Trimethylsilane Plasma-Nanocoated Silver Nanowires for Improved Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. AgNW Preparation
2.2. Plasma Nanocoating Deposition
2.3. Plasma Coating Thickness Assessment
2.4. Sheet Resistance and Optical Transmittance Measurement
2.5. Surface Wettability Evaluation
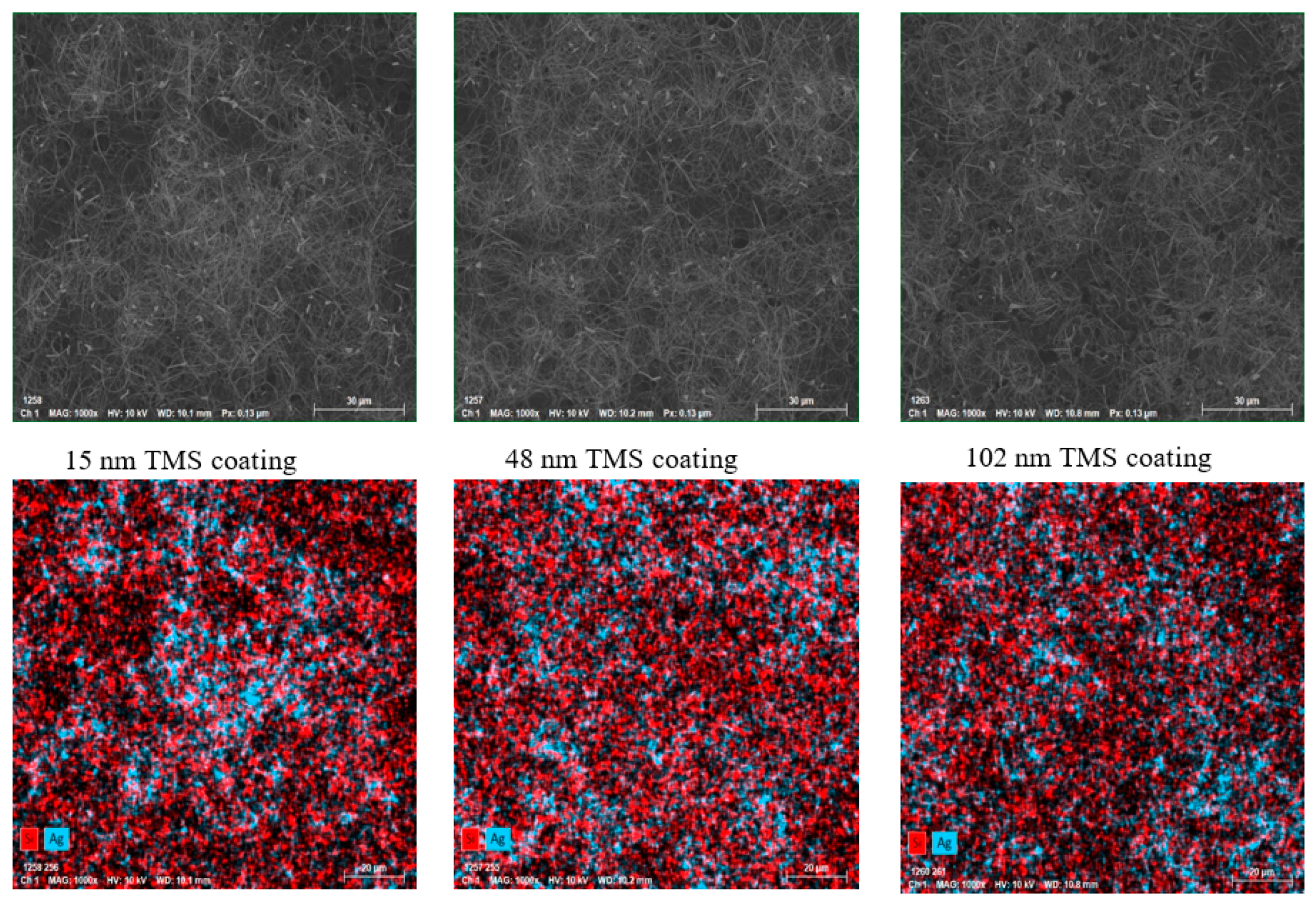
2.6. SEM and EDS Analysis
2.7. Statistical Analysis
3. Results
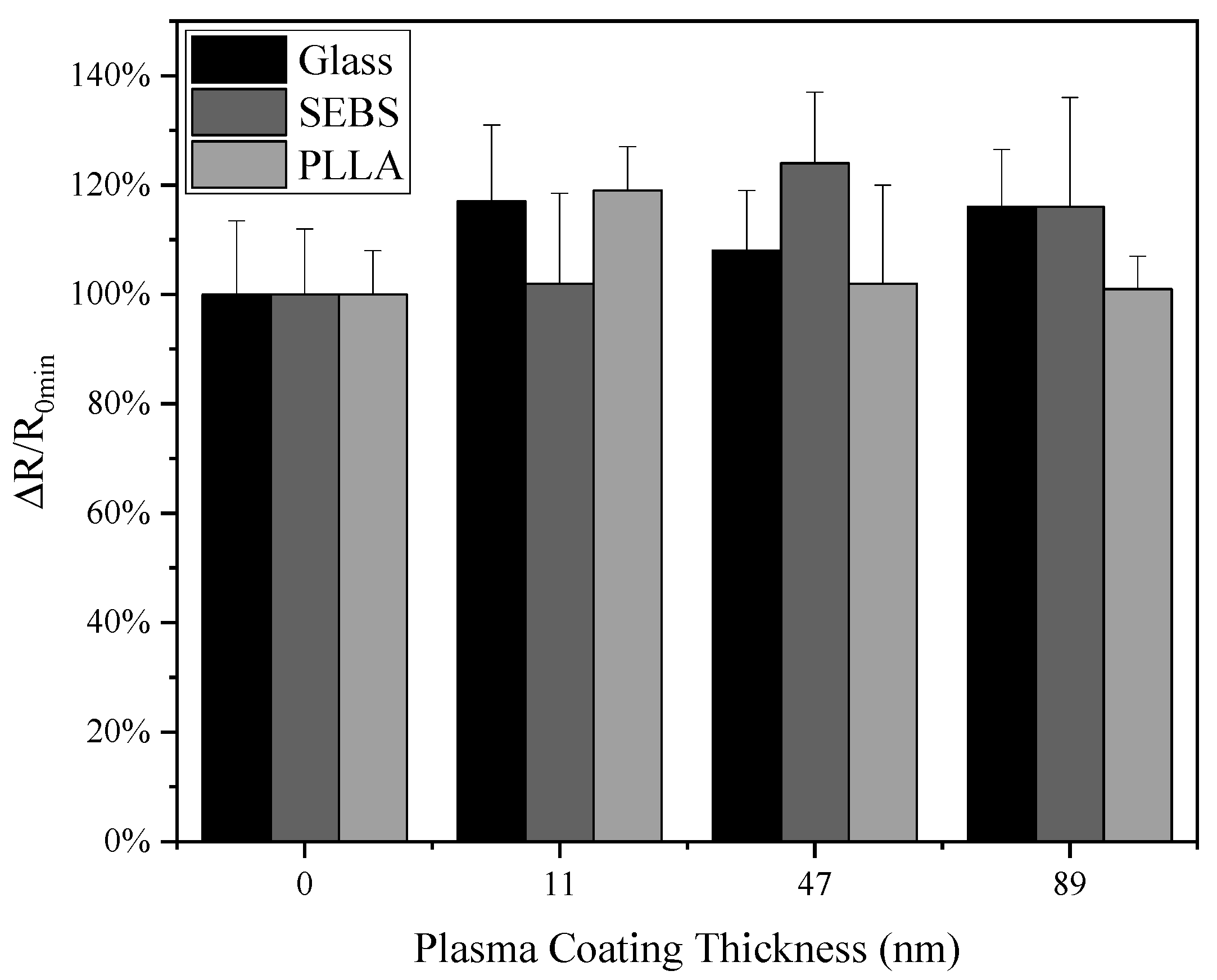
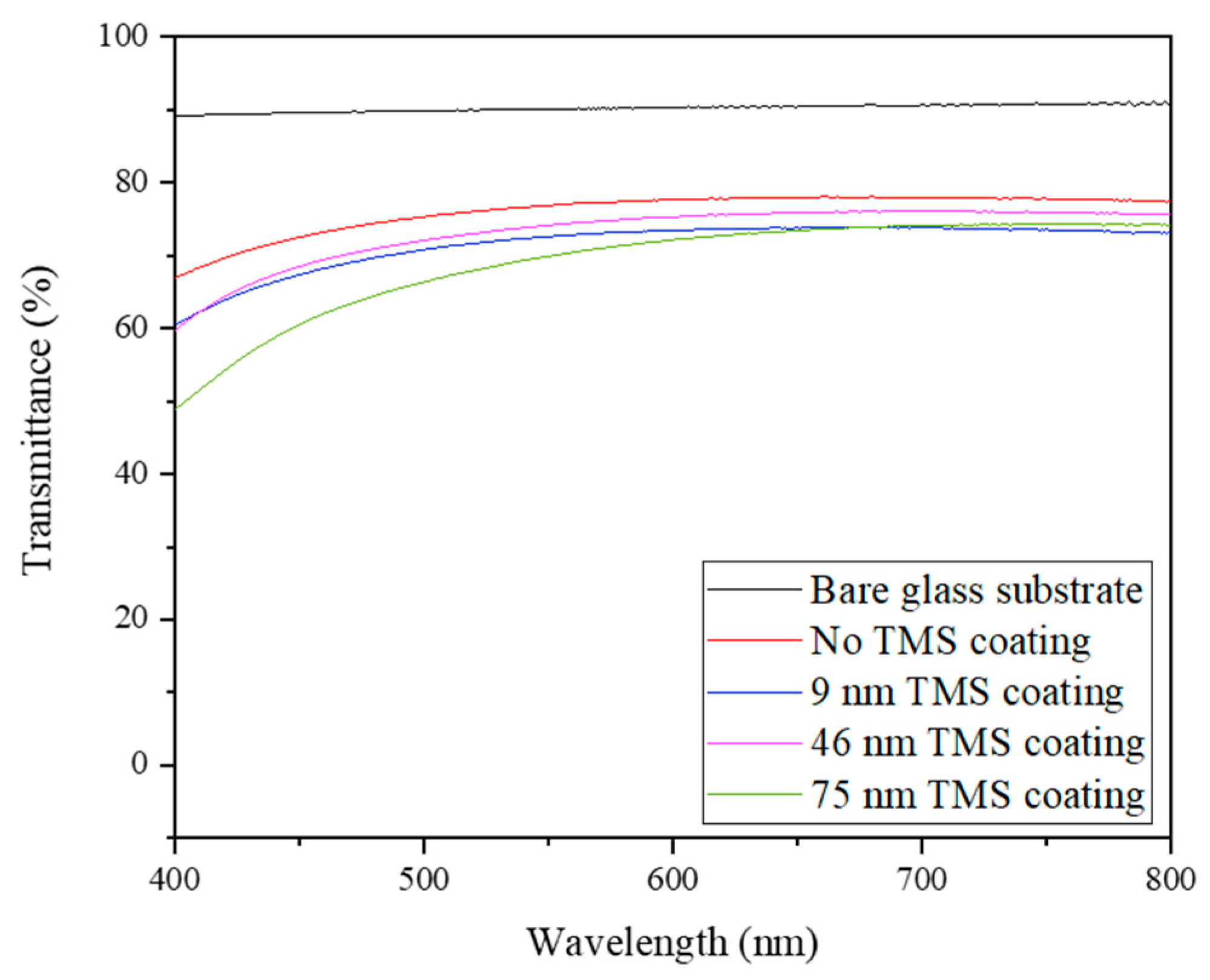
3.1. Resistance and Transmittance of AgNWs Right after TMS Plasma Nanocoating Deposition
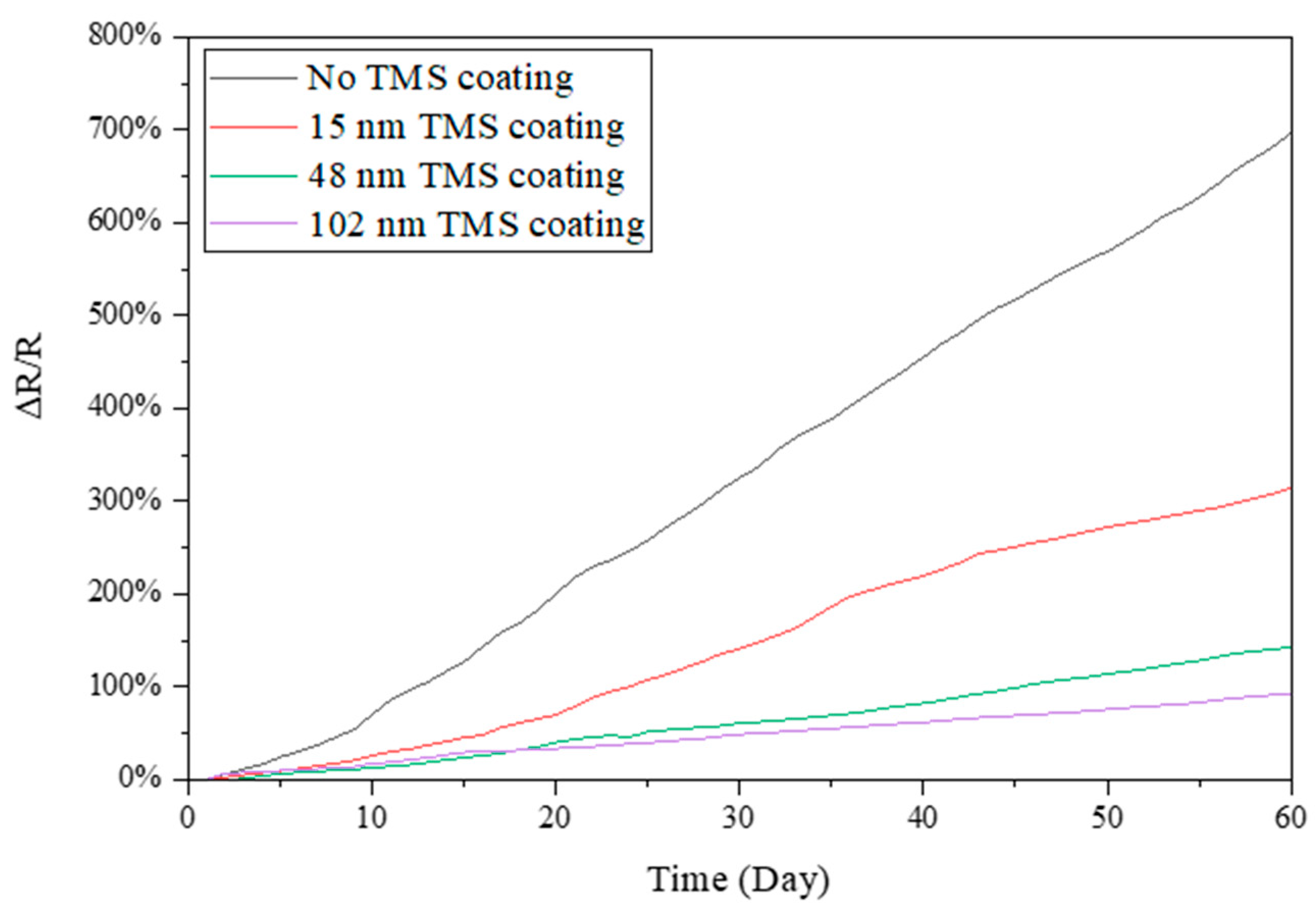
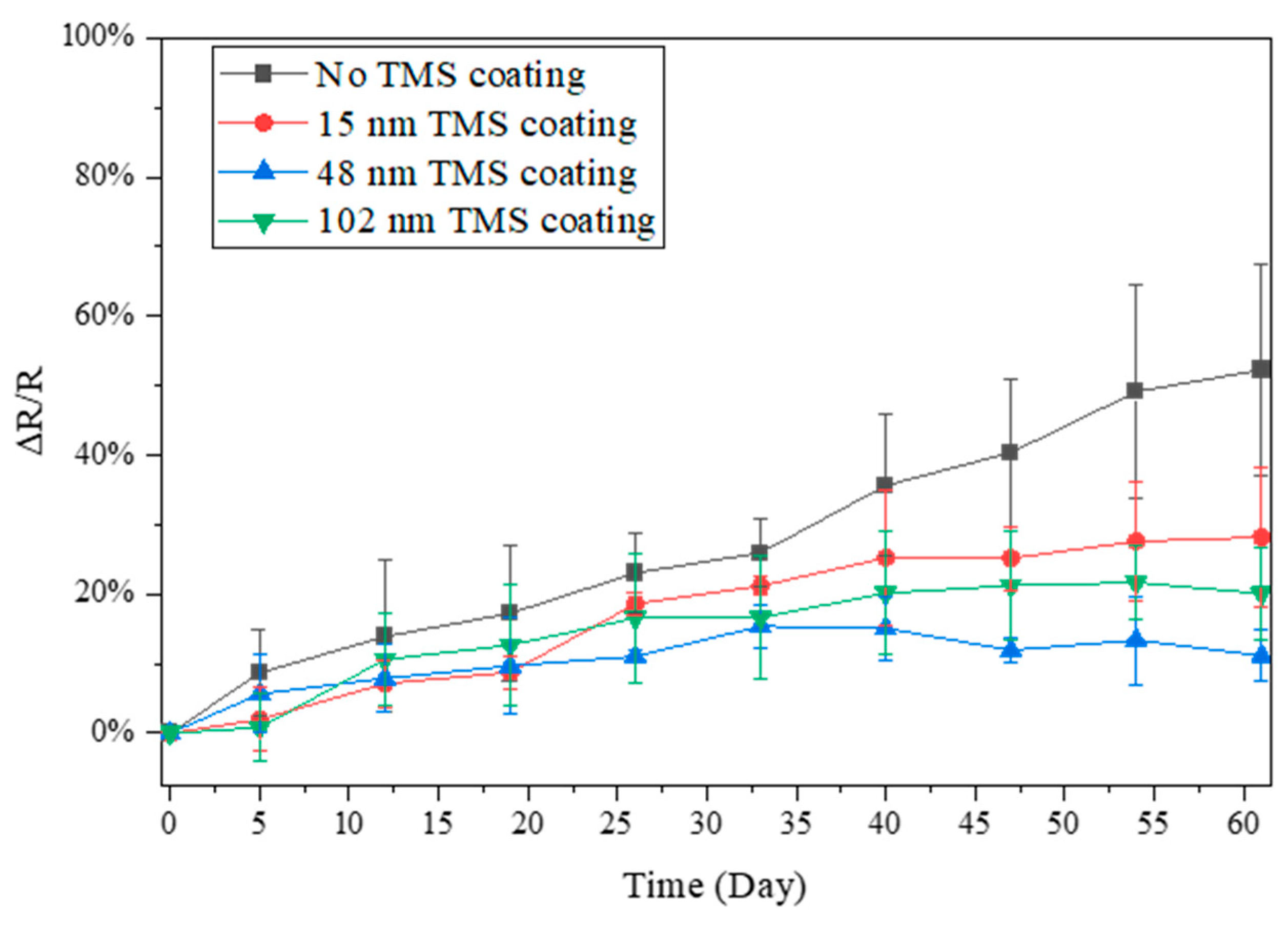
3.2. Electrical Resistance Stability in Summer Season
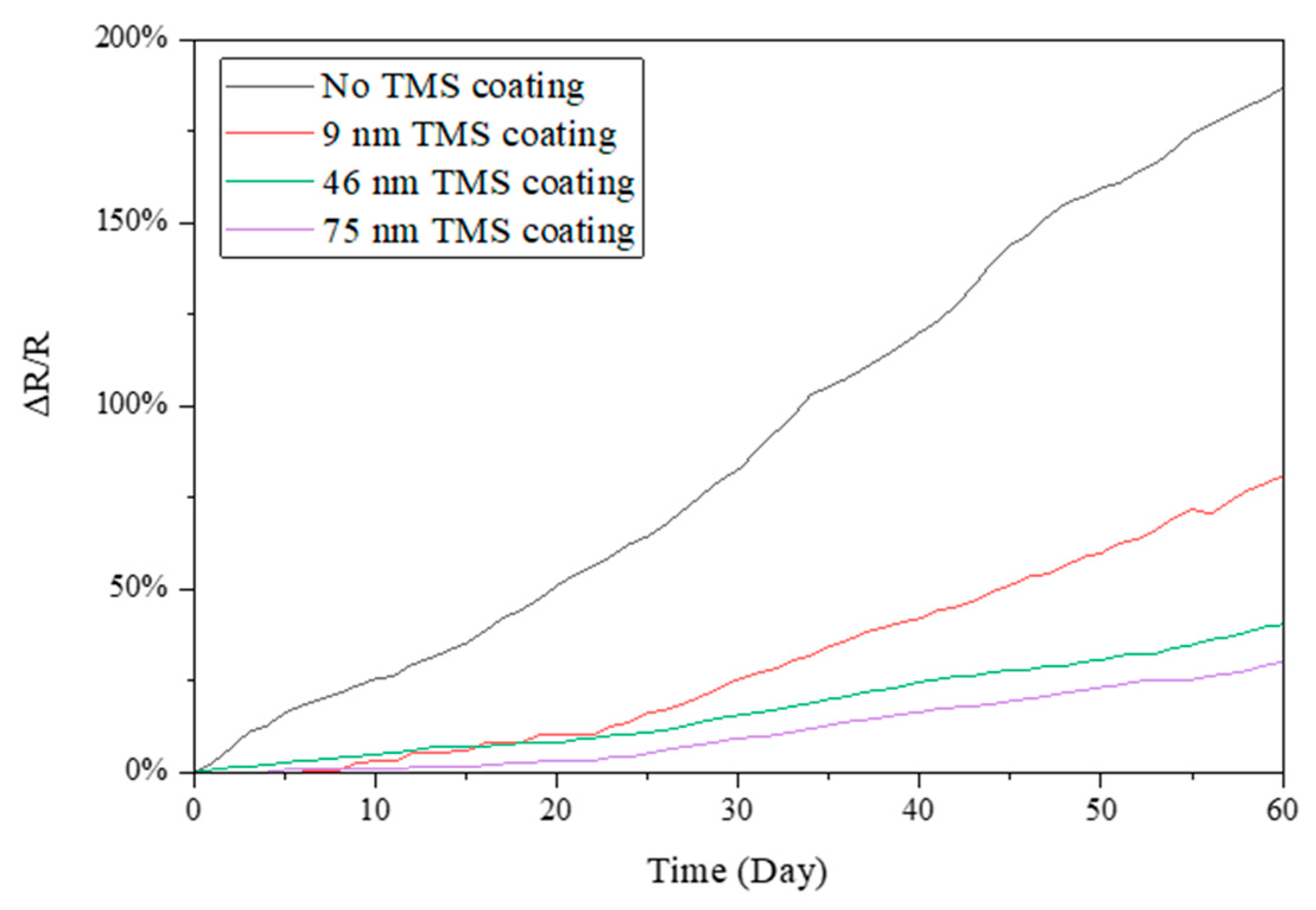
3.3. Electrical Resistance Stability in Winter Season
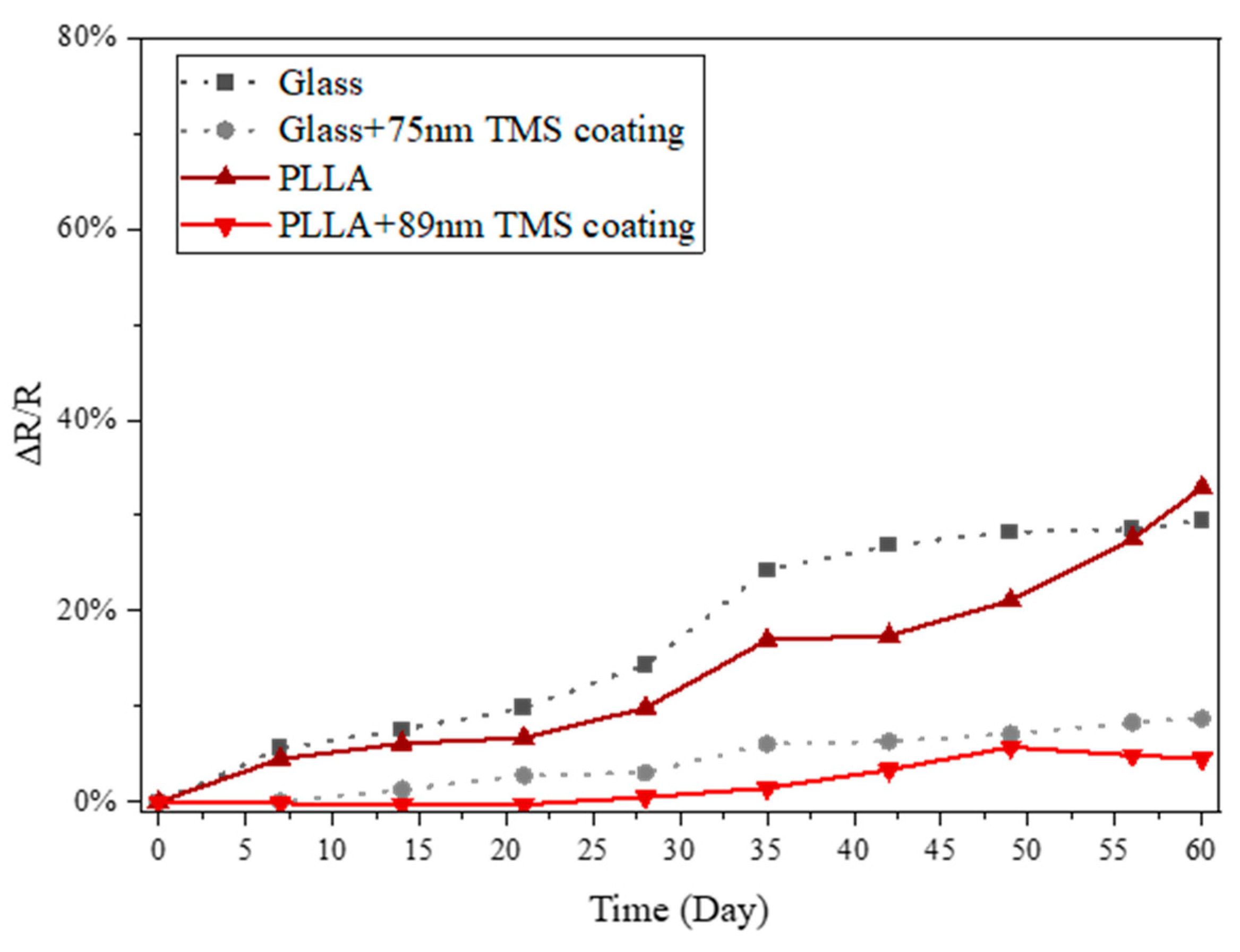
3.4. Humidity Influence
3.5. Surface Morphology of TMS-Coated AgNWs on SEBS
3.6. Surface Wettability Change in TMS Plasma-Nanocoated AgNWs
4. Discussion
4.1. Effects of TMS Plasma Nanocoatings on AgNWs
4.2. Surface Protection of AgNWs for Long-Term Stability
4.3. Factors Affecting the Stability of AgNWs
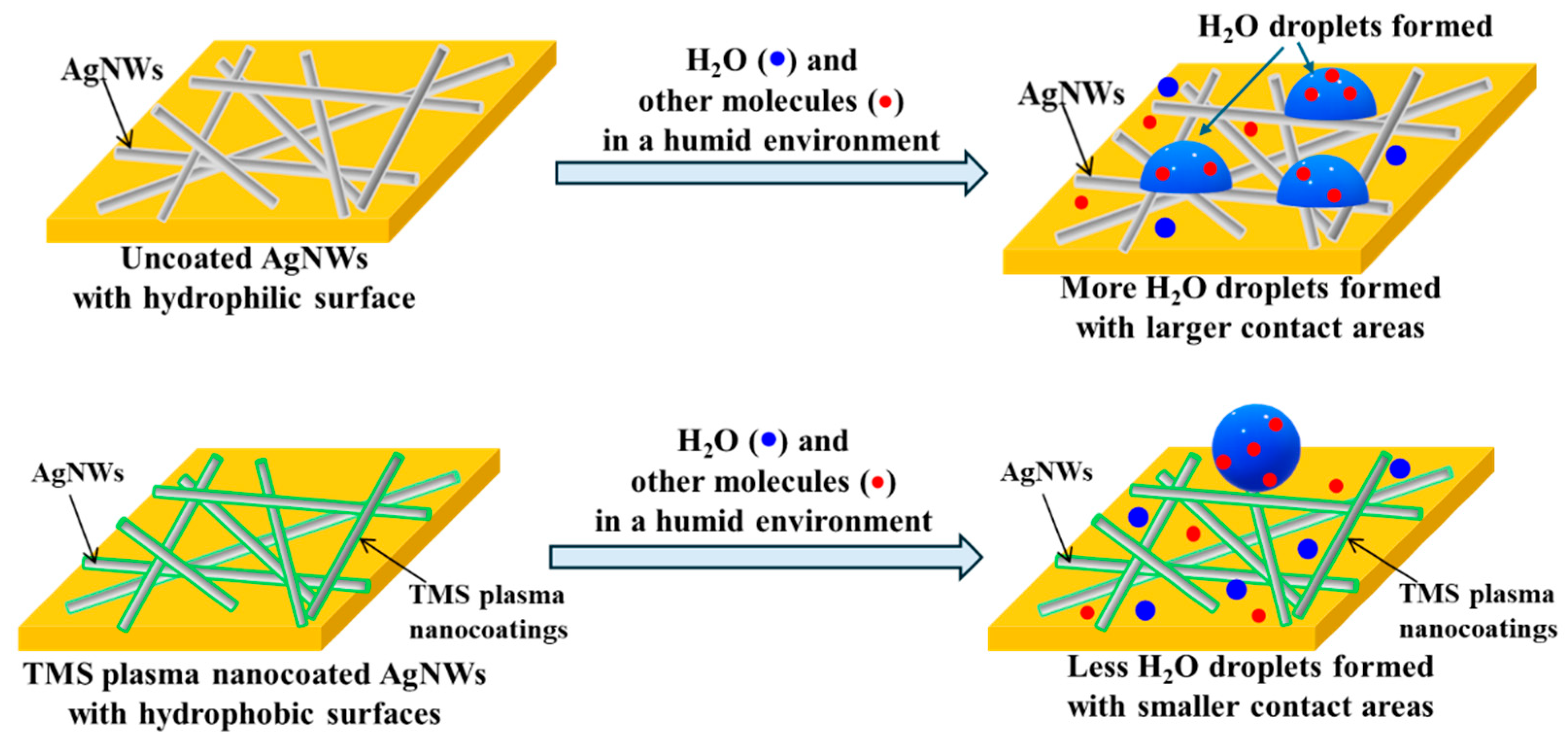
4.4. Protection Mechanism of AgNWs by TMS Plasma Nanocoatings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.-E.; Kim, S.; Lee, D.-Y.; Kim, E.; Hwang, J. Fabrication of silver nanowire transparent electrodes using electrohydrodynamic spray deposition for flexible organic solar cells. J. Mater. Chem. A 2013, 1, 14286–14293. [Google Scholar] [CrossRef]
