Fabrication of Soft Biodegradable Foam with Improved Shrinkage Resistance and Thermal Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
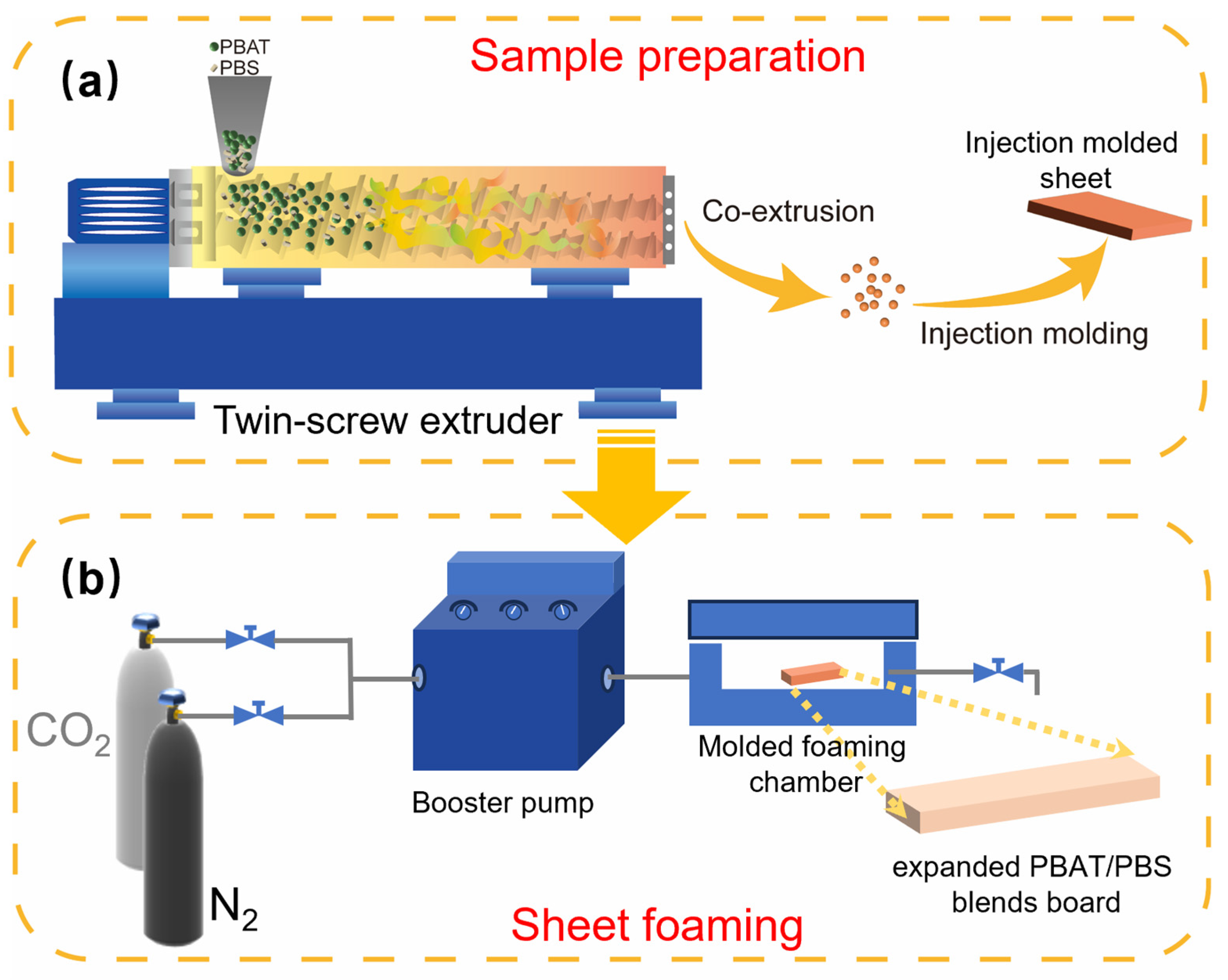
2.2. Sample Preparation
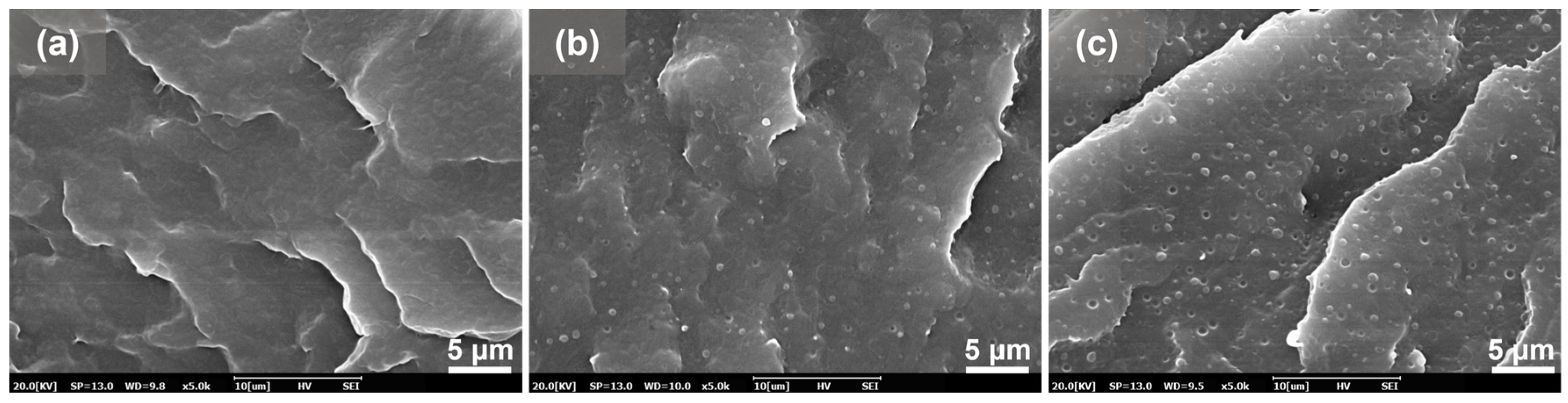
2.3. Dispersion Morphology Characterization
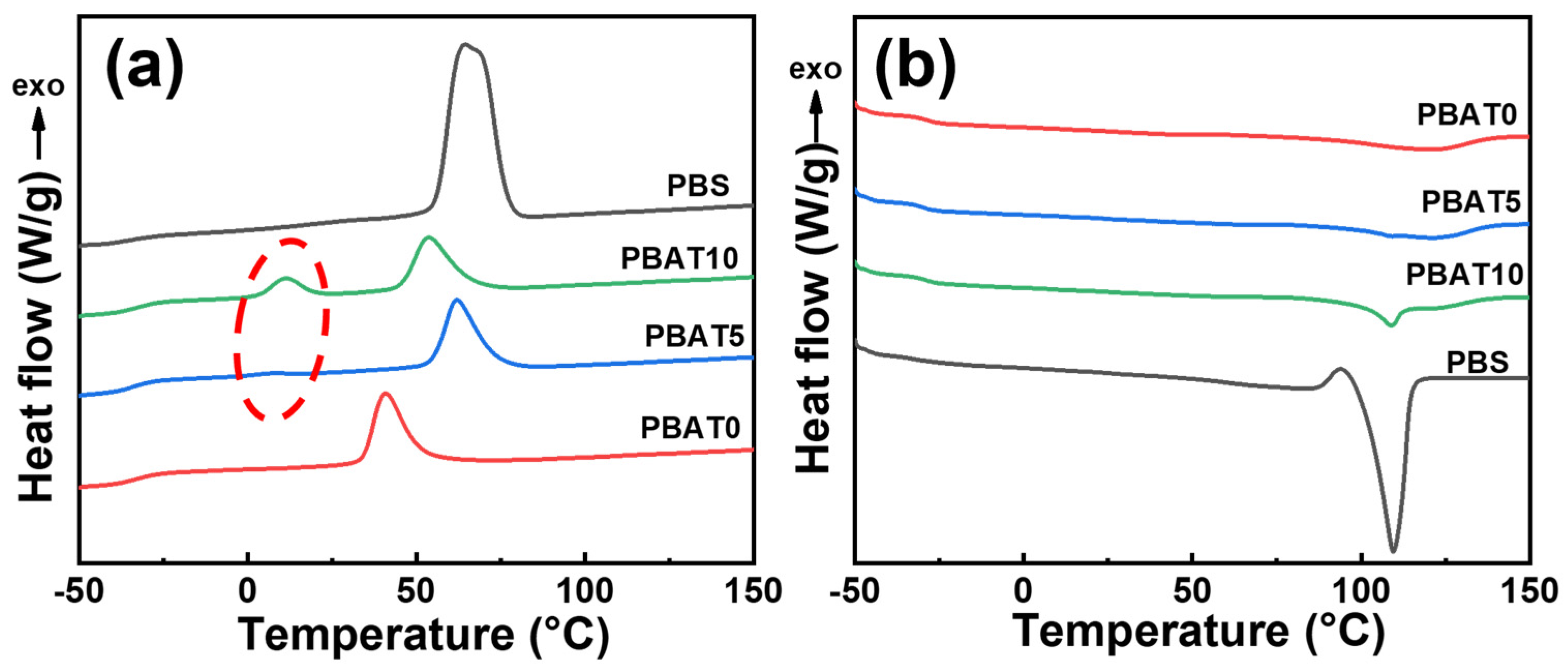
2.4. Crystallization Behavior
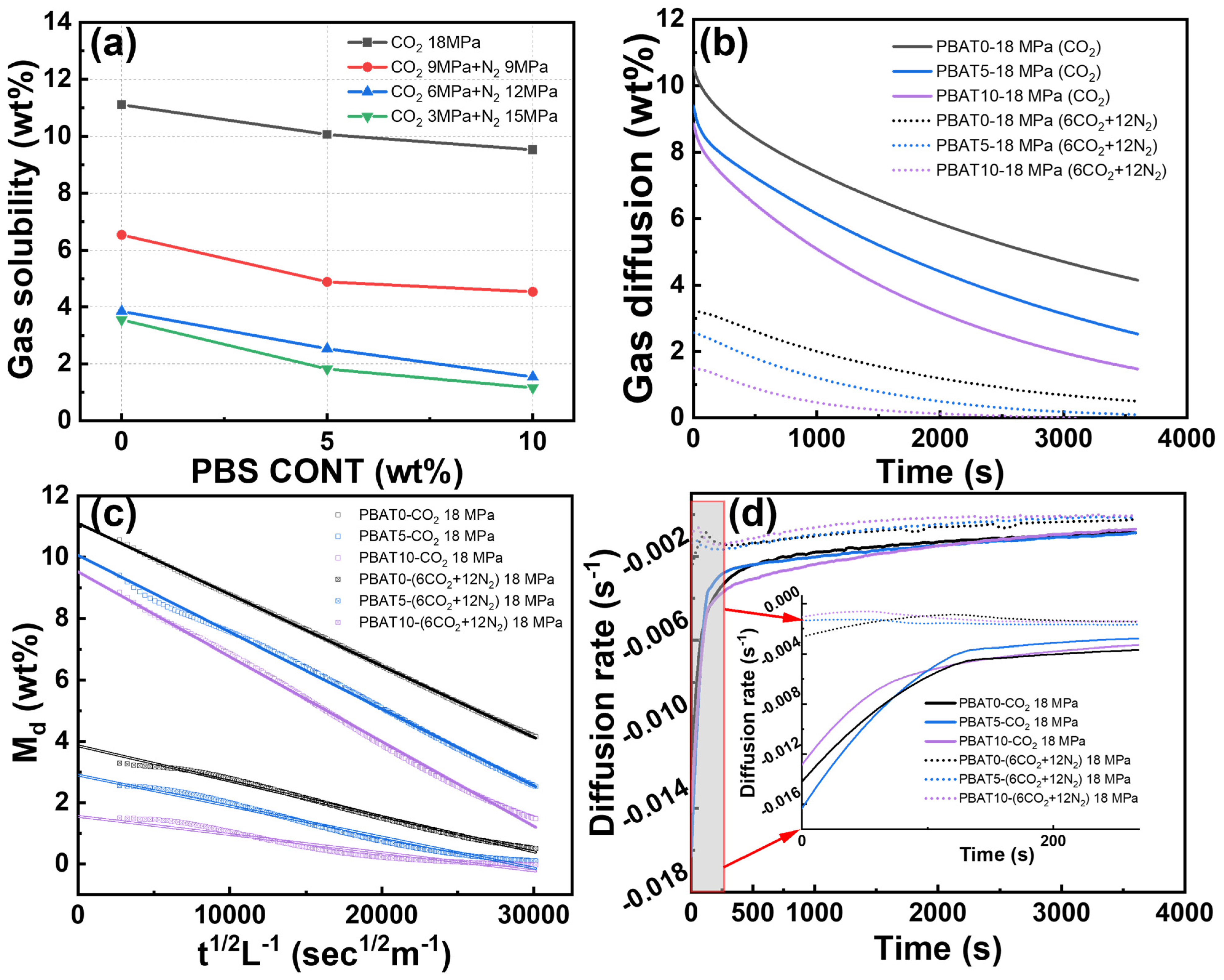
2.5. Gas Solubility Test
2.6. Foam Morphology and Structure Characterization
2.7. Mechanical and Thermal Properties
3. Results and Discussion
3.1. Compatibility of PBAT/PBS Blends
3.2. Non-Isothermal Crystallization and Subsequent Melting Behavior
3.3. Gas Solubility and Diffusivity
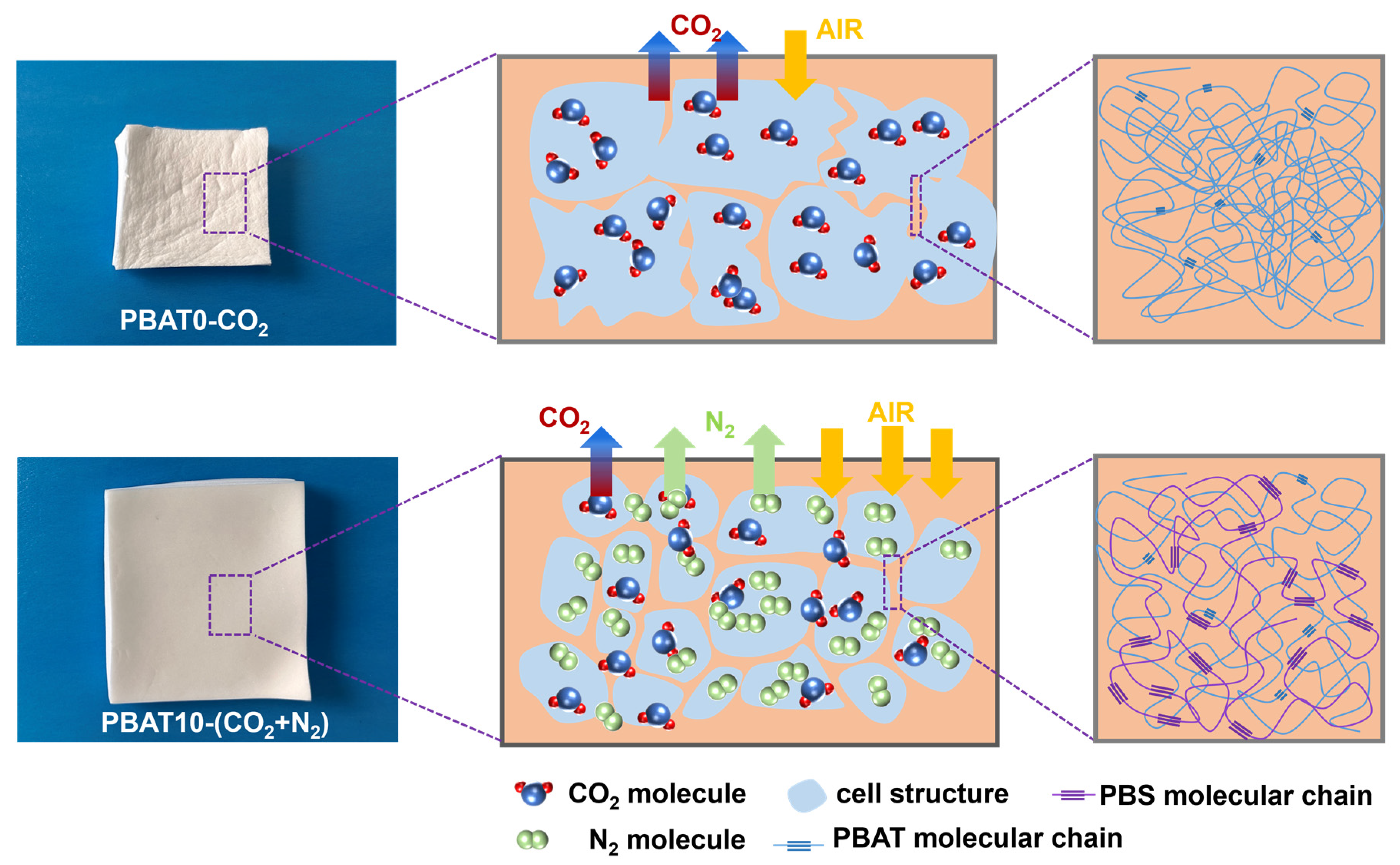
3.4. Foaming Behaviors of PBAT/PBS Blends Blown with CO2
3.5. Foaming Behaviors of PBAT/PBS Blends with Mixed CO2/N2 Gas
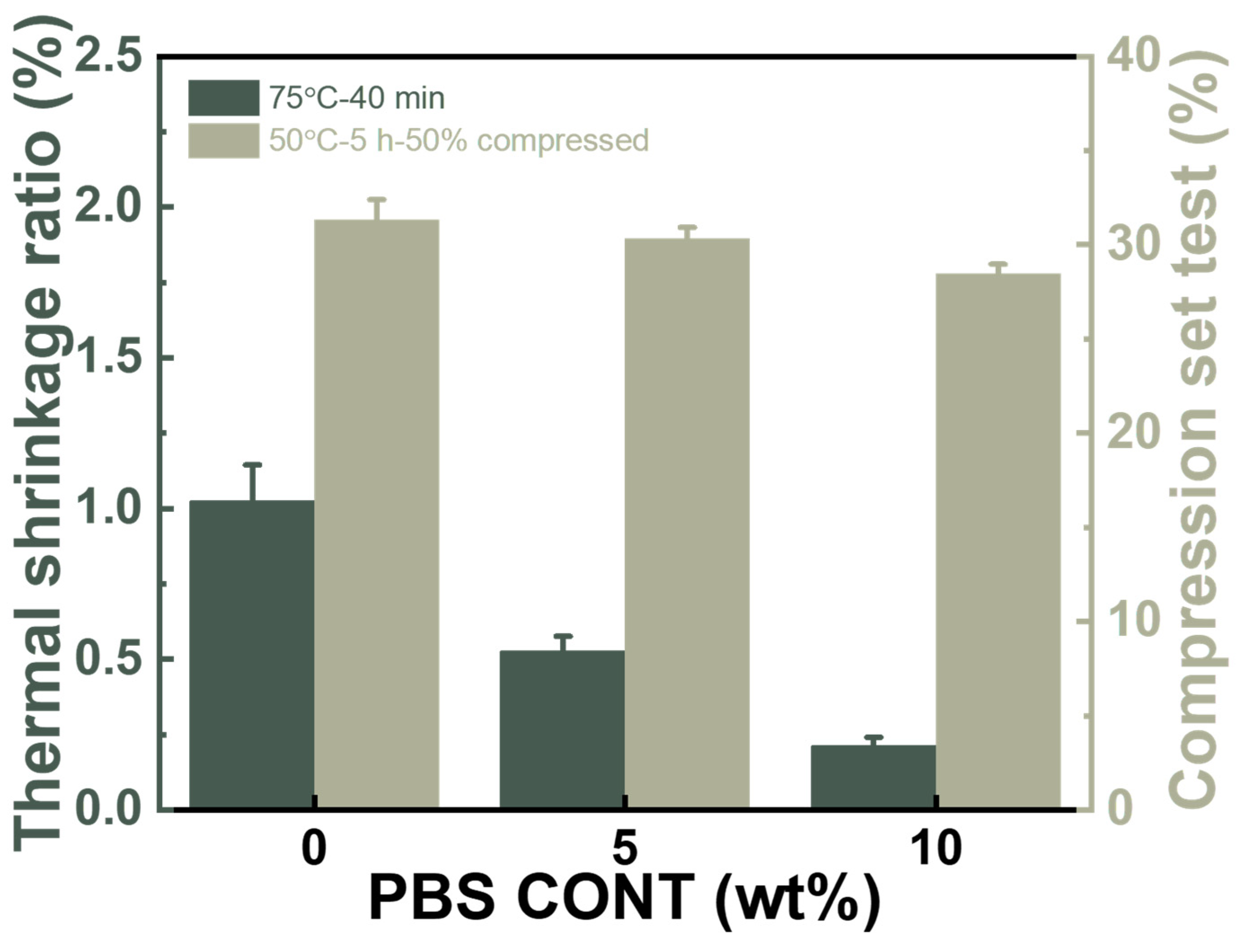
3.6. Thermo-Mechanical Properties of the Foams of PBAT/PBS Blends
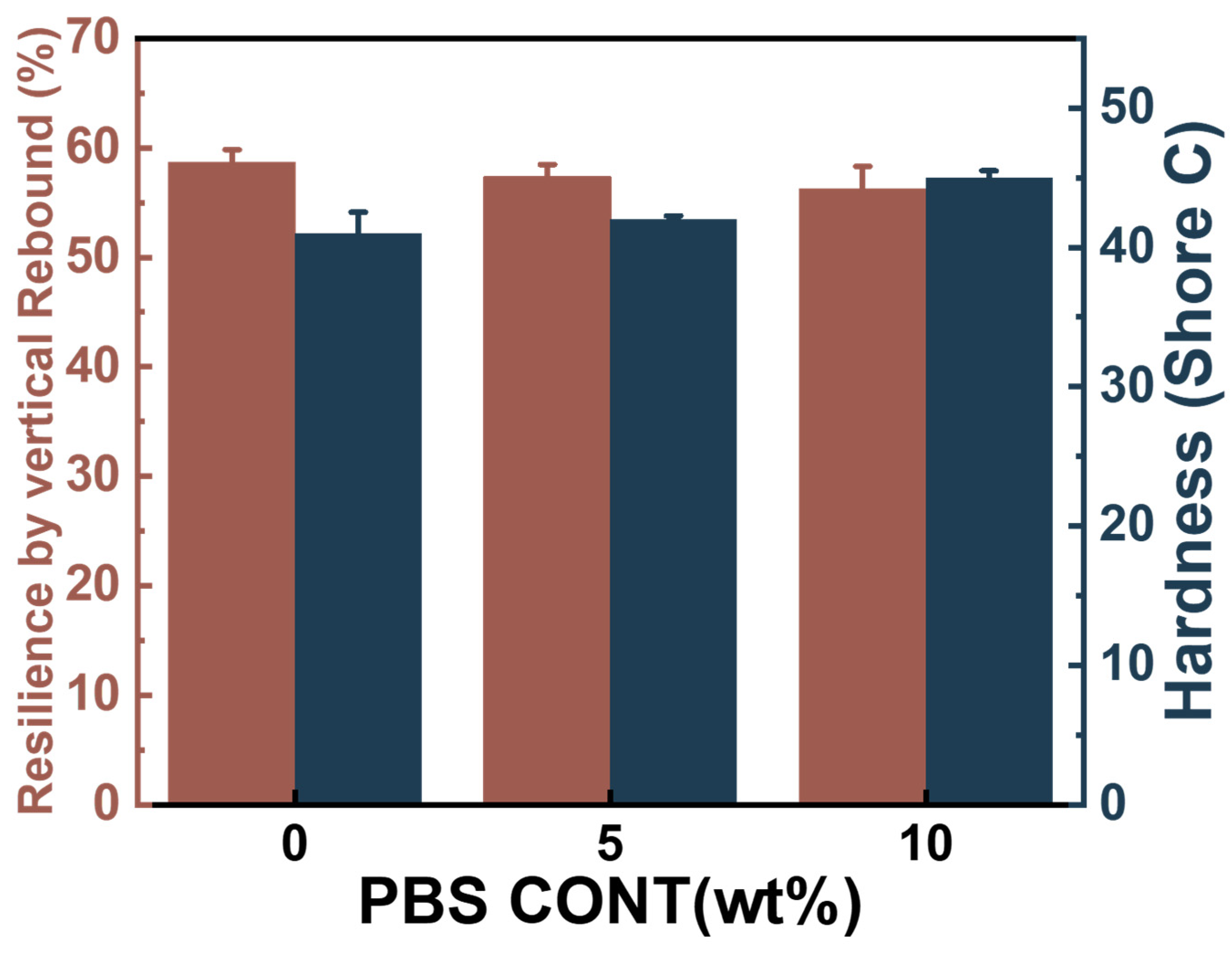
3.7. Resilience and Hardness of the Foams of PBAT/PBS Blends
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, J.; Chen, B.; Zhou, M.; Liu, H.; Li, Y.; Tian, F.; Wang, Z.; Wang, L.; Zhai, W. A convenient and efficient path to bead foam parts: Restricted cell growth and simultaneous inter-bead welding. J. Supercrit. Fluids 2023, 194, 105852. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Li, Y.; Yao, Z.; Cao, K.; Zeng, C. TPU/PLA blend foams: Enhanced foamability, structural stability, and implications for shape memory foams. J. Appl. Polym. Sci. 2018, 136, 47416. [Google Scholar] [CrossRef]
- Li, Y.; Gong, P.; Liu, Y.; Niu, Y.; Park, C.B.; Li, G. Environmentally Friendly and Zero-Formamide EVA/LDPE Microcellular Foams via Supercritical Carbon Dioxide Solid Foaming. ACS Appl. Polym. Mater. 2021, 3, 4213–4222. [Google Scholar] [CrossRef]
