1. Introduction
The first veneer is suspected to have been manufactured at around 3000 B.C. in ancient Egypt. In fact, a type of plywood has been found in coffins dating back to this period, possibly using albumin glues. The modern form of plywood has been used since 1860 in a variety of different applications, initially in furniture items and later as a construction material. During World War I, plywood was used in the production of aircrafts [
1,
2]. Currently, plywood remains a highly valuable material. In fact, in 2021, the global plywood market revenue was at USD 52 billion [
3].
These wood-based panels consist of an assembly of layers glued together with the direction of the grain in adjacent layers usually at right angles [
4,
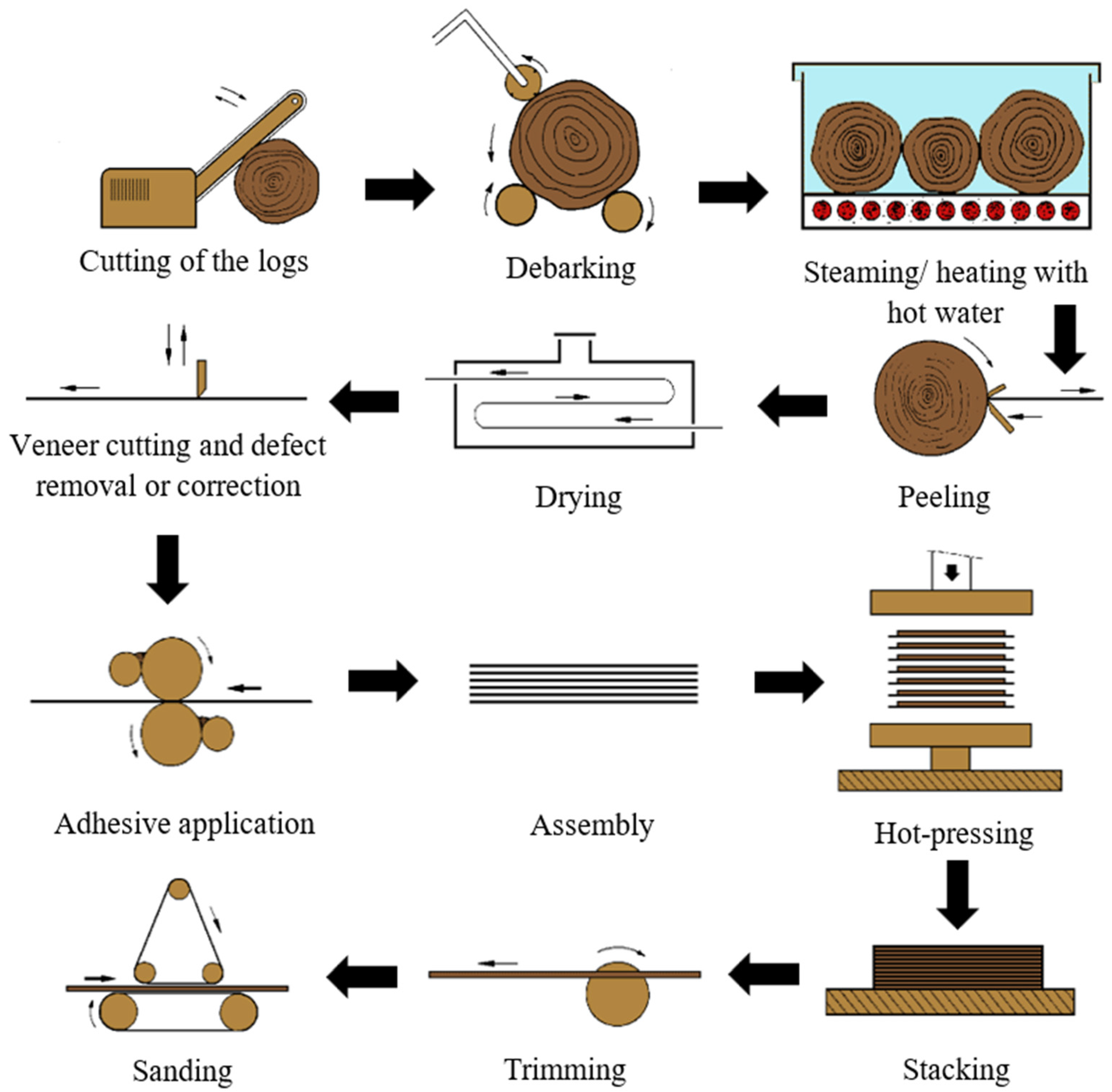
5]. The manufacturing process consists mainly of eight steps: log preparation, heating with steam/hot water, peeling, drying, veneer grading and cutting, adhesive application, assembly of veneer into panels, pressing, and finishing [
6]. A schematic version of the plywood production process is shown in
Figure 1.
The quality of the veneers largely influences the final plywood performance. Thus, the production process begins with the preparation of the logs. As the bark cannot be cut into useful veneer, it is removed. Then, the logs are heated with steam and/or hot water, increasing their flexibility and allowing them to easily transformed into veneers with less strains and internal stresses. The temperature of this process varies depending on the wood species [
6].
Veneering follows as the logs are transformed into continuous sheets by rotating a bolt against a sharp knife. These sheets are then dried to a moisture content between 6 and 12%. Then, the veneer is graded, and the sheets are separated according to their quality. In this step, the patching of veneer sheets may also take place. Thus, defects, such as knots, are patched [
6].
The adhesive application occurs on both sides of alternate veneers, usually through the means of a roller coater. The type of adhesive also depends on the final application of the boards, as phenol–formaldehyde resins are commonly used in exterior grade plywood, whilst urea-formaldehyde (UF) or melamine-urea-formaldehyde (MUF) resins are used in interior applications. The grammage of adhesive is selected according to the veneer wood species and the smoothness of its surface, the grade of the panel, as well as the applied adhesive [
6]. For example, spread rates of 160 to 200 g/m
2 can be applied; however, for low porosity veneers, rates of 130 to 140 g/m
2 have been found to be appropriate. These adhesive formulations are composed of a resin, as well as fillers and/or diluents, which adjust wetting and avoid excessive adhesive penetration, allowing for a uniform joint thickness. For exterior grade plywood, CaCO
3 or non-swelling fillers, such as coconut shell flour, are used in combination with phenol–formaldehyde resins. On the other hand, for interior applications, rye or wheat flour are used alongside UF or MUF resins. Nevertheless, an excessive amount of fillers may deteriorate the plywood’s performance [
4,
7].
In spite of the current advances in automation, plywood panels are mostly assembled manually [
6]. Then, these are submitted to pressing, where pressures of 0.8 to 1.5 N/mm
2 are commonly applied for softwood veneers; on the other hand, for hardwood species, these values can range from 1.5 to 2.5 N/mm
2. Pressure requirements also vary according to the number of veneers and their thickness. Pressing temperatures have also been reported to range from 100 to 165 °C, depending on the chosen adhesive. These conditions result in adhesive temperatures at the bond line from 60 to 120 °C [
4,
8]. Lastly, the plywood is trimmed to size and sanded according to a target thickness [
4]. A wide range of finishes may also be applied, such as non-slip surfaces and various colours [
6].
As mentioned previously, plywood can be used for both interior, humid, and exterior applications [
4]. Nevertheless, for exterior wood products, phenolic resins, usually resoles, are mostly applied. These resins allow these boards to retain their properties even after being repeatedly exposed to moisture, drying, or extreme weather [
4,
7].