- Netzer, N.L.; Gunawidjaja, R.; Hiemstra, M.; Zhang, Q.; Tsukruk, V.V.; Jiang, C. Formation and Optical Properties of Compression-Induced Nanoscale Buckles on Silver Nanowires. ACS Nano 2009, 3, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Yoshioka, A.; Naito, T.; Cuya, J.; Ido, Y.; Okawa, R.; Jeyadevan, B.; Yamaguchi, H. Field induced anisotropic thermal conductivity of silver nanowire dispersed-magnetic functional fluid. Exp. Therm. Fluid Sci. 2016, 79, 111–117. [Google Scholar] [CrossRef]
- Kobler, A.; Beuth, T.; Klöffel, T.; Prang, R.; Moosmann, M.; Scherer, T.; Walheim, S.; Hahn, H.; Kübel, C.; Meyer, B.; et al. Nanotwinned silver nanowires: Structure and mechanical properties. Acta Mater. 2015, 92, 299–308. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, J.; Jin, C.; Peng, L.M.; Tang, D.; Cheng, H. In situ electrical measurements of polytypic silver nanowires. Nanotechnology 2008, 19, 085711. [Google Scholar]
- Cheng, D.; Kim, W.Y.; Min, S.K.; Nautiyal, T.; Kim, K.S. Magic structures and quantum conductance of [110] silver nanowires. Phys. Rev. Lett. 2006, 96, 096104. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Chang, M.-H.; Lee, E.-J.; Ihm, D.-W.; Kim, J.-Y. Improved electrical conductivity of PEDOT-based electrode films hybridized with silver nanowires. Synth. Met. 2014, 195, 69–74. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Li, R.; Jiang, G.; Yu, H.; Chen, T. Effect of silver nanowires on electrical conductance of system composed of silver particles. J. Mater. Sci. 2007, 42, 3172–3176. [Google Scholar] [CrossRef]