- Ranganathan, P.; Chen, C.-W.; Chou, Y.-L.; Chang, Y.-Y.; Yan, H.-C.; Rwei, S.-P. Development of Anti-Shrinkage and High-Elastic Thermoplastic Poly(Ether-Ester) Elastomer Foams Via Supercritical Carbon Dioxide Foaming. ACS Appl. Polym. Mater. 2023, 5, 9796–9806. [Google Scholar] [CrossRef]
- Yang, F.; Sun, H.; Mao, Z.; Tao, Y.; Zhang, J. Facile fabrication of EVA cellular material with hydrophobic surface, high solar reflectance and low thermal conductivity via chemical foaming. Microporous Mesoporous Mater. 2021, 328, 111460. [Google Scholar] [CrossRef]
- Brzozowski, Z.K.; Kijeńska, D.; Zatorski, W. New achievements in fire-safe polyurethane foams. Des. Monomers Polym. 2012, 5, 183–193. [Google Scholar] [CrossRef][Green Version]
- Jiang, J.; Zhou, M.; Li, Y.; Chen, B.; Tian, F.; Zhai, W. Cell structure and hardness evolutions of TPU foamed sheets with high hardness via a temperature rising foaming process. J. Supercrit. Fluids 2022, 188, 105654. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, G.; Wang, Z.; Zhang, A.; Zhao, G. High performance plant-derived thermoplastic polyester elastomer foams achieved by manipulating charging order of mixed blowing agents. Int. J. Biol. Macromol. 2023, 252, 126261. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Dong, G.; Wang, G.; Zhao, J.; Zhao, G. Super-Elastic and Dimensionally Stable Polyether Block Amide Foams Fabricated by Microcellular Foaming with CO2&N2 as Co-Blowing Agents. Macromol. Mater. Eng. 2024, 309, 2300437. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Dutta, A. Energy issues in the utilization of CO2 in the synthesis of chemicals: The case of the direct carboxylation of alcohols to dialkyl-carbonates. Catal. Today 2017, 281, 345–351. [Google Scholar] [CrossRef]
