Rapid, Selective, and Ultra-Sensitive Field Effect Transistor-Based Detection of Escherichia coli
Abstract
:1. Introduction
| Biomarker | LOD | Range | Detection Time | Reference |
|---|---|---|---|---|
| Carcinoembryonic antigen (CEA) | 72 ag/mL | 1 fg/mL–1 ng/mL | N/A | [43] |
| CA125 breast cancer biomarker | 0.01 U/mL | 0.01–1000 U/mL | ~20 min | [44] |
| Prostate-specific antigen (PSA) | 1 fg/mL | 1 fg/mL–100 ng/mL | ~2 min | [45] |
| SARS-CoV-2 S1 antigen | 4.12 fg/mL | 0.1 fg/mL–5.0 pg/mL | ~2–3 min | [46] |
2. Experimental Section
2.1. Materials
2.2. Fabrication of BN-rGO FETs
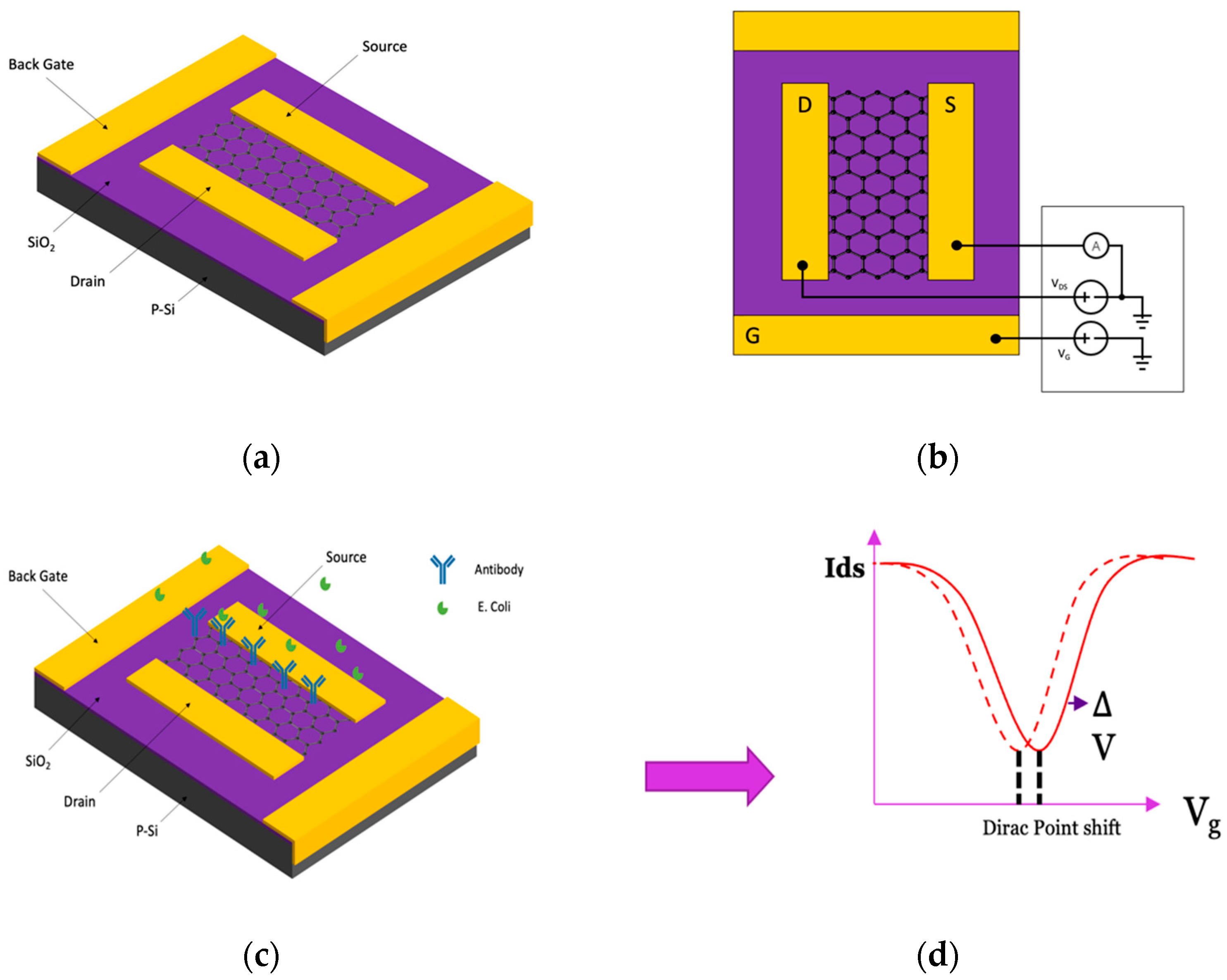
2.3. Working Principle of BN-rGO FET Biosensor
