Plant Extracts for Production of Functionalized Selenium Nanoparticles
Abstract
:1. Introduction
2. Characterization of SeNPs
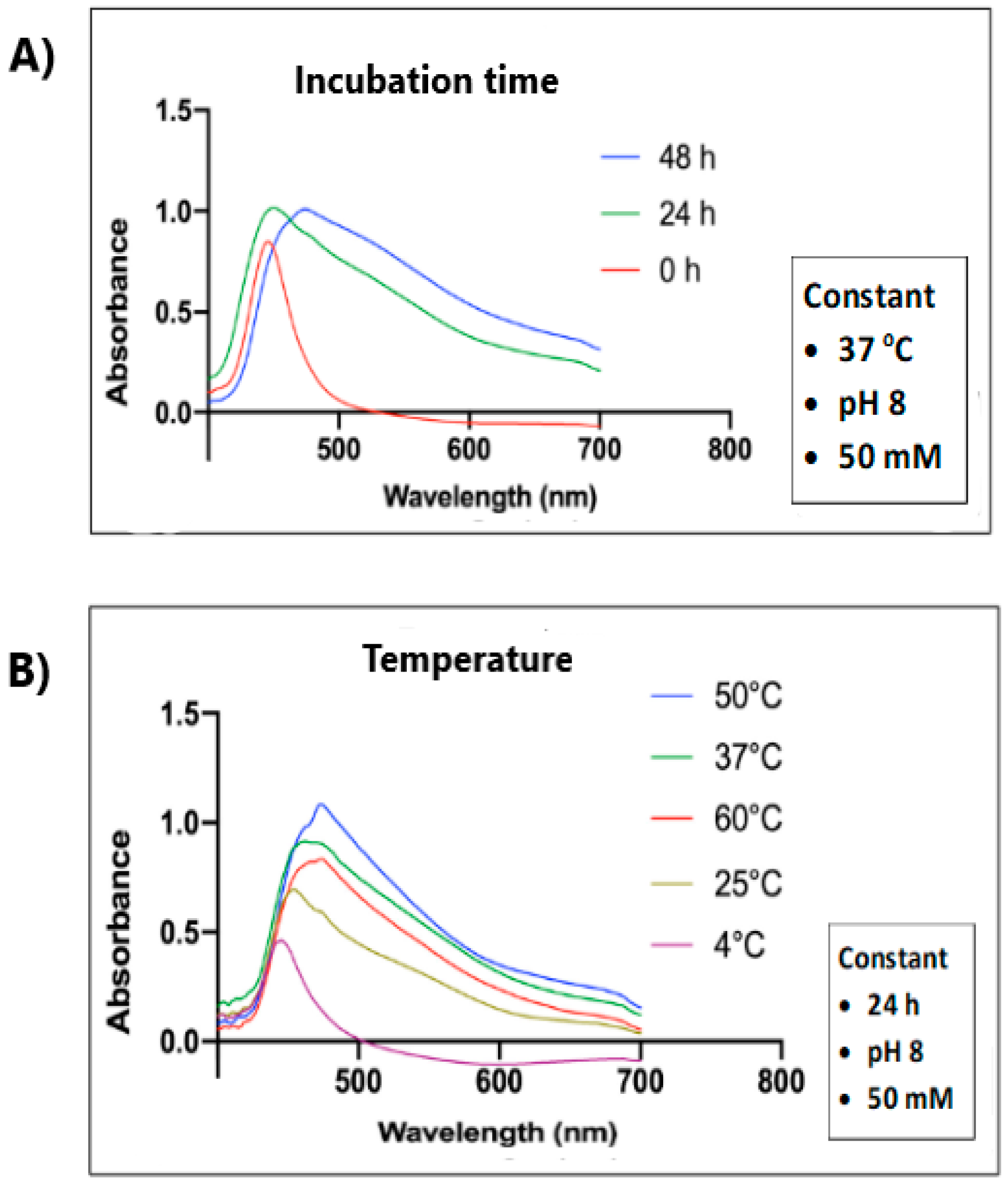
2.1. UV–Vis Spectra of SeNPs
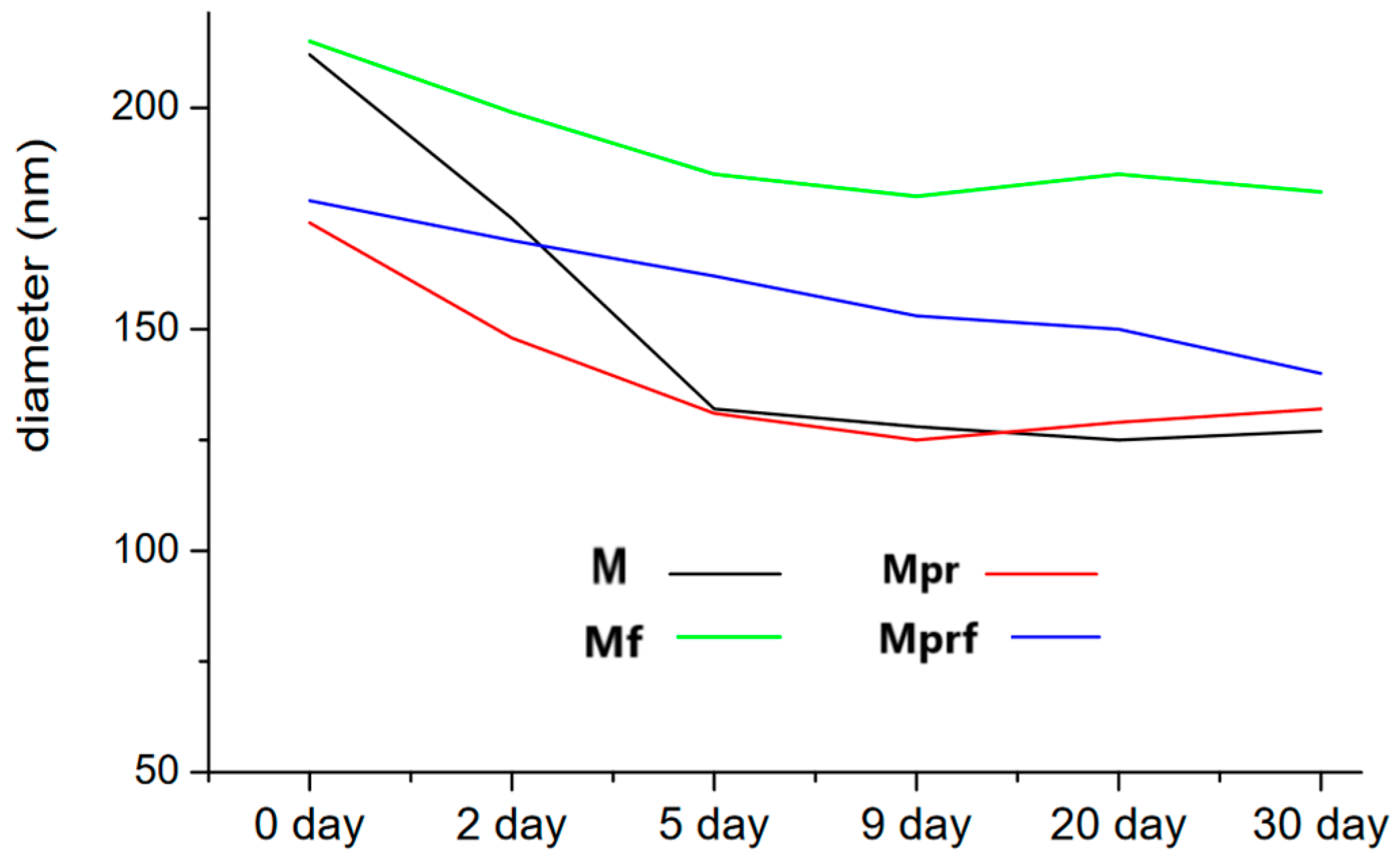
2.2. Particle Size, Distribution, and Morphology
2.3. Surface Composition and Charge
| Plant Material | Synthesis Conditions | Characteristics | Properties | Ref. |
|---|---|---|---|---|
| Broccoli (Brassica oleraccea) leaves | 30 mM Na2SeO3 + extract (30 mL), stirred for 2–3 days | 10–28 nm (SEM) average 15.2 nm (TEM) | Antioxidant, anticarcinogenic | [21] |
| Okra (Abelmoschus esculentus) | Na2SeO3 (0.08 g dissolved in 50 mL of water) + extract stirred for 48 h | 17.3 nm (DLS) 46.15 nm (TEM) ZP: −64 mV | Antibacterial | [22] |
| Mandarin peel-derived pectins functionalized with olive pomace | 0.1 M Na2SeO3 (1 mL) + 5 mL of 1% olive pomace (5%) + pectins (15 mg), stirred for 20 min | 171–217 nm PDI: 22.7 ZP: −22.5 mV | Antioxidant | [23] |
| Herbs (lemon balm, hop, raspberry, sage, blackberry) | 0.1 M Na2SeO3 (2.5 mL) + extract (2.5 mL), stirred for 60 min | 74.0–96.8 nm PDI: 0.103–0.132 | Antibacterial, antioxidant | [27] |
| Walnut leaves | 0.01 M Na2SeO3 (15 mL) + extract (5 mL), heated with microwaves (800 W) for 4 min | 208 nm PDI: 0.206 ZP: −24.7 mV | Antibacterial | [33] |
| Withania somnifera | 0.050 M Na2SeO3 + extract (100 mL) | 45–90 nm | Antioxidant, photocatalytic | [34] |
| Amphipterygium glaucum leaves | 0.01 M Na2SeO3 (10 mL) + extract (80 µL), stirred for 24 h at 40 °C | 8.0 nm PDI: 0.236 | Antifungal | [35] |
| Crocus caspius | Na2SeO3 (17.3 g in 100 mL) + extract (5 mL), stirred for 48 h | average 23.47 nm ZP: −44.75 mV | Antimicrobial, antifungal, photocatalytic | [36] |
| Moringa oleifera leaves | 0.05 M Na2SeO3 (5 mL) + extract (20 mL), stirred for 48 h at 37 °C | 20–250 nm | Antioxidant, antidiabetic | [37] |
| Black and green tea, herbs (chamomile, mint) | 0.1 M Na2SeO3 (2.5 mL) + extract (2.5 mL), stirred for 60 min | 54.8–108 nm | Antioxidant | [38] |
| Lycium barbarum + green tea | 25 mM Na2SeO3 (0.5 mL) + extract (2 mg/L) + 1 mL of tea infusion, dialyzed overnight | average 260 nm PDI: 0.242ZP: −24.1 mV | Antioxidant, neuroprotective agent | [40] |
| Black, green, red, and white tea | 0.1 M Na2SeO3 (2.5 mL) + extract (7.7 mL), stirred for 60 min | 3.9–12.5 nm PDI: 0.165–0.381 | Antioxidant | [41] |
| Elaeagnus indica | 50 mM of H2SeO3 + extract (200 mL), stirred for 24 h | av. 14 nm | Antimicrobial, photocatalytic | [42] |
| Asteriscus graveolens aerial parts | 0.01 M H2SeO3 (25 mL) extract (75 mL), incubated for 24 h | 21.6 nm PDI: 1.00 ZP: −24.1 mV | Anticancer | [44] |
| Vaccium artostaphylos L. fruits | 0.1 M Na2SeO3 (9 mL) + extract (1 mL), stirred for 24 h | average 50 nm (SEM) 246 nm (DLS) PDI: 0.267 ZP: −11.5 mV | Antibacterial | [49] |
| Lemon and grapefruit juice and peels | Na2SeO3 (8–12 mM) + extracts, pH 7, stirred at 70 °C for 2 h | 1100–3500 nm (DLS) PDI: 0.127 | Antibacterial | [50] |
| Ginger and onion | Na2SeO3 (10 g) + extract (100 mL), stirred at 60 °C for 3–12 h | 90–114 nm | Antimicrobial | [52] |
| Cacao bean shell (Theobroma cacao L.) | Na2SeO3 (0.14 g) + extract (50 mL), heated in the microwave oven (788.6 W) for 15.6 min | 1–3 nm | Antioxidant | [54] |
| Diospyros montana bark | 0.3 M Na2SeO3 + 10 mL of extract, stirred for 24 h | 120–200 nm (SEM) 20–200 nm (TEM) 140.4 nm (DLS) PDI: 0.418 | Antioxidant, antibacterial, antiproliferative | [59] |
| Terminalia arjuna bark | 0.35 M of Na2SeO3 (10 mL) + extract (10 mL), stirred for 24 h at 37 °C | 100–150 nm ZP: −26.1 mV | Antioxidant, antimicrobial, anticancer | [94] |
| Orthosiphon stamineus leaves + curcumin | 20 mM of Na2SeO3 (45 mL) + 5 mL of extract + curcumin (5 mg/mL), stirred for 30 min | 100 nm | Tissue engineering | [95] |
| Hibiscus esculentus L. | 0.01 M Na2SeO3 + extract (10 mL), stirred for 24 h at 45–50 °C | 50.1 nm (SEM) 266.3 nm (DLS) ZP: 51.3 nm | Anticancer, antibacterial, antifungal | [98] |
