The Identification of Leidenfrost Phenomenon Formation on TiO2-Coated Surfaces and the Modelling of Heat Transfer Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup Section
2.2. Boiling Crisis and Leidenfrost Temperature Formation
2.3. Modelling Methodology of Heat Transfer
3. Results and Discussion
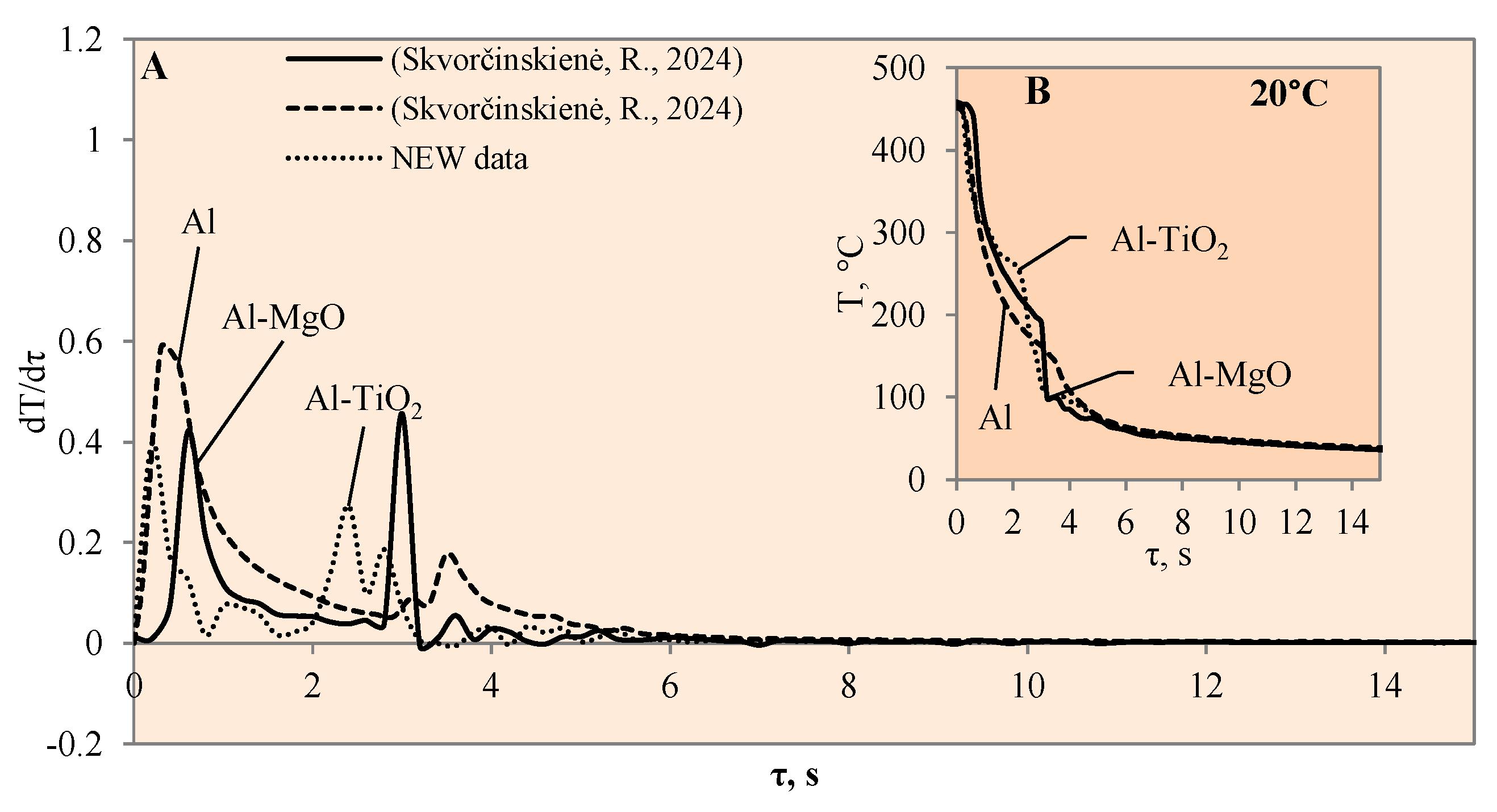
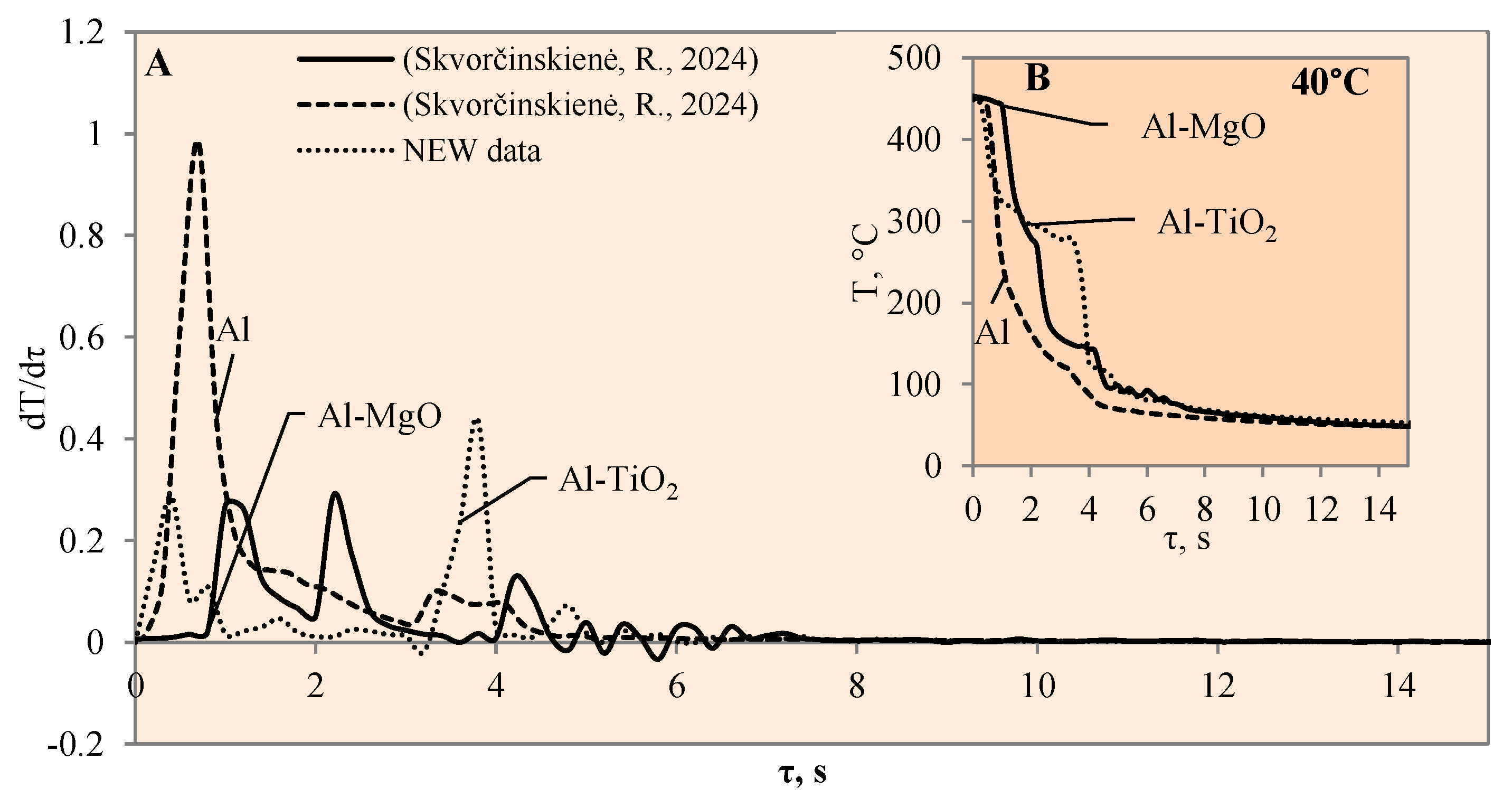
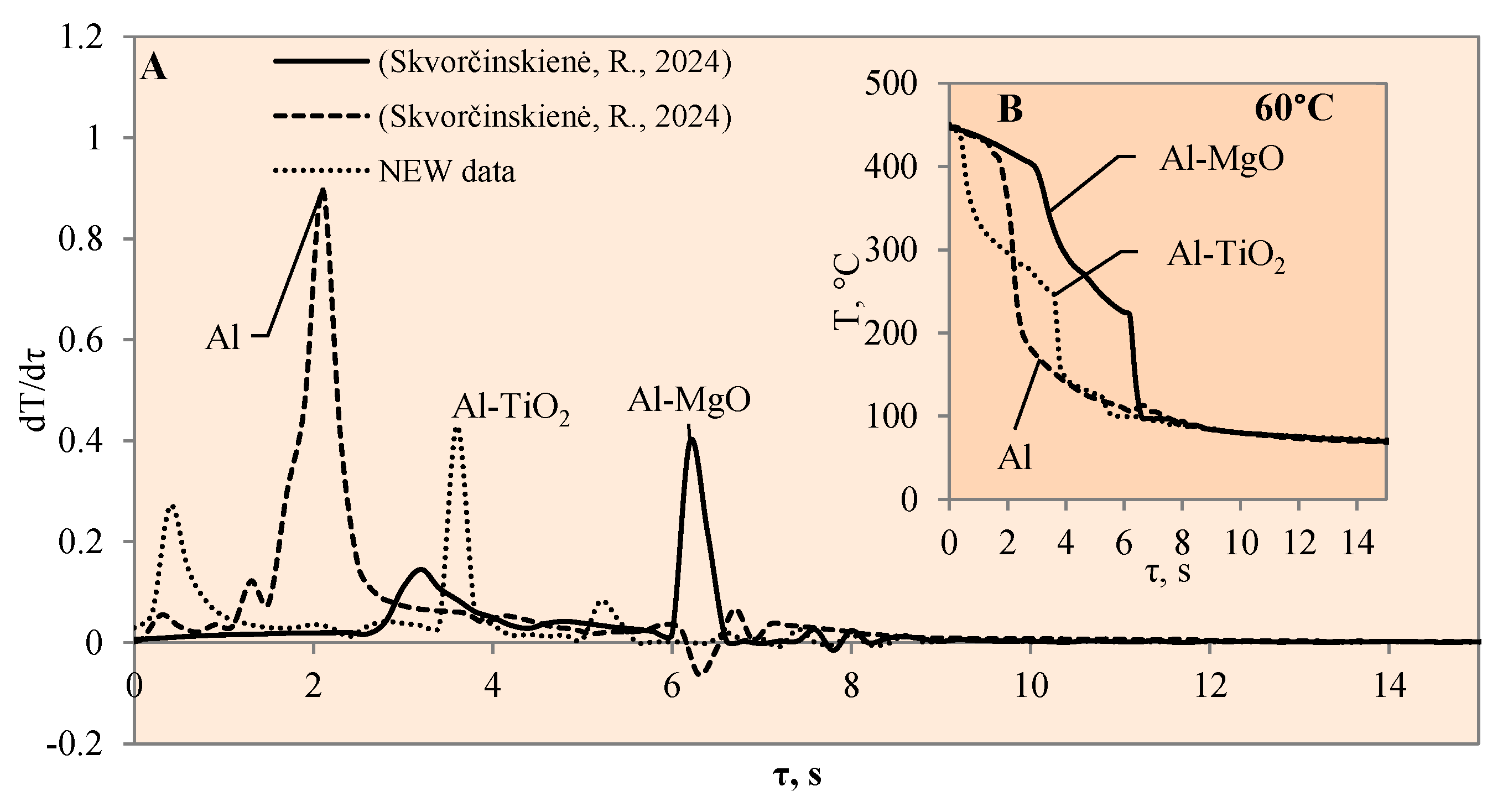
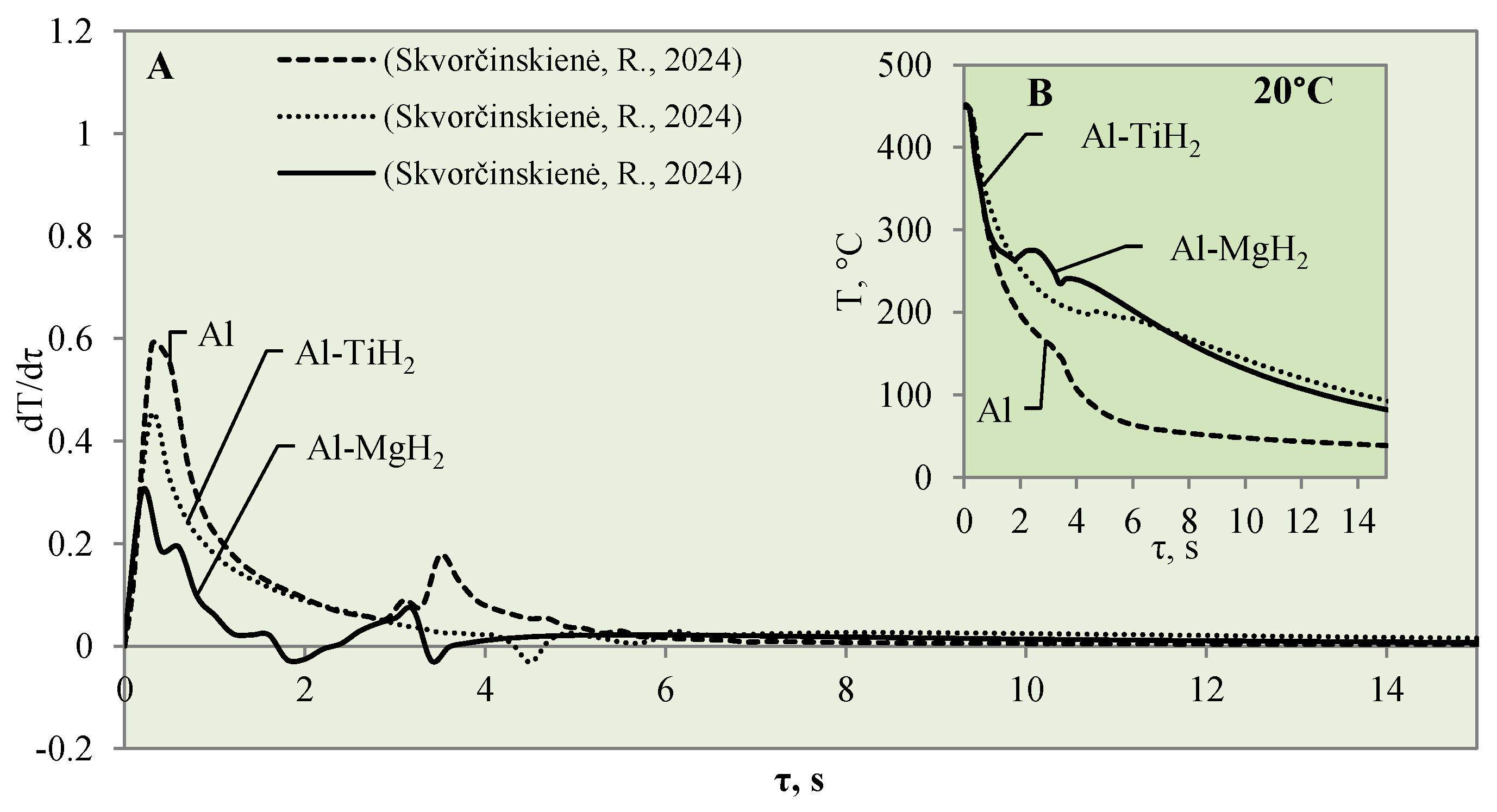
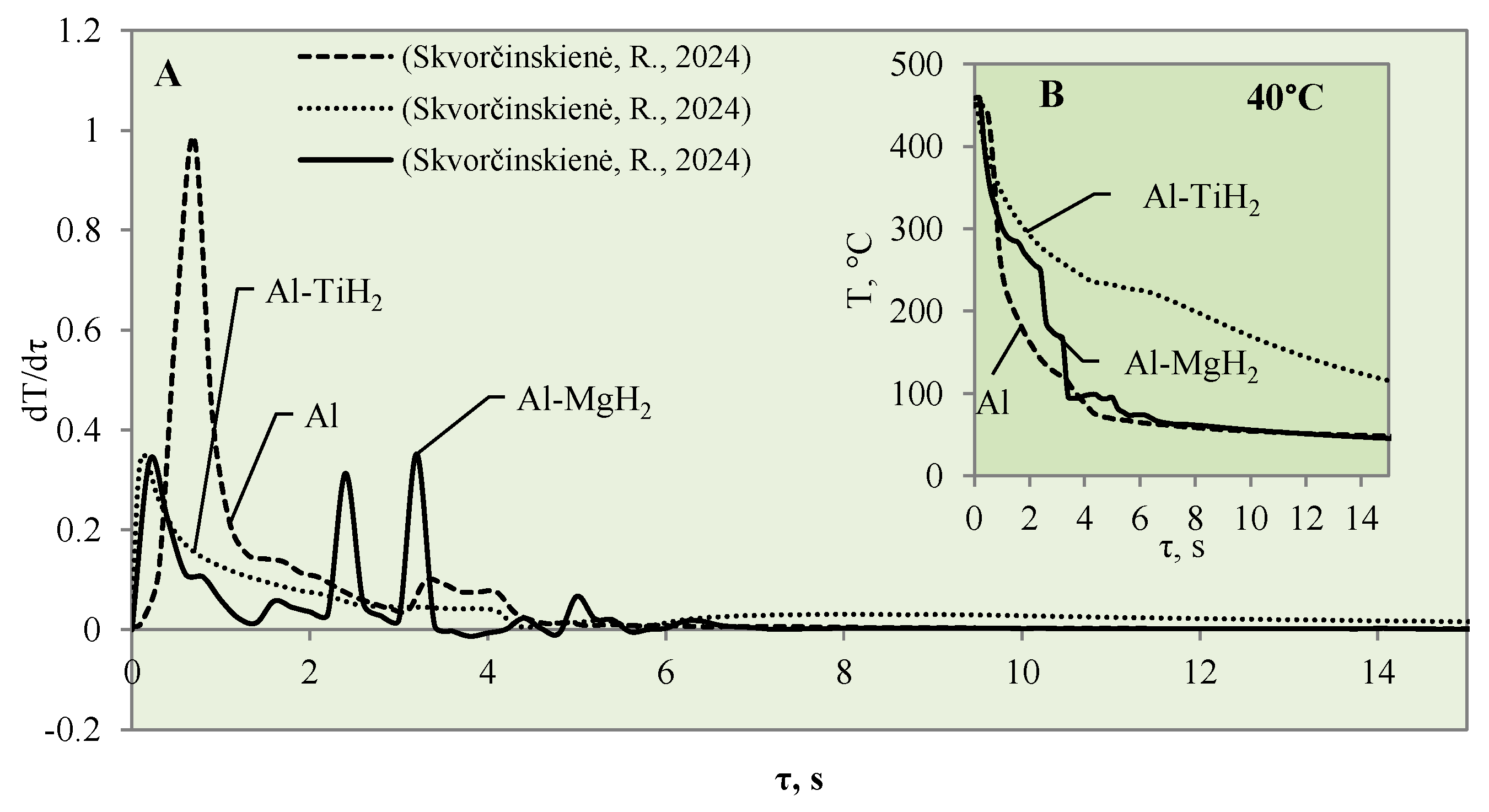