2.4. Antibody Functionalization and Device Passivation
2.5. Immunodetection
2.6. Device Configuration
3. Results and Discussion
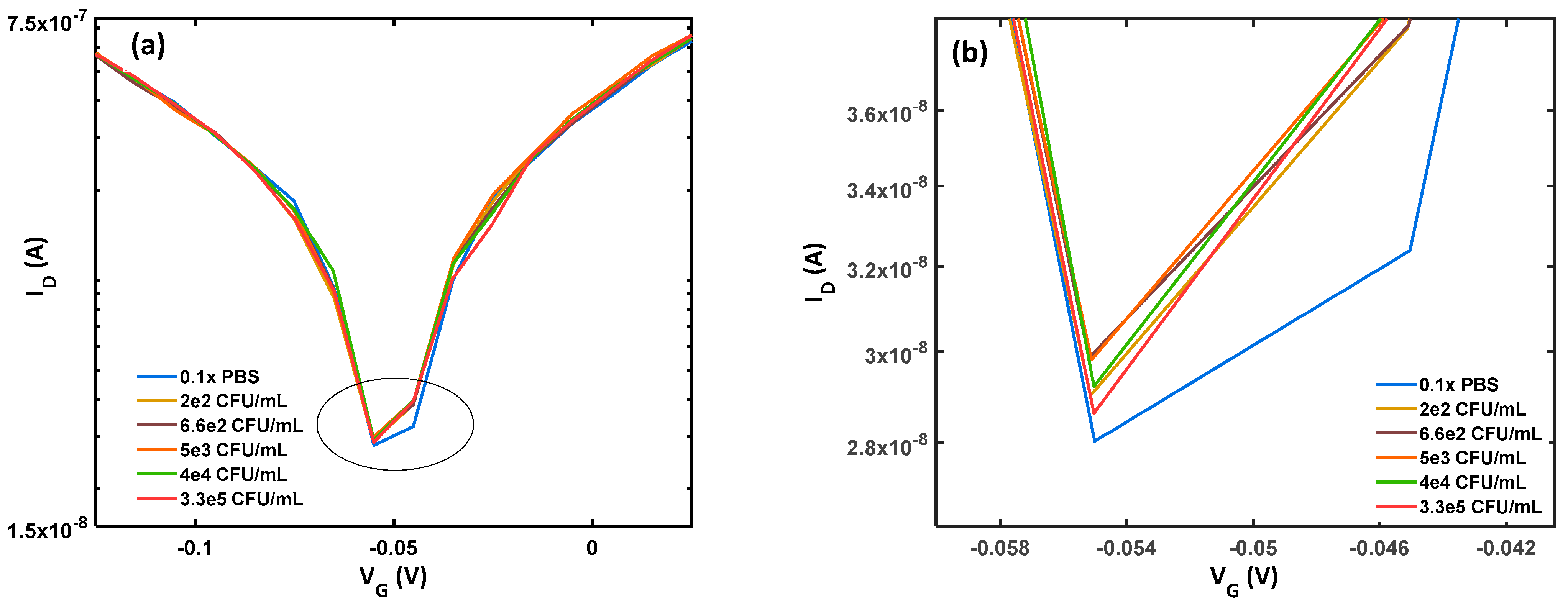
3.1. Sensing Performance
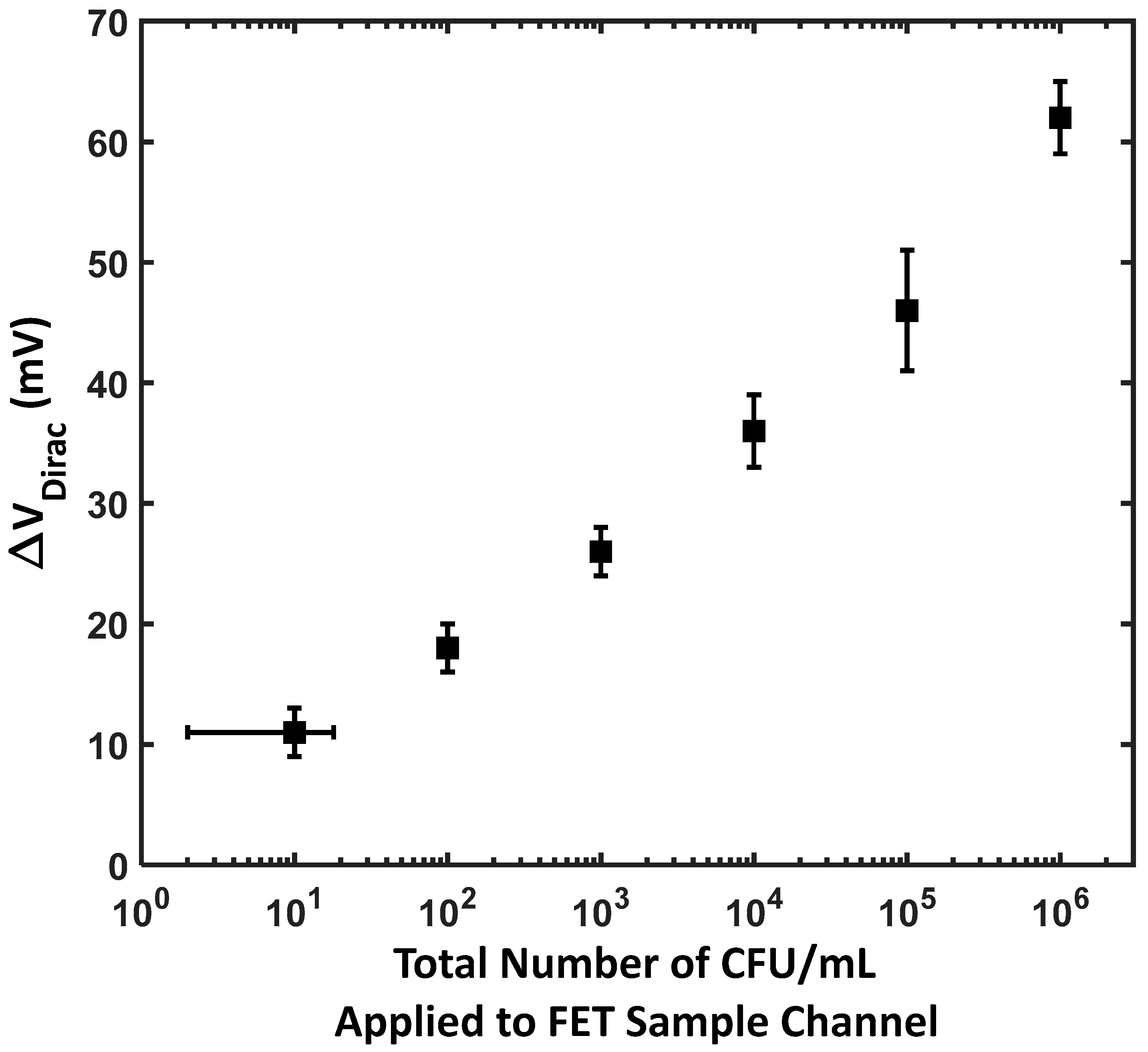
3.2. Sensitivity
3.3. Selectivity and Specificity
3.4. Performance Compared to the State of the Art
| Method | LOD (CFU/mL) | Range (CFU/mL) | Sample | Detection Time | Reference |
|---|---|---|---|---|---|
| Carbon dots–Fe3O4 nanomaterial | 6.88 | 10–108 | Milk and water | ~35–40 min | [28] |
| Portable microfluidic biosensor with finger actuation | 10 | 102–108 | Buffer | ~2.5 h | [27] |
| rGO-based field-effect transistor | 1.4 | 1.4–1.47 | Buffer | ~46 s | [26] |
| Graphene-based field-effect transistor | 1 | 1–107 | River water | <3 min | [29] |
| BN-GO gel-functionalized field-effect transistor | 10 | 10–108 | Buffer | <2 min | This work |
4. Conclusions and Future Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costigan, C.; Raftery, T.; Carroll, A.G.; Wildes, D.; Reynolds, C.; Cunney, R.; Dolan, N.; Drew, R.J.; Lynch, B.J.; O’Rourke, D.J.; et al. Neurological involvement in children with hemolytic uremic syndrome. Eur. J. Pediatr. 2022, 181, 501–512. [Google Scholar] [CrossRef] [PubMed]
- WHO. E. coli. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/e-coli (accessed on 22 May 2024).