3. Applications
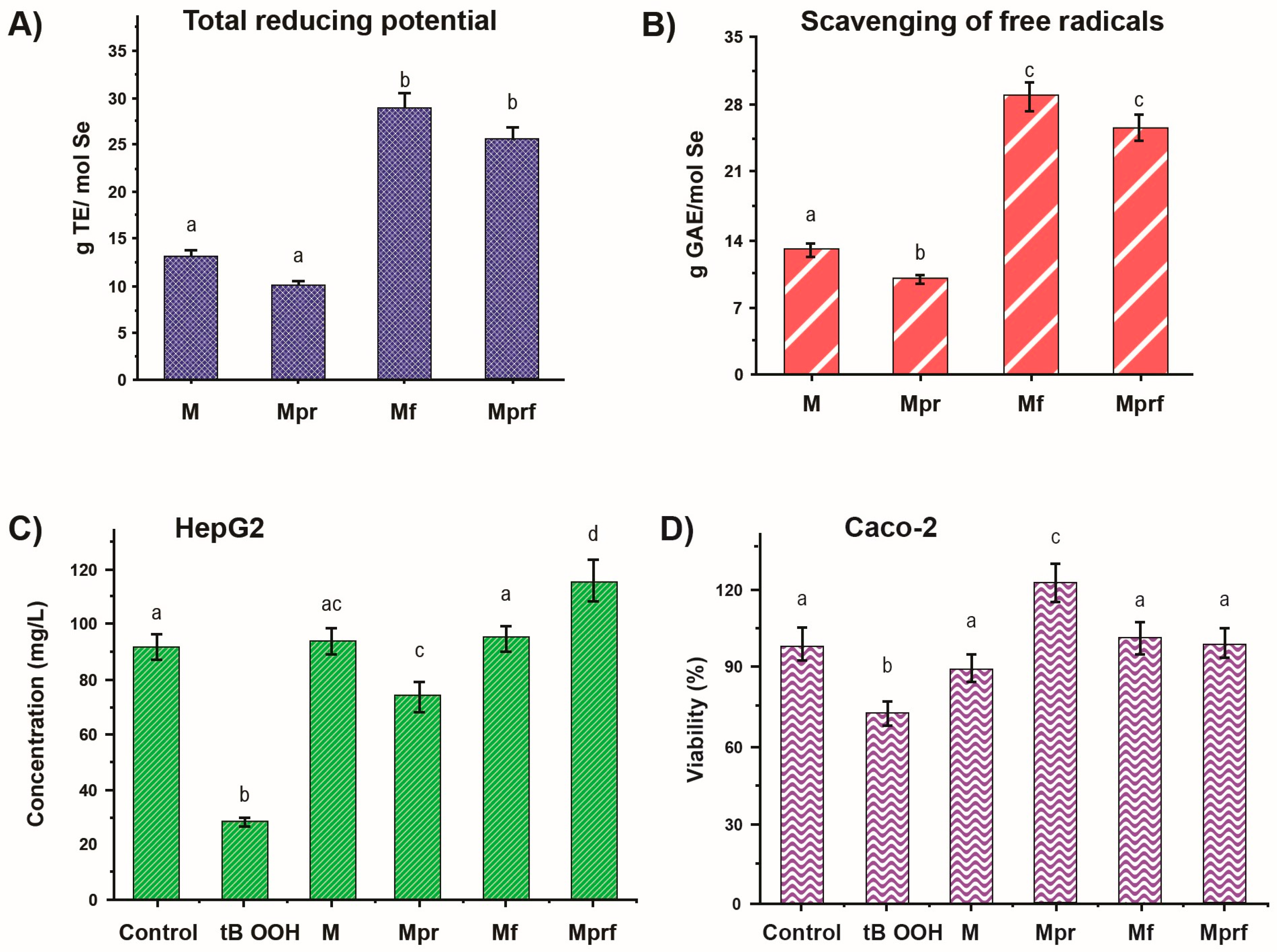
3.1. Antioxidant Activity
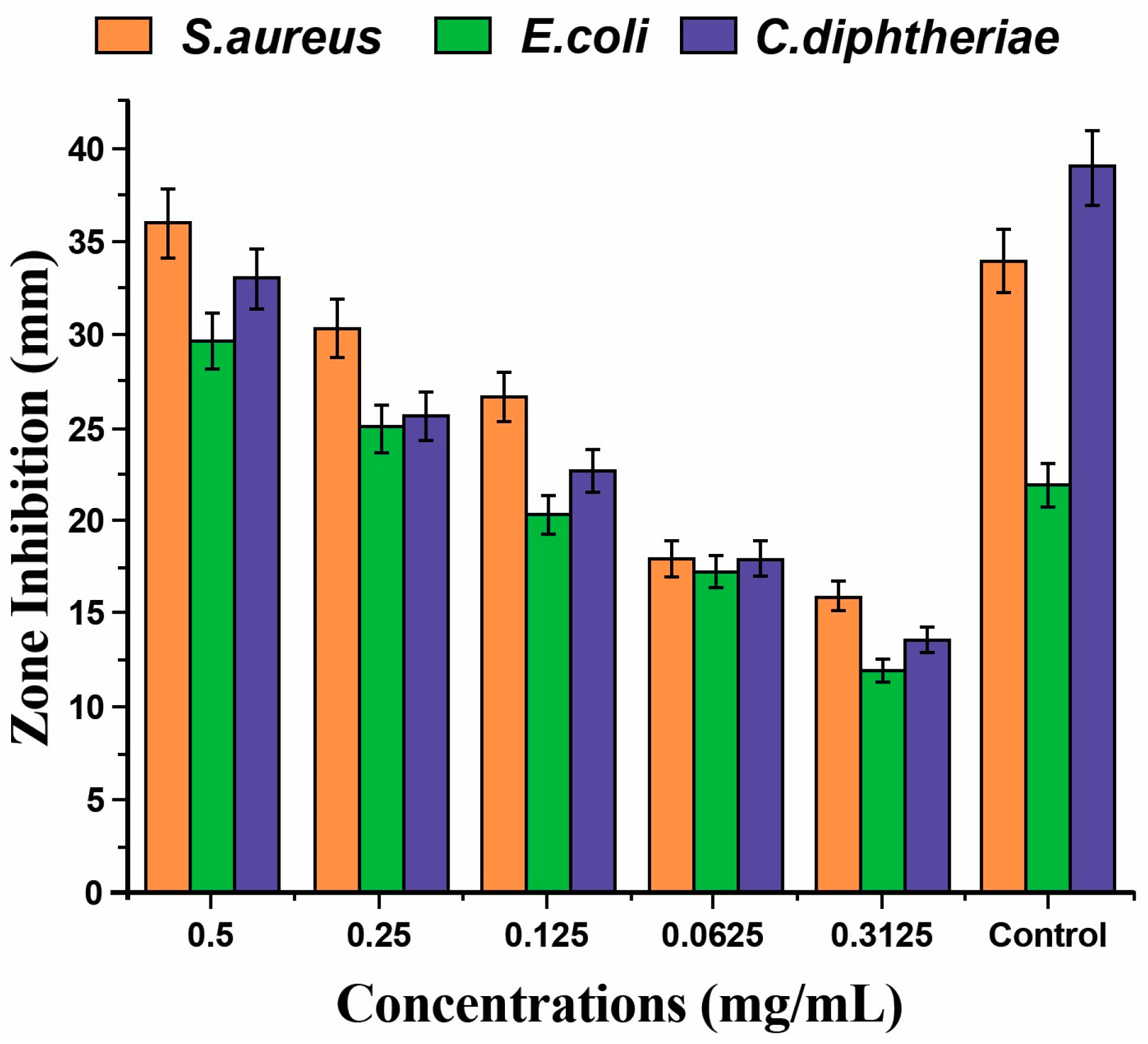
3.2. Antimicrobial Activity
3.3. Antidiabetic Activity
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of nanotechnology in the medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2021, 19, 9–14. [Google Scholar] [CrossRef]
- Moulick, D.; Mukherjee, A.; Das, A.; Roy, A.; Majumdar, A.; Dhar, A.; Pattanaik, B.K.; Chowardhara, B.; Ghosh, D.; Upadhyay, M.K.; et al. Selenium—An environmentally friendly micronutrient in agroecosystem in the modern era: An overview of 50-year findings. Ecotoxicol. Environ. Saf. 2024, 270, 115832. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Cominetti, C.; Seale, L.A. Editorial: Selenium, Human Health and Chronic Disease. Front. Nutr. 2022, 8, 827759. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Sentkowska, A. Selenium Species in Diabetes Mellitus Type 2. Biol. Trace Elem. Res. 2024, 202, 2993–3004. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Mejia, B.S.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.C.; et al. Selenium, antioxidants, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 112, 1642–1652. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzynska, K. The influence of Synthesis Conditions on the Antioxidant Activity of Selenium Nanoparticles. Molecules 2022, 27, 2486. [Google Scholar] [CrossRef]
- Sampath, S.; Sundaram, V.; Manjusha, M.; Dlamini, Z.; Lawrance, A.V. Selenium nanoparticles: A Comprehensive Examination of Synthesis Techniques and Their Diverse Applications in Medical Research and Toxicology Studies. Molecules 2024, 29, 801. [Google Scholar] [CrossRef]
- Miu, B.A.; Dinischitou, A. New Green Approaches in Nanoparticles Synthesis: An Overview. Molecules 2022, 27, 6472. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Selenium Nanoparticles: Green Synthesis and Biomedical Application. Molecules 2023, 28, 8125. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-Synthesized Nanoparticles and Their Role in Silencing the Biofilm Signaling Cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar]
- dos Santos Souza, L.M.; Dibo, M.; Sarmiento, J.J.P.; Seabra, A.B.; Medeiros, L.P.; Lourenço, L.M.; Takayama Kobayashi, R.K.; Nakazato, G. Biosynthesis of selenium nanoparticles using combinations of plant extracts and their antibacterial activity. Curr. Green Chem. 2022, 5, 100303. [Google Scholar]
- Hussain, A.; Lakhan, M.N.; Hanan, A.; Soomro, I.A.; Ahmed, M.; Bibi, F.; Irum Zehra, I. Recent progress on green synthesis of selenium nanoparticles—A review. Mater. Today Sustain. 2023, 23, 100420. [Google Scholar] [CrossRef]
- Zhang, T.; Qi, M.; Wu, Q.; Xiang, P.; Tang, D.; Li, Q. Recent research progress on the synthesis and biological effects of selenium nanoparticles. Front. Nutr. 2023, 10, 1183487. [Google Scholar] [CrossRef]
- Karthik, K.K.; Cheriyan, B.V.; Rajeshkumar, S.; Gopalakrishnan, M. A review on selenium nanoparticles and their biomedical applications. Biomed. Technol. 2024, 6, 61–74. [Google Scholar]
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2022, 12, 467–480. [Google Scholar] [CrossRef]
- Dhanraj, G.; Rajeshkumar, S. Anticarcinogenic Effect of Selenium Nanoparticles Synthesized Using Brassica oleracea. J. Nanomater. 2021, 2021, 8115585. [Google Scholar] [CrossRef]
- Ghaderi, R.S.; Adibian, F.; Sabouri, Z.; Davoodi, J.; Kazemi, M.; Amel Jamehdar, S.; Meshkat, Z.; Soleimanpour, S.; Daroudi, M. Green synthesis of selenium nanoparticle by Abelmoschus esculentus extract and assessment of its antibacterial activity. Mater. Technol. 2022, 37, 1289–1297. [Google Scholar] [CrossRef]
- Golub, N.; Galić, E.; Radić, K.; Jagodić, A.M.; Predović, N.; Katelan, K.; Tesla, L.; Pedisić, S.; Vinković, T.; Vitali Čepo, D. Phyto-Assisted Synthesis of Nanoselenium–Surface Modification and Stabilization by Polyphenols and Pectins Derived from Agricultural Wastes. Foods 2023, 12, 1117. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ul Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 873–886. [Google Scholar] [CrossRef]