- Caglioti, L.; Micskei, K.; Tacconi, L.; Zucchi, C.; Pályi, G. Carbon dioxide A C-1 source for chemical industry. Chim. Oggi-Chem. Today 2009, 27, 18–22. [Google Scholar]
- Ni, G.-L.; Zhu, X.; Mi, H.-Y.; Feng, P.-Y.; Li, J.; Jing, X.; Dong, B.; Liu, C.; Shen, C. Skinless porous films generated by supercritical CO2 foaming for high-performance complementary shaped triboelectric nanogenerators and self-powered sensors. Nano Energy 2021, 87, 106148. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Wu, M.; Weng, Z.; Ren, Q.; Xiao, P.; Wang, L.; Zheng, W. Multifunctional polyether block amides/carbon nanostructures piezoresistive foams with largely linear range, enhanced and humidity-regulated microwave shielding. Chem. Eng. J. 2023, 455, 140860. [Google Scholar] [CrossRef]
- Shah, A.A.; Kato, S.; Shintani, N.; Kamini, N.R.; Nakajima-Kambe, T. Microbial degradation of aliphatic and aliphatic-aromatic co-polyesters. Appl. Microbiol. Biotechnol. 2014, 98, 3437–3447. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Cividanes, L.S.; Gouveia, R.F.; Lona, L.M.F. An overview on properties and applications of poly(butylene adipate-co-terephthalate)–PBAT based composites. Polym. Eng. Sci. 2019, 59, E7–E15. [Google Scholar] [CrossRef]
- Li, C.; Cui, Q.; Li, Y.; Zhang, K.; Lu, X.; Zhang, Y. Effect of LDPE and biodegradable PBAT primary microplastics on bacterial community after four months of soil incubation. J. Hazard. Mater. 2022, 429, 128353. [Google Scholar] [CrossRef]
- Dammak, M.; Fourati, Y.; Tarrés, Q.; Delgado-Aguilar, M.; Mutjé, P.; Boufi, S. Blends of PBAT with plasticized starch for packaging applications: Mechanical properties, rheological behaviour and biodegradability. Ind. Crops Prod. 2020, 144, 112061. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Xu, Z.; Yang, C.; Zhao, G. Structure-tunable poly(butylene adipate-co-terephthalate) foams with enhanced mechanical performance derived by microcellular foaming with carbon dioxide as blowing agents. J. CO2 Util. 2023, 72, 102495. [Google Scholar] [CrossRef]