- Fahad, S.; Yu, H.; Wang, L.; Abdin, Z.U.; Haroon, M.; Ullah, R.S.; Nazir, A.; Naveed, K.-U.; Elshaarani, T.; Khan, A. Recent progress in the synthesis of silver nanowires and their role as conducting materials. J. Mater. Sci. 2018, 54, 997–1035. [Google Scholar] [CrossRef]
- Yang, L.; Huang, X.; Wu, H.; Liang, Y.; Ye, M.; Liu, W.; Li, F.; Xu, T.; Wang, H. Silver Nanowires: From Synthesis, Growth Mechanism, Device Fabrications to Prospective Engineered Applications. Eng. Sci. 2023, 23, 808. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.-Y.; Peumans, P.; Cui, Y. Scalable Coating and Properties of Transparent, Flexible, Silver Nanowire Electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Rathmell, A.R.; Nguyen, M.; Chi, M.; Wiley, B.J. Synthesis of Oxidation-Resistant Cupronickel Nanowires for Transparent Conducting Nanowire Networks. Nano Lett. 2012, 12, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.Y.; Chung, M.H.; Kim, J.; Kim, J.H. A one-step roll-to-roll process of stable AgNW/PEDOT: PSS solution using imidazole as a mild base for highly conductive and transparent films: Optimizations and mechanisms. J. Mater. Chem. C 2015, 3, 5859–5868. [Google Scholar] [CrossRef]
- Ye, S.; Rathmell, A.R.; Chen, Z.; Stewart, I.E.; Wiley, B.J. Metal Nanowire Networks: The Next Generation of Transparent Conductors. Adv. Mater. 2014, 26, 6670–6687. [Google Scholar] [CrossRef] [PubMed]