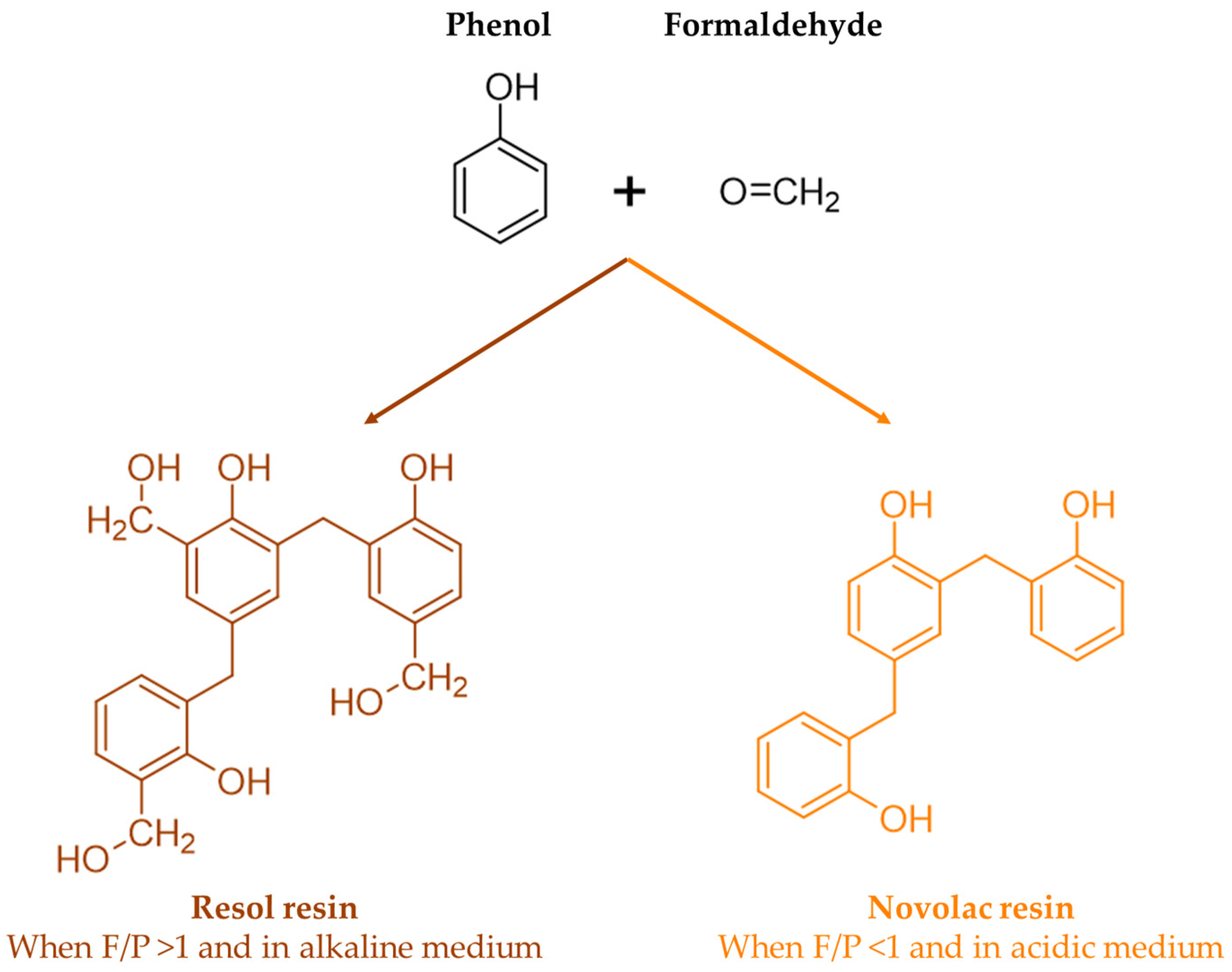
In the synthesis of phenol–formaldehyde (PF) resins, phenols condense with formaldehyde at the 2-, 4-, or 6-position in the presence of acid or alkali catalysts. Thus, methylolphenol or phenolic alcohol is formed, and then dimethylolphenol is formed. The reaction proceeds in a second stage where methylol groups react with other available phenols or methylolphenols, resulting first in the formation of linear polymers and then in highly branched structures [
9]. As numerous condensation products can be formed, the structure of these resins is still not fully understood [
10].
The chemistry of phenolic resins is influenced by a series of factors such as the formaldehyde-to-phenol molar ratio, the used catalyst (acid, base, metal salt, or enzyme), the physical state of the resin (liquid, solid, dispersion), as well as its type (thermoplastic, named novolacs, or thermosetting and resols) [
4].
When a base catalyst combined with an excess of formaldehyde-to-phenol ratio is used, a resol resin is obtained. On the other hand, when less than an equimolar amount of formaldehyde is used under acidic conditions, a novolac resin is produced. These reactions are shown in
Figure 2 [
7].
Unlike resols, novolac resins contain no reactive methylol groups. Therefore, these resins need to be mixed with compounds that can release formaldehyde and form methylene bridges, such as paraformaldehyde or hexamethylenetetramine, in order to crosslink. On the other hand, acidification or heating causes resols to crosslink through their uncondensed phenolic alcohol groups and, possibly, through the release of formaldehyde by the breakdown of ether links [
11].
The main advantages of PF resins are their resistance to water, weather, and high temperatures, as well as their high mechanical performance. Nonetheless, their high price and toxicity has made phenol alternatives, such as technical lignins, increasingly more attractive [
9].
As the most abundant renewable source of phenolic compounds of natural origin, lignin has been increasingly researched as an eco-friendly alternative to petroleum-based compounds [
12]. The large amounts of lignin waste have been proposed for adhesive production since the beginning of the wood pulping industry. However, only 1 to 2% of the global lignin production is used for obtaining value-added products. Lignosulphonates (LS), derived from the sulphite pulping process, account for 70% of the total market for commercial lignin [
13,
14,
15,
16]. Currently, the most successful industrial attempts comprise using lignin in combination with phenol–formaldehyde resins or urea–formaldehyde (UF) resins [
17,
18].
Many approaches have been suggested to increase the reactivity of lignin molecules and, consequently, facilitate their incorporation in resins. These include phenolation, hydrolysis, oxidation, treatment with ionic liquids, and, lastly, methylolation [
19,
20,
21,
22,
23,
24]. Lignin methylolation, or hydroxymethylation, introduces hydroxymethyl groups (–CH
2OH) onto lignin molecules. This reaction usually takes place with formaldehyde in an alkaline medium. The resultant lignin can be directly incorporated as a phenol substitute in the synthesis of PF resol resins for wood adhesives [
21].
Ghorbani and co-workers studied four commercial spruce LS for different sulphite pulping processes as partial 40% (
w/
w) phenol in lignin–phenol–formaldehyde (LPF) resol resins. Although displaying the lowest average MW and dispersity, sodium LS, when incorporated into the LPF resin for gluing beech veneer strips, resulted in the best curing and tensile shear strength development under hot pressing. On the other hand, calcium and magnesium LS were less suited as phenol replacements, since the obtained LPF adhesives had poor performance. The authors also claimed that the phenolation of sodium and ammonium LS, which had the most promising characteristics, did not significantly improve the performance of the obtained LPF resins [
25]. Nevertheless, the moisture resistance of the LPF resins was not assessed, although this property is frequently reported to be compromised when LS are used in wood adhesives. These resins were also not applied in the production of plywood.
Studies have also shown that impurities in crude lignin, specifically elemental sugars, lowered the reactivity of lignin towards formaldehyde for the incorporation in LPF resins, thus leading to longer pressing times. The strength and water resistance of the obtained resins were also hindered [
26,
27].
Alonso and co-workers used methylolated softwood ammonium LS as a phenol substitute in the synthesis of a LPF resin. The optimum operating conditions for this synthesis were studied and defined as a phenol substitution of 35% (
w/
w), a NaOH-to-phenol and LS molar ratio of 0.6, and a formaldehyde-to-phenol and LS molar ratio of 2.5. The authors claimed that the obtained LPF resin complied with the specifications necessary for use in plywood. Its characteristics were also similar to those of the commercial PF resol resin used as a reference [
28]. Nonetheless, this study did not include the production of plywood nor any testing of plywood samples.
Lastly, Akhtar et al. used herbaceous lignosulphonates as phenol substitutes in PF resins for exterior plywood. These boards where submitted to three separate pre-immersion treatments prior to shear testing: 24 h in water at room temperature, 2 h in water at 70 °C, and immersion in boiling water for 8 h. The authors concluded that the pretreatments did not significantly affect the shear testing results. However, the increase in LS content resulted in a significant decrease in board performance. For a 70% phenol substitution, a 50% decrease in shear load was observed. Nonetheless, the authors concluded that these boards obeyed Pakistani standard PS.871:1970 (plywood for general purposes) [
29]. It should be noted that these boards were not assessed according to the more demanding current European standard EN 314-1 [
30].
Other types of technical lignins have also been used as phenol substitutes in the production of plywood with higher degrees of success.
Ghorbani et al. achieved a 40% (
w/
w) phenol substitution using pine kraft lignin in exterior grade plywood, which complied with the requirements of EN 314-2 bonding class 3 for exterior conditions. When this lignin sample was previously methylolated, no significant improvements in terms of bonding strength were detected [
31,
32].