- Wang, L.; Wei, X.; Zhou, H.; Wang, X.; Hu, J. An Ultra-High Volume Expansion Ratio and No-Shrinkage Poly(Butylene Adipate-co-Terephthalate) Foam: Compression and Resilience Properties. J. Polym. Environ. 2024, 1–16. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Yu, L.; Yu, Z.; Li, L.; Zhang, Z. Exploring the Polybutylene Adipate Terephthalate/Thermoplastic Polyether Ester Elastomer Blend-Modified Foam: The Frontier of High-Elasticity Sustainable Foam. ACS Appl. Polym. Mater. 2023, 5, 8822–8832. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Chen, P.; Xu, Z.; Zhao, L.; Hu, D. Supercritical CO2 foaming and shrinkage resistance of thermoplastic polyurethane/modified magnesium borate whisker composite. J. CO2 Util. 2022, 57, 101887. [Google Scholar] [CrossRef]
- Lan, B.; Li, P.; Yang, Q.; Gong, P. Dynamic self generation of hydrogen bonding and relaxation of polymer chain segment in stabilizing thermoplastic polyurethane microcellular foams. Mater. Today Commun. 2020, 24, 101056. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Chen, Y.; Ge, Y.; Liu, T. Effect of chain relaxation on the shrinkage behavior of TPEE foams fabricated with supercritical CO2. Polymer 2022, 256, 125262. [Google Scholar] [CrossRef]
- Long, H.; Xu, H.; Shaoyu, J.; Jiang, T.; Zhuang, W.; Li, M.; Jin, J.; Ji, L.; Ying, H.; Zhu, C. High-Strength Bio-Degradable Polymer Foams with Stable High Volume-Expansion Ratio Using Chain Extension and Green Supercritical Mixed-Gas Foaming. Polymers 2023, 15, 895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cai, C.; Liu, Q.; Hu, S. Enhancing the Melt Strength of Poly(Lactic Acid) via Micro-Crosslinking and Blending with Poly(Butylene Adipate-co-Butylene Terephthalate)for the Preparation of Foams. J. Polym. Environ. 