- Dao, V.H.; Mapleback, B.J. Generation of highly porous silver nanowire networks by plasma treatment and their direct application as supercapacitor electrodes. Nanoscale 2020, 12, 11868–11877. [Google Scholar] [CrossRef] [PubMed]
- Tokuno, T.; Nogi, M.; Karakawa, M.; Jiu, J.; Nge, T.T.; Aso, Y.; Suganuma, K. Fabrication of silver nanowire transparent electrodes at room temperature. Nano Res. 2011, 4, 1215–1222. [Google Scholar] [CrossRef]
- Ahn, Y.; Jeong, Y.; Lee, Y. Improved Thermal Oxidation Stability of Solution-Processable Silver Nanowire Transparent Electrode by Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2012, 4, 6410–6414. [Google Scholar] [CrossRef]
- Khaligh, H.H.; Liew, K.; Han, Y.; Abukhdeir, N.M.; Goldthorpe, A.I. Silver nanowire transparent electrodes for liquid crystal-based smart windows. Sol. Energy Mater. Sol. Cells 2015, 132, 337–341. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, B.; Ling, Y.; Fei, Q.; Chen, Z.; Li, X.; Guo, P.; Jeon, N.; Goswami, S.; Liao, Y.; et al. Multiscale porous elastomer substrates for multifunctional on-skin electronics with passive-cooling capabilities. Proc. Natl. Acad. Sci. USA 2020, 117, 205–213. [Google Scholar] [CrossRef]
- Im, H.-G.; Jung, S.-H.; Jin, J.; Lee, D.; Lee, J.; Lee, D.; Lee, J.-Y.; Kim, I.-D.; Bae, B.-S. Flexible Transparent Conducting Hybrid Film Using a Surface-Embedded Copper Nanowire Network: A Highly Oxidation-Resistant Copper Nanowire Electrode for Flexible Optoelectronics. ACS Nano 2014, 8, 10973–10979. [Google Scholar] [CrossRef]