These studies all report up to 40% (
w/
w) phenol substitution without the loss of the resin’s performance. However, Kalami and co-workers have reported 100% (
w/
w) phenol substitution. In this study, the applied lignin was a byproduct of the bioethanol process obtained through a dilute acid pretreatment and the enzymatic hydrolysis of corn stover. When the LPF resin was used to produce poplar veneer single-lap-joint samples, there was no significant difference between the shear strengths made with the LPF adhesives and those made with the commercial phenol–resorcinol–formaldehyde resin. These conclusions were also maintained after a pretreatment in boiling water for 4 h, subsequent drying at 65 °C for 20 h, and a final immersion in boiling water for another 4 h [
33]. Although these single-lap-joint samples were submitted to a treatment similar to EN 314-2 bonding class 3 for exterior conditions, plywood was not manufactured.
Patents have also been filed by companies, such as UPM, which report a phenol substitution of up to 50% (
w/
w) in plywood production. For this purpose, kraft lignin was previously modified through alkylation, phenolation, and methylolation [
34]. UPM also claims that their lignin products can replace up to 80% (
w/
w) of phenol in PF resins for plywood whilst meeting the requirements of EN 314-2 bonding class 3 for exterior conditions. For this purpose, a high pressing factor of upwards of 240 s/mm was employed, whereas values of about 35 to 145 s/mm have been reported in industrial plywood production [
2,
29,
35].
In this study, lignosulphonate–phenol–formaldehyde resoles were synthesized considering a phenol replacement of 30% (w/w) by LS. Two LS samples of softwood and hardwood origin were studied and compared. Their performance was evaluated, in terms of single-lap-joint bonding, through the Automated Bonding Evaluation System (ABES). Then, the most promising were used to produce plywood samples, whose performance was assessed according to EN 314-2 bonding class 3 for exterior conditions. This study aims to address a gap in the literature where kraft lignin is more commonly studied as a phenol substitute, while LS are dismissed for having poor moisture resistance. Similarly noteworthy, plywood was manufactured and tested according to the current European standard EN 314, which is seldom taken into consideration in the literature. As such, this study effectively addresses the implications of using LS as a partial phenol replacement in exterior-grade plywood.
2. Materials and Methods
2.1. Materials
Thick spent sulfite liquor (HLS) from the acidic magnesium-based sulfite pulping process of Eucalyptus globulus (hardwood) was supplied by Caima-Indústria de Celulose SA (Constância, Portugal). Spray-dried sodium lignosulphonate (SLS) (STARLIG®NA98S) from the sodium bisulfite pulping process of Picea abies (softwood) was supplied by LignoStar International BV (The Hague, The Netherlands). Poplar veneers were supplied by Laminar—Indústria de Madeiras e Derivados, S.A. (Vila Nova de Gaia, Portugal). Unless stated otherwise, all other chemicals were provided by Euroresinas—Indústrias Químicas, S.A. (Sines, Portugal), a company from Sonae Arauco Group.
2.2. Physico-Chemical Characterization of the LS Samples
For the determination of the dry matter content, the samples were dried in a ventilated oven at 105 °C until a constant mass was reached [
36]. The ash content of the samples was determined gravimetrically after ignition in a muffle furnace at 525 °C, according to ISO 1762 [
37].
For the spent sulphite liquor sample, the pH, density, and viscosity were also determined. Further, pH values were evaluated using an InLab Routine Pro combined pH glass electrode with an integrated temperature probe (Mettler Toledo, Columbus, OH, USA). The density and viscosity determinations were conducted using a hydrometer (Ludwig Schneider, Wertheim, Germany) and a Brookfield Model DV-II + viscometer with spindle 2 at 60 rpm (AMETEK Brookfield, Middleboro, MA, USA), respectively.
In order to determine the lignosulphonate content, 0.5 g of HLS was diluted with deionized water in a 1000 mL volumetric flask. In this case, the pH was previously adjusted to 5. A similar solution was prepared for the SLS, taking into account the solids content of the HLS. Then, 0.5 mL of these solutions was transferred to a quartz cuvette and diluted to 3.0 mL with deionized water [
38]. The LS content of the samples was calculated from the absorbance at a wavelength of 232.5 nm, considering an extinction coefficient of 24.5 L g
−1 cm
−1 [
39,
40]. A GENESYS 10S UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA) was used.
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
The infrared spectra were recorded using a VERTEX 70 FTIR spectrometer (Bruker, Billerica, MA, USA) in the transmittance mode with a high-sensitivity DLaTGS detector at room temperature. The samples were measured in ATR mode, with an A225/Q PLATINUM ATR Diamond crystal (Bruker, Billerica, MA, USA) with a single reflection accessory. The spectra were recorded from 4000 to 500 cm−1 with a resolution of 4 cm−1. The samples were previously dried at 105 °C.
2.4. Resin Synthesis
The resins were manufactured in round bottom flasks of 2000 mL equipped with a thermometer and mechanical stirrer. Temperature control was accomplished by means of a heating blanket. The synthesis process was essentially divided into two stages: methylolation and condensation.
The synthesis process began with the addition of phenol, water, and an appropriate amount of 50% (w/w) NaOH resulting in a pH of 9 to 10. Then, methylolation began with the addition of formaldehyde 50% (w/w) over a 30 min time period whilst maintaining the temperature at 60 °C. A formaldehyde-to-phenol ratio between 2.5 and 3.5 was considered. The mixture was then heated slowly to 95 °C and left to react for 15 min. Thereafter, the condensation began at 85 °C with the addition of 50% (w/w) NaOH, resulting in a pH of 10.5 to 12.
At this stage, the condensation of the resin was allowed to progress until a viscosity of about 250 to 450 cP was reached. Lastly, the reaction was stopped through cooling of the reaction mixture to room temperature.
When LS were used as a partial phenol substitute, they were added alongside phenol, considering a 10, 20, or 30% (w/w) substitution rate. This was determined considering that the concentration of LS in the HLS and SLS sample was 33% and 80%, respectively. The formaldehyde content of the resins was also reduced and determined according to a formaldehyde-to-LS solids ratio between 0.15 and 0.20.
2.5. Methylolation
Firstly, the jacketed reactor was loaded with the LS sample, and deionized water was added to adjust the final concentration of lignosulphonate in the reaction medium to approximately 280 g/L. Then, the pH was adjusted to 9.6 using a NaOH solution at 50% (w/w). Lastly, formaldehyde 50% (w/w) was added according to a formaldehyde-to-LS solids ratio of 0.17 and the reaction mixture was heated to the desired temperature. The mixture was left to react for 3 h at 60 °C or 1 h at 70 °C.
2.6. Resin Characterization
pH—The pH of the obtained resins was measured at 25 °C using a pH glass electrode with an integrated temperature probe.
Viscosity (cP)—The viscosity of the resins was measured at a constant temperature of 25 °C using a Brookfield DV-II programmable viscometer.
Density (g·cm−3)—The determination of the resin’s density is based on the weight/volume ratio and was conducted using a hydrometer.
Solids content (%)—The solids content was determined by drying 2 g of resin for 2 h in an oven at 135 °C. Three replicates were caried out for each resin.
Free formaldehyde content (%)—The free formaldehyde content of the obtained resins was not determined. This was due to the rapid precipitation of the resins upon the addition of acid, which is required for the adjustment of a sample’s pH as stated in ISO 11402:2004 [
41].
Water tolerance (%)—For the determination of the water tolerance of the resins, 5 mL of resin was placed inside a test tube and water was added gradually. When the solution turned turbid, the consumed water volume was registered and the water tolerance was determined as follows:
where
is the volume, in mL, of water added.