2016, 25, 1335–1341. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, D.; Wei, S.; Xi, Z.; Zhen, W.; Zhao, L. Effects of chain composition of PBAT on the supercritical CO2 foaming and degradation behavior. J. CO2 Util. 2023, 72, 102500. [Google Scholar] [CrossRef]
- Yang, Q.; Li, S.; Wang, X. Strategy for the Preparation of PBAT/Starch Blended Foam with High Resilience and Shrinkage Resistance. J. Polym. Environ. 2024, 1–12. [Google Scholar] [CrossRef]
- Hu, D.; Xue, K.; Liu, Z.; Xu, Z.; Zhao, L. The essential role of PBS on PBAT foaming under supercritical CO2 toward green engineering. J. CO2 Util. 2022, 60, 101965. [Google Scholar] [CrossRef]
- Behera, K.; Veluri, S.; Chang, Y.-H.; Yadav, M.; Chiu, F.-C. Nanofillers-induced modifications in microstructure and properties of PBAT/PP blend: Enhanced rigidity, heat resistance, and electrical conductivity. Polymer 2020, 203, 122758. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Wang, W.; Gong, P.; Yang, Q.; Park, C.B.; Li, G. Ultra-fast degradable PBAT/PBS foams of high performance in compression and thermal insulation made from environment-friendly supercritical foaming. J. Supercrit. Fluids 2022, 181, 105512. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Jiang, J.; Tian, F.; Gao, N.; Zhai, W. Microextrusion Foaming of Polyetheretherketone Fiber: Mechanical and Thermal Insulation Properties. Adv. Eng. Mater. 2021, 24, 2101110. [Google Scholar] [CrossRef]
- ASTM D2240; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D2632; Standard Test Method for Rubber Property—Resilience by Vertical Rebound. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D395; Standard Test Methods For Rubber Property—Compression Set. ASTM International: West Conshohocken, PA, USA, 2014.