- Deng, B.; Hsu, P.C.; Chen, G.C.; Chandrashekar, B.N.; Liao, L.; Ayitimuda, Z.; Wu, J.X.; Guo, Y.F.; Lin, L.; Zhou, Y.; et al. Roll-to-Roll Encapsulation of Metal Nanowires between Graphene and Plastic Substrate for High-Performance Flexible Transparent Electrodes. Nano Lett. 2015, 15, 4206–4213. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lee, J.; Jeong, S.; Yang, S.; Ko, J.-H.; Im, H.-G.; Baek, S.-W.; Lee, J.-Y.; Bae, B.-S. High-performance hybrid plastic films: A robust electrode platform for thin-film optoelectronics. Energy Environ. Sci. 2013, 6, 1811–1817. [Google Scholar] [CrossRef]
- Liu, C.-H.; Yu, X. Silver nanowire-based transparent, flexible, and conductive thin film. Nanoscale Res. Lett. 2011, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-T.; Huang, S.-X. Transparent conductive silver nanowire electrodes with high resistance to oxidation and thermal shock. RSC Adv. 2014, 4, 59226–59232. [Google Scholar] [CrossRef]
- Yang, K.C.; Sung, D.I.; Shin, Y.J.; Yeom, G.Y. Highly oxidation-resistant silver nanowires by CxFy polymers using plasma treatment. Nanotechnology 2019, 30, 285702. [Google Scholar]
- Li, J.; Tao, Y.; Chen, S.; Li, H.; Chen, P.; Wei, M.-Z.; Wang, H.; Li, K.; Mazzeo, M.; Duan, Y. A flexible plasma-treated silver-nanowire electrode for organic light-emitting devices. Sci. Rep. 2017, 7, 16468. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lai, W.; Feng, F.; Huang, W. Post-Treatment of Screen-Printed Silver Nanowire Networks for Highly Conductive Flexible Transparent Films. Adv. Mater. Interfaces 2021, 8, 2100548. [Google Scholar] [CrossRef]