- Magnus, T.; Röther, J.; Simova, O.; Meier-Cillien, M.; Repenthin, J.; Möller, F.; Gbadamosi, J.; Panzer, U.; Wengenroth, M.; Hagel, C.; et al. The neurological syndrome in adults during the 2011 northern German E. coli serotype O104:H4 outbreak. Brain 2012, 135, 1850–1859. [Google Scholar] [CrossRef] [PubMed]
- Trachtman, H.; Austin, C.; Lewinski, M.; Stahl, R.A.K. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat. Rev. Nephrol. 2012, 8, 658–669. [Google Scholar] [CrossRef] [PubMed]
- WHO. Outbreak of Haemolytic Uraemic Syndrome in Germany. 2011. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2011_05_27-en (accessed on 22 May 2024).
- Vanesse, P.; Georgery, H.; Duprez, T.; Gerard, L.; Collienne, C.; Verroken, A.; Crombé, F.; Morelle, J.; Hantson, P. Severe Neurological Involvement in an Adult with Shiga Toxin-Producing Escherichia coli-Hemolytic Uremic Syndrome Treated with Eculizumab. Case Rep. Nephrol. Dial. 2023, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Public Health Notice—United States Outbreak of E. coli Infections Linked to Romaine Lettuce with Implications for Canadians—Canada.ca. 2019. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2019/outbreak-united-states-e-coli-infections-romaine-lettuce.html (accessed on 22 May 2024).
- di Toma, A.; Brunetti, G.; Chiriacò, M.S.; Ferrara, F.; Ciminelli, C. A Novel Hybrid Platform for Live/Dead Bacteria Accurate Sorting by On-Chip DEP Device. Int. J. Mol. Sci. 2023, 24, 7077. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, H.; Wang, W. Multiplex real-time PCR assay for detection of Escherichia coli O157:H7 and screening for non-O157 Shiga toxin-producing E. coli. BMC Microbiol. 2017, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.M.; Grieve, C.M. Detection and quantification of Escherichia coli O157:H7 in environmental samples by real-time PCR. J. Appl. Microbiol. 2003, 94, 421–431. [Google Scholar] [CrossRef]
- Chen, J.; Tang, J.; Liu, J.; Cai, Z.; Bai, X. Development and evaluation of a multiplex PCR for simultaneous detection of five foodborne pathogens. J. Appl. Microbiol. 2012, 112, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Hariri, S. Detection of Escherichia coli in Food Samples Using Culture and Polymerase Chain Reaction Methods. Cureus 2022, 14, e32808. [Google Scholar] [CrossRef]
- Blumenfeld, N.R.; Bolene, M.A.E.; Jaspan, M.; Ayers, A.G.; Zarrandikoetxea, S.; Freudman, J.; Shah, N.; Tolwani, A.M.; Hu, Y.; Chern, T.L.; et al. Multiplexed reverse-transcriptase quantitative polymerase chain reaction using plasmonic nanoparticles for point-of-care COVID-19 diagnosis. Nat. Nanotechnol. 2022, 17, 984–992. [Google Scholar] [CrossRef]
- Park, J.Y.; Lim, M.C.; Park, K.; Ok, G.; Chang, H.J.; Lee, N.; Park, T.J.; Choi, S.W. Detection of E. coli O157:H7 in Food Using Automated Immunomagnetic Separation Combined with Real-Time PCR. Processes 2020, 8, 908. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, D.; Yan, C.; Chen, W.; Ren, J.; Jiang, Y.; Jiang, L.; Xue, F.; Ji, D.; Tang, F.; et al. Rapid and accurate detection of Escherichia coli O157:H7 in beef using microfludic wax printed paper based Elisa. Analyst 2020, 145, 3106–3115. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Hou, N.; Jin, M.; Qiu, Z.; Wang, J.; Zhang, B.; Wang, X.; Wang, J.; Zhou, D.; Li, J. A novel enzyme-linked immunosorbent assay for detection of Escherichia coli O157:H7 using immunomagnetic and beacon gold nanoparticles. Gut Pathog. 2014, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Rooney, A.M.; Raphenya, A.R.; Melano, R.G.; Seah, C.; Yee, N.R.; MacFadden, D.R.; McArthur, A.G.; Schneeberger, P.H.H.; Coburn, B. Performance Characteristics of Next-Generation Sequencing for the Detection of Antimicrobial Resistance Determinants in Escherichia coli Genomes and Metagenomes. mSystems 2022, 7, e00022-22. [Google Scholar] [CrossRef] [PubMed]
- Manzanas, C.; Morrison, E.; Kim, Y.S.; Alipanah, M.; Adedokun, G.; Jin, S.; Osborne, T.Z.; Fan, Z.H. Molecular testing devices for on-site detection of E. coli in water samples. Sci. Rep. 2023, 13, 4245. [Google Scholar] [CrossRef] [PubMed]
- Daley, K.; Truelstrup Hansen, L.; Jamieson, R.C.; Hayward, J.L.; Piorkowski, G.S.; Krkosek, W.; Gagnon, G.A.; Castleden, H.; MacNeil, K.; Poltarowicz, J.; et al. Chemical and microbial characteristics of municipal drinking water supply systems in the Canadian Arctic. Environ. Sci. Pollut. Res. 2018, 25, 32926–32937. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Chang, Y.; Sawtelle, S.D.; Wipf, M.; Duan, X.; Reed, M.A. Silicon nanowire field-effect transistors—A versatile class of potentiometric nanobiosensors. IEEE Access 2015, 3, 287–302. [Google Scholar] [CrossRef]
- Kwong Hong Tsang, D.; Lieberthal, T.J.; Watts, C.; Dunlop, I.E.; Ramadan, S.; del Rio Hernandez, A.E.; Klein, N. Chemically Functionalised Graphene FET Biosensor for the Label-free Sensing of Exosomes. Sci. Rep. 2019, 9, 13946. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Moudgil, A.; Singh, S.; Mishra, N.; Mishra, P.; Das, S. MoS2/TiO2 Hybrid Nanostructure-Based Field-Effect Transistor for Highly Sensitive, Selective, and Rapid Detection of Gram-Positive Bacteria. Adv. Mater. Technol. 2020, 5, 1900615. [Google Scholar] [CrossRef]
- Vu, C.A.; Chen, W.Y. Field-Effect Transistor Biosensors for Biomedical Applications: Recent Advances and Future Prospects. Sensors 2019, 19, 4214. [Google Scholar] [CrossRef]
- Du, M.; Ma, J.; Zhang, Z.; Wu, G.; Wu, J.; Wang, H.; Xie, X.; Wang, C. Direct, ultrafast, and sensitive detection of environmental pathogenic microorganisms based on a graphene biosensor. Anal. Chim. Acta 2023, 1279, 341810. [Google Scholar] [CrossRef]
- Shang, Y.; Xing, G.; Liu, X.; Lin, H.; Lin, J.-M. Fully Integrated Microfluidic Biosensor with Finger Actuation for the Ultrasensitive Detection of Escherichia coli O157:H7. Anal. Chem. 2022, 94, 16787–16795. [Google Scholar] [CrossRef]
- Lin, X.; Mei, Y.; He, C.; Luo, Y.; Yang, M.; Kuang, Y.; Ma, X.; Zhang, H.; Huang, Q. Electrochemical Biosensing Interface Based on Carbon Dots-Fe3O4 Nanomaterial for the Determination of Escherichia coli O157:H7. Front. Chem. 2021, 9, 769648. [Google Scholar] [CrossRef]
- Wei, S.; Dou, Y.; Li, T. Ultra-sensitive and label-free detection of Escherichia coli O157:H7 using graphene-based field effect transistor modified with heat-denatured casein. Microchem. J. 2023, 193, 109049. [Google Scholar] [CrossRef]
- Waleed, M. Modeling and Simulation of Electrochemical DNA Biosensors in CMOS Technology. Master’s Thesis, King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia, 2007. [Google Scholar]