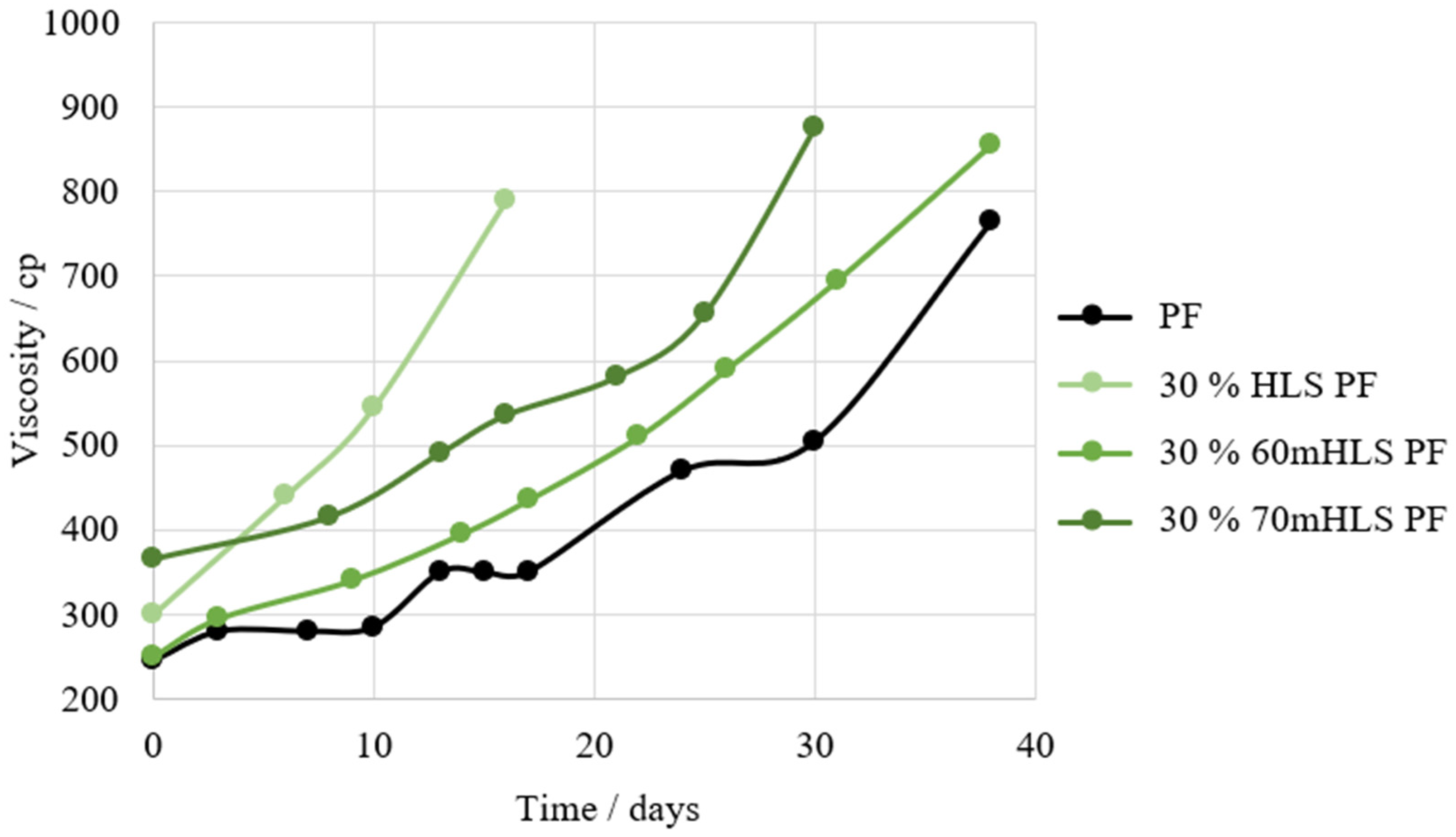
Shelf-life (days)—The increase in viscosity of the resins over the course of several days at 25 °C was measured using a Brookfield DV-II programmable viscometer. The shelf-life of the resins was established as the number of days required for a viscosity above 700 cP to be reached.
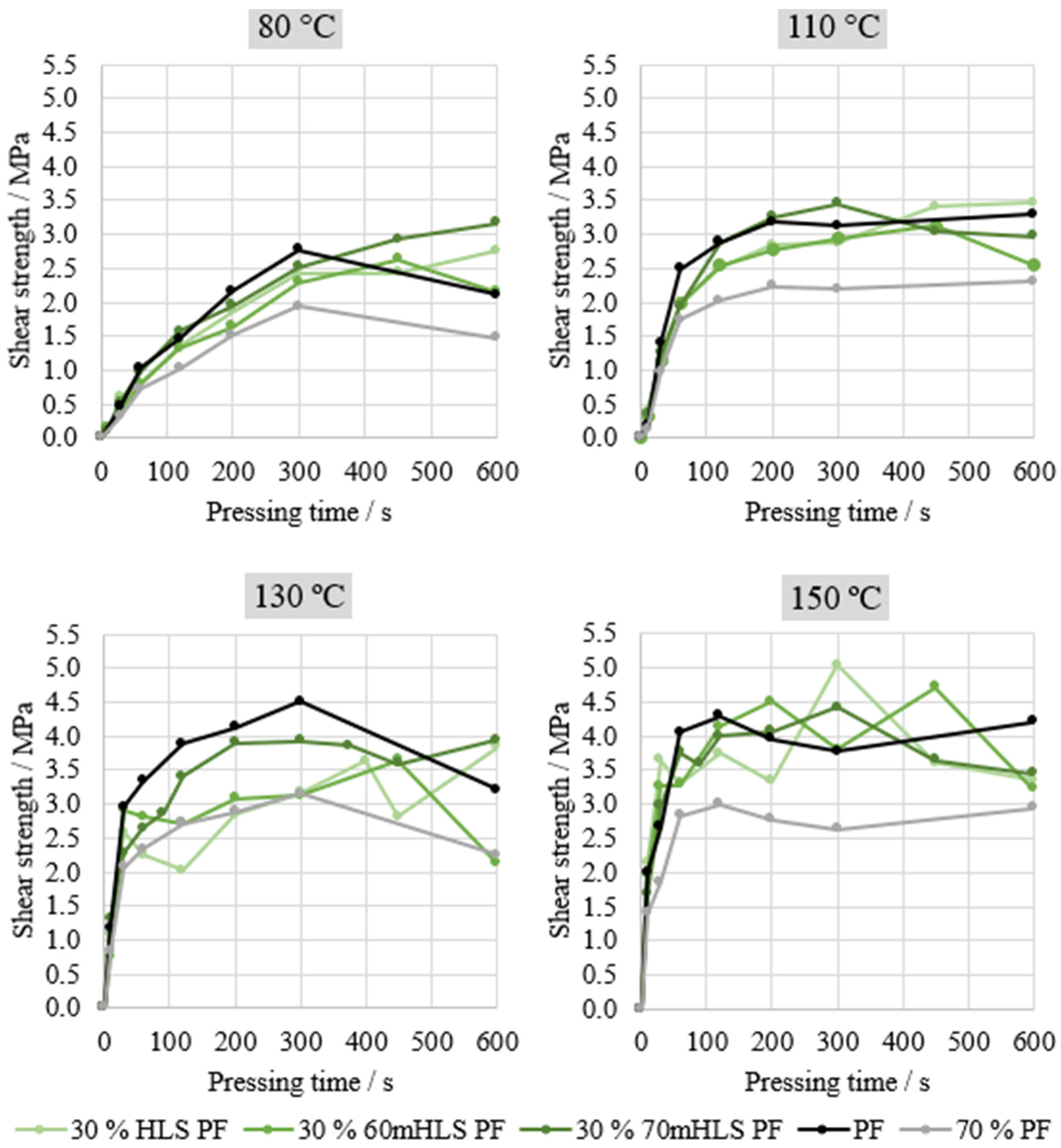
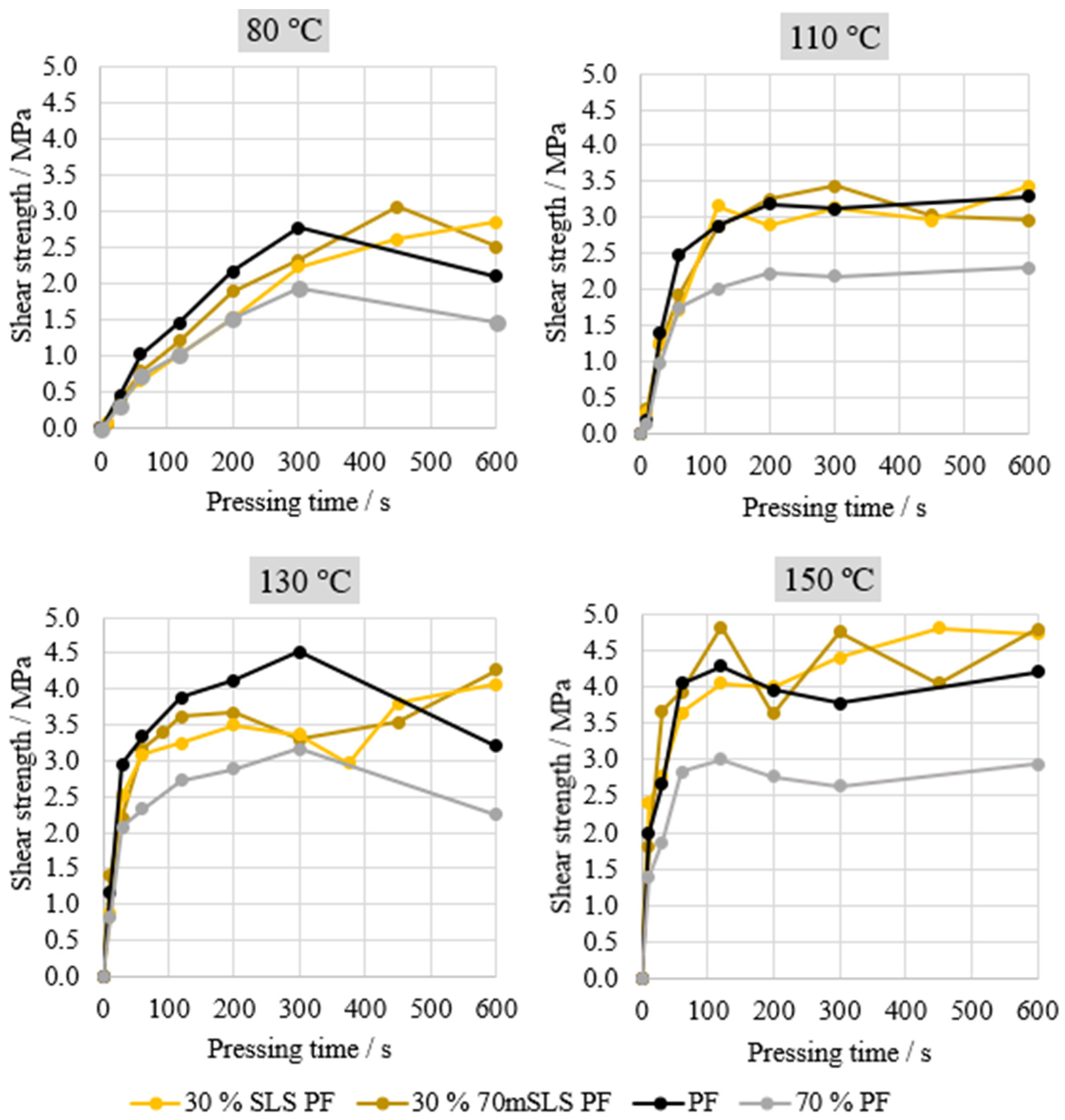
Automated Bonding Evaluation System (ABES)—The bonding performance to a wood substrate of the manufactured resins was evaluated by testing the samples in shear mode. For this test, two wood veneer strips (
Fagus sylvatica), 0.5 mm thick, 20 mm wide, and 117 mm in length, were used. These strips were previously stored at a controlled temperature (25 °C) and relative humidity (65%) in order to stabilize them and achieve an equilibrium moisture content between 8 and 11% (dry basis). These wood test pieces were glued manually together with a spatula (10 mg in 100 mm