- Jiu, J.; Wang, J.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; Suganuma, K.; Hara, M.; Nakazawa, E.; Uchida, H. The effect of light and humidity on the stability of silver nanowire transparent electrodes. RSC Adv. 2015, 5, 27657–27664. [Google Scholar] [CrossRef]
- Jin, Y.; Deng, D.; Cheng, Y.; Kong, L.; Xiao, F. Annealing-free and strongly adhesive silver nanowire networks with long-term reliability by introduction of a nonconductive and biocompatible polymer binder. Nanoscale 2014, 6, 4812–4818. [Google Scholar] [CrossRef]
- Choi, D.Y.; Kang, H.W.; Sung, H.J.; Kim, S.S. Annealing-free, flexible silver nanowire–polymer composite electrodes via a continuous two-step spray-coating method. Nanoscale 2013, 5, 977–983. [Google Scholar] [CrossRef]
- Zilberberg, K.; Gasse, F.; Pagui, R.; Polywka, A.; Behrendt, A.; Trost, S.; Heiderhoff, R.; Görrn, P.; Riedl, T. Highly Robust Indium-Free Transparent Conductive Electrodes Based on Composites of Silver Nanowires and Conductive Metal Oxides. Adv. Funct. Mater. 2013, 24, 1671–1678. [Google Scholar] [CrossRef]