- Ranjitha, V.R.; Rai, R. Selenium nanostructure: Progress towards green synthesis and functionalization for biomedicine. J. Pharm. Investig. 2021, 51, 117–135. [Google Scholar] [CrossRef]
- Sentkowska, A.; Konarska, J.; Szmytke, J.; Grudniak, A. Herbal Polyphenols as Selenium Reducers in the Green Synthesis of Selenium Nanoparticles: Antibacterial and Antioxidant Capabilities of the Obtained SeNPs. Molecules 2024, 29, 1686. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological Function of Plant Tannin and Its Application in Animal Health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yang, L.; Wang, Y.; Rensing, C.; Zheng, S. Proteins enriched in charged amino acids control the formation and stabilization of selenium nanoparticles in Comamonas testosterone S44. Sci. Rep. 2018, 8, 4766. [Google Scholar] [CrossRef]
- Sentkowska, A. The potential of traditionally used medicinal plants for the synthesis of selenium nanoparticles. Nat. Prod. Res. 2023, 37, 2055–2059. [Google Scholar] [CrossRef]
- Hashem, A.H.; Salem, S.S. Green and ecofriendly biosynthesis of selenium nanoparticles using Urtica dioica (stinging nettle) leaf extract: Antimicrobial and anticancer activity. Biotechnol. J. 2021, 17, e2100432. [Google Scholar] [CrossRef]
- Sheikhlou, K.; Allahyari, S.; Sabouri, S.; Najian, Y.; Jafarizadeh-Malmiri, H. Walnut leaf extract-based green synthesis of selenium nanoparticles via microwave irradiation and their characteristics assessment. Open Agric. 2020, 5, 227–235. [Google Scholar] [CrossRef]
- Alagesan, V.; Venugopal, S. Green synthesis of selenium nanoparticles using leaves extract of Withania somnifera and its biological applications and photocatalytic activities. BioNanoScience 2019, 9, 105–116. [Google Scholar] [CrossRef]
- Lazcano-Ramírez, H.G.; Garza-García, J.J.O.; Hernández-Díaz, J.A.; León-Morales, J.M.; Macías-Sandoval, A.S.; García-Morales, S. Antifungal Activity of Selenium Nanoparticles Obtained by Plant-Mediated Synthesis. Antibiotics 2023, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.R.; Abbastabar, M.; Nosratabadi, M.; Ebrahimzadeh, M.A. High antimicrobial, cytotoxicity, and catalytic activities of biosynthesized selenium nanoparticles using Crocus caspius extract. Arab. J. Chem. 2023, 16, 104705. [Google Scholar] [CrossRef]
- Tarmizi, A.A.; Nik Ramli, N.N.; Adam, S.H.; Abdul Mutalib, M.; Mokhtar, M.H.; Tang, S.G.H. Phytofabrication of Selenium Nanoparticles with Moringa oleifera (MO-SeNPs) and Exploring Its Antioxidant and Antidiabetic Potential. Molecules 2023, 28, 5322. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzynska, K. Antioxidant Properties of Selenium Nanoparticles Synthesized Using Tea and Herb Water Extracts. Appl. Sci. 2023, 13, 1071. [Google Scholar] [CrossRef]
- Lin, X.; Mu, J.; Chen, Z.; Zhang, Y.; Ye, X.; Gao, X.; Chen, W.; Luo, Y.; Li, B. Stabilization and functionalization of selenium nanoparticles mediated by green tea and Pu-Erh tea polysaccharides. Ind. Crops Prod. 2023, 194, 116312. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Ding, D.; Zhang, L.; Luis Muehlmann, A.; Deng, S.; Wang, X.; Li, W.; Zhang, W. Synthesis and antioxidant properties of Lycium barbarum polysaccharides capped selenium nanoparticles using tea extract. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1463–1470. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzynska, K. Does the Type Matter? Verification of Different Tea Types’ Potential in the Synthesis of SeNPs. Antioxidants 2022, 11, 2489. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, R.; Jafarizadeh-Malmiri, H. Green approach in gold, silver and selenium nanoparticles using coffee bean extract. Open Agric. 2020, 5, 761–767. [Google Scholar] [CrossRef]
- Baluken, P.; Kamiloglu, A.; Kutlu, N. Green Synthesis of Selenium Nanoparticles using Green Coffee Beans: An Optimization Study. Chem. Biodivers. 2024, 21, e202301250. [Google Scholar] [CrossRef] [PubMed]