- MacKin, C.; Hess, L.H.; Hsu, A.; Song, Y.; Kong, J.; Garrido, J.A.; Palacios, T. A current-voltage model for graphene electrolyte-gated field-effect transistors. IEEE Trans. Electron Devices 2014, 61, 3971–3977. [Google Scholar] [CrossRef]
- Wu, G.; Meyyappan, M.; Lai, K.W.C. Simulation of Graphene Field-Effect Transistor Biosensors for Bacterial Detection. Sensors 2018, 18, 1715. [Google Scholar] [CrossRef]
- Kim, K.Y.; Park, B.G. Transient simulation of field-effect biosensors how to avoid charge screening effect. In Proceedings of the International Conference on Simulation of Semiconductor Processes and Devices, SISPAD, Udine, Italy, 4–6 September 2019. [Google Scholar] [CrossRef]
- Cao, W.; Kang, J.; Liu, W.; Banerjee, K. A compact current-voltage model for 2D semiconductor based field-effect transistors considering interface traps, mobility degradation, and inefficient doping effect. IEEE Trans. Electron Devices 2014, 61, 4282–4290. [Google Scholar] [CrossRef]
- Marin, E.; Medina-Rull, A.; Toral-Lopez, A.; Cuesta-Lopez, J.; Ruiz, G.; Mir, E.; Godoy, L.; Bartolomeo, D.; Pasadas, F.; El Grour, T.; et al. Compact Modeling of Two-Dimensional Field-Effect Biosensors. Sensors 2023, 23, 1840. [Google Scholar] [CrossRef]
- Bala Tripura Sundari, B.; Arya Raj, K. DC, frequency characterization of Dual Gated Graphene FET (GFET) Compact Model and its Circuit Application—Doubler Circuit. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 012016. [Google Scholar] [CrossRef]
- Ushiba, S.; Okino, T.; Miyakawa, N.; Ono, T.; Shinagawa, A.; Kanai, Y.; Inoue, K.; Takahashi, K.; Kimura, M.; Matsumoto, K. State-space modeling for dynamic response of graphene FET biosensors. Jpn. J. Appl. Phys. 2020, 59, SGGH04. [Google Scholar] [CrossRef]
- Sharma, P.; Mišković, Z.L. Ionic screening of charged impurities in electrolytically gated graphene: A partially linearized Poisson-Boltzmann model. J. Chem. Phys. 2015, 143, 134118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Peng, S.; Wang, W.; Xu, G.; Ji, Z.; Lu, N.; Li, L.; Jin, Z.; Liu, M. Surface-potential-based physical compact model for graphene field effect transistor. J. Appl. Phys. 2016, 120, 084509. [Google Scholar] [CrossRef]
- Novodchuk, I.; Kayaharman, M.; Ibrahim, K.; Al-Tuairqi, S.; Irannejad, M.; Abdel-Rahman, E.; Sanderson, J.; Bajcsy, M.; Yavuz, M. B/N co-doped graphene oxide gel with extremely-high mobility and ION/IOFF for large-area field effect transistors. Carbon 2020, 158, 624–630. [Google Scholar] [CrossRef]
- Novodchuk, I.; Kayaharman, M.; Ausri, I.R.; Karimi, R.; Tang, X.S.; Goldthorpe, I.A.; Abdel-Rahman, E.; Sanderson, J.; Bajcsy, M.; Yavuz, M. An ultrasensitive heart-failure BNP biosensor using B/N co-doped graphene oxide gel FET. Biosens. Bioelectron. 2021, 180, 113114. [Google Scholar] [CrossRef] [PubMed]
- Novodchuk, I.; Kayaharman, M.; Prassas, I.; Soosaipillai, A.; Karimi, R.; Goldthorpe, I.A.; Abdel-Rahman, E.; Sanderson, J.; Diamandis, E.P.; Bajcsy, M.; et al. Electronic Detection of SARS-CoV-2 N-Protein before the Onset of Symptoms. Biosens. Bioelectron. 2022, 210, 114331. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Wei, T.; Wang, K.; Zhao, Z.; Cao, J.; Liu, Y.; Zhang, Z. Carbon Nanotube Field-Effect Transistor Biosensor with an Enlarged Gate Area for Ultra-Sensitive Detection of a Lung Cancer Biomarker. ACS Appl. Mater. Interfaces 2023, 15, 27299–27306. [Google Scholar] [CrossRef]
