 | 252 | 256 | 321 | 5.441 |
| c–BN 0.5% | 178 | 190 | 196 | 220 | 260 | 267 | 326 | 0.284 |
| c–BN 1% | 172 | 183 | 190 | 210 | 246 | 255 | 300 | 0.412 |
| c–BN 2% | 176 | 189 | 196 | 220 | 264 | 265 | 338 | 1.315 |
| c–BN 5% | 180 | 193 | 200 | 223 | 263 | 271 | 312 | 0.735 |
| stearic acid | 180 | 191 | 198 | 221 | 258 | 274 | - | 0.146 |
| behenyl alcohol | 185 | 196 | 203 | 228 | 270 | 287 | - | 0.055 |
| bromocresol purple | 244 | 247 | 249 | 256 | 557 | 257 | - | 48.409 |
| Sample Code | Tmonset [°C] | Tm1 [°C] | Tm2 [°C] | Tmendset [°C] | ΔTm [°C] | Heat of Fusion [J/g] | Tsonset [°C] | Ts1 [°C] | Ts2 [°C] | Tsendset [°C] | ΔTs [°C] | Heat of Crystallization [J/g] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BN 0% | 54 | 58 | 66 | 69 | 15 | 194.22 | 65 | 63 | 51 | 48 | 17 | 186.06 |
| h–BN 0.5% | 54 | 59 | 67 | 69 | 14 | 185.90 | 66 | 63 | 51 | 47 | 19 | 178.27 |
| h–BN 1% | 55 | 59 | 66 | 69 | 14 | 186.97 | 66 | 64 | 50 | 47 | 19 | 179.89 |
| h–BN 2% | 54 | 58 | 66 | 69 | 15 | 175.89 | 66 | 64 | 50 | 47 | 19 | 169.90 |
| h–BN 5% | 55 | 59 | 66 | 69 | 14 | 179.06 | 66 | 63 | 50 | 47 | 19 | 172.72 |
| c–BN 0.5% | 54 | 59 | 67 | 69 | 16 | 184.30 | 65 | 63 | 51 | 45 | 20 | 177.98 |
| c–BN 1% | 53 | 57 | 66 | 69 | 16 | 189.83 | 65 | 63 | 51 | 45 | 20 | 182.73 |
| c–BN 2% | 53 | 58 | 66 | 68 | 16 | 163.33 | 66 | 63 | 51 | 45 | 21 | 160.30 |
| c–BN 5% | 53 | 58 | 67 | 69 | 16 | 192.34 | 65 | 63 | 51 | 45 | 20 | 186.71 |
| stearic acid | 68 | – | 71 | 75 | 7 | 201.77 | 68 | 67 | – | 62 | 5 | 207.51 |
| behenyl alcohol | 70 | – | 72 | 76 | 6 | 233.16 | 70 | 68 | – | 61 | 9 | 232.92 |
| Sample Code | Stored Heat [J/g] |
|---|---|
| BN 0% | 334.86 |
| h–BN 0.5% | 329.55 |
| h–BN 1% | 330.93 |
| h–BN 2% | 330.70 |
| h–BN 5% | 310.55 |
| c–BN 0.5% | 344.52 |
| c–BN 1% | 337.31 |
| c–BN 2% | 304.41 |
| c–BN 5% | 359.87 |
| Sample | 25 °C | 75 °C | ΔE | ||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | ||
| BN 0% | 36.00 | 50.75 | 27.75 | 40.25 | 55.00 | 42.00 | 15.47 |
| h–BN 0.5% | 46.50 | 52.75 | 26.50 | 55.50 | 36.25 | 38.00 | 22.03 |
| h–BN 1% | 49.00 | 52.75 | 24.50 | 61.00 | 33.75 | 34.25 | 24.50 |
| h–BN 2% | 51.00 | 50.75 | 24.75 | 68.25 | 22.75 | 33.75 | 34.10 |
| h–BN 5% | 56.75 | 42.75 | 16.00 | 72.75 | 16.00 | 19.25 | 31.34 |
| c–BN 0.5% | 31.00 | 42.50 | 23.75 | 26.00 | 35.50 | 28.25 | 9.71 |
| c–BN 1% | 29.25 | 39.75 | 23.75 | 25.75 | 29.50 | 25.50 | 10.97 |
| c–BN 2% | 30.00 | 40.00 | 23.25 | 21.75 | 22.50 | 19.50 | 19.71 |
| c–BN 5% | 28.25 | 28.75 | 16.75 | 30.00 | 15.25 | 19.25 | 13.84 |
| Sample Name | Density [g/cm3] | Specific Heat Capacity [J/g*K] | Thermal Diffusivity [mm2/s] |
|---|---|---|---|
| BN 0% | 0.872 | 1.694 | 0.171 |
| h–BN 0.5% | 0.879 | 1.690 | 0.191 |
| h–BN 1% | 0.885 | 1.687 | 0.196 |
| h–BN 2% | 0.898 | 1.677 | 0.203 |
| h–BN 5% | 0.935 | 1.652 | 0.210 |
| c–BN 0.5% | 0.885 | 1.686 | 0.216 |
| c–BN 1% | 0.898 | 1.686 | 0.196 |
| c–BN 2% | 0.924 | 1.675 | 0.177 |
| c–BN 5% | 0.997 | 1.648 | 0.198 |
| Sample Code | Thermal Conductivity [W/m*K] |
|---|---|
| BN 0% | 0.252 |
| h–BN 0.5% | 0.284 |
| h–BN 1% | 0.313 |
| h–BN 2% | 0.306 |
| h–BN 5% | 0.324 |
| c–BN 0.5% | 0.324 |
| c–BN 1% | 0.297 |
| c–BN 2% | 0.274 |
| c–BN 5% | 0.326 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paprota, N.; Szumera, M.; Pielichowska, K. The Impact of Boron Nitride Additive on Thermal and Thermochromic Properties of Organic Thermochromic Phase Change Materials. Materials 2024, 17, 3632. https://doi.org/10.3390/ma17153632
Paprota N, Szumera M, Pielichowska K. The Impact of Boron Nitride Additive on Thermal and Thermochromic Properties of Organic Thermochromic Phase Change Materials. Materials. 2024; 17(15):3632. https://doi.org/10.3390/ma17153632
Chicago/Turabian StylePaprota, Natalia, Magdalena Szumera, and Kinga Pielichowska. 2024. "The Impact of Boron Nitride Additive on Thermal and Thermochromic Properties of Organic Thermochromic Phase Change Materials" Materials 17, no. 15: 3632. https://doi.org/10.3390/ma17153632