- Aly Khalil, A.M.; Saied, E.; Mekky, A.E.; Saleh, A.M.; Al Zoubi, O.M.; Hashem, A.H. Green biosynthesis of bimetallic selenium-gold nanoparticles using Pluchea indica leaves and their biological applications. Front. Bioeng. Biotechnol. 2024, 11, 1294170. [Google Scholar] [CrossRef] [PubMed]
- Zeebaree, S.Y.S.; Zeebaree, A.Y.S.; Zebari, O.I.H. Diagnosis of the multiple effects of selenium nanoparticles decorated by Asteriscus graveolens components in inhibiting HepG2 cell proliferation. Sustain. Chem. Pharm. 2020, 15, 100210. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.C.; Jiang, W.; Hu, J.N. Angelica sinensis polysaccharides modified selenium nanoparticles for effective prevention of acute liver injury. Int. J. Biol. Macromol. 2024, 263, 130321. [Google Scholar] [CrossRef] [PubMed]
- Anu, K.; Devanesan, S.; Prasanth, R.; AlSalhi, M.S.; Ajithkumar, S.; Singaravelu, G. Biogenesis of selenium nanoparticles and their anti-leukemia activity. J. King Saud. Univ. 2020, 32, 2520–2526. [Google Scholar] [CrossRef]
- Hassan, H.U.; Raja, N.I.; Abasi, F.; Mehmood, A.; Qureshi, R.; Manzoor, Z.; Shahbaz, M.; Proćków, J. Comparative Study of Antimicrobial and Antioxidant Potential of Olea ferruginea Fruit Extract and Its Mediated Selenium Nanoparticles. Molecules 2022, 27, 5194. [Google Scholar] [CrossRef]
- Khudier, M.A.A.; Hammadi, H.A.; Atyia, H.T.; Al-Karagoly, H.; Albukhaty, S.; Sulaiman, G.M.; Dewir, Y.H.; Mahood, H.B. Antibacterial activity of green synthesized selenium nanoparticles using Vaccinium arctostaphylos (L.) fruit extract. Cogent Food Agric. 2023, 9, 2245612. [Google Scholar] [CrossRef]
- Alvi, G.B.; Iqbal, M.S.; Saeed Ghaith, M.M.; Bilal Ahmed, A.H.; Qadir, M.I. Biogenic selenium nanoparticles (SeNPs) from citrus fruit have anti-bacterial activities. Sci. Rep. 2021, 11, 4811. [Google Scholar] [CrossRef]
- Sribenjarat, P.; Jirakanjanakit, N.; Jirasripogpun, K. Selenium nanoparticles biosynthesized by garlic extract as antimicrobial agent. Sci. Eng. Health Stud. 2020, 14, 22–31. [Google Scholar]
- Menon, S.; Shrudhi Devi, K.S.; Agarval, H.; Shanmugam, V.K. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloids Interface Sci. Commun. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Martinez-Esquivias, F.; Guzmán-Flores, J.M.; Perez-Larios, A. Antimicrobial activity of green synthesized Se nanoparticles using ginger and onion extract: A laboratory and silico analysis. J. Part. Sci. Technol. 2023, 41, 319–329. [Google Scholar] [CrossRef]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef]
- Mellinas, C.; Jiménez, A.; Garrigós, M.C. Microwave-assisted green synthesis and antioxidant activity of selenium nanoparticles using Theobroma cacao L. bean shell extract. Molecules 2019, 4, 4048. [Google Scholar] [CrossRef]
- Salem, M.F.; Abd-Elraoof, W.A.; Tayel, A.A.; Alzuaibr, F.M.; Abonama, O.M. Antifungal application of biosynthesized selenium nanoparticles with pomegranate peels and nanochitosan as edible coatings for citrus green mold protection. J. Nanobiotechnol. 2022, 20, 182. [Google Scholar] [CrossRef]
- Salem, S.S.; Badawy, M.S.E.M.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green Biosynthesis of Selenium Nanoparticles Using Orange Peel Waste: Characterization, Antibacterial and Antibiofilm Activities against Multidrug-Resistant Bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef]
- Ali, B.M.H.; Lamia, A.M.; Almashhedy, L.A.M. Green Synthesis and Characterization of Selenium Nanoparticles Using Aqueous Extract of Peel Solanum melongena L. IOP Conf. Ser. Earth Environ. Sci. 2023, 1158, 10200. [Google Scholar] [CrossRef]