2) with the resin formulation (composed of the resin, wheat flour, and calcium carbonate). After the set temperature was reached, the strips were mounted in the system with an overlapping area of 20 × 5 mm
2 and pressed together at 1.2 N·mm
−2 for a chosen period of time. The system is digitally controlled and pneumatically driven, and a standard loading rate of 1 kN·s
−1 was used [
42].
2.7. Plywood Production
Plywood samples were manufactured using poplar wood veneers bonded with the LPF resins. The adhesive mixture was composed of resin, wheat flour, and calcium carbonate. Five-layer plywood with a size of 50 cm × 50 cm were assembled by spreading the glue mixture on the veneer surface with a glue spread of 180 g/m2. The adhesive mixture was spread on top of the first sheet and on the bottom of the third, fourth, and fifth veneer. The plywood was pressed in a parallel plate hot-press, ITALPRESSE GL/90 (ITALPRESS, Bergamo, Italy), at 130 °C for 10 min, considering a target thickness of 10.5 mm. As a control, plywood bonded with a commercial PF adhesive was also prepared.
2.8. Plywood Characterization
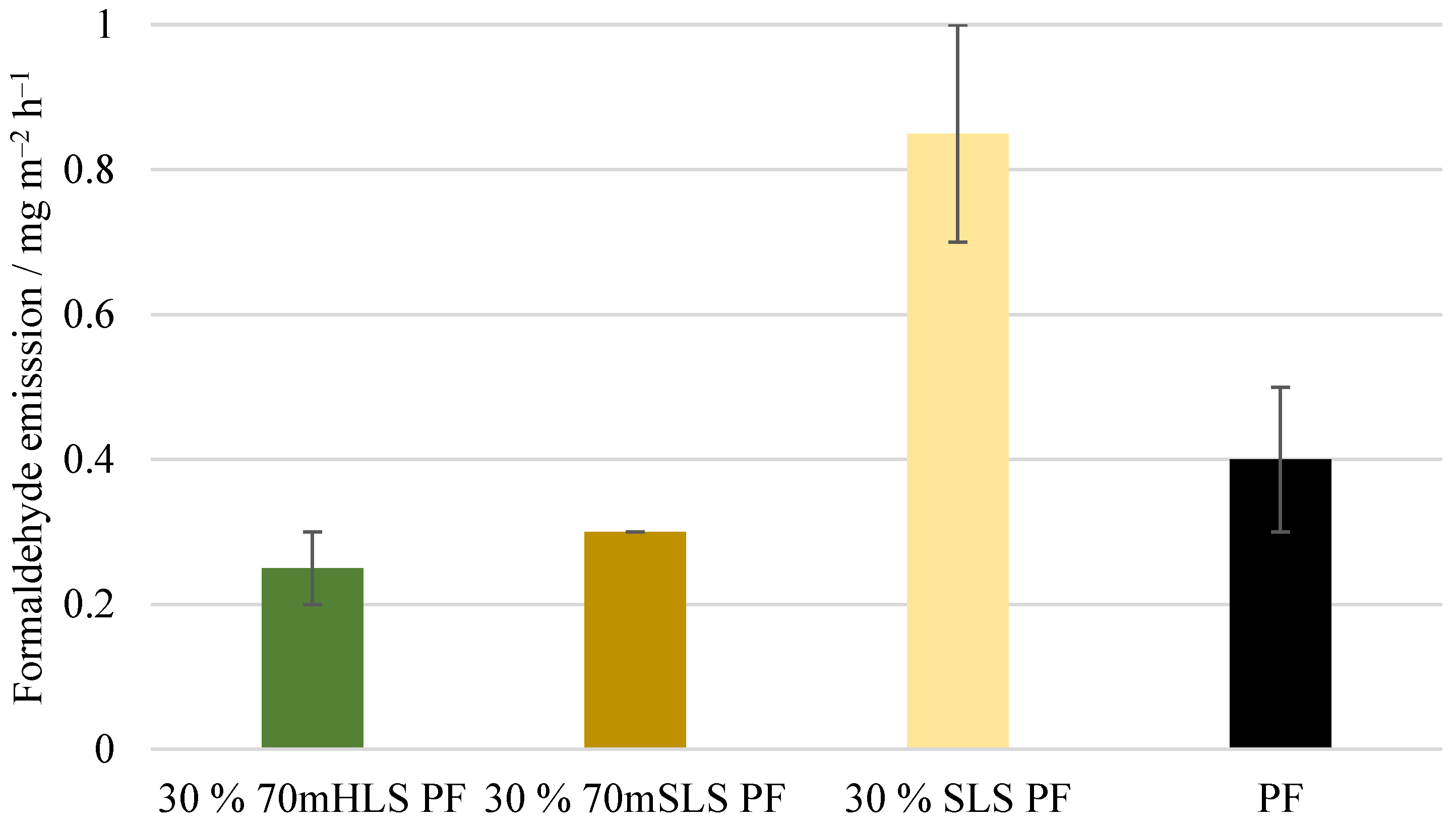
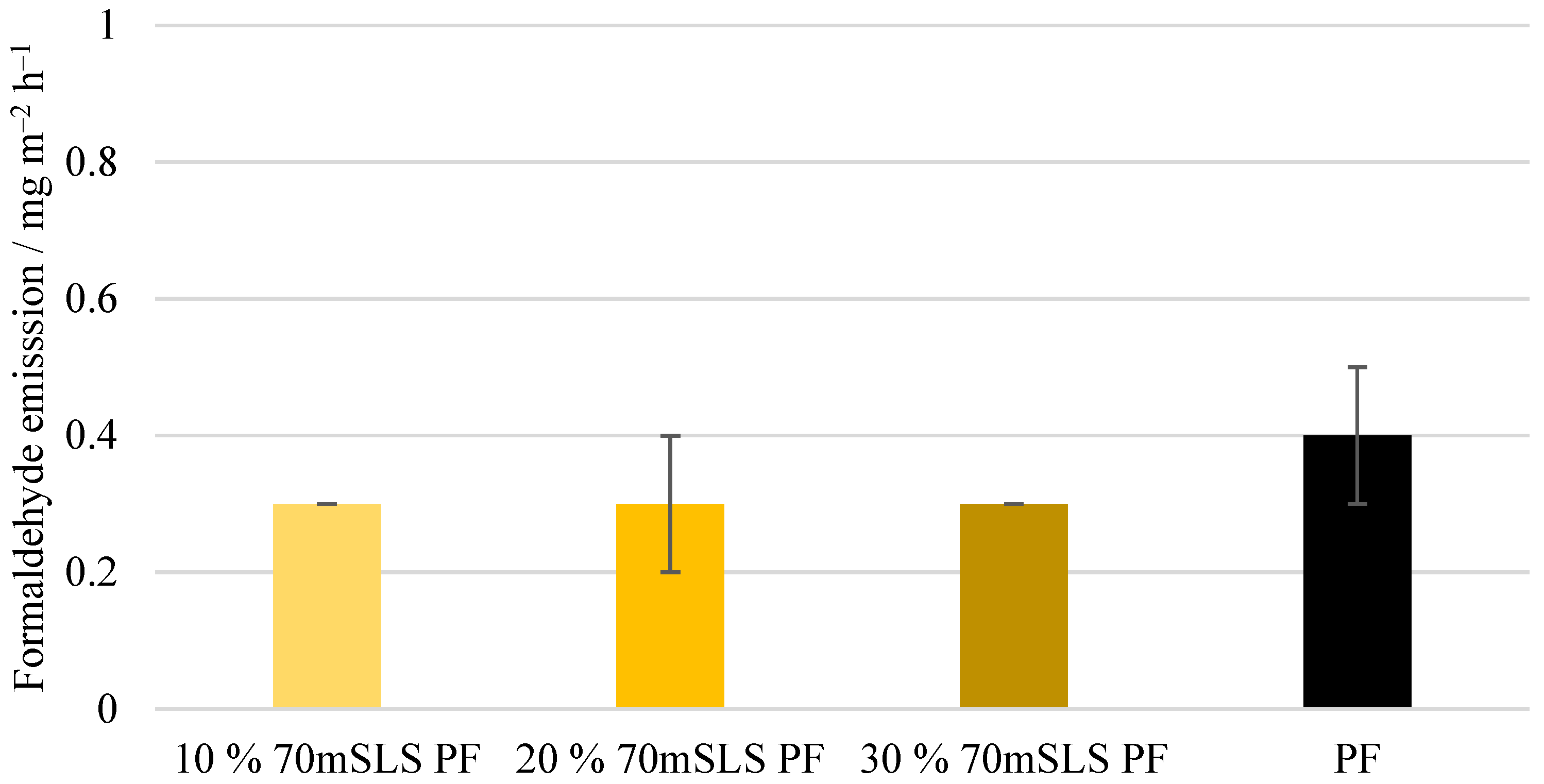
The following physico-mechanical properties were studied in accordance with their respective European standards: density (EN 323), moisture content (EN 322), formaldehyde release through the gas analysis method (EN ISO 12460-3), modulus of elasticity and bending strength (EN 310), and bonding quality (EN 314-1). Before testing, the test pieces were subjected to the pretreatments according to class 3—exterior conditions (EN 314-2) [
30,
32,
43,
44,
45,
46]. Thus, the plywood samples, shown in