- Wu, S. Formation of dispersed phase in incompatible polymer blends: Interfacial and rheological effects. Polym. Eng. Sci. 1987, 27, 335–343. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Tailoring the toughness of sustainable polymer blends from biodegradable plastics via morphology transition observed by atomic force microscopy. Polym. Degrad. Stab. 2020, 173, 109066. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Novel tunable super-tough materials from biodegradable polymer blends: Nano-structuring through reactive extrusion. RSC Adv. 2019, 9, 2836–2847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, T.; Li, B.; Li, H.; Cao, Z.; Jin, G.; Zhao, L.; Xin, Z. Foaming and dimensional stability of LDPE foams with N2, CO2, i-C4H10 and CO2-N2 mixtures as blowing agents. J. Supercrit. Fluids 2020, 164, 104930. [Google Scholar] [CrossRef]
- Sun, Y.; Ueda, Y.; Suganaga, H.; Haruki, M.; Kihara, S.-i.; Takishima, S. Pressure drop threshold in the foaming of low-density polyethylene, polystyrene, and polypropylene using CO2 and N2 as foaming agents. J. Supercrit. Fluids 2015, 103, 38–47. [Google Scholar] [CrossRef]
- Wang, D.; Cai, Z.; Huang, X.; Wang, L. Study on the Dissolution and Diffusion of Supercritical Carbon Dioxide in Polystyrene Melts Based on Adsorption and Diffusion Mechanism. ACS Omega 2021, 6, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Jiang, J.; Park, C.B. A review on physical foaming of thermoplastic and vulcanized elastomers. Polym. Rev. 2021, 62, 95–141. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Z.; Yu, J.; Zhao, Y.; Pan, H.; Bian, J.; Han, L.; Wang, Z.; Zhang, H. Preparation of shrink-resistant environmentally friendly foam. J. CO2 Util. 2024, 82, 102769. [Google Scholar] [CrossRef]