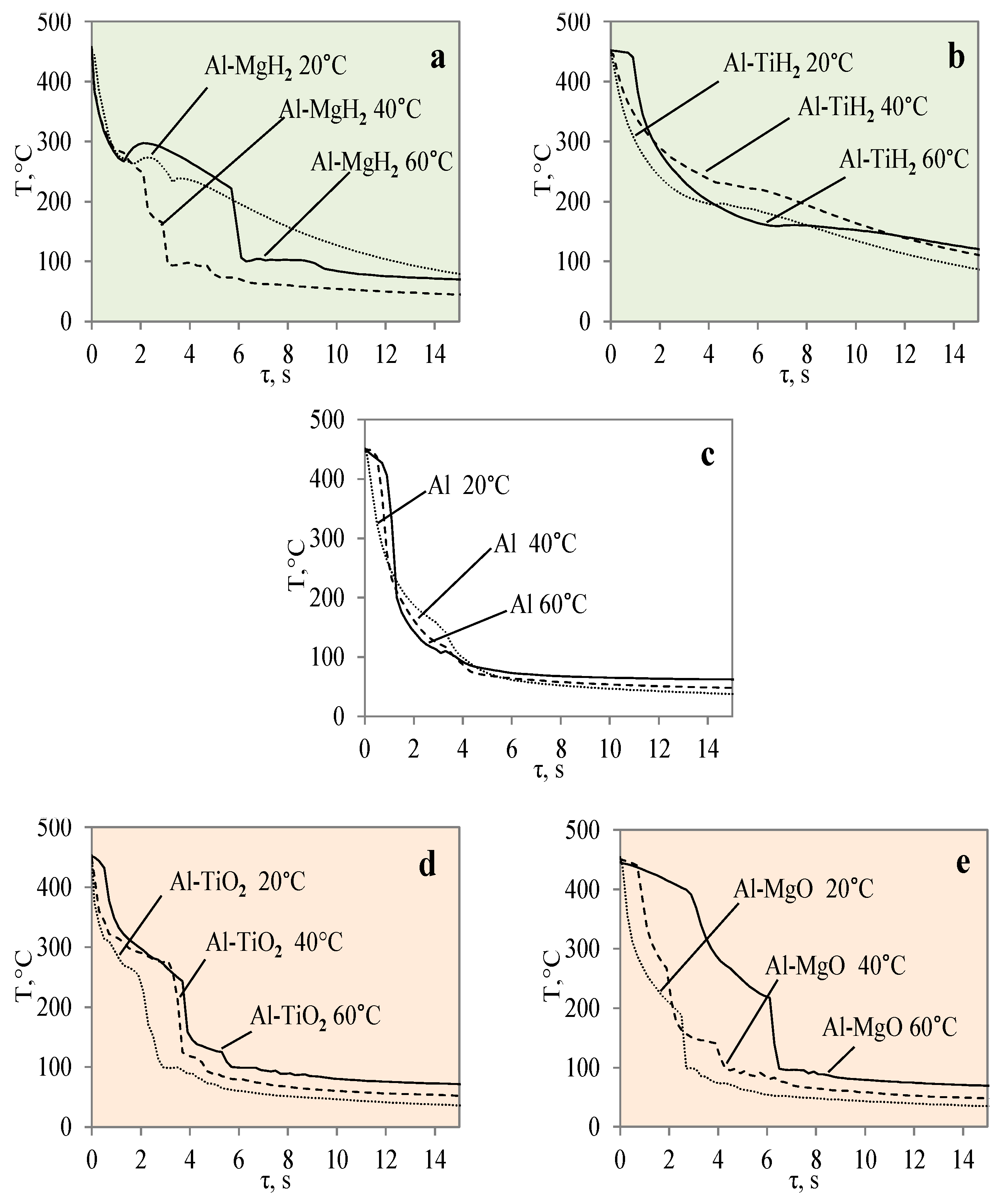
3.1. Sample Cooling Dynamics and Vapour Film Formation
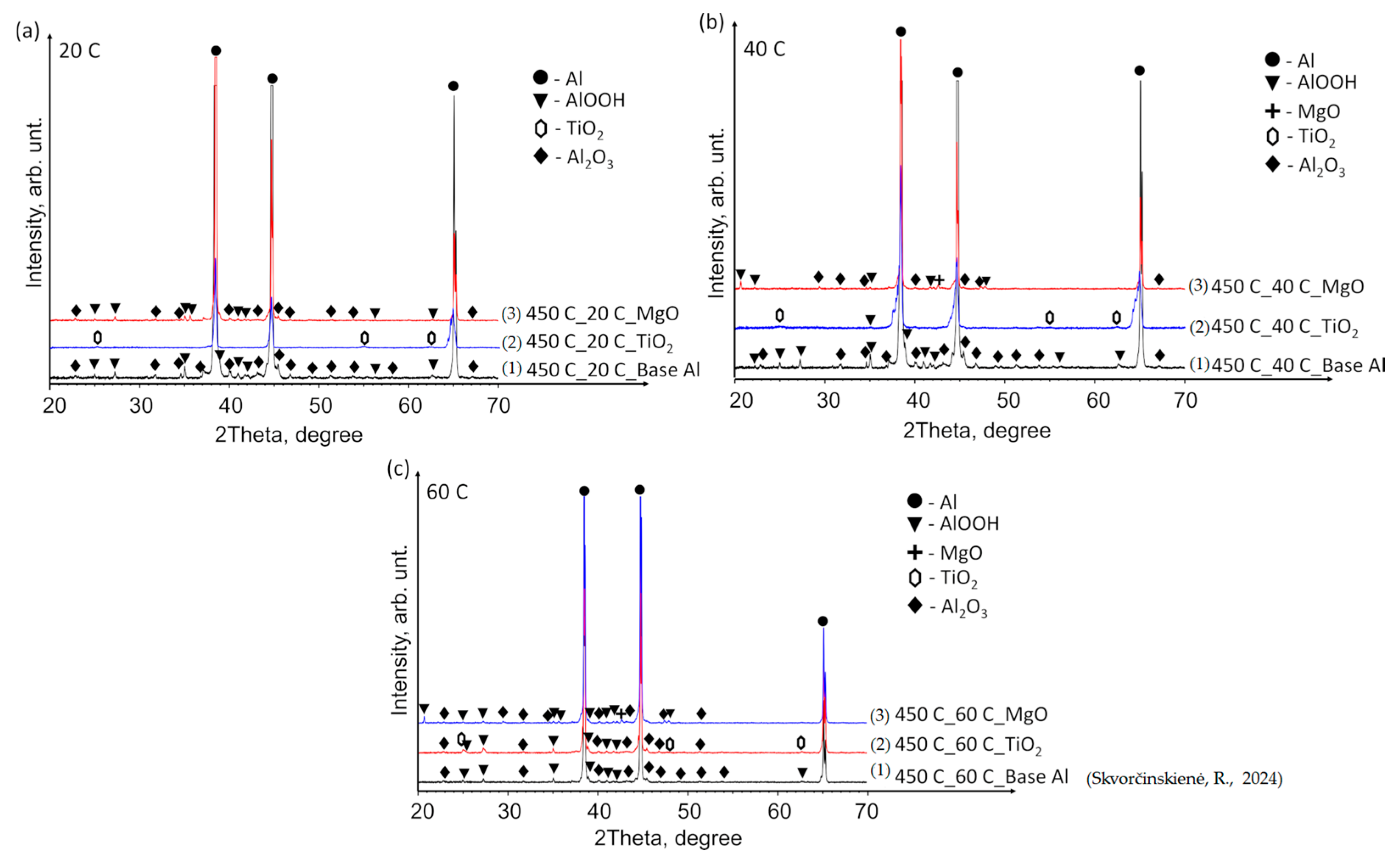
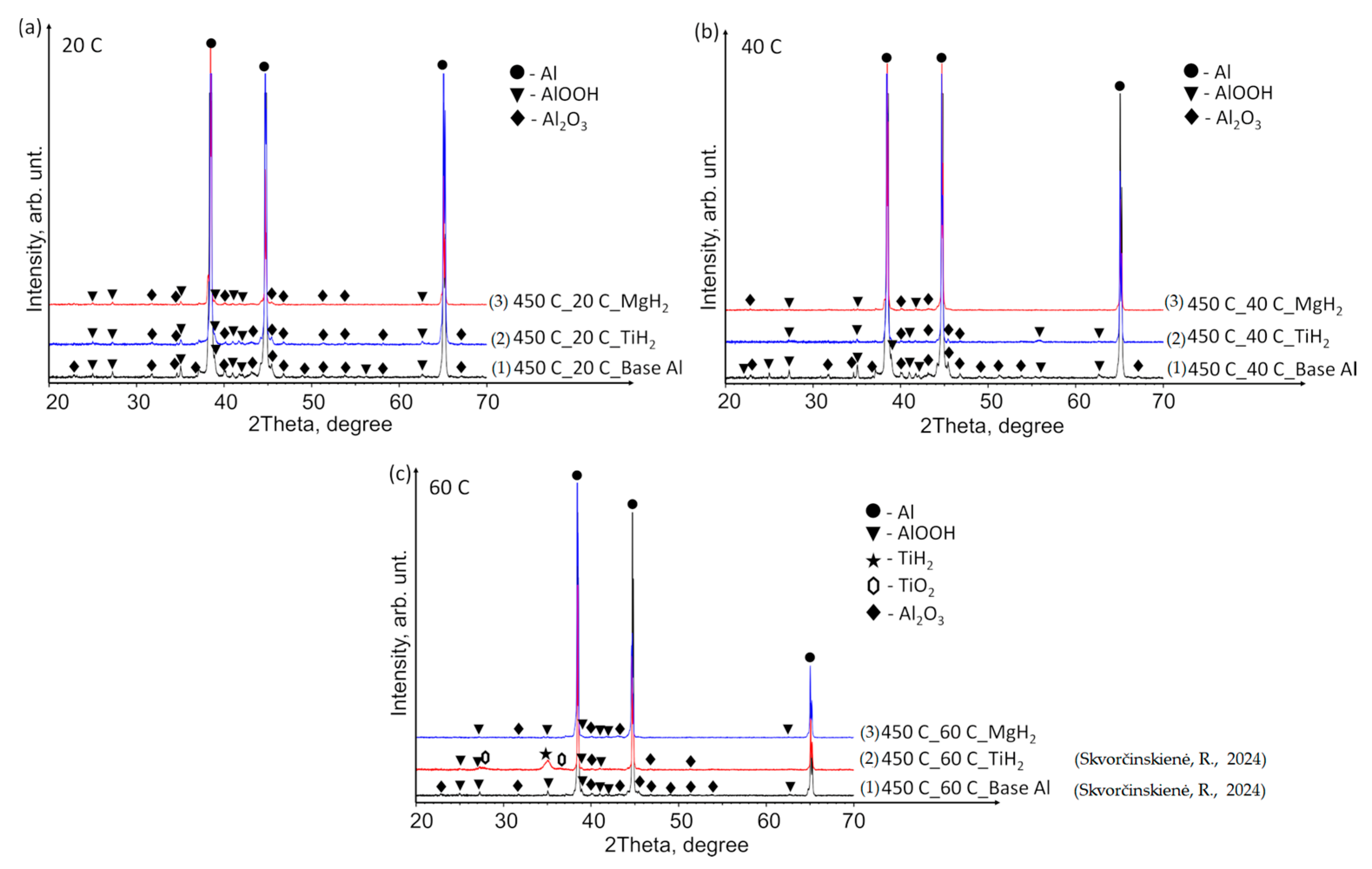
3.2. XRD Analysis Results
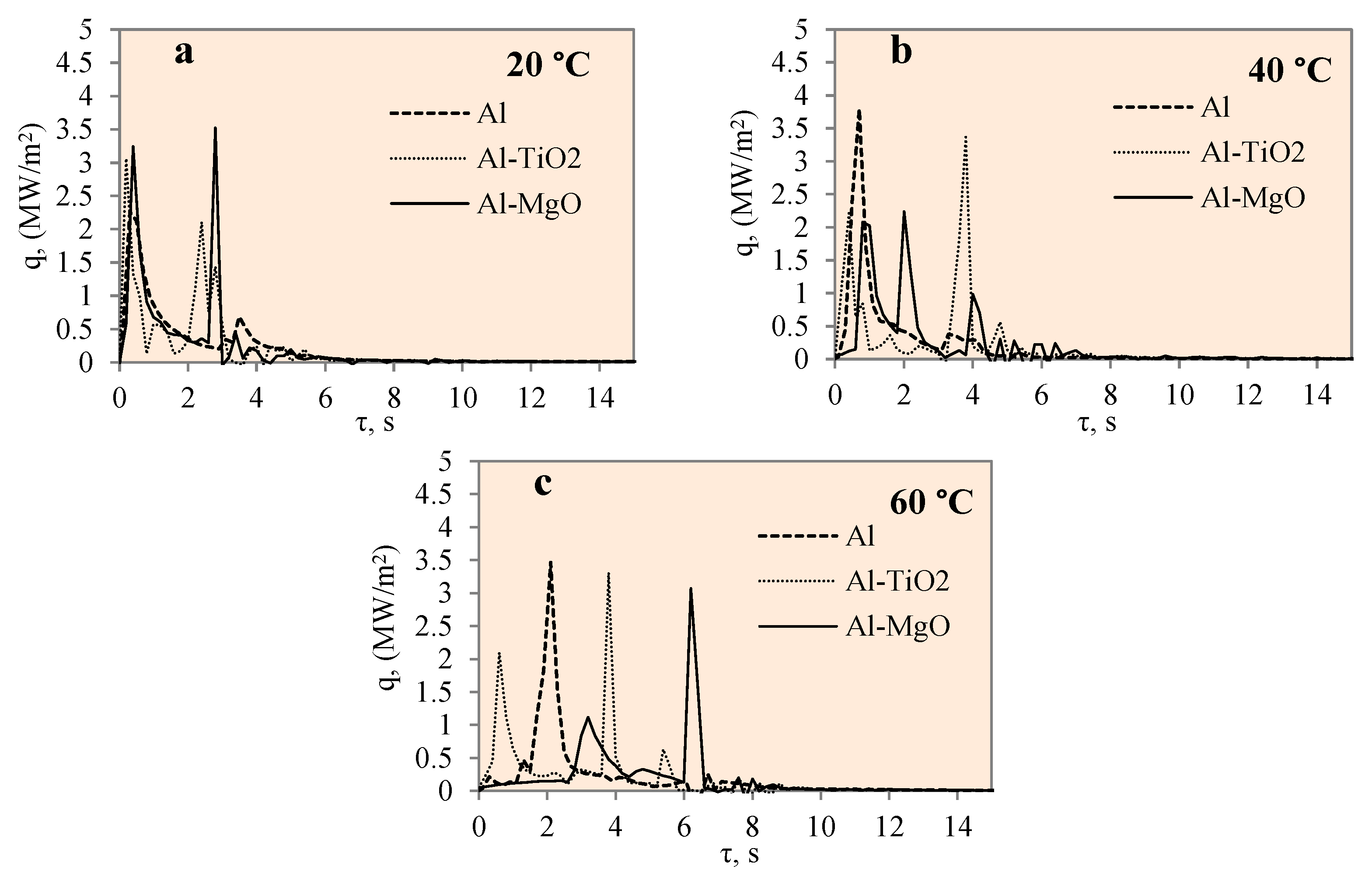
3.3. Heat Transfer Modelling Results
4. Conclusions
- The experiments with the Al–TiO2 coating did not produce the expected Leidenfrost effect and no vapour film formed around the specimen at any of the water temperatures (14*–20, 40 or 60 °C). However, the cooling dynamics of the Al–TiO2 specimen showed a decrease in surface temperature variation in temperature ranges of 350 °C to 290 °C (in 40 °C water) and of 310 °C to 250 °C (in 60 °C water). It is likely that the heated specimen body temperature of 450 °C was too low for the appearance of the LP.