- Puri, A.; Patil, S. Biogenic Synthesis of Selenium Nanoparticles using Diospyros montana Bark Extract: Characterization, Antioxidant, Antibacterial, and Antiproliferative Activity. Biosci. Biotechnol. Res. Asia 2022, 19, 423–441. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin–Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent advances in green synthesized nanoparticles: From production to application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Pradnya, B.; Nikam, M.; Salunkhe, J.D.; Minkina, M.; Rajput, V.D.; Kim, B.S.; Satish, J.; Patil, V. A review on green synthesis and recent applications of red nano selenium. Results Chem. 2022, 4, 100581. [Google Scholar]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium Nanoparticles for Biomedical Applications: From Development and Characterization to Therapeutics. Adv. Helthc. Mater. 2021, 10, 2100598. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esquivias, F.; Gutiérrez-Angulo, M.; Pérez-Larios, A.; Sánchez-Burgos, J.A.; Becerra-Ruiz, J.S.; Guzmán-Flores, J.M. Anticancer Activity of Selenium Nanoparticles In Vitro Studies. Anticancer Agents Med. Chem. 2022, 22, 658–1673. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Chen, Y.; Gandahi, J.A.; Quazi, I.H.; Sun, J.; Wang, T.; Liu, Z.; Zou, H. Cross-Talk Between Selenium Nanoparticles and Cancer Treatment through Autophagy. Biol. Trace Elem. Res. 2024, 202, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Deepa, T.; Mohan, S.; Manimaran, P. A crucial role of selenium nanoparticles for future perspectives. Results Chem. 2022, 4, 100367. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, J.; Xu, J.F.; Pi, J. The Advancing of Selenium Nanoparticles Against Infectious Diseases. Front. Pharmacol. 2021, 12, 682284. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Wang, X.; Han, L.; Lu, X. The Developments of Surface-Functionalized Selenium Nanoparticles and Their Applications in Brain Diseases Therapy. Biomimetics 2023, 8, 259. [Google Scholar] [CrossRef]
- Pereira, A.G.; Gerolis, L.G.L.; Gonçalves, L.S.; Costa Moreira, L.M.; Gastelois, P.L.; Neves, M.J. Radiolytic synthesis and characterization of selenium nanoparticles: Comparative biosafety evaluation with selenite and ionizing radiation. World J. Microbiol. Biotechnol. 2022, 38, 33. [Google Scholar] [CrossRef]
- Jagadeesh, P.; Rangappa, S.M.; Siengchin, S. Advanced characterization techniques for nanostructured materials in biomedical applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 122–143. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, H.S.N.; Liyanage, S.H.; Rathnayake, K.; Patel, U.; Yan, M. Analytical Methods for Characterization of Nanomaterial Surfaces. Anal Chem. 2021, 93, 1889–1911. [Google Scholar] [CrossRef]
- Srivastava, N.; Mukhopadhyay, M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst. Eng. 2015, 38, 1723–1730. [Google Scholar] [CrossRef]
- Lin, Z.H.; Chris-Wang, C.R. Evidence on the size-dependent absorption spectral evolution of selenium nanoparticles. Mater. Chem. Phys. 2005, 92, 591–594. [Google Scholar] [CrossRef]
- Sarkar, J.; Mridha, D.; Davoodbasha, M.A.; Banerjee, J.; Chanda, S.; Ray, K.; Roychowdhury, T.; Acharya, K.; Sarkar, J. A State of the Art Systemic Review on Selenium Nanoparticles: Mechanisms and Factors Influencing Biogenesis and Its Potential Applications. Biol. Trace Elem. Res. 2023, 201, 5000–5036. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Qiu, W.Y.; Sun, L.; Ding, Z.C.; Ya, J.K. Preparation, characterization, and antioxidant capacities of selenium nanoparticles stabilized using polysaccharide-protein complexes from Corbicula fluminea. Food Biosen. 2018, 26, 177–184. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Liu, X.; Han, Z.; Chen, S. Constructing Selenium Nanoparticles with Enhanced Storage Stability and Antioxidant Activities via Conformational Transition of Curdlan. Foods 2023, 12, 563. [Google Scholar] [CrossRef]
- Cheng, B.; Liu, J.; Li, X.; Yue, L.; Cao, X.; Li, J.; Wang, C.; Wang, Z. Bioavailability of selenium nanoparticles in soil and plant: The role of particle size. Environ. Exp. Bot. 2024, 220, 105682. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Nobbmann, U.L. Polydispersity—What Does It Mean for DLS and Chromatography. 2014. Available online: https://www.malvernpanalytical.com (accessed on 10 May 2024).