- Mayousse, C.; Celle, C.; Fraczkiewicz, A.; Simonato, J.-P. Stability of silver nanowire based electrodes under environmental and electrical stresses. Nanoscale 2014, 7, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Lee, K.; Kim, S.Y.; Lee, H.; Park, J.; Choi, K.H.; Kim, H.K.; Kim, D.G.; Lee, D.Y.; Nam, S.; et al. High-performance, transparent, and stretchable electrodes using graphene–metal nanowire hybrid structures. Nano Lett. 2013, 13, 2814–2821. [Google Scholar] [CrossRef]
- Liang, J.; Li, L.; Tong, K.; Ren, Z.; Hu, W.; Niu, X.; Chen, Y.; Pei, Q. Silver Nanowire Percolation Network Soldered with Graphene Oxide at Room Temperature and Its Application for Fully Stretchable Polymer Light-Emitting Diodes. ACS Nano 2014, 8, 1590–1600. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.; Ahn, Y.; Jeong, Y.; Lee, D.Y.; Lee, Y. Highly stable and flexible silver nanowire–graphene hybrid transparent conducting electrodes for emerging optoelectronic devices. Nanoscale 2013, 5, 7750–7755. [Google Scholar] [CrossRef]
- Kleber, C.; Wiesinger, R.; Schnöller, J.; Hilfrich, U.; Hutter, H.; Schreiner, M. Initial oxidation of silver surfaces by S2−and S4+ species. Corros. Sci. 2008, 50, 1112–1121. [Google Scholar] [CrossRef]
- Andrieux-Ledier, A.; Tremblay, B.; Courty, A. Stability of self-ordered thiol-coated silver nanoparticles: Oxidative environment effects. Langmuir 2013, 29, 13140–13145. [Google Scholar] [CrossRef]
- Graedel, T.; Franey, J.; Gualtieri, G.; Kammlott, G.; Malm, D. On the mechanism of silver and copper sulfidation by atmospheric H2S and OCS. Corros. Sci. 1985, 25, 1163–1180. [Google Scholar] [CrossRef]