Figure 3, were immersed in water for 24 h at 20 °C. Then, these where immersed in boiling water for 4 h and dried for 16 to 20 h at 60 °C. Afterwards, the samples were once again immersed in boiling water for 4 h. Lastly, the samples were cooled in water at 20 °C [
32]. The requirements for EN 314-2 are shown in
Table 1.
4. Conclusions
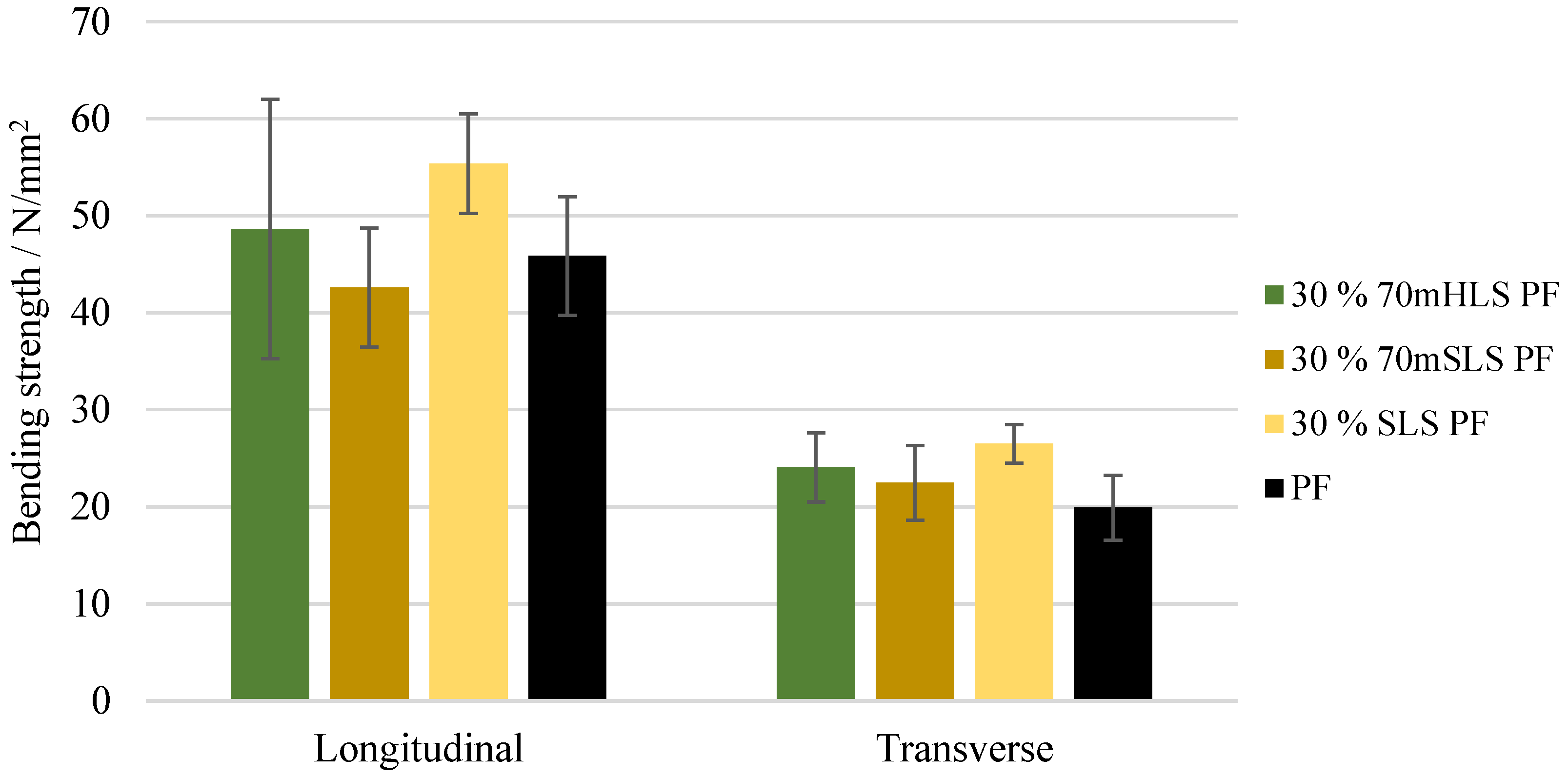
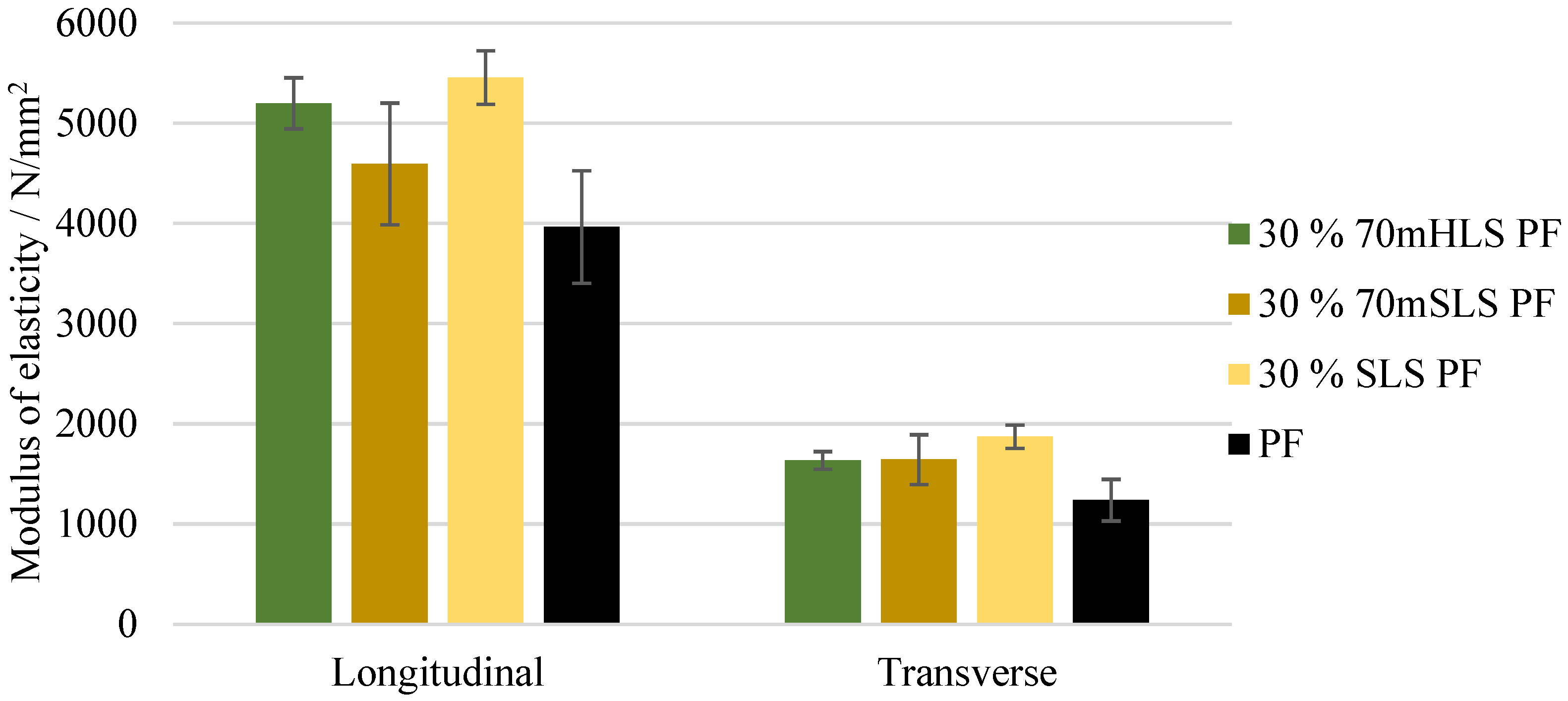
Hardwood and softwood lignosulphonates (LS), HLS and SLS, respectively, were applied as partial phenol substitutes to produce lignosulphonate–phenol–formaldehyde resins for exterior plywood. At a 30% (w/w) phenol substitution, the physico-chemical characteristics of the original commercial phenol–formaldehyde resin were maintained.
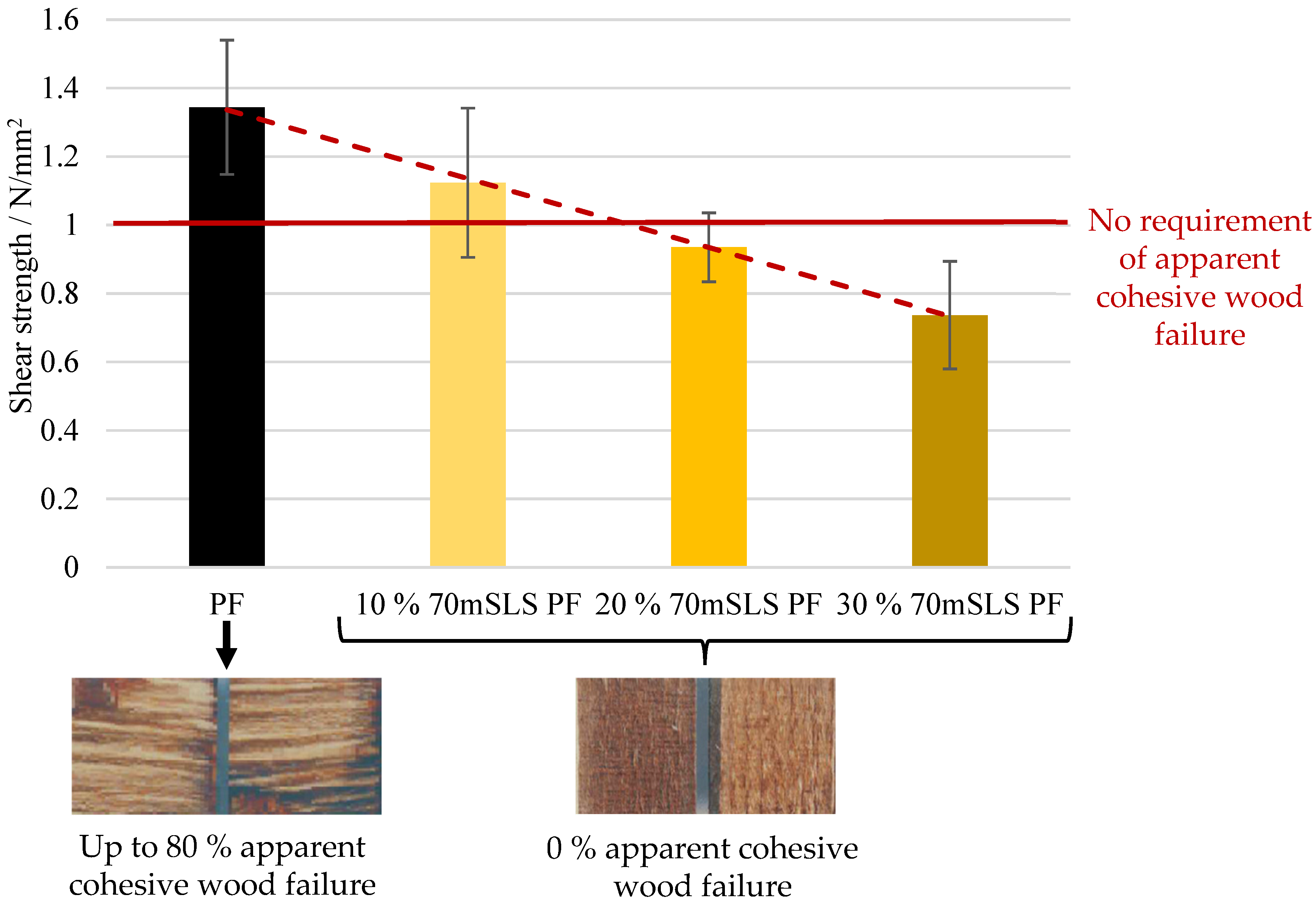
Based on ABES (Automated Bonding Evaluation System) evaluation, three resins were selected as the most promising: the LPF resins using SLS as a phenol substitute without previous methylolation and the resins using SLS and HLS, previously methylolated at 70 °C.