- The performed XRD analysis showed that no substantial structural changes occurred in the polished aluminium specimens. The curves for the Al–TiO2, Al–MgO and Al–MgH2 films show high similarity to the curve for the base Al substrate. The results for Al–TiH2 show that aluminium was only present in the specimens that were heated up and immersed in water at 60 °C. The results could be affected by the adhesion process between the film and the base of the specimen.
- The highest heat flux values on the surface of the Al–TiO2 specimens ranged from ~3.4 to ~3.3 MW/m2 without a Leidenfrost effect even being formed. The maximum heat flux values for the Al–MgO specimens were in the range of ~0.39 to 3.6 MW/m2. Al–MgO was also remarkable in terms of its Leidenfrost phenomenon formation at all water temperatures. The maximum heat flux peak values for Al–TiH2 were in the range of ~0.8 to ~1.4 MW/m2, while for Al–MgH2, the highest heat flux values ranged from ~4.3 to ~2.7 MW/m2, respectively.
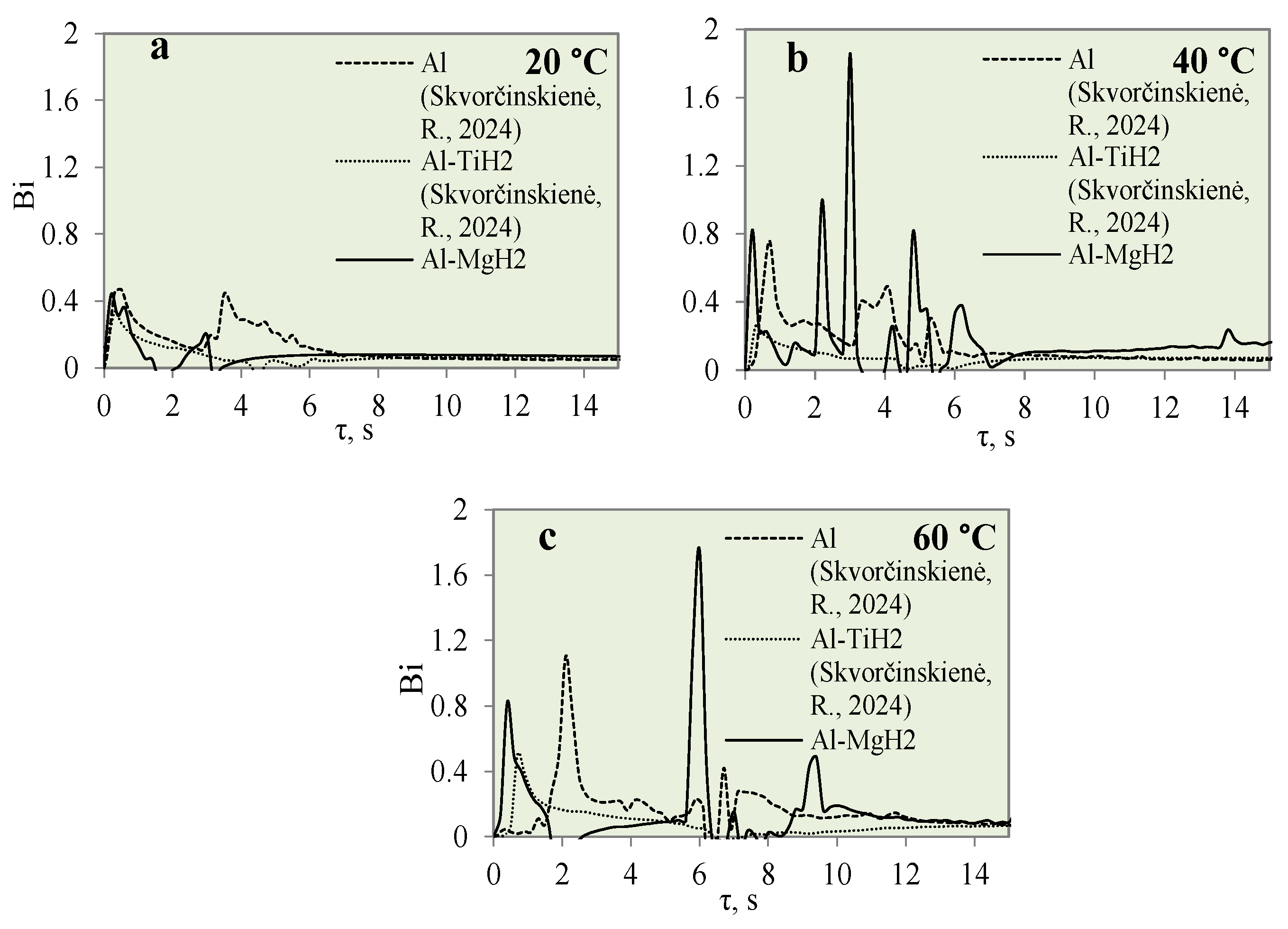
- The calculated Bi number values for TiO2 increased from 0.9 to 1.4 to 1.5 at water temperatures of 20, 40 and 60 °C, respectively. Increasing the water temperature impacts heat transfer and results in the dominance of convection transfer on the surface of the specimen over heat transfer via internal conduction. The higher Bi values of the Al–TiO2 and Al–MgO oxide materials show that the hydrides Al–TiH2 and Al–MgH2 have superior insulating properties.