- Levard, C.; Reinsch, B.C.; Michel, F.M.; Oumahi, C.; Lowry, G.V.; Brown, G.E. Sulfidation Processes of PVP-Coated Silver Nanoparticles in Aqueous Solution: Impact on Dissolution Rate. Environ. Sci. Technol. 2011, 45, 5260–5266. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Colman, B.P.; Dale, A.L.; Truong, L.; Yang, X.Y.; Bone, A.J.; Brown, G.E.; Tanguay, R.L.; Di Giulio, R.T.; et al. Sulfidation of Silver Nanoparticles: Natural Antidote to Their Toxicity. Environ. Sci. Technol. 2013, 47, 13440–13448. [Google Scholar] [CrossRef]
- Elechiguerra, J.; Gutierrez, D.; Camacho-Bragado, A.; Yacaman, M.J. Corrosion at the nanoscale: The case of silver nanowires and nanoparticles. Chem. Mater. 2005, 17, 6042–6052. [Google Scholar] [CrossRef]
- Chan, Y.; Yu, Q. Electrochemical characterization of plasma polymer coatings in corrosion protection of aluminum alloys. J. Vac. Sci. Technol. A 2005, 23, 991–997. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Zhao, G.; Ling, Y.; Yan, Z.; Yu, Q. Trimethylsilane Plasma-Nanocoated Silver Nanowires for Improved Stability. Materials 2024, 17, 3635. https://doi.org/10.3390/ma17153635
Liao Y, Zhao G, Ling Y, Yan Z, Yu Q. Trimethylsilane Plasma-Nanocoated Silver Nanowires for Improved Stability. Materials. 2024; 17(15):3635. https://doi.org/10.3390/ma17153635
Chicago/Turabian StyleLiao, Yixuan, Ganggang Zhao, Yun Ling, Zheng Yan, and Qingsong Yu. 2024. "Trimethylsilane Plasma-Nanocoated Silver Nanowires for Improved Stability" Materials 17, no. 15: 3635. https://doi.org/10.3390/ma17153635