- Wang, Y.; Pi, C.; Feng, X.; Hou, Y.; Zhao, L.; Wei, Y. The influence of nanoparticle properties on oral bioavailability of drugs. Int. J. Nanomed. 2020, 15, 6295–6310. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Q.; Zeng, G.; Yang, G.; Wang, G.; Zhou, L. A Review of Selenium (Se) Nanoparticles: From Synthesis to Applications. Part. Part. Syst. Charact. 2023, 40, 2300098. [Google Scholar] [CrossRef]
- Chen, N.; Yao, P.; Zhang, W.; Zhang, Y.; Xin, N.; Wei, H.; Zhang, T.; Zhao, C. Selenium nanoparticles: Enhanced nutrition and beyond. Crit. Rev. Food Sci. Nutr. 2023, 63, 12360–12371. [Google Scholar] [CrossRef] [PubMed]
- Sindhu Devi, M.; Srinivasan, S.; Muthuvel, A. Selenium nanomaterial is a promising nanotechnology for biomedical and environmental remediation: A detailed review. Biocatal. Agric. Biotechnol. 2023, 51, 102766. [Google Scholar]
- Chauhan, P.; Chaudhary, S. Role of surface modification on selenium nanoparticles: Enumerating the optical, thermal and structural properties. Opt. Mater. 2019, 97, 109380. [Google Scholar] [CrossRef]
- Indhira, D.; Aruna, A.; Manikandan, K.; Albeshr, M.F.; Alrefaei, A.F.; Vinayagam, R.; Kathirvel, A.; Priyan, S.R.; Kumar, G.S.; Srinivasan, R. Antimicrobial and Photocatalytic Activities of Selenium Nanoparticles Synthesized from Elaeagnus indica Leaf Extract. Processes 2023, 11, 1107. [Google Scholar] [CrossRef]
- Memon, S.; Devi, K.S.; Santiya, R.; Rajeshkumar, S.; Kumar, V. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf. B Biointerfaces 2018, 170, 280–292. [Google Scholar] [CrossRef]
- Buacheen, P.; Chaipuang, A.; Karinchai, J.; Nuchuchua, O.; Imsumran, A.; Wongnoppavich, A.; Pimpha, N.; Pitchakarn, P. Stabilization of Antioxidant and Anti-Inflammatory Activities of Nano-Selenium Using Anoectochilus burmannicus Extract as a Potential Novel Functional Ingredient. Nutrients 2023, 15, 1018. [Google Scholar] [CrossRef]
- Puri, A.; Mohite, P.; Patil, S.; Chidrawar, V.R.; Ushir, Y.V.; Dodiya, R.; Singh, S. Facile green synthesis and characterization of Terminalia arjuna bark phenolic–selenium nanogel: A biocompatible and green nanobiomaterial for multifaceted biological applications. Front. Chem. 2023, 11, 1273360. [Google Scholar] [CrossRef]
- Naaziya, M.; Biju, T.S.; Prakash, F.A.; Veeraraghavan, V.P.; Gayathri, R.; Kavitha, S. Synthesis, Characterization and in-vitro Biological Studies of Curcumin decorated Biogenic Selenium Nanoparticles. NanoLife 2024, 14, 2350013. [Google Scholar] [CrossRef]
- Galić, E.; Radić, K.; Golub, N.; Mlinar, J.; Čepo, D.V.; Vinković, T. Functionalization of selenium nanoparticles with olive polyphenols—Impact on toxicity and antioxidative activity. Acta Pharm. 2023, 73, 617–631. [Google Scholar] [CrossRef]
- Lunkov, A.; Konovalova, M.; Shagdarova, B.; Zhuikova, Y.; Il’ina, A.; Varlamov, V. Synthesis of Selenium Nanoparticles Modified by Quaternary Chitosan Covalently Bonded with Gallic Acid. Polymers 2023, 15, 2123. [Google Scholar] [CrossRef]
- Tuyen, N.N.K.; Huy, V.K.; Duy, N.H.; Au, H.; Nan, N.M.; Dat, N.M.; Huong, Q.T.; Tang, N.P.; Anh, N.P.; Thy, L.T.; et al. Green Synthesis of Selenium Nanorods Using Muntigia calabura Leaf Extract: Effect of pH on Characterization and Bioactivities. Waste Biomass Valor. 2024, 15, 1987–1998. [Google Scholar] [CrossRef]
- Qiu, W.Y.; Wang, Y.Y.; Wang, M.; Yan, J.K. Construction, stability, and enhanced antioxidant activity of pectin-decorated selenium nanoparticles. Colloids Surf. B Biointerfaces 2018, 170, 692–700. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Moradsomarein, M.; Lalerdi, F.S.; Alizadeh, S.R. Biogenic synthesis of selenium nanoparticles using Hibiscus esculentus L. extract: Catalytic degradation of organic dye and its anticancer, antibacterial and antifungal activities. Eur. J. Chem. 2023, 14, 144–154. [Google Scholar] [CrossRef]
- Xiao, X.; Deng, H.; Lin, X.; Ali, A.S.M.; Viscardi, A.; Guo, Z.; Qiao, L.; He, Y.; Han, J. Selenium nanoparticles: Properties, preparation methods, and therapeutic applications. Chem. Biol. Interact. 2023, 378, 110483. [Google Scholar] [CrossRef]
- Azmoonfar, R.; Moslehi, M.; Shahbazi-Gahrouei, D. Radioprotective Effect of Selenium Nanoparticles: A Mini Review. IET Nanobiotechnol. 2024, 2024, 5538107. [Google Scholar] [CrossRef]
- Karami, M.; Asri-Rezaie, S.; Dormanesh, B.; Nazarizadeh, A. Comparative study of radioprotective effects of selenium nanoparticles and sodium selenite in irradiation induced nephropathy of mice model. Int. J. Radiat. Biol. 2017, 94, 17–27. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Bahreyni Toossi, M.T.; Vaziri-Nezamdoost, F.; Khademi, S.; Darroudi, M.; Azimian, H. Comparison of Radioprotective Effects of Colloidal Synthesis of Selenium Nanoparticles in Aqueous Rosemary Extract and Rosemary in Chinese Hamster Ovary (CHO) Cells. J. Nanostruct. 2022, 12, 711–717. [Google Scholar]
- Mostafavi, E.; Medina-Cruz, D.; Truong, L.B.; Kaushik, A.; Iravani, S. Selenium-based nanomaterials for biosensing applications. Mater. Adv. 2022, 3, 7742–7756. [Google Scholar] [CrossRef] [PubMed]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ye, C.; Ma, H.; Zhang, Z.; Wang, J.; Zhang, Z.H.; Zhao, X.; Ho, C.T. Research Advances in Preparation, Stability, Application, and Possible Risks of Nanoselenium: Focus on Food and Food-Related Fields. J. Agric. Food Chem. 2023, 71, 8731–8745. [Google Scholar] [CrossRef]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamini, B.C. Advances in green synthesis of selenium nanoparticles and their application in food packaging. Food Sci. Technol. 2021, 56, 2640–2650. [Google Scholar] [CrossRef]