- Approximated polynomial equations were newly proposed for each of the investigated materials, Al, Al–TiO2, Al–MgO, Al–TiH2 and Al–MgH2, and their cooling dynamics. The presented equations make it possible to foresee the cooling rate for a given material, predict the formation of the Leidenfrost temperature and indicate the threshold of the phenomenon. The obtained results could be used for heat and mass transfer models and for selecting the right materials in the industrial sector.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paraschiv, L.S.; Paraschiv, S. Contribution of Renewable Energy (Hydro, Wind, Solar and Biomass) to Decarbonization and Transformation of the Electricity Generation Sector for Sustainable Development. Energy Rep. 2023, 9, 535–544. [Google Scholar] [CrossRef]
- Pan, X.; Shao, T.; Zheng, X.; Zhang, Y.; Ma, X.; Zhang, Q. Energy and Sustainable Development Nexus: A Review. Energy Strategy Rev. 2023, 47, 101078. [Google Scholar] [CrossRef]
- Maramizonouz, S.; Nadimi, S. Drag Force Acting on Ellipsoidal Particles with Different Shape Characteristics. Powder Technol. 2022, 412, 117964. [Google Scholar] [CrossRef]
- Kim, H.; Liou, M.F. Flow Simulation and Drag Decomposition Study of N3-X Hybrid Wing-Body Configuration. Aerosp. Sci. Technol. 2019, 85, 24–39. [Google Scholar] [CrossRef]
- Aljallis, E.; Sarshar, M.A.; Datla, R.; Sikka, V.; Jones, A.; Choi, C.H. Experimental Study of Skin Friction Drag Reduction on Superhydrophobic Flat Plates in High Reynolds Number Boundary Layer Flow. Phys. Fluids 2013, 25, 025103. [Google Scholar] [CrossRef]
- Shen, X.; Ceccio, S.L.; Perlin, M. Influence of Bubble Size on Micro-Bubble Drag Reduction. Exp. Fluids 2006, 41, 415–424. [Google Scholar] [CrossRef]
- Kumagai, I.; Takahashi, Y.; Murai, Y. Power-Saving Device for Air Bubble Generation Using a Hydrofoil to Reduce Ship Drag: Theory, Experiments, and Application to Ships. Ocean Eng. 2015, 95, 183–194. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Zhang, Y.; Zhao, R.; Cui, H.; Zhai, T.; Liu, W.; Liu, Z. High Heat Flux Dissipation of Membrane-Venting Heat Sink with Thin Film Boiling. Int. J. Heat Mass Transf. 2024, 221, 125078. [Google Scholar] [CrossRef]
- Wang, H.; Wang, K.; Liu, G. Drag Reduction by Gas Lubrication with Bubbles. Ocean Eng. 2022, 258, 111833. [Google Scholar] [CrossRef]
- Jetly, A.; Vakarelski, I.U.; Yang, Z.; Thoroddsen, S.T. Giant Drag Reduction on Leidenfrost Spheres Evaluated from Extended Free-Fall Trajectories. Exp. Therm. Fluid Sci. 2019, 102, 181–188. [Google Scholar] [CrossRef]
- Puzina, Y.Y.; Kryukov, A.P.; Levashov, V.Y. Evolution of Liquid–Vapour Interface during Film Boiling on Sphere. Int. J. Therm. Sci. 2024, 196, 108683. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Patankar, N.A. The Thermo-Wetting Instability Driving Leidenfrost Film Collapse. Proc. Natl. Acad. Sci. USA 2020, 117, 13321–13328. [Google Scholar] [CrossRef] [PubMed]
- Prasad, D.; Dash, S. Evaporation of Leidenfrost Droplets on Microtextured Substrates. Int. J. Therm. Sci. 2024, 197, 108743. [Google Scholar] [CrossRef]
- Kim, E.; Chun, C.; Kim, M.H.; Jo, H. Effect of Thermal Properties and Thickness for Various Metals on the Leidenfrost Temperature. Int. J. Therm. Sci. 2024, 196, 108697. [Google Scholar] [CrossRef]
- Cai, C.; Mudawar, I. Review of the Dynamic Leidenfrost Point Temperature for Droplet Impact on a Heated Solid Surface. Int. J. Heat Mass Transf. 2023, 217, 124639. [Google Scholar] [CrossRef]
- Sahoo, V.; Lo, C.W.; Lu, M.C. Leidenfrost Suppression and Contact Time Reduction of a Drop Impacting on Silicon Nanowire Array-Coated Surfaces. Int. J. Heat Mass Transf. 2020, 148, 118980. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Lin, Y.Z.; Shieh, J.; Hsu, C.C. Fast Spreading of Liquid on Leidenfrost Vapor Layer Surface. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132311. [Google Scholar] [CrossRef]
- Lee, G.C.; Kim, S.H.; Kang, J.; Kim, M.H.; Jo, H. Leidenfrost Temperature on Porous Wick Surfaces: Decoupling the Effects of the Capillary Wicking and Thermal Properties. Int. J. Heat Mass Transf. 2019, 145, 118809. [Google Scholar] [CrossRef]
- Du, J.; Li, Y.; Wang, X.; Wu, X.; Min, Q. Dynamics and Heat Transfer of Water Droplets Impacting on Heated Surfaces: The Role of Surface Structures in Leidenfrost Point. Int. J. Heat Mass Transf. 2023, 212, 124241. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, W.; Peng, Y.; Jiang, L. Dynamic Leidenfrost Temperature Increase of Impacting Droplets Containing High-Alcohol Surfactant. Int. J. Heat Mass Transf. 2018, 118, 1160–1168. [Google Scholar] [CrossRef]
- Lim, E.; Ng, B.T.; Hung, Y.M.; Tan, M.K. Graphene-Mediated Suppression of Leidenfrost Effect for Droplets on an Inclined Surface. Int. J. Therm. Sci. 2022, 174, 107426. [Google Scholar] [CrossRef]
- Ashouri, M.; Kayhani, M.H.; Nazari, M. An Experimental Study of Wall Effect on a Hot Settling Sphere in a Newtonian-Fluid-Contained Block Using Photography. Processes 2023, 11, 248. [Google Scholar] [CrossRef]