- Park, K.W.; Kim, G.H.; Chowdhury, S.R. Improvement of compression set property of ethylene vinyl acetate copolymer/ethylene-1-butene copolymer/organoclay nanocomposite foams. Polym. Eng. Sci. 2008, 48, 1183–1190. [Google Scholar] [CrossRef]
- Li, N.; Fan, D.; Shi, Z.; Xie, Y.; Li, M.; Tang, T. Effect of ion-crosslinking on supercritical CO2 foaming behavior and foam properties of EVA/ZnO composites. Compos. Commun. 2021, 25, 100760. [Google Scholar] [CrossRef]
- Zhong, W.P.; Yu, Z.; Zhu, T.; Zhao, Y.; Phule, A.D.; Zhang, Z.X. Influence of different ratio of CO2/N2 and foaming additives on supercritical foaming of expanded thermoplastic polyurethane. Express Polym. Lett. 2022, 16, 318–336. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.J. Controlling properties of thermoplastic polyurethane-based foam through adopting different type of oligomers. Macromol. Res. 2023, 32, 273–280. [Google Scholar] [CrossRef]
- Huang, G.; Li, S.; Li, Y.; Wu, X.; Feng, X.; Gui, Y.; Deng, J.; Wang, C.; Pan, K. Preparation and characterization of microcellular foamed thermoplastic polyamide elastomer composite consisting of EVA/TPAE1012. J. Appl. Polym. Sci. 2021, 138, 50952. [Google Scholar] [CrossRef]
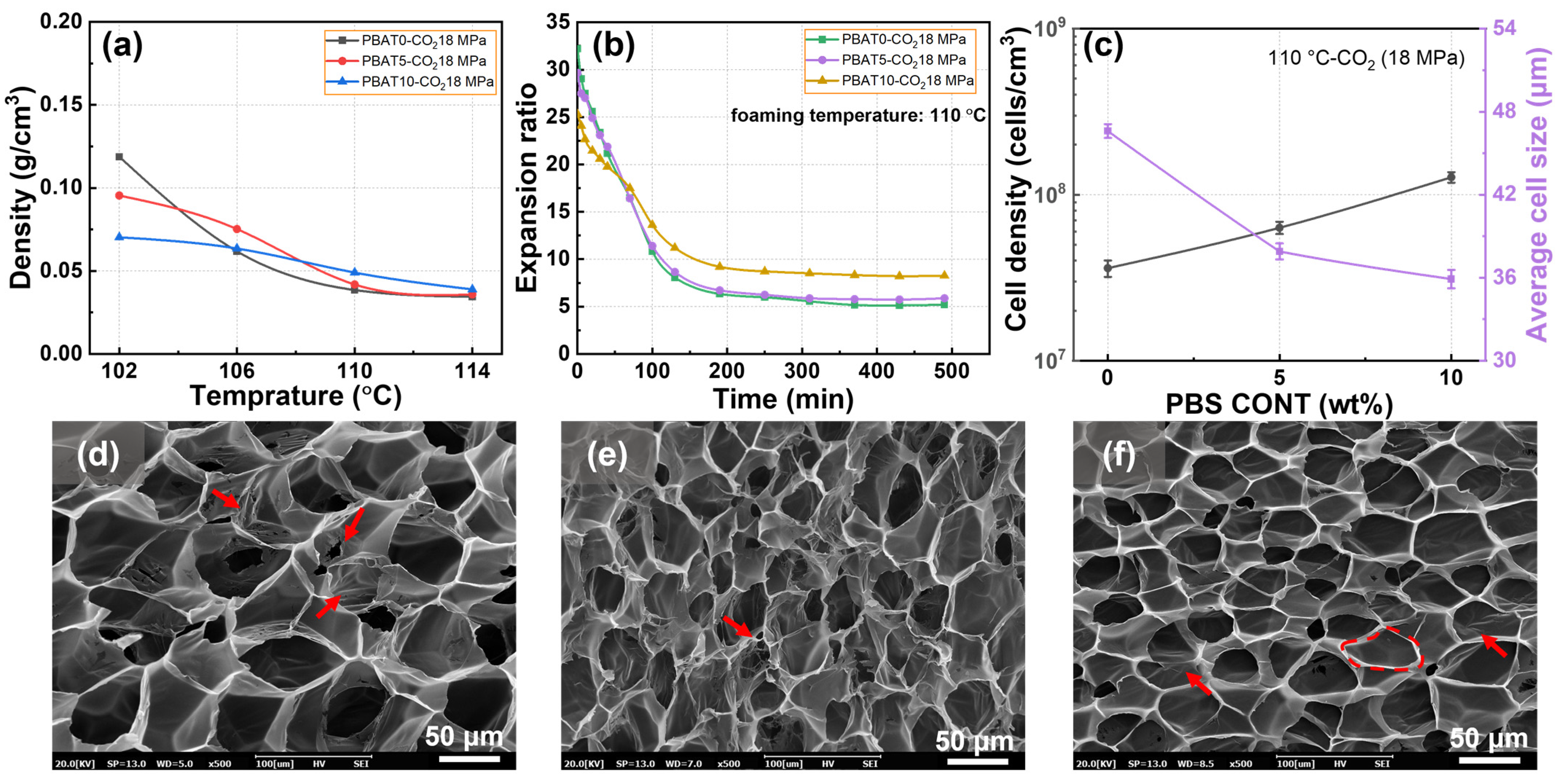
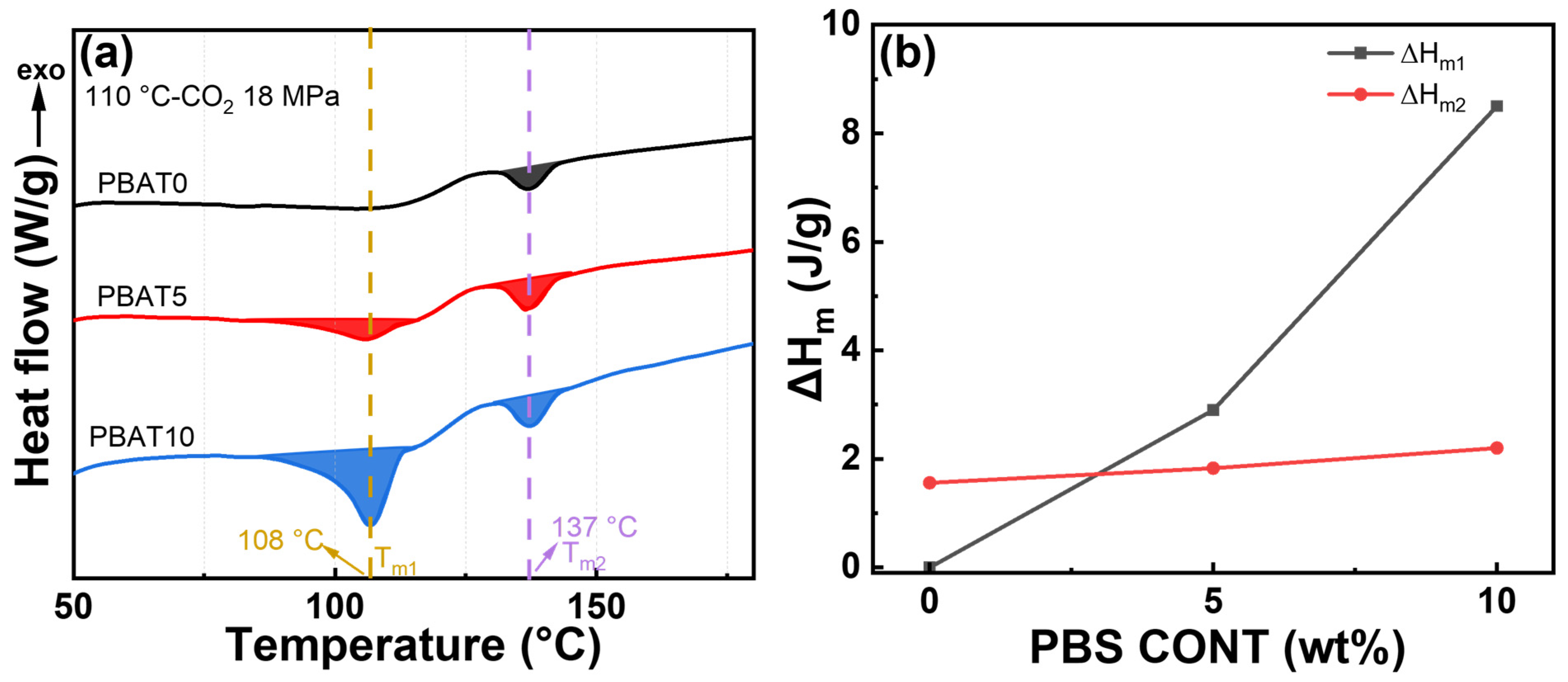
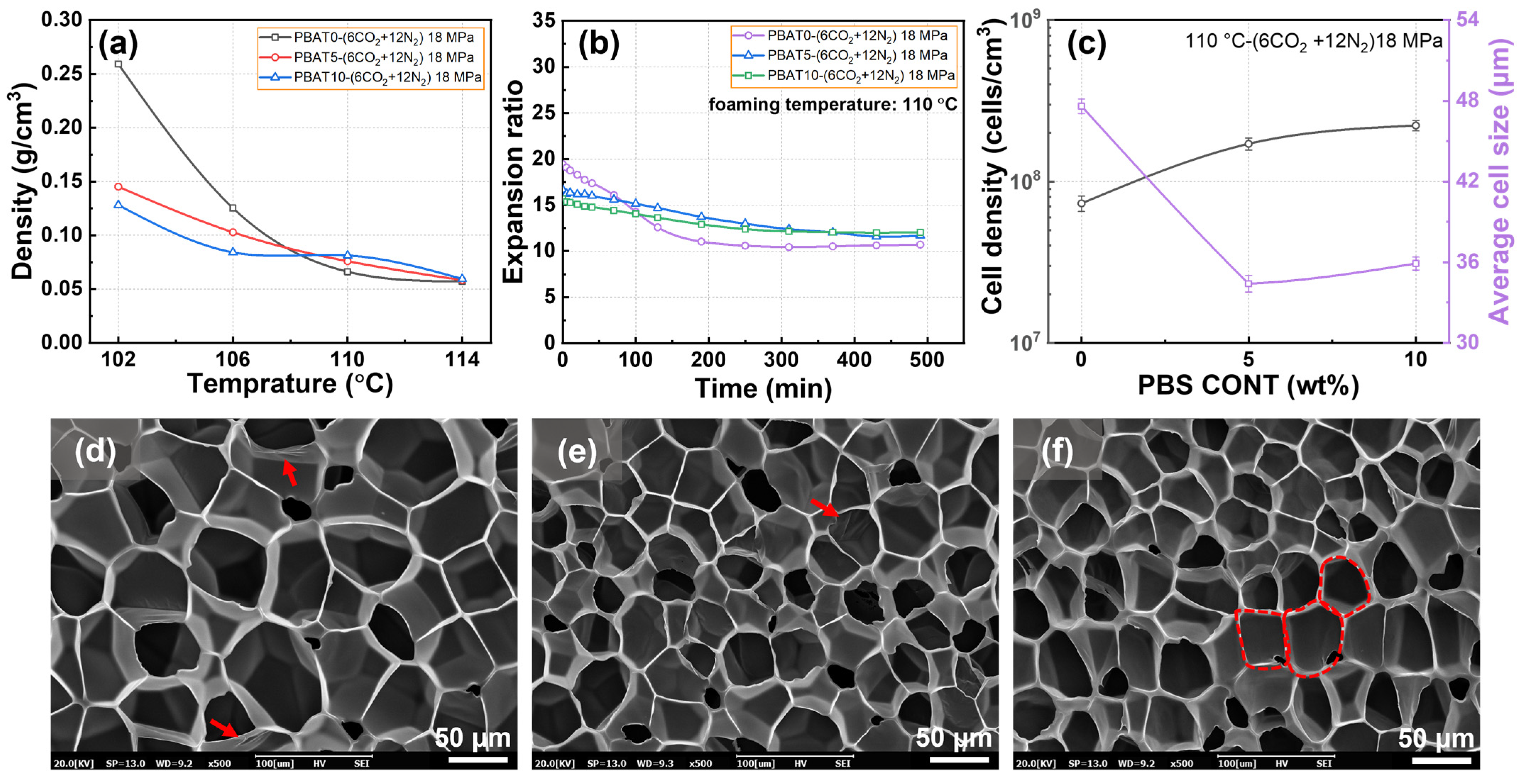
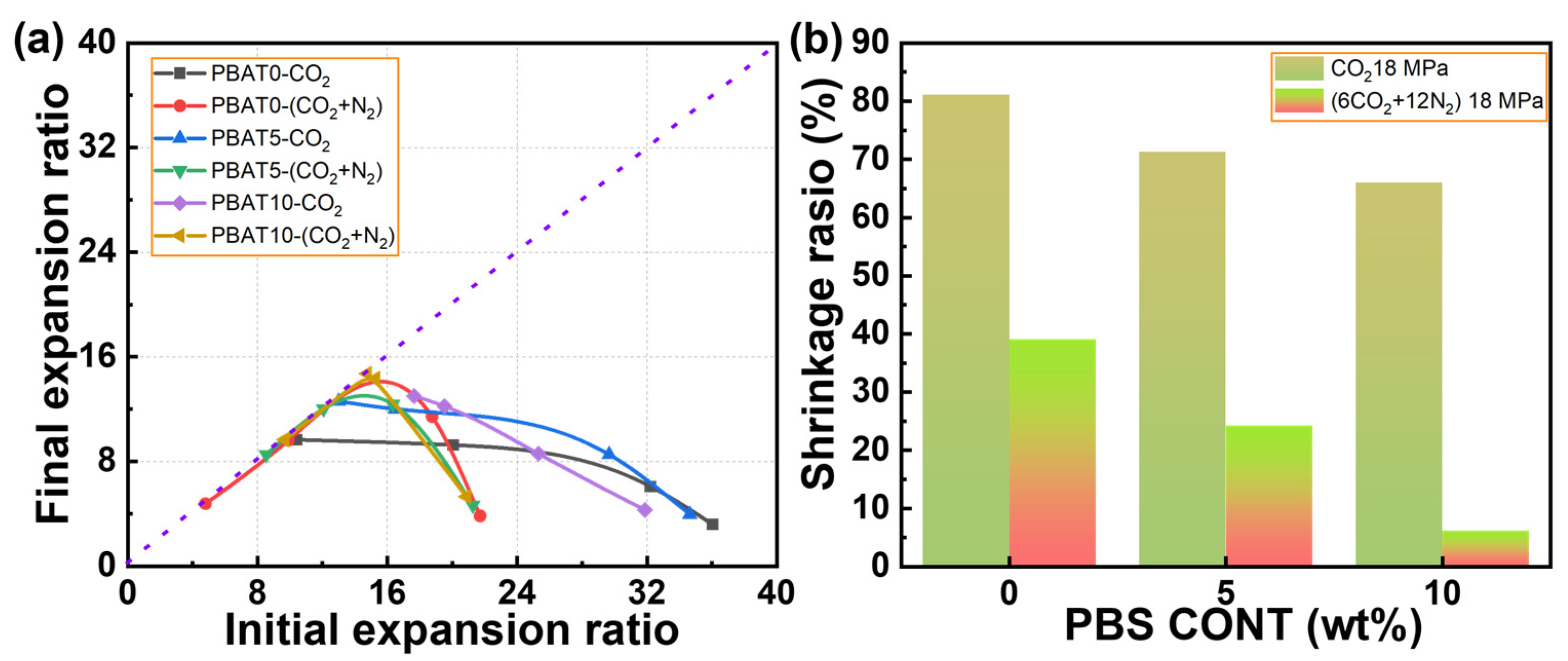
| Sample | Tc1 (°C) | ΔHc1 (J/g) | Tc2 (°C) | ΔHc2 (J/g) | Tm1 (°C) | Tm2 (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|---|---|