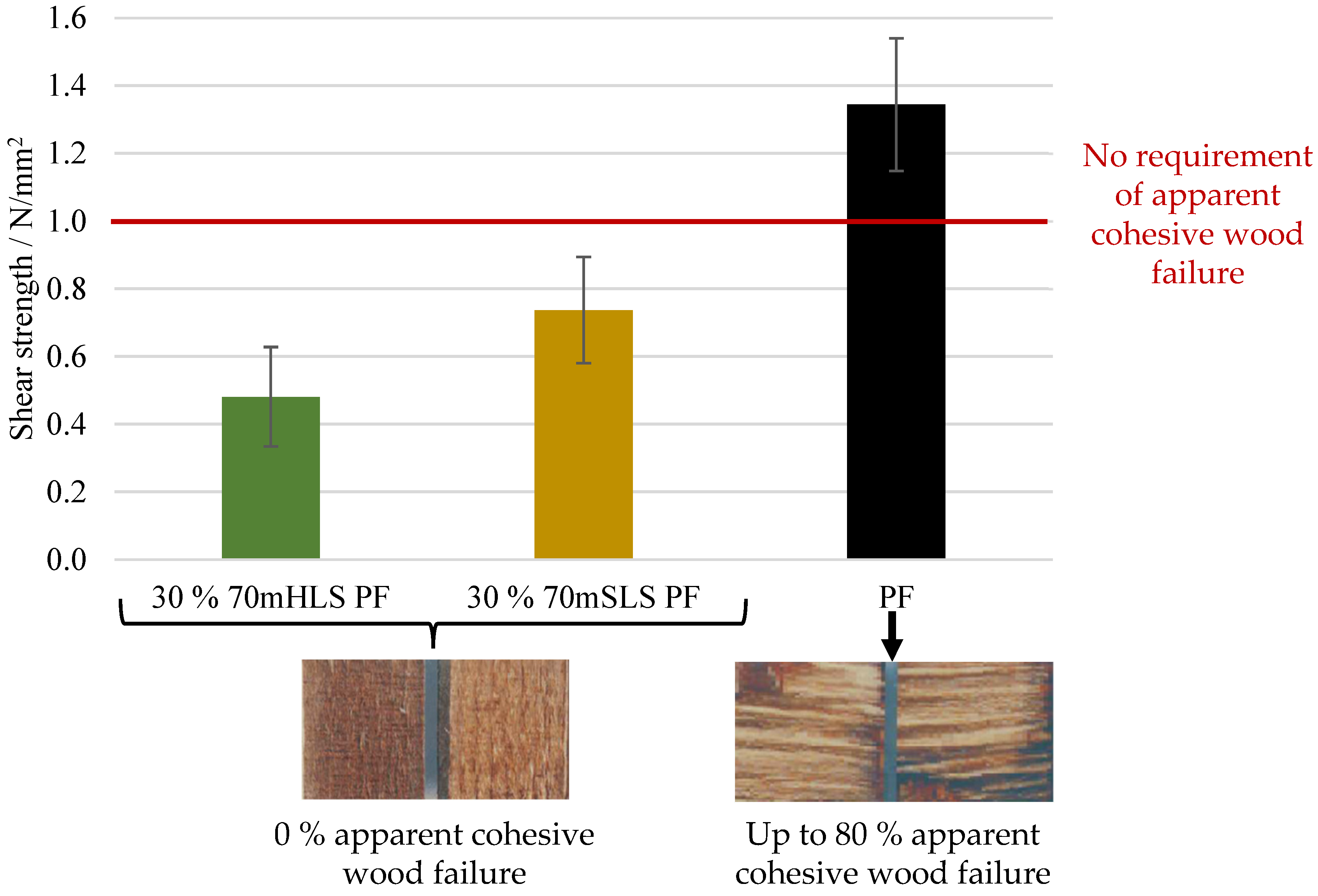
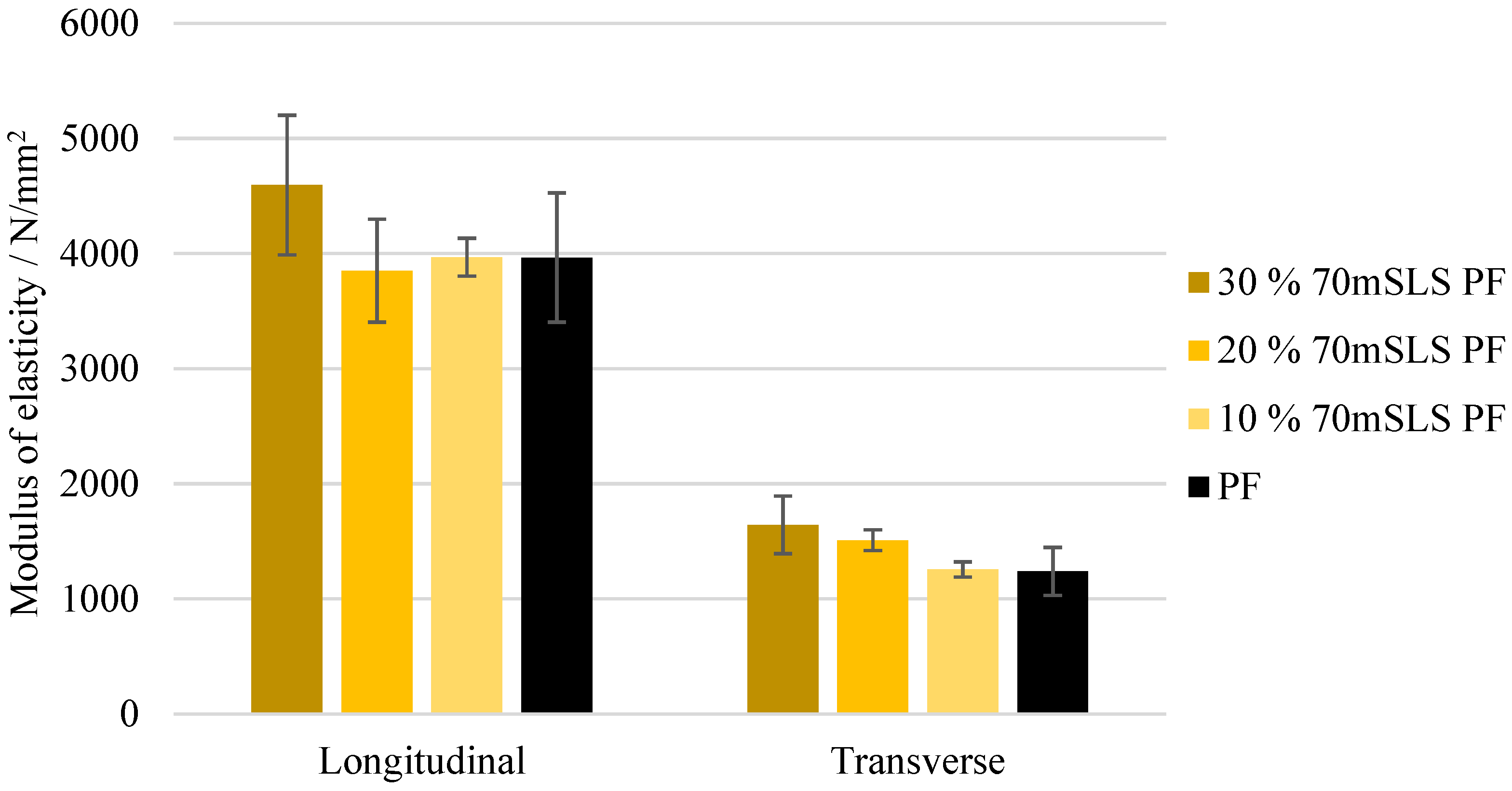
At 30% (w/w) phenol replacement, the methylolated SLS sample resulted in the lowest decrease in plywood performance. Nonetheless, when this sample was used without prior methylolation, the plywood samples suffered delamination during immersion in boiling water prior to shear testing, as required in EN 314-2 bonding quality class 3 for exterior conditions.
None of the LPF boards obeyed the requirements of this standard. In fact, even at 10% (w/w) phenol substitution, a significant decrease in board performance was already observed.
Despite the insufficient performance of the plywood samples produced, this study provides new insights on the application of lignosulphonates in PF resins for the manufacture of exterior plywood. The results also reinforce the necessity of the modification of LS prior to its incorporation in phenolic resins in order to overcome their tendency to confer low moisture resistance to the final adhesive.
Future studies should attempt to further improve these results, testing other LS modifications, such as phenolation. Additional crosslinkers may also be added in order to increase phenol substitution without the loss of the LPF resin’s performance.