- Ji, H.; Wang, Z.; Wang, S.; Wang, C.; Zhang, K.; Zhang, Y.; Han, L. Highly Stable InSe-FET Biosensor for Ultra-Sensitive Detection of Breast Cancer Biomarker CA125. Biosensors 2023, 13, 193. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, D.; Xu, Y.; Yin, Z.; Dou, W.; Habiba, U.M.E.; Pan, C.; Zhang, Z.; Mou, H.; Deng, H.; et al. DNA-based functionalization of two-dimensional MoS2 FET biosensor for ultrasensitive detection of PSA. Appl. Surf. Sci. 2021, 548, 149169. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Nate Ahn, S.; Choudhry, H. Carbon nanotube field-effect transistor (CNT-FET)-based biosensor for rapid detection of SARS-CoV-2 (COVID-19) surface spike protein S1. Bioelectrochemistry 2022, 143, 107982. [Google Scholar] [CrossRef]
- Novodchuk, I.; Bajcsy, M.; Yavuz, M. Graphene-based field effect transistor biosensors for breast cancer detection: A review on biosensing strategies. In Carbon; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 172, pp. 431–453. [Google Scholar] [CrossRef]
- Campos, R.; Jérô Me Borme, J.; Guerreiro, J.R.; Machado, G.; Fátima, M.; Cerqueira, F.; Petrovykh, D.Y.; Alpuim, P. Attomolar Label-Free Detection of DNA Hybridization with Electrolyte-Gated Graphene Field-Effect Transistors. ACS Sens. 2019, 4, 286–293. [Google Scholar] [CrossRef]
- Eswaran, M.; Chokkiah, B.; Pandit, S.; Rahimi, S.; Dhanusuraman, R.; Aleem, M.; Mijakovic, I. A Road Map toward Field-Effect Transistor Biosensor Technology for Early Stage Cancer Detection. Small Methods 2022, 6, 2200809. [Google Scholar] [CrossRef]
- Wang, S.; Hossain, M.Z.; Shinozuka, K.; Shimizu, N.; Kitada, S.; Suzuki, T.; Ichige, R.; Kuwana, A.; Kobayashi, H. Graphene field-effect transistor biosensor for detection of biotin with ultrahigh sensitivity and specificity. Biosens. Bioelectron. 2020, 165, 112363. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Domingues, T.; Borme, J.; Guerreiro, J.R.; Ipatov, A.; Abreu, C.M.; Martins, M.; Alpuim, P.; Prado, M. Influence of the Electrolyte Salt Concentration on DNA Detection with Graphene Transistors. Biosensors 2021, 11, 24. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaidan, L.; Novodchuk, I.; H.Xu, A.; Nica, A.; Takaloo, S.; Lloyd, C.; Karimi, R.; Sanderson, J.; Bajcsy, M.; Yavuz, M. Rapid, Selective, and Ultra-Sensitive Field Effect Transistor-Based Detection of Escherichia coli. Materials 2024, 17, 3648. https://doi.org/10.3390/ma17153648
Zaidan L, Novodchuk I, H.Xu A, Nica A, Takaloo S, Lloyd C, Karimi R, Sanderson J, Bajcsy M, Yavuz M. Rapid, Selective, and Ultra-Sensitive Field Effect Transistor-Based Detection of Escherichia coli. Materials. 2024; 17(15):3648. https://doi.org/10.3390/ma17153648
Chicago/Turabian StyleZaidan, Liena, Inna Novodchuk, Alexander H.Xu, Alexandru Nica, Saeed Takaloo, Christopher Lloyd, Reza Karimi, Joe Sanderson, Michal Bajcsy, and Mustafa Yavuz. 2024. "Rapid, Selective, and Ultra-Sensitive Field Effect Transistor-Based Detection of Escherichia coli" Materials 17, no. 15: 3648. https://doi.org/10.3390/ma17153648