| PBAT0 | 40.8 | 19.54 | - | - | 120.0 | - | 14.2 | 12.5 |
| PBAT5 | 3.5 | 1.3 | 62.0 | 19.68 | 108.0 | 123.2 | 16.06 | 14.1 |
| PBAT10 | 11.4 | 4.3 | 53.6 | 16.76 | 109.0 | 124.1 | 20.04 | 17.6 |
| PBS | 64.5 | 60.0 | - | - | 109.6 | - | 51.6 | 46.8 |
| Samples | Gas Ratio (CO2:N2) (MPa) | Cell Size (μm) | Cell Density (Cells/cm3) |
|---|---|---|---|
| PBAT0 | 18:0 | 46.6 | 3.6 × 107 |
| 9:9 | 45.3 | 8.8 × 107 | |
| 6:12 | 47.6 | 7.3 × 107 | |
| 3:15 | 45.8 | 1.1 × 108 | |
| PBAT5 | 18:0 | 37.9 | 6.3 × 107 |
| 9:9 | 36.8 | 7.8 × 107 | |
| 6:12 | 34.4 | 1.7 × 108 | |
| 3:15 | 32.7 | 3.8 × 108 | |
| PBAT10 | 18:0 | 35.9 | 1.3 × 108 |
| 9:9 | 34.6 | 3.6 × 108 | |
| 6:12 | 35.9 | 2.2 × 108 | |
| 3:15 | 33.1 | 3.8 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, F.; Huang, H.; Li, Y.; Zhai, W. Fabrication of Soft Biodegradable Foam with Improved Shrinkage Resistance and Thermal Stability. Materials 2024, 17, 3712. https://doi.org/10.3390/ma17153712
Tian F, Huang H, Li Y, Zhai W. Fabrication of Soft Biodegradable Foam with Improved Shrinkage Resistance and Thermal Stability. Materials. 2024; 17(15):3712. https://doi.org/10.3390/ma17153712
Chicago/Turabian StyleTian, Fangwei, Hanyi Huang, Yaozong Li, and Wentao Zhai. 2024. "Fabrication of Soft Biodegradable Foam with Improved Shrinkage Resistance and Thermal Stability" Materials 17, no. 15: 3712. https://doi.org/10.3390/ma17153712
APA StyleTian, F., Huang, H., Li, Y., & Zhai, W. (2024). Fabrication of Soft Biodegradable Foam with Improved Shrinkage Resistance and Thermal Stability. Materials, 17(15), 3712. https://doi.org/10.3390/ma17153712