- Abd-Elraoof, W.A.; Tayel, A.A.; El-Far, S.W.; Walid Abukhatwah, O.M.; Diab, A.M.; Abonama, O.M.; Assas, M.A.; Abdella, A. Characterization and antimicrobial activity of a chitosan-selenium nanocomposite biosynthesized using Posidonia oceanica. RSC Adv. 2023, 13, 26001–26014. [Google Scholar] [CrossRef] [PubMed]
- Kuršvietiené, L.; Mongirdiené, A.; Bernatoniené, J.; Šulinskiené, J.; Stanevičiené, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, W.; Wang, J. Theranostic applications of selenium nanomedicines against lung cancer. J. Nanobiotechnol. 2023, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- İpek, P.; Baran, A.; Hatipoğlu, A.; Baran, M.F. Cytotoxic potential of selenium nanoparticles (SeNPs) derived from leaf extract of Mentha longifolia L. Int. J. Agric. Environ. Food Sci. 2024, 8, 169–175. [Google Scholar] [CrossRef]
- Dana, P.; Pimpha, N.; Chaipuang, A.; Thumrongsiri, N.; Tanyapanyachon, P.; Taweechaipaisankul, A.; Chonniyom, W.; Watcharadulyarat, N.; Sathornsumetee, S.; Saengkrit, N. Inhibiting Metastasis and Improving Chemosensitivity via Chitosan-Coated Selenium Nanoparticles for Brain Cancer Therapy. Nanomaterials 2022, 12, 2606. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Biswas, J.; Sen, T.; Bhattacharya, S. Chemoprotective and chemosensitizing properties of selenium nanoparticle (Nano-Se) during adjuvant therapy with cyclophosphamide in tumour-bearing mice. Mol. Cell. Biochem. 2017, 424, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Gharbavi, M.; Johari, B.; Mousazadeh, N.; Rahimi, B.; Leilan, M.P.; Eslami, S.S.; Sharafi, A. Hybrid of niosomes and bio-synthesized selenium nanoparticles as a novel approach in drug delivery for cancer treatment. Mol. Biol. Rep. 2020, 47, 6517–6529. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.U. Biomedical Potential of Plant-Based Selenium Nanoparticles: A Comprehensive Review on Therapeutic and Mechanistic Aspects. Int. J. Nanomed. 2021, 16, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Al-Otaibi, W.A.; Saber, T.; Al Motwaa, S.M.; Alshallash, K.S.; Elhady, M.; Badr, N.F.; Abdel-Rahman, M.A. Antimicrobial, Antiviral, and In-Vitro Cytotoxicity and Mosquitocidal Activities of Portulaca oleracea-Based Green Synthesis of Selenium Nanoparticles. J. Funct. Biomater. 2022, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Díaz, J.A.; Garza-García, J.J.; León-Morales, J.M.; Zamudio-Ojeda, A.; Arratia-Quijada, J.; Velázquez-Juárez, G.; López-Velázquez, J.C.; García-Morales, S. Antibacterial Activity of Biosynthesized Selenium Nanoparticles Using Extracts of Calendula officinalis against Potentially Clinical Bacterial Strains. Molecules 2021, 26, 5929. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.R.; Tabatabaei, R.R.; Aminian, F.; Ranjbar, S.; Ashrafi, F.; Ranjbar, R. Antibacterial and antibiofilm effects of green synthesized selenium nanoparticles on clinical Klebsiella pneumoniae isolates. J. Basic Microbiol. 2023, 63, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.B.; Medina-Cruz, D.; Mostafavi, E.; Rabiee, N. Selenium Nanomaterials to Combat Antimicrobial Resistance. Molecules 2021, 26, 3611. [Google Scholar] [CrossRef] [PubMed]
- Serov, D.A.; Khabatova, V.; Vodeneev, V.; Li, R.; Gudkov, S.V. A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials 2023, 16, 5363. [Google Scholar] [CrossRef] [PubMed]
- Pescuma, M.; Aparicio, F.; Zysler, R.D.; Lima, E.; Zapata, C.; Marfetán, J.A.; Vélez, M.L.; Ordoñez, O.F. Biogenic selenium nanoparticles with antifungal activity against the wood-rotting fungus Oligoporus pelliculosus. Biotechnol. Rep. 2023, 37, e00787. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mehak, A.; Fatima, N.; Ajmal, M.; Ali, K.; Mustafa, N.; Abasi, F. Antifungal activity of green synthesized selenium nanoparticles and their effect on physiological, biochemical, and antioxidant defense system of mango under mango malformation disease. PLoS ONE 2023, 18, e0274679. [Google Scholar] [CrossRef]
- Macías Sánchez, K.L.; González Martínez, H.D.R.; Carrera Cerritos, R.; Martínez Espinosa, J.C. In Vitro Evaluation of the Antifungal Effect of AgNPs on Fusarium oxysporum f. sp. lycopersici. Nanomaterials 2023, 13, 1274. [Google Scholar] [CrossRef]
- Li, F.; Huang, T.; Pasic, P.; Easton, C.D.; Voelcker, N.H.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J.; Thissen, H. One step antimicrobial coatings for medical device applications based on low fouling polymers containing selenium nanoparticles. Chem. Eng. J. 2023, 467, 143546. [Google Scholar] [CrossRef]
- Wang, Q.; Larese-Casanova, P.; Webster, T.J. Inhibition of various gram-positive and gram-negative bacteria growth on selenium nanoparticle coated paper towels. Int. J. Nanomed. 2015, 10, 2885–2894. [Google Scholar]
- Huang, J.; Xie, L.; Song, A.; Zhang, C. Selenium status and its antioxidant role in metabolic diseases. Oxid. Med. Cell Longev. 2022, 6, 7009863. [Google Scholar] [CrossRef]
- Steinbremmer, H.; Dundas, L.H.; Rayman, M.P. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022, 50, 10222. [Google Scholar]
- Gutiérrez, R.M.P.; Gómez, J.T.; Urby, R.B.; Soto, J.G.C.; Parra, H.R. Evaluation of Diabetes Effects of Selenium Nanoparticles Synthesized from a Mixture of Luteolin and Diosmin on Streptozotocin-Induced Type 2 Diabetes in Mice. Molecules 2022, 27, 5642. [Google Scholar] [CrossRef]
- Pérez Gutiérrez, R.M.; Gómez, J.T.; Martínez Jerónimo, F.F.; Paredes-Carrera, S.P.; Sánchez-Ochoa, J.C. Effects of Selenium Nanoparticles Using Potential Natural Compounds Naringenin and Baicalin for Diabetes. Biointerface Res. Appl. Chem. 2023, 13, 597. [Google Scholar]