- Cheol, L.G.; Noh, H.; Yeom, H.; Jo, H.; Kim, T.K.; Kim, M.; Sridharan, K.; Park, H.S. Zirconium-Silicide Coating on Zircaloy-4 Substrate for Accident Tolerance: Effects on Oxidation Resistance and Boiling. Ann. Nucl. Energy 2019, 126, 350–358. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, H.S.; Kim, J.; Kim, M.H.; Park, H.S. Experimental Study of Water Droplets on Over-Heated Nano/Microstructured Zirconium Surfaces. Nucl. Eng. Des. 2014, 278, 367–376. [Google Scholar] [CrossRef]
- Misyura, S.Y. The Effect of Weber Number, Droplet Sizes and Wall Roughness on Crisis of Droplet Boiling. Exp. Therm. Fluid Sci. 2017, 84, 190–198. [Google Scholar] [CrossRef]
- Talari, V.; Behar, P.; Lu, Y.; Haryadi, E.; Liu, D. Leidenfrost drops on micro/nanostructured surfaces. Front. Energy 2018, 12, 22–42. [Google Scholar] [CrossRef]
- Jun, Y.K.; Cheol, L.G.; Kaviany, M.; Sun, P.H.; Moriyama, K.; Hwan, K.M. Control of Minimum Film-Boiling Quench Temperature of Small Spheres with Micro-Structured Surface. Int. J. Multiph. Flow 2018, 103, 30–42. [Google Scholar]
- Avedisian, C.T.; Koplik, J. Leidenfrost Boiling of Methanol Droplets on Hot Porous/Ceramic Surfaces. Int. J. Heat Mass Transf. 1987, 30, 379–393. [Google Scholar] [CrossRef]
- Werner, R.; Mayhew, E.; Kim, K.; Kweon, C.B.; Michael, J.B. Examining Surface Wetting and Leidenfrost Transition of Jet Fuels and Bicomponent Mixtures. Exp. Therm. Fluid Sci. 2024, 154, 111167. [Google Scholar] [CrossRef]
- Skvorčinskienė, R.; Eimontas, J.; Bašinskas, M.; Vorotinskienė, L.; Urbonavičius, M.; Kiminaitė, I.; Maziukienė, M.; Striūgas, N.; Zakarauskas, K.; Makarevičius, V. Magnesium Hydride: Investigating Its Capability to Maintain Stable Vapor Film. Energies 2024, 17, 661. [Google Scholar] [CrossRef]
- Skvorčinskienė, R.; Urbonavičius, M.; Vorotinskienė, L.; Bašinskas, M.; Zakarauskas, K.; Maziukienė, M.; Striūgas, N.; Buidovas, P.; Milčius, D.; Varnagiris, Š. Application of TiH2 Dehydrogenation for Vapour Layer Formation under Boiling Crisis Conditions. Appl. Therm. Eng. 2024, 247, 122935. [Google Scholar] [CrossRef]
- Lopez, R.J. Newton’s Law of Cooling. In Maple via Calculus; Birkhäuser: Boston, MA, USA, 1994. [Google Scholar]
- Mills, A.F.; Irwin, R.D. Basic Heat and Mass Transfer; University of California at Los Angeles: Los Angeles, CA, USA, 1995; pp. 254–921. [Google Scholar]
- Incropera, F.P.; De Witt, D.P. Incropera, Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 1985. [Google Scholar]
- Tong, W.; Qiu, L.; Jin, J.; Sun, L.; Duan, F. Unique lift-off of droplet impact on high temperature nanotube surfaces. Appl. Phys. Lett. 2017, 111, 091605. [Google Scholar] [CrossRef]
- Liu, M.; Lu, K.; Li, X.; Liu, H.; Jing, D. Light-Induced Enhancement of Critical Heat Flux on TiO2 Coatings with Specific Surface Topology. Appl. Therm. Eng. 2020, 174, 115333. [Google Scholar] [CrossRef]





| Surface of Specimens | Specimen Temperature, °C | Water Temperature, °C | Measurement Time, s |
|---|---|---|---|
| Al–TiO2 | 450 | 20 | 15 |
| 40 | |||
| 60 |
| Materials | Specimens, Temperature, °C | Water Temperature, °C | ||
|---|---|---|---|---|
| 14*–20 | 40 | 60 | ||
| polished Al | 450 | No v | 374 v | 331 v |
| Al–TiO2 | No | No | No | |
| Al–MgO | 439 v | 441 v | 373 v | |
| Al–TiH2 | No v | No v | 385 v | |
| Al–MgH2 | No v | No v | 384 v | |
| Twater,° C | Tspeciment,°C | BiAl | BiAl–TiO2 | BiAl–MgO | BiAl–TiH2 | BiAl–MgH2 |
|---|---|---|---|---|---|---|
| 14*–20 | 450 | 0.46 | 0.9 | 1.6 | 0.33 | 0.43 |
| 40 | 0.93 | 1.4 | 0.77 | 0.25 | 1.8 | |
| 60 | 1.05 | 1.5 | 1.66 | 0.50 | 1.74 |
| Material | Approximation |
|---|---|
| Al | Ts = 5 × 10−25τ6 − 1·10−19τ5 + 9 × 10−15τ4 − 4 × 10−10τ3 + 9 × 10−6τ2 − 0.0844τ + 345.48 |
| Al–TiO2 | Ts = 10−24 × τ6‒ 2·10−19τ5 + 2 × 10−14τ4 ‒ 6 × 10−10 τ3+ 1 × 10−5τ2 ‒ 0.1063τ + 461.57 |
| Al–MgO | Ts = 3 × 10−25τ6 − 8·10−20τ5 + 7 × 10−15τ4 − 3 × 10−10τ3 + 8 × 10−6τ2 − 0.0861τ + 408.54 |
| Al–TiH2 | Ts = 2 × 10−24τ6 − 4·10−19τ5 + 2 × 10−14τ4 − 8 × 10−10τ3 + 1 × 10−6τ2 − 0.1006τ + 462.09 |
| Al–MgH2 | Ts = 6 × 10−26τ6 − 2·10−20τ5 + 2 × 10−15τ4 − 1 × 10−10τ3 + 4 × 10−6τ2 − 0.0553τ + 388.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maziukienė, M.; Striūgas, N.; Vorotinskienė, L.; Skvorčinskienė, R.; Urbonavičius, M. The Identification of Leidenfrost Phenomenon Formation on TiO2-Coated Surfaces and the Modelling of Heat Transfer Processes. Materials 2024, 17, 3687. https://doi.org/10.3390/ma17153687
Maziukienė M, Striūgas N, Vorotinskienė L, Skvorčinskienė R, Urbonavičius M. The Identification of Leidenfrost Phenomenon Formation on TiO2-Coated Surfaces and the Modelling of Heat Transfer Processes. Materials. 2024; 17(15):3687. https://doi.org/10.3390/ma17153687
Chicago/Turabian StyleMaziukienė, Monika, Nerijus Striūgas, Lina Vorotinskienė, Raminta Skvorčinskienė, and Marius Urbonavičius. 2024. "The Identification of Leidenfrost Phenomenon Formation on TiO2-Coated Surfaces and the Modelling of Heat Transfer Processes" Materials 17, no. 15: 3687. https://doi.org/10.3390/ma17153687
APA StyleMaziukienė, M., Striūgas, N., Vorotinskienė, L., Skvorčinskienė, R., & Urbonavičius, M. (2024). The Identification of Leidenfrost Phenomenon Formation on TiO2-Coated Surfaces and the Modelling of Heat Transfer Processes. Materials, 17(15), 3687. https://doi.org/10.3390/ma17153687