- Abozaid, O.A.R.; El-Sonbaty, S.M.; Hamam, N.M.; Farrag, M.A.; Kodous, A.S. Chitosan-encapsulated nano-selenium targeting TCF7L2, PPARγ, and CAPN10 genes in diabetic rats. Biol. Trace Elem. Res. 2022, 201, 306–323. [Google Scholar] [CrossRef]
- Anuse, S.S.; Sumathi, V.; Uma, C.; Sangeetha, D.; Sivagurunathan, P.; Kumar, D.J.M. Antidiabetic effect of Acacia catechu mediated selenium nanoparticles. Uttar Pradesh Zool. Soc. 2022, 43, 115–120. [Google Scholar] [CrossRef]
- Pérez Gutiérrez, R.M.; Soto Contreras, J.G.; Martínez Jerónimo, F.F.; de la Luz Corea Téllez, M.; Borja-Urby, R. Assessing the Ameliorative Effect of Selenium Cinnamomum verum, Origanum majorana, and Origanum vulgare Nanoparticles in Diabetic Zebrafish (Danio rerio). Plants 2022, 11, 893. [Google Scholar] [CrossRef]
- Puri, A.; Mohite, P.; Ansari, Y.; Mukerjee, N.; Alharbi, H.M.; Upaganlawar, A.; Thorat, N. Plant-derived selenium nanoparticles: Investigating unique morphologies, enhancing therapeutic uses, and leading the way in tailored medical treatments. Mater. Adv. 2024, 5, 3602. [Google Scholar] [CrossRef]
- Nyabadza, A.; McCarthy, F.; Makhesana, M.; Heidarinassab, S.; Plouze, A.; Vazquez, M.; Brabazon, D. A review of physical, chemical and biological synthesis methods of bimetallic nanoparticles and applications in sensing, water treatment, biomedicine, catalysis and hydrogen storage. Adv. Colloid Interface Sci. 2023, 321, 103010. [Google Scholar] [CrossRef] [PubMed]
- Malyugina, S.; Skalickova, S.; Skladanka, J.; Slama, P.; Horky, P. Biogenic Selenium Nanoparticles in Animal Nutrition: A Review. Agriculture 2021, 11, 1244. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Z.; Hu, H.; Zhou, Q.; Liu, W.; Li, X.; Liu, Z.; Wang, Y.; Ma, Y. A point-of-care selenium nanoparticle-based test for the combined detection of anti-SARS-CoV-2 IgM and IgG in human serum and blood. Lab A Chip 2020, 20, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamin, B.C. Selenium nanoparticles–enhanced potato starch film for active food packaging application. Int. J. Food Sci. Technol. 2022, 55, 6512–6521. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and Use of Selenium Nanoparticles as Fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef] [PubMed]
- Urbankova, L.; Skalickova, S.; Pribilova, M.; Ridoskova, A.; Pelcova, P.; Skladanka, J.; Horky, P. Effects of Sub-Lethal Doses of Selenium Nanoparticles on the Health Status of Rats. Toxics 2021, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Muthukumaran, A. A novel one-pot green synthesis of selenium nanoparticles and evaluation of its toxicity in zebrafish embryos. Artif. Cells Nanomed. Biotechnol. 2016, 44, 471–477. [Google Scholar] [CrossRef]
- Tehrani, H.A.M.; Keyhanfar, M.; Behbahani, M. Synthesis and characterization of algae-coated selenium nanoparticles as a novel antibacterial agent against Vibrio harveyi, a Penaeus vannamei pathogen, aquaculture. Aquaculture 2020, 534, 736260. [Google Scholar] [CrossRef]

| Strain | Plant Material | Activity | Ref. |
|---|---|---|---|
| Gram-positive bacteria | |||
| Staphylococcus aureus | Abelmoschus esculentus | MIC = 32 µg/mL | [22] |
| Withanua somnifera | ZOI = 19.66 mm | [34] | |
| Crocus caspius | MIC = 17.08 µg/m | [36] | |
| Coffee beans | ZOI = 8 mm | [42] | |
| Pluchea indica | MIC = 31.25 µg/m | [44] | |
| Olea ferruginea | MIC = 11.33 µg/mL | [48] | |
| Vaccinium arctostaphylos | ZOI = 36 mm | [49] | |
| Onion | MIC = 10.67 µg/mL | [53] | |
| Dispros montana | ZOI = 34.16 mm | [59] | |
| Bacillus subtilis | Withanua somnifera | ZOI = 12 mm | [30] |
| Pluchea indica | MIC = 3.9 µg/m | [44] | |
| Cassica auriculata | ZOI = 27 mm | [47] | |
| Olea ferruginea | MIC = 11.33 µg/mL | [48] | |
| Grapefruit juice | ZOI = 19 mm | [50] | |
| Lemon juice | ZOI = 24 mm | [50] | |
| Dispros montana | ZOI = 44.14 | [59] | |
| Streptococcus mutants | Abelmoschus esculentus | MIC = 128 µg/mL | [22] |
| Enterococcus faecalis | Crocus caspius | MIC = 136.66 µg/mL | [36] |
| Corynebacterium diphtheriae | Vaccinium arctostaphylos | ZOI = 25.77 mm | [49] |
| Acinetobacter baumannii | Crocus caspius | MIC = 17.08 µg/mL | [36] |
| Micrococcus luteus | Grapefruit juice | ZOI = 18 mm | [50] |
| Lemon juice | ZOI = 22 mm | [50] | |
| Gram-negative bacteria | |||
| Escherichia coli | Abelmoschus esculentus | MIC = 256 µg/mL | [22] |
| Crocus caspius | MIC = 68.33 µg/mL | [36] | |
| Coffee beans | ZOI = 7.1 mm | [42] | |
| Pluchea indica | ZOI = 20.2 mm | [44] | |
| Cassica auriculata | ZOI = 29 mm | [47] | |
| Grapefruit juice | ZOI = 19 mm | [50] | |
| Lemon juice | ZOI = 24 mm | [50] | |
| Klebsiella pneumonia | Withanua somnifera | ZOI = 12.0 mm | [34] |
| Grapefruit juice | ZOI = 20 mm | [50] | |
| Lemon juice | ZOI = 24 mm | [50] | |
| Dispros montana | ZOI = 48.0 mm | [59] | |
| Pseudomonas aeruginosa | Abelmoschus esculentus | MIC = 128 µg/mL | [23] |
| Crocus caspius | MIC = 34.17 µg/ | [36] | |
| Pluchea indica | MIC = 15.62 µg/m | [44] | |
| Proteus mirabilis | Crocus caspius | MIC = 136.66 µg/mL | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyrzynska, K. Plant Extracts for Production of Functionalized Selenium Nanoparticles. Materials 2024, 17, 3748. https://doi.org/10.3390/ma17153748
Pyrzynska K. Plant Extracts for Production of Functionalized Selenium Nanoparticles. Materials. 2024; 17(15):3748. https://doi.org/10.3390/ma17153748
Chicago/Turabian StylePyrzynska, Krystyna. 2024. "Plant Extracts for Production of Functionalized Selenium Nanoparticles" Materials 17, no. 15: 3748. https://doi.org/10.3390/ma17153748
APA StylePyrzynska, K. (2024). Plant Extracts for Production of Functionalized Selenium Nanoparticles. Materials, 17(15), 3748. https://doi.org/10.3390/ma17153